Aberrant Salience Network Functional Connectivity in Resting-State and Fear-Related Autobiographical Memory Recall in Female Adolescents with Borderline Personality Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Sociodemographic and Clinical Assessment
2.3. EEG Recordings
2.4. EEG Functional Connectivity Analyses
2.5. Statistical Analyses
3. Results
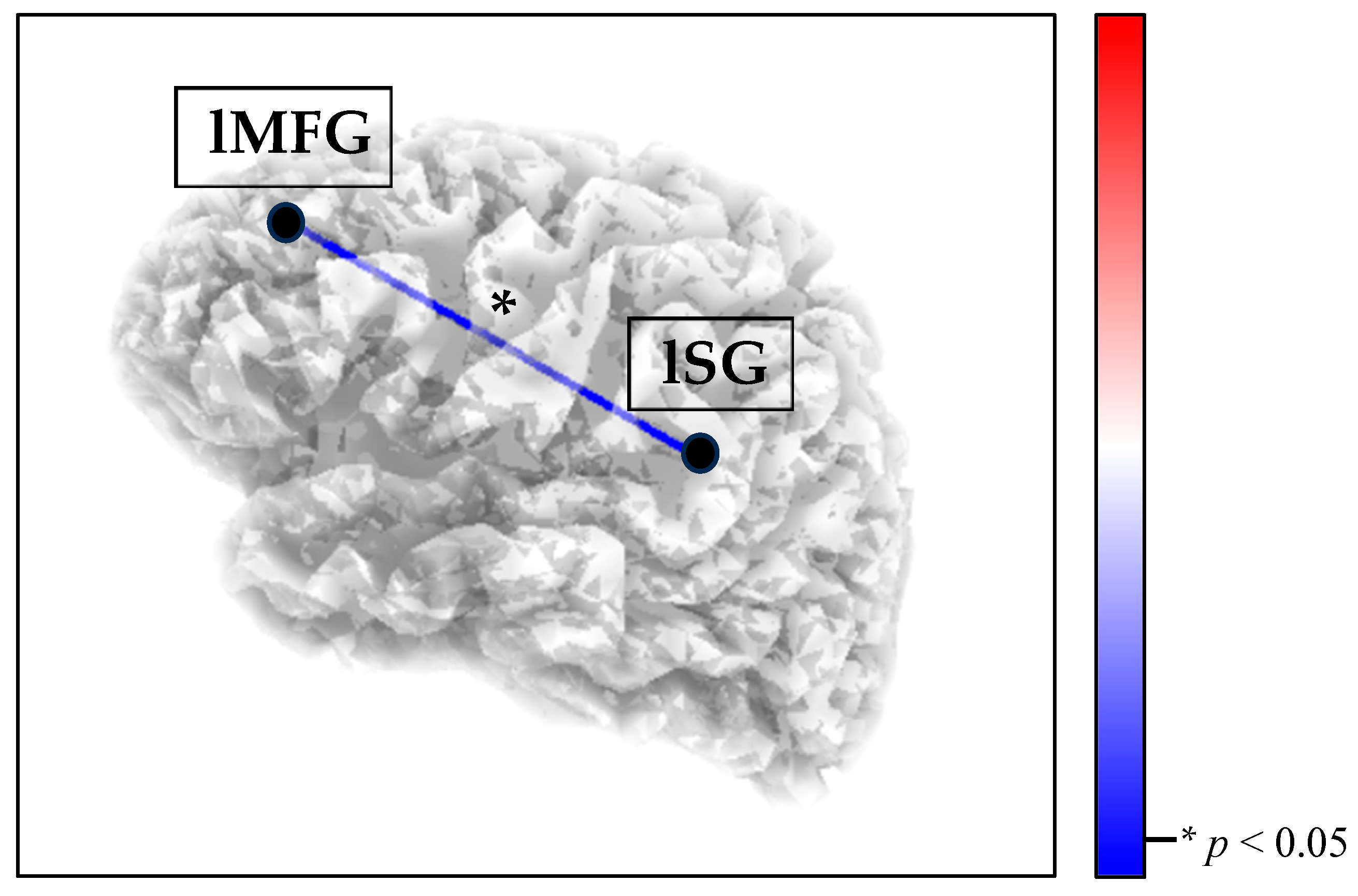
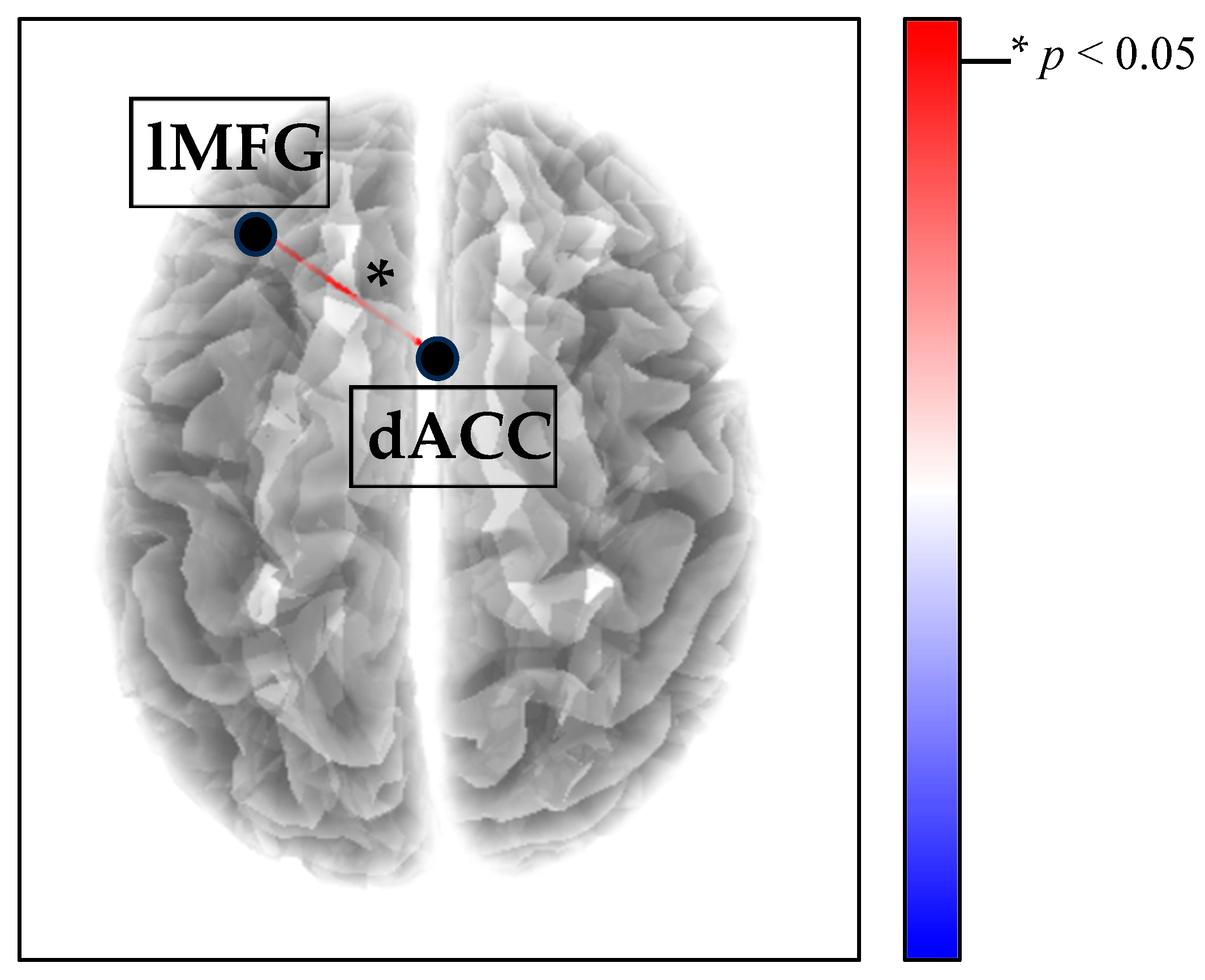
3.1. Between-Group Functional Connectivity Results
Within Functional Connectivity Results
3.2. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanen, A.; Sharp, C.; Hoffman, P. Prevention and early intervention for borderline personality disorder: A novel public health priority. World Psychiatry 2017, 16, 215–216. [Google Scholar] [CrossRef]
- Zimmerman, M.; Gazarian, D. Is research on borderline personality disorder underfunded by the national institute of health? Psychiatry Res. 2014, 220, 941–944. [Google Scholar] [CrossRef]
- Iliakis, E.A.; Sonley, A.K.I.; Ilagan, G.S.; Choi-Kain, L.W. Treatment of borderline personality disorder: Is supply adequate to meet public health needs? Psychiatr. Serv. 2019, 70, 772–781. [Google Scholar] [CrossRef]
- Gunderson, J.G. The emergence of a generalist model to meet public health needs for patients with borderline personality disorder. Am. J. Psychiatry 2016, 173, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Reichl, C.; Kaess, M. Self-harm in the context of borderline personality disorder. Curr. Opin. Psychol. 2021, 37, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wertz, J.; Caspi, A.; Ambler, A.; Arseneault, L.; Belsky, D.W.; Danese, A.; Fisher, H.L.; Matthews, T.; Richmond-Rakerd, L.S.; Moffitt, T.E. Borderline symptoms at age 12 signal risk for poor outcomes during the transition to adulthood: Findings from a genetically sensitive longitudinal cohort study. J. Am. Acad. Child. Adolesc. Psychiatry 2020, 59, 1165–1177.e1162. [Google Scholar] [CrossRef] [PubMed]
- Winsper, C.; Marwaha, S.; Lereya, S.T.; Thompson, A.; Eyden, J.; Singh, S.P. Clinical and psychosocial outcomes of borderline personality disorder in childhood and adolescence: A systematic review. Psychol. Med. 2015, 45, 2237–2251. [Google Scholar] [CrossRef]
- Winsper, C. Borderline personality disorder: Course and outcomes across the lifespan. Curr. Opin. Psychol. 2021, 37, 94–97. [Google Scholar] [CrossRef]
- Ruocco, A.C.; Marceau, E.M. Update on the neurobiology of borderline personality disorder: A review of structural, resting-state and task-based brain imaging studies. Curr. Psychiatry Rep. 2024, 26, 807–815. [Google Scholar] [CrossRef]
- Leichsenring, F.; Fonagy, P.; Heim, N.; Kernberg, O.F.; Leweke, F.; Luyten, P.; Salzer, S.; Spitzer, C.; Steinert, C. Borderline personality disorder: A comprehensive review of diagnosis and clinical presentation, etiology, treatment, and current controversies. World Psychiatry 2024, 23, 4–25. [Google Scholar] [CrossRef]
- Xiao, Q.; Fu, Y.; Yi, X.; Ding, J.; Han, Z.; Zhang, Z.; Tan, Z.; Wang, J.; Wu, Z.; Pi, J.; et al. Altered cortical thickness and emotional dysregulation in adolescents with borderline personality disorder. Eur. J. Psychotraumatol. 2023, 14, 2163768. [Google Scholar] [CrossRef]
