Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
Objectives
2. Materials and Methods
2.1. Search Strategies
2.2. Inclusion and Exclusion Criteria
2.3. Screening and Study Selection
2.4. Data Extraction and Analysis
2.5. Quality Assessment of Included Studies
3. Results
3.1. Search Result
3.2. Study and Sample Characteristics
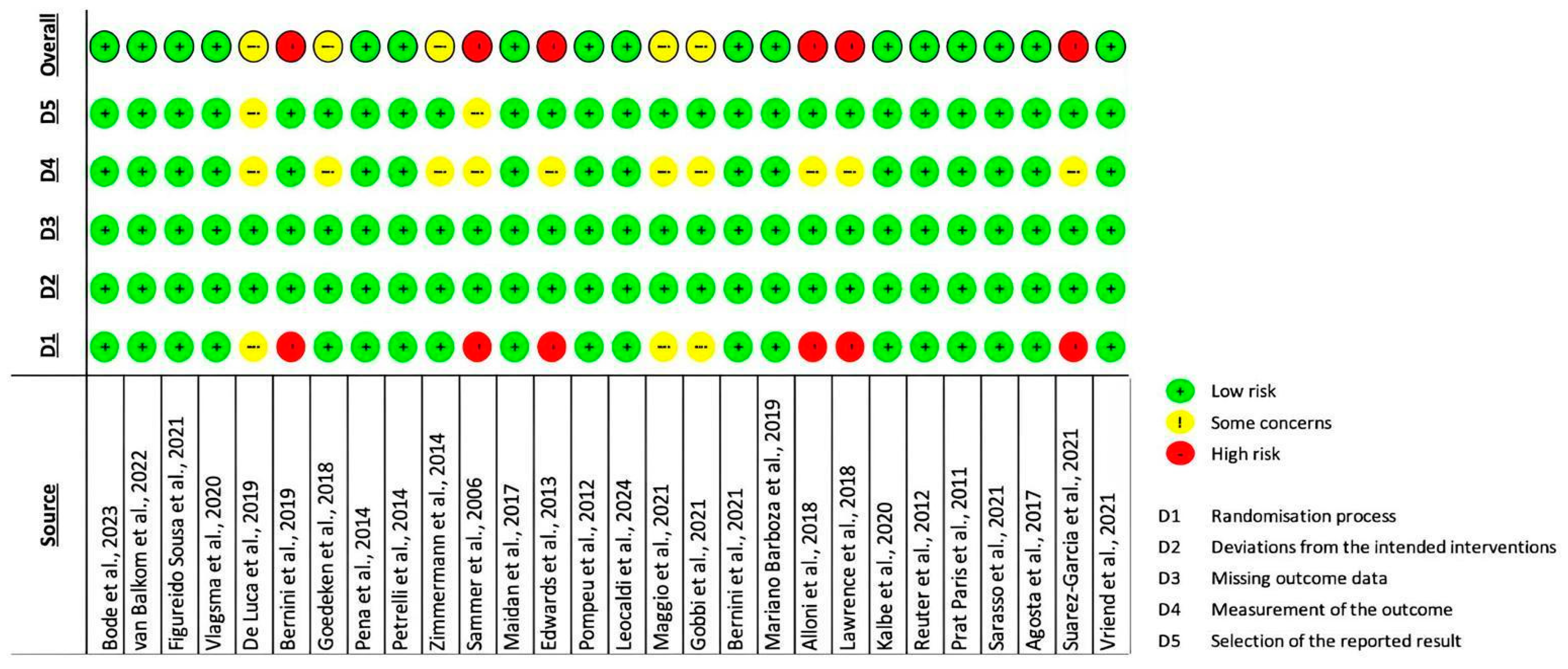
3.3. Quality Appraisal
3.4. Data Extraction
3.4.1. Assessment of Cognitive Functions
3.4.2. Cognitive Training
3.4.3. Effect of Cognitive Training on Cognitive Functioning
4. Discussion
5. Conclusions
Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS Clinical Diagnostic Criteria for Parkinson’s Disease: MDS-PD Clinical Diagnostic Criteria. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Lahuerta-Martín, S.; Llamas-Ramos, R.; Llamas-Ramos, I. Effectiveness of Therapies Based on Mirror Neuron System to Treat Gait in Patients with Parkinson’s Disease—A Systematic Review. J. Clin. Med. 2022, 11, 4236. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Wenning, G.; Poewe, W. The Diagnosis of Parkinson’s Disease. Lancet Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Uitti, R.J.; Berry, K.; Yasuhara, O.; Eisen, A.; Feldman, H.; McGeer, P.L.; Calne, D.B. Neurodegenerative “overlap” Syndrome: Clinical and Pathological Features of Parkinson’s Disease, Motor Neuron Disease, and Alzheimer’s Disease. Park. Relat. Disord. 1995, 1, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Morris, H.R.; Robbins, T.W.; Goedert, M.; Hardy, J.; Ben-Shlomo, Y.; Bolam, P.; Burn, D.; Hindle, J.V.; Brooks, D. Parkinson’s Disease--the Debate on the Clinical Phenomenology, Aetiology, Pathology and Pathogenesis. J. Park. Dis. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, A.; Arkadir, D.; Israel, Z.; Bergman, H. Akineto-Rigid vs. Tremor Syndromes in Parkinsonism. Curr. Opin. Neurol. 2009, 22, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kann, S.J.; Chang, C.; Manza, P.; Leung, H.-C. Akinetic Rigid Symptoms Are Associated with Decline in a Cortical Motor Network in Parkinson’s Disease. Npj Park. Dis. 2020, 6, 19. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force Report on the Hoehn and Yahr Staging Scale: Status and Recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. the index of Adl: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Cova, I.; Di Battista, M.E.; Vanacore, N.; Papi, C.P.; Alampi, G.; Rubino, A.; Valente, M.; Meco, G.; Contri, P.; Di Pucchio, A.; et al. Validation of the Italian Version of the Non Motor Symptoms Scale for Parkinson’s Disease. Park. Relat. Disord. 2017, 34, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Kassubek, J.; Csoti, I.; Ehret, R.; Herbst, H.; Wellach, I.; Winkler, J.; Jost, W.H. Differentiation of Atypical Parkinson Syndromes. J. Neural Transm. 2017, 124, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Dysautonomia and Cognitive Dysfunction in Parkinson’s Disease. Mov. Disord. 2007, 22 (Suppl. S17), S374–S378. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Non-Motor Symptoms in Parkinson’s Disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Orgeta, V.; McDonald, K.R.; Poliakoff, E.; Hindle, J.V.; Clare, L.; Leroi, I. Cognitive Training Interventions for Dementia and Mild Cognitive Impairment in Parkinson’s Disease. Cochrane Database Syst. Rev. 2020, 2020, CD011961. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.; Borgnis, F.; Pozzo, T. Mirror Neurons and Their Relationship with Neurodegenerative Disorders. J. Neurosci. Res. 2020, 98, 1070–1094. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, D.; Marinus, J.; Visser, M.; van Rooden, S.M.; Stiggelbout, A.M.; Middelkoop, H.A.M.; van Hilten, J.J. Cognitive Impairment in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1182–1187. [Google Scholar] [CrossRef]
- Goffredo, M.; Baglio, F.; DE Icco, R.; Proietti, S.; Maggioni, G.; Turolla, A.; Pournajaf, S.; Jonsdottir, J.; Zeni, F.; Federico, S.; et al. Efficacy of Non-Immersive Virtual Reality-Based Telerehabilitation on Postural Stability in Parkinson’s Disease: A Multicenter Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 689–696. [Google Scholar] [CrossRef]
- Cochen De Cock, V.; Dotov, D.; Damm, L.; Lacombe, S.; Ihalainen, P.; Picot, M.C.; Galtier, F.; Lebrun, C.; Giordano, A.; Driss, V.; et al. BeatWalk: Personalized Music-Based Gait Rehabilitation in Parkinson’s Disease. Front. Psychol. 2021, 12, 655121. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.; Aarsland, D.; Biundo, R.; Dobkin, R.; Goldman, J.; Lewis, S. Management of Psychiatric and Cognitive Complications in Parkinson’s Disease. BMJ 2022, 379, e068718. [Google Scholar] [CrossRef] [PubMed]
- Burchill, E.; Watson, C.J.; Fanshawe, J.B.; Badenoch, J.B.; Rengasamy, E.; Ghanem, D.A.; Holle, C.; Conti, I.; Sadeq, M.A.; Saini, A.; et al. The Impact of Psychiatric Comorbidity on Parkinson’s Disease Outcomes: A Systematic Review and Meta-Analysis. Lancet Reg. Health—Eur. 2024, 39, 100870. [Google Scholar] [CrossRef]
- Weintraub, D.; Mamikonyan, E. The Neuropsychiatry of Parkinson Disease: A Perfect Storm. Am. J. Geriatr. Psychiatry 2019, 27, 998–1018. [Google Scholar] [CrossRef]
- Santangelo, G.; D’Iorio, A.; Maggi, G.; Cuoco, S.; Pellecchia, M.T.; Amboni, M.; Barone, P.; Vitale, C. Cognitive Correlates of “Pure Apathy” in Parkinson’s Disease. Park. Relat. Disord. 2018, 53, 101–104. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical Diagnostic Criteria for Dementia Associated with Parkinson’s Disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Papagno, C.; Trojano, L. Cognitive and Behavioral Disorders in Parkinson’s Disease: An Update. I: Cognitive Impairments. Neurol. Sci. 2018, 39, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. Evolution of Cognitive Dysfunction in an Incident Parkinson’s Disease Cohort. Brain 2007, 130, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and Clinical Heterogeneity of Cognitive Impairment and Dementia in Patients with Parkinson’s Disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson Disease-Associated Cognitive Impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Bek, J.; Gowen, E.; Vogt, S.; Crawford, T.J.; Poliakoff, E. Combined Action Observation and Motor Imagery Influences Hand Movement Amplitude in Parkinson’s Disease. Park. Relat. Disord. 2019, 61, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, P.; Cui, S.; He, Y.; Tan, Y.; Chen, S. Effect of Long-Term Tai Chi Training on Parkinson’s Disease: A 3.5-Year Follow-up Cohort Study. J. Neurol. Neurosurg. Psychiatr. 2023, 95, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Lantos, P.L.; Cairns, N.J. Overlap between Neurodegenerative Disorders. Neuropathology 2005, 25, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Calleo, J.; Burrows, C.; Levin, H.; Marsh, L.; Lai, E.; York, M.K. Cognitive Rehabilitation for Executive Dysfunction in Parkinson’s Disease: Application and Current Directions. Park. Dis. 2012, 2012, 512892. [Google Scholar] [CrossRef]
