Abstract
Nutrient deficiency in food crops is seriously affecting human health, especially those in the rural areas, and nanotechnology may become the most sustainable approach to alleviating this challenge. There are several ways of fortifying the nutrients in food such as dietary diversification, use of drugs and industrial fortification. However, the affordability and sustainability of these methods have not been completely achieved. Plants absorb nutrients from fertilizers, but most conventional fertilizers have low nutrient use and uptake efficiency. Nanofertilizers are, therefore, engineered to be target oriented and not easily lost. This review surveys the effects of the addition of macro- and nanonutrients to soil, the interaction, and the absorption capability of the plants, the environmental effect and food content of the nutrients. Most reports were obtained from recent works, and they show that plants nutrients could be enriched by applying nanoparticulate nutrients, which are easily absorbed by the plant. Although there are some toxicity issues associated with the use of nanoparticles in crop, biologically synthesized nanoparticles may be preferred for agricultural purposes. This would circumvent the concerns associated with toxicity, in addition to being pollution free. This report, therefore, offers more understanding on the application of nanotechnology in biofortification of plant nutrients and the future possibilities offered by this practice. It also highlights some of the ills associated with the introduction of nanomaterials into the soil for crop’s improvement.
1. Introduction
The quest to apply nanotechnology in agriculture arises from the fact that human population is constantly on the rise, which necessitates the need for more food. Population survey has estimated about 9.6 billion people by the end of 2050 [1]. Farm lands are losing their fertility due to human activities on them and societal change in lifestyle. This invariably affects the production of crops and could lead to famine and hunger, thus concerted efforts are necessary to improve plants for enhanced production. Nanotechnology serves as the latest technology for precision agriculture, whereby strategies are formulated and channeled towards meeting with food demands of the increasing human population.
There is a diversion from the traditional ways of crop production to technologies that could increase agricultural productivities with required nutrients, cost effective and efficient resource use that guarantees nutrient security, uplifts the value of production, boosts farmers’ economy, delivers agri-value chain to rural partakers and supports pollution free environment [2]. This technology uses improved materials to add value to agriculture, by exploiting the nanoscale properties. Nanotechnology, as applied to agriculture, is bridging the gap in nutrient loss and fortification of crops. Farmers are using this science in the nano-regime to boost the quality and quantity of agricultural produce. The application of nanotechnology in agriculture includes nanobiotechnology, livestock, nanotoxicology, agrochemicals, hydroponics, biotechnology, etc.
Urban agriculture that makes use of recent nanotechnologies has the potential to contribute immensely to food security and healthy nutrition. Although there are associated risks from chemicals which may have emanated from soils, water or air [3], the ultimate goals for application of nanomaterials in agriculture spans reducing hazard chemicals, nutrient losses, pest control and crop yield improvement [4].
The nutrients needed by the plants are fortified in the fertilizers, with the belief that they could be absorbed by the plants. The lack of the micronutrients is manifested by abnormal growth of the plant parts; however, sometimes the soil may not be deficient of the micronutrient, rather the roots are unable to absorb and translocate the nutrients due to small root pore size. It is, therefore, imperative to explore the strategies of improving crop quality and their essential nutrients to meet the food demands of the growing populace.
The use of chemical fertilizers is an age long practice and has tremendously increased crop yields. However, they lead to soil mineral imbalance, destroy the soil structure, soil fertility and general ecosystem, which are serious impediments in the long term. To deal with the situation, it is pertinent to develop smart materials that can release nutrients to targeted areas and contribute to clean environment. Recent studies have shown that graphene is a promising material that could serve as a carrier for plant nutrients. It is capable of slow and controlled release of nutrient for the plants benefit, and ultimately increases the amount of crop production with low environmental impact [5].
Nanotechnology seems to be the alternative that could revolutionize this field of agriculture as the entire nanotechnology industry had grown to $1 trillion in 2015 [6].
Plants remain the primary source of nourishment for humans, and food quality determines the health of majority of the people. Staple foods are usually high calorific foods, consumed regularly in high quantity, which becomes dominant part of a standard diet in a community. As a result, there is high correlation between staple foods and nutrition of its consumers, especially among the rural and poor communities who rarely have other sources of nutrient supplementation. Lack of essential micronutrients in food is common in such areas. These have become a global issue with serious adverse effects [7,8,9,10,11]. About 50% of children do not get the necessary vitamins and minerals and they become vulnerable, thus impairing their intelligence and mental capabilities. According to Clemens [12], high percentage of child death and global disease concern are traced to iron and zinc deficiencies. Several methods have been postulated to combat nutrient deficiencies such as dietary diversification, use of drugs and industrial fortification [13]. However, Sharma et al. noted that these interventions have not been fully successful owing to economic level of the people, social content or some technicalities in the method adopted [14]. Consumption of diverse food sources, although recommended as a sustainable solution, is unaffordable to the poor populace, who are at risk of malnutrition. The use of industries for the fortification of food nutrients has not been very successful, except for iodized salt. Biofortification is a concept of increasing the nutrient content of food crops during their cultivation [15]. The uniqueness of biofortification over other interventions for combating micronutrient deficiencies is that it is affordable and available for everyone. This is because these fortified crops are staple foods widely consumed by many people. As a result, it neither incurs extra cost nor is affected by the social behavior of the people. Non-staple foods such as animal products and vegetables, although high in vitamins and minerals, are very expensive. Significant number of the poor spend their income on staple foods for provision of energy, with little left for fruits and vegetables and animal-based proteins [14].
Biofortification strategies exist in enhancing nutritional content of crops and these strategies, according to Stein et al. (2007), include agronomic and breeding methods [16]. Under agronomic interventions, the use of fertilizers including inorganic, organic and biofertilizers are highlighted. Inorganic fertilizers usually with sizes more than 100 nm are easily lost due to leaching and volatilization, while organic matter utilization is hampered by its low mineral content and long-period of nutrient release. Numerous attempts to increase the efficiency of nutrient uptake of crops and thus biofortify them have not been so successful. Thus, the time is rife to apply nanotechnology in solving some of these problems.
This research work is focused on exploring recent available literature on the use of nanoparticles in biofortification. The global importance of nutrient fortification of crops in tackling malnutrition is discussed, and methods of biofortification and the use of nanoparticles in biofortification are highlighted.
2. Nutrient Fortification, Its Relevance and Types
Humans derive their nutrition from food, and require over 20 minerals and 40 nutrients for healthy living. Unfortunately, human diets most often contain less of the required essential nutrients, thus leading to malnutrition [17]. A fortified food reduces incidence of heart disease, anemia, blindness, incidence of cancer and early death [7]. Poor maternal health, low intellectual capacity and stumpy educational ability are consequences of micronutrient deficiencies. It also leads to reduced work ability and earning power, with untold consequences for sustainable national development [15].
To tackle this global menace, there is need to fortify the crops. According to Smith and Bouis et al. [18,19], several options to tackle micronutrient deficiencies exist: supplementation, dietary variation, industrial fortification, and biofortification. Dietary variation or diversification can be seen as consumption of different dietary sources (including fruits, vegetables and animal and animal-based products) at the community or household level, targeted at addressing micronutrient deficiencies [20]. Arimond and Ruel [21] noted that children’s nutritional status and growth is positively influenced by dietary diversification. The problem with dietary diversification is that foodstuffs containing high micronutrients are not easily accessible because the affordability and availability in rural communities is low. Notwithstanding, these animals and animal products also rely on nutrients in plants for their nourishment [12]. thus, if these plants are not enriched in micronutrients, it becomes a vicious cycle of deficiency in humans [22].
Supplementation, however, involves the intake of micronutrients in the form of capsules, tablets or syrup. In fortification and supplementation, manufacturing and/or distribution infrastructure is required, which in the long run may not benefit many, especially those in rural communities. Vitamin A capsules intervention, which started in the 1990s, is an example of supplementation [23]. Moreover, supplementation can easily lead to overdose. Murgia et al. opined that overdose of iron can exacerbate diseases [24,25]. To be effective supplementation, as seen during some immunization, requires annually costly campaigns and these drugs or fortified foods do not always reach the most targeted and most affected rural community [26].
Gibson and Hotz [27] noted that staples and other food sources can be modified or altered to improve the micronutrient content or bioavailability. In most resource poor settings, starch-based diets with limited access to animal-based products or fruits and vegetables are predominant. Strategies such as plant breeding, agricultural biofortification and genetic engineering during food processing are means of dietary diversification. This is because sustainable availability of staple food supplies preconditions food security at household or community level. In addition, increased and sustainable yields from agricultural production will increase the potential access to adequate food and food diversification.
Industrial fortification of food materials has been practiced for a number of years, such as iodized salt or vitamin D enriched milk. The crucial goal of the fortification strategy is to reduce diseases and death rates associated with micronutrient malnutrition. In commercial food fortification, micronutrients are introduced during the processing of the foods, which provides the appropriate nutritional levels to humans when consumed. However, those concerned do not necessarily need to change their diet. According to Pandav et al., only 29% of the populace has no access to iodized salt globally; and, since 2003, the number of iodine-sufficient countries has increased from 46% to 68% [28]. Other forms of fortification may include enrichment of wheat flour with zinc, iron, vitamin B and folic acid and introduction of vitamin A to edible oils and sugar. To avoid micronutrient deficiencies in preschool and breastfeeding children, it is recommended that they take adequate breast milk, powder and other food formula rich in micronutrient. Supplementation with vitamin A, according to Edejer et al. [29], is one of the most efficient strategies for improving children survival. Vitamin A is known to be associated with a reduced risk of diarrhea and causes of mortality [30].
Fortified foods may only be accessible to urban consumers, who can easily see and buy them. It is also very essential at crisis period, where food supply is inadequate and unbalanced. Thus, these fortified diets rich in minerals and vitamins are distributed to avoid malnutrition. However, it may be difficult to get to the rural consumers who cannot afford or have access to them. Thus, the need for biofortification of crops is conceived as a strategy for nutrient fortification in crops or staples while in the field. Dubock [31] noted that the primary priority in fortification should constitute fortification of locally available food sources, while food supplementation should be an interim measure. Biofortification is intended to cater to the poor populace, low-income earners and everyone at large.
3. Biofortification of Crops
Malnutrition increase has resulted from consuming specific type of food without diversifying them, especially consumption of staples high in calories [13]. Biofortification is a novel technique to address this. Biofortification means growing varieties that are rich in minerals and vitamins. A typical example is the development of new variety of sweet potatoes rich in vitamin A. With biofortification, mass accessibility to better nutrient rich food is guaranteed. The target in biofortification is to produce staple crops at low cost that are sustainable and have high nutritional value, able to reduce the consequential side effects of micronutrient deficiencies. Although biofortified staple crops deliver low level of essential nutrients and vitamins per day compared to supplements or industrially enriched foods, they can satisfy the individual daily requirement of micronutrients [15]. They offer the rural consumers the ability to obtain rich nutrient foods within the community, unlike with industrial or commercial fortified foods. There are different biofortification techniques: agronomic biofortification, conventional breeding, and nutritional genetic modification [16].
3.1. Crop Breeding and Genetic Modification as Biofortification Tool
Biofortification using crop breeding involves the science of improving micronutrient content of staple crops, using the conventional breeding methods and current biotechnology [32]. Crops are bred and genetically modified to improve absorption capacity and nutrient content. Plant breeding methods, which tend to enhance the micronutrient content of various cereals, legumes and tubers, exist. Biofortification by breeding has several advantages as a policy: it leads to development of staples consumed daily and ardently caters for the poor. Again, these developed varieties can be used over time, thus reducing cost and making it sustainable.
