Abstract
Mitochondrial division inhibitor-1 (mdivi-1), a non-specific inhibitor of Drp1-dependent mitochondrial fission, is neuroprotective in numerous preclinical disease models. These include rodent models of Alzheimer’s disease and ischemic or traumatic brain injury. Among its Drp1-independent actions, the compound was found to suppress mitochondrial Complex I-dependent respiration but with less resultant mitochondrial reactive oxygen species (ROS) emission compared with the classical Complex I inhibitor rotenone. We employed two different methods of quantifying Trolox-equivalent antioxidant capacity (TEAC) to test the prediction that mdivi-1 can directly scavenge free radicals. Mdivi-1 exhibited moderate antioxidant activity in the 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate) (ABTS) assay. Half-maximal ABTS radical depletion was observed at ~25 μM mdivi-1, equivalent to that achieved by ~12.5 μM Trolox. Mdivi-1 also showed antioxidant activity in the α, α-diphenyl-β-picrylhydrazyl (DPPH) assay. However, mdivi-1 exhibited a reduced capacity to deplete the DPPH radical, which has a more sterically hindered radical site compared with ABTS, with 25 μM mdivi-1 displaying only 0.8 μM Trolox equivalency. Both assays indicate that mdivi-1 possesses biochemical antioxidant activity but with modest potency relative to the vitamin E analog Trolox. Future studies are needed to evaluate whether the ability of mdivi-1 to directly scavenge free radicals contributes to its mechanisms of neuroprotection.
Keywords:
ROS; oxidative stress; free radical; scavenger; superoxide; oxygen; mitochondria; Complex I; mitochondrial fission; neurons 1. Introduction
Mitochondrial division inhibitor-1 (mdivi-1) crosses the blood–brain barrier and confers neuroprotection in animal models of traumatic brain injury [1,2,3,4] and focal and global ischemia [5,6,7], with protective effects in the latter comparable to that of hypothermia [7]. As mdivi-1 also mitigates chronic neurodegeneration in many additional disease models, e.g., of Alzheimer’s disease [8] and Parkinson’s disease [9,10], there is widespread interest in this compound as a preclinical drug candidate.
Mdivi-1 was originally identified as a yeast mitochondrial fission inhibitor from a chemical library screen [11]. Widely referred to as a Drp1-specific inhibitor, mdivi-1 negligibly inhibits the GTPase activity of recombinant human mitochondrial fission protein Drp1 [11,12,13,14], although it does preserve elongated mitochondrial morphology in a variety of mammalian injury models [11,15,16]. Mdivi-1 appears to attenuate mitochondrial fission, at least in some cases, by preventing the phosphorylation-dependent translocation of Drp1 to mitochondria [14,17,18]. However, although mdivi-1 may behave like a Drp1 antagonist in a variety of scenarios, many Drp1-independent mdivi-1 effects on mammalian cells have been described [13,14,19,20,21,22,23,24,25,26]. Thus, the compound is clearly not a specific inhibitor of Drp1. Because mdivi-1 is so broadly neuroprotective, interest in defining the potentially multiple mechanisms of action is high.
Several studies, using a variety of disease models, have shown mdivi-1 antioxidant effects, e.g., [14,18,27,28]. We previously identified mitochondrial Complex I as a Drp1-independent site of action [13]. Most Complex I inhibitors stimulate reactive oxygen species (ROS) generation [29]. However, in contrast to the classical Complex I inhibitor rotenone, mdivi-1 failed to increase ROS accumulation by primary rat cortical neurons despite impairing respiration [13]. Furthermore, compared with rotenone, mdivi-1 negligibly stimulated ROS production by isolated brain mitochondria oxidizing Complex I-linked substrates. However, like rotenone, mdivi-1 attenuated reverse electron transfer (RET)-stimulated ROS production from the quinone (Q) site of brain mitochondria [13], supporting a Complex I-dependent mechanism of action [29].
Given the divergent effects of mdivi-1 on ROS measurements relative to classical Complex I inhibitors, we wondered whether mdivi-1 has intrinsic biochemical antioxidant activity potentially contributing to the observed differences. In this study, we employed the ABTS and DPPH free radical depletion assays to test the prediction that mdivi-1 can directly scavenge free radicals. The results are consistent with this prediction. However, they indicate that mdivi-1’s antioxidant capacity is relatively modest compared with the reference antioxidant Trolox.
2. Materials and Methods
2.1. Chemicals
The following chemicals and enzymes (catalogue numbers in parentheses) were purchased from Millipore-Sigma (St. Louis, MO, USA): 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, #648471), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, #A1888), 2,2-diphenyl-1-picrylhydrazyl (DPPH, #D9132), potassium persulfate (#216224), xanthine (#X0626), L-glutathione reduced (#G4251), xanthine oxidase from bovine milk (#X1875), Cu/Zn superoxide dismutase from bovine erythrocytes (#S5395), and horseradish peroxidase (#P8375). AmplexTM UltraRed Reagent (#A36006) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Other chemicals (e.g., KCl, K2HPO4, MgCl2, etc.) were obtained at high purity (≥99%) from Millipore-Sigma.
Mdivi-1 was purchased from Millipore-Sigma (#M0199) and prepared as a 50 mM stock in dimethyl sulfoxide (DMSO). A 1:500 dilution of DMSO (0.2%), equivalent to the highest tested mdivi-1 concentration (100 μM), was used as the vehicle control for all experiments.
2.2. Absorbance Spectra
To assess the absorbance spectra of mdivi-1 and DMSO vehicle, we measured optical density (O.D.) every 10 nm from 340 nm to 750 nm using a Spectramax ABS spectrophotometer (Molecular Devices, San Jose, CA, USA).
2.3. ABTS Radical Depletion Assay
To measure the antioxidant activity of mdivi-1, we performed an assay as described in [30] that is based on the inhibition of the absorbance of the radical cation of ABTS (ABTS•+). ABTS (7 mM) was prepared in water, and ABTS•+ (Figure 1A) was produced by combining ABTS with 2.45 mM potassium persulfate and allowing the mixture to react at room temperature in the dark for 16–24 h prior to experimental use. Trolox standard and mdivi-1 compound were mixed with ABTS•+, and absorbance at 734 nm was determined on a Varian Cary 100 Bio spectrophotometer (Walnut Creek, CA, USA) after a 6 min incubation. The antioxidant activity of mdivi-1 was calculated as the percentage inhibition of ABTS•+ solution absorbance equated against a Trolox standard curve.