- Leung, I.H.K.; Walton, C.C.; Hallock, H.; Lewis, S.J.G.; Valenzuela, M.; Lampit, A. Cognitive Training in Parkinson Disease: A Systematic Review and Meta-Analysis. Neurology 2015, 85, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.J.; Gasson, N.; Bucks, R.S.; Troeung, L.; Loftus, A.M. Cognitive Training and Noninvasive Brain Stimulation for Cognition in Parkinson’s Disease: A Meta-Analysis. Nehurorehabilitation Neural Repair 2017, 31, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.; Venneri, A. Cognitive Rehabilitation in Parkinson’s Disease: A Systematic Review. J. Park. Dis. 2018, 8, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Luengos, I.; Balboa-Bandeira, Y.; Lucas-Jiménez, O.; Ojeda, N.; Peña, J.; Ibarretxe-Bilbao, N. Effectiveness of Cognitive Rehabilitation in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Latella, D.; Maggio, M.G.; Di Lorenzo, G.; Maresca, G.; Sciarrone, F.; Militi, D.; Bramanti, P.; Calabrò, R.S. Computer Assisted Cognitive Rehabilitation Improves Visuospatial and Executive Functions in Parkinson’s Disease: Preliminary Results. NRE 2019, 45, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Bernini, S.; Alloni, A.; Panzarasa, S.; Picascia, M.; Quaglini, S.; Tassorelli, C.; Sinforiani, E. A Computer-Based Cognitive Training in Mild Cognitive Impairment in Parkinson’s Disease. NRE 2019, 44, 555–567. [Google Scholar] [CrossRef]
- Leocadi, M.; Canu, E.; Sarasso, E.; Gardoni, A.; Basaia, S.; Calderaro, D.; Castelnovo, V.; Volontè, M.A.; Filippi, M.; Agosta, F. Dual-Task Gait Training Improves Cognition and Resting-State Functional Connectivity in Parkinson’s Disease with Postural Instability and Gait Disorders. J. Neurol. 2024, 271, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Luca, A.; Cicero, C.E.; Calabrò, R.S.; Drago, F.; Zappia, M.; Nicoletti, A. Effectiveness of Telerehabilitation plus Virtual Reality (Tele-RV) in Cognitive e Social Functioning: A Randomized Clinical Study on Parkinson’s Disease. Park. Relat. Disord. 2024, 119, 105970. [Google Scholar] [CrossRef]
- Bernini, S.; Panzarasa, S.; Barbieri, M.; Sinforiani, E.; Quaglini, S.; Tassorelli, C.; Bottiroli, S. A Double-Blind Randomized Controlled Trial of the Efficacy of Cognitive Training Delivered Using Two Different Methods in Mild Cognitive Impairment in Parkinson’s Disease: Preliminary Report of Benefits Associated with the Use of a Computerized Tool. Aging Clin. Exp. Res. 2021, 33, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Alloni, A.; Quaglini, S.; Panzarasa, S.; Sinforiani, E.; Bernini, S. Evaluation of an Ontology-Based System for Computerized Cognitive Rehabilitation. Int. J. Med. Inform. 2018, 115, 64–72. [Google Scholar] [CrossRef]
- Sarasso, E.; Agosta, F.; Piramide, N.; Gardoni, A.; Canu, E.; Leocadi, M.; Castelnovo, V.; Basaia, S.; Tettamanti, A.; Volontè, M.A.; et al. Action Observation and Motor Imagery Improve Dual Task in Parkinson’s Disease: A Clinical/FMRI Study. Mov. Disord. 2021, 36, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Gatti, R.; Sarasso, E.; Volonté, M.A.; Canu, E.; Meani, A.; Sarro, L.; Copetti, M.; Cattrysse, E.; Kerckhofs, E.; et al. Brain Plasticity in Parkinson’s Disease with Freezing of Gait Induced by Action Observation Training. J. Neurol. 2017, 264, 88–101. [Google Scholar] [CrossRef]
- Bode, M.; Sulzer, P.; Schulte, C.; Becker, S.; Brockmann, K.; Elben, S.; Folkerts, A.-K.; Ophey, A.; Schlenstedt, C.; Witt, K.; et al. Multidomain Cognitive Training Increases Physical Activity in People with Parkinson’s Disease with Mild Cognitive Impairment. Park. Relat. Disord. 2023, 113, 105330. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Kaesberg, S.; Barbe, M.T.; Timmermann, L.; Fink, G.R.; Kessler, J.; Kalbe, E. Effects of Cognitive Training in Parkinson’s Disease: A Randomized Controlled Trial. Park. Relat. Disord. 2014, 20, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Sammer, G.; Reuter, I.; Hullmann, K.; Kaps, M.; Vaitl, D. Training of Executive Functions in Parkinson’s Disease. J. Neurol. Sci. 2006, 248, 115–119. [Google Scholar] [CrossRef]
- Kalbe, E.; Folkerts, A.-K.; Ophey, A.; Eggers, C.; Elben, S.; Dimenshteyn, K.; Sulzer, P.; Schulte, C.; Schmidt, N.; Schlenstedt, C.; et al. Enhancement of Executive Functions but Not Memory by Multidomain Group Cognitive Training in Patients with Parkinson’s Disease and Mild Cognitive Impairment: A Multicenter Randomized Controlled Trial. Park. Dis. 2020, 2020, 4068706. [Google Scholar] [CrossRef] [PubMed]
- Reuter, I.; Mehnert, S.; Sammer, G.; Oechsner, M.; Engelhardt, M. Efficacy of a Multimodal Cognitive Rehabilitation Including Psychomotor and Endurance Training in Parkinson’s Disease. J. Aging Res. 2012, 2012, 235765. [Google Scholar] [CrossRef] [PubMed]
- Vriend, C.; Van Balkom, T.D.; Berendse, H.W.; Van Der Werf, Y.D.; Van Den Heuvel, O.A. Cognitive Training in Parkinson’s Disease Induces Local, Not Global, Changes in White Matter Microstructure. Neurotherapeutics 2021, 18, 2518–2528. [Google Scholar] [CrossRef]
- Van Balkom, T.D.; Berendse, H.W.; Van Der Werf, Y.D.; Twisk, J.W.R.; Peeters, C.F.W.; Hoogendoorn, A.W.; Hagen, R.H.; Berk, T.; Van Den Heuvel, O.A.; Vriend, C. Effect of Eight-Week Online Cognitive Training in Parkinson’s Disease: A Double-Blind, Randomized, Controlled Trial. Park. Relat. Disord. 2022, 96, 80–87. [Google Scholar] [CrossRef]
- Vlagsma, T.T.; Duits, A.A.; Dijkstra, H.T.; Van Laar, T.; Spikman, J.M. Effectiveness of ReSET; a Strategic Executive Treatment for Executive Dysfunctioning in Patients with Parkinson’s Disease. Neuropsychol. Rehabil. 2020, 30, 67–84. [Google Scholar] [CrossRef]
- Sousa, N.M.F.; Neri, A.C.D.M.; Brandi, I.V.; Brucki, S.M.D. Impact of Cognitive Intervention on Cognitive Symptoms and Quality of Life in Idiopathic Parkinson’s Disease: A Randomized and Controlled Study. Dement. Neuropsychol. 2021, 15, 51–59. [Google Scholar] [CrossRef]
