Abstract
In this work, we developed a portable device with low production and operation costs for generating an ambient air low-current arc (AALCA) that is transferred to the surface of a treated liquid. It was possible to generate a stable discharge, irrespective of the conductivity of the treated liquid, as a sequence of corona, repeating spark, and low-current arc discharges. The estimated concentration of reactive oxygen and nitrogen species (RONS) in plasma-treated water (PTW) produced using AALCA treatment was two orders of magnitude higher than that of PTW produced using conventional He nonequilibrium atmospheric pressure plasma jets or dielectric barrier discharges. The strong bactericidal effect of the treatment using AALCA and the water treated using AALCA was confirmed by survival tests of Escherichia coli. Further, the possibility of treating a continuous flow of liquid using AALCA was demonstrated.
1. Introduction
Recently, treatment of liquids using various atmospheric pressure plasmas has attracted much attention owing to its wide range of possible applications, such as synthesis of nanocarbons and nanoparticles, sterilization of microorganisms, growth enhancement of plants, plasma farming, cancer therapy, and water purification [1,2,3,4,5,6,7,8,9,10,11,12,13]. Typically, low-temperature nonequilibrium atmospheric pressure plasma jets (NEAPPJs) or dielectric barrier discharges (DBDs) are used for activation of liquids by plasma [5,12,13,14,15,16]. However, in both the case of atmospheric pressure plasma jet (APPJ) and DBD, the production rate of reactive oxygen and nitrogen species (RONS), which play key roles in bactericidal effect and plant growth promotion, is low and irradiation of the liquid takes a long time (from several minutes to hours) due to the low density of the plasma [5,12,13,14,15,16,17,18,19,20,21]. Moreover, in most cases, plasma is generated using pure gases (e.g., argon, helium, or oxygen) at a high voltage, so a substantial amount of electrical energy is required to sustain the discharge and only a limited volume (typically several milliliters) of medium can be treated at one time [4,7,17,19,20,21,22]. The low efficiency, the requirement of pure gases, and the limited volume of liquid that can be treated make plasma-treated water (PTW) expensive and its use in medicine and agriculture impossible. The most promising method for effective and low-cost production of PTW is treating liquids with high-density plasma generated by using air as a process gas [3,18,23,24,25,26]. Furthermore, it may be possible to scale up production and reduce the cost of PTW by applying plasmas generated in ambient air to treat a continuous flow of liquid. Several works have reported on treating a liquid flow using air as the process gas, however, the major drawback of these studies is the considerably low production rate of RONS, which limits the wide application of the produced PTW [13,15,18,27,28].
Typically, cold nonequilibrium plasmas are used for biomedical and agricultural applications to avoid thermal damage of the treated target [12,13,29,30]. However, in the case of using liquids treated by plasma, a plasma-treated medium (PTM) could be stored after treatment to reduce the temperature and for use after storage considering the presence of the long-living RONS [31,32]. For that reason, thermal and warm plasmas could be used for irradiation of medium following the cooling of PTM and use in biomedical applications. Considering the high efficiency of arc or gliding arc discharges in the decomposition of CO2 and other gases and the effective production of RONS, this type of discharge could also be employed for the effective generation and fast delivery of RONS to the target liquid [3,25,33,34]. There are many works on the generation of arc and discharges and other types of plasmas inside or on the surface of highly conductive liquids using various configurations and experimental conditions, however, generation of high-density plasma transferred to the surface of deionized water (DI) or other liquids with low electrical conductivity in ambient air is still challenging owing to the relatively high electric currents required for the stable generation of the discharge [10,11,18,23,35,36,37,38,39]. It appears that it is possible to generate a low-current arc in ambient air that can be transferred to the surface of a liquid that has low electrical conductivity by using a high-voltage pin electrode as the anode and the target liquid as the cathode. In this work, we developed a simple and compact generator for direct treatment of liquids using an ambient air low-current arc (AALCA) and analyzed the discharge process and production rate of RONS.