- Safar, K.; Sato, J.; Ruocco, A.C.; Korenblum, M.S.; O’Halpin, H.; Dunkley, B.T. Disrupted emotional neural circuitry in adolescents with borderline personality traits. Neurosci. Lett. 2019, 701, 112–118. [Google Scholar] [CrossRef]
- American Psychiatric Association [APA]. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text rev.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Rivnyák, A.; Pohárnok, M.; Péley, B.; Láng, A. Identity diffusion as the organizing principle of borderline personality traits in adolescents-a non-clinical study. Front. Psychiatry 2021, 12, 683288. [Google Scholar] [CrossRef] [PubMed]
- Bech, M.; Elklit, A.; Simonsen, E. Autobiographical memory in borderline personality disorder-a systematic review. Personal. Ment. Health 2015, 9, 162–171. [Google Scholar] [CrossRef]
- Tulving, E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Cili, S.; Stopa, L. Autobiographical Memory and the Self: Relationship and Implications for Cognitive-Behavioural Therapy; Routledge Focus: London, UK, 2018; p. 142. [Google Scholar]
- Bluck, S.; Alea, N.; Habermas, T.; Rubin, D. A tale of three functions: The self—Reported uses of autobiographical memory. Soc. Cogn. 2005, 23, 91–117. [Google Scholar] [CrossRef]
- Talarico, J.M.; LaBar, K.S.; Rubin, D.C. Emotional intensity predicts autobiographical memory experience. Mem. Cogn. 2004, 32, 1118–1132. [Google Scholar] [CrossRef]
- Dolcos, F.; LaBar, K.S.; Cabeza, R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc. Natl. Acad. Sci. USA 2005, 102, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.J.; Safer, M.A.; Lench, H.C. Remembering and misremembering emotions. In Judgments Over Time: The Interplay of Thoughts, Feelings, and Behaviors; Sanna, L.J., Chang, E.C., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 271–290. [Google Scholar]
- Tailby, C.; Rayner, G.; Wilson, S.; Jackson, G. The spatiotemporal substrates of autobiographical recollection: Using event-related ica to study cognitive networks in action. Neuroimage 2017, 152, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.C.; Tsang, H.L.M.; Han, M.; Zhang, J.; Maes, M.; Klugah-Brown, B.; Bore, M.C.; Becker, B. Neural systems underlying autobiographical memory dysregulations in depressive and at-risk individuals: A neuroimaging meta-analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The role of the salience network in cognitive and affective deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef]
- Rakesh, D.; Allen, N.B.; Whittle, S. Longitudinal changes in within-salience network functional connectivity mediate the relationship between childhood abuse and neglect, and mental health during adolescence. Psychol. Med. 2023, 53, 1552–1564. [Google Scholar] [CrossRef]
- Chahal, R.; Miller, J.G.; Yuan, J.P.; Buthmann, J.L.; Gotlib, I.H. An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: Implications for trajectories of internalizing symptoms. Dev. Psychopathol. 2022, 34, 557–571. [Google Scholar] [CrossRef]
- Sarkheil, P.; Ibrahim, C.N.; Schneider, F.; Mathiak, K.; Klasen, M. Aberrant functional connectivity profiles of brain regions associated with salience and reward processing in female patients with borderline personality disorder. Brain Imaging Behav. 2020, 14, 485–495. [Google Scholar] [CrossRef]