- Pompeu, J.E.; Mendes, F.A.D.S.; Silva, K.G.D.; Lobo, A.M.; Oliveira, T.D.P.; Zomignani, A.P.; Piemonte, M.E.P. Effect of Nintendo WiiTM-Based Motor and Cognitive Training on Activities of Daily Living in Patients with Parkinson’s Disease: A Randomised Clinical Trial. Physiotherapy 2012, 98, 196–204. [Google Scholar] [CrossRef]
- Gobbi, L.T.B.; Pelicioni, P.H.S.; Lahr, J.; Lirani-Silva, E.; Teixeira-Arroyo, C.; Santos, P.C.R.D. Effect of Different Types of Exercises on Psychological and Cognitive Features in People with Parkinson’s Disease: A Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2021, 64, 101407. [Google Scholar] [CrossRef] [PubMed]
- Barboza, N.M.; Terra, M.B.; Bueno, M.E.B.; Christofoletti, G.; Smaili, S.M. Physiotherapy Versus Physiotherapy Plus Cognitive Training on Cognition and Quality of Life in Parkinson Disease: Randomized Clinical Trial. Am. J. Phys. Med. Rehabil. 2019, 98, 460–468. [Google Scholar] [CrossRef]
- Goedeken, S.; Potempa, C.; Prager, E.M.; Foster, E.R. Encoding Strategy Training and Self-Reported Everyday Prospective Memory in People with Parkinson Disease: A Randomized-Controlled Trial. Clin. Neuropsychol. 2018, 32, 1282–1302. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.D.; Hauser, R.A.; O’Connor, M.L.; Valdés, E.G.; Zesiewicz, T.A.; Uc, E.Y. Randomized Trial of Cognitive Speed of Processing Training in Parkinson Disease. Neurology 2013, 81, 1284–1290. [Google Scholar] [CrossRef]
- Suárez-García, D.M.A.; Birba, A.; Zimerman, M.; Diazgranados, J.A.; Lopes Da Cunha, P.; Ibáñez, A.; Grisales-Cárdenas, J.S.; Cardona, J.F.; García, A.M. Rekindling Action Language: A Neuromodulatory Study on Parkinson’s Disease Patients. Brain Sci. 2021, 11, 887. [Google Scholar] [CrossRef]
- Peña, J.; Ibarretxe-Bilbao, N.; García-Gorostiaga, I.; Gomez-Beldarrain, M.A.; Díez-Cirarda, M.; Ojeda, N. Improving Functional Disability and Cognition in Parkinson Disease: Randomized Controlled Trial. Neurology 2014, 83, 2167–2174. [Google Scholar] [CrossRef]
- París, A.P.; Saleta, H.G.; De La Cruz Crespo Maraver, M.; Silvestre, E.; Freixa, M.G.; Torrellas, C.P.; Pont, S.A.; Nadal, M.F.; Garcia, S.A.; Bartolomé, M.V.P.; et al. Blind Randomized Controlled Study of the Efficacy of Cognitive Training in Parkinson’s Disease. Mov. Disord. 2011, 26, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Gschwandtner, U.; Benz, N.; Hatz, F.; Schindler, C.; Taub, E.; Fuhr, P. Cognitive Training in Parkinson Disease: Cognition-Specific vs Nonspecific Computer Training. Neurology 2014, 82, 1219–1226. [Google Scholar] [CrossRef]
- Maidan, I.; Rosenberg-Katz, K.; Jacob, Y.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Disparate Effects of Training on Brain Activation in Parkinson Disease. Neurology 2017, 89, 1804–1810. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Gasson, N.; Johnson, A.R.; Booth, L.; Loftus, A.M. Cognitive Training and Transcranial Direct Current Stimulation for Mild Cognitive Impairment in Parkinson’s Disease: A Randomized Controlled Trial. Park. Dis. 2018, 2018, 4318475. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Philips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in Frontotemporal Dementia and Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A Brief Cognitive Test Battery for Dementia Screening. Int. J. Geriatr. Psychiatr. 2006, 21, 1078–1085. [Google Scholar] [CrossRef]
- Kalbe, E.; Reinhold, N.; Brand, M.; Markowitsch, H.J.; Kessler, J. A New Test Battery to Assess Aphasic Disturbances and Associated Cognitive Dysfunctions—German Normative Data on the Aphasia Check List. J. Clin. Exp. Neuropsychol. 2005, 27, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Reitan, R.M. Trail Making Test. Manual for Administration, Scoring, and Interpretation; Indiana University Press: Indianapolis, IN, USA, 1956. [Google Scholar]
- Spinnler, H.; Tognoni, G. Standardizzazione e Taratura Italiana Di Test Neuropsicologici. Ital. J. Neurol. Sci. 1987, 8, 44–46. [Google Scholar]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Weiss, A.A. The Weigl-Goldstein-Scheerer Color-Form Sorting Test: Classification of Performance. J. Clin. Psychol. 1964, 20, 103–107. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at Bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Bousfield, W.A.; Sedgewick, C.H.W. An Analysis of Sequences of Restricted Associative Responses. J. Gen. Psychol. 1944, 30, 149–165. [Google Scholar] [CrossRef]
- Wilson, B.A.; Evans, J.J.; Alderman, N.; Burgess, P.W.; Emslie, H. Behavioural Assessment of the Dysexecutive Syndrome. In Methodology of Frontal and Executive Function; Routledge: London, UK, 1997; ISBN 978-0-203-34418-7. [Google Scholar]
- Raven, J.; Raven, J. Raven Progressive Matrices. In Handbook of Nonverbal Assessment; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 223–237. ISBN 978-0-306-47715-7. [Google Scholar]
- Berg, W.; Byrd, D. The Tower of London Spatial Problem-Solving Task: Enhancing Clinical and Research Implementation. J. Clin. Exp. Neuropsychol. 2002, 24, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Bean, J. Rey Auditory Verbal Learning Test, Rey AVLT. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 2174–2175. ISBN 978-0-387-79948-3. [Google Scholar]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. CVLT, California Verbal Learning Test: Adult Version: Manual; Psychological Corporation: San Antonio, TX, USA, 1987. [Google Scholar]
- Corsi, P.M. Human Memory and the Medial Temporal Region of the Brain; McGill University: Montreal, QC, Canada, 1973. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Blackburn, H.L.; Benton, A.L. Revised Administration and Scoring of the Digit Span Test. J. Consult. Psychol. 1957, 21, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.; Leech, L.; Kaplan, E.; Winocur, G.; Shulman, K.; Delis, D. Clock Drawing: A Neuropsychological Analysis; Oxford Press: New York, NY, USA, 1994; Volume 15. [Google Scholar]
- Benton, A.L.; Varney, N.; Hamsher, K. Visuospatial Judgment: A Clinical Test. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Rey, A. L’examen Psychologique Dans Les Cas d’encephalopathie Traumatique.(Les Problems.). Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Schuhfired GmbH. Cogniplus; Schuhfired GmbH: Modling, Austria, 2007. [Google Scholar]