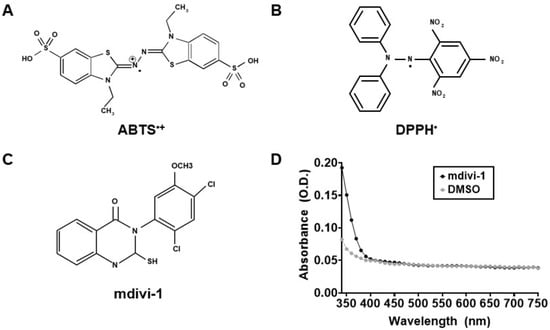
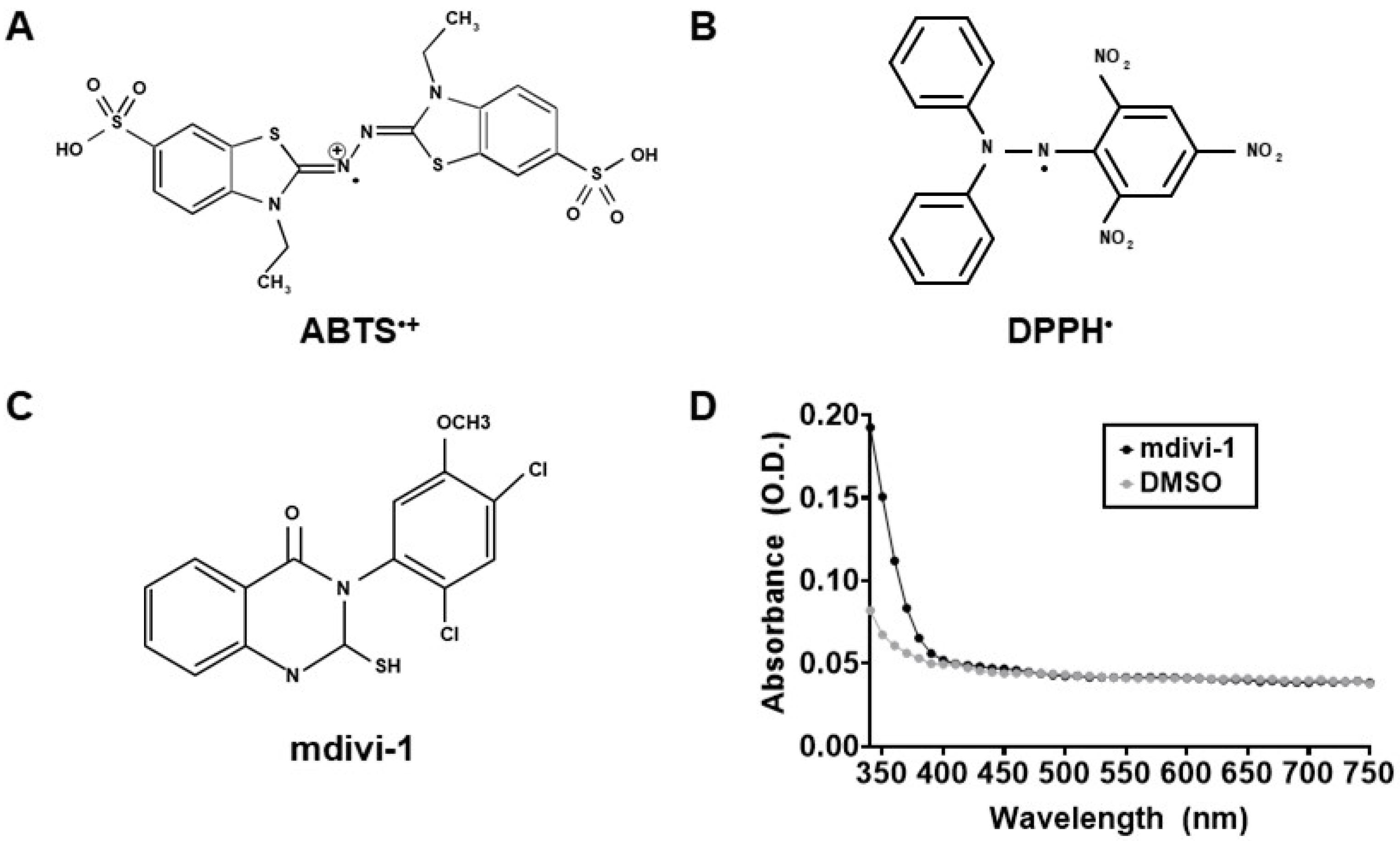
Figure 1.
Mitochondrial division inhibitor-1 (mdivi-1) structure, absorbance, and the free radicals used to measure its antioxidant activity. (A) 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical. (B) 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical. (C) Mdivi-1 structure. (D) Absorbance spectra of mdivi-1 (100 μM) and dimethyl sulfoxide (DMSO, 0.2%) in solution. O.D., optical density.
2.4. DPPH Radical Depletion Assay
To measure the antioxidant activity of mdivi-1, we also performed an assay based on the inhibition of the absorbance of the radical DPPH• (Figure 1B), as previously described [31]. Mdivi-1 and Trolox were diluted from 50 mM stocks, prepared in DMSO and water, respectively, to 2x stocks in absolute ethanol. Then, 1 mL of 100 mM DPPH made in absolute ethanol was added to 1 mL of 2x mdivi-1 or Trolox to obtain the final reaction mixtures with the indicated concentrations of compounds. The reaction mixtures were vortexed vigorously for 10 s and allowed to stand at room temperature, protected from light, for 30 min. Absorbance was determined at 517 nm on a Varian Cary 100 Bio spectrophotometer. Mdivi-1 antioxidant activity was calculated as the percentage inhibition of DPPH• solution absorbance equated against a Trolox standard curve.
2.5. Amplex UltraRed Assay
The hydrogen peroxide (H2O2)-sensitive fluorescent indicator Amplex UltraRed (Waltham, MA, USA), an Amplex Red variant with increased sensitivity [32], was used to test for mdivi-1 H2O2-scavenging ability using an enzymatic H2O2-generating system. This system consisted of xanthine oxidase, its substrate xanthine, and superoxide dismutase [33]. Amplex UltraRed (5 μM), horseradish peroxidase (10 U/mL), superoxide dismutase 1 (SOD1, 40 U/mL), xanthine (100 μM), and test compound (DMSO, mdivi-1, Trolox, or reduced glutathione) were added to KCl assay medium consisting of 125 mM KCl, 2 mM K2HPO4, 0.1 mM EGTA, 1 mM MgCl2, and 20 mM HEPES, pH 7.0. Fluorescence was monitored at 37 °C using a Thermo Fisher VarioskanTM Lux multimode microplate reader (excitation 560 nm; emission 585 nm). After two minutes of baseline recording to evaluate background Amplex UltraRed oxidation in the absence and presence of test compounds, xanthine oxidase (1.33 mU/mL) was added to initiate H2O2 production, and fluorescence was monitored for an additional five minutes. Amplex UltraRed oxidation rate was calculated as the slope of the first three minutes of fluorescence recordings, which was linear, as determined by linear regression analysis.
2.6. Data Analysis and Statistics
Statistical analyses were performed using GraphPad Prism Version 9.1 software (San Diego, CA, USA). Experimental results are expressed as means ± standard error of the mean (SEM) of 3–4 replicates. One- or two-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons post hoc test was used to test for differences among groups. Results with p < 0.05 are considered statistically significant. The [agonist] vs. response–variable slope non-linear fit model of GraphPad Prism 9.1 was used to determine the effective concentration (EC50) values for mdivi-1 and Trolox. The EC50 values give the concentration of antioxidant required to decrease the initial concentration of free radical by 50%.