- Doll, A.; Sorg, C.; Manoliu, A.; Meng, C.; Wöller, A.; Förstl, H.; Zimmer, C.; Wohlschläger, A.; Riedl, V. Shifted intrinsic connectivity of central executive and salience network in borderline personality disorder. Front. Hum. Neurosci. 2013, 7, 727. [Google Scholar] [CrossRef]
- Quattrini, G.; Pini, L.; Pievani, M.; Magni, L.R.; Lanfredi, M.; Ferrari, C.; Boccardi, M.; Bignotti, S.; Magnaldi, S.; Cobelli, M.; et al. Abnormalities in functional connectivity in borderline personality disorder: Correlations with metacognition and emotion dysregulation. Psychiatry Res. Neuroimaging 2019, 283, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, G.; Magni, L.R.; Lanfredi, M.; Pedrini, L.; Carcione, A.; Riccardi, I.; Corbo, D.; Gasparotti, R.; Rossi, R.; Pievani, M.; et al. Aberrant structural connectivity of the triple network system in borderline personality disorder is associated with behavioral dysregulation. J. Clin. Med. 2022, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Orth, L.; Zweerings, J.; Ibrahim, C.N.; Neuner, I.; Sarkheil, P. Altered functional connectivity during evaluation of self-relevance in women with borderline personality disorder. NeuroImage Clin. 2020, 27, 102324. [Google Scholar] [CrossRef]
- Liotti, G.; Farina, B. Painful incoherence: The self in borderline personality disorder. In The Self in Understanding and Treating Psychological Disorders; Kyrios, M., Moulding, R., Doron, G., Bhar, S.S., Nedeljkovic, M., Mikulincer, M., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 169–178. [Google Scholar]
- Chmiel, J.; Kurpas, D. Neural correlates of borderline personality disorder (bpd) based on electroencephalogram (eeg)-a mechanistic review. Int. J. Mol. Sci. 2025, 26, 8230. [Google Scholar] [CrossRef]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef]
- Whitton, A.E.; Deccy, S.; Ironside, M.L.; Kumar, P.; Beltzer, M.; Pizzagalli, D.A. Electroencephalography source functional connectivity reveals abnormal high-frequency communication among large-scale functional networks in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 50–58. [Google Scholar] [CrossRef]
- Khaleghi, N.; Hashemi, S.; Peivandi, M.; Ardabili, S.Z.; Behjati, M.; Sheykhivand, S.; Danishvar, S. Eeg-based functional connectivity analysis of brain abnormalities: A systematic review study. Inform. Med. Unlocked 2024, 47, 101476. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. User’s Guide for the SCID-5-PD (Structured Clinical Interview for DSM-5 Personality); American Psychiatric Association Publishing: Washington, DC, USA, 2016; p. 112. [Google Scholar]
- Gratz, K.L.; Roemer, L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Harrison, A.; Sullivan, S.; Tchanturia, K.; Treasure, J. Emotional functioning in eating disorders: Attentional bias, emotion recognition and emotion regulation. Psychol. Assess. 2010, 40, 1887–1897. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): Initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.L.; Dixon-Gordon, K.L.; Walters, K.N. Borderline personality features moderate emotion reactivity and emotion regulation in response to a fear stressor. J. Exp. Psychopathol. 2013, 4, 451–470. [Google Scholar] [CrossRef]