In conventional breeding, crops such as legumes and cereals with high micronutrient content are selected, purified and multiplied [33]. Then, newly improved food crops of varying nutritional contents can be conventionally developed and isolated from the varieties of the same plant. Breeding of crops is principally committed to increasing micronutrients and vitamin A content in the common food crops [34]. Crops produced through conventional breeding have gained more acceptance than those from gene modification. Collection of such high nutrient rich varieties has been on the increase since the 1960s in seed banks. According to Sharma et al. [13], orange fleshed sweet potato (OFSP) and quality protein maize have been well accepted and can be seen as interesting examples of conventionally bred biofortified crops. Iron beans and zinc rice are other examples. It is obvious that not all micronutrients can be enhanced in crops through conventional breeding. As a result, this necessitates the role of genetic nutrient modification in nutrient enhancement.
Genetic engineering of crops has facilitated modification of crops in unique ways and is a tool for addressing global agricultural challenges. Improved knowledge of DNA led to speedy advancement of agricultural biotechnology as a field. Agricultural biotechnology uses modern biology techniques to alter living beings or their components for cogent purpose in crops. As a result, it permits infusion of genes from the wild. Such cannot be done using conventional breeding. The overall objective of nutritional genetic modification is to integrate high micronutrient traits in already proven highest-yielding varieties [13]. This will motivate farmers to cultivate that new variety. Bilski et al. reported that improving the Fe, Zn, and Se content of crops by utilizing the plant genetic makeup and applying biotechnological process could solve nutritional inadequacies in human foods; unfortunately, it is an expensive approach and involves a lot of time [35]. However, once these crop varieties are obtained, no more resources are invested and generations yet unborn will benefit from them. In soil low or lacking in these essential micronutrients, it becomes difficult for the crops to obtain enough micronutrients, which makes agronomic biofortification using fertilizers essential.
Although plant breeding is the most practiced sustainable method in fortification [24,36,37], development of new genotypes enriched with micronutrient is a long-time venture [38]. In addition, available micronutrient in the soil limits the effectiveness of new genotypes in increasing micronutrient content [39,40]. Moreover, these genetically-modified micronutrient-rich crops may not be adopted by many. Consequent upon these limitations, agronomic biofortification is an alternative mechanism to increase micronutrients content in staples to overcome the limitations accruing from crop breeding biofortification technique.
3.2. Agronomic Biofortification
Agronomic method of biofortification of crops with micronutrients is envisaged as a fast and easy way out of the inadequacies of these essential minerals in soils and plants. It involves cultivation of varieties that are rich in minerals and vitamins. This method uses fertilization as a strategy to increase micronutrient content of cultivated crops such as cereals and legumes. It is pertinent to emphasize that the agronomic biofortification method could be more beneficial in developing countries [40].
To improve micronutrient content of crops using agronomic biofortification, White and Broadley [41] suggested the use of phytoavailable micronutrient fertilizers, routine correction of the soil alkalinity, crop rotational methods of planting and strategic introduction of symbiotic soil microorganisms. Graham et al. also listed the following as agricultural tools for enhancing nutrient content of crops: fertilizers, cropping systems and soil amendment [42]. Others have stated that micronutrient fertilization, in addition to boosting crop yield, enhances crop nutritional quality, thus addressing the attendant human micronutrient deficiency and health challenge [22,39,40,43,44].
It has been observed that micronutrient content of crops decreases even when the yield is high, probably because of continuous mining of these nutrients without replenishment by especially high yielding varieties [14,42,45]. Therefore, Dimkpa and Bindraban advised that the success of any biofortification program will depend on adequate available micronutrients in the soil for plant absorption or supplied externally through micronutrient fertilizer [46]. This is because the complex interaction required in transporting nutrients from the soil to edible portion of the crop need to be surmounted [47]. There is evidence of increasing nutrient and crop yield by application of micronutrient fertilizers [48]. Bilski et al. showed increased Fe, Zn and Se content of six cereal plants by growing them on coal combustion residues that was naturally rich in these nutrients [35]. The progress of agronomic fortification is a function of the application methods, fertilizer type and packaging and the crop developmental stage during application [39,45].
Conventional fertilizers are readily available for plant uptake but also easily lost through leaching, which is a major challenge. NPK and other agrochemicals have been found to have low use efficiency by plants because of fixation, leaching, microbial degradation, photolysis and volatilization [46,49]. As such, quantities of these inputs are usually lower than minimum effective doses that reach the crops. Thus, repeated applications are required to attain maximum yield. This pollutes the environment, including underground water sources. Use of fertilizers, which are usually in the form of salts or in ionic form, results in ready availability and also rapid loss from the soil. However, the challenge to sustainably produce crops with high nutritional values amidst the unfavorable biophysical conditions and other limiting factors becomes the focus of the researchers. Along this line, the need for innovative fertilizers is recently presented [50].
Moreover, arable land and water resources are increasingly limited. Therefore, to continue the development of agricultural sector, use of modern technologies to increase resource use efficiency with the least damage to environment must be pursued [51].
4. Inorganic Fertilizers
The need for inorganic fertilizers arises in order to supplement the soil nutrients needed for crop production. The soil could be deficient in some nutrients because, e.g. constant use of the soil, which negates recycling; post-harvest practices, which may take away the nutrients with the harvested crops; etc. However, for sustainability and excellent crop production, there is need for plant supplements, which could be in form of organic manure or inorganic fertilizers. Inorganic fertilizers are synthetic fertilizers made from petroleum, the formulation of which could either be single nutrients or combination of different nutrients. In the case of different nutrients, they are referred to as multi-nutrient fertilizers and they contain majorly nitrogen, phosphorus and potassium among other nutrients and can be complete or balanced. They are balanced when they are of the same ratio and complete when they are needed in a particular formula. The soil supplies most of the nutrients needed for plant growth, but sometimes the nutrients are depleted during harvest and need to be replaced using fertilizers.
Fertilizers help plants to grow and absorb the appropriate nutrients required in crops. Most conventional fertilizers contain the macronutrients, namely nitrogen, phosphorus and potassium, with little calcium, sulfur and magnesium. Other micronutrients such as zinc, iron, copper and manganese become lacking, making the crops deficient. A typical fertilizer with all nutrients should have the following: N (2–4%), P (0.3–1%), K (1.5–5%), S (0.15–0.8%), Ca (0.2–1.5%), Mg (0.15–1%), Zn (10–100 ppm), Fe (20–500 ppm), Mn (15–250 ppm), Cl (4–50 ppm), Co (2.5–50 ppm), Cu (5–75 ppm), and Mo (0.03–10 ppm) [52]. The confronting issues are: How much of the nutrients are absorbed by the plants? Are they in a manner that could be absorbable? Do the crops get the complete nutrients that fulfils the human requirement when consumed?
There is no doubt that inorganic fertilizers are made having full knowledge of the necessary ingredients needed by the plants. With this complete formula of ingredients, when applied to the soil, they are dispersed by water molecules and the nutrients are broken down into various forms that are needed by the plants. There are various soil reactions and mechanisms that determine the quantity absorbed by the plants.
Soil test is a preliminary operation that must be done before application of fertilizers. If the soil is acidic, using urea fertilizer, for example, the NH3 released from the reaction of the urea fertilizers with sufficient water molecules is absorbed in the form of ammonium ions and, when they are basic, there is no change. In neutral or near neutral pH (7–8), the ammonia may escape into the environment, causing pollution such as greenhouse gases. These are the challenges that need to be addressed.
4.1. Nitrogen Fertilizers
Nitrogen, as a single nutrient, is needed by the soil to enhance crop productivity. It is commercially sold in solid or liquid forms as anhydrous ammonia, urea, and urea–ammonium nitrates [53]. It is applied into the soil and reacts with water to release the fertilizer as ammonium ions, which are further nitrified by bacteria to nitrate ions needed by the plants. Farmers sometimes apply fertilizers in excess quantity so that they can get bountiful harvest, but such practices may be of disadvantage to the soil, as it will change the structure and concentration of the soil. It may also lead to eutrophication when the excess nitrogen compounds are leached into water bodies.
There could be addition of nitrification inhibitors, stabilizers or additives when the nitrogen fertilizers are applied to reduce the loss of NH3 formed during the course of reaction of the fertilizers with the soil and water molecules. Such compounds may include NBPT [N-(n-butyl) thiophosphoric triamide], boric acid, which can be used with urea or urea–ammonium nitrate fertilizers and they function to slow the action of urease bacteria that transforms the fertilizers to NH3 [54]. Such action allows the fertilizers to be well absorbed by the soil, giving room for reaction with enough water molecules. US environmental protection agency approved dicyandiamide (DCD) and 3, 4-dimethypyrazole phosphate (DMPP), in the late twentieth century, as a nitrification inhibitor [55]. The best form of the nitrogen fertilizers is to be in ammonium ions, which are easily absorbed by the soil. In the form of nitrates, they are difficult to be absorbed by clay soil or organic matter, while, in the form of ammonia, they tend to escape into the atmosphere.
Special farm practices are also important in the application of these fertilizers. When they are applied on the surface, they tend to be converted to NH3 and escape very easily. The best practice, therefore, requires the tilling of the soil and applying them in the inner part with adequate water. The water molecules will enable them to be converted to ammonium ions but not too much water that may eventually lead to leaching.
Urea is the most widely used nitrogen fertilizer due to its high nitrogen content, compatibility with other nutrients, easy handling and application.
4.2. Phosphorus Fertilizers
Phosphorus is a major plant nutrient responsible for protein synthesis and it is an integral part of the nucleic acid structure of plants. It is involved in cell division, development of new tissue and complex energy transformations in the plant. Phosphate compounds act as energy reservoir, obtained from photosynthesis and carbohydrates metabolism, which is later released for plant growth and reproduction [56]. When there is required quantities of phosphorus in the soil, it promotes plant root growth and quickens maturity of crops. However, soils deficient in phosphorus can lead to accumulation of sugars in plants and exhibit reddish-purple color due to anthocyanin pigments. Phosphorus is needed mostly in the early stages of crop production, as a study has shown that cereals have the capacity of taking up to 75% of their P requirements in the first 5–6 weeks after crop emergence [56].
Apart from the inorganic phosphorus fertilizers obtained from rock minerals apatite, other sources of phosphorus fertilizers may include bone meal, and industrial wastes such as basic slag and Thomas slag. The phosphorous fertilizers are grouped into three types depending on their solubility: water soluble (monobasic calcium phosphate and ammoniated superphosphates), citric soluble (dicalcium phosphate, Thomas slag, basic slag, defluorinated phosphate, and fused magnesium phosphate) and sparingly soluble phosphate (tricalcium phosphates) [57]. Phosphorus can never be too much in the soil for plant absorption, as it is slowly absorbed and greatly needed for an overall growth and health of the crops.
4.3. Potassium Fertilizers
Plant processes and development cannot function properly without potassium. It is one of the three major nutrients required for quality assurance, excellent appearance and great harvest of crops. It is key to various processes ranging from reproduction, growth, photosynthesis, protein synthesis, enzyme activation, water and stomata regulation among others [58]. When there is deficiency of potassium in the soil for plant absorption, the plants become prone to diseases, resistant to wind and temperature changes and the overall growth and development process of the crop is affected [58]. Other symptoms may include appearance of spots under the leaves, curling of the leaf tips, interveinal chlorosis, etc.
