A New Approach for Economical Pretreatment of Corncobs
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Analytical Method
2.2.1. Analysis of the Soluble Protein
2.2.2. Analysis of the Metal Ions
2.2.3. Analysis of Corncob Surface Morphology
2.3. Experimental Method
3. Results and Discussion
3.1. Effect of Reuse of Pretreatment Solution on Soluble Protein Removal in Corncob
3.1.1. Effect of pH Change of the Pretreatment Solution on Soluble Protein Removal in Corncob
3.1.2. Effect of Reuse Round of Pretreatment Liquid on Soluble Protein Removal in Corncob
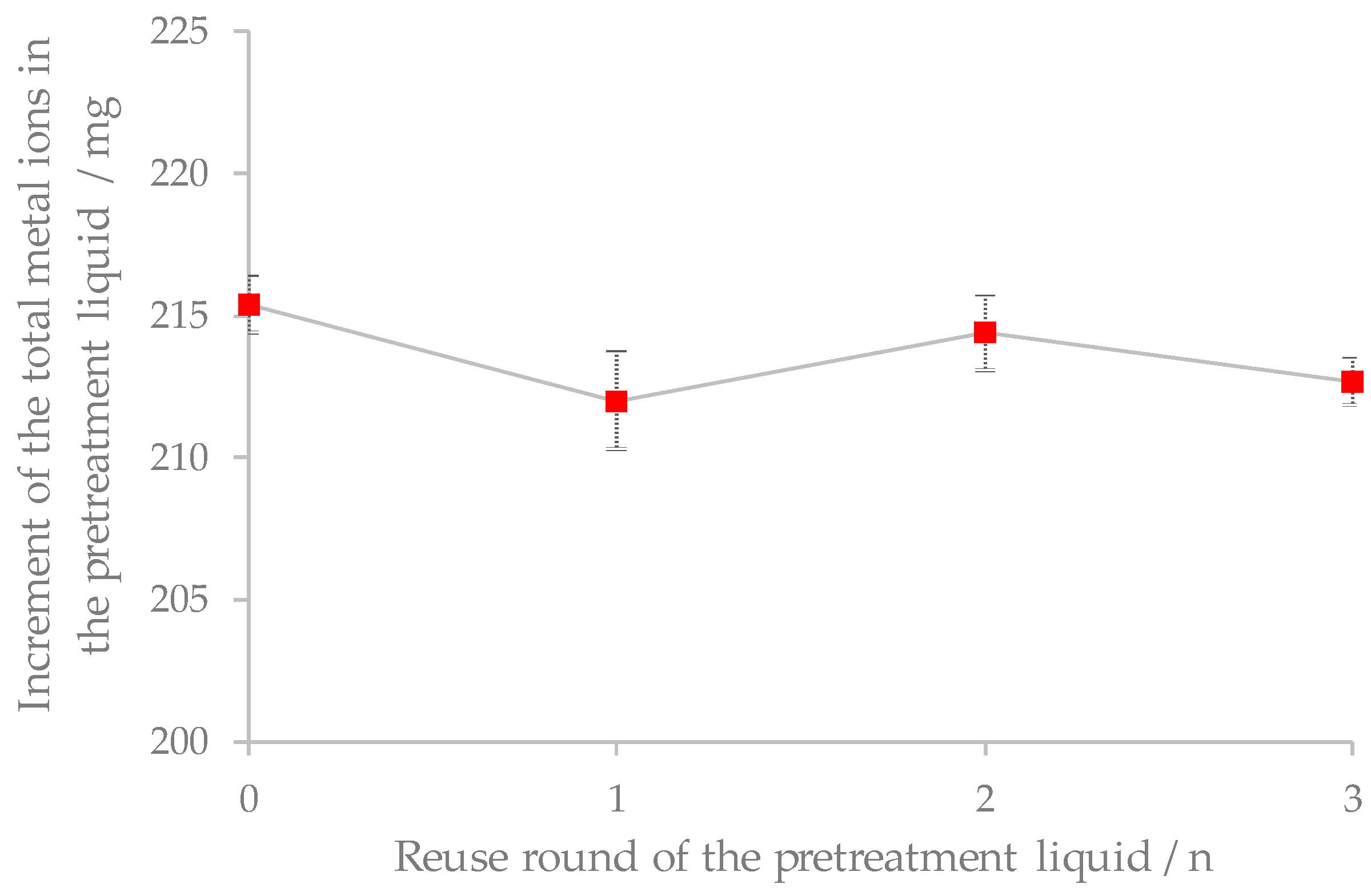
3.2. Effect of Reuse of Pretreatment Liquid on Removal of Metal Ions in Corncob
3.3. Selection of Reuse Times of the Corncob Pretreatment Liquid
3.4. Morphology Comparison of the Corncob after Pretreatment Liquid and New Liquid Pretreatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised Heterogeneous Catalysts for Sustainable Biomass Valorization. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef] [PubMed]
- Ambursa, M.M.; Ali, T.H.; Lee, H.V.; Sudarsanam, P.; Bhargava, S.K.; AbdHamid, S.B. Hydrodeoxygenation of Dibenzofuran to Bicyclic Hydrocarbons Using Bimetallic Cu–Ni Catalysts Supported on Metal Oxides. Fuel 2016, 180, 767–776. [Google Scholar] [CrossRef]
- Yu, N.; Zhu, Z.Y.; Liu, Y.; Zhang, J.Y.; Zhang, Y.M. Chromatographic Analysis and Preparation of l-arabinose from Corncob by Acid Hydrolysis. Ind. Crops Prod. 2017, 95, 163–169. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of Hydroxymethylfurfural from Cellulose using Green Processes: A Promising Biochemical and Biofuel Feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Meena, S.; Navatha, S.; Devi Prabhavathi, B.L.A.; Prasad, R.B.N.; Pandey, A.; Sukumaran, R.K. Evaluation of Amberlyst15 for Hydrolysis of Alkali Pretreated Rice Straw and Fermentation to Ethanol. Biochem. Eng. J. 2015, 102, 149–165. [Google Scholar] [CrossRef]
- Mardawati, E.; Andoyo, R.; Syukra, K.A.; Kresnowati, M.; Bindar, Y. Production of Xylitol from Corn Cob Hydrolysate through Acid and Enzymatic Hydrolysis by Yeast. Earth Environ. Sci. 2018, 141, 12–19. [Google Scholar] [CrossRef]
- Joshi, S.S.; Zodge, A.D.; Pandare, K.V.; Kulkarni, B. Efficient Conversion of Cellulose to Levulinic Acid by Hydrothermal Treatment Using Zirconium Dioxide as a Recyclable Solid Acid Catalyst. Ind. Eng. Chem. Res. 2014, 53, 18796–18805. [Google Scholar] [CrossRef]
- Lu, T.; Hou, Y.C.; Wu, W.Z.; Niu, M.G.; Wang, Y.P. Formic Acid and Acetic Acid Production from Corn Cob by Catalytic Oxidation Using O2. Fuel Process. Technol. 2018, 171, 133–139. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Han, D.; Zhang, W.; Zhang, Z.; Ye, X.; Tian, L.; Dong, Y.; Zhu, Q.; Chen, Y. Correlation of Hepatitis B Surface Antigen Level with Response to Telbivudine in Naive Patients with Chronic Hepatitis B. Hepatol. Res. 2014, 44, 187–193. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Stohs, S. Inhibition by Natural Dietary Substances of Gastrointestinal Absorption of Starch and Sucrose in Rats and Pigs: 1. Acute Studies. Int. J. Med. Sci. 2007, 4, 196–202. [Google Scholar] [CrossRef]