- Alloni, A.; Sinforiani, E.; Zucchella, C.; Sandrini, G.; Bernini, S.; Cattani, B.; Pardell, D.T.; Quaglini, S.; Pistarini, C. Computer-Based Cognitive Rehabilitation: The CoRe System. Disabil. Rehabil. 2017, 39, 407–417. [Google Scholar] [CrossRef]
- Rendell, P.; Henry, J. A Review of Virtual Week for Prospective Memory Assessment: Clinical Implications. Brain Impair.—BRAIN IMPAIR 2009, 10, 14–22. [Google Scholar] [CrossRef]
- Baller, G.; Kaesberg, S.; Kessler, J.; Kalbe, E. NeuroVitalis—Ein neuropsychologisches Gruppenprogramm zur Steigerung der geistigen Leistungsfähigkeit in zwei Schwierigkeitsstufen. Prax. Ergother. 2012, 25, 349–350. [Google Scholar]
- Ojeda, N.; Peña, J.; Bengoetxea, E.; Guerrero, A.; Sanchez, P.; Elizagárate, E.; Ezcurra, J.; Eguíluz, J. REHACOP: A Cognitive Rehabilitation Programme in Psychosis. Rev. De Neurol. 2012, 54, 337–342. [Google Scholar] [CrossRef]
- Frazzitta, G.; Maestri, R.; Bertotti, G.; Riboldazzi, G.; Boveri, N.; Perini, M.; Uccellini, D.; Turla, M.; Comi, C.; Pezzoli, G.; et al. Intensive Rehabilitation Treatment in Early Parkinson’s Disease: A Randomized Pilot Study with a 2-Year Follow-Up. Neurorehabil. Neural Repair 2015, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martín, P.; Rodríguez-Blázquez, C.; Alvarez, M.; Arakaki, T.; Arillo, V.C.; Chaná, P.; Fernández, W.; Garretto, N.; Martínez-Castrillo, J.C.; Rodríguez-Violante, M.; et al. Parkinson’s Disease Severity Levels and MDS-Unified Parkinson’s Disease Rating Scale. Park. Relat. Disord. 2015, 21, 50–54. [Google Scholar] [CrossRef]
- Walton, C.C.; Naismith, S.L.; Lampit, A.; Mowszowski, L.; Lewis, S.J.G. Cognitive Training in Parkinson’s Disease: A Theoretical Perspective. Neurorehabil. Neural Repair 2017, 31, 207–216. [Google Scholar] [CrossRef] [PubMed]
- de Sena, I.G.; da Costa, A.V.; dos Santos, I.K.; de Araújo, D.P.; da Silva Gomes, F.T.; da Paiva Cavalcanti, J.R.L.; Knackfuss, M.I.; de Andrade, M.F.; Melo, P.K.M.; Fonseca, I.A.T. Feasibility and Effect of High-Intensity Training on the Progression of Motor Symptoms in Adult Individuals with Parkinson’s Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0293357. [Google Scholar] [CrossRef]
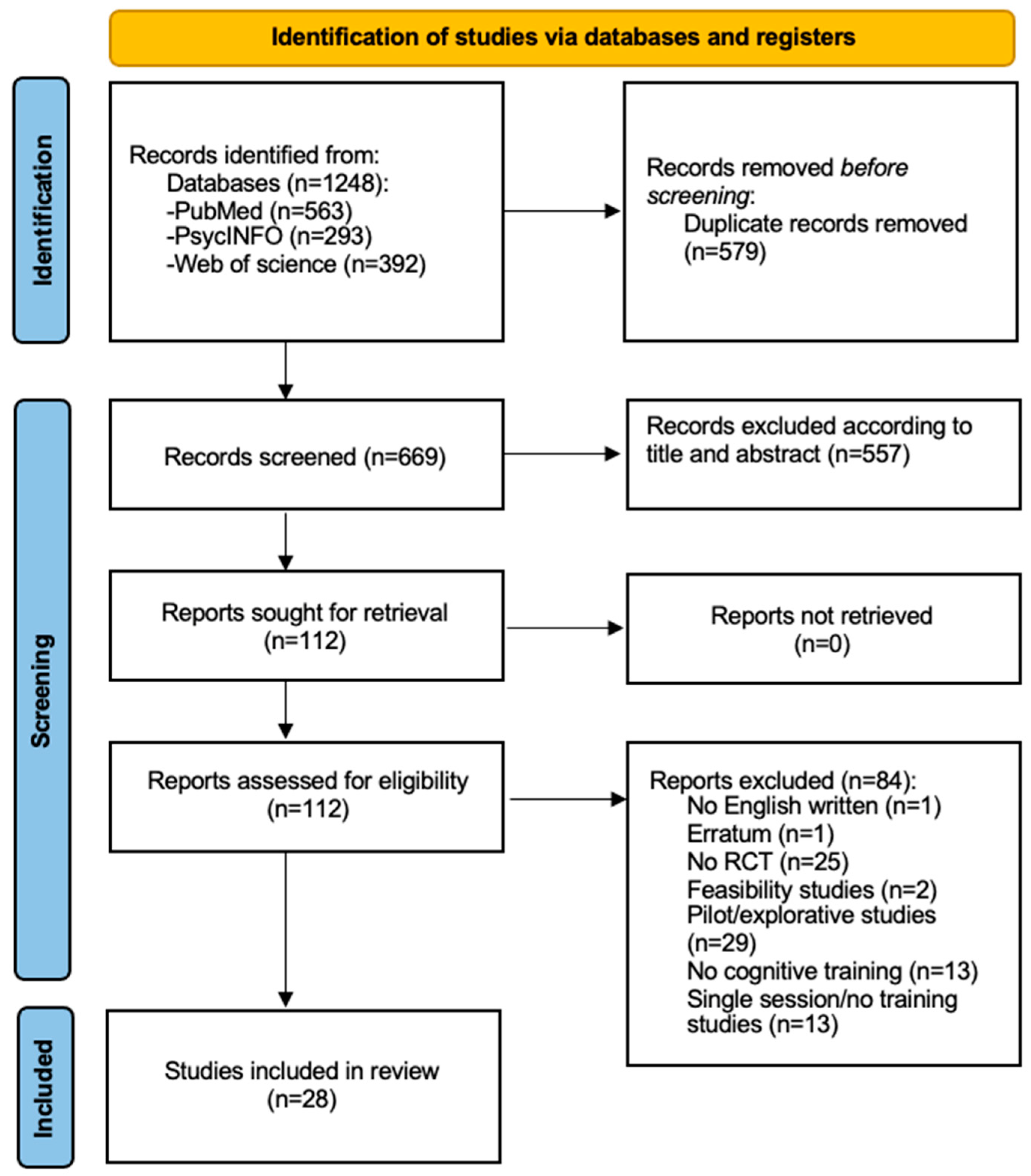
| Database | Research String |
|---|---|
| PsycINFO | (“Parkinson’s disease” OR “Parkinson” OR “Parkinson’s” OR “Parkinsons” OR “PD” OR “Idiopathic Parkinson’s disease” OR “Idiopathic Parkinson” OR “Idiopathic Parkinson’s” OR “IPD” OR “Parkinson’s disease Idiopathic” OR “Idiopathic Parkinson disease”) AND (“cognitive training” OR “cognitive rehabilitation” OR “cognitive intervention” OR “attention training” OR “brain training” OR “reasoning training” OR “mnemonic training” OR “speed and processing training” OR “executive function* training” OR “mirror neurons” OR “action observation” OR “motor imagery”) |
| PubMed | (“Parkinson’s disease” OR “Parkinson” OR “Parkinson’s” OR “Parkinsons” OR “PD” OR “Idiopathic Parkinson’s disease” OR “Idiopathic Parkinson” OR “Idiopathic Parkinson’s” OR “IPD” OR “Parkinson’s disease Idiopathic” OR “Idiopathic Parkinson disease”) AND (“cognitive training” OR “cognitive rehabilitation” OR “cognitive intervention” OR “attention training” OR “brain training” OR “reasoning training” OR “mnemonic training” OR “speed and processing training” OR “executive function* training” OR “mirror neurons” OR “action observation” OR “motor imagery”) |
| Web of science | (TS = (“Parkinson’s disease” OR “Parkinson” OR “Parkinson’s” OR “Parkinsons” OR “PD” OR “Idiopathic Parkinson’s disease” OR “Idiopathic Parkinson” OR “Idiopathic Parkinson’s” OR “IPD” OR “Parkinson’s disease Idiopathic” OR “Idiopathic Parkinson disease”)) AND TS = (“cognitive training” OR “cognitive rehabilitation” OR “cognitive intervention” OR “attention training” OR “brain training” OR “reasoning training” OR “mnemonic training” OR “speed and processing training” OR “executive function* training” OR “mirror neurons” OR “action observation” OR “motor imagery”) |
| Source | Study Design | Experimental Treatment | Control Treatment | Results |
|---|---|---|---|---|
| Bode et al., 2023 [51] | Multicentre randomised controlled trials | NEUROvitalis Parkinson training (CT) Duration: 6 weeks Intensity: twice a week Type of treatment: standardised programme targeting executive functions, memory, attention, and visuo-cognition through group and individual tasks. Psycho-educative elements on cognitive functions and strategies to enhance targeted functions were also included in each session. | Low-intensity physical activity programme (PT) Duration: 6 weeks Intensity: twice a week Type of treatment: active control training aimed to improve motor function, but not cognition. Sessions included warm-up exercises, stretching, flexibility, loosening up, and relaxation, as well as psychoeducation and homework. | Motor outcomes: CT group displayed more periods of physical activity after training vs. the PT group. Cognitive outcomes: CT group:
↑ attention with an unstable time effect. |
| van Balkom et al., 2022 [57] | Double-blind randomised controlled trials | Computerised cognitive training Duration: 8 weeks Intensity: three sessions a week Type of treatment: intervention consisted of 13 training games that focused on attention, processing speed and executive functions, and had an adaptive difficulty based on the performance, based on the Braingymmer online CT platform | Active computer-based control group Duration: 8 weeks Intensity: three sessions a week Type of treatment: intervention consisted of three games without difficulty adjustments. | Cognitive outcomes: Computerised cognitive training group:
|
| Sousa et al., 2021 [59] | Randomised controlled trials with placebo | Paper-pencil cognitive training Duration: 4 weeks Intensity: twice a week Type of treatment: group training that emphasised attention and executive dysfunction, plus all the activities of the general rehabilitation programme. Paper-and-pencil tasks focused on the repeated practice of structured exercises. In the same session, three levels of difficulty were offered. | General rehabilitation programme Duration: 4 weeks Intensity: twice a week Type of treatment: various group activities, including: physiotherapy dance, re-education in writing, speech therapy, information groups, manual skills workshops, and physical activity. | Cognitive outcomes: Paper-pencil cognitive training: ↑ in attention (especially shifting attention and processing speed), executive functions (verbal fluency) and global measures in the ACE-III battery Autonomy and QoL outcomes: Paper-pencil cognitive training: ↑ in QoL |