Potash or potassium fertilizers can be obtained from several sources either organically or inorganically. Soil potash could be improved from compost made by agricultural wastes or food by-products such as banana peels. Wood ash obtained from burning of wood can also be a good source of potash and can be used to enrich the soil. Majority of inorganic potash used in agriculture can be obtained from potassium chloride (KCl). Other contributors are potassium sulfate, potassium nitrate, and potassium–magnesium salts. Commercially available potassium and other fertilizers are shown in Table 1. To process the fertilizer, potassium bearing ores are obtained from natural mineral deposits and crushed to reduce the sizes. Clay deposits are further removed and flotation process of separating the potassium from other compounds is carried out. Other methods such as sizing, refining, and crystallization are also carried out to obtain the fertilizers in different stages.

Table 1.
Some macronutrients fertilizers and their composition Data from [52].
4.4. Secondary and Micronutrients
Secondary nutrients are also needed in large amounts similarly to nitrogen, phosphorus and potassium: calcium (Ca), sulfur (S) and magnesium (Mg). Micronutrients on the other hand are needed in small quantities and necessary for crop development. Iron (Fe), boron (B), copper (Cu), zinc (Zn), manganese (Mn), molybdenum (Mo), nickel (Ni), chlorine (Cl) and molybdenum are important micronutrients. Their deficiency can impair crop yield, cause low absorption of other nutrients and structural problems. In as much as nitrogen, phosphorus and potassium are the principal nutrients for crop production, their excesses may lead to imbalance in the micronutrients. For instance, when nitrogen and potassium are used in excess, they lead to deficiency in magnesium; and when phosphorus is in excess, it causes imbalance in zinc content.
These secondary and micronutrients can be blended with the primary plants nutrients (NPK), and applied to crops; and their quantities should be able to correct the soil deficiencies as well as be bio-available. These nutrients behave differently in soils; some of them may be in adequate forms but may not be available for plant absorption (e.g., Fe and Mn). Boron on the other hand can be difficult to accumulate, especially in sandy soils due to its high mobility [58].
However, their excesses are detrimental to the growth of crops, as too much calcium in the form of calcium sulfate or chloride can lead to higher pH from the anions (Cl− and SO42−) and thereby result to some other nutritional problems. Higher levels of boron in starter fertilizers could also lead to toxicity in some sensitive crops such as beans and grains [59].
5. Biofertilizers
Continuous farming and use of agricultural lands, over a period of time, lead to depletion of the nutrients contained in the soil. Hence, the frequent resuscitation of the lands using fertilizers is necessary. When the soil is impoverished, there is low crop production, which leads to poor harvest, hunger and malnutrition. Farmers find it easy and convenient to use chemical fertilizers for crop improvement, but their cost and inherent environmental pollution calls for urgent attention. Biofertilizers or green fertilizers are natural ways of enriching the soil by using dead plant materials or animal wastes, which are fed on by microbes to give the required nutrients for efficient crop production.
The microorganisms are key in this process of natural fertilization because, without them, the plant or animal materials are not in the absorbable form by the growing crops and therefore are of no use to the soil [60]. Alternatively, biofertilizers can be microorganisms that activate the soil and plant natural processes for efficient nutrient uptake, high crop yield and quality, and tolerance to abiotic stress. Such microorganisms are majorly bacteria that are contained in the soil and are called plant growth-promoting rhizobacteria, including blue-green algae, phosphorus-potassium solubilizing organisms, azotobacter, and Rhizopium [60]. They fix the atmospheric nitrogen and interact with the decaying organic matter to make available nutrients for the plant growth. They are symbiotic in behavior, as plants cannot grow well without them, and they also depend on plants for existence. Apart from making nutrients available for plants, they also get involved in protecting the plants against some pathogenic attack.
Other methods of the biofertilization involve the rotational practice of cereal–legume with combined crop–livestock agricultural system. This method works to enhance the soil nutrients through fixing of the atmospheric nitrogen by legumes and the livestock wastes combines with the cereal–legumes to improve the soil texture and provide appropriate environment for the action of the microbes. Organic farming or biofertilization generally gives high-value crops but can be practiced in a small way, especially by rural farmers and the prices of the produce are high.
6. Nanofertilizers
According to recent research works, nanotechnology has the possibility to revolutionize agricultural systems [46,51,61]. It enables the platform for the use of elegant delivery structure for agrochemicals which is safe, target bound and has easy mode of delivery. Nanofertilizers, due to their high surface area to volume ratio, are more effective than most of the latest polymeric type conventional fertilizers. Their nature could also allow slow release and promote efficient nutrient uptake by the crops. This technology, therefore, offers the platform for sustainable and novel nutrient delivery systems, which will exploit the nanoporous surfaces of the plant parts on plant surfaces. With encapsulated nanoparticles, nanoclays and zeolites, there is increase in the efficiency of applied fertilizer, restoration of soil fertility and plant health and reduction of environmental pollution and agroecology degradation [51].
The components of nanofertilizers may include zinc oxide nanoparticles (ZnONPs), silica, iron and titanium dioxide, ZnS/ZnCdSe core–shell quantum dots (QDs), InP/ZnS core–shell QDs, Mn/ZnSe QDs, gold nanorods, Al2O3, TiO2, CeO2, and FeO [62]. The success of using nanomaterials as fertilizers in plant growth depends on the species of the plants and some other factors such as the size, concentration, composition and chemical properties of nanomaterials [4]. The vast knowledge of the fields of biology, biotechnology, material science, and engineering is key to development of new technologies needed to expand the field on nano-agriculture for efficient crop production.
There are shortcomings associated with the conventional fertilizers, as most of the nutrients are lost through leaching and they go further to pollute the underground water aquifers. In other words, chemical fertilizers lead to environmental consequences such as greenhouse gas emissions and hypoxia and these problems need urgent attention; hence, the search for alternatives such as the nanofertilizers [63]. With nanofertilizer, there is slow release of the nutrients, which minimizes leaching of the nutrients among other interesting properties.
Nanomaterials are of unique properties resulting from their low particle size, large surface to volume ratio and excellent optical properties. Such properties, among others, afford nanofertilizers the opportunities in plant development, nutrient security and diverse farm practices. Since the great revolution of nanotechnology applications in the early 21st century, several fields of endeavor are making use of this novel science in creating novel products. Human population is growing and likewise crop production should also grow. Fertilizers have helped in making crops abundant for human consumption; and taking advantage of nanoscience, improved varieties of fertilizers in form of nanofertilizers can be processed. They get easily absorbed by the soil and enhance the quality of the soil, thereby improving the growth of the plant. The mainstream application of nanotechnology concentrates on electronics, optical devices, water purification, and health care with little awareness on agriculture. Conventional fertilizers do not possess all the nutrients required for plant growth and nutritional composition; on that premise and owing to the active nature of nanoparticulate materials, it becomes an interesting venture to engineer materials to give nanofertilizers that can address nutrient problems and environmental issues associated with fertilizers [64].
Nanofertilizers are more advantageous to the conventional fertilizers because they can triple the effectiveness of the nutrients, reduce the requirement of chemical fertilizers, make the crops drought and disease resistant and are less hazardous to the environment. They can easily get absorbed by plants due to their high surface area to volume ratio. The sizes and morphologies of nanoparticles are however strong factors that determine the level of bio-accessibility by the plants from the soil. The nanoparticles may not be activated instantly to be taken up by plants, rather series of reactions ranging from oxidation and recombination may take place to provide the plants with the right micronutrients. Since the nutrients are in nanoscale, the fortification of the plant with such nanonutrients seems to be an interesting option. The plants not only grow but also accumulate such nutrients, which bridges the gap of nutrient deficiency. Moreover, nanofertilizers could be engineered in such a way as to address particular deficient nutrients in plants. This is possible because the atoms on the surfaces of nanomaterials could be structured to obtain characteristic different properties.
Li et al. [65] reported that metals and anionic nanoparticles are highly adsorbed by porous materials, or the soil, which makes them overly available as food nutrients or even contaminants when not desirable. In addition, in recent times, some researchers have developed and patented a nanofertilizer called “Nano-Leucite Fertilizer”, which is eco-friendly and could reduce nutrient loss in food, with overall increase in crop and food production [66]. In a nutshell, nanofertilizers might be the best thing that could happen in agricultural revolution, as they have the potentials of enhancing soil fertility in nutrient deficient soil. However, it may be seen as “one more tool in the toolkit”. Table 2 shows some approved nanofertilizers currently used around the world [62,67].

Table 2.
Some approved nanofertilizers used in the world today and their compositions. Data from [62,67].
6.1. Zeolite-Based Nanofertilizer System for Sustainable Agriculture
Zeolites are mineral materials that are safe to the soil and function well to conserve available soil nutrients, for efficient crop production and reduction in environmental hazards associated with agricultural activities [68]. Natural zeolite, which comprises over fifty mineral types of basically alkali and alkaline earth aluminosilicates, has been developed into nanofertilizer in recent times. This is mainly due to its availability and inexpensive nature, and it has been applied in the cultivation of maize [69,70]. Apart from providing some minerals for the plants, it is a smart carrier and regulator of major mineral fertilizers. Zeolite acts as a carrier of nitrogen and potassium fertilizers, leading to reduced application quantities with enhanced productivity [71]. Zeolite compound was found to mix with humus materials and enhance the productivity of crops. Zeolite nanocomposites of nitrogen, phosphorus and potassium (NPK), in addition to other nutrients and micronutrients of amino acids, mannose, Ca, Fe, and Zn, have been reported, which have aided crop growth and are absorbed in those crops, as explained in Figure 1 [6]. One disadvantage of zeolite is its inability to absorb and adsorb anions, thus it is enhanced in this perspective with biopolymers [68].
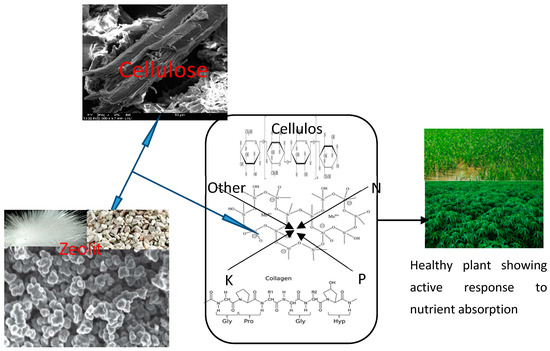
Figure 1.
Negatively charged zeolite platform enhanced by natural polymers and other nutrients for optimum crop performance. Adapted from [74,75].
The three-dimensional crystal structure of zeolite allows high cationic exchange, and, coupled with its excellent porosity and extensive surface area, it is capable of retaining positive and negative nutrient ions for a long time. These exposed surfaces have excellent interactions with cations and polar molecules due to the effectiveness of the hydrated negative inorganic ions contained therein. All these properties have positioned zeolite as one of the most sought materials to be used in fertilizers. It also aids in the gradual release of the nutrients into the soil and absorption by the plants, which will avoid the case of nutrient loss as in conventional systems [72]. As compounds of interest to researchers in different fields, the International Agency for Research on Cancer (IARC) and Food and Drug Administration (FDA) have declared zeolites non-toxic, which enables their extensive use in agriculture and food respectively in recent times [70].
