Characterizing the Microenvironment of Cerebral Arteriovenous Malformations to Test Novel Treatment Modalities
Abstract
1. Introduction
2. The Physiology of Brain Arteriovenous Malformation Formation
2.1. Angiogenesis
2.2. Pathophysiology of Syndromic-Related Brain Arteriovenous Malformations
2.2.1. Hereditary Hemorrhagic Telangiectasias
2.2.2. Wyburn–Mason Syndrome
2.2.3. Sturge–Weber Syndrome
2.3. Pathophysiology of Sporadic Brain Arteriovenous Malformations
2.4. Pathophysiology of Acquired Brain Arteriovenous Malformations
3. Animal Models of Brain AVMs
4. Targeted Therapeutic Approaches for the Treatment of AVMs
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.J.; Ding, D.; Derdeyn, C.P.; Lanzino, G.; Friedlander, R.M.; Southerland, A.M.; Lawton, M.T.; Sheehan, J.P. Brain arteriovenous malformations: A review of natural history, pathobiology, and interventions. Neurology 2020, 95, 917–927. [Google Scholar] [CrossRef]
- Al-Shahi, R.; Fang, J.S.; Lewis, S.C.; Warlow, C.P. Prevalence of adults with brain arteriovenous malformations: A community based study in Scotland using capture-recapture analysis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 547–551. [Google Scholar] [CrossRef]
- Berman, M.F.; Sciacca, R.R.; Pile-Spellman, J.; Stapf, C.; Connolly, E.S., Jr.; Mohr, J.P.; Young, W.L. The epidemiology of brain arteriovenous malformations. Neurosurgery 2000, 47, 389–396; discussion 397. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, E.A.; Dabus, G.; Meyers, P.M.; Kan, P.T.; Frosen, J.; Lanzino, G.; Welch, B.G.; Volovici, V.; Gonzalez, F.; Fifi, J.; et al. Most Promising Approaches to Improve Brain AVM Management: ARISE I Consensus Recommendations. Stroke 2024, 55, 1449–1463. [Google Scholar] [CrossRef]
- Daou, B.J.; Palmateer, G.; Thompson, B.G.; Maher, C.O.; Hayman, J.A.; Lam, K.L.; Wahl, D.R.; Kim, M.; Pandey, A.S. Stereotactic Radiosurgery for Brain Arteriovenous Malformations: Evaluation of Obliteration and Review of Associated Predictors. J. Stroke Cerebrovasc. Dis. 2020, 29, 104863. [Google Scholar] [CrossRef]
- Morales-Valero, S.F.; Bortolotti, C.; Sturiale, C.; Lanzino, G. Are parenchymal AVMs congenital lesions? Neurosurg. Focus 2014, 37, E2. [Google Scholar] [CrossRef]
- Walcott, B.P.; Winkler, E.A.; Zhou, S.; Birk, H.; Guo, D.; Koch, M.J.; Stapleton, C.J.; Spiegelman, D.; Dionne-Laporte, A.; Dion, P.A.; et al. Identification of a rare BMP pathway mutation in a non-syndromic human brain arteriovenous malformation via exome sequencing. Hum. Genome Var. 2018, 5, 18001. [Google Scholar] [CrossRef]
- Nagai, Y.; Anan, M.; Fujiki, M. Cerebral Arteriovenous Malformations as Acquired Lesions: Case Reports and Review of the Literature. J. Stroke Cerebrovasc. Dis. 2020, 29, 105157. [Google Scholar] [CrossRef]
- Park, H.; Koh, E.J.; Lee, E.J.; Cheon, J.E.; Kim, S.K. An acquired cerebral arteriovenous malformation after brain abscess treatment: Case report and a review of the literature. Childs Nerv. Syst. 2021, 37, 2923–2926. [Google Scholar] [CrossRef]
- Florian, I.A.; Beni, L.; Moisoiu, V.; Timis, T.L.; Florian, I.S.; Balasa, A.; Berindan-Neagoe, I. ‘De Novo’ Brain AVMs-Hypotheses for Development and a Systematic Review of Reported Cases. Medicina 2021, 57, 201. [Google Scholar] [CrossRef]
- Winkler, E.A.; Kim, C.N.; Ross, J.M.; Garcia, J.H.; Gil, E.; Oh, I.; Chen, L.Q.; Wu, D.; Catapano, J.S.; Raygor, K.; et al. A single-cell atlas of the normal and malformed human brain vasculature. Science 2022, 375, eabi7377. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Guo, Y.; Moore, W.V.; He, L.G.; Zang, M.; Clements, M.A.; Yan, Y. Thioredoxin-interacting protein promotes high-glucose-induced macrovascular endothelial dysfunction. Biochem. Biophys. Res. Commun. 2017, 493, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Chen, M.B.; Yang, A.C.; Yousef, H.; Lee, D.; Chen, W.; Schaum, N.; Lehallier, B.; Quake, S.R.; Wyss-Coray, T. Brain Endothelial Cells Are Exquisite Sensors of Age-Related Circulatory Cues. Cell Rep. 2020, 30, 4418–4432.e4. [Google Scholar] [CrossRef]
- Kalucka, J.; de Rooij, L.; Goveia, J.; Rohlenova, K.; Dumas, S.J.; Meta, E.; Conchinha, N.V.; Taverna, F.; Teuwen, L.A.; Veys, K.; et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779.e20. [Google Scholar] [CrossRef]
- Choi, E.J.; Chen, W.; Jun, K.; Arthur, H.M.; Young, W.L.; Su, H. Novel brain arteriovenous malformation mouse models for type 1 hereditary hemorrhagic telangiectasia. PLoS ONE 2014, 9, e88511. [Google Scholar] [CrossRef]
- Matsubara, S.; Bourdeau, A.; terBrugge, K.G.; Wallace, C.; Letarte, M. Analysis of endoglin expression in normal brain tissue and in cerebral arteriovenous malformations. Stroke 2000, 31, 2653–2660. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Tran, M.N.; Achrol, A.S.; Ha, C.; Burchard, E.; Choudhry, S.; Zaroff, J.; Lawton, M.T.; Castro, R.; McCulloch, C.E.; et al. Polymorphisms in transforming growth factor-beta-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke 2005, 36, 2278–2280. [Google Scholar] [CrossRef]
