Abstract
Oral cancer continues to be a leading cause of death worldwide, and its prevalence is particularly high in developing countries, where people chew tobacco and betel nut on a regular basis. Radiation-, chemo-, targeted-, immuno-, and hormone-based therapies along with surgery are commonly used as part of a treatment plan. However, these treatments frequently result in various unwanted short- to long-term side effects. As a result, there is an urgent need to develop treatment options for oral cancer that have little or no adverse effects. Numerous bioactive compounds derived from various plants have recently attracted attention as therapeutic options for cancer treatment. Antioxidants found in medicinal plants, such as vitamins E, C, and A, reduce damage to the mucosa by neutralizing free radicals found in various oral mucosal lesions. Phytochemicals found in medicinal plants have the potential to modulate cellular signalling pathways that alter the cellular defence mechanisms to protect normal cells from reactive oxygen species (ROS) and induce apoptosis in cancer cells. This review aims to provide a comprehensive overview of various medicinal plants and phytoconstituents that have shown the potential to be used as oral cancer therapeutics.
1. Introduction
According to Globocan 2020, oral cancer is a common malignancy, with 177,757 deaths and 377,713 new cases reported each year worldwide. Oral cavity cancer is very common in South-Central Asia (e.g., India, Pakistan, and Sri Lanka), with more than one-third of the new cases (135,929) and one-fifth of the deaths (75,290) occurring in India alone [1]. The most common oral cancer is oral squamous cell carcinoma (OSCC), which has a wide range of clinical manifestations and accounts for more than 90% of all oral cancers [2]. The major risk factors for oral cancer include chronic inflammation, human papillomavirus or Candida infections, alcohol and tobacco use, ultraviolet radiation, immunosuppression, genetic susceptibility, and diet. Despite the availability of novel therapeutic options, the 5-year survival rate for oral cancer in most countries remains below 50%. Tobacco and alcohol usage are two of the most significant risk factors for oral cancer [3]. Oral inflammation may also have a role in the pathophysiology of oral cancers due to the involvement of several inflammatory pathways, such as cyclooxygenase (COX)-2, phosphatidylinositol 3-kinase (PI3K/Akt), mitogen-activated protein kinases (MAPK), nuclear factor-κB (NF-κB), Janus kinase/signal transducer and activator of transcription (JAK/STAT) [4]. It has been reported that oral cancer patients have significantly higher levels of Candida albicans genotype A strains than noncancer patients, and these strains have also been linked to leukoplakic lesions [5]. Immunosuppression has also been linked to the growth of oral cancer in patients undergoing bone marrow transplantation and renal transplantation [6].
Despite several advancements in treatment options for oral cancer, the survival rates have not increased considerably over the last few decades. Therefore, new and effective treatments to prevent the development of oral cancer are required. In recent years, several authors have reported the potential of natural products and phytochemicals derived from plants in the treatment of oral diseases [7,8,9,10,11]. Several natural compounds have been investigated for their ability to cause apoptosis in human cancer cells [12]. These phytocompounds may cause cell death by inducing apoptosis and arresting the cell cycle and could be exploited for the treatment of oral cancer with less systemic toxicity and side effects in humans [13]. Traditional medicines are used by a significant proportion of the global population to treat a variety of diseases [14,15,16,17,18,19,20,21]. Curcumin, lycopene, ginseng, anthocyanins, and artemisinin are some of the potential compounds that have shown promising results against OSCC and other tumours.
To the best of our knowledge, a comprehensive review that considers the role of antioxidant plant extracts and their constituents in treating oral cancer has not been reported in the literature. Thus, this review provides information on medicinal plants and phytochemicals derived from them for the treatment of oral cancer and assesses studies that have examined their anticancer properties in vitro and in vivo.
2. Methodology
In this study, the anticancer properties of medicinal plants and their phytoextracts in oral cancer treatment were reviewed using the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) 2020 guidelines [22]. Studies were selected based on the following inclusion criteria: (i) both endemic and cosmopolitan species were included based on less-frequently reviewed literature; (ii) original studies showing chemopreventive properties against oral cancer were selected; (iii) studies available in full text articles in English were included; and (iv) in vitro, in vivo and clinical trial studies showing authentic data were included (studies published in high-ranking journals were preferred).
The exclusion criteria were as follows: (i) Studies published in local languages (except English) were excluded; (ii) studies not having full text available were excluded; (iii) studies examining other types of cancer, e.g., lung cancer, colon cancer, etc., were excluded; and (iv) in vivo and in vitro studies not following ethical guidelines were excluded.
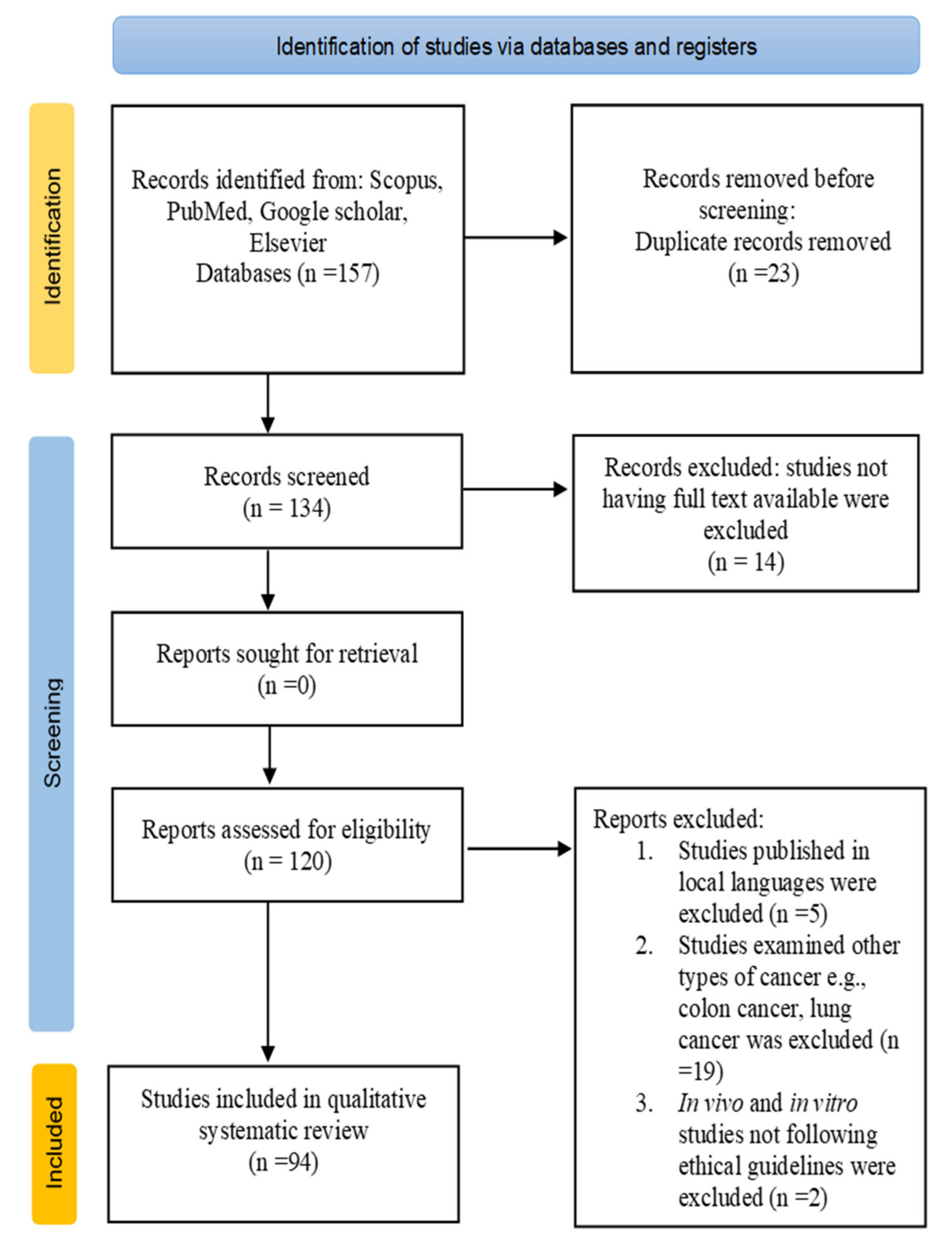
An electronic literature search was carried out in the following databases: Scopus, PubMed, Google Scholar, and Elsevier using the following keywords alone or in combination: oral cancer, carcinoma, types of oral cancer, medicinal plants, phytoextracts, anticancer agents, apoptosis, cell cycle arrest, and oral squamous cell carcinoma in vivo and in vitro. The literature search was performed from 24 March 2021 to 12 July 2021. Studies within the period from 2001 to 2021 were included in the review. A total of 157 studies were found from database searches, 23 duplicate records were removed, 14 studies with no full text were removed, and a total of 94 were selected for systematic review.
The name of medicinal plants was followed according to the plant list database [23]. After the selection of studies, the following data were collected: distribution of plants, source of phytoextract, anticancer properties of medicinal plants and phytoextracts, concentration of extract, the results of in vivo, in vitro and clinical trial studies.
The PRISMA flow diagram (Figure 1) shows the number of records identified, selection process, inclusion and exclusion criteria, and number of studies selected for the literature review.

Figure 1.
PRISMA flow diagram for the selection process of studies included in the current review.
3. Types of Oral Cancer
3.1. Oral Squamous Cell Carcinoma (OSCC)
OSCC dominates with an 84–97% occurrence rate among all oral cancer cases worldwide and is the 10th most common malignancy in females and the 6th most common malignancy in males. Approximately 50% of OSCC tumours report that a dysfunctional p53 gene plays an important role in checkpoint controls and apoptosis mechanisms. Potentially malignant disorders (PMDs), such as candidal leukoplakia, lichen planus, erythroplakia, inflammation, and dyskeratosis congenita, were indicators in the preclinical phase of oral cancer. OSCC commonly develops on the tongue, lips, and mouth surface [24]. The main therapeutic strategy in the treatment of OSCC includes a combination of radiation therapy and surgery. During treatment, patients were reported to have negative side effects in terms of oral function and appearance. In recent years, for OSCC treatment, many researchers have studied drugs obtained from medicinal plants that have fewer side effects and a better survival rate [25,26].