2. Materials and Methods
2.1. Experimental Setup
The experimental setup is presented in Figure 1. A plasma discharge was generated in ambient air between the needle electrode (cut of stainless-steel wire, 751487, Nilaco, Tokyo, Japan) and the surface of the liquid by applying a positive high voltage to a needle electrode (anode). A grounded ring electrode (Ni wire, NI-311386, Nilaco, Tokyo, Japan) was introduced into the liquid, and during the discharge, the grounded liquid was used as a cathode. Using the needle electrode as the anode allowed us to avoid strong heating and melting of the electrode.
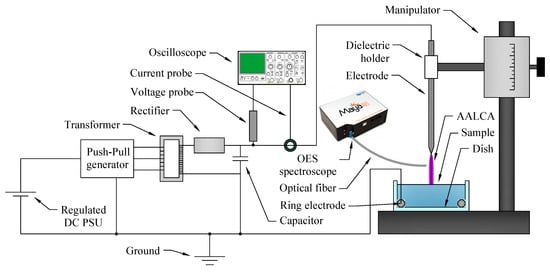
Figure 1.
Experimental setup.
A high voltage was produced by a custom power supply consisting of a regulated direct current power supply (DC PSU), a push–pull generator, a high-voltage transformer, a diode rectifier, and a reservoir capacitor. The voltage and current waveforms during the discharge were monitored using a high-voltage probe (P6015A, Tektronix, Beaverton, OR, USA) and a current probe (2877, Pearson Electronics Inc., Palo Alto, CA, USA), and stored in a digital oscilloscope (TBS2000, Tektronix Beaverton, OR, USA). The discharge gap between the surface of the liquid and the pin of the needle electrode was set at 8.5 mm using a micromanipulator.
The optical emission spectrum from the plasma was recorded using a multichannel spectrometer (Maya 2000 Pro, Ocean Optics, Largo, FL, USA) with a measurement range of 200–665 nm and a quartz optical fiber (QP1000-2-SR, Ocean Optics, Largo, FL, USA).
2.2. Investigation of Properties of the Treated Water
DI water (18.2 MΩ∙cm at 25 °C) was produced by a Direct-Q 3UV (Millipore, Tokyo, Japan) system. To compare the performance of the developed AALCA to commonly used plasma sources, DI water was treated using AALCA, a commercially available NEAPPJ (PN-110/120TPG, NU Global Co., Ltd., Nagoya, Japan) [40], and a high-density nonequilibrium atmospheric pressure radical source (NEAPRS, Tough Plasma FPA10, Fuji MFG Co., Ltd., Tokyo, Japan) [4,31,41].
The concentration of H2O2 in the produced PTW was measured using high-performance liquid chromatography (HPLC) with an electrochemical detector (ECD-700s, Eicom, Kyoto, Japan) and a column (5020-07146 Inertsil CX, 5 µm, 4.6 × 250 mm, GL Sciences, Torrance, CA, USA). Concentrations were estimated from the measured HPLC spectra of PTW using the calibration curve obtained from H2O2 (081-04215, FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) diluted to various concentrations.
Concentrations of NO2− and NO3− were measured using ion chromatography with a conductivity detector (CDD-10Avp, Shimadzu, Kyoto, Japan) and a column (Shim-pac IC-A3, 4.6 × 150 mm, Shimadzu, Kyoto, Japan). Concentrations were estimated from data measured by the ion chromatography spectra of PTW using the calibration curve obtained from a reference solution (228-33603-93, Shimadzu, Kyoto, Japan) diluted to various concentrations. The composition of the reference solution is presented in Table 1.

Table 1.
Composition of reference solution.
The pH value of PTW was measured using a pH meter (S SevenCompactTM pH/Ion, METTLER TOLEDO, Columbus, DC USA) and a probe (InLab Pure Pro-ISM, METTLER TOLEDO, Columbus, DC, USA). The pH meter and probe were calibrated using a standard buffer solution set (101-S, Horiba, Kyoto, Japan). The conductivity of PTW was measured using a conductivity measurement cell (LAQUA 3551-10D, Horiba, Kyoto, Japan).
2.3. Microorganism Culturing and Colony Counting
A survival test of Escherichia coli was performed to evaluate the bactericidal efficacy of direct treatment using AALCA and the bactericidal effect of the produced PTW. E. coli (O1:K1:H7) was precultured in 3 mL of nutrient broth (DifcoTM, BD, San Jose, CA, USA) at 250 rpm with a regulated temperature of 30 °C for 17 h. Then, E. coli was separated by centrifugation at 5000 × g for 3 min and diluted with DI water to obtain a concentration of bacteria of approximately 1 × 107 mL−1.