- Simon, M.; Franke, D.; Ludwig, M.; Aliashkevich, A.F.; Koster, G.; Oldenburg, J.; Bostrom, A.; Ziegler, A.; Schramm, J. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J. Neurosurg. 2006, 104, 945–949. [Google Scholar] [CrossRef]
- Pan, P.; Weinsheimer, S.; Cooke, D.; Winkler, E.; Abla, A.; Kim, H.; Su, H. Review of treatment and therapeutic targets in brain arteriovenous malformation. J. Cereb. Blood Flow. Metab. 2021, 41, 3141–3156. [Google Scholar] [CrossRef]
- Vetiska, S.; Walchli, T.; Radovanovic, I.; Berhouma, M. Molecular and genetic mechanisms in brain arteriovenous malformations: New insights and future perspectives. Neurosurg. Rev. 2022, 45, 3573–3593. [Google Scholar] [CrossRef]
- Bharatha, A.; Faughnan, M.E.; Kim, H.; Pourmohamad, T.; Krings, T.; Bayrak-Toydemir, P.; Pawlikowska, L.; McCulloch, C.E.; Lawton, M.T.; Dowd, C.F.; et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: Quantitative assessment. Stroke 2012, 43, 72–78. [Google Scholar] [CrossRef]
- Corti, P.; Young, S.; Chen, C.Y.; Patrick, M.J.; Rochon, E.R.; Pekkan, K.; Roman, B.L. Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 2011, 138, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, R.K.; Chaloupka, J.C.; Putman, C.M.; Sze, G.K.; Merriam, M.M.; Lee, G.K.; Fayad, P.B.; Awad, I.A.; White, R.I., Jr. MR of hereditary hemorrhagic telangiectasia: Prevalence and spectrum of cerebrovascular malformations. AJNR Am. J. Neuroradiol. 1998, 19, 477–484. [Google Scholar] [PubMed]
- Nishida, T.; Faughnan, M.E.; Krings, T.; Chakinala, M.; Gossage, J.R.; Young, W.L.; Kim, H.; Pourmohamad, T.; Henderson, K.J.; Schrum, S.D.; et al. Brain arteriovenous malformations associated with hereditary hemorrhagic telangiectasia: Gene-phenotype correlations. Am. J. Med. Genet. A 2012, 158A, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- McAllister, K.A.; Grogg, K.M.; Johnson, D.W.; Gallione, C.J.; Baldwin, M.A.; Jackson, C.E.; Helmbold, E.A.; Markel, D.S.; McKinnon, W.C.; Murrell, J.; et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 1994, 8, 345–351. [Google Scholar] [CrossRef]
- Vincent, P.; Plauchu, H.; Hazan, J.; Faure, S.; Weissenbach, J.; Godet, J. A third locus for hereditary haemorrhagic telangiectasia maps to chromosome 12q. Hum. Mol. Genet. 1995, 4, 945–949. [Google Scholar] [CrossRef]
- Johnson, D.W.; Berg, J.N.; Gallione, C.J.; McAllister, K.A.; Warner, J.P.; Helmbold, E.A.; Markel, D.S.; Jackson, C.E.; Porteous, M.E.; Marchuk, D.A. A second locus for hereditary hemorrhagic telangiectasia maps to chromosome 12. Genome Res. 1995, 5, 21–28. [Google Scholar] [CrossRef]
- Gallione, C.J.; Richards, J.A.; Letteboer, T.G.; Rushlow, D.; Prigoda, N.L.; Leedom, T.P.; Ganguly, A.; Castells, A.; Ploos van Amstel, J.K.; Westermann, C.J.; et al. SMAD4 mutations found in unselected HHT patients. J. Med. Genet. 2006, 43, 793–797. [Google Scholar] [CrossRef]
- Shameem, Y.; Irshad, S.; Mirza, N.; Hassan, N. Wyburn-Mason Syndrome: A Narrative Review. Cureus 2024, 16, e68070. [Google Scholar] [CrossRef]
- So, J.M.; Mishra, C.; Holman, R.E. Wyburn-Mason Syndrome; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vanaman, M.J.; Hervey-Jumper, S.L.; Maher, C.O. Pediatric and inherited neurovascular diseases. Neurosurg. Clin. N. Am. 2010, 21, 427–441. [Google Scholar] [CrossRef]
- Kasasbeh, A.S.; Kalaria, A.; Comi, A.M.; Lo, W.; Lin, D.D.M. Atypical Intracerebral Developmental Venous Anomalies in Sturge-Weber Syndrome: A Case Series and Review of Literature. Pediatr. Neurol. 2020, 104, 54–61. [Google Scholar] [CrossRef]
- Nakashima, M.; Miyajima, M.; Sugano, H.; Iimura, Y.; Kato, M.; Tsurusaki, Y.; Miyake, N.; Saitsu, H.; Arai, H.; Matsumoto, N. The somatic GNAQ mutation c.548G>A (p.R183Q) is consistently found in Sturge-Weber syndrome. J. Hum. Genet. 2014, 59, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanam, A.; Ardern-Holmes, S.L. Sturge-Weber syndrome: From the past to the present. Eur. J. Paediatr. Neurol. 2014, 18, 257–266. [Google Scholar] [CrossRef]
- Yeom, S.; Comi, A.M. Updates on Sturge-Weber Syndrome. Stroke 2022, 53, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, H.; Liu, Y.; Xu, L.; Hu, M.; Yang, Y.; Wang, N.; Wu, Y.; Guo, W. GNAQ R183Q somatic mutation contributes to aberrant arteriovenous specification in Sturge-Weber syndrome through Notch signaling. FASEB J. 2023, 37, e23148. [Google Scholar] [CrossRef] [PubMed]
- Krah, K.; Mironov, V.; Risau, W.; Flamme, I. Induction of vasculogenesis in quail blastodisc-derived embryoid bodies. Dev. Biol. 1994, 164, 123–132. [Google Scholar] [CrossRef]