3.2. Oral Verrucous Carcinoma (VC)
Oral VC is a rare subtype of oral squamous cell carcinoma with cytodynamic features and specific morphologies. Oral VC accounts for 2% to 16% of all oral malignancies [27]. In early 1948, Lauren V. Ackermann first described oral VC, which is also referred to as “Ackermann’s tumour” [28]. Common oral cavity VC sites include the gingiva, mandibular alveolar crest, buccal mucosa, and tongue, and a common nonoral site is the glottic larynx. Drinking alcohol, the use of tobacco in inhaled and smokeless forms, and invariably poor oral hygiene are shown to be major causes in affected patients [29]. Oral VC is a low-grade malignancy that rarely metastasizes and has slow growth and a high degree of differentiation. Its proliferative and invasive outgrowing nature might induce the destruction of adjacent tissue, such as bone and cartilage. Numerous therapeutic protocols, including chemotherapy, lasers, and radiotherapy, have been employed in the treatment of oral VC (28).
3.3. Oral Melanoma
Oral melanoma, a metastatic cancer, is an aggressive lethal skin cancer and extremely rare (0.2–8%) among all malignant melanomas [30]. Melanoma is a type of cancerous tumour that results from the uncontrolled proliferation of pigment-producing cells called melanocytes [31]. The oral and nasal cavities are locations with the best prognosis. The first case of oral melanoma was identified in 1885 [32]. This type of cancer frequently affects males and occurs mostly in different sites of the oral cavity, such as the maxillary alveolar mucosa, gingiva, and hard palate. As the third most common malignancy, malignant melanoma represents only 3 to 5% of all cutaneous malignancies. The diagnosis for patients with oral melanoma is much worse than that for patients with cutaneous lesions, and the 5-year survival rate is approximately 15–38%. Oral malignant melanoma occurs on oral sites, including the floor of the mouth, lips, tongue and buccal mucosa. The palate is reported to be the most common site at 40% of cases, followed by the buccal gingiva, with one-third of cases [30].
3.4. Lymphoma
Lymphoma is a heterogeneous malignant tumour of the lymphatic system that results from the proliferation of lymphoid cells and their precursors. According to the alterations in their behavioural patterns and histology, they were divided into two groups: non-Hodgkin’s and Hodgkin’s lymphoma. Hodgkin’s lymphoma arises mostly in lymph nodes (>90%) [33]. Compared to squamous cell carcinoma, oral cavity lymphoma signifies the 3rd most commonly occurring malignancy in the oral cavity, and 4% of all patients with AIDS (acquired immune deficiency syndrome) suffer from lymphomas. The oral manifestations of lymphomas are difficult to diagnose because of their resemblance to the clinical features of other diseases, such as osteomyelitis, periodontal disease, and other malignancies [34]. Primary sites of oral manifestations of lymphomas in the oral cavity include the gingiva, floor of the mouth, palate, tongue, cheek and lips. Diagnosis is based on a combination of blood tests, selective biopsies, physical examination, and diagnostic imaging [33].
4. Plants with Beneficial Effects against Oral Cancer
4.1. Ocimum sanctum L.
Ocimum sanctum, which is known as Holy Basil or Tulsi, is a sacred herb belonging to the Labiatae family, and it is richly cultivated worldwide. O. sanctum is reported to have numerous medicinal properties, such as anticaries, antifungal, anticancer, antibacterial and antiviral properties. Holy basil contains compounds such as flavonoids, alkaloids, ursolic acid, tannins, carbohydrates, and eugenol [35]. A study reported that O. sanctum has a cytotoxic effect against the KB oral cancer mouth cell line (mouth epidermal carcinoma cells), with IC50 values of 10 µg/mL (light leaf aqueous extract) and 20 µg/mL (dark leaf aqueous extract) [36]. In a recent study, O. sanctum leaf ethanolic extract was examined for its antiinvasive effect on head and neck squamous cell carcinoma (HNSCC) cell lines (HN4, HN12, HN30, and HN31). In the cytotoxicity assay, O. sanctum ethanolic extract (0.8 mg/mL) treatment showed a decrease in the viability of HNSCC cell lines HN30 (40%), HN31 (53%), HN4 (52%), and HN12 (40%) compared to the controls (p < 0.05). O. sanctum ethanolic extract at a concentration of 0.4 mg/mL inhibited matrix metalloproteinase (MMP)-2 activity in HN12 and HN4 cells by 71% and 65%, respectively, and MMP-9 activity in HN12 and HN4 cells by 85% and 44%, respectively. The invasion activity of HN12 and HN4 cells was inhibited by 30% in comparison with that of the control (p < 0.05), but no significant changes were observed in HN31 and HN30 cells [37]. In a recent study, the anticancer activity of O. sanctum was examined in the OSCC cell line Ca9–22. In a treatment with O. sanctum aqueous extract, the highest permissive concentration (HPC) value was 30 mg/L and minimum inhibitory concentration (MIC) was 5 mg/L, and in a treatment with the dry extract, the HPC value was 35 mg/L and MIC value was 5 mg/L. A neutral red uptake (NRU) assay showed that the lethal concentration (LC) values for the dry extract were 30.19 mg/L (LC75), 23.44 mg/L (LC50), and 16.59 mg/L (LC25) and those for the aqueous extract were 20.89 mg/L (LC75), 14.79 mg/L (LC50), and 10.23 (LC25). In a 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, the LC values for the dry extract were 29.51 mg/L (LC75), 20.89 mg/L (LC50), and 12.58 mg/L (LC25) and those for the aqueous extract were 26.91 mg/L (LC75), 14.79 mg/L (LC50), and 7.41 mg/L (LC25) [35]. In an in vivo study, the anticancer activity of vicenin-2 (a bioactive compound found in O. sanctum) was examined in DMBA (7,12-dimethylbenz [a] anthracene)-induced OSCC hamsters. With the DMBA treatment, 100% tumour incidence was observed and cytokine levels (tumour necrosis factor-alpha [TNF-α], interleukin [IL]-1β, IL-6) were upregulated. Vicenin-2 treatment (30 mg/kg) with DMBA-induced OSCC hamsters improved antioxidant levels, inhibited lipid peroxidation, and halted tumour incidence. DMBA-induced hamsters treated with vicenin-2 showed a lack of production of proinflammatory cytokine (TNF-α, IL-1β, and IL-6) and inhibited immunohistochemical expression of cyclin D1, B-cell lymphoma 2 (Bcl-2), and proliferating cell nuclear antigen (PCNA). Vicenin-2 treatment activates apoptotic Bax expression, which further inhibits the expression of antiapoptotic Bcl-2 [38].
4.2. Curcuma longa L.
Curcuma longa (Turmeric), a medicinal herb belonging to the Zingiberaceae family, is native to Southeast Asia. Turmeric is known for its medicinal properties, such as antimicrobial, anti-inflammatory, hepatoprotective, antiseptic, and antimutagenic properties, and it is used in the treatment of oral cancer and periodontal diseases [39]. Turmeric contains chemical compounds known as curcuminoids, including bisdemethoxycurcumin-curcumin, curcumin, and dimethoxy-curcumin. A recent study reported that curcumin is the chief component of turmeric and has an antitumour effect in HNSCC [40]. In a recent study, the antitumour effect of curcumin (with copper supplementation) treatment with OSCC cell Lines H314 and ORL-115 obtained from cancer patients was examined. In the presence of 250 µM copper, a decrease in curcumin concentrations was observed, which inhibited 50% of cell viability in OSCC (IC50), 25 μM to 5.3 μM at 48 h and 50 μM to 40.3 μM at 24 h. With an increase in copper levels in OSCC cells treated with curcumin, an increase in Nrf2 levels and significant induction of intracellular ROS were observed. Curcumin along with copper treatment at 24 h compromised cell membrane integrity as a late event in apoptosis, and this treatment at 6 h induced apoptotic DNA fragmentation [41]. In another in vitro study, the effect of curcumin on epithelial-mesenchymal transition (EMT) induced by hepatocyte growth factor (HGF) in the OSCC cell lines Ca9–22 and HSC4 was observed. The results showed that curcumin treatment in OSCC cells inhibited cell motility and HGF-induced EMT via c-Met blockade. Curcumin treatment reduced phosphorylated c-mesenchymal epithelial transition/extracellular signal-regulated kinase (Met/ERK) pathway expression, which inhibited the HGF-induced increase in vimentin levels [42].
4.3. Vaccinium corymbosum L.
Vaccinium corymbosum is commonly known as blueberry, belongs to the Ericaceae family and is distributed among different countries, such as Canada, North America, northeastern United States as wild blueberries (lowbush) and British Columbia as cultivated blueberries (highbush). Blueberries contain phytochemicals, such as anthocyanins, which show potential antioxidant and anticancer effects. In some studies, the mechanism of anticancer activity has been reported, such as oxidative stress, products of oxidative stress such as increased apoptosis, DNA damage, inhibition of cell proliferation and production of proinflammatory molecules [43]. A study reported the chemopreventive efficacy and impact on angiogenesis and invasion of blueberries analysed by the ability to target PI3K/Akt, MAPK, NF-κB, and transforming growth factor β signalling in a hamster buccal pouch (HBP) carcinogenesis model. Administration of blueberry (200 mg/kg) inhibited the growth and progression of DMPA-induced OSCC by reducing the expression of the PI3K/Akt and TGF-β pathways. NF-κB activation is suppressed by preventing NF-κB p65 nuclear translocation, and in the MAPK pathway, no significant changes were observed [44]. In another study, blueberry and its main compound malvidin were reported to suppress STAT-3 phosphorylation in the SCC131 oral cancer cell line and induce mitochondrial-mediated apoptosis and cell cycle arrest in G1/S phase. Blueberry treatment of DMBA-painted hamsters at a 200 mg/kg concentration increased the tumour growth delay to 68.22% and reduced the multiplicity (0.87 ± 0.22) and tumour burden (26.27±2.82) [45]. The anticancer effects of pterostilbene (a polyphenolic compound found in blueberry fruit) on cisplatin-resistant human oral cancer (CAR) cells were reported in a recent study. pterostilbene treatment of CAR cells at concentrations of 25, 50, 70 and 100 µM (48 h) detected DNA breaks in cells, and an increase in the number of TUNEL (terminal deoxynucleotidyl transferase-mediated d-UTP nick end labelling)-positive cells was observed, indicating that pterostilbene mediates apoptosis of CAR cells through caspase-dependent signalling. Treatment of CAR cells with 50 and 75 µM pterostilbene (24 h) reduced the mRNA expression of multidrug resistance protein 1 (MDR1) and the phosphorylation of AKT at the Ser473 site [46].