3. Results
3.1. Mdivi-1 and DMSO Absorbance Spectra
Mdivi-1 (structure given in Figure 1C) was prepared in DMSO, a solvent reported to not interfere with the ABTS or DPPH radical depletion assays that rely on 734 nm and 517 nm absorbance measurements, respectively [30,34]. Because we noticed previously that the spectral properties of mdivi-1 at 340 nm interferes with NADH-based Complex I assays [13], we measured broad absorbance spectra from 340 to 750 nm to ensure that mdivi-1 absorbance did not also interfere with these antioxidant activity assays. Both mdivi-1 and its vehicle, DMSO, showed little absorbance at wavelengths ≥400 nm in solution (Figure 1D), confirming that neither interfered with the ABTS and DPPH decoloration assays.
3.2. Antioxidant Capacity of Mdivi-1 Measured by ABTS Radical Depletion Assay
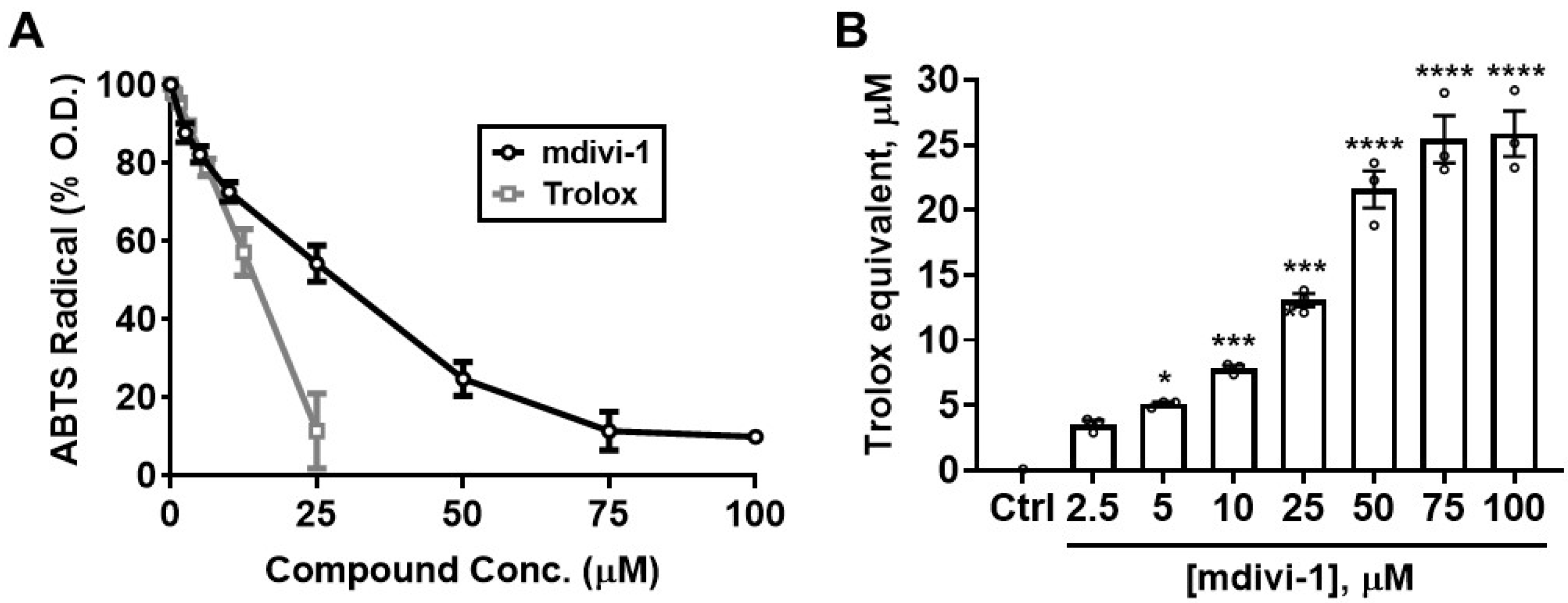
To determine whether mdivi-1 possesses the intrinsic ability to scavenge free radicals, we first employed the ABTS radical cation assay [30]. As seen in Figure 2A, Trolox (gray squares) dose-dependently depleted ABTS•+ absorbance, with an EC50 of 12.88 μM. Although not as potent as Trolox, mdivi-1 nevertheless showed significant and dose-dependent free radical-scavenging activity over the range of concentrations typically employed to inhibit mitochondrial fission in cell-based experiments (Figure 2A; black circles). The calculated EC50 for mdivi-1 was 24.38 μM, approximately twice that of Trolox. Figure 2B shows the Trolox-equivalent antioxidant capacity (TEAC) of mdivi-1 for the range of concentrations used in this experiment.
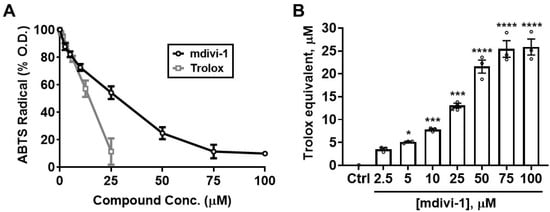
Figure 2.
Mdivi-1 dose-dependently depletes ABTS•+. (A) Mdivi-1 (2.5–100 μM) or Trolox (0.78–25 μM) was added to ABTS•+ and antioxidant activity was monitored by the ABTS•+ decolorization assay as a decrease in optical density (O.D.), expressed as a percentage of the initial O.D. Each value is depicted as the mean ± SEM; n = 3 separate experiments. (B) Trolox-equivalent antioxidant capacity (TEAC) of mdivi-1 for the depletion of ABTS•+. Bar graphs represent mean ± SEM from n = 3 separate experiments. One-way ANOVA, F-value (7, 16) = 95.29, p < 0.0001, was followed by Bonferroni’s multiple comparisons post hoc test comparing mdivi-1 treatment to the vehicle control (Ctrl). * p < 0.05, *** p < 0.001, **** p < 0.0001.