- El-Farargy, A.F.; Ghonium, A.A. Synthesis of some New C-Nucleosides from L-Arabinose and D-Glucose. ARKIVOC 2008, 13, 278–285. [Google Scholar]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernández, B.; Penedo, S. Efficient Conversion of Levulinic Acid into Alkyl Levulinates Catalyzed by Sulfonic Mesostructured Silicas. Appl. Catal. A 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of Pretreatment and Downstream Processing Technologies on Economics and Energy in Cellulosic Ethanol Production. Biotechnol. Biofuels 2011, 4, 27–46. [Google Scholar] [PubMed]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.E.; Rausch, K.D.; Sinqh, V. Improvement of Sugar Yields from Corn Stover Using Sequential Hot Water Pretreatment and Disk Milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.B.; Liu, R.H.; Yu, H.Q.; Chen, H.Z.; Yu, B.; Harada, H.; Li, Y.Y. Enhanced Anaerobic Ruminal Degradation of Bulrush through Steam Explosion Pretreatment. Ind. Eng. Chem. Res. 2008, 47, 5899–5905. [Google Scholar] [CrossRef]
- Keshav, P.K.; Naseeruddin, S.; Rao, L.V. Improved Enzymatic Saccharification of Steam Exploded Cotton Stalk Using Alkaline Extraction and Fermentation of Cellulosic Sugars into Ethanol. Bioresour. Technol. 2016, 214, 363–370. [Google Scholar] [CrossRef]
- Narra, M.; Balasubramanian, V.; James, J.P. Enhanced Enzymatic Hydrolysis of Mild Alkali Pre-Treated Rice Straw at High-Solid Loadings Using in-House Cellulases in a Bench Scale System. Bioprocess. Biosyst. Eng. 2016, 39, 993–1003. [Google Scholar] [CrossRef]
- Qin, W.J.; Chen, Y.F.; Zhao, H.Y.; Wang, R.S.; Xiao, D.G. Optimization of Pretreatment Conditions for Corn Cob with Alkali Liquor. Trans. Chin. Soc. Agric. Eng. 2010, 26, 248–253. [Google Scholar]
- Lu, Y.J.; Guo, L.J.; Zhang, X.M.; Ji, C.M. Hydrogen Production by Supercritical Water Gasification of Biomass: Explore the Way to Maximum Hydrogen Yield and High Carbon Gasification Efficiency. Int. J. Hydrog. Energy 2012, 37, 3177–3185. [Google Scholar] [CrossRef]
- Shi, J.; Sharma-Shivappa, R.R.; Chinn, M.; Howell, N. Effect of Microbial Pretreatment on Enzymatic Hydrolysis and Fermentation of Cotton Stalks for Ethanol Production. Biomass Bioenergy 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M.; Karim, M.R. Production of Xylose from Meranti Wood Sawdust by Dilute Acid Hydrolysis. Appl. Biochem. Biotechnol. 2014, 174, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, M.; Fan, X.G.; Zhu, X.T.; Xu, T.; Yuan, Q.P. An Environmentally Friendly and Efficient Method for Xylitol Bioconversion with High Temperature Steaming Corncob Hydrolysate by Adapted Candida Tropicalis. Process Biochem. 2011, 46, 1619–1626. [Google Scholar] [CrossRef]