| Vlagsma et al., 2020 [58] | Multicentre randomised controlled trials | ReSET training Duration: 7–14 weeks Intensity: once/twice a week Type of treatment: individual treatment, to improve or stabilise the participants’ level of independence and QoL, by teaching the patient strategies to compensate for impairments in EF in everyday life situations. Three modules: “Information and awareness”, “Goal setting and planning”, and “Initiative, execution and regulation”. | CogniPlus training Duration: 7–14 weeks Intensity: once/twice a week Type of treatment: six subtests of Cogniplus were individually administered to patients; five subtests aimed at training aspects of attention, and one subtest aimed at training working memory. | Cognitive outcomes: RESET training group:
Both groups: no significant effects on the level of participation in societal domains |
| De Luca et al., 2019 [43] | Randomised controlled trials | Computerised cognitive training (CACR) with ERICA platform Duration: 8 weeks Intensity: three sessions a week Type of treatment: training with ERICA, an Italian computerised cognitive tool, comprising a series of specific cognitive exercises. | Standard cognitive training (SCT) Duration: 8 weeks Intensity: three sessions a week Type of treatment: face-to-face interaction between therapist and patient, and paper-and pencil-activities. | Cognitive outcomes: CACR group ↑ visual-spatial and executive domains vs. SCT |
| Bernini et al., 2019 [44] | Open not blind randomised controlled trials | CoRe cognitive training + standard physical training (G1) Duration: 4 weeks Intensity: three sessions a week Type of treatment: computer-based logical-executive patient-tailored tasks CoRE and physical rehabilitation. Standard physical rehabilitation comprised cardiovascular warm-up activities, active and passive exercises, stretching, postural changes, and exercises operating on balance and postural control. | Standard physical training (G2) Duration: 4 weeks Intensity: three sessions a week Type of treatment: same standard physical rehabilitation of G1. | Cognitive outcomes: G1 group: ↑ MoCA and executive tests vs. G2 Both G1 and G2 groups: no post-training improvement was maintained 6 months later |
| Goedeken et al., 2018 [63] | Single-blind randomised controlled trials | Implementation intention training (II) Duration: 3 days Intensity: 3 days Type of treatment: computer-based prospective memory test. Participants encountered activities for which they made decisions; also, they encountered prospective memory tasks that they had to remember to “perform” another related task. | Verbal rehearsal training (VR) Duration: 3 days Intensity: 3 days Type of treatment: patients recited the prospective memory tasks they encountered aloud at least three times and studied them for 30 s. Participants were instructed to use their strategy as much as possible in their everyday lives to help them remember to do things. | Cognitive outcomes: VR group ↓ self-reported everyday prospective memory vs. II group. |
| Peña et al., 2014 [66] | Randomised controlled trials | REHACOP cognitive training Duration: 13 weeks Intensity: three sessions a week Type of treatment: structured group format programme using paper-pencil tasks with a gradual level of cognitive effort and demand. It trained different cognitive domains and included one module for ADL. | Occupational training (active control group) Duration: 13 weeks Intensity: three sessions a week Type of treatment: occupational group activities; including drawing, reading the daily news, and constructing using different materials. | Cognitive outcomes: REHACOP group ↑ in visual memory, TOM, functional disability, and processing speed vs. Occupational training group |
| Petrelli et al., 2014 [52] | Randomised controlled trials | 1. NEUROvitalis training Duration: 6 weeks Intensity: twice a week Type of treatment: structured training programme that includes individual tasks, group tasks and group games each focusing on specific cognitive functions, and with a corresponding psychoeducational part. 2. Mentally Fit training Duration: 6 weeks Intensity: twice a week Type of treatment: unstructured, not domain-specific “brain jogging” program. Domains were not addressed in focused sessions, individual and group tasks or conversations. | Control waiting list group Duration: N.A. Intensity: N.A. Type of treatment: no training between test sessions. | Cognitive outcomes: NEUROvitalis training group: ↑ verbal short-term memory and executive functions (working memory) Mentally Fit training group:
|
| Zimmermann et al., 2014 [68] | Parallel single-blind randomised controlled trials | CogniPlus training Duration: 4 weeks Intensity: three sessions a week Type of treatment: CogniPlus training programme, specifically aimed to improve focused attention, working memory, executive functions, and inhibition. The level of difficulty was adapted automatically by the program itself, or manually if necessary. | Nintendo Wii exergames training Duration: 4 weeks Intensity: three sessions a week Type of treatment: Nintendo Wii, a game console with movement-capturing controllers. The patients were seated, so that they could not fall. In each session, the patients played four sports games from Wii Sports Resort: Table Tennis, Swordplay, Archery, and Air Sports. The level of difficulty was adapted automatically by the game. | Cognitive outcomes: Both groups: ↑ attention, working memory, inhibition, and planning Nintendo Wii exergames training group: ↑ attention vs. CogniPlus training group |
| Sammer et al., 2006 [53] | Randomised controlled trials | Executive functions training Duration: 3–4 weeks Intensity: 10 sessions Type of treatment: cognitive training in which all methods were designed to improve working memory abilities associated with executive functions. Speech production was encouraged by requesting patients to tell short stories. A set of photos was used to train working memory and to produce short stories. | Standard treatment Duration: 3–4 weeks Intensity: 10 sessions Type of treatment: standard training, including: occupational therapy, physiotherapy, and physical treatment. | Cognitive outcomes: Executive functions training group: ↑ core executive abilities (rule shift, and organising performance of a task) maintained in the after-treatment measurement Standard treatment: no significant improvement |