3.3. Antioxidant Capacity of Mdivi-1 Measured by DPPH Radical Depletion Assay
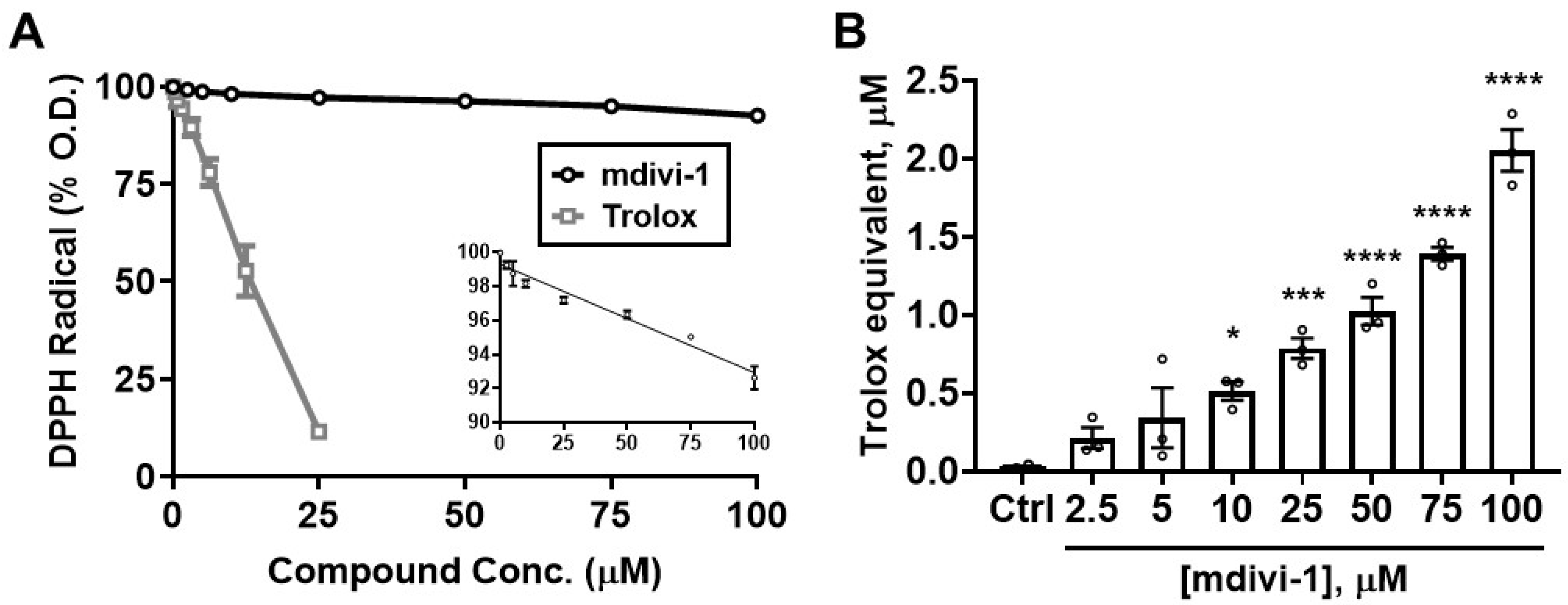
To determine whether the ability of mdivi-1 to scavenge ABTS•+ extends to other radicals, we next tested the compound in the DPPH radical decoloration assay [31]. As seen in the ABTS•+ assay, Trolox efficiently depleted DPPH radical absorbance, with an EC50 of 11.75 μM, close to that for ABTS•+ depletion (12.88 μM, Figure 3A; gray squares). However, mdivi-1 exhibited a relatively weak ability to deplete the DPPH radical compared with either that of the reference antioxidant Trolox (Figure 3A; black circles) or with its own ability to scavenge ABTS•+ (Figure 2).
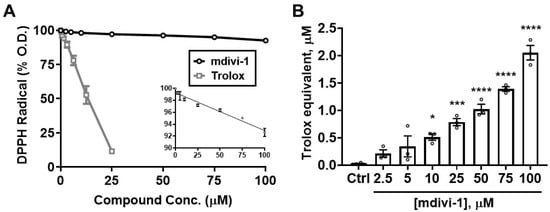
Figure 3.
Mdivi-1 weakly but dose-dependently depletes DPPH radical. (A) Mdivi-1 (2.5–100 μM) or Trolox (0.78–25 μM) was added to DPPH radical and antioxidant activity was monitored by the DPPH decolorization assay as a decrease in optical density (O.D.), expressed as a percentage of the initial O.D. Each value is depicted as the mean ± SEM from n = 3 separate experiments. The inset in (A) shows the means ± SEM for the mdivi-1 group on a compressed scale (DPPH Radical (% O.D.), y-axis vs. Compound Conc. (μM), x-axis), fit by linear regression analysis (R2 = 0.9139, p < 0.001). (B) Trolox-equivalent antioxidant capacity (TEAC) of mdivi-1 for the depletion of DPPH. Bar graphs represent means ± SEM from n = 3 separate experiments. One-way ANOVA, F-value (7, 16) = 48.44, p < 0.0001, was followed by Bonferroni’s multiple comparisons post hoc test comparing mdivi-1 treatment to Ctrl. * p < 0.05, *** p < 0.001, **** p < 0.0001.
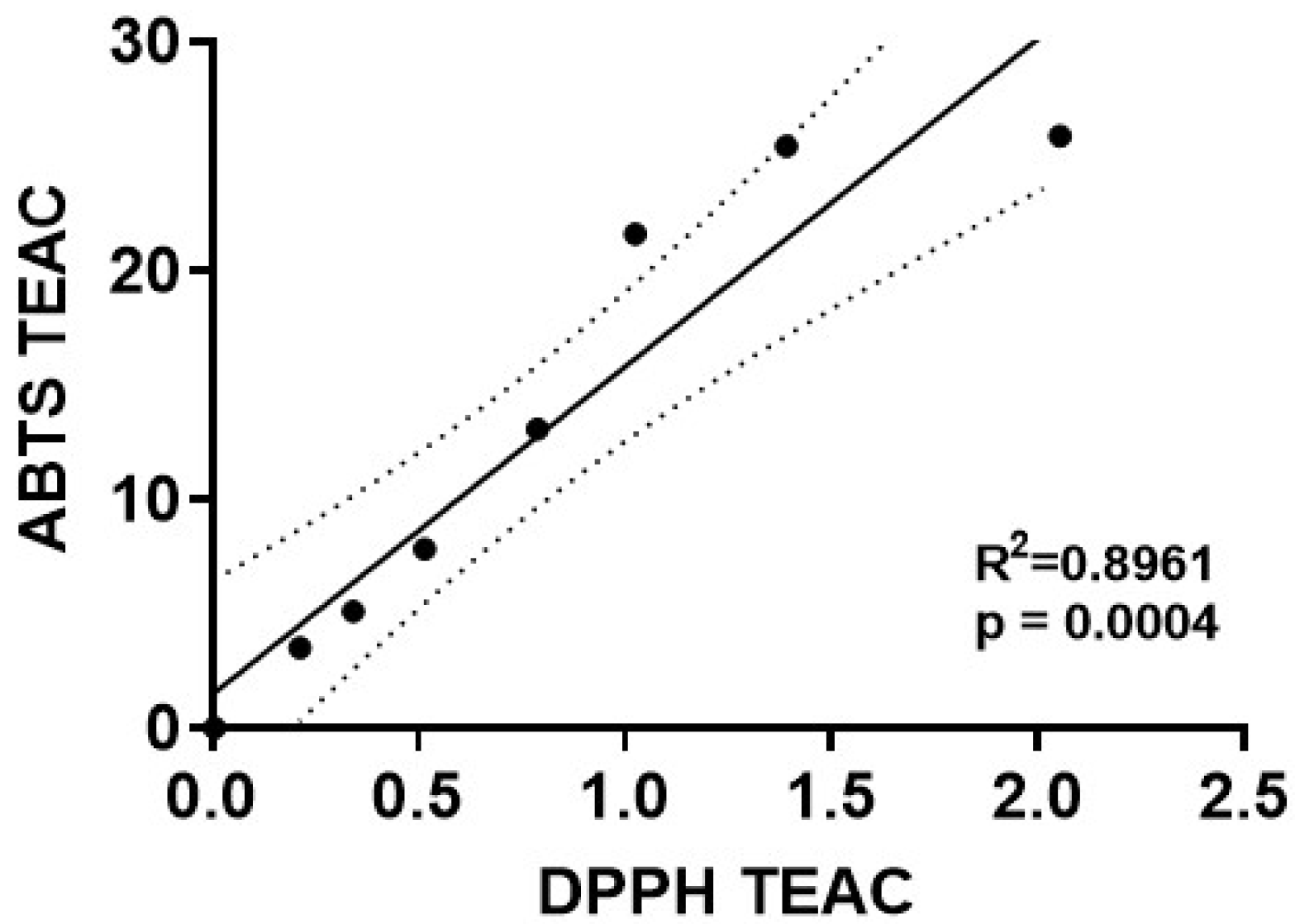
We next performed a regression analysis to directly compare the Trolox-equivalent antioxidant capacity values for mdivi-1 obtained using the ABTS radical assay with those obtained by the DPPH radical depletion assay. A significant linear correlation of mdivi-1’s TEAC was found between the ABTS and DPPH assays (Figure 4; R2 = 0.8961, p = 0.0004). However, for every mdivi-1 concentration tested, the compound depleted ABTS•+ to a much greater extent than the DPPH radical. Therefore, although mdivi-1 showed significant antioxidant activity in both free radical depletion assays, the antioxidant strength, calculated as Trolox equivalency, was much lower when measured by the DPPH radical decoloration assay.