4.4. Vaccinium macrocarpon Aiton
Vaccinium macrocarpon (cranberry), or the “wonder fruit”, is well known for its substantial therapeutic potential because of the availability of resveratrol and bioactive flavonoids, including anthocyanins, proanthocyanidins (PACs), and flavanols [47]. In another in vitro study, the antiproliferative effect of cranberry seed extract was analysed in the OSCC cell lines SCC25 and CAL27. Cranberry extract treatment of CAL27 cells upregulated key regulators of apoptosis, namely, caspase-8 (+181%) and caspase-2 (+327%), and a study of the relative change in proliferation between Days 1 and 3 showed that the GImax value of 70 µg/mL cranberry extract reduced SCC25 proliferation by 36.3% in comparison to the baseline treatment control. Cranberry extract treatment of CAL25 cells upregulated the expression of c-myc (+29%), ODC (+371%), and p53 (+44%) [48]. In a recent in vitro study, the anticancer effect of V. macrocarpon hydroalcoholic fruit extract against the KB oral cancer cell line was examined, and the results showed that V. macrocarpon extract killed 50% of oral cancer cells, with an IC50 value of 3.564 µg/mL. A study reported that V. macrocarpon extract treatment of a normal fibroblast cell line (L929) resulted in a higher cell viability percentage and had an antiproliferative effect on the KB cell line [47].
4.5. Momordica charantia L.
Momordica charantia (bitter melon) contains bioactive compounds that show anticancer activities, such as phenolic acids, triterpene glycosides, triterpenoids, sterols, lectins, and flavonoids. Bitter melon is used in traditional folk medicine and cultivated in subtropical and tropical regions, including in India, tropical Africa, Indonesia, and China. Bitter melon extract and its components show anticancer activities by inhibiting the cell cycle, cancer stem cells, metastasis, and angiogenesis and enhancing reactive oxygen species generation [49]. In a recent study, the chemopreventive effect of bitter melon extract (BME) was analysed in HNSCC induced by the carcinogen 4-nitroquinoline 1-oxide (4-NQO). BME 30% v/v (600 mg/mouse) treatment reduced the occurrence of 4-NQO-induced carcinogenesis. BME treatment suppressed the expression of the immune checkpoint gene PDCD1/PD1 and the proinflammatory genes IL1b, IL23a, and s100a9, which were reported to have elevated expression during oral cancer development. BME treatment led to an 8.1-fold downregulation of MMP9 compared to that in 4-NQO-induced oral cancer tissues (6.8-fold upregulation of MMP9) [50]. In another study, the effects of BME on the metabolic pathways, lipid metabolism and glycolysis of human oral cancer cells were analysed. BME treatment of Cal27 and JHU022 oral cancer cell lines downregulated the protein and mRNA expression levels of glycolytic genes phosphofructo kinase (platelet) (PFKB), pyruvate kinase muscle (PKM), pyruvate dehydrogenase kinase 3 (PDK3), glucose transporter-1 (SLC2A1/GLUT-1) and lactate dehydrogenase alpha (LDHA). BME treatment also led to a significant reduction in the mRNA and protein levels of genes involved in fatty acid biogenesis, including the genes for ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), and fatty acid synthase (FASN). BME induced mitochondrial reactive oxygen species generation and CCAAT/enhancer-binding protein-homologous protein (CHOP) expression, which are associated with endoplasmic reticulum (ER) stress, and facilitated cell death via apoptosis in oral cancer [51].
4.6. Azadirachta indica A. Juss
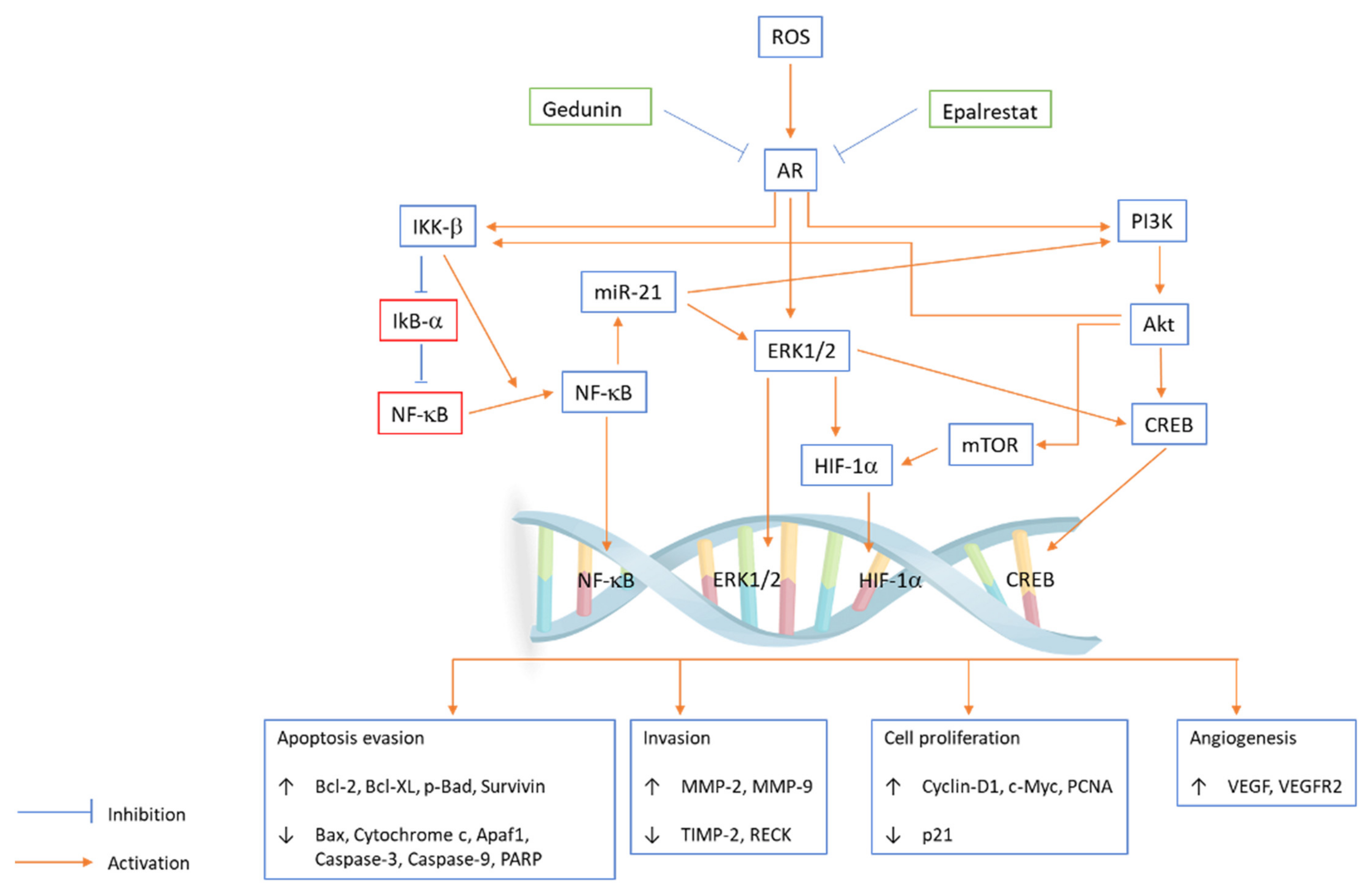
Azadirachta indica (neem), belonging to the family Meliaceae, is a large perennial tree that is largely distributed in India. The neem tree is well known for its medicinal properties, such as insecticidal, antioxidant, antifungal, antitumour, and antibacterial properties [52]. Studies on different neem tree products, such as the limonoids nimbolide and azadirachtin and neem leaf glycoprotein, reported that they had anticarcinogenic properties against OSCC. In an in vitro study, the potential of gedunin alone or with epalrestat (AR inhibitor) to prevent hallmarks of cancer by inhibiting the downstream PI3K/Akt/mTOR/ERK/NF-κB signalling axis and aldose reductase (AR) in the oral cancer cell line SCC131 was examined. Gedunin and epalrestat treatment downregulated proangiogenic and proinvasive proteins in SCC131 cells and inhibited ROS generation and ARase expression (Figure 2). G1/S phase cell cycle arrest is associated with autophagy cell death following apoptosis [53]. Aqueous neem leaf extract is reported to have chemopreventive effects by regulating the enzymatic breakdown of glutathione in the oral mucosa [54].
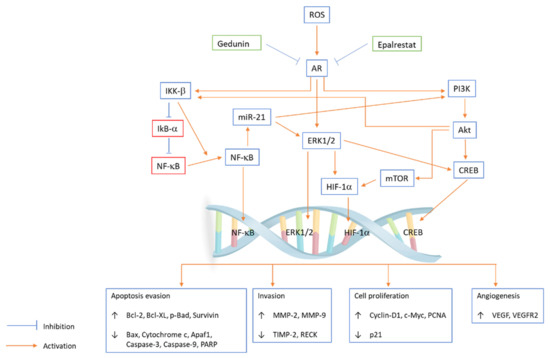
Figure 2.