- Johnson, J. Effect of emotions on learning, memory, and disorders associated with the changes in expression levels: A narrative review. Brain Circ. 2024, 10, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.M.; Feike, M.; Stangier, U. Mental imagery and social pain in adolescents-analysis of imagery characteristics and perspective-a pilot study. Children 2021, 8, 1160. [Google Scholar] [CrossRef]
- Dejonckheere, E.; Demeyer, F.; Geusens, B.; Piot, M.; Tuerlinckx, F.; Verdonck, S.; Mestdagh, M. Assessing the reliability of single-item momentary affective measurements in experience sampling. Psychol. Assess. 2022, 34, 1138–1154. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Armstrong, J.G.; Putnam, F.W.; Carlson, E.B.; Libero, D.Z.; Smith, S.R. Development and validation of a measure of adolescent dissociation: The adolescent dissociative experiences scale. J. Nerv. Ment. Dis. 1997, 185, 491–497. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Guile, J.M.; Boissel, L.; Alaux-Cantin, S.; de La Riviere, S.G. Borderline personality disorder in adolescents: Prevalence, diagnosis, and treatment strategies. Adolesc. Health Med. Ther. 2018, 9, 199–210. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. Eeglab: An open source toolbox for analysis of single-trial eeg dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Imperatori, C.; Massullo, C.; De Rossi, E.; Carbone, G.A.; Theodorou, A.; Scopelliti, M.; Romano, L.; Del Gatto, C.; Allegrini, G.; Carrus, G. Exposure to nature is associated with decreased functional connectivity within the distress network: A resting state eeg study. Front. Psychol. 2023, 14, 1171215. [Google Scholar] [CrossRef] [PubMed]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. The iclabel dataset of electroencephalographic (eeg) independent component (ic) features. Data Brief 2019, 25, 104101. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Pernier, J.; Bertrand, O.; Echallier, J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Ferree, T.C. Spherical splines and average referencing in scalp electroencephalography. Brain. Topogr. 2006, 19, 43–52. [Google Scholar]
- Chmiel, J.; Nadobnik, J.; Smerdel, S.; Niedzielska, M. Resting-state electroencephalogram (eeg) as a biomarker of learning disabilities in children-a systematic review. J. Clin. Med. 2025, 14, 5902. [Google Scholar] [CrossRef] [PubMed]
- Wantzen, P.; Clochon, P.; Doidy, F.; Wallois, F.; Mahmoudzadeh, M.; Desaunay, P.; Christian, M.; Guile, J.M.; Guenole, F.; Eustache, F.; et al. Eeg resting-state functional connectivity: Evidence for an imbalance of external/internal information integration in autism. J. Neurodev. Disord. 2022, 14, 47. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ganzetti, M.; Wenderoth, N.; Mantini, D. Detecting large-scale brain networks using eeg: Impact of electrode density, head modeling and source localization. Front. Neuroinform. 2018, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, S.; Yousefi Rezaii, T.; Beheshti, S.; Delpak, A.; Meshgini, S. A systematic review of eeg source localization techniques and their applications on diagnosis of brain abnormalities. J. Neurosci. Methods 2020, 339, 108740. [Google Scholar] [CrossRef]
- Miljevic, A.; Bailey, N.W.; Vila-Rodriguez, F.; Herring, S.E.; Fitzgerald, P.B. Electroencephalographic connectivity: A fundamental guide and checklist for optimal study design and evaluation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 546–554. [Google Scholar] [CrossRef]