The reduction of zeolites to nanoforms can be done by the top-down approach of ball milling, which reduces their sizes and enhances the surface area for proper interaction or adsorption and desorption of ions [73]. This property enables holding some ions and releasing some in a mechanistic way appropriate for crop improvement and it is also environmentally friendly. Nanozeolite therefore is strategized for improved properties, hence promoting the excellent use of other macro- and micronutrients. In general agriculture, their functions are not only limited to enhancing the soil nutrients, regulating soil acidity, enabling quality seed germination or acting as pesticides, but extends to supplementing the nutritional requirements of most animals and exhibiting wound healing properties [70]. Manikandan and Subramanian reported that there was high uptake of nitrogen by plants, but low concentration on the soil and roots when urea fertilizers were applied with nanozeolite (nanozeourea), because they aided the slow release of the nutrients [60]. Such practice prevents the over concentration of nitrogen in the soil, which could be leached to cause eutrophication and greenhouse effect. Zeolite-based agricultural systems are highly promising but there has been limited interest in them over the years [60]. With the recent uncovering of potentials exhibited by nanozeolites, it is clear that they can change the agricultural sector for the better.
6.2. Synthesis and Role of Zinc/Zinc Oxide Nanoparticles in Fertilizers
The primary sources of zinc micronutrients for fertilizer fortification are zinc oxides (ZnO) and zinc sulfates (ZnSO4H2O or ZnSO4.7H2O). The use of zinc in living organisms cannot be overemphasized as it is contained in most enzymes, necessary for hormones and chlorophyll regulation, and for carbohydrate metabolism. In the nanoparticles form, ZnO can be absorbed, metabolized and accumulated in plant systems. Zinc is a micronutrient and are therefore needed in small quantity; higher concentrations of ZnO NPs can harm the development of plants, leading to inherent aberration in seed germination, root growth and seedling biomass [76]. Nevertheless, its deficiency could hamper the growth and overall development of plants.
When there is deficiency of zinc in the soil, it leads to low or no zinc absorption in the crop, thereby causing malnutrition in food and humans. One third of the human population has been affected by zinc deficiency according to WHO reports due to low zinc content in most foods especially the cereals [77,78,79]. Deficiency of zinc in our food together with other micronutrients causes hidden hunger diseases. Since the body cannot produce this micronutrient, it has a required daily amount, which, when not reached for a long time, leads to serious illness. Hence, such could be avoided by fertilizing crops with adequate micronutrients.
The mixture of ZnO and zinc sulfate is used as fertilizer, since the oxide is not completely soluble in water. Sadeghzadeh [78] reported that zinc deficiency in soils has been a problem that grossly affects crop productivity, especially in alkaline soils.
In the application of these micronutrients to the soil, it is usually difficult to have uniform concentrations due to the amount needed. They are most suitably applied with macronutrients, which act as carriers, although zinc oxide and zinc sulfate can be moderately efficient alone as micronutrient, unlike only zinc [80]. The dissolution and absorption of the micronutrients also depends on the carriers, especially when they are liquids, as 2.0% of zinc could be dissolved in polyphosphate, ammonia, and anhydrous ammonium nitrate (AAN), whereas only 0.05% of zinc is absorbed in orthophosphate [80]. Soil factors and properties such as mineral composition, ionic strength, and organic materials also play important roles in the absorption and bioavailability of the nutrients. Calcareous soils with high pH value and calcium carbonate (CaCO3) content affects the solubility and amount of zinc absorbed into the soil [81]. The presence of the calcium carbonate exchanges Zn for calcium and precipitates zinc carbonate. This continues to hamper crop production, even when micronutrients are applied to counter the reaction of the minerals in the soil. Another hindrance to micronutrient absorption is the nature of soils, as sandy soils hardly retain nutrients applied to them because they are easily leached out, therefore, making the micronutrients unavailable for plant absorption. Soil pH is another key factor that determines the availability of micronutrients in the soil. As the soil pH increases, micronutrients availability decreases except for molybdenum. The mechanism in the case of zinc is that, with increasing soil pH, the Zn is adsorbed on the soil surface together with other clay and inorganic materials, and can be precipitated as Zn(OH)2, ZnCO3 and Zn2SiO4 [78]. With such behavior, the solubility of the entire system is compromised, hence leading to inefficient desorption of the minerals into the soil for the plant uptake.
Nevertheless, nanozinc oxide has immense applications in agriculture as it is not only applied as fertilizers in crop production, but also extends to mechanistic blockage of excess UV radiation during plant growth, aids in the soil recovery of lost nutrients, and is applied in genetic modification of crops and nanofoods, as recommended diets to some ailing patients [66].
6.3. Iron Oxide Nanoparticles and Their Role in Plant Nutrient Fortification
Iron is among the Earth’s abundant elements and it majorly occurs as oxides of magnetite (Fe3O4), maghemite (g-Fe2O3), and hematite (α-Fe2O3), among others. It is used in many applications due to its inexpensive nature, yet it has not been extensively exploited in the field of agriculture. Its low toxicity, availability, and superparamagnetic properties have positioned iron in recent times as one of the transition metal nanoparticles of current interest for improved agricultural production. However, the shortcomings of iron, being pyrophoric and extremely reactive, hinder its application [82].
Most foods are deficient in iron, which arises from poor iron content of the soil unavailable for the plants to absorb. In calcareous soils, the applied iron fertilizers are transformed and may not be available for plant absorption. To solve the problem, iron-chelated fertilizers are used. Iron fertilization of crops in form of γ-Fe2O3 nanoparticles is gradually gaining attention, as it holds prospects towards elimination of iron deficiency in soils and crops. These nanoparticles increase seed germination, help the root to grow and also enhance water content in the chlorophyll. Due to the dynamic nature of nanoparticles, iron is released from γ-Fe2O3NPs when applied to the soil and they migrate from the roots to other parts tissues to supply the nutritional contents. Hu et al. reported an increase in the iron concentration of C. maxima shoots when the plants were exposed to both γ-Fe2O3NPs and Fe3+ treatment compared to controls and Fe(II)-EDTA treated plants [83]. Moreover, when the roots were assessed, they observed that there were no significant changes in the Fe levels among all Fe treated and control plants, which indicated that the iron had been transported to other parts. Most researchers are of the opinion that nutrients get to other parts of the plant through the root, making it a major pathway for nutrient absorption; however, it has also been reported that nutrients can be translocated from the leaves to various parts of the plant tissues. Corredor et al. observed that carbon-coated iron NPs sprinkled on the leaves migrated from the leaves to other parts of the plant [83,84].
6.4. Copper and Copper Oxide Nanoparticles (CuO NPs)
Copper is seen as the third most important metal due to its daily use, and it is important for most living creatures [85]. Cu is an important micronutrient required by plants and should be administered at very low doses. Bulk CuO has a long history in agriculture, especially as fungicide, and has contributed to environmental pollution due to its insolubility, thereby easily being eroded into water bodies. This property limits its usage but the development of nanotechnology offers new hope for the application of copper as the environmental risks are reduced due to existence of copper in 0, +1 and +2 oxidation states, which exhibit different physicochemical properties [34].
In agriculture, copper oxide nanoparticles have found use in fertilizers, plant growth regulators, pesticides, herbicides and as additives for soil remediation [86]. A study on the accumulation of CuO NPs on lettuce and cabbage at concentration of up to 250 mg/L shows reduced water content and growth of the vegetables [86]. Since they are leafy vegetables, in addition to absorbing CuO NPs from the soil, they are also vulnerable to atmospheric pollution, as the nanoparticles are deposited on their leaves, which is the part ingested by humans. The dosage on the vegetables tends to increase, since the absorption is from both the leaves and soils. The absorbed quantities also depend on the type of plant, the soil and environmental factors, as it has been reported that 0.3 mg/L Cu2+ released from 1000 mg/L of copper nanoparticles increases plant growth and is not toxic to the plant [87]. However, some reactions may lead to release of copper ions inside the plant cells that could be toxic. Toxicity of copper nanomaterials results from their solubility in the medium of application and redox processes arising from their interactions with other substances [3].
CuO NPs tend to be more toxic than copper nanoparticles due to their oxidative nature, even at low concentrations, but positively impact on the photosynthetic process of the plant. CuO NPs have also been reported to exhibit antimicrobial activities, which affect the microbial reactions in the soil as some are resistant while others are not [3]. The resistance could be a result of release of Cu2+ ions, which bind with the cellular membrane of microorganisms thereby causing damage.
6.5. Titanium Dioxide Nanoparticles (TiO2 NPs)
TiO2 nanoparticles (less than 4% concentration) have been reported to enhance nitrogen fixation and promote the photosynthesis in spinach, thereby improving the overall growth efficiency of the plant [88]. In the reports of Asli and Neumann [89], TiO2 NPs of about 30 nm applied in Zea mays were not translocated because the sizes of the nanoparticles were more than the pore diameter (6.6 nm) of the root cells. In another study by Du et al. [90] on penetration of TiO2 NPs in wheat plant, some of the nanoparticles passed through the root cells while some did not. Based on that, it could be seen that the nanoparticles were polydispersed and the smaller particle sizes less than 20 nm could penetrate the root cells, while the bigger particles formed agglomerates in the soil medium and could not penetrate the root cells. The presence of the nanoparticles in the soil could have contributed to changing the concentration of the soil enzymes, thereby inhibiting their activities and leading to toxicity. Since they were not translocated, their presence in the soil would affect the soil environment and the ecosystem.
6.6. Cerium Oxide Nanoparticles (CeO2NPs)
Cerium oxide nanoparticles (CeO2 NPs) are one of the sought after nanomaterials needed in agriculture for crop improvement and nutritional effect [91]. Their impact on crops depends on the concentration applied, the soil composition and the plant species. When the concentration of the nanoparticles applied to the soil is in minute quantity, they bring about enhanced crop development and nutritional value. However, they have some detrimental effects in higher concentrations, which depends on the nature of the plants. There are some reports supporting the effect of the higher concentrations of CeO2 NPs on plants. One is the case of enhanced growth of lettuce (Lactuca sativa L.) when treated with 100 mg kg−1 CeO2 NPs, but the growth of the plant was hindered at a higher concentration of 1000 mg kg−1 CeO2 NPs [92]. A similar situation was reported in soybean plant by Cao et al. [93]. They reported that CeO2 NPs greatly improved the photosynthetic rate of soybean under high moisture contents of the soil, but not under limited soil water conditions. The simple mechanism is that, at low moisture content of the soil, the plant stomata are closed, resulting from drought, which hinders transpiration and the uptake of CO2 by the plant [91]. Other mechanisms that portray the uptake of CeO2 NPs by plants explain that positively charged CeO2 NPs are better adsorbed by plant roots than negatively charged CeO2 NPs. This enhances the transport of Ce to other plant parts as the positive surface charge is easily dissolved and attracted to the negative surface charge in the plant rhizosphere. These results are correct within the ambits of laboratory or small-scale application, while there could be different results when applied to the field due to different reactions involved in the soil.
6.7. Noble Metal Nanoparticles
Noble metal nanomaterials have been used in agriculture and, among them, silver is the most widely studied. Silver has a long history of antimicrobial effect and that property has been utilized to remove unwanted microorganisms in the soils, plants and hydroponic systems with improved effect from nanosilver [94]. Apart from promoting plant growth and seed germination, silver has also been applied in the control of fungi, rot and various plant diseases [95]. On the other hand, there could be some negative influence. Masrahi et al. reported that oxidative dissolution of Ag and polyvinyl pyrollidone (PVP) coated silver nanoparticles in the soil system negatively affect the nitrification processes [96]. Other noble metals such as gold nanoparticles have not been well utilized in plant production, as they are not among the micronutrient required in crops. However, they have been limitedly applied as pesticides and also support rapid plant growth. Gold nanoparticles at lower concentration in the soil have been reported to enhance the shoot to length ratio in Lactuca sativa seeds, without affecting the microbial concentration or causing any toxicity [87]. In other studies, they improved the seed germination, growth rate and yield with early flowering, high pod length, excellent chlorophyll and sugar content and enhanced free radical scavenging due to increased flavonoids content [87,97]. The mechanism of free radical scavenging follows that plants contain varied biomolecules such as flavonoids, phenolic substances, etc., and these substances by virtue of their functional groups and antioxidant properties can complex with the metallic ions in the plant and scavenge the free radicals that causes oxidative stress [60,98].