- Yamaguchi, T.P.; Dumont, D.J.; Conlon, R.A.; Breitman, M.L.; Rossant, J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 1993, 118, 489–498. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Moses, M.A. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells 1997, 15, 180–189. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Siefert, S.A.; Sarkar, R. Matrix metalloproteinases in vascular physiology and disease. Vascular 2012, 20, 210–216. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, S.; Liu, B.; Zhang, Q.; Li, Y.; Liu, J.; Shen, Y.; Ding, X.; Lin, J.; Wu, Y.; et al. Perturbations of BMP/TGF-beta and VEGF/VEGFR signalling pathways in non-syndromic sporadic brain arteriovenous malformations (BAVM). J. Med. Genet. 2018, 55, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Santos, T.; Amar, A.; Tahara, S.M.; Chen, T.C.; Giannotta, S.L.; Hofman, F.M. MicroRNA-18a improves human cerebral arteriovenous malformation endothelial cell function. Stroke 2014, 45, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.; Tan, W.L.; Ng, P.Y.; Lim, J. Hypoxia inducible factor-1alpha and expression of vascular endothelial growth factor and its receptors in cerebral arteriovenous malformations. J. Clin. Neurosci. 2005, 12, 794–799. [Google Scholar] [CrossRef]
- Sonstein, W.J.; Kader, A.; Michelsen, W.J.; Llena, J.F.; Hirano, A.; Casper, D. Expression of vascular endothelial growth factor in pediatric and adult cerebral arteriovenous malformations: An immunocytochemical study. J. Neurosurg. 1996, 85, 838–845. [Google Scholar] [CrossRef]
- Koizumi, T.; Shiraishi, T.; Hagihara, N.; Tabuchi, K.; Hayashi, T.; Kawano, T. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 2002, 50, 117–124; discussion 124–126. [Google Scholar] [CrossRef]
- Han, C.; Choe, S.W.; Kim, Y.H.; Acharya, A.P.; Keselowsky, B.G.; Sorg, B.S.; Lee, Y.J.; Oh, S.P. VEGF neutralization can prevent and normalize arteriovenous malformations in an animal model for hereditary hemorrhagic telangiectasia 2. Angiogenesis 2014, 17, 823–830. [Google Scholar] [CrossRef]
- Sandalcioglu, I.E.; Wende, D.; Eggert, A.; Muller, D.; Roggenbuck, U.; Gasser, T.; Wiedemayer, H.; Stolke, D. Vascular endothelial growth factor plasma levels are significantly elevated in patients with cerebral arteriovenous malformations. Cerebrovasc. Dis. 2006, 21, 154–158. [Google Scholar] [CrossRef]
- Kim, G.H.; Hahn, D.K.; Kellner, C.P.; Hickman, Z.L.; Komotar, R.J.; Starke, R.M.; Mack, W.J.; Mocco, J.; Solomon, R.A.; Connolly, E.S., Jr. Plasma levels of vascular endothelial growth factor after treatment for cerebral arteriovenous malformations. Stroke 2008, 39, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T.; Jacobowitz, R.; Spetzler, R.F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J. Neurosurg. 1997, 87, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lawton, M.T.; Du, R.; Shwe, Y.; Chen, Y.; Shen, F.; Young, W.L.; Yang, G.Y. Expression of hypoxia-inducible factor-1 and vascular endothelial growth factor in response to venous hypertension. Neurosurgery 2006, 59, 687–696; discussion 96. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Miyachi, S.; Sahara, Y.; Nakai, K.; Okamoto, T.; Hattori, K.; Kobayashi, N.; Negoro, M.; Yoshida, J. The relationship between venous hypertension and expression of vascular endothelial growth factor: Hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surg. Neurol. 2007, 68, 277–284; discussion 84. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wen, G.; Lawton, M.T.; Boudreau, N.J.; Bollen, A.W.; Yang, G.Y.; Barbaro, N.M.; Higashida, R.T.; Dowd, C.F.; Halbach, V.V.; et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 2003, 34, 925–931. [Google Scholar] [CrossRef]
- Bicer, A.; Guclu, B.; Ozkan, A.; Kurtkaya, O.; Koc, D.Y.; Necmettin Pamir, M.; Kilic, T. Expressions of angiogenesis associated matrix metalloproteinases and extracellular matrix proteins in cerebral vascular malformations. J. Clin. Neurosci. 2010, 17, 232–236. [Google Scholar] [CrossRef]
- Neyazi, B.; Herz, A.; Stein, K.P.; Gawish, I.; Hartmann, C.; Wilkens, L.; Erguen, S.; Dumitru, C.A.; Sandalcioglu, I.E. Brain arteriovenous malformations: Implications of CEACAM1-positive inflammatory cells and sex on hemorrhage. Neurosurg. Rev. 2017, 40, 129–134. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sugita, Y.; Nakashima, S.; Okada, Y.; Yoshitomi, M.; Kimura, Y.; Miyoshi, H.; Morioka, M.; Ohshima, K. Alternatively Activated Macrophages Play an Important Role in Vascular Remodeling and Hemorrhaging in Patients with Brain Arteriovenous Malformation. J. Stroke Cerebrovasc. Dis. 2016, 25, 600–609. [Google Scholar] [CrossRef]
- Guo, Y.; Tihan, T.; Kim, H.; Hess, C.; Lawton, M.T.; Young, W.L.; Zhao, Y.; Su, H. Distinctive distribution of lymphocytes in unruptured and previously untreated brain arteriovenous malformation. Neuroimmunol. Neuroinflamm. 2014, 1, 147–152. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Z.; Degos, V.; Shen, F.; Choi, E.J.; Sun, Z.; Kang, S.; Wong, M.; Zhu, W.; Zhan, L.; et al. Persistent infiltration and pro-inflammatory differentiation of monocytes cause unresolved inflammation in brain arteriovenous malformation. Angiogenesis 2016, 19, 451–461. [Google Scholar] [CrossRef]