| Maidan et al., 2017 [69] | Randomised controlled trials | Treadmill training + Virtual reality (TT + VR) Duration: 6 weeks Intensity: three sessions a week Type of treatment: patients walked on a treadmill while reacting to a virtual environment that included real-life challenges requiring continual adjustment of steps and provided visual and auditory feedback. | Treadmill training (TT) Duration: 6 weeks Intensity: 3threesessions a week Type of treatment: active control intervention in which patients walked on a treadmill, with similar intensity and duration as the experimental group, but without the VR simulation. | Motor outcome: TT + VR group ↓ falls incidents Neurophysiological outcome: TT + VR group:
|
| Edwards et al., 2013 [64] | Randomised trial | Cognitive speed of processing training (SOPT) Duration: 20 h Intensity: three sessions a week Type of treatment: A self-administered version of SOPT, InSight, was completed by participants at home. InSight included five exercises designed to improve information processing speed in realistic visual contexts and four additional exercises. | Control waiting list group Duration: N.A. Intensity: N.A. Type of treatment: no contact. | Cognitive outcomes: SOPT group:
|
| Pompeu et al., 2012 [60] | Parallel prospective single-blind randomised clinical trial | Wii-based exergames cognitive and motor training Duration: 7 weeks Intensity: twice a week and an additional session was performed 60 days after the end of training. Type of treatment: balance training by playing 10 Wii Fit games. The cognitive demands of the games were attention to solving the tasks, working memory and performance management. | Active balance control group Duration: 7 weeks Intensity: twice a week Type of treatment: balance exercise therapy developed considering the games chosen for the experimental group. The control group performed balance exercises that were equivalent to the motor demands of the experimental group but without the provision of external cues, feedback and cognitive stimulation. | Motor outcomes: Both groups:
Both groups: ↑ global cognition maintained 60 days after training ends Autonomy and QoL outcomes: Both groups: ↑ ADL maintained 60 days after training ends |
| Leocadi et al., 2024 [45] | Randomised clinical/fMRI study | DUAL-TASK + AOT-MI training Duration: 6 weeks Intensity: Not specified Type of treatment: gait/balance training consisting of AOT and MI in combination with observed-imagined exercises. | DUAL-TASK training Duration: 6 weeks Intensity: Not specified Type of treatment: participants performed the same number of exercises as the experimental group combined with watching landscape videos instead of observation/imagination. | Cognitive outcomes: Both groups: ↑ accuracy in a task relying on set-shifting (specific for the attentive–executive domain) DUAL-TASK + AOT-MI group: no specific effect on cognition Neurophysiological outcomes: DUAL-TASK + AOT-MI group: ↑ substantial brain functional changes vs. DUAL-TASK group |
| Maggio et al., 2021 [46] | Randomised clinical study | 1. Tele-VR cognitive training (EG1) Duration: 6 weeks Intensity: three sessions a week Type of treatment: remote programme using two cognitive rehabilitation apps on smartphones; that offered science-based brain training enhancing cognitive performance across multiple cognitive domains. 2. Tele-VR cognitive and socio-cognitive training (EG2) Duration: 6 weeks Intensity: three sessions a week Type of treatment: remote program via one cognitive rehabilitation app, and one social-cognitive rehabilitation app; in which the patient overcame social challenges with audiovisual feedback. | Not-VR cognitive training (aCG) Duration: 6 weeks Intensity: three sessions a week Type of treatment: conventional training conducted using paper-pencil exercises performed independently at home and evaluated by the therapist at the end of the rehabilitation programme. Worksheets containing cognitive exercises, targeting both cognitive and emotional-social components and including various types of exercises were used. | Cognitive outcomes: Both EG1 and EG2 groups: ↑ in the subjective perception of memory performance, MoCA and FAB scores vs. aCG group EG2 group: ↑ MoCA and FAB scores vs. aCG group aCG group: ↑ executive-attentive and visuospatial domains Socio-emotional outcomes: Both EG1 and EG2 groups: ↑ mood and TOM vs. aCG group |
| Gobbi et al., 2021 [61] | Randomised controlled trials with crossover features | 1. Multimodal training Duration: 32 weeks Intensity: twice a week Type of treatment: training for improving/maintaining all components of functional capacity. Individuals enrolled in the Multimodal exercise group in the first year were switched to the Functional Mobility or Mental/Leisure group in the second year, and Mental/Leisure or Functional Mobility for the third year. 2. Functional Mobility training Duration: 32 weeks Intensity: twice a week Type of treatment: training to improve/maintain balance and locomotion parameters as well as functional capacity and participants’ QoL. | Mental/Leisure training Duration: 32 weeks Intensity: twice a week Type of treatment: cognitive and leisure activities. This programme included two periods, including three sub-periods each. The sub-periods, based on different leisure dimensions (social, manual, and artistic), were always combined with intellectual and social aspects, such as social activities, math problem-solving, card and memory games, drawing, debates, and lectures. | Cognitive outcomes: Multimodal training group:
Multimodal training group: ↓ physical stress |