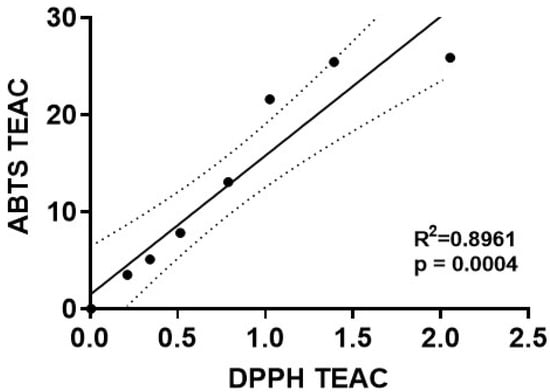
Figure 4.
Correlation of mdivi-1’s dose-dependent antioxidant capacity values measured by two different radical depletion assays. The Trolox-equivalent antioxidant capacity (TEAC) of mdivi-1 in the ABTS•+ (y-axis) and DPPH radical (x-axis) depletion assays was found to be significantly correlated by linear regression analysis (R2 = 0.8961, p = 0.0004). Dotted line depicts 95% confidence bands for the best-fit line. Note that the axes have different scales.
Mdivi-1, like the physiological antioxidant glutathione, has a thiol group that may quench peroxides. DPPH radical is quenched by antioxidants either by electron transfer, which is very fast, or by hydrogen atom transfer, which is slow and diffusion-controlled [35,36]. Glutathione reacts with DPPH radical primarily by the latter mechanism and exhibits an almost seven-fold slower reaction rate with DPPH than the reference antioxidant Trolox, resulting in an underestimation of its antioxidant capacity when measured by the standard DPPH protocol [35].
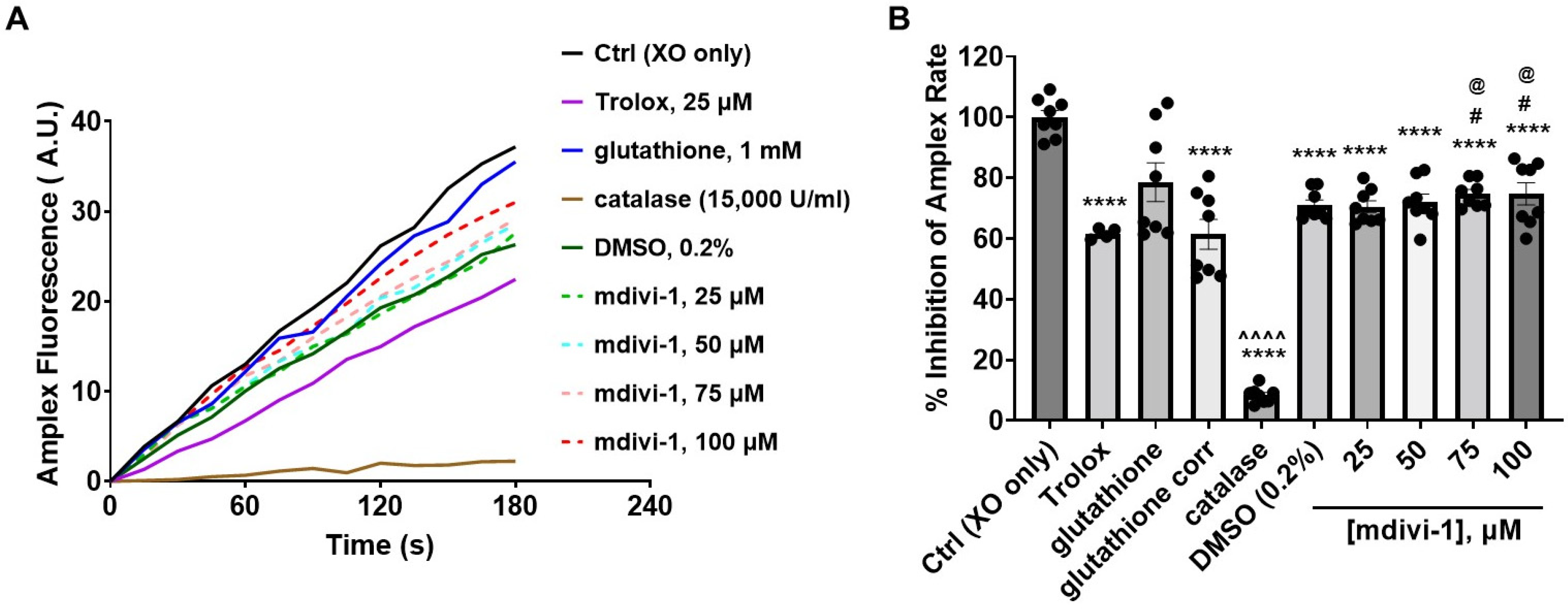
Because mdivi-1, like glutathione, may react with peroxides, we tested whether the hydrogen peroxide (H2O2)-sensitive fluorescent indicator Amplex UltraRed (hereafter called Amplex) together with an enzymatic H2O2-generating system [33] could be used to evaluate mdivi-1’s H2O2-scavenging ability. The oxidation of the xanthine substrate by the xanthine oxidase enzyme generates superoxide, which can be rapidly converted to H2O2 by Cu/Zn superoxide dismutase (SOD1). These reactions yield an H2O2-dependent linear increase in Amplex fluorescent product catalyzed by horseradish peroxidase [33].