- Thomas, J.M.; Surendran, S.; Abraham, M.; Sasankan, D.; Bhaadri, S.; Rajavelu, A.; Kartha, C.C. Gene expression analysis of nidus of cerebral arteriovenous malformations reveals vascular structures with deficient differentiation and maturation. PLoS ONE 2018, 13, e0198617. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, S.I.; Vetiska, S.; Bonilla, X.; Boudreau, E.; Jauhiainen, S.; Rezai Jahromi, B.; Khyzha, N.; DiStefano, P.V.; Suutarinen, S.; Kiehl, T.R.; et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N. Engl. J. Med. 2018, 378, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Kushamae, M.; Aoki, T.; Yamaguchi, T.; Kitazato, K.; Abekura, Y.; Kawamata, T.; Mizutani, T.; Miyamoto, S.; Takagi, Y. KRAS G12D or G12V Mutation in Human Brain Arteriovenous Malformations. World Neurosurg. 2019, 126, e1365–e1373. [Google Scholar] [CrossRef] [PubMed]
- Priemer, D.S.; Vortmeyer, A.O.; Zhang, S.; Chang, H.Y.; Curless, K.L.; Cheng, L. Activating KRAS mutations in arteriovenous malformations of the brain: Frequency and clinicopathologic correlation. Hum. Pathol. 2019, 89, 33–39. [Google Scholar] [CrossRef]
- Hong, T.; Yan, Y.; Li, J.; Radovanovic, I.; Ma, X.; Shao, Y.W.; Yu, J.; Ma, Y.; Zhang, P.; Ling, F.; et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019, 142, 23–34. [Google Scholar] [CrossRef]
- Al-Olabi, L.; Polubothu, S.; Dowsett, K.; Andrews, K.A.; Stadnik, P.; Joseph, A.P.; Knox, R.; Pittman, A.; Clark, G.; Baird, W.; et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 2018, 128, 1496–1508. [Google Scholar] [CrossRef]
- Shoemaker, L.D.; Fuentes, L.F.; Santiago, S.M.; Allen, B.M.; Cook, D.J.; Steinberg, G.K.; Chang, S.D. Human brain arteriovenous malformations express lymphatic-associated genes. Ann. Clin. Transl. Neurol. 2014, 1, 982–995. [Google Scholar] [CrossRef]
- Shoemaker, L.D.; McCormick, A.K.; Allen, B.M.; Chang, S.D. Evidence for endothelial-to-mesenchymal transition in human brain arteriovenous malformations. Clin. Transl. Med. 2020, 10, e99. [Google Scholar] [CrossRef]
- Fish, J.E.; Flores Suarez, C.P.; Boudreau, E.; Herman, A.M.; Gutierrez, M.C.; Gustafson, D.; DiStefano, P.V.; Cui, M.; Chen, Z.; De Ruiz, K.B.; et al. Somatic Gain of KRAS Function in the Endothelium Is Sufficient to Cause Vascular Malformations That Require MEK but Not PI3K Signaling. Circ. Res. 2020, 127, 727–743. [Google Scholar] [CrossRef]
- Giarretta, I.; Sturiale, C.L.; Gatto, I.; Pacioni, S.; Gaetani, E.; Porfidia, A.; Puca, A.; Palucci, I.; Tondi, P.; Olivi, A.; et al. Sonic hedgehog is expressed in human brain arteriovenous malformations and induces arteriovenous malformations in vivo. J. Cereb. Blood Flow. Metab. 2021, 41, 324–335. [Google Scholar] [CrossRef]
- Wang, S.; Deng, X.; Wu, Y.; Zhou, S.; Yang, J.; Huang, Y. Understanding the pathogenesis of brain arteriovenous malformation: Genetic variations, epigenetics, signaling pathways, and immune inflammation. Hum. Genet. 2023, 142, 1633–1649. [Google Scholar] [CrossRef]
- Mansur, A.; Radovanovic, I. Vascular malformations: An overview of their molecular pathways, detection of mutational profiles and subsequent targets for drug therapy. Front. Neurol. 2023, 14, 1099328. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alfayate, R.; Grasso, G. State of the Art and Future Direction in Diagnosis, Molecular Biology, Genetics, and Treatment of Brain Arteriovenous Malformations. World Neurosurg. 2022, 159, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Shaligram, S.S.; Do Prado, L.B.; He, L.; Su, H. The role of mural cells in hemorrhage of brain arteriovenous malformation. Brain Hemorrhages 2021, 2, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.A.; Huang, A.Y.; Smith, E.; Konczyk, D.J.; Smits, P.J.; Sudduth, C.L.; Stapleton, C.; Patel, A.; Alexandrescu, S.; Warman, M.L.; et al. Somatic mutations in intracranial arteriovenous malformations. PLoS ONE 2019, 14, e0226852. [Google Scholar] [CrossRef]
- Murphy, P.A.; Lam, M.T.; Wu, X.; Kim, T.N.; Vartanian, S.M.; Bollen, A.W.; Carlson, T.R.; Wang, R.A. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 10901–10906. [Google Scholar] [CrossRef]
- ZhuGe, Q.; Zhong, M.; Zheng, W.; Yang, G.Y.; Mao, X.; Xie, L.; Chen, G.; Chen, Y.; Lawton, M.T.; Young, W.L.; et al. Notch-1 signalling is activated in brain arteriovenous malformations in humans. Brain 2009, 132 Pt 12, 3231–3241. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Wang, Y.; Li, H.; Zheng, J.; Duan, R.; Zhao, J. Receptors of the Notch signaling pathway are associated with hemorrhage of brain arteriovenous malformations. Mol. Med. Rep. 2014, 9, 2233–2238. [Google Scholar] [CrossRef]
- Xu, H.; Huo, R.; Li, H.; Jiao, Y.; Weng, J.; Wang, J.; Yan, Z.; Zhang, J.; Zhao, S.; He, Q.; et al. KRAS mutation-induced EndMT of brain arteriovenous malformation is mediated through the TGF-beta/BMP-SMAD4 pathway. Stroke Vasc. Neurol. 2023, 8, 197–206. [Google Scholar] [CrossRef]
- Winkler, E.; Wu, D.; Gil, E.; McCoy, D.; Narsinh, K.; Sun, Z.; Mueller, K.; Ross, J.; Kim, H.; Weinsheimer, S.; et al. Endoluminal Biopsy for Molecular Profiling of Human Brain Vascular Malformations. Neurology 2022, 98, e1637–e1647. [Google Scholar] [CrossRef]