- Carbone, G.A.; Farina, B.; Lo Presti, A.; Adenzato, M.; Imperatori, C.; Ardito, R.B. Lack of mental integration and emotion dysregulation as a possible long-term effect of dysfunctional parenting: An eeg study of functional connectivity before and after the exposure to attachment-related stimuli. J. Affect. Disord. 2025, 375, 222–230. [Google Scholar] [CrossRef]
- Massullo, C.; Panno, A.; Carbone, G.A.; Della Marca, G.; Farina, B.; Imperatori, C. Need for cognitive closure is associated with different intra-network functional connectivity patterns: A resting state eeg study. Soc. Neurosci. 2022, 17, 143–153. [Google Scholar] [CrossRef]
- Zinn, M.L.; Zinn, M.A.; Jason, L.A. Intrinsic functional hypoconnectivity in core neurocognitive networks suggests central nervous system pathology in patients with myalgic encephalomyelitis: A pilot study. Appl. Psychophysiol. Biofeedback 2016, 41, 283–300. [Google Scholar] [CrossRef]
- Hata, M.; Kazui, H.; Tanaka, T.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Kanemoto, H.; Yoshiyama, K.; et al. Functional connectivity assessed by resting state eeg correlates with cognitive decline of alzheimer’s disease—An eloreta study. Clin. Neurophysiol. 2016, 127, 1269–1278. [Google Scholar] [CrossRef]
- de la Salle, S.; Choueiry, J.; Shah, D.; Bowers, H.; McIntosh, J.; Ilivitsky, V.; Knott, V. Effects of ketamine on resting-state eeg activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front. Pharmacol. 2016, 7, 348. [Google Scholar] [CrossRef]
- Kitaura, Y.; Nishida, K.; Yoshimura, M.; Mii, H.; Katsura, K.; Ueda, S.; Ikeda, S.; Pascual-Marqui, R.D.; Ishii, R.; Kinoshita, T. Functional localization and effective connectivity of cortical theta and alpha oscillatory activity during an attention task. Clin. Neurophysiol. Pract. 2017, 2, 193–200. [Google Scholar] [CrossRef]
- Mazza, M.; Losurdo, A.; Testani, E.; Marano, G.; Di Nicola, M.; Dittoni, S.; Gnoni, V.; Di Blasi, C.; Giannantoni, N.M.; Lapenta, L.; et al. Polysomnographic findings in a cohort of chronic insomnia patients with benzodiazepine abuse. J. Clin. Sleep Med. 2014, 10, 35–42. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain. Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Hayashi, N.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Sakamoto, T.; Iwata, M.; Kimura, K.; et al. Short-term meditation modulates eeg activity in subjects with post-traumatic residual disabilities. Clin. Neurophysiol. Pract. 2019, 4, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.G.; Lin, Y.H.; Dadario, N.B.; Kim, S.J.; Young, I.M.; Bai, M.Y.; Dhanaraj, V.; Fonseka, R.D.; Hormovas, J.; Tanglay, O.; et al. Anatomy and white matter connections of the middle frontal gyrus. World Neurosurg 2021, 150, e520–e529. [Google Scholar] [CrossRef]
- Rubinstein, D.Y.; Camarillo-Rodriguez, L.; Serruya, M.D.; Herweg, N.A.; Waldman, Z.J.; Wanda, P.A.; Sharan, A.D.; Weiss, S.A.; Sperling, M.R. Contribution of left supramarginal and angular gyri to episodic memory encoding: An intracranial eeg study. Neuroimage 2021, 225, 117514. [Google Scholar] [CrossRef]
- von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Fiebelkorn, I.C.; Szczepanski, S.M.; Lin, J.J.; Parvizi, J.; Knight, R.T.; Kastner, S. Neural mechanisms of sustained attention are rhythmic. Neuron 2018, 99, 854–865.e855. [Google Scholar] [CrossRef]