Of all the treatments, only glutathione increased Amplex fluorescence when incubated in the enzyme/substrate reaction mixture in the absence of xanthine oxidase. The auto-oxidation of glutathione is known to interfere with H2O2 detection by Amplex Red [37]. Once xanthine oxidase was added to trigger H2O2 production, mdivi-1, the DMSO vehicle, Trolox, and the H2O2-eliminating enzyme catalase (used as a positive control) all significantly inhibited the Amplex oxidation rate (Figure 5A,B). For the glutathione treatment, the background Amplex oxidation rate (not shown) was subtracted from the rate measured following xanthine oxidase addition. The resultant rate (shown as glutathione corrected (corr) in Figure 5B) was also significantly suppressed compared with the xanthine oxidase (XO) only control (Ctrl) condition (solid black trace in Figure 5A). Though the mdivi-1 solutions, like glutathione, significantly inhibited Amplex oxidation relative to Ctrl, the level of inhibition was not dose dependent across the tested mdivi-1 concentration range (25–100 μM, dashed lines in Figure 5A). In addition, the extent of inhibition did not differ from the DMSO vehicle control. Thus, the significant effect of DMSO confounded the measurement of the peroxide-scavenging ability of mdivi-1 using this assay.
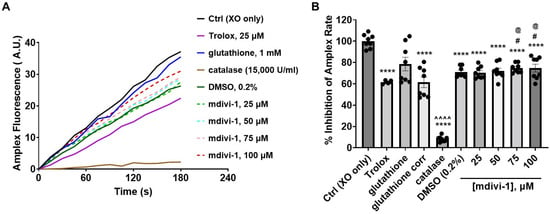
Figure 5.
Effect of mdivi-1 on Amplex UltraRed oxidation rate. (A) Fluorescence in arbitrary units (A.U.) of Amplex UltraRed (Amplex) oxidation product monitored over time following xanthine oxidase (XO) addition in the absence (Ctrl, control) or presence of mdivi-1 (25–100 μM), DMSO (0.2%, vehicle for mdivi-1), Trolox (25 μM), glutathione (1 mM) or catalase (15,000 U/mL). Glutathione was prepared from powder in the aqueous Amplex assay medium and Trolox was diluted from a 50 mM stock prepared in water. Traces are means of quadruplicate wells and are representative of the experiment run on two different days. Trolox was only included on one of the days (depicted). (B) Amplex UltraRed oxidation rates expressed as a percentage of the Ctrl rate. Data are mean ± SEM, n = 4 for Trolox, and n = 8 for all others. An uncorrected representative glutathione trace is shown in (A), and both the uncorrected and corrected (corr, i.e., background-subtracted) rates are given in (B). Only the corrected rates were used for statistical analysis. A two-way mixed-model ANOVA followed by Bonferroni’s multiple comparisons post hoc test was used to test for differences among treatments, with treatment and experiment day as factors. Both treatment (F-value (8, 58) = 97.25, p < 0.0001) and the experiment day (F-value (1, 58) = 10.50, p = 0.002) had a significant effect, accounting for 92.18% and 1.244% of the total variation, respectively. **** p < 0.0001 compared to Ctrl, # p < 0.05 compared to Trolox, @ p < 0.05 compared to glutathione corr, ^^^^ p < 0.0001 compared to all other groups.
We also attempted to evaluate whether mdivi-1 can scavenge superoxide using a combination of xanthine, xanthine oxidase, and the fluorescent indicator dihydroethidium. However, in our hands, this method lacked sufficient sensitivity for accurate rate determination in cell-free assays (data not shown).
4. Discussion
Mdivi-1 is a promising preclinical drug candidate for both acute and chronic neurodegenerative disorders. The compound is referred to as a specific Drp1 inhibitor in many, if not most, of the ~430 peer reviewed journal articles in which it has been mentioned to date (see [15,38] for review). However, several biological effects of mdivi-1 are now described in Drp1-deficient cells, indicating Drp1-independent mechanisms of action [38]. These include antioxidant effects [14].
The very specific goal of the experiments described in this short report was to determine whether mdivi-1 can directly scavenge free radicals, an ability that could potentially contribute to its Drp1-independent biological activities in cells. The findings obtained by two of the most widely used assays to evaluate antioxidant strength demonstrate that mdivi-1 can scavenge free radicals in cell-free systems. We found a linear relationship across a range of mdivi-1 concentrations in the TEAC values obtained by the ABTS and DPPH radical depletion assays. However, a limitation of our study is that mdivi-1 far more effectively scavenges ABTS radicals than DPPH radicals, hindering the interpretation of its antioxidant capacity.
Though still one of the most widely used assays for evaluating antioxidant activity, a re-evaluation of the DPPH•-based method found that despite measuring free radical-scavenging ability, it is a poor method for ranking antioxidant strength [35]. The main reasons for this are that TEAC values determined by the DPPH assay are influenced by whether a compound has bulky ring adducts and/or multiple phenolic rings that limit accessibility to the sterically hindered DPPH radical site (Figure 1B) and are additionally influenced by the nature of the compound’s dominant antioxidant mechanism (fast vs. slow) [39]. The DPPH method, relative to the ABTS method, is also more sensitive to pH effects [39]. Vanillic acid and resorcinol, in addition to the aforementioned glutathione, are examples of antioxidants that exhibit low antioxidant capacity relative to Trolox in the DPPH assay [35]. Various other compounds were found to have weaker antioxidant capacity when measured by the DPPH radical depletion assay as compared to the ABTS radical depletion assay [40,41,42,43].
Another limitation of our study is that neither ABTS•+ nor DPPH• are free radicals found in mammals. Mdivi-1 has a thiol group (see Figure 1C) that most likely quenches peroxides and sulphenic oxidations. Mdivi-1′s ability to scavenge physiological ROS may be superior or inferior to its ability to scavenge ABTS•+ or DPPH•. With this caveat in mind, we explored the use of an enzymatic system that generates H2O2 combined with detection by Amplex UltraRed, an assay occasionally used to evaluate antioxidant activity [44,45]. Unfortunately, the significant effect of the DMSO solvent obscured the interpretation of the results. DMSO has known antioxidant properties, including the ability to scavenge hydroxyl radicals [46] and inhibit lipid peroxidation [47]. A drawback of the Amplex (Ultra) Red assay is that the antioxidant test compound may interact with one or more of the enzymes (e.g., xanthine oxidase, horseradish peroxidase) or the fluorescent indicator, making results difficult to interpret (see [37,44,45]). For this reason, methods that directly monitor radical disappearance, such as the ABTS and DPPH decolorization assays, are generally preferred. Because we saw a significant DMSO vehicle effect, we did not attempt to determine whether the effects observed in the Amplex assay were due to H2O2 scavenging or the inhibition of enzyme activity.