- Adhicary, S.; Fanelli, K.; Nakisli, S.; Ward, B.; Pearce, I.; Nielsen, C.M. Rbpj Deficiency Disrupts Vascular Remodeling via Abnormal Apelin and Cdc42 (Cell Division Cycle 42) Activity in Brain Arteriovenous Malformation. Stroke 2023, 54, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Hermanto, Y.; Takagi, Y.; Ishii, A.; Yoshida, K.; Kikuchi, T.; Funaki, T.; Mineharu, Y.; Miyamoto, S. Immunohistochemical Analysis of Sox17 Associated Pathway in Brain Arteriovenous Malformations. World Neurosurg. 2016, 87, 573–583.e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Shabani, Z.; Schuerger, J.; Su, H. Cellular loci involved in the development of brain arteriovenous malformations. Front. Hum. Neurosci. 2022, 16, 968369. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Peng, Z.; Zhang, L.; Yang, J.; Guo, K.; Tang, X.; Ye, J.; Zhang, F.; Huang, A.; Yu, J.; et al. Neuroinflammation and hypoxia promote astrocyte phenotypic transformation and propel neurovascular dysfunction in brain arteriovenous malformation. J. Neuroinflamm. 2025, 22, 124. [Google Scholar] [CrossRef]
- Thomas, J.M.; Sasankan, D.; Surendran, S.; Abraham, M.; Rajavelu, A.; Kartha, C.C. Aberrant regulation of retinoic acid signaling genes in cerebral arterio venous malformation nidus and neighboring astrocytes. J. Neuroinflamm. 2021, 18, 61. [Google Scholar] [CrossRef]
- Nakisli, S.; Lagares, A.; Nielsen, C.M.; Cuervo, H. Pericytes and vascular smooth muscle cells in central nervous system arteriovenous malformations. Front. Physiol. 2023, 14, 1210563. [Google Scholar] [CrossRef]
- Dalton, A.; Dobson, G.; Prasad, M.; Mukerji, N. De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br. J. Neurosurg. 2018, 32, 305–311. [Google Scholar] [CrossRef]
- Andaluz, N.; Myseros, J.S.; Sathi, S.; Crone, K.R.; Tew, J.M., Jr. Recurrence of cerebral arteriovenous malformations in children: Report of two cases and review of the literature. Surg. Neurol. 2004, 62, 324–330; discussion 30–31. [Google Scholar] [CrossRef]
- Hashimoto, N.; Nozaki, K. Do cerebral arteriovenous malformations recur after angiographically confirmed total extirpation? Crit. Rev. Neurosurg. 1999, 9, 141–146. [Google Scholar] [CrossRef]
- Kader, A.; Goodrich, J.T.; Sonstein, W.J.; Stein, B.M.; Carmel, P.W.; Michelsen, W.J. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J. Neurosurg. 1996, 85, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Galletti, F.; Costa, C.; Cupini, L.M.; Eusebi, P.; Hamam, M.; Caputo, N.; Siliquini, S.; Conti, C.; Moschini, E.; Lunardi, P.; et al. Brain arteriovenous malformations and seizures: An Italian study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Cellerini, M.; Mangiafico, S.; Villa, G.; Ammannati, F.; Giordano, G.P. Disappearance of cerebral arteriovenous malformations after partial endovascular embolisation: Four cases with follow-up. Neuroradiology 2003, 45, 916–920. [Google Scholar] [CrossRef]
- Lasjaunias, P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interv. Neuroradiol. 1997, 3, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Walker, E.J.; Shen, F.; Oh, S.P.; Arthur, H.M.; Young, W.L.; Su, H. Minimal homozygous endothelial deletion of Eng with VEGF stimulation is sufficient to cause cerebrovascular dysplasia in the adult mouse. Cerebrovasc. Dis. 2012, 33, 540–547. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Z.; Han, Z.; Jun, K.; Camus, M.; Wankhede, M.; Mao, L.; Arnold, T.; Young, W.L.; Su, H. De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 2014, 45, 900–902. [Google Scholar] [CrossRef]
- Walker, E.J.; Su, H.; Shen, F.; Choi, E.J.; Oh, S.P.; Chen, G.; Lawton, M.T.; Kim, H.; Chen, Y.; Chen, W.; et al. Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann. Neurol. 2011, 69, 954–962. [Google Scholar] [CrossRef]
- Shaligram, S.S.; Zhang, R.; Zhu, W.; Ma, L.; Luo, M.; Li, Q.; Weiss, M.; Arnold, T.; Santander, N.; Liang, R.; et al. Bone Marrow-Derived Alk1 Mutant Endothelial Cells and Clonally Expanded Somatic Alk1 Mutant Endothelial Cells Contribute to the Development of Brain Arteriovenous Malformations in Mice. Transl. Stroke Res. 2022, 13, 494–504. [Google Scholar] [CrossRef]
- Hwan Kim, Y.; Vu, P.N.; Choe, S.W.; Jeon, C.J.; Arthur, H.M.; Vary, C.P.H.; Lee, Y.J.; Oh, S.P. Overexpression of Activin Receptor-Like Kinase 1 in Endothelial Cells Suppresses Development of Arteriovenous Malformations in Mouse Models of Hereditary Hemorrhagic Telangiectasia. Circ. Res. 2020, 127, 1122–1137. [Google Scholar] [CrossRef]
- Krebs, L.T.; Starling, C.; Chervonsky, A.V.; Gridley, T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis 2010, 48, 146–150. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Cuervo, H.; Ding, V.W.; Kong, Y.; Huang, E.J.; Wang, R.A. Deletion of Rbpj from postnatal endothelium leads to abnormal arteriovenous shunting in mice. Development 2014, 141, 3782–3792. [Google Scholar] [CrossRef]