PI3K/Akt/mTOR/ERK/NF-κB signalling axis pathway plays an important role in regulating a broad range of cellular functions, including cell survival, growth, proliferation, invasion, apoptosis, angiogenesis, cell cycle and migration. Gedunin and epalrestat inhibit ROS generation and AR expression in the SCC131 cell line, and the coactivation of ERK and Akt is coupled with IKK/NF-κB signalling abrogation. Epalrestat and gedunin influence subcellular localization and phosphorylation, which modulate the expression of transcription factors and key oncogenic signalling kinases. Gedunin and epalrestat inhibit AR-mediated ROS signalling, which leads to the upregulation of Bcl-2, Bcl-XL, p-Bad, survivin, MMP-2, MMP-9, cyclin D1, c-Myc, PCNA, VEGF, and VEGFR2 and downregulation of Bax, cytochrome c, Apaf1, caspase-3, caspase-9, PARP, TIMP-2, RECK and p21.
4.7. Senegalia Catechu (L. f.) P.J.H. Hurter & Mabb.
Senegalia catechu (formerly known as Acacia catechu) is commonly known as black cutch, khair and babul and belongs to the Leguminosae family. It is native to East Africa and Asian countries, especially India. Bioactive compounds present in S. catechu, such as catechin, kaempferol, rutin, mesquitol, phenol, and aromadendrin, were isolated from the leaves, stems, roots, and bark and showed various biological activities. S. catechu shows various biological activities, including antifungal, vermifuge, astringent, anti-inflammatory, antimicrobial agent, antioxidant, chemopreventive activities, and are involved in oral health maintenance and wound healing. The cytotoxic and anticancer effects of S. catechu have been reported in COLO-205, HT-1080, and HeLa cell lines in vitro [55]. In a recent study, the ethanolic extract of S. catechu bark showed cytotoxic activity against the HSCC cell line SCC-25 with an IC50 value of 52.09 µg/mL. S. catechu extract treatment significantly upregulated the expression of apoptotic marker genes bcl-2, cytochrome c (Cyt-c), bax, and caspase-8. Ethidium bromide (EB)/acridine orange (AO) and propidium iodide (PI) staining showed nuclear membrane distortion and membrane blebbing, thus confirming that S. catechu extract induced apoptosis induction in SCC-25 cells. S. catechu extract at a 25 µg/mL concentration shows cell cycle arrest with 25% cell accumulation at S phase [56]. In another study, S. catechu seed ethanol extract was analysed for cytotoxic activity against the HSCC cell line SCC-25. Ethanolic seed extract treatment of the SCC-25 cell line caused cytotoxicity, with an IC50 value of 100 µg/mL. S. catechu seed extract treatment applied to SCC-25 cells resulted in downregulation of Bcl-2 gene expression and upregulation of apoptotic gene marker expression, including cytochrome c, Bax, caspase 8 and 9. Nuclear membrane distortion and membrane blebbing were observed based on PI and EB/AO staining, indicating that A. catechu induced apoptosis in SCC-25 cells [57].
4.8. Dracaena cinnabari Balf.f.
Dracaena cinnabari belongs to the Asparagaceae family and is commonly known as dragon’s blood tree, Damm Alakhwain (Yemen). It is native to the southern coast of Yemen and has been used since ancient times as a traditional folk medicine in different countries. Various biological activities of D. cinnabari have been reported, including anticancer, antifungal, antioxidant and cytotoxic activities [58]. In a recent in vitro study, D. cinnabari extract was analysed for its apoptosis-inducing and cytotoxic effects against the OSCC (H400) cell line, with an IC50 value of 5.9 µg/mL. Upregulation of caspase 9, caspase 8, caspase 3/7 activity and depolarization of mitochondrial membrane potential (MMP) were observed with D. cinnabari treatment. In the apoptotic protein array, the results showed that the Bcl-2 protein family regulates MMP by upregulating Bid, Bax, and Bad and downregulating Bcl-2. D. cinnabari extract treatment (48 and 47 h) increased the number (p < 0.05) of H400 cells in S phase, indicating apoptosis or cell cycle arrest [59]. In a recent in vivo study, the chemopreventive potential of a D. cinnabari resin methanol extract against a 4NQO-induced oral cancer animal model was observed. D. cinnabari extract treatment (1000 mg/kg) inhibited the expression of Bcl-2, p53, Ki-67 and cyclin D1 proteins and induced apoptosis by downregulating the Cox-2, Tp53, Bcl-2, cyclin D1, and epidermal growth factor receptor (EGFR) genes and upregulating the Casp3 and Bax genes [60]. In another study, the chemopreventive effect of D. cinnabari methanolic extract treatment on 4NQO-induced tongue SCC in rats was analysed. In an in vitro study, the D. cinnabari methanolic extract showed a cytotoxic effect on H103 cells, with an IC50 value of 5.5 µg/mL in a time- and dose-dependent manner. After treatment of H103 cells, morphological changes and cell migration reduction were observed, apoptosis was induced through the intrinsic (mitochondrial) pathway, and G2/M and S phase cell cycle arrest was observed. In an in vivo study, D. cinnabari methanolic extract-treated rats showed incidences of SCC of 500 mg/kg (28.6%), 100 mg/kg (57.1%) and 1000 mg/kg (14.3%) compared to 4NQO-induced cancer rats (85.7%) [61].
4.9. Piper nigrum L.
Piper nigrum (black pepper), also known as ‘King of spices’, belongs to the Piperaceae family and is cultivated among tropical regions of India and Sri Lanka. It is known for its pungent flavour due to the presence of essential oils, volatile chemical compounds, and alkaloids (e.g., piperine) [62]. Black pepper is reported to have biological activities, such as anticancer, anti-larvicidal, pesticide, anti-Alzheimer’s, antidepressant, antiviral, and anti-inflammatory activities. A recent study reported that piperine presents cytotoxic activity against the human oral squamous carcinoma (HOSC) KB cell line [63]. In a recent in vivo study, the selective potential and cytotoxicity of the extracts of four Piper species, i.e., Piper truncatum (PT), Piper arboretum (PA), Piper cernnum (PC), and Piper mollicomum (PM), were analysed in OSCC cell lines (SCC9, SCC25, and SCC4). The three fractions PM (-L-D), PCa (-L-D) and PC (-L-D) (crude methanolic extract of leaves) with OSCC cell treatment showed toxicity, with IC50 values of 47.2, 94.2, and 47.5 µg/mL, respectively. An in vivo toxicology analysis of the PC-L-D fraction showed no significant alterations and less than 5% haemolysis [64]. Original photographs of a few important discussed plants are shown in Figure 3.

Figure 3.
Medicinal plants used in oral cancer treatment.
4.10. Zingiber Officinale Roscoe
Zingiber officinale (ginger) belongs to the Zingiberaceae family. It is cultivated mostly in Africa, India, South America, Nigeria, Thailand, and the Philippines and has been consumed worldwide as an herbal medicine and spice. Various bioactive compounds have been identified, such as terpene and phenolic compounds, gingerols, shogaols, and paradols, which have various bioactivities, including anticancer, antioxidant, antimicrobial, and anti-inflammatory activities [65]. In a recent in vitro study, zerumbone (a bioactive sesquiterpene found in Zingiber species) was analysed for its chemopreventive effect on OSCC cell lines. Zerumbone treatment of normal keratinocyte cells and OSCC cells shows cytotoxicity with IC50 values of 25 µM and 5 µM. Zerumbone inhibits the activation of the phosphatidylinositol-3-kinase-mammalian target of rapamycin (PI3K-mTOR) and chemokine C–X–C motif receptor 4 (CXCR4-RhoA) signalling pathways, leading to reduced OSCC cell viability. Zerumbone treatment (30 µM) inhibited invasion and migration, induced apoptosis in OSCC cells, and induced G2/M phase cell cycle arrest [66]. In another in vivo study, the cell proliferation and inflammatory effects of (6)-Shogaol ((6)-SHO) in DMBA-induced HBP carcinogenesis were examined by inhibiting the translocation of AP-1 and NF-κB. (6)-SHO treatment in DMBA-induced hamsters shows degradation of IκB-α, aberrant activation of AP-1, inhibition of nuclear factor kappa-B kinase subunit beta (IKKβ), c-jun, c-fos and NF-κB and upregulation of cell proliferative markers (PCNA, Ki-67 and cyclin D1) and inflammatory markers (interleukin-1 and -6, COX-2, TNF-α, inducible nitric oxide synthase (iNOS)). The results showed a reduction in the cell proliferative response and inflammation in DMBA-induced hamsters [67]. In another in vivo study, the chemopreventive effect of (6)-gingerol was analysed in DMBA-induced HBP carcinogenesis models. Oral supplementation with 20 mg/kg (6)-gingerol reduced the tumour incidence, tumour volume and tumour burden compared to DMBA treatment (1346.84 ± 81.19) and tumour volume (429.19 ± 28.29). (6)-Gingerol treatment induces HBP proapoptotic markers, inhibits cell proliferation markers: cyclin D1, inflammatory markers (interleukin [IL]-1β, TNF-α, cyclooxygenase-2, IL-6, inducible nitric oxide synthase) and proliferating cell nuclear antigen. Treatment with (6)-gingerol prevents HBP carcinogenesis by enhancing nuclear factor erythroid-2-related factor-2 expression, as DMBA-induced hamsters showed depleted Nrf2 signalling [68].
The medicinal plants and phytoextracts discussed in this review are demonstrated in Table 1.

Table 1.
Role of medicinal plants in oral cancer.
5. Phytoextracts/Phytoconstituents for the Treatment of Oral Cancer
5.1. Curcumin
Curcumin is a nontoxic polyphenol compound found in Curcuma longa turmeric and is a well-known anticancer agent due to its effect on various biological pathways involved in oncogene expression, apoptosis, metastasis, tumorigenesis, and mutagenesis [39]. A study showed that curcumin has a stimulatory effect on the extrinsic apoptotic pathway activated by binding of Fas ligand and TNF-α “death activators” to their conforming cell surface receptors. It induces apoptosis in cancer cells at the G2 cell cycle phase by upregulating p53 gene expression [69]. A recent study reported that curcumin has an IC50 value of 10 µM, which reduces the progression and migration of TSCC cells (squamous cell carcinoma of the tongue), inhibits tumorigenesis, and promotes apoptosis [70]. Curcumin was found to be effective in suppressing cyclooxygenase 2 (p = 0.03) and NF-κB (p < 0.01) expression in experimentally induced oral squamous cell carcinoma [71].