Mahakham et al. reported that exposure of 5–15 mg/L of phytosynthesized gold nanoparticles to maize aged seeds significantly improved their germination and physiology without any toxicity [99]. The results obtained reveal active presence of the nanoparticles in the seed tissues, but were not translocated to the root or shoots, possibly due to the low concentration used. On the contrary, as applied in tobacco plants, gold nanoparticles have been observed to be absorbed in both the root and the shoot of the plant, suggesting more bioavailability of the nanomaterials to some plant species than the others [100].
Platinum nanoparticles are another group of noble metal nanomaterials with interesting applications in plant growth. They have been reported to practically affect the growth mechanism of plants by increasing the length and weight of the plant root system [101]. The reactions of the nanoparticles are such that, if there is agglomeration, it becomes difficult for the nanoparticles to be absorbed and bio-accessibility is hampered. Astafurova et al. [101] reported enhanced plant protective mechanisms due to increased flavonoids concentration resulting from nanoplatinum application. In another study of platinum nanoparticles uptake using Sinapis alba and Lepidium sativum plants, both plant species showed uptake of considerable amounts in the roots and shoots at different concentrations [102]. In this study, both the metal salts and nanoparticles were bioavailable with no recorded phytotoxicity and the Sinapis alba plant recorded higher nanoparticles translocation with increasing concentration than Lepidium sativum.
Ngo et al. [103] used some nanocrystalline metals (Fe, Co, and Cu) to treat soybean seeds, and recorded improvements in the chlorophyll index, number of nodules and amount of crops. Nanocobalt powder exhibited the most interesting crop growth and development among the three nanometals investigated.
6.8. Selenium Nanoparticles (SeNPs)
Selenium can exist in different forms including oxyhalides, selenides, halides, oxides, acids, oxyacids, selenoenzymes and selenium nucleic acids [104]. Selenium occurs in the same group as sulfur and can exist in oxidation states of −2, 0, +2, +4 and +6. They differ in some reactions with the sulfur due to the unstable state of the +6 oxidation state and the poor shielding effect of the d-orbitals introduced in the selenium. The most stable oxidation state of the selenides is the −2 state and, in this form, they react with the highly electropositive elements.
Over one billion humans are suffering from selenium malnutrition, thus its supplementation or fertilization in plants and animals is necessary for human wellbeing [105]. It had been reported to be toxic, but recent research has shown that its toxicity depends on the concentration [106,107]. Selenium can be obtained as supplements from food or meat, and the best method of enrichment to satisfy human requirements is to enhance its level in agricultural crops by means of spraying or addition as selenite or selenates in fertilizers [108]. Phosphate fertilizers, sewage waste and farmyard manure are rich in selenium and can become sources of this micronutrient [106]. Selenium nanoparticles have interesting physicochemical properties and are greatly bioavailable with good physiological functions such as excellent antimicrobial, anti-cancer, antioxidant, etc. activities. They have been greatly applied as supplements in plants and food and are widely used in nanomedicines due to their low toxicity profiles [109]. They act as detoxifying agents when they contact heavy metals such as mercury, cadmium, and lead and such property has enabled them to guard living organisms against several diseases. Selenium compounds can easily functionalize with ligands. For example, selenocystin binding with glutathione peroxidase can neutralize the free radicals present in cells, thereby eliminating the harmful effect of the radicals. However, SeNPs hardly interact with most compounds and as a result are slowly released into living systems [104]. Inorganic forms of selenium seem to be more bioavailable than the organic ones, as demonstrated with tomatoes and strawberries. These crops scavenge for selenium in soils and store them in the edible parts, and the quantities of the inorganic forms are found to be more than the organic ones. Such actions reduce the inorganic selenium in the soils, which is more cost-effective [108].
On a general note, selenium fertilization can interestingly improve the production of biochemical compounds such as amino acids, flavonoids, glucosinolates, protein, and phenolics compounds [107]. Schiavon et al. [110] reported enhanced flavonoids and phenolic compounds in selenium-biofortified tomato fruit. Dinkova-Kostova also reported an increase in glucosinolates, which hydrolyzes to form isothiocyanates that possess excellent anticancer properties [111].
6.9. Carbon-Based Nanomaterials in Plant Fortification
Organic or carbon-based nanomaterials, including fullerenes, fullerols, single-walled carbon nanotubes (SWCNTs), and multi-walled carbon nanotubes (MWCNTs), have been used in crop production and they behave differently. Generally, carbon nanotubes (CNTs) are highly hydrophobic and do not dissolve in aqueous media, but form aggregates due to high van der Waals forces, while SWCNTS are hydrophilic and can hardly penetrate the cell walls of the plants because of their large sizes. Fullerol, which has been reported to be hydrophilic and of smaller size, can be transported into the plant tissues by apoplastic means [112]. On the other hand, fullerenes and MWCNTs can easily interact with the hydrophobic components of the natural organic matters and possibly find their way into plant tissues. However, they have been reported to block the pores of the cell wall of Allium cepa, making their translocation insignificant [113,114,115]. Overall, carbonaceous materials find their way into the plant tissues [116,117].
MWCNTs have been reported to enhance the seed germination rate of tomato plant and decrease cell concentration of cultured rice cell suspensions. However, there are no observable physiological change or toxicity when applied to crops such as cucumber, lettuce, corn, rape, wheat, etc. [114,118]. Lahiani et al. reported that there were no toxic effects associated with long-term exposure of crops to MWCNTs, rather there was positive impact on the general growth of the plants compared to the untreated crops [119]. In the case of single-walled carbon nanohorns (SWCNHs) used to assess the germination of six crops (rice, tomatoes, barley, soybean, corn, and switchgrass), Lahiani et al. [112] found that, at higher concentration of the SWCNHs (100 µg/mL), all crops germinated except tomatoes. In contrast, at lower concentration (25 µg/mL) of the nanomaterials, the tomato crop showed the greatest rate of germination, while there was no result of early germination for soybean. Overall, no toxicity was recorded and the results showed that different plants respond differently to varying concentrations of the nanomaterials. To control some of the problems associated with the different carbon nanomaterials, they could be functionalized with magnetic nanoparticles, which delivers multiple functions of filling internal space with plant. This protects the chemicals and directs the movement of nanocarriers within the plant structure [94].
6.10. Nanosilicon Dioxide
Silicon has not been well recognized as an essential micronutrient needed for plant growth compared to others. However, it offers outstanding beneficial activities to plants ranging from enhanced plant growth and yield to resistance to biotic and abiotic factors [120]. It regulates plant physiological activities and acts as physicomechanical barrier. Plants that lack silicon minerals are structurally weak and poorly developed. Nano-silicon dioxide is currently one of the interesting new inorganic materials studied due to its extraordinary characteristics of excellent chemical purity, ultrafine particle size, enhanced surface adsorption and energy, excellent dispersion and high thermal resistance.
Siddiqui and Al-Whaibi reported the improved seed germination of Lycopersicum esculentum using 8 g/L of 12 nm nano-silicon oxide [120]. It is also reported that nanostructured silicon dioxide helps to reduce the transpiration rate of plants, and improve the green coloration and shoot expansion of plants [121]. Yeo et al. reported the reduced sodium uptake and transpirational bypass flow by Oryza sativa due to treatment with silicon [122]. In the work of Faryadi and Sheikhahmadi, nano-silicon dioxide supplements affected the weights of eggs and increased the bone ash content and calcium in laying quails [123].
Since there is a drift from primitive to modern agriculture to meet the food demands of the population, nano-silicon dioxide has been reported to possess the potentials of improving the growth rates of different plants and vegetables with short maturity time. It is a new material that can lead to excellent food safety and interesting agricultural experience. Table 3 summarizes the concentration dosage of some of the nanoparticles and their merits and demerits to plant and food production, while Figure 2 emphasizes the need for biofortification of plant crops.

Table 3.
Effects of some of the engineered nanomaterials on agricultural crops.
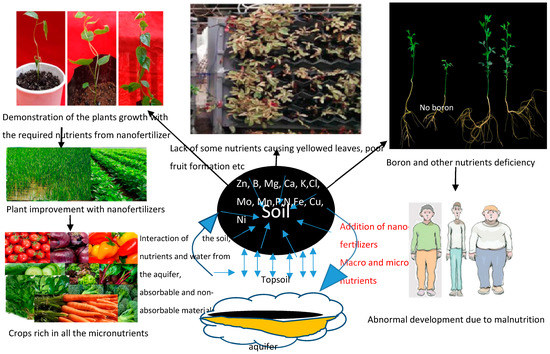
Figure 2.
Schematic diagram of influence of micronutrients on crops Adapted from [137,138,139,140].
7. General Synthesis of Nanomaterials
There are two major ways of synthesizing nanomaterials: the top-down and bottom-up approaches. Top-down methods are physical means, which are expensive and consume a lot of energy and time, whereas bottom-up approaches are wet-chemical or biological means and are preferred by most researchers. Top-down methods are physical means and include the mechanical/ball milling, photolithography, sputtering, chemical etching, etc., while bottom-up approaches involve physical, chemical and biological means and include sonochemical, microwave, photochemical, vapor deposition, sol-gel, chemical and electrochemical deposition, atomic and molecular condensation, spray, laser pyrolysis, etc. [141].
The biological method is one of the emerging green methods of synthesis because it uses eco-friendly materials and aqueous solvents, saves time and energy, and is cost effective, sustainable and non-toxic. The ultimate objectives of using biological means of synthesis, phytonanotechnology or green nanotechnology is to reduce the environmental and human risks associated with other methods of engineering nanomaterials. They provide nanomaterials that serve the essential needs of the present century. Biological means of synthesis consist of the microorganism-mediated method and plant-assisted green nanotechnology. More focus and interest have been give to the use of the plant method, as all plant parts ranging, including the roots, barks, leaf, stem, fruits, sap, and even fruit wastes, are applied in fabricating nanomaterials for various uses. Plants are considered as natural reservoirs for various components such as flavonoids, phenolics, terpenoids, carbohydrates, proteins, saponins, and acids that have potentials for reducing, stabilizing and capping metal or metal oxide nanoparticles as well as functionalizing carbon-based nanomaterials. Since these biocomponents in plants have functional groups that can act as organic ligands, they serve as electron donors and effectively reduce the bulk metal or metal oxides to their nanoparticulate forms. This method provides nanoparticles that have interesting characteristics including small sizes, eco-friendly nature, biocompatibility, low toxicity, simple reaction procedures and enhanced surface morphologies with unlimited applications.