- Larrivee, B.; Prahst, C.; Gordon, E.; del Toro, R.; Mathivet, T.; Duarte, A.; Simons, M.; Eichmann, A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 2012, 22, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem. 2011, 286, 30034–30046. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kim, S.; Yao, D.C.; Savarraj, J.P.J.; Choi, H.A.; Chen, P.R.; Kim, E. Soluble Endoglin Stimulates Inflammatory and Angiogenic Responses in Microglia That Are Associated with Endothelial Dysfunction. Int. J. Mol. Sci. 2022, 23, 1225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Zhao, H.; Chandakkar, P.; Chatterjee, P.K.; Papoin, J.; Blanc, L.; Metz, C.N.; Campagne, F.; Marambaud, P. A mouse model of hereditary hemorrhagic telangiectasia generated by transmammary-delivered immunoblocking of BMP9 and BMP10. Sci. Rep. 2016, 5, 37366. [Google Scholar] [CrossRef]
- Tillet, E.; Bailly, S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front. Genet. 2014, 5, 456. [Google Scholar] [CrossRef]
- Townson, S.A.; Martinez-Hackert, E.; Greppi, C.; Lowden, P.; Sako, D.; Liu, J.; Ucran, J.A.; Liharska, K.; Underwood, K.W.; Seehra, J.; et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem. 2012, 287, 27313–27325. [Google Scholar] [CrossRef]
- Saito, T.; Bokhove, M.; Croci, R.; Zamora-Caballero, S.; Han, L.; Letarte, M.; de Sanctis, D.; Jovine, L. Structural Basis of the Human Endoglin-BMP9 Interaction: Insights into BMP Signaling and HHT1. Cell Rep. 2017, 19, 1917–1928. [Google Scholar] [CrossRef]
- Park, E.S.; Kim, S.; Huang, S.; Yoo, J.Y.; Korbelin, J.; Lee, T.J.; Kaur, B.; Dash, P.K.; Chen, P.R.; Kim, E. Selective Endothelial Hyperactivation of Oncogenic KRAS Induces Brain Arteriovenous Malformations in Mice. Ann. Neurol. 2021, 89, 926–941. [Google Scholar] [CrossRef]
- Couto, J.A.; Huang, A.Y.; Konczyk, D.J.; Goss, J.A.; Fishman, S.J.; Mulliken, J.B.; Warman, M.L.; Greene, A.K. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am. J. Hum. Genet. 2017, 100, 546–554. [Google Scholar] [CrossRef]
- Chen, W.; Choi, E.J.; McDougall, C.M.; Su, H. Brain arteriovenous malformation modeling, pathogenesis, and novel therapeutic targets. Transl. Stroke Res. 2014, 5, 316–329. [Google Scholar] [CrossRef]
- Darsaut, T.E.; Magro, E.; Bojanowski, M.W.; Chaalala, C.; Nico, L.; Bacchus, E.; Klink, R.; Iancu, D.; Weill, A.; Roy, D.; et al. Surgical treatment of brain arteriovenous malformations: Clinical outcomes of patients included in the registry of a pragmatic randomized trial. J. Neurosurg. 2023, 138, 891–899. [Google Scholar] [CrossRef]
- Huang, P.W.; Peng, S.J.; Pan, D.H.; Yang, H.C.; Tsai, J.T.; Shiau, C.Y.; Su, I.C.; Chen, C.J.; Wu, H.M.; Lin, C.J.; et al. Vascular compactness of unruptured brain arteriovenous malformation predicts risk of hemorrhage after stereotactic radiosurgery. Sci. Rep. 2024, 14, 4011. [Google Scholar] [CrossRef]
- Frenzel, T.; Lee, C.Z.; Kim, H.; Quinnine, N.J.; Hashimoto, T.; Lawton, M.T.; Guglielmo, B.J.; McCulloch, C.E.; Young, W.L. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: Pilot study of adverse events and tolerance. Cerebrovasc. Dis. 2008, 25, 157–163. [Google Scholar] [CrossRef]
- Muster, R.; Ko, N.; Smith, W.; Su, H.; Dickey, M.A.; Nelson, J.; McCulloch, C.E.; Sneed, P.K.; Clarke, J.L.; Saloner, D.A.; et al. Proof-of-concept single-arm trial of bevacizumab therapy for brain arteriovenous malformation. BMJ Neurol. Open 2021, 3, e000114. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.M.; Dekeuleneer, V.; Coulie, J.; Marot, L.; Bataille, A.C.; Hammer, F.; Clapuyt, P.; Jeanjean, A.; Dompmartin, A.; Vikkula, M. Case report study of thalidomide therapy in 18 patients with severe arteriovenous malformations. Nat. Cardiovasc. Res. 2022, 1, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lekwuttikarn, R.; Lim, Y.H.; Admani, S.; Choate, K.A.; Teng, J.M.C. Genotype-Guided Medical Treatment of an Arteriovenous Malformation in a Child. JAMA Dermatol. 2019, 155, 256–257. [Google Scholar] [CrossRef]
- Edwards, E.A.; Phelps, A.S.; Cooke, D.; Frieden, I.J.; Zapala, M.A.; Fullerton, H.J.; Shimano, K.A. Monitoring Arteriovenous Malformation Response to Genotype-Targeted Therapy. Pediatrics 2020, 146, e20193206. [Google Scholar] [CrossRef]
- Cooke, D.L.; Frieden, I.J.; Shimano, K.A. Angiographic evidence of response to trametinib therapy for a spinal cord arteriovenous malformation. J. Vasc. Anom. 2021, 2, e018. [Google Scholar] [CrossRef]
- Kalailingam, P.; Rannikmae, K.; Hausman-Kedem, M.; Musolino, P.L.; Ruigrok, Y.M. Genetic Insights Into Hemorrhagic Stroke and Vascular Malformations: Pathogenesis and Emerging Therapeutic Strategies. Stroke 2025, 56, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Naylor, R.M.; Ghozy, S.A.; Thirupathi, K.; Rinaldo, L.; Kallmes, D.F.; Kadirvel, R. Advances in sporadic brain arteriovenous malformations: Novel genetic insights, innovative animal models and emerging therapeutic approaches. J. Cereb. Blood Flow. Metab. 2025, 45, 793–799. [Google Scholar] [CrossRef]