| Bernini et al., 2021 [47] | Three arm double-blind randomised controlled trials | CoRe cognitive training (CCT) Duration: 3 weeks Intensity: four sessions a week Type of treatment: CoRe, a software tool, administered 11 tasks targeting several cognitive abilities. These tasks were computerised versions of existing paper-and-pencil exercises or were created to meet specific requirements. The individual patient’s performance was analysed to set the appropriate difficulty level which progressively increased. | 1. Paper-pencil cognitive training (PCT) Duration: 3 weeks Intensity: four sessions a week Type of treatment: same training programme as the CCT group but using the paper-and-pencil version of the tasks. The increasing levels of difficulty were managed by the therapist. 2. Unstructured activity training (CG) Duration: 3 weeks Intensity: four sessions a week Type of treatment: unstructured activities that served as a behavioural placebo treatment. | Cognitive outcomes: CCT group: ↑ MoCA scores, attention, and processing speed domains vs. PCT and CG groups PCT group: ↑ attention/processing speed domain vs. CG group |
| Mariano Barboza et al., 2019 [62] | Randomised clinical trial | Cognitive–Motor training (CMG) Duration: 16 weeks Intensity: twice a week Type of treatment: intervention performed in two parts: the same protocol used in the MG and, at the end of each therapy session, 30 min of cognitive paper-pencil tasks with gradually increased difficulty. The participants received three more activities to perform at home, which were reviewed in the next session. | Motor training (MG) Duration: 16 weeks Intensity: twice a week Type of treatment: protocol focused on balance training, sensory integration, agility and motor coordination, exploration of limits of stability, anticipatory and reactive postural adjustments, functional independence, and gait improvement. The therapy sessions were divided into four blocks with a gradual increase in exercise complexity. | Cognitive outcomes: Both CMG and MG groups: ↑ short-term memory and visuospatial function |
| Alloni et al., 2018 [48] | Single-blind randomised controlled trials | CoRe cognitive training (G1) Duration: 4 weeks Intensity: three sessions a week Type of treatment: CoRe system (Cognitive Rehabilitation); a software tool that automatically generates patient-tailored exercises using a big set of stimuli organised into an ontology. | Sham training (G2) Duration: 4 weeks Intensity: three sessions a week Type of treatment: only sham intervention; no cognitive training. | Cognitive outcomes: G1 group: ↑ executive and memory functions vs. G2 (not maintained after the discharge) |
| Lawrence et al., 2018 [70] | Randomised controlled trials | 1. Tailored cognitive training Duration: 4 weeks Intensity: three sessions a week Type of treatment: patients completed individualised activities on Smartbrain Pro; an interactive computer-based training programme designed to train each cognitive domain. Performance was automatically monitored by the programme to adjust individual difficulty levels for each activity. 2. Tailored cognitive training + tDCS Duration: 4 weeks Intensity: 3 sessions a week + tDCS once a week Type of treatment: same as tailored cognitive training, plus 20 min of tDCS. | 1. Standard cognitive training Duration: 4 weeks Intensity: three sessions a week Type of treatment: computer-based training. Predetermined programme comprising 10 activities, two activities per cognitive domain 2. Standard cognitive training + tDCS Duration: 4 weeks Intensity: three sessions a week + tDCS once a week Type of treatment: same as standard cognitive training, plus 20 min of stimulation. 3. Only tDCS Duration: 4 weeks Intensity: once a week Type of treatment: 20 min of stimulation. 4. Waiting list Duration: N.A. Intensity: N.A. Type of treatment: participants completed baseline, post-intervention, and 12-week follow-up neuropsychological assessments but did not complete cognitive training or tDCS. | Cognitive outcomes: Standard cognitive training group: ↑ memory Tailored cognitive training group: ↑ attention and working memory tDCS group: ↑ attention, working memory and memory Standard cognitive training + tDCS group: ↑ executive function, attention and working memory Tailored cognitive training + tDCS group: ↑ executive function, attention/working memory, and memory Autonomy and QoL outcomes: Standard cognitive training group: ↑ ADL and QoL Tailored cognitive training group: ↑ QoL Standard cognitive training + tDCS group: ↑ ADL |
| Kalbe et al., 2020 [54] | Multicentre randomised controlled trials | NEUROvitalis Parkinson training (CT) Duration: 6 weeks Intensity: twice a week Type of treatment: intervention targeting executive functions, memory, attention, and visuocognition. Each session is characterised by several training elements: psychoeducation group tasks and activity games, individual exercises, and homework. | Low-intensity physical activity training (CG) Duration: 6 weeks Intensity: twice a week Type of treatment: intervention aimed to be beneficial for PD patients but to have minimal effects on cognition. The main trained domains are stretching, flexibility, loosening up, and relaxation; also, psychoeducation on PD symptoms, therapy options and homework were conducted. | Cognitive outcomes: CT group: ↑ executive functions (especially verbal fluency), but not memory CG group: ↑ working memory |
| Reuter et al., 2012 [55] | Blind randomised study | Cognitive, transfer, and psychomotor training (Group C): Duration: 3–4 weeks + prosecution at home Intensity: four cognitive training sessions a week + three transfer training sessions a week + three psychomotor training sessions a week + three cognitive training sessions a week at home, two transfer training sessions a week and two psychomotor training sessions a week. Type of treatment: cognitive training in addition to transfer and psychomotor training, with a prosecution at home. The cognitive training employed a computer-based programme, and it included training of different cognitive functions. For transfer training, patients were asked to practise competence in tasks of daily routines. Psychomotor training included games and tasks designed to learn how to perform motor sequences. Also, mental imagery and aerobic training were employed. | 1. Transfer and cognitive training (Group B) Duration: 3–4 weeks + prosecution at home Intensity: four cognitive training sessions a week + three transfer training sessions a week + three cognitive training sessions a week at home, two transfer training sessions a week, and two relaxation training a week. Type of treatment: same cognitive and transfer training as Group C, with a prosecution at home. While at home, instead of the same psychomotor training of Group C, participants performed relaxation training. 2. Only cognitive training (Group A) Duration: 3–4 weeks + prosecution at home Intensity: four cognitive training sessions a week + three cognitive training sessions a week at home, two transfer training sessions a week, and two relaxation training a week. Type of treatment: same cognitive training of Group C and B, with a prosecution at home. | Cognitive outcomes: Group C: ↑ cognitive performance vs. Group B and A Group B: ↑ cognitive performance vs. Group A |