5.2. Nimbolide
Nimbolide is a type of limonoid derived from Azadirachta indica (neem tree) that inhibits the proliferation of carcinogenic cells by inducing apoptosis. The aqueous extract of neem leaves regulates the enzymatic breakdown of glutathione and thereby exerts an anticarcinogenic effect in the oral mucosa [54]. In a recent study, the chemopreventive use of nimbolide against SCC4 and EAhy926 oral cancer cell lines was analysed. Nimbolide treatment upregulated reversion-inducing cysteine-rich protein with kazal motifs (RECK) by reducing hypoxia-inducible factor (HIF-1α), and miR-21 expression led to the downregulation of the chief mediators of angiogenesis and invasion. Nimbolide inhibits DMBA-induced hamster buccal pouch carcinomas that are closely related to human OSCC cells in gene expression signatures, metastasis, precancerous lesions and histology [72]. Nimbolide treatment activates apoptosis by inhibiting the cytoprotective autophagy shielding effect through modulation of the glycogen synthase kinase (GSK)-3β and Akt phosphorylation status in oral cancer cells SCC131 and SCC4 as well as in miR-126, ncRNAs, and homeobox transcript antisense intergenic RNA (HOTAIR) [73]. Supercritical CO2 neem leaf extract and its main bioactive compound nimbolide triggered the disruption of cell migration and signalling and efficiently reduced the levels of procancer inflammatory cytokines. The use of nimbolide and SCNE decreased cyclooxygenase-2 expression and NFkBp65 and downregulated pERK1/2, pSTAT3 and pAKT in SCC4 cells [74].
5.3. Resveratrol
Resveratrol is a type of natural phytoalexin produced from red wine, strawberries and grapes. It is reported to have antioxidant and in vivo and in vitro anticarcinogenic activity through mediating cell cycle arrest and various signalling pathways. Moreover, resveratrol induces cell apoptosis in oral cancer. A study on resveratrol suggested its use as a chemopreventive agent because the compound efficiently inhibited metastasis and invasion of the OSCC KB cell line in vitro [75]. A study reported that resveratrol shows an inhibitory effect on the growth of OSCC cells by enhancing the expression of cyclin A2, cyclin B1, and phospho-cdc2 (Tyr15) and the induction of apoptosis and cell cycle arrest in G2/M phase. The IC50 values of resveratrol after 48 h of treatment against the cell lines SCC-25, YD-38 and SCC-VII were 0.7, 1.0, and 0.5 μg/mL, respectively [76] Resveratrol can possibly decrease the metastasis and invasion of OSCC in oral cancer patients because it proficiently inhibits lysophosphatidic acid (LPA)-induced oral cancer cell invasion and epithelial-mesenchymal transition (EMT) by downregulating TWIST1 and SLUG (transcription factor) expression [77]. A recent study reported that Rab coupling protein (RCP) induces OSCC invasion and EMT by expressing Zeb1 and MT1-MMP. Resveratrol was reported to inhibit RCP-induced OSCC invasion by downregulating Zeb1 and β1 integrin expression [78].
5.4. Anthocyanin
Anthocyanins are natural polyphenolic pigments responsible for purple, red and blue colours in vegetables, fruits such as purple cabbage, grapes, and berries, and exhibit anticarcinogenic activity [79]. In a recent study, SCC4, SCC9, and SCC25 OSCC cell lines treated with anthocyanin showed morphological changes, including apoptotic cells, nuclear condensation, and fragmentation. Cell cycle arrest of SCC25 cells in the S-G2/M and G0/G1 stages with simultaneous upregulation of the sub-G1 fraction was observed using flow cytometry, which indicated cell death by apoptosis. Further involvement of caspase-3 activities in anthocyanin-induced apoptosis was verified using immunofluorescence analysis, which showed that in anthocyanin (IC50 and IC80 concentration)-treated SCC25 cells, the % caspase-3 expression level was between 1.5- and 3-fold (IC50—134%, IC80—267.5%) in comparison with control cells (89.6%) [80]. Furthermore, in a different study, anthocyanin showed a reduction in the viability of OSCC cells at a 250 µg/mL concentration at 48 h and inhibited the invasion and migration abilities of the OSCC cell lines SCC15 and Tca8113. Anthocyanin treatment in OSCC cells led to a significant increase in the protein expression of caspase-1, IL-1β, NLRP3 (nucleotide-binding oligomerization domain-like receptor pyrin domain-3) and was correlated with the activation of pyroptosis, and the administration of a caspase-1 inhibitor increased the invasiveness, cell viability, and migration compared to the anthocyanin-treated group [81].
5.5. Piperine
Piperine (1-piperidine) is an alkaloid found in the roots and fruits of Piper longum L. and P. nigrum L., and it is reported to have potential therapeutic activities, such as antioxidant, anticancer, immunomodulatory, and antimutagenic activities [82]. A study conducted to verify the chemoprevention efficacy of piperine during hamster buccal pouch carcinogenesis induced by DMBA found that tumour formation in hamster buccal pouches painted with DMBA alone was 100% after 14 weeks, the tumour burden was 1399.7 mm3 and the mean tumour volume was 378.3 mm3. Piperine oral administration completely prevents OSCC formation in hamster buccal pouches painted with DMBA (at a dose of 50 mg/kg body weight) alternately for 14 weeks. Piperine also restored the status of detoxifying agents, antioxidants, and lipid peroxidation in DMBA-painted hamsters [83]. A mechanism by which piperine induces apoptosis is suggested via the reduction in mitochondrial membrane potential (MMP) and liberation of ROS following cell cycle arrest and caspase-3 activation. The study results showed that the viability of the HOSC KB cell line was reduced significantly (p < 0.001) by treatment with various piperine concentrations (25 mM (90.14%), 50 mM (76.59%), 100 mM (52.39%), 200 mM (25.26%), and 300 mM (18.96%)), and the IC50 value of piperine in KB cells was 124 µM. A cell cycle study showed that piperine reduced the DNA content and arrested cells in the G2/M phase [63]. A recent study performed in silico docking simulations and showed that inside the ATP binding site in cyclin-dependent kinase 2, there is hydrogen bonding between residue Ser5 and piperine, which predicts protein-piperine hydrophobic interactions and antitumour molecular mechanisms. In vivo assays with supercritical fluid (SCF) inhibited the expression of the cell cycle proteins cyclin A and CDK2 and the antiapoptotic protein Bcl-xL and increased the expression of the proapoptotic proteins p53 and Bax [84]. In a recent study, the findings of molecular docking analysis showed an optimal hydrogen bonding length between the ligand piperine and cell cycle proteins, including cyclin D (2.62), cyclin T (1.5), CDK4 (3.26), and CDK2 (3.19) [85].
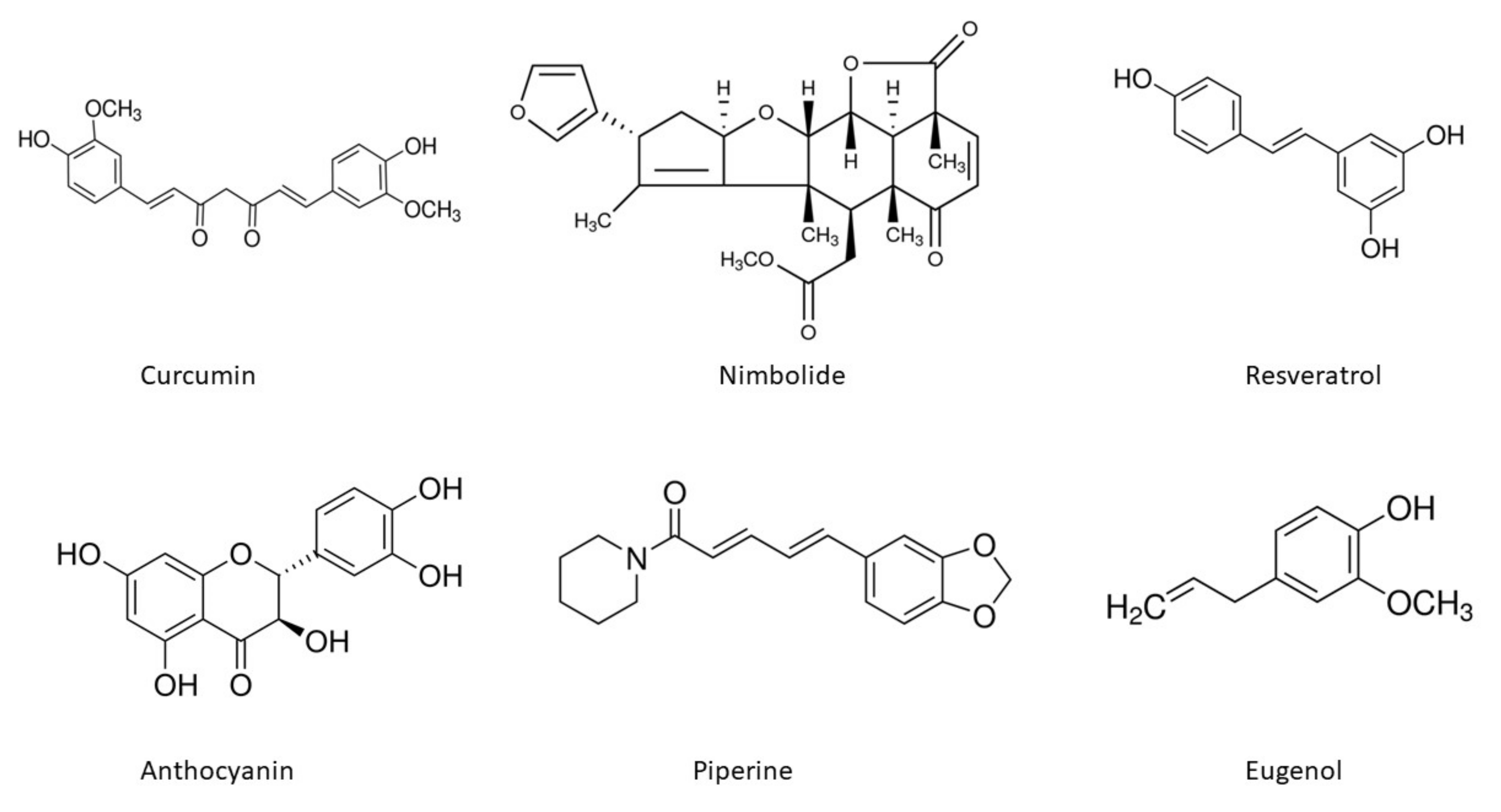
The chemical structures of the important phytoconstituents discussed in the review paper are shown in Figure 4.