Both antioxidant and pro-oxidant effects of mdivi-1 have been reported in vitro and in vivo [15,38]. These opposing mdivi-1 actions may result from the conditions under which it interacts with Complex I or other protein targets rather than from direct free radical interactions. We found previously that although mdivi-1 fails to increase ROS in primary cortical neurons, it stimulates ROS to the same extent as the Complex I inhibitor rotenone in both wild-type and Drp1 knockout mouse embryonic fibroblasts [13]. Future work is needed to determine whether mdivi-1 binds to the Complex I Q-site, like rotenone, or exhibits an alternative mechanism of Complex I inhibition. Interestingly, idebenone, widely considered an antioxidant, is a compound that, like mdivi-1, both inhibits Complex I activity and exhibits relatively weak antioxidant activity in cell-free assays [48,49]. Recent work found that the expression level of NAD(P)H quinone dehydrogenase 1 (NQO1), an enzyme that can reduce idebenone to idebenol, dictates whether the compound behaves as an antioxidant or pro-oxidant in cells [50,51]. Future elucidation of the precise Drp1-independent targets of mdivi-1 may similarly identify enzymes that modify its redox behavior in cells.
5. Conclusions
In summary, using two of the most widely employed free radical depletion assays for evaluating antioxidant capacity, our study demonstrated that mdivi-1 can directly scavenge free radicals. Mdivi-1 exhibited significant antioxidant activity in both assays but showed a superior ability to scavenge ABTS•+ compared with the DPPH• radical that has a sterically hindered reaction site. Given that the antioxidant potency of mdivi-1 is ~two-fold lower than the vitamin E analog Trolox, even as determined by the ABTS•+ depletion assay, it seems unlikely that biochemical antioxidant activity is the major mechanism responsible for its neuroprotection. Nevertheless, the ability of mdivi-1 to engage in redox reactions independently of actions on its putative target Drp1 is another variable to consider when attributing biological effects to specific molecular mechanisms.
Author Contributions
Conceptualization, E.A.B. and B.M.P.; methodology, E.A.B. and N.Z.; formal analysis, E.A.B., N.Z. and J.W.; investigation, E.A.B. and N.Z.; writing—original draft preparation, E.A.B. and B.M.P.; writing—review and editing, J.W.; supervision, B.M.P.; funding acquisition, B.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health grant number NS096538 (B.M.P.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fischer, T.D.; Hylin, M.J.; Zhao, J.; Moore, A.N.; Waxham, M.N.; Dash, P.K. Altered Mitochondrial Dynamics and TBI Pathophysiology. Front. Syst. Neurosci. 2016, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Xia, S.X.; Li, Q.Q.; Gao, Y.; Shen, X.; Ma, L.; Zhang, M.Y.; Wang, T.; Li, Y.S.; Wang, Z.F.; et al. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res. 2016, 1630, 134–143. [Google Scholar] [CrossRef]
- Fan, L.F.; He, P.Y.; Peng, Y.C.; Du, Q.H.; Ma, Y.J.; Jin, J.X.; Xu, H.Z.; Li, J.R.; Wang, Z.J.; Cao, S.L.; et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol. Med. 2017, 112, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, C.; Wang, H.; Zhang, X.; Li, Q.; Gu, Z.; Shi, X.; Cui, Y.; Wang, T.; Chen, X.; et al. Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int. J. Biochem. Cell Biol. 2018, 94, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, S.; Li, Y.; Che, L.; Zhao, Q. A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci. Lett. 2013, 535, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, P.; Wei, J.; Fan, R.; Zuo, Y.; Shi, M.; Wu, H.; Zhou, M.; Lin, J.; Wu, M.; et al. Inhibition of Drp1 by Mdivi-1 attenuates cerebral ischemic injury via inhibition of the mitochondria-dependent apoptotic pathway after cardiac arrest. Neuroscience 2015, 311, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Yang, Z.; Yu, T.; Zheng, G.; Fang, X.; Huang, Z.; Jiang, L.; Tang, W. Inhibition of dynamin-related protein 1 has neuroprotective effect comparable with therapeutic hypothermia in a rat model of cardiac arrest. Transl. Res. 2018, 194, 68–78. [Google Scholar] [CrossRef]
- Wang, W.; Yin, J.; Ma, X.; Zhao, F.; Siedlak, S.L.; Wang, Z.; Torres, S.; Fujioka, H.; Xu, Y.; Perry, G.; et al. Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum. Mol. Genet. 2017, 26, 4118–4131. [Google Scholar] [CrossRef] [Green Version]
- Bido, S.; Soria, F.N.; Fan, R.Z.; Bezard, E.; Tieu, K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-alpha-synuclein rat model of Parkinson’s disease. Sci. Rep. 2017, 7, 7495. [Google Scholar] [CrossRef] [Green Version]
- Rappold, P.M.; Cui, M.; Grima, J.C.; Fan, R.Z.; de Mesy-Bentley, K.L.; Chen, L.; Zhuang, X.; Bowers, W.J.; Tieu, K. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat. Commun. 2014, 5, 5244. [Google Scholar] [CrossRef]
- Cassidy-Stone, A.; Chipuk, J.E.; Ingerman, E.; Song, C.; Yoo, C.; Kuwana, T.; Kurth, M.J.; Shaw, J.T.; Hinshaw, J.E.; Green, D.R.; et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 2008, 14, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallat, A.; Uchiyama, L.F.; Lewis, S.C.; Fredenburg, R.A.; Terada, Y.; Ji, N.; Nunnari, J.; Tseng, C.C. Discovery and characterization of selective small molecule inhibitors of the mammalian mitochondrial division dynamin, DRP1. Biochem. Biophys. Res. Commun. 2018, 499, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Clerc, P.; Roelofs, B.A.; Saladino, A.J.; Tretter, L.; Adam-Vizi, V.; Cherok, E.; Khalil, A.; Yadava, N.; Ge, S.X.; et al. The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev. Cell 2017, 40, 583–594.e586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, C.; Wang, L.; Zhang, J.; Xiang, X.; Wu, Y.; Zhang, Z.; Li, Q.; Tian, K.; Xue, M.; Liu, L.; et al. Mdivi-1 attenuates oxidative stress and exerts vascular protection in ischemic/hypoxic injury by a mechanism independent of Drp1 GTPase activity. Redox. Biol. 2020, 37, 101706. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Gallo, G. To mdivi-1 or not to mdivi-1: Is that the question? Dev. Neurobiol. 2017, 77, 1260–1268. [Google Scholar] [CrossRef]
- Rosdah, A.A.; Holien, J.K.; Delbridge, L.M.; Dusting, G.J.; Lim, S.Y. Mitochondrial fission—A drug target for cytoprotection or cytodestruction? Pharmacol. Res. Perspect 2016, 4, e00235. [Google Scholar] [CrossRef]