- Farina, B.; Liotti, M.; Imperatori, C. The role of attachment trauma and disintegrative pathogenic processes in the traumatic-dissociative dimension. Front. Psychol. 2019, 10, 933. [Google Scholar] [CrossRef]
- Farina, B.; Meares, R. The traumatic disintegration dimension. In Dissociation and the Dissociative Disorders: Past, Present, Future, 2nd ed.; Dorahy, M.J., Gold, S.N., O’Neil, J.A., Eds.; Routledge: New York, NY, USA, 2023; pp. 50–65. [Google Scholar]
- Farina, B.; Imperatori, C. Are traumatic disintegration, detachment, and dissociation separate pathogenic processes related to attachment trauma? A working hypothesis for clinicians and researchers. Psychopathology 2024, 57, 236–247. [Google Scholar] [CrossRef]
- Bozzatello, P.; Morese, R.; Valentini, M.C.; Rocca, P.; Bosco, F.; Bellino, S. Autobiographical memories, identity disturbance and brain functioning in patients with borderline personality disorder: An fmri study. Heliyon 2019, 5, e01323. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Pineda, P.; Ferrer, M.; Calvo, N.; Costa, X.; Pozuelo-López, M.Á.; Ramos-Quiroga, J.A.; Tarragona, B.; Fuentes-Claramonte, P.; Salvador, R.; Pomarol-Clotet, E. Brain functional correlates of recall of life events in medication-naïve adolescents with borderline personality disorder. Neuropsychobiology 2024, 83, 49–60. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.-Q. A new perspective on the anterior cingulate cortex and affective pain. Neurosci. Biobehav. Rev. 2018, 90, 200–211. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H.; Hurley, R.A.; Hayman, L.A.; Taber, K.H. Anterior cingulate cortex: Unique role in cognition and emotion. J. Neuropsychiatr. Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef]
- Stevens, F.L. The anterior cingulate cortex in psychopathology and psychotherapy: Effects on awareness and repression of affect. Neuropsychoanalysis 2016, 18, 53–68. [Google Scholar] [CrossRef]
- Crespo-García, M.; Wang, Y.; Jiang, M.; Anderson, M.C.; Lei, X. Anterior cingulate cortex signals the need to control intrusive thoughts during motivated forgetting. J. Neurosci. 2022, 42, 4342–4359. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Pusil, S.; Pereda, E.; Maestú, F.; Barceló, F. Dynamic low frequency eeg phase synchronization patterns during proactive control of task switching. Neuroimage 2019, 186, 70–82. [Google Scholar] [CrossRef]
- Gulbinaite, R.; van Rijn, H.; Cohen, M.X. Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Front. Hum. Neurosci. 2014, 8, 761. [Google Scholar] [CrossRef]
- Imperatori, C.; Brunetti, R.; Farina, B.; Speranza, A.M.; Losurdo, A.; Testani, E.; Contardi, A.; Della Marca, G. Modification of eeg power spectra and eeg connectivity in autobiographical memory: A sloreta study. Cogn. Process. 2014, 15, 351–361. [Google Scholar] [CrossRef]
- Etemadi, M.; Aghebati, A.; Ayatmehr, F.; Ashoori, A. Predicting borderline personality traits in adolescents based on parenting styles and emotion regulation strategies. JPCP 2020, 8, 133–142. [Google Scholar] [CrossRef]
- van der Kaap-Deeder, J.; Brenning, K.; Neyrinck, B. Emotion regulation and borderline personality features: The mediating role of basic psychological need frustration. Pers. Individ. Differ. 2021, 168, 110365. [Google Scholar] [CrossRef]