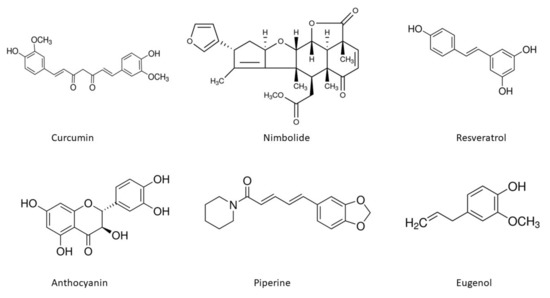
Figure 4.
Chemical structures of phytoextracts useful in oral cancer treatment.
5.6. Eugenol
Eugenol is a chief phytochemical present in clove oil extracted from cloves. Anticancer activities and eugenol-induced apoptosis molecular mechanisms have been reported against various cancers, such as leukaemia, melanoma, skin tumours, and osteosarcoma. However, a few studies have documented the oral cancer activities of eugenol. A study conducted to assess the mechanism of cytotoxicity induced by eugenol towards OSCC cells reported that eugenol induced nonapoptotic cell death in OSCC cells based on the metabolic profile. Eugenol treatment in OSCC cells induced oxidative stress by increasing the oxidized form of glutathione (69%) and the methionine sulfoxide/methione ratio (37%), increasing glycolytic metabolites and polyamines, and decreasing ATP utilization based on a 53% decrease in the ADP/ATP ratio and a 70% decrease in the AMP/ATP ratio [86]. In another study, the pathological and synergistic effects of oral mucositis were analysed using an N-succinyl chitosan gel delivery system of microemulsified sodium hyaluronate (0.2% w/v), eugenol (10% v/v), and honey (10% v/v). Compared with chitosan gel, N-succinyl chitosan mediated the efflux of eugenol and showed better results in rat buccal mucosal tissue ex vivo penetration studies, with an enhancement ratio of 1.71, and eugenol release from N-succinyl chitosan was 87.45 ± 0.14% in PBS buffer (pH-6.4) after 8 h in an in vitro study. N-succinyl chitosan orogel reduced inflammation in the oral mucosa of animals (p < 0.05) compared to the disease control [87]. In a recent in vitro study, the anticancer effect of eugenol extracted from Cinnamomum verum was analysed in an OSCC cell line. Eugenol treatment of OSCC 25 cells using the MTT cytotoxic assay showed an IC50 value of 24.71 µM, with concentrations ranging from 1.9 µg/mL to 1000 µg/mL. In a DNA fragmentation assay (48 h) of a 25 µM eugenol treatment of SCC cells, the agarose gel electrophoresis results showed a ladder pattern in eugenol-treated cells and the absence of a ladder pattern in untreated cells or negative controls, indicating apoptosis. A cell cycle analysis showed that a 25 µM eugenol treatment of the SSC 25 cell line resulted in a subsequent increase in the sub-G0 population and S phase arrest [88]. The roles of the phytoconstituents discussed in this review are demonstrated in Table 2.

Table 2.
Role of phytoextract in oral cancer.
6. Safety of Phytoextracts in Oral Cancer Treatment
In a recent phase I randomized clinical trial (RCT), the safety of curcuminoids (Curcuma longa) and an extract of Bidens pilosa L. as a mucoadhesive formulation (FITOPROT) for the treatment and prevention of oral mucositis induced by chemoradiotherapy in patients with HNSCC was investigated. A total of 20 healthy adult participants were divided into 2 groups: Group I received B. pilosa extract 20% v/v and curcuminoids 10 mg/mL (FITOPROT), and Group II received B. pilosa extract 40% v/v and curcuminoids 20 mg/mL (FITOPROT). The treatments were administered for ten consecutive days, three times daily. The results showed no change in micronuclei frequencies (p > 0.05) or cellular genotoxic effects (p > 0.05). In biochemical assays, no difference was found in pro45 inflammatory cytokine levels (p > 0.05) [89]. In another phase I CT, the effect of APG-157, a botanical drug containing curcumin and other polyphenols, on oral cancer was determined under the US Food and Drug Administration’s Botanical Drug Development division. In this study, 12 oral cancer patients and 13 normal controls were selected, and two transoral doses of 200 mg and 100 mg were given every hour for a 3 h period. After 3 h of treatment, reductions in IL-6, IL-8, and IL-1β levels and bacteroid species and peaks in circulating curcumin were observed in cancer subjects [90].
7. Antioxidants and Anticancer Activity Relationship
Antioxidants are compounds that inhibit oxidation, and they are commonly found in vegetables, medicinal plants, fruits (e.g., blueberries and cranberries) and grains. Antioxidants have a stable aromatic ring system that facilitates the delocalization of unpaired electrons in reactive oxygen species. These compounds eliminate the unpaired electron status of ROS by donating or accepting an electron to neutralize ROS and mitigate excessive ROS-induced oxidative stress by converting and scavenging ROS into less reactive species. A reduction in ROS levels can reverse the initiation of carcinogenesis [91]. Excess ROS can cause damage to RNA, DNA, and proteins, which further cause genetic alterations in cells, resulting in mutagenesis and eventually cancer. Low levels of ROS are essential in biological functions, including cell proliferation, growth, differentiation and cell survival. Studies have reported that ROS participate in both the p38 mitogen-activated protein kinase (p38MAPK) tumour suppressing pathway and Ras-Raf-MEK1/2-ERK1/2 oncogenic signalling. Some anticancer drugs cause cancer cell death by inducing ROS generation; for example, doxorubicin, an anthracycline-based anticancer drug, causes cancer cell death by accumulating hydroxyl radicals [92]. Some natural compounds also exhibit anticancer effects by inducing ROS; for example, curcumin treatment of OSCC cells induces apoptosis and induces intracellular ROS [41]. Gedunin and epalrestat treatment inhibits ROS generation, and piperine causes apoptosis by inducing ROS generation following cell cycle arrest [53]. Resveratrol induces the ROS-p38-p53 pathway by upregulating phosphorylated p38 MAPK gene expression [92]. The reason for cell death in cancer cells is due to the low tolerance thresholds of redox homeostasis; however, normal cells can tolerate drug-induced ROS stress [93]. Natural compounds show harmful or beneficial effects independent of their oxidative properties. Dietary antioxidants eliminate excess oxygen metabolites, which help reinforce our antioxidant system. Synergistic action between exogenous and endogenous antioxidants is often observed, which provides a balance between antioxidation protection and oxidant production and is believed to play an important role in maintaining a healthy biological system [94].
8. Conclusions and Future Perspectives
Since ancient times, plants and their bioactive substances have been used for medicinal purposes. Medicinal herbs are a gift from nature to humanity, and they have been demonstrated to possess significant anticancer properties. Phytochemicals found in some medicinal plant species have shown the ability to suppress the progression and development of oral cancer. Natural dietary phytoconstituents will continue to be a promising and active research topic in coming years. Further studies should be performed to explore promising phytochemicals from plants to assess their potential uses, mechanisms of action, pharmacokinetic profiles, metabolism, pharmacodynamic responses, toxicities, polymorphisms, drug-drug interactions, and dosage regimens for use as standard herbal medicines. Large-scale, well-controlled clinical trials are required to evaluate their efficacies, side effects, and safety. Furthermore, there is a need to further investigate the synergistic effects of phytochemical compound and chemotherapeutic drug combinations in cancer cells. Thus, the chemopreventive and anticancer properties of phytochemicals have attracted the interest of researchers in recent years because of their ability to provide anticancer treatment with low intrinsic toxicity and high effectiveness.