In the application of the biological method of synthesis, the plant-assisted method in particular is seriously gaining attention because it is cost effective, easily manipulated, of high purity, sustainable, parametric efficient and greens. It has thus far outweighed other options. For instance, in a typical synthesis of SeNPs, sodium selenite, selenous acid or selenium oxide can be used as the precursor compound and plants substrates from Vitis vinifera fruits, Bougainvillea spectabilis wild flower, etc. have been used [142,143]. The synthesis involves different concentrations of the precursor compound and the plant extract. The solution is mixed and stir-heated at a temperature the plant biocomponents can withstand—not too high as to render them inactive. After a period, there could be observable color changes, indicative of nanoparticles formation and the solution is centrifuged, washed and dried to get the nanoparticles. Surega reported different positive influences of silver nanoparticles synthesized by different plant extracts on improving crop production [144]. From the results, there was interesting development on the root and shoot and the fruit yield, among others. Although there are many reports on plant-mediated synthesis of nanoparticles, some gaps still exist on their application in crop improvement.
8. Future of Nanotechnology in Plant Improvement
Sustainable agriculture, food availability and nutrient security are among the key sustainable development goals of the century. It is therefore imperative to harness the advantages of nanotechnology in achieving the feat by improving the nutrient availability of plants and minimizing their losses on agricultural soils. Many processes occur in the ecosystem during plant production and subsequent consumption of the food and their digestion. During these processes, whatever is added to the soil to improve their production and nutrient enhancement may translate to the crop harvested and also be extended to the nutritional level in organisms afterwards. The knowledge of this phenomenon, therefore, sensitizes the new technologies of using nanomaterials in soil improvement and crop production. Nanochemicals, by the virtue of their quantum sizes and surface area, have improved properties and interesting applications. However, they may also have some toxic effects on the environment when applied as fertilizers, pesticides, nanodelivery tools, food packaging, etc. Figure 3 points out the possible beneficial nanoparticles and their demerits if not properly used.
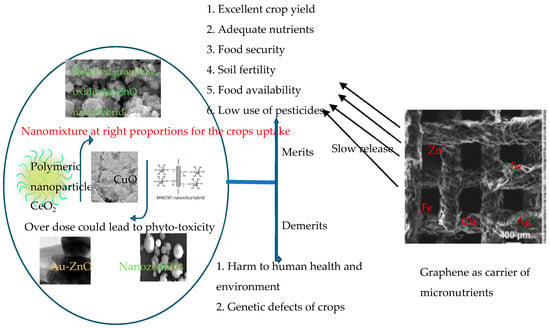
Figure 3.
Improving the quality of soil and crops using appropriate nanoparticles Adapted from [148,149,150,151].
The argument now is: Do the pros outweigh the cons? The answer is obviously “yes”, as there are new developments towards using eco-friendly materials for more acceptable nanoagriculture. Moreover, plants may absorb the required materials and leave the rest in the soil. However, it is not clear whether the toxic materials are absorbed or become non-toxic, and, regardless, the toxic component would still be part of the ecosystem. To clear all doubts and apply the tenets of nanotechnology towards sustainable agriculture and green chemistry, there is a drift from the use of toxic chemicals in synthesis of nanomaterials to the use of biological organisms. These biological organisms have special functional groups that affect the transformation of materials into their nanoforms and also possess capping and stabilizing effects.
The merit of this novel technology towards agriculture is that there is reclamation of lost nutrients in the soil; herb and pest control using green nanochemicals; supply of macro- and micronutrients for proper plant development; nutrient absorption; security in the food crops; nanofood and nutrient delivery; food packaging; etc. It therefore entails an overall development of the agricultural sector starting from the field to the wellbeing of individuals or organisms that feed on the crops. This is a future technology to be embraced in all ramifications. It is gaining serious attention already, but there is still much work to be done.
Apart from crop improvement, another agricultural area of application of this smart technology is in the use of nanobarcodes of gold and silver stripes in crop and food packaging, which reveals all the information about the product. This is important due to the people’s fear of using nanoproducts.
In biosensing, graphene oxide has been applied in the enhanced detection of aflatoxins in food materials [145]. Zhang et al [146] immobilized hemoglobin with silica coated gold nanorods, which act as biosensor. The applications of nanomaterials in biosensing are relatively new and, in agriculture, the activities are more beneficial before and after crop planting or in the food. Further attempts to develop the application of nanocomposites have led to increased hydrophilicity of synthesized gelatin–gold–single-walled nanotubes with biocompatible surface that not only could be applied in sensing but could also improve nutrient absorption by plant cell and lead to excellent growth [147].
9. Biosafety of Nanomaterials in Sustainable Agriculture
Assessment of safety of nanomaterials is always a difficult issue as the materials depend on many factors: substances or salts used, process method, biological substrates, size and structure, and reactions in medium of applications [101]. During toxicity tests, the prepared stable nanoparticles may become unstable due to aggregation, which modifies the required administered doses. Such behavior would rip the nanoparticles of their unique behaviors and they become different materials [152].
The commonly studied nanomaterials include fullerene (C70); fullerol (C60(OH)20); carbon nanotubes; and ZnO, TiO2, Fe3O4, CeO2, Au, Ag, Cu, and Fe nanoparticles. They influence in one or more ways soil fertility and plant nutrients fortification [114].
Nanomaterials may not be completely safe depending on the method of synthesis. Chemically-, physically- and biologically-synthesized nanomaterials have being reported as toxic. Chemically-synthesized nanoparticles pose greater toxicity due to the slow release of the chemical agents used in their synthesis. The biologically-engineered nanomaterials are more biocompatible and relatively safer to the environment and organisms.
Since the synthesis of nanomaterials involve different methods and reaction conditions, the materials are formed in various sizes and morphologies, which gives them different properties. As a result, there are many contrasting results obtained from these nanomaterials. Different factors affect their behavior and toxicity: particle sizes, the materials of fabrication, etc. Nano-scaled materials can be safe but, when in contact with other media, some inconsistent reactions may be triggered, making them unsafe. To make this new technology safe and sustainable, there is a recent trend from using chemical methods for nanomaterial synthesis to biological methods. This is necessary because the materials of synthesis greatly affect the nanoparticles, which need to be observed individually. Individual bionanomaterials are composed of biomolecules that may be harmful, and they behave differently when used in material synthesis. It is therefore important to continue to study the behaviors of these nanomaterials and their decomposition products in organisms to ascertain their level of toxicity.
Fullerene (C60) NPs have been reported to be non-toxic to microorganisms in the soil, but are toxic in aquatic environments, which clearly indicates that the environment affects the reactions and behaviors of nanomaterials [96,153].
At the moment, chemicalized nanoparticles may contribute some toxicity to the environment or where applied due to the medium of preparation, but the toxicity of the bionanomaterials are still under intensive study. Thus far, the level of toxicity of bionanomaterials is still uncertain due to the different materials used in the preparation and the various reactions with the medium of application. However, it is promising to use these nanoparticles for future development of the agrarian sector.
Researchers at the School of Agricultural, Food and Biosystems Engineering (ETSIAAB) from Universidad Politécnica de Madrid (UPM) have recently reported that zinc oxide nanoparticles serve as the source of zinc micronutrient and can be well utilized as fertilizer feedstock without excessive toxicity [154,155]. On the other hand, Garcia-Gomez et al. reported more lethal effects of using zinc oxide nanoparticles in acidic soil than in calcareous soils [156]. In their experiment, using tomato and bean plants, there was higher photosynthetic pigmentation of the plants with increasing zinc concentration in the calcareous soil. In a nutshell, the toxicity of zinc oxide nanoparticles greatly depends on the soil pH.
Plants interaction with zinc ions and zinc oxide nanoparticles are different. Previous studies have shown that there are decreased macro- and micronutrients in plants when exposed to high concentrations of ZnO NPs, due to blockage of the roots by the nanoparticles, which has not been observed using zinc ions [157,158,159]. They block the uptake of other nutrients while becoming concentrated on the roots. Zhang et al. showed higher Zn levels in the roots of Schoenoplectus tabernaemontani than in the shoots, which reflects poor mineral translocation potential to other parts of the plants as a result of excess ZnO nanoparticles treatment of the soils [160]. Theey carried out their experiment out using zinc ions, and it observed that the roots accumulate more zinc in the nanoparticulate form than in the zinc ions treatment. Further reasons are that ZnO NPs achieves higher sorption into the plant organs with lower mobility than the Zn2+ ions [129]. Zinc in any form, however, should not be used in excess in the soil to avoid toxicity due to chlorosis and root morphology impairment [157,161].
In general, metal and metal oxide nanoparticles are more detrimental to soil microorganisms than organic nanomaterials. ZnO-NPs has been reported to affect the soil microbial metabolism and reduce the numbers of the nutrient fixing bacteria such as the azotobacter, and P- and K-solubilizing bacteria [130,162]. CuO NPs are also reported to inhibit the root growth of wheat plants [163,164]. Copper nanoparticles can affect the photosynthetic, respiratory and growth processes of the plant at higher concentration and would pose dangers on humans when such crops are consumed. Lu et al. [165], used SiO2 and TiO2 NPs to enhance the activities of nitrifying bacteria and the uptake of fertilizer and water in soybean. In spinach, TiO2 NPs was used to improve the chlorophyll concentration, enhancing the plant photosynthesis and dry weight [88,141]. They did not constitute any toxic effects, rather they caused obstruction to the apoplastic flow through the cell walls.
AgNPs are reported to be toxic at higher concentrations of ≥60–100 μg/mL. At low concentrations of about 30 μg/mL, they cannot penetrate the roots of plants and do not cause any harm [126]. In a study conducted by Krishnaraj et al. (2012) on Bacopa monnieri plant, the biologically synthesized AgNPs offered less or no toxicity and positively affected protein and carbohydrate synthesis with lower phenol contents [166]. These reports generally imply that nanoparticles affect the crop nutrient quality in diverse ways. In cucumber, ZnO added to the soil has improved the starch and protein content of the plant, while reducing the copper and molybdenum micronutrient content [167,168]. However, in CeO2 modulated soil, the nutritional content of the wheat grains used in the study was not detrimentally affected by the nanoparticles. In other crops such as rice, soybean, tomatoes and cucumber, there was high accumulation of cerium, which shows different transport mechanism in plants and root storage of engineered nanomaterials [168,169].
The use of nanomaterials to boost plants nutrient and agricultural production is obviously gaining attention, but there should be more attention on the safety of these materials, as there is a thin line between deficiency and toxicity [115]. Although there are successful developments in the use of nanotechnologies in the field of agriculture, we cannot entirely rule out the dangers inherent in their usage as well. Modern research has adopted this technology as a way towards unparalleled development and, therefore, should also invest more in evaluating the safety of the materials to improve on their processing, characterization and overall application standards.
10. Conclusions
Despite the reports on the endangering nature of some nanoparticles, nanotechnology remains in the frontline as the alternative to change the agricultural sector for the better. Its advantages could be greater than those of nuclear energy. The reasons are not far-fetched and include: their manipulative ability that enhances the physicochemical properties; their high carrier system use, bioavailability, and easy processability and engineering; and their low toxicity compared to other compounds. To make this field of study more lucrative and applicable to agriculture without hassles, the plant-mediated biological methods of synthesis, which utilize raw materials such as waste vegetables, plant extracts, flowers, plant barks, roots, fruit peels and leather cuttings, should be exploited and expanded. There should be regulations on the nanoproducts to protect the environment, the health of the users of such products and the entire public health. Nanotechnology industries should be made to provide product information for their nanomaterials. Despite the problems and challenges that could be associated with the nanomaterials, it is now time to take it out of the laboratory stage into the field. In agriculture, nanomaterials should be introduced in the nursery stage of crop production and monitored. They should also be applied during land preparation to supply the required nutrients to the soil and for biosensing, among others. Such practices could greatly improve nutritional health and sanitation, food security and sustainability, and the environment, especially in developing countries.