| Prat Paris et al., 2011 [67] | Blind multicentre randomised controlled trials | Cognitive training (CTG) Duration: 4 weeks Intensity: three sessions a week Type of treatment: interactive multimedia software and paper-and-pencil exercises. Computer-aided training employed the SmartBrain tool, designed to stimulate specific and non-specific cognitive domains. Participants received a pack with 20 cognitive homework exercises designed to stimulate specific and nonspecific cognitive areas. | Speech therapy Duration: 4 weeks Intensity: three times a week group sessions + once-a-week individual tutored session Type of treatment: speech therapy aimed to make participants aware of their speech and communication difficulties. | Cognitive outcomes: CTG group: ↑ in attention, information processing speed, memory, visuospatial and visuoconstructive abilities, semantic verbal fluency, and executive functions vs. Speech therapy group Autonomy and QoL outcomes: CTG group: no significant improvements in QoL |
| Sarasso et al., 2021 [49] | Randomised clinical/fMRI study | DUAL-TASK + AOT-MI training Duration: 6 weeks Intensity: three sessions a week Type of treatment: patients performed a gait/balance training consisting of AOT-MI combined with practising the observed-imagined exercises | DUAL-TASK training Duration: 6 weeks Intensity: three sessions a week Type of treatment: patients performed the same number of exercises combined with watching landscape videos instead of observation/imagination, and exercises were increasingly difficult, including the dual-task. | Motor outcomes: Both groups: ↑ in mobility during TUG-COG, TUG-MAN, and TUG (maintained 2 months after training) DUAL-TASK + AOT-MI group: ↑ change in TUG-COG mean, and peak of turning velocity during TUG and TUG-COG (maintained at follow-up) vs. DUAL-TASK group |
| Agosta et al., 2017 [50] | Prospective randomised study | AOT Group Duration: 4 weeks Intensity: three sessions a week Type of treatment: physical therapy training: during each training session, two video clips showing strategies useful in circumventing FoG episodes, were presented twice. Overall, subjects in the AOT group were presented with six video clips, repeated each week. The complexity of actions increased, and auditory cues were associated with the movements. After each video clip observation, patients were asked to imitate the observed actions repetitively and accurately at the beat of the auditory cues. | Landscape Group Duration: 4 weeks Intensity: three sessions a week Type of treatment: physical therapy training, during each training session they watched video clips containing sequences of static pictures of landscapes without any living representations for the same time length. During training sessions, patients performed the same movements/actions used for the AOT group in the same order and amount of time, following the physical therapist’s instructions. | Motor outcomes: Both groups:
Both groups: ↑ quality of life AOT group: ↑ quality of life vs. Landscape group (after short-term follow-up) |
| Suarez-Garcia et al., 2021 [65] | Randomised blinded sham-controlled study | PD-atDCS Group Duration: 5 consecutive days Intensity: three phases Type of treatment: three phases of a protocol with stimulation; pre-stimulation phase, stimulation phase, and post-stimulation phase. Both before and after the stimulation protocol, participants completed a PWA task involving action-verb and object-noun conditions. During the stimulation phase, participants received 20 min of online stimulation while completing a cognitive training protocol. The post-stimulation phase was identical in structure and duration to the pre-stimulation phase. | PD-stDCS Group Duration: 5 consecutive days Intensity: three phases Type of treatment: same three phases of the experimental treatment; except for receiving a sham stimulation, lasting 1 min and not effective. | Cognitive outcomes: PD-atDCS group:
|
| Vriend et al., 2021 [56] | Double-blind randomised controlled trials | Computerised cognitive training Duration: 8 weeks Intensity: three sessions a week Type of treatment: home-based computer intervention employed 13 training games with adaptive difficulty that focused on different cognitive functions and were adapted from the Braingymmer online platform. | Computerised active control group Duration: 8 weeks Intensity: three sessions a week Type of treatment: home-based, computer intervention that employed three low-threshold games with constant difficulty primarily based on “crystallised intelligence” factors, i.e., solitaire, hangman, and trivia questions. | Cognitive outcomes: Computerised cognitive training group: faster responses on the ToL task vs. Computerised active control group Neurophysiological outcomes: Computerised cognitive training group: no effect on network topology neither on the global or subnetwork level |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gattoni, M.F.; Gobbo, S.; Feroldi, S.; Salvatore, A.; Navarro, J.; Sorbi, S.; Saibene, F.L. Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review. Brain Sci. 2025, 15, 61. https://doi.org/10.3390/brainsci15010061
Gattoni MF, Gobbo S, Feroldi S, Salvatore A, Navarro J, Sorbi S, Saibene FL. Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review. Brain Sciences. 2025; 15(1):61. https://doi.org/10.3390/brainsci15010061
Chicago/Turabian StyleGattoni, Marina Francesca, Silvia Gobbo, Sarah Feroldi, Anna Salvatore, Jorge Navarro, Sandro Sorbi, and Francesca Lea Saibene. 2025. "Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review" Brain Sciences 15, no. 1: 61. https://doi.org/10.3390/brainsci15010061
APA StyleGattoni, M. F., Gobbo, S., Feroldi, S., Salvatore, A., Navarro, J., Sorbi, S., & Saibene, F. L. (2025). Identification of Cognitive Training for Individuals with Parkinson’s Disease: A Systematic Review. Brain Sciences, 15(1), 61. https://doi.org/10.3390/brainsci15010061