Author Contributions
Conceptualization and Supervision: M.K., M.M., R., N.K. and S.P. (Sunil Puri); writing—original draft preparation: S.P.B., A.K.S., M.T., A.S., N.K.G., A.P., S.R., S.C., S.D., A.T., M.S. (Minnu Sasi), T.I., P.C.P., S.P. (Suraj Prakash) and S.S.; writing—review and editing: M.S. (Marisennayya Senapathy), M.K., M.N., R., S.P., S.N., R.A., A.D., M.M.A.-D., M.S. (Munisha Sharma) and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the University of Kiel and Schleswig-Holstein for their support through the OA program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hema, K.N.; Smitha, T.; Sheethal, H.S.; Mirnalini, S.A. Epigenetics in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Yasmine, G.; Elnaaj, A. Global incidence and risk factors of oral cancer. Harefuah 2017, 156, 645–649. [Google Scholar]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [Green Version]
- Irani, S. New insights into oral cancer—Risk factors and prevention: A review of literature. Int. J. Prev. Med. 2020, 11, 202. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression induced by chronic inflammation and the progression to oral squamous cell carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Prakash, S.; Bhatia, R.; Negi, M.; Singh, J.; Bishnoi, M.; Kondepudi, K.K. Generation of structurally diverse pectin oligosaccharides having prebiotic attributes. Food Hydrocoll. 2020, 108, 10. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Saurabh, V.; Sasi, M.; Punia, S.; Potkule, J.; Maheshwari, C.; Changan, S.; Radha, B.B.; Singh, S.; et al. Delineating the inherent functional descriptors and biofunctionalities of pectic polysaccharides. Carbohydr. Polym. 2021, 269, 5988. [Google Scholar] [CrossRef]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S.; et al. Custard Apple (Annona squamosa L.) Leaves: Nutritional Composition 8319, Phytochemical Profile, and Health-Promoting Biological Activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S.; et al. Cottonseed: A sustainable contributor to global protein requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Kumar, M.; Potkule, J.; Patil, S.; Mageshwaran, V.; Radha, S.V.; Berwal, M.K.; Mahapatra, A.; Saxena, S.; Ashtaputre, N.; Souza, C.D. Evaluation of detoxified cottonseed protein isolate for application as food supplement. Toxin Rev. 2021. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Dwivedi, J.; Jain, P.K.; Satpathy, S.; Patra, A. Medicinal plants for treatment of cancer: A brief review. Pharmacogn. J. 2016, 8, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.K.; Waske, S. Study of Medicinal Plants used by Local Herbal Healers in South Block of Seoni District (M.P.). Int. J. Theor. Appl. Sci. 2018, 10, 95–99. [Google Scholar]
- Radha, P.S.; Chandel, K.; Pundir, A.; Thakur, M.S.; Chauhan, B.; Simer, K.; Dhiman, N.; Shivani Thakur, Y.S.; Kumar, S. Diversity of ethnomedicinal plants in Churdhar Wildlife Sanctuary of district Sirmour of Himachal Pradesh, India. J. Appl. Pharm. Sci. 2019, 9, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Radha, P.S.; Kumar, V. Phytochemical screening of medicinal plants used by tribal migratory shepherds in Western Himalaya. Ann. Biol. 2019, 35, 11–14. [Google Scholar]
- Radha, S.P.; Pundir, A. Review on Ethnomedicinal Plant: Trillium govanianum Wall. Ex D. Don. Int. J. Theor. Appl. Sci. 2019, 11, 4–9. [Google Scholar]
- Radha, J.S.; Srivastava, S.; Negi, V. Ethnobotanical study of medicinal plants used in shikari devi wildlife sanctuary of Himachal Pradesh, India. Med. Plants 2020, 12, 666–673. [Google Scholar] [CrossRef]
- Radha, R.; Chauhan, P.; Puri, S.; Thakur, M.; Rathour, S.; Sharma, A.K.; Pundir, A. A study of wild medicinal plants used in Nargu Wildlife Sanctuary of district Mandi in Himachal Pradesh, India. J. Appl. Pharm. Sci. 2021, 11, 135–144. [Google Scholar] [CrossRef]
- Radha, K.M.; Puri, S.; Pundir, A.; Bangar, S.P.; Changan, S.; Choudhary, P.; Parameswari, E.; Alhariri, A.; Samota, M.K.; Damale, R.D.; et al. Evaluation of Nutritional, Phytochemical, and Mineral Composition of Selected Medicinal Plants for Therapeutic Uses from Cold Desert of Western Himalaya. Plants 2021, 10, 1429. [Google Scholar] [CrossRef]
- Radha, I.; Janjua, S.; Ali, M.; Thakur, M.; Jamwal, R.; Rathour, S.; Kumar, P.A.; Kumari, N.; Puri, S.; Pundir, A.; et al. Documenting Traditional Knowledge before they are Forgotten: A Study on the Ethnomedicinal uses of Wild Plants by Rural People of Jubbarhatti in District Shimla. Int. J. Theor. Appl. Sci. 2021, 13, 37–51. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Home–The Plant List. 2013. Available online: http://www.theplantlist.org/ (accessed on 22 May 2021).
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126. [Google Scholar] [CrossRef]
- Yakop, F.; Abd Ghafar, S.A.; Yong, Y.K.; Saiful Yazan, L.; Mohamad, H.R.; Lim, V.; Eshak, Z. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sens. Int. 2020, 1, 100046. [Google Scholar] [CrossRef]
- Franklyn, J.; Janakiraman, R.; Tirkey, A.; Thankachan, C.; Muthusami, J. Oral verrucous carcinoma: Ten-year experience from a Tertiary Care Hospital in India. Indian J. Med Paediatr. Oncol. 2017, 38, 452. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.D.; Liu, O.S.; Tang, Z.G. Oral verrucous carcinoma: A retrospective clinical study of 29 Chinese patients. Int. J. Clin. Exp. Med. 2017, 10, 5228–5232. [Google Scholar]
- Shergill, A.K.; Solomon, M.C.; Carnelio, S.; Kamath, A.T.; Aramanadka, C.; Shergill, G.S. Verrucous carcinoma of the oral cavity: Current concepts. Int. J. Sci. Study 2015, 3, 114–118. [Google Scholar]
- Padhye, A.; D’Souza, J. Oral malignant melanoma: A silent killer. J. Indian Soc. Periodontol. 2011, 15, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of melanoma. Exon Publ. 2017, 3–22. [Google Scholar] [CrossRef]
- Zito, P.M.; Mazzoni, T. Cancer, Melanoma, Oral. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30020648 (accessed on 22 May 2021).
- Guevara-Canales, J.O.; Morales-Vadillo, R.; de Faria, P.E.; Sacsaquispe-Contreras, S.J.; Leite, F.P.; Chaves, M.G. Systematic review of lymphoma in oral cavity and maxillofacial region. Acta Odontol. Latinoam. AOL 2011, 24, 245–250. Available online: https://pubmed.ncbi.nlm.nih.gov/22550817/ (accessed on 22 May 2021).
- Domingues, T.; Silva, B.; Belo, C.; Ferreira, T.; Leite, G.B.; De Menezes Pontes, J.R.; Antunes, H.S. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience 2016. [Google Scholar] [CrossRef] [Green Version]
- Luke, A.M.; Patnaik, R.; Kuriadom, S.T.; Jaber, M.; Mathew, S. An in vitro study of Ocimum sanctum as a chemotherapeutic agent on oral cancer cell-line. Saudi J. Biol. Sci. 2021, 28, 887–890. [Google Scholar] [CrossRef]
- Shivpuje, P.; Ammanangi, R.; Bhat, K.; Katti, S. Effect of Ocimum sanctum on Oral Cancer Cell Line: An in vitro Study. J. Contemp. Dent. Pract. 2015, 16, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Niyomtham, N.; Yingyongnarongkul, B.E.; Koontongkaew, S. Ethanolic Extract of Ocimum sanctum Leaves Reduced Invasion and Matrix Metalloproteinase Activity of Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2020, 21, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Wang, H. Anticancer activity of Vicenin-2 against 7,12 dimethylbenz[a]anthracene-induced buccal pouch carcinoma in hamsters. J. Biochem. Mol. Toxicol. 2021, 35, e22673. [Google Scholar] [CrossRef]
- Sood, S.; Nagpal, M. Role of curcumin in systemic and oral health: An overview. J. Nat. Sci. Biol. Med. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.M.; Patel, V.; Shyur, L.F.; Lee, W.L. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine 2016, 23, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Sakamoto, T.; Zhengguang, L.; Yasui, H.; Hamada, H.; Kubo, H.; Nakajima, M. Curcumin inhibits epithelial-mesenchymal transition in oral cancer cells via c-Met blockade. Oncol. Lett. 2020, 19, 4177–4182. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Arjmandi, B. Evidence for Anti-Cancer Properties of Blueberries: A Mini-Review. Anti Cancer Agents Med. Chem. 2013, 13, 1142–1148. [Google Scholar] [CrossRef]
- Baba, A.B.; Kowshik, J.; Krishnaraj, J.; Sophia, J.; Dixit, M.; Nagini, S. Blueberry inhibits invasion and angiogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters via suppression of TGF-β and NF-κB signaling pathways. J. Nutr. Biochem. 2016, 35, 37–47. [Google Scholar] [CrossRef]
- Baba, A.B.; Nivetha, R.; Chattopadhyay, I.; Nagini, S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem. Toxicol. 2017, 109, 534–543. [Google Scholar] [CrossRef]
- Chang, H.P.; Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Juan, Y.N.; Tsao, J.W.; Chiu, H.Y.; Yang, J.S. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. Int. J. Oncol. 2018, 52, 1504–1514. [Google Scholar] [CrossRef]
- Ankola, A.; Kumar, V.; Thakur, S.; Singhal, R.; Smitha, T.; Sankeshwari, R. Anticancer and antiproliferative efficacy of a standardized extract of Vaccinium macrocarpon on the highly differentiating oral cancer KB cell line athwart the cytotoxicity evaluation of the same on the normal fibroblast L929 cell line. J. Oral Maxillofac. Pathol. 2020, 24, 258. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, K.; Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid. Based Complementary Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Sur, S.; Ray, R.B. Bitter Melon (Momordica Charantia), a Nutraceutical Approach for Cancer Prevention and Therapy. Cancers 2020, 12, 2064. [Google Scholar] [CrossRef]