Author Contributions
All authors contributed equally to the writing of this review article and consent to its publication.
Acknowledgments
The authors wish to thank the management of NWU, South Africa for the platform that enabled this interesting work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nation Department of Economic and Social Affairs. World Population Projected to Reach 9.6 Billion by 2050; United Nation Department of Economic and Social Affairs: New York, NY, USA, 2015. [Google Scholar]
- Subramanian, K.S.; Tarafdar, J.C. Prospects of nanotechnology in Indian farming. Indian J. Agric. Sci. 2011, 81, 887–893. [Google Scholar]
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper Oxide Nanoparticle Foliar Uptake, Phytotoxicity, and Consequences for Sustainable Urban Agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, T.; Kumar, R. Bio-Nanotechnology and its Role in Agriculture and Food Industry. J. Mol. Genet. Med. 2018, 12, 1–5. [Google Scholar]
- Kabiri, S.; Degryse, F.; Tran, D.N.H.; Da-Silva, R.C.; Mclaughlin, M.J.; Losic, D. Graphene oxide; A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325. [Google Scholar] [CrossRef] [PubMed]
- Harper, T. The Year of the Trillion Dollar Nanotechnology Market? AZoNetwork UK Ltd.: Manchester, UK, 2015. [Google Scholar]
- Hossain, S.M.; Mohiuddin, A.K.M. Study on Biofortification of Rice by Targeted Genetic Engineering. Int. J. Agric. Res. Innov. Technol. 2012, 2, 25–35. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Food Fortification Programs to Alleviate Micronutrient Deficiencies. J. Food Process Technol. 2013, 4, 257–267. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrient fortification of food and its impact on woman and child health: A systematic review. Syst. Rev. 2013, 2, 67. [Google Scholar] [CrossRef]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The prevalence of micronutrient deficiencies and inadequacies in the middle east and approaches to interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Burchi, F.; Fanzo, J.; Frison, E. The Role of Food and Nutrition System Approaches in Tackling Hidden Hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Vitolins, M.Z. Food Fortification and Supplement Use-Are There Health Implications? Crit. Rev. Food Sci. Nutr. 2016, 56, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aggarwal, P.; Kaur, A. Biofortification: A new approach to eradicate hidden hunger. Food Rev. Int. 2017, 33, 1–21. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Nestel, P.; Meenakshi, J.V.; Qaim, M.; Sachdev, H.P.S.; Bhutta, Z.A. Plant breeding to control zinc deficiency in India: How cost-effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar] [CrossRef]
- WHO. Nutrition for Health and Development; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Smith, I.F. Micronutrient interventions: Options for Africa. Food Nutr. Bull. 2000, 21, 532–537. [Google Scholar] [CrossRef]
- Bouis, H. An Overview of the landscape and approach for Biofortification in Africa. Afr. J. Food Agric. Nutr. Dev. 2017. [Google Scholar] [CrossRef]
- Gibson, R.S.; Anderson, V.P. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr. Bull. 2009, 30, 108–143. [Google Scholar] [CrossRef]
- Arimond, M.; Ruel, M.T. Dietary Diversity Is Associated with Child Nutritional Status: Evidence from 11 Demographic and Health Surveys. J. Nutr. 2004, 134, 2579–2585. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Watts, M.J.; Young, S.D.; Chilimba, A.D.C.; Ander, E.L. Dietary mineral supplies in Malawi: Spatial and socioeconomic assessment. BMC Nutr. 2015, 1, 42. [Google Scholar] [CrossRef]
- Semba, R.D. The vitamin A story: Lifting the shadow of death. Vitamin A Story Lift Shad Death 2012, 104, 1–207. [Google Scholar]
- Murgia, I.; Arosio, P.; Tarantino, D.; Soave, C. Biofortification for combating ‘hidden hunger’ for iron. Trends Plant Sci. 2012, 17, 47–55. [Google Scholar] [CrossRef]
- Rawat, R.; Nguyen, P.H.; Ali, D.; Saha, K.; Alayon, S.; Kim, S.S.; Ruel, M.; Menon, P. Learning How Programs Achieve their Impact: Embedding Theory-Driven Process Evaluation and Other Program Learning Mechanisms in Alive & Thrive. Food Nutr. Bull. 2013, 34, S212–S225. [Google Scholar]
- Gilligan, D.O. Biofortification, agricultural technology adoption, and nutrition policy: Some lessons and emerging challenges. CESifo Econ Stud. 2012, 58, 405–421. [Google Scholar] [CrossRef]
- Gibson, R.S.; Hotz, C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br. J. Nutr. 2001, 85, S159. [Google Scholar] [CrossRef]
- Pandav, C.S.; Yadav, K.; Srivastava, R.; Pandav, R.; Karmarkar, M.G. Iodine deficiency disorders (IDD) control in India. Indian J. Med. Res. 2013, 138, 418–433. [Google Scholar]
- Tan-Torres, E.T.; Aikins, M.; Black, R.; Wolfson, L.; Hutubessy, R.; Evans, D.B. Cost effectiveness analysis of strategies for child health in developing countries. BMJ 2005, 331, 1177. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.K. Vitamin a supplementation for preventing death and illness in children 6 months to 5 years of age. In Cochrane Database of Systematic Reviews; Tovey, D., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dubock, A. An overview of agriculture, nutrition and fortification, supplementation and biofortification: Golden Rice as an example for enhancing micronutrient intake. Agric. Food Secur. 2017, 6, 59. [Google Scholar] [CrossRef]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Lucca, P.; Hurrell, R.; Potrykus, I. Fighting Iron Deficiency Anemia with Iron-Rich Rice. J. Am. Coll. Nutr. 2002, 21, 184S–190S. [Google Scholar] [CrossRef]
- Pandya-Lorch, S.F.R. About IFPRI and the 2020 Vision Initiative; International Food Policy Research Institute: Washington, DC, USA, 2012. [Google Scholar]
- Bilski, J.; Jacob, D.; Soumaila, F.; Kraft, C.; Farnsworth, A. Agronomic Biofortification of Cereal Crop Plants with Fe, Zn, and Se, by the Utilization of Coal Fly Ash as Plant Growth Media. Adv. Biores. 2012, 3, 130–136. [Google Scholar]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Melash, A.A.; Mengistu, D.K.; Aberra, D.A. Linking Agriculture with Health through Genetic and Agronomic Biofortification. Agric. Sci. 2016, 07, 295–307. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic Biofortification of Cereal Grains with Iron and Zinc. In Advances in Agronomy; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 55–91. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.P. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D.; Welch, R.M.; Saunders, D.A.; Ortiz-Monasterio, I.; Bouis, H.E.; Bonierbale, M.; de Haan, S.; Burgos, G.; Thiele, G.; Liria, R.; et al. Nutritious Subsistence Food Systems. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; pp. 1–74. [Google Scholar]
- Duffner, A.; Weng, L.; Hoffland, E.; van der Zee, S.E.A.T.M. Multi-surface Modeling to Predict Free Zinc Ion Concentrations in Low-Zinc Soils. Environ. Sci. Technol. 2014, 48, 5700–5708. [Google Scholar] [CrossRef] [PubMed]
- Ros, G.H.; Van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Jones, K.M.; de Brauw, A. Using Agriculture to Improve Child Health: Promoting Orange Sweet Potatoes Reduces Diarrhea. World Dev. 2015, 74, 15–24. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Fortification of micronutrients for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36, 7. [Google Scholar] [CrossRef]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Raj, T.G.; Khan, N.A. Designer nanoparticle: Nanobiotechnology tool for cell biology. Nano Converg. 2016, 3, 22. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–3. [Google Scholar]
- McKenzie, R. Crop Nutrition and Fertilizer Requirements Essential Plant Nutrients. Agri-Facts 1998, 540, 1–7. [Google Scholar]
- Monreal, C.M.; Derosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Rech, I.; Polidoro, J.C.; Pavinato, P.S. Additives incorporated into urea to reduce nitrogen losses after application to the soil. Pesqui Agropecu Bras 2017, 52, 194–204. [Google Scholar] [CrossRef]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Monostori, T. Crop Production; University of Szeged, Faculty of Agriculture Andrássy út 15: Hódmezővásárhely, Hungary, 2014; pp. 15–20. [Google Scholar]
- Diatta, J.; Borowiak, K.; Szczepaniak, W. Evaluation of fertilizers solubility and phosphate release in slightly acidic arable soil. Arch. Agron. Soil Sci. 2018, 64, 1131–1141. [Google Scholar] [CrossRef]
- Hagin, J.; Tucker, B. Fertilization of Dryland and Irrigated Soils; Advanced Series in Agricultural Sciences; Springer: Berlin/Heidelberg, Germany, 1982; pp. 120–140. [Google Scholar]
- Olson-Rutz, K.; Jones, C. Soil Nutrient Management for Forages; Department of Land Resources and Environmental Sciences, Montana State University: Bozeman, MT, USA, 2015; pp. 1–10. [Google Scholar]
- Manikandan, A.; Subramanian, K. Evaluation of Zeolite Based Nitrogen Nano-fertilizers on Maize Growth, Yield and Quality on Inceptisols and Alfisols. Int. J. Plant Soil Sci. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Rai, A.; Rai, S.; Rakshit, A. Mycorrhiza-mediated phosphorus use efficiency in plants. Environ. Exp. Biol. 2013, 11, 107–117. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suppan, S. Nanomaterials in Soil; Institute for Agriculture and Trade Policy: Washington, DC, USA, 2013. [Google Scholar]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2017, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.; Zhang, Y.; Cai, J.; Zhang, W.; Pan, B. Arsenate Adsorption by Hydrous Ferric Oxide Nanoparticles Embedded in Cross-linked Anion Exchanger: Effect of the Host Pore Structure. ACS Appl. Mater. Interfaces 2016, 8, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Kamran, A.; Haroon, Z.K.; Muhammad, Z.; Imdad, H.; Zeeshan, A. Nano-zinc oxide as a future fertilizer. Weekly Technology Times, 27 April 2016. [Google Scholar]
- Azam, F. Added nitrogen interaction in the soil-plant system—A review. Pakistan J. Agron. 2002, 1, 54–59. [Google Scholar]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Suppan, S. Applying Nanotechnology to Fertilizer the Institute for Agriculture and Trade Policy Works Locally and Globally at the Intersection of Policy and Practice to Ensure Fair and Sustainable Food, Farm and Trade Systems. October 2017. Available online: iatp.org (accessed on 23 January 2019).