- Bozzatello, P.; Blua, C.; Brandellero, D.; Baldassarri, L.; Brasso, C.; Rocca, P.; Bellino, S. Gender differences in borderline personality disorder: A narrative review. Front. Psychiatry 2024, 15, 1320546. [Google Scholar] [CrossRef]
- Qian, X.; Townsend, M.L.; Tan, W.J.; Grenyer, B.F.S. Sex differences in borderline personality disorder: A scoping review. PLoS ONE 2022, 17, e0279015. [Google Scholar] [CrossRef] [PubMed]
- Dattola, S.; Foresta, F.L.; Bonanno, L.; De Salvo, S.; Mammone, N.; Marino, S.; Morabito, F.C. Effect of sensor density on eloreta source localization accuracy. In Neural Approaches to Dynamics of Signal Exchanges; Smart Innovation, Systems and Technologies; Esposito, A., Faundez-Zanuy, M., Morabito, F., Pasero, E., Eds.; Springer: Singapore, 2020; pp. 403–414. [Google Scholar]
- Amiri, S.; Mirfazeli, F.S.; Grafman, J.; Mohammadsadeghi, H.; Eftekhar, M.; Karimzad, N.; Mohebbi, M.; Nohesara, S. Alternation in functional connectivity within default mode network after psychodynamic psychotherapy in borderline personality disorder. Ann. Gen. Psychiatry 2023, 22, 18. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; Aracil-Bolaños, I.; Carmona, I.F.C.; Soler, J.; Schmidt, C.; Elices, M.; Pomarol-Clotet, E.; Salvador, R.; Vega, D.; Pascual, J.C. A functional connectivity study to investigate the role of the right anterior insula in modulating emotional dysfunction in borderline personality disorder. Psychosom. Med. 2022, 84, 64–73. [Google Scholar] [CrossRef]
- Schlumpf, Y.R.; Nijenhuis, E.R.S.; Klein, C.; Jäncke, L.; Bachmann, S. Functional connectivity changes in the delta frequency band following trauma treatment in complex trauma and dissociative disorder patients. Front. Psychiatry 2022, 13, 889560. [Google Scholar] [CrossRef] [PubMed]

| Instrument | Type | Brief Description |
|---|---|---|
| SCID-5-PD | Clinical Interview | Semi-structured clinical interview used to assess personality disorders according to DSM-5 criteria. |
| K-SADS-PL-5 | Clinical Interview | Semi-structured interview for children and adolescents assessing current and past psychiatric disorders according to DSM-5 criteria. |
| DERS | Self-report | 36-item questionnaire assessing difficulties in emotion regulation across multiple domains. |
| BIS-11 | Self-report | 30-item questionnaire measuring impulsivity in adults across attentional, motor, and non-planning domains. |
| A-DES | Self-report | 30-item questionnaire assessing dissociative experiences in adolescents. |
| CTQ-SF | Self-report | 28-item questionnaire measuring childhood abuse and neglect, including a scale to detect underreporting. |
| Variables | BPD (N = 24) | HCs (N = 15) | Test | p |
|---|---|---|---|---|
| Age—M ± SD | 16.04 ± 1.26 | 16.57 ± 1.15 | KS = 1.063 | p = 0.208 |
| Females—N (%) | 24 (100) | 14 (93.33) | χ2 = 1.642 | p = 0.200 |
| Alcohol use—N (%) | 7 (29.17) | 0 (0) | χ2 = 5.332 | p = 0.021 |
| Nicotine use—N (%) | 16 (66.67) | 2 (13.33) | χ2 = 10.565 | p = 0.001 |
| Cannabis use—N (%) | 10 (41.67) | 0 (0) | χ2 = 8.405 | p = 0.004 |
| Other substances use—N (%) | 1 (4.17) | 0 (0) | χ2 = 0.641 | p = 0.423 |
| Self-harm—N (%) | 24 (100) | - | - | - |
| Suicidal risk | ||||