- Sur, S.; Steele, R.; Aurora, R.; Varvares, M.; Schwetye, K.E.; Ray, R.B. Bitter Melon Prevents the Development of 4-NQO–Induced Oral Squamous Cell Carcinoma in an Immunocompetent Mouse Model by Modulating Immune Signaling. Cancer Prev. Res. 2018, 11, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, S.; Nakanishi, H.; Flaveny, C.; Ippolito, J.E.; McHowat, J.; Ford, D.A.; Ray, R.B. Inhibition of the key metabolic pathways, glycolysis and lipogenesis, of oral cancer by bitter melon extract. Cell Commun. Signal. 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Prakash, S.; Radha, K.N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; Pradhan, P.C.; et al. Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Tanagala, K.K.K.; Baba, A.B.; Kowshik, J.; Reddy, G.B.; Nagini, S. Gedunin, A Neem Limonoid in Combination with Epalrestat Inhibits Cancer Hallmarks by Attenuating Aldose Reductase-Driven Oncogenic Signaling in SCC131 Oral Cancer Cells. Anti Cancer Agents Med. Chem. 2018, 18, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Popli, D.B.; Sircar, K.; Chowdhry, A. A review of the anticancer activity of Azadirachta indica (Neem) in oral cancer. J. Oral Biol. Craniofac. Res. 2020, 10, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Antioxidant, Anti-inflammatory, and Chemoprotective Properties of Acacia catechu Heartwood Extracts. Phytother. Res. 2015, 29, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, T.; Ezhilarasan, D.; Vijayaragavan, R.; Bhullar, S.K.; Rajendran, R. Acacia catechu ethanolic bark extract induces apoptosis in human oral squamous carcinoma cells. J. Adv. Pharm. Technol. Res. 2017, 8, 143–149. [Google Scholar] [CrossRef]
- Lakshmi, T.; Ezhilarasan, D.; Nagaich, U.; Vijayaragavan, R. Acacia catechu ethanolic seed extract triggers apoptosis of SCC-25 cells. Pharmacogn. Mag. 2017, 13, 405–411. [Google Scholar] [CrossRef]
- Al-Fatimi, M. Ethnobotanical survey of Dracaena cinnabari and investigation of the pharmacognostical properties, antifungal and antioxidant activity of its resin. Plants 2018, 7, 91. [Google Scholar] [CrossRef] [Green Version]
- Alabsi, A.M.; Lim, K.L.; Paterson, I.C.; Ali-Saeed, R.; Muharram, B.A. Cell Cycle Arrest and Apoptosis Induction via Modulation of Mitochondrial Integrity by Bcl-2 Family Members and Caspase Dependence in Dracaena cinnabari—Treated H400 Human Oral Squamous Cell Carcinoma. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Al-Afifi, N.; Alabsi, A.; Kaid, F.; Bakri, M.; Ramanathan, A. Prevention of oral carcinogenesis in rats by Dracaena cinnabari resin extracts. Clin. Oral Investig. 2018, 23, 2287–2301. [Google Scholar] [CrossRef]
- Al-Afifi, N.A.; Alabsi, A.M.; Shaghayegh, G.; Ramanathan, A.; Ali, R.; Alkoshab, M.; Bakri, M.M. The in vitro and in vivo antitumor effects of Dracaena cinnabari resin extract on oral cancer. Arch. Oral Biol. 2019, 104, 77–89. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahamad, M.S.; Jafri, A.; Afzal, M.; Arshad, M. Piperine Triggers Apoptosis of Human Oral Squamous Carcinoma Through Cell Cycle Arrest and Mitochondrial Oxidative Stress. Nutr. Cancer 2017, 69, 791–799. [Google Scholar] [CrossRef]
- Macedo, A.L.; da Silva, D.P.D.; Moreira, D.L.; de Queiroz, L.N.; Vasconcelos, T.R.A.; Araujo, G.F.; Kaplan, M.A.C.; Pereira, S.S.C.; de Almeida, E.C.P.; Valverde, A.L.; et al. Cytotoxicity and selectiveness of Brazilian Piper species towards oral carcinoma cells. Biomed. Pharmacother. 2019, 110, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zainal, N.S.; Gan, C.P.; Lau, B.F.; Yee, P.S.; Tiong, K.H.; Abdul Rahman, Z.A.; Patel, V.; Cheong, S.C. Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine 2018, 39, 33–41. [Google Scholar] [CrossRef]
- Annamalai, G.; Suresh, K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed. Pharmacother. 2018, 98, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, J.; Wang, F. [6]-Gingerol impedes 7,12-dimethylbenz(a)anthracene-induced inflammation and cell proliferation-associated hamster buccal pouch carcinogenesis through modulating Nrf2 signaling events. J. Biochem. Mol. Toxicol. 2021, 35, e22689. [Google Scholar] [CrossRef]
- Alok, A.; Singh, I.; Singh, S.; Jha, A. Curcumin: Pharmacological actions and its role in head and neck squamous cell carcinoma—A review. J. Indian Acad. Oral Med. Radiol. 2017, 29, 115. [Google Scholar] [CrossRef]
- Ardito, F.; Perrone, D.; Giuliani, M.; Testa, N.F.; Muzio, L.L. Effects of Curcumin on Squamous Cell Carcinoma of Tongue: An In Vitro Study. Curr. Top. Med. Chem. 2018, 18, 233–243. [Google Scholar] [CrossRef]
- Maulina, T.; Hadikrishna, I.; Hardianto, A.; Sjamsudin, E.; Pontjo, B.; Yusuf, H.Y. The therapeutic activity of curcumin through its anti-cancer potential on oral squamous cell carcinoma: A study on Sprague Dawley rat. SAGE Open Med. 2019, 7, 205031211987598. [Google Scholar] [CrossRef]
- Kowshik, J.; Mishra, R.; Sophia, J.; Rautray, S.; Anbarasu, K.; Reddy, G.D.; Dixit, M.; Mahalingam, S.; Nagini, S. Nimbolide upregulates RECK by targeting miR-21 and HIF-1α in cell lines and in a hamster oral carcinogenesis model. Sci. Rep. 2017, 7, 2045. [Google Scholar] [CrossRef] [Green Version]
- Sophia, J.; Kowshik, J.; Dwivedi, A.; Bhutia, S.K.; Manavathi, B.; Mishra, R.; Nagini, S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/ GSK-3β signalling pathway in oral cancer. Cell Death Dis. 2018, 9, 1087. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.; Gonzales, C.B.; De La Chapa, J.J.; Cabang, A.B.; Fountzilas, C.; Patel, M.; Orozco, S.; Wargovich, M.J. The Highly Pure Neem Leaf Extract, SCNE, Inhibits Tumorigenesis in Oral Squamous Cell Carcinoma via Disruption of Pro-tumor Inflammatory Cytokines and Cell Signaling. Front. Oncol. 2019, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Yang, G.; Xiang, W.; Pei-Jun, W.; Bin, Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J. Cancer Res. Clin. Oncol. 2014, 140, 371–374. [Google Scholar] [CrossRef]
- Yu, X.D.; Yang, J.L.; Zhang, W.L.; Liu, D.X. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumor Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Lee, H.Y. Effect of Resveratrol on Oral Cancer Cell Invasion Induced by Lysophosphatidic Acid. J. Dent. Hyg. Sci. 2018, 18, 188–193. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, K.H.; Jeong, B.Y.; Park, C.G.; Lee, H.Y. Experimental & Molecular Medicine Zeb1 for RCP-induced oral cancer cell invasion and its suppression by resveratrol. Exp. Mol. Med. 2020, 52, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.M.; Frond, A.D.; Ştirbu, I.; Rugina, D.; Socaciu, C. Anthocyanins-Smart Molecules for Cancer Prevention. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018. [Google Scholar]
- Madanakumar, A.J.; Lawarence, B.; Manoj, G.S.; Kumaraswamy, M. Purified Anthocyanin from in vitro Culture of Bridelia retusa (L.) Spreng. Capable of Inhibiting the Growth of Human Oral Squamous Cell Carcinoma Cells. Pharmacogn. J. 2018, 10, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Yue, E.; Tuguzbaeva, G.; Chen, X.; Qin, Y.; Li, A.; Sun, X.; Dong, C.; Liu, Y.; Yu, Y.; Zahra, S.M.; et al. Anthocyanin is involved in the activation of pyroptosis in oral squamous cell carcinoma. Phytomedicine 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2017, 25, 4918–4928. [Google Scholar] [CrossRef]
- Manoharan, S.; Balakrishnan, S.; Menon, V.P.; Alias, L.M.; Reena, A.R. Chemopreventive & efficacy of curcumin and piperine during 7,12-dimethylbenz [a]anthracene-induced hamster buccal pouch carcinogenesis. Singap. Med J. 2009, 50, 139–146. Available online: https://europepmc.org/article/med/19296028 (accessed on 22 May 2021).
- Grinevicius, V.M.; Andrade, K.S.; Mota, N.S.; Bretanha, L.C.; Felipe, K.B.; Ferreira, S.R.; Pedrosa, R.C. CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem. Toxicol. 2019, 132, 110644. [Google Scholar] [CrossRef]
- Rekha, U.V.; Anita, M.; Jayamathi, G.; Sadhana, K.; Deepa, S.; Hussain, S.; Bhuvaneswari, J.; Ramya, V.; Selvaraj, J.; Naveenraj, N.S. Molecular docking analysis of piperine with CDK2, CDK4, Cyclin D and Cyclin T proteins. Bioinformation 2020, 16, 359–362. [Google Scholar] [CrossRef]
- Koh, T.; Murakami, Y.; Tanaka, S.; Machino, M.; Onuma, H.; Kaneko, M.; Sugimoto, M.; Soga, T.; Tomita, M.; Sakagami, H. Changes of Metabolic Profiles in an Oral Squamous Cell Carcinoma Cell Line Induced by Eugenol. In Vivo 2013, 27, 233–243. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23422484 (accessed on 22 May 2021). [PubMed]
- Dhawan, N.; Kumar, K.; Kalia, A.N.; Arora, S. N-Succinyl Chitosan as Buccal Penetration Enhancer for Delivery of Herbal Agents in Treatment of Oral Mucositis. Curr. Drug Deliv. 2014, 11, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Narasimhan, M.; Balaji, T.M.; Chamundeeswari, D.P.; Sakthisekaran, D. In Vitro Anticancer Effects of Cinnamomum verum J. Presl, Cinnamaldehyde, 4 Hydroxycinnamic Acid and Eugenol on an Oral Squamous Cell Carcinoma Cell Line. J. Contemp. Dent. Pract. 2020, 21, 1027–1033. [Google Scholar] [CrossRef]
- Dos Santos Filho, E.X.; Arantes, D.A.C.; Oton Leite, A.F.; Batista, A.C.; Mendonça, E.F.; de Marreto, R.N.; Naves, L.N.; Lima, E.M.; Valadares, M.C. Randomized clinical trial of a mucoadhesive formulation containing curcuminoids (Zingiberaceae) and Bidens pilosa Linn (Asteraceae) extract (FITOPROT) for prevention and treatment of oral mucositis—Phase I study. Chem. Biol. Interact. 2018, 291, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H. A randomized, phase 1, placebo-controlled trial of APG-157 in oral cancer demonstrates systemic absorption and an inhibitory effect on cytokines and tumor-associated microbes. Cancer 2020, 126, 1668–1682. [Google Scholar] [CrossRef]
- Fernando, W.; Rupasinghe, H.P.V.; Hoskin, D.W. Dietary phytochemicals with antioxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Ou-Yang, F.; Hou, M.F.; Huang, H.W.; Wang, H.R.; Li, K.T.; Fayyaz, S.; Shu, C.W.; Chang, H.W. Oxidative stress-modulating drugs have preferential anticancer effects—Involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration. Semin. Cancer Biol. 2019, 58, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).