- Coffman, S.A.; Peterson, K.; Contillo, N.; Fargen, K.M.; Wolfe, S.Q. A comprehensive review on the development of sporadic cerebral arteriovenous malformations: From Padget to next-generation sequencing. J. Neurosurg. 2024, 141, 323–332. [Google Scholar] [CrossRef]
- Scherschinski, L.; Rahmani, R.; Srinivasan, V.M.; Catapano, J.S.; Oh, S.P.; Lawton, M.T. Genetics and Emerging Therapies for Brain Arteriovenous Malformations. World Neurosurg. 2022, 159, 327–337. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Conti, A.; Bortolotti, C.; Germano, A.; Alafaci, C.; Vinci, S.L.; D’Angelo, R.; Sidoti, A. Methylome analysis of endothelial cells suggests new insights on sporadic brain arteriovenous malformation. Heliyon 2024, 10, e35126. [Google Scholar] [CrossRef]
- Palmieri, M.; Curro, A.; Tommasi, A.; Di Sarno, L.; Doddato, G.; Baldassarri, M.; Frullanti, E.; Giliberti, A.R.; Fallerini, C.; Spinazzola, A.; et al. Cell-free DNA next-generation sequencing liquid biopsy as a new revolutionary approach for arteriovenous malformation. JVS Vasc. Sci. 2020, 1, 176–180. [Google Scholar] [CrossRef]
- Zenner, K.; Jensen, D.M.; Cook, T.T.; Dmyterko, V.; Bly, R.A.; Ganti, S.; Mirzaa, G.M.; Dobyns, W.B.; Perkins, J.A.; Bennett, J.T. Cell-free DNA as a diagnostic analyte for molecular diagnosis of vascular malformations. Genet. Med. 2021, 23, 123–130. [Google Scholar] [CrossRef]
- Zimmermann, W.H.; Melnychenko, I.; Eschenhagen, T. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 2004, 25, 1639–1647. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 2015, 4, e05098. [Google Scholar] [CrossRef][Green Version]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–109. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Kim, H.M.; Kwak, S.; Huh, C.; Chung, H.Y. The Formation of Human Arteriovenous Malformation Organoids and Their Characteristics. Cells 2024, 13, 1955. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.T.; He, Z.; Wimmer, R.A.; Seimiya, M.; Nikoloff, J.M.; Penninger, J.M.; Gray Camp, J.; Treutlein, B. Fate and state transitions during human blood vessel organoid development. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Salewskij, K.; Penninger, J.M. Blood Vessel Organoids for Development and Disease. Circ. Res. 2023, 132, 498–510. [Google Scholar] [CrossRef]
- Cai, H.; Tian, C.; Chen, L.; Yang, Y.; Sun, A.X.; McCracken, K.; Tchieu, J.; Gu, M.; Mackie, K.; Guo, F. Vascular network-inspired diffusible scaffolds for engineering functional midbrain organoids. Cell Stem Cell 2025, 32, 824–837.e5. [Google Scholar] [CrossRef]
- Kistemaker, L.; van Bodegraven, E.J.; de Vries, H.E.; Hol, E.M. Vascularized human brain organoids: Current possibilities and prospects. Trends Biotechnol. 2025, 43, 1275–1285. [Google Scholar] [CrossRef]
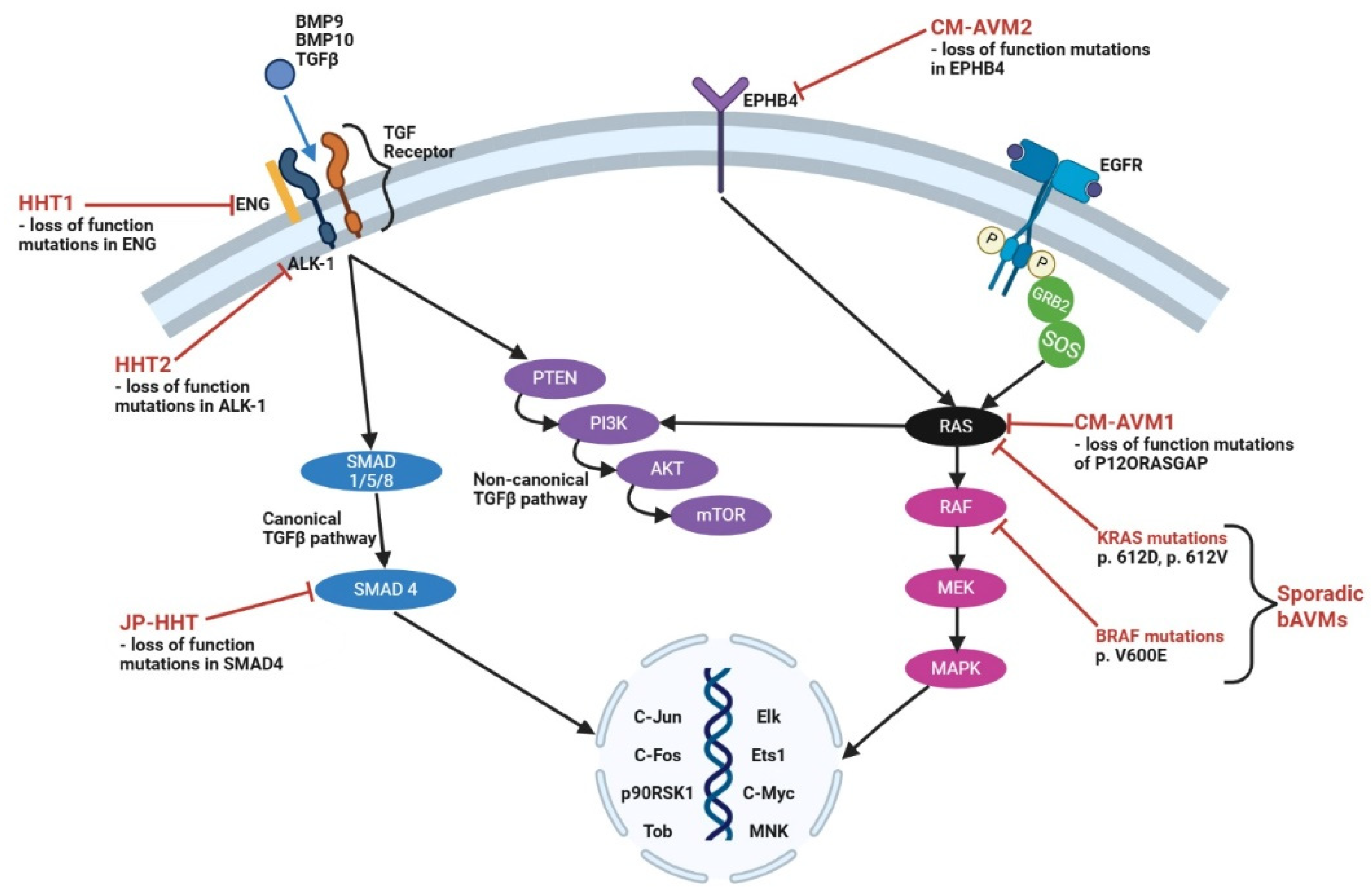

| Study | Genes | Mechanism of Action |
|---|---|---|
| Fish et al., 2020 [71] | MEK-ERK | Upstream KRAS activation increases MEK kinase activation. |
| Giarretta et al., 2021 [72]; Shoemaker et al., 2014 [69] | Shh COUP-TFII | Induced AVM-like properties of vessels. Gli1 and COUP-TFII. |
| Wang et al., 2023 [73] | ACVRL1 | Mutation linked to HHT. Found links to sporadic bAVMs. |
| Mansur and Radovanovic, 2023 [74]; Pérez-Alfayate et al., 2022 [75] | VEGF | Upregulated signaling in HHT activates MAPK-ERK pathway. Role in endothelial cell function. |
| Mansur and Radovanovic, 2023 [74]; Wang et al., 2023 [73]; Pan et al., 2021 [76]; Pérez-Alfayate et al., 2022 [75]; Goss et al., 2019 [77]; Fish et al., 2020 [71]; Nikolaev et al., 2018 [64] | KRAS | Increased downstream ERK phosphorylation and angiogenic signaling. Enhanced cell migratory behavior. Somatic KRAS activating mutations: KRAS G12V, KRAS G12D, KRAS G12C, and BRAF. Altered endothelial morphogenesis and growth dynamics. |
| Murphy et al., 2008 [78]; ZhuGe et al., 2009 [79]; Li et al., 2014 [80]; Pérez-Alfayate et al., 2022 [75]; | NOTCH | Abnormal gain or loss of NOTCH function. Increased expression of NOTCH-1 and downstream target HES-1 are observed in human bAVM tissue compared to control vessels. Alk1 knockout mice have decreased Notch signaling. Connects Alk1 and Notch signaling during vascular morphogenesis. Abnormal NOTCH-1 expression in bAVM hemorrhage. |
| Zhang et al., 2016 [53]; Mansur and Radovanovic, 2023 [74]; Pan et al., 2021 [20] | Alk1 | Cx3cr1+ microglia and Ccr2+ macrophages are present in AVM lesions of an Alk1 deficient mouse model. LOF mutation in HHT patients. |
| Wang et al., 2023 [73]; Xu et al., 2023 [81] | TGF-β | Mutated in ECs, essential for bAVM initiation. Low doses TGF-β stimulate proliferation and migration of ECs through ALK1. High doses of TGF-β result in quiescent endothelium. End-MT in bAVM tissues. |
| Mansur and Radovanovic, 2023 [74]; Wang et al., 2023 [73] | RAS-MAPK Ex. RASA1 | Mutated in ECs, essential for bAVM initiation. LOF mutation in RASA1 specifically causes abnormal activation of RAS-MAPK pathway and increases cellular proliferation, growth, differentiation, motility. |