- Pointner, M.; Kuttner, P.; Obrlik, T.; Jäger, A.; Kahr, H. Composition of Corncobs as a Substrate for Fermentation of Biofuels. Agron. Res. 2014, 12, 391–396. [Google Scholar]
- Narra, M.; Macwan, K.; Vyas, B.; Harijan, M.R.; Shah, D.; Balasubramanian, V.; Prajapati, A. A Bio-refinery Concept for Production of Bio-methane and Bio-ethanol from Nitric Acid Pre-treated Corncob and Recovery of a High Value Fuel from a Waste Stream. Renew. Energy 2018, 127, 1–10. [Google Scholar] [CrossRef]
- Guo, L.; You, W.N.; Zhao, X.; Hu, Y.D.; Wu, L.Y. Pretreatment Technology of Desalination and Deproteinization of Corn Cobs. Chem. Ind. Eng. Prog. 2017, 36, 1927–1932. [Google Scholar]
- Deng, L.L.; Pan, X.Q.; Sheng, J.P.; Shen, L. Optimization of experimental conditions for the determination of water-soluble protein in apple pulp using coomassie brilliant blue method. Food Sci. 2009, 33, 185–189. [Google Scholar]



| Reuse of Pretreatment Liquid (n) | Volume of Previous Used Liquid (mL) | Volume of Deionized Water (mL) | Solution pH after Pretreatment | Concentration of the Soluble Protein (mg·L−1) | Increment of the Soluble Proteins (mg) |
|---|---|---|---|---|---|
| 0 | 0 | 300 | 3.1 | 228 | 68.4 |
| 1 | 225 | 75 | 3.2 | 395 | 67.2 |
| 2 | 228 | 72 | 3.1 | 532 | 69.5 |
| 3 | 226 | 74 | 3.1 | 628 | 68.2 |
| Reuse Times of the Pretreatment Liquid/(n) | Concentration of Metal Ions after Pretreatment/(mg·L−1) | Increment of Metal Ions after Pretreatment/(mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Mg2+ | Na+ | Total Amount | K+ | Ca2+ | Mg2+ | Na+ | Total Amount | |
| 0 | 638.3 | 59.3 | 16.9 | 3.2 | 717.7 | 191.5 | 17.8 | 5.1 | 1.0 | 215.4 |
| 1 | 1107.6 | 102.2 | 29.1 | 6.0 | 1244.9 | 188.7 | 17.3 | 4.9 | 1.1 | 212.0 |
| 2 | 1477.1 | 136.7 | 39.1 | 7.7 | 1660.6 | 190.6 | 17.7 | 5.1 | 1.0 | 214.4 |
| 3 | 1743.6 | 161.2 | 46.2 | 9.1 | 1960.1 | 189.2 | 17.5 | 5.0 | 1.0 | 212.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, Y.; Qi, P.; Guo, L. A New Approach for Economical Pretreatment of Corncobs. Appl. Sci. 2019, 9, 504. https://doi.org/10.3390/app9030504
Wang Y, Hu Y, Qi P, Guo L. A New Approach for Economical Pretreatment of Corncobs. Applied Sciences. 2019; 9(3):504. https://doi.org/10.3390/app9030504
Chicago/Turabian StyleWang, Yan, Yanci Hu, Pengfei Qi, and Lei Guo. 2019. "A New Approach for Economical Pretreatment of Corncobs" Applied Sciences 9, no. 3: 504. https://doi.org/10.3390/app9030504
APA StyleWang, Y., Hu, Y., Qi, P., & Guo, L. (2019). A New Approach for Economical Pretreatment of Corncobs. Applied Sciences, 9(3), 504. https://doi.org/10.3390/app9030504