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Karaca, M.; Demir, H.; Onus, N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Guo, J. Synchrotron radiation, soft-X-ray spectroscopy and nanomaterials. Int. J. Nanotechnol. 2004, 1, 193–225. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Suarato, G.; Rosalia Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Tarmizi, E.Z.M.; Baqiah, H.; Talib, Z.A.; Kamari, H.M. Preparation and physical properties of polypyrrole/zeolite composites. Results Phys. 2018, 11, 793–800. [Google Scholar] [CrossRef]
- Singh, N.B.; Amist, N.; Yadav, K.; Singh, D.; Pandey, J.K.; Singh, S.C. Zinc Oxide Nanoparticles as Fertilizer for the Germination, Growth and Metabolism of Vegetable Crops. J. Nanoeng. Nanomanuf. 2013, 3, 353–364. [Google Scholar] [CrossRef]
- Biesalski, H.K. Hidden Hunger; Springer: Berlin, Germany, 2013. [Google Scholar]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health 2003, 16, 230. [Google Scholar]
- Mortvedt, J.J.; Giordano, P.M. Crop Response to Zinc Oxide Applied in Liquid and Granular Fertilizers. J. Agric. Food Chem. 1967, 15, 118–122. [Google Scholar] [CrossRef]
- Milani, N.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G.; Stacey, S.P.; McLaughlin, M.J. Fate of zinc oxide nanoparticles coated onto macronutrient fertilizers in an alkaline calcareous soil. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Phull, A.R.; Ali, J.S. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar]
- Hu, J.; Guo, H.; Li, J.; Wang, Y.; Xiao, L.; Xing, B. Interaction of γ-Fe2O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina, C.; Ibarra, M.R.; De La Fuente, J.M.; Rubiales, D.; Pérez-De-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ. Toxicol. Chem. 2018, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Sahid, M.; Pierart, A.; Sobanksa, S. Potential Contamination of Copper Oxide Nanoparticles and Possible Consequences on Urban Agriculture. Environ. Sci. Technol. 2017, 78, 5774–5782. [Google Scholar]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. J. Nanosci. Nanotechnol. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of Nano-TiO2 on Strength of Naturally Aged Seeds and Growth of Spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Gui, X.; Zhang, Z.; Liu, S.; Ma, Y.; Zhang, P.; He, X.; Li, Y.; Zhang, J.; Li, H.; Rui, Y.; et al. Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS ONE 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (: Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Saurabh, S.; Singh, B.K.; Yadav, S.M.; Gupta, A.K. Applications of Nanotechnology in Agricultural and their Role in Disease Management. Res. J. Nanosci. Nanotechnol. 2015, 5, 15. [Google Scholar]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Masrahi, A.; VandeVoort, A.R.; Arai, Y. Effects of silver nanoparticle on soil-nitrification processes. Arch. Environ. Contam. Toxicol. 2014, 66, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013, 461, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of gold nanomaterials to plants: Importance of particle size and surface coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Astafurova, T.; Zotikova, A.; Morgalev, Y.; Verkhoturova, G.; Postovalova, V.; Kulizhskiy, S.; Mikhailova, S. Effect of platinum nanoparticles on morphological parameters of spring wheat seedlings in a substrate-plant system. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012004. [Google Scholar] [CrossRef]
- Asztemborska, M.; Steborowski, R.; Kowalska, J.; Bystrzejewska-Piotrowska, G. Accumulation of platinum nanoparticles by Sinapis alba and Lepidium sativum plants. Water Air Soil Pollut. 2015, 226, 126. [Google Scholar] [CrossRef]
- Ngo, Q.B.; Dao, T.H.; Nguyen, H.C.; Tran, X.T.; Van Nguyen, T.; Khuu, T.D.; Huynh, T.H. Effects of nanocrystalline powders (Fe, Co and Cu) on the germination, growth, crop yield and product quality of soybean (Vietnamese species DT-51). Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 015016. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected MajorRisks; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Uttam, S.; Abioye, F.L.S. Selenium in the Soil-Plant Environment: A Review. Int. J. Appl. Agric. Sci. 2017, 3, 1–18. [Google Scholar]
- Meetu, G.; Shikha, G. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar]
- Carvalho, K.M.; Gallardo-Williams, M.T.; Benson, R.F.; Martin, D.F. Effects of selenium supplementation on four agricultural crops. J. Agric. Food Chem. 2003, 51, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Dall’acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.M.A. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanumlycopersicon L.). Aquat. Toxicol. 2012. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T. Chemoprotection against cancer by isothio-cyanates: A focus on the animal models and the protective mechanisms. Top. Curr. Chem. 2013, 329, 179–201. [Google Scholar] [PubMed]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural Organic Matter Stabilizes Carbon Nanotubes in the Aqueous Phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.A.S.; Ke, P.C. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, C.; Ma, X.; White, J.C. Fullerene-enhanced accumulation of p, p’-DDE in agricultural crop species. Environ. Sci. Technol. 2012, 46, 9315–9323. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2017, 66, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Avestan, S.; Naseri, L.A.; Hassanzade, A.; Sokri, S.M.; Barker, A.V. Effects of nanosilicon dioxide application on in vitro proliferation of apple rootstock. J. Plant Nutr. 2016, 39, 850–855. [Google Scholar] [CrossRef]
- Yeo, A.R.; Flowers, S.A.; Rao, G.; Welfare, K.; Senanayake, N.; Flowers, T.J. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 1999, 22, 559–565. [Google Scholar] [CrossRef]
- Faryadi, S.; Sheikhahmadi, A. Effect of nanosilicon dioxide on growth performance, egg quality, liver histopathology and concentration of calcium, phosphorus and silicon in egg, liver and bone in laying quails. Appl. Nanosci. 2017, 7, 765–772. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front Chem. 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; White, J.C.; Xing, B. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef]
- Raddy, R. Efficacy of Nano Zinc Particle on Growth. Master’s Thesis, University of Agricultural Sciences, Bangalore, India, 2014. [Google Scholar]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zarafshar, M.; Akbarinia, M.; Askari, H.; Hosseini, S.M.; Rahaie, M.; Struve, D. Toxicity Assessment of SiO2 Nanoparticles to Pear Seedlings. Int. J. Nanosci. Nanotechnol. 2015, 11, 13–22. [Google Scholar]
- Sidkey, N.M.; Ismail, A.A.; Arafa, R.A.; Fathy, R.M. Impact of Silver and Selenium Nanoparticles Synthesized by Gamma Irradiation and Their Physiological Response on Early Blight Disease of Potato. J. Chem. Pharm. Res. 2016, 8, 934–951. [Google Scholar]
- Aslani, F.; Bagheri, S.; Julkapli, N.M.; Juraimi, A.S.; Sadat, F.; Hashemi, G.; Baghdadi, A. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 1–28. [Google Scholar] [CrossRef]
- Sztrik, A. Production of Nano-Size Elemental Selenium Particles and Its Investigation in the Soil-Plant-Animal System. Ph.D. Thesis, School of Crop Production, Horticulture and Food Science, University of Debrecen, Debrecen, Hungary, 2016; pp. 1–26. [Google Scholar]
- Ragavan, P.; Ananth, A.; Rajan, M.R. Impact of Selenium Nanoparticles on Growth, Biochemical Characteristics and Yield of Cluster Bean Cyamopsis tetragonoloba. J. Environ. Agric. Biotechnol. 2017, 2, 2917–2926. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Kannan, N.; Manokari, M.; Ravidran, C.P. In vitro regeneration of shoots and ex-vitro rooting of an important medicinal plant Passiflora foetida L through segment cultures. J. Genetic Eng. Biotechnol. 2015, 13, 209–214. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yieemwattana, S. Role of specific plant characteristics on thermal and carbon sequestration properties of living walls in tropical climate. Build. Environ. 2017, 115, 67–79. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Imran, M.; Rana, M.S.; Jiang, C. Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol. Environ. Safety 2018, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, T.D.A.; Barcala-Jorge, A.S.; Andrade, J.M.O.; Ferreira, E.C.N.; Crespo, T.S.; Batista-Jorge, G.C.; Vieira, C.A.; Lelis, D.D.F.; Paraíso, A.F.; et al. Obesity and malnutrition similarly alter the renin–angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017, 48, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. J. Adv. Appl. Sci. Technol. 2014, 1, 89–93. [Google Scholar]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V. Biogenic Synthesis and Characterization of Selenium Nanoparticles Using the Flower of Bougainvillea spectabilis Willd. Int. J. Sci. Res. 2015, 4, 690–695. [Google Scholar]
- Surega, R. Green Synthesis of Bioactive Silver Nanoparticles Using Plant Extracts and Their Antinemic Properties. Ph.D. Thesis, Dept of Nematology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University Coimbatore, Coimbatore, India, 2015. [Google Scholar]
- Zhang, J.-J.; Li, Z.; Zhao, S.; Lu, Y. Size-dependent modulation of graphene oxide–aptamer interactions for an amplified fluorescence-based detection of aflatoxin B1 with a tunable dynamic range. Analyst 2016, 141, 4029–4034. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Liu, Y.-G.; Jiang, L.-P.; Zhu, J.-J. Synthesis, characterization of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin. Electrochem. Commun. 2008, 10, 355–358. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Gu, M.-M.; Zheng, T.-T.; Zhu, J.-J. Synthesis of Gelatin-Stabilized Gold Nanoparticles and Assembly of Carboxylic Single-Walled Carbon Nanotubes/Au Composites for Cytosensing and Drug Uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P.; Dong, L.; Ruan, Y.; Lu, H. Highly conductive, mechanically strong graphene monolith assembled by three-dimensional printing of large graphene oxide. J.Col. Interf. Sci. 2019, 534, 12–19. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.-M.; Zhu, W.-Y.; Zhang, C.-H.; Tang, H.; Jiang, J.-H. Conjugated polymer nanoparticles-based fluorescent biosensor for ultrasensitive detection of hydroquinone. Anal. Chim. Acta 2018, 1012, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, S.; Sen, S. Rapid ultrasound assisted hydrothermal synthesis of highly pure nanozeolite X from fly ash for efficient treatment of industrial effluent. Chemosphere 2018, 210, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Neena, G.; Venugopal, B.; Honey, J.; Mathiazhagan, A.; Rani, J. Nanosilica decorated multiwalled carbon nanotubes (CS hybrids) in natural rubber latex. Polymer 2019, 161, 170–180. [Google Scholar]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Fortner, J.D.; Sayes, C.M.; Colvin, V.L.; Hughe, J.B. Bacterial Cell Association and Antimicrobial Activity of a C 60 Water Suspension. Environ. Toxicol. Chem. 2005, 24, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- AG Chemgroup. Study Finds Potential Use for Zinc Oxide Nanoparticles for Fertilizer Feedstock; AG Chemgroup: Praha, Czech Republic, 2017. [Google Scholar]
- de Madrid, U.P. Will we be able to use zinc oxide nanoparticles as fertilizers. Science Daily, 11 October 2017. [Google Scholar]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef]
- Zhang, D.; Hua, T.; Xiao, F.; Chen, C.; Gersberg, R.M.; Liu, Y.; Stuckey, D.; Ng, W.J.; Tan, S.K. Phytotoxicity and bioaccumulation of ZnO nanoparticles in Schoenoplectus tabernaemontani. Chemosphere 2015, 120, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, F.; Armengaud, P.; Seditas, T.J.; Danku, J.; Salt, D.E.; Amtmann, A. Analysis of the Root System Architecture of Arabidopsis Provides a Quantitative Readout of Crosstalk between Nutritional Signals. Plant Cell 2014, 26, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Yao, J.; Sun, J.; Zhang, C.; Liu, W.; Zhu, M.; Ceccanti, B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015, 94, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Joan, E.; McLean, D.E.; Latta, E.M. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation and induction of oxidative stress in sand-grown wheat. Nanoparticle Res. 2012, 14, 1125–1140. [Google Scholar] [CrossRef]
- Tang, Y.; He, R.; Zhao, J.; Nie, G.; Xu, L.; Xing, B. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ. Pollut 2016, 212, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Wu, G.R.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 22, 168–172. [Google Scholar]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Yanga, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Rico, C.M.; Morales, M.I.; Barrios, A.C.; McCreary, R.; Hong, J.; Lee, W.Y.; Nunez, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J. Agric. Food Chem. 2013, 61, 11278–11285. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).