| Suicidal ideation N (%) | 13 (54.17) | - | - | - |
| Suicidal attempt N (%) | 10 (41.67) | - | - | - |
| Other psychiatric disorders * N (%) | 23 (95.83) | - | - | - |
| Psychiatric medication use—N (%) | 22 (91.67) | - | - | - |
| DERS—M ± SD | 148.75 ± 12.34 | 94.13 ± 19.61 | KS = 3.038 | p < 0.001 |
| BIS-11—M ± SD | 82.52 ± 11.00 | 63.47 ± 9.15 | KS = 1.025 | p = 0.001 |
| Attentional impulsivity—M ± SD | 23.09 ± 4.27 | 17.53 ± 2.72 | KS = 1.177 | p < 0.001 |
| Motor—M ± SD | 27.26 ± 4.61 | 19.80 ± 3.39 | KS = 1.899 | p = 0.001 |
| Non-planning—M ± SD | 32.13 ± 4.36 | 26.20 ± 4.92 | KS = 1.696 | p = 0.006 |
| A-DES—M ± SD | 4.97 ± 2.38 | 2.42 ± 0.74 | KS = 2.279 | p < 0.001 |
| CTQ-SF—M ± SD | 54.17 ± 15.54 | 29.87 ± 4.69 | KS = 2.658 | p < 0.001 |
| PA—M ± SD | 6.52 ± 2.28 | 5.20 ± 0.56 | KS = 0.987 | p = 0.284 |
| EA—M ± SD | 14.87 ± 5.29 | 6.27 ± 1.94 | KS = 2.532 | p < 0.001 |
| SA—M ± SD | 9.70 ± 6.03 | 5.00 ± 0.00 | KS = 1.646 | p = 0.009 |
| PN—M ± SD | 7.44 ± 2.62 | 5.40 ± 0.63 | KS = 1.266 | p = 0.081 |
| EN—M ± SD | 15.65 ± 4.99 | 8.00 ± 2.51 | KS = 2.253 | p < 0.001 |
| MD—M ± SD | 0.46 ± 1.06 | 0.47 ± 0.92 | KS = 0.304 | p = 1.000 |
| 1. | 2. | 3. | 4. | ||
|---|---|---|---|---|---|
| 1. A-DES | rho | - | |||
| p | - | ||||
| 2. CTQ-SF | rho | 0.450 | - | ||
| p | 0.005 | - | |||
| 3. BIS-11 | rho | 0.303 | 0.671 | - | |
| p | 0.068 | <0.001 | - | ||
| 4. DERS | rho | 0.570 | 0.743 | 0.649 | - |
| p | <0.001 | <0.001 | <0.001 | - | |
| 5. RS theta FC between lMFG and lSG | rho | −0.414 | −0.479 | −0.431 | −0.520 |
| p | 0.010 | 0.002 | 0.007 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rossi, E.; Di Maggio, C.; Imperatori, C.; Covuccia, M.; Carbone, G.A.; Terrinoni, A.; Massullo, C.; Guidetti, V.; Brinciotti, M.; Biscione, G.; et al. Aberrant Salience Network Functional Connectivity in Resting-State and Fear-Related Autobiographical Memory Recall in Female Adolescents with Borderline Personality Disorder. Brain Sci. 2025, 15, 1146. https://doi.org/10.3390/brainsci15111146
De Rossi E, Di Maggio C, Imperatori C, Covuccia M, Carbone GA, Terrinoni A, Massullo C, Guidetti V, Brinciotti M, Biscione G, et al. Aberrant Salience Network Functional Connectivity in Resting-State and Fear-Related Autobiographical Memory Recall in Female Adolescents with Borderline Personality Disorder. Brain Sciences. 2025; 15(11):1146. https://doi.org/10.3390/brainsci15111146
Chicago/Turabian StyleDe Rossi, Elena, Chiara Di Maggio, Claudio Imperatori, Marilina Covuccia, Giuseppe A. Carbone, Arianna Terrinoni, Chiara Massullo, Vincenzo Guidetti, Mario Brinciotti, Giulia Biscione, and et al. 2025. "Aberrant Salience Network Functional Connectivity in Resting-State and Fear-Related Autobiographical Memory Recall in Female Adolescents with Borderline Personality Disorder" Brain Sciences 15, no. 11: 1146. https://doi.org/10.3390/brainsci15111146
APA StyleDe Rossi, E., Di Maggio, C., Imperatori, C., Covuccia, M., Carbone, G. A., Terrinoni, A., Massullo, C., Guidetti, V., Brinciotti, M., Biscione, G., & Farina, B. (2025). Aberrant Salience Network Functional Connectivity in Resting-State and Fear-Related Autobiographical Memory Recall in Female Adolescents with Borderline Personality Disorder. Brain Sciences, 15(11), 1146. https://doi.org/10.3390/brainsci15111146