| Mansur and Radovanovic, 2023 [74]; Pan et al., 2021 [20]; Pérez-Alfayate et al., 2022 [75] | Endoglin (ENG) | LOF mutations. ENG is a receptor for TGF-β and BMPs which are predominantly expressed in ECs. LOF undoes BMP/Alk1 signal cascade which suppresses endothelial cell migration and proliferation. |
| Wang et al., 2023 [73]; Pan et al., 2021 [20] | SMAD4 | LOF mutation in HHT patients. Linked to juvenile polyposis. |
| Mansur and Radovanovic, 2023 [74]; Xu et al., 2023 [81] | BMP9, BMP10 | Mutated in HHT patients. Plays important role in EC function and angiogenesis. BMP9 and BMP10 are probably the natural ligands for the ENG/ALK1 signaling pathway. |
| Winkler et al., 2022 [82] | PLVAP, ANGPT2 | Marker of fenestrated endothelium normally confined to developmental angiogenesis, the brain’s circumventricular organs and choroid plexus. |
| Adhicary et al., 2023 [83] | Rbpj | GTPase-mediated cellular function in brain ECs. Deficient expression increased Cdc-42 activity in isolated ECs. Disrupted cell polarity and focal adhesion properties. |
| Hermanto et al., 2016 [84]; Shoemaker et al., 2014 [69] | Sox17 | Downstream pathways implicated in bAVM. High expression in thick-walled veins and arteries. |
| Studies | Cell Type | Role in AVM Biology |
|---|---|---|
| Winkler et al., 2022 [11]; Wang et al., 2023 [73] | Macrophages | Perivascular macrophages (28.3% of bAVMs), IBA1+P2RY12− MΦ. Significantly increased in bAVM tissue. |
| Winkler et al., 2022 [11] | Monocytes | AIF1+-P2RY12− monocytes over-represented in ruptured bAVMs. |
| Winkler et al., 2022 [11] | Microglia | Discrete areas have numerous IBA1+ PSRY12+ monocytes. |
| Wang et al., 2023 [73] | Neutrophils | Significantly increased in bAVM tissue. |
| Winkler et al., 2022 [11]; Shabani et al., 2022 [85] | T lymphocytes | CD4+, CD8+, Tregs found in immune cell clusters associated with cerebrovasculature. Predominant detection in unruptured bAVM tissue. |
| Winkler et al., 2022 [11] | Natural killer cells | Found in immune cell clusters associated with cerebrovasculature. |
| Winkler et al., 2022 [11] | Plasmacytoid Dendritic Cells | Found in immune cell clusters associated with cerebrovasculature. |
| Tu et al., 2025 [86]; Shabani et al., 2022 [85]; Thomas et al., 2021 [87] | Astrocytes | Promotion of angiogenesis and vascular instability (hemorrhagic risk). Aberrant expressions of ALDH1A2 and CYR61 in abnormal neighboring astrocytes. |
| Winkler et al., 2022 [11]; Mansur and Radovanovic, 2023 [74]; Nikolaev et al., 2018 [64]; Shabani et al., 2022 [85] | Endothelial Cells | CLDN5+ within bAVM cell population. Clusters with suppressed venule and capillary cell identities. Alk1, Eng, and SMAD transcription factors work to suppress migration. VEGF and ET-1. KRAS mutations. |
| Winkler et al., 2022 [11] | Fibromyocytes | CCL19+ within bAVM cell population. |
| Winkler et al., 2022 [11] | Smooth Muscle Cells | TAGLN+ within bAVM cell population. |
| Winkler et al., 2022 [11] | Perivascular Fibroblasts | COL1A2+ within bAVM cell population. |
| Winkler et al., 2022 [11]; Pan et al., 2021 [76]; Nakisli et al., 2023 [88]; Shabani et al., 2022 [85] | Mural Cells | KCNJ8+ Pericyte number and coverage reduced. PDGF-B/PDGFR-disruption. Notch signaling pathway. BMP/ALK/SMAD pathway. RAS/MAPK pathway. |
| Winkler et al., 2022 [11] | Perivascular Fibroblasts | DCN+ APOD+ |
| Shoemaker et al., 2020 [70]; Xu et al., 2023 [81] | Mesenchymal Cells | The result of endothelial–mesenchymal transition signaling within bAVMs. |
| Animal Model | Features |
|---|---|
| Engf/f mice [96] |
|
| Eng or Alk1 conditional knockout mice [16,112] |
|
| Eng or Alk1 conditional knockout mice [100] |
|
| KrasG12D or KrasG12V transgenic mice [71,110] |
|
| Int3 transgenic mice [78] |
|
| KrasG12D zebrafish [71] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wazhi, K.; Lam, F.C.; Guru, S.; Hori, Y.S.; AbuReesh, D.; Shoemaker, L.; Park, D.J.; Chang, S.D. Characterizing the Microenvironment of Cerebral Arteriovenous Malformations to Test Novel Treatment Modalities. Brain Sci. 2025, 15, 1145. https://doi.org/10.3390/brainsci15111145
Wazhi K, Lam FC, Guru S, Hori YS, AbuReesh D, Shoemaker L, Park DJ, Chang SD. Characterizing the Microenvironment of Cerebral Arteriovenous Malformations to Test Novel Treatment Modalities. Brain Sciences. 2025; 15(11):1145. https://doi.org/10.3390/brainsci15111145
Chicago/Turabian StyleWazhi, Kavin, Fred C. Lam, Santosh Guru, Yusuke S. Hori, Deyaldeen AbuReesh, Lorelei Shoemaker, David J. Park, and Steven D. Chang. 2025. "Characterizing the Microenvironment of Cerebral Arteriovenous Malformations to Test Novel Treatment Modalities" Brain Sciences 15, no. 11: 1145. https://doi.org/10.3390/brainsci15111145
APA StyleWazhi, K., Lam, F. C., Guru, S., Hori, Y. S., AbuReesh, D., Shoemaker, L., Park, D. J., & Chang, S. D. (2025). Characterizing the Microenvironment of Cerebral Arteriovenous Malformations to Test Novel Treatment Modalities. Brain Sciences, 15(11), 1145. https://doi.org/10.3390/brainsci15111145