- Zhan, L.; Lu, Z.; Zhu, X.; Xu, W.; Li, L.; Li, X.; Chen, S.; Sun, W.; Xu, E. Hypoxic preconditioning attenuates necroptotic neuronal death induced by global cerebral ischemia via Drp1-dependent signaling pathway mediated by CaMKIIalpha inactivation in adult rats. FASEB J. 2019, 33, 1313–1329. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, L.; Mo, Y.; Huang, Y.; Li, W.; Peng, Q.; Huang, L.; Ai, Y. Mdivi-1 attenuates lipopolysaccharide-induced acute lung injury by inhibiting MAPKs, oxidative stress and apoptosis. Pulm. Pharmacol. Ther. 2020, 62, 101918. [Google Scholar] [CrossRef]
- Koch, B.; Barugahare, A.A.; Lo, T.L.; Huang, C.; Schittenhelm, R.B.; Powell, D.R.; Beilharz, T.H.; Traven, A. A Metabolic Checkpoint for the Yeast-to-Hyphae Developmental Switch Regulated by Endogenous Nitric Oxide Signaling. Cell Rep. 2018, 25, 2244–2258.e2247. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Alberdi, E.; Matute, C. Mitochondrial Division Inhibitor 1 (mdivi-1) Protects Neurons against Excitotoxicity through the Modulation of Mitochondrial Function and Intracellular Ca(2+) Signaling. Front. Mol. Neurosci. 2018, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Quintela-López, T.; Sánchez-Gómez, M.V.; Gaminde-Blasco, A.; Alberdi, E.; Matute, C. Mitochondrial division inhibitor 1 disrupts oligodendrocyte Ca(2+) homeostasis and mitochondrial function. Glia 2020, 68, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, J.; Roginskaya, V.; McDermott, L.A.; Edwards, R.P.; Stolz, D.B.; Llambi, F.; Green, D.R.; Van, H.B. Novel combination of mitochondrial division inhibitor 1 (mdivi-1) and platinum agents produces synergistic pro-apoptotic effect in drug resistant tumor cells. Oncotarget 2014, 5, 4180–4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Wang, G.; Chwa, J.; Oh, M.E.; Abeywardana, T.; Yang, Y.; Wang, Q.A.; Jiang, L. Mitochondrial division inhibitor (mdivi-1) decreases oxidative metabolism in cancer. Br. J. Cancer 2020, 122, 1288–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucantoni, F.; Dussmann, H.; Prehn, J.H.M. Metabolic Targeting of Breast Cancer Cells With the 2-Deoxy-D-Glucose and the Mitochondrial Bioenergetics Inhibitor MDIVI-1. Front. Cell Dev. Biol. 2018, 6, 113. [Google Scholar] [CrossRef]
- Fang, C.T.; Kuo, H.H.; Yuan, C.J.; Yao, J.S.; Yih, L.H. Mdivi-1 induces spindle abnormalities and augments taxol cytotoxicity in MDA-MB-231 cells. Cell Death Discov. 2021, 7, 118. [Google Scholar] [CrossRef]
- So, E.C.; Hsing, C.H.; Liang, C.H.; Wu, S.N. The actions of mdivi-1, an inhibitor of mitochondrial fission, on rapidly activating delayed-rectifier K(+) current and membrane potential in HL-1 murine atrial cardiomyocytes. Eur. J. Pharmacol. 2012, 683, 1–9. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Zhu, S.; Wang, C.; Dai, J.; Zhang, G.; Zheng, B.; Xu, S.; Wang, L.; Zhang, T.; et al. Mdivi-1 Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats, Possibly via Inhibition of Drp1-Activated Mitochondrial Fission and Oxidative Stress. Neurochem. Res. 2017, 42, 1449–1458. [Google Scholar] [CrossRef]
- Ojha, R.; Tantray, I.; Rimal, S.; Mitra, S.; Cheshier, S.; Lu, B. Regulation of reverse electron transfer at mitochondrial complex I by unconventional Notch action in cancer stem cells. Dev. Cell 2022, 57, 260–276.e269. [Google Scholar] [CrossRef]
- Drose, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Zhu, A.; Romero, R.; Petty, H.R. Amplex UltraRed enhances the sensitivity of fluorimetric pyruvate detection. Anal. Biochem. 2010, 403, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinta, S.J.; Rane, A.; Yadava, N.; Andersen, J.K.; Nicholls, D.G.; Polster, B.M. Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Radic. Biol. Med. 2009, 46, 939–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. A high correlation indicating for an evaluation of antioxidant activity and total phenolics content of various chilli varieties. J. Food Sci. Technol. 2015, 52, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Schaich, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Ganapathi, M.R.; Hermann, R.; Naumov, S.; Brede, O. Free electron transfer from several phenols to radical cations of non-polar solvents. Phys. Chem. Chem. Phys. 2000, 2, 4947–4955. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. Detection of hydrogen peroxide with Amplex Red: Interference by NADH and reduced glutathione auto-oxidation. Arch. Biochem. Biophys. 2004, 431, 138–144. [Google Scholar] [CrossRef]
- Rosdah, A.A.; Smiles, W.J.; Oakhill, J.S.; Scott, J.W.; Langendorf, C.G.; Delbridge, L.M.D.; Holien, J.K.; Lim, S.Y. New perspectives on the role of Drp1 isoforms in regulating mitochondrial pathophysiology. Pharmacol. Ther. 2020, 213, 107594. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Oliveira, S.d.; Souza, G.A.d.; Eckert, C.R.; Silva, T.A.; Sobral, E.S.; Fávero, O.A.; Ferreira, M.J.P.; Romoff, P.; Baader, W.J. Evaluation of antiradical assays used in determining the antioxidant capacity of pure compounds and plant extracts. Quim. Nova 2014, 37, 497–503. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Alternat. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [Green Version]
- Schlieve, C.R.; Lieven, C.J.; Levin, L.A. Biochemical activity of reactive oxygen species scavengers do not predict retinal ganglion cell survival. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3878–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, J.; Jové, M.; Boada, J.; Bellmunt, M.J.; Pamplona, R.; Portero-Otín, M. Dietary antioxidants interfere with Amplex Red-coupled-fluorescence assays. Biochem. Biophys. Res. Commun. 2009, 388, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, R.C., Jr. Oxidation of DMSO and methanesulfinic acid by the hydroxyl radical. Free Radic. Biol. Med. 1995, 18, 271–277. [Google Scholar] [CrossRef]
- Sanmartín-Suárez, C.; Soto-Otero, R.; Sánchez-Sellero, I.; Méndez-Álvarez, E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods 2011, 63, 209–215. [Google Scholar] [CrossRef]
- Jaber, S.; Polster, B.M. Idebenone and neuroprotection: Antioxidant, pro-oxidant, or electron carrier? J. Bioenerg. Biomembr. 2015, 47, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Gueven, N.; Ravishankar, P.; Eri, R.; Rybalka, E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol. 2021, 38, 101812. [Google Scholar] [CrossRef]
- Varricchio, C.; Beirne, K.; Heard, C.; Newland, B.; Rozanowska, M.; Brancale, A.; Votruba, M. The ying and yang of idebenone: Not too little, not too much—Cell death in NQO1 deficient cells and the mouse retina. Free Radic. Biol. Med. 2020, 152, 551–560. [Google Scholar] [CrossRef]
- Jaber, S.M.; Ge, S.X.; Milstein, J.L.; VanRyzin, J.W.; Waddell, J.; Polster, B.M. Idebenone Has Distinct Effects on Mitochondrial Respiration in Cortical Astrocytes Compared to Cortical Neurons Due to Differential NQO1 Activity. J. Neurosci. 2020, 40, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).