To investigate the bactericidal effect of treatment using AALCA, the prepared E. coli suspension was treated with AALCA under various conditions. To investigate the bactericidal effect of the produced PTW, a water sample was treated with AALCA and a 0.3 mL E. coli suspension was mixed with 2.7 mL of the produced PTW and incubated at 250 rpm with a regulated temperature of 30 °C for up to 24 h. The 0.3 mL E. coli suspension was mixed with 2.7 mL of pristine DI water as the control.
Then, the analyzed suspensions of E. coli were diluted, and samples of 0.1 mL were spread onto nutrient agar medium (DifcoTM, BD, San Jose, CA, USA) and cultured at 37 °C for 24 h. The number of E. coli that survived after the treatment was estimated using a colony-forming units (CFU) assay.
3. Results and Discussion
3.1. Generation of the Discharge
It was found that it was possible to generate various types of discharges between the pin electrode and the liquid depending on the input power. In the case of a low input power of 6 W supplied to the push–pull generator, it was possible to generate a stable positive corona. During the corona discharge, the current was negligible, resulting in a charge of the reservoir capacitor at the high-voltage side and stabilization of the voltage at about 11.25 kV, as shown in Figure 2a. A photo of the corona discharge is shown in Figure 2b. With an increase in input power to 6.5 W, the voltage during the corona discharge increased to about 12 kV, resulting in the generation of occasional spark discharges. The moment of breakdown and the number of consequent spark discharges were random. After breakdown, the voltage dropped to 1–2 kV and a short current pulse was observed during the spark discharge. After termination of the spark discharge, the current dropped to 0 following oscillation and the voltage increased to the initial value. Owing to a low input power, it took several tens of milliseconds to charge the reservoir capacitor and reach the initial voltage. A further increase of power resulted in an increase in the number of occasional spark discharges, and finally at an input power of 9.5 W, consequent spark discharges were observed, as shown in Figure 2c,d. In the case of the 9.5 W input power, the spark discharges were similar to those observed for the lower input powers. On the basis of the current and voltage (I and V) waveforms, the type of discharge was the same. Some variation of peak current and voltage and frequency observed in the I and V waveforms were related to the migration of the discharge spot on the surface of the liquid. In the case of repeating spark discharges, the sparks had a scanning area of about 5 mm in diameter on the surface of the liquid, as shown in Figure 2d.

Figure 2.
I & V waveforms (a) and photo (b) of corona discharge, I and V waveforms (c) and photo (d) of spark discharges at 9.5 W of input power, I and V waveforms (e) and photo (f) of spark discharges at 23 W of input power, and I & V waveforms (g) and photo (h) of ambient air low-current arc (AALCA) at 25 W of input power.
A further increase of power up to 23 W did not change the discharge type and the same type of repeating sparks was observed, however, the increase of the input power resulted in some changes of the current and voltage waveforms, as shown in Figure 2e. An increase of input power resulted in an increase of peak current during the spark discharge, which decreased the time required for charging the reservoir capacitor, causing an increase of frequency of the spark discharges. On the other hand, the increase of input power resulted in stabilization of the spot of the spark and the repeating spark discharges looked like steady glow plasma (Figure 2f).
Increasing the input power to values above 23 W resulted in the occasional formation of low-current arc discharges, and a stable repulsing low-current arc discharge was generated at an input power of 25 W. Compared with the repeating spark discharges, the repulsing low-current arc discharge featured a lower peak voltage of about 2.5 kV, continuous current during the discharge, and high stability of the current and voltage waveforms, as shown in Figure 2g. A photo of AALCA at the input power of 25 W is presented in the Figure 2h. In the present setup, a low-pass filter was not employed, resulting in an AC ripple of voltage, which can be observed in Figure 2e,g.
In the case of the direct application of an input power of 25 W, the discharges occurred in the same order as described above, that is, as a sequence of a corona discharge, repeating sparks with increasing frequency, and finally, a low-current arc discharge. At the moment of voltage application, a corona discharge was generated first and was followed by repeating spark discharges for several seconds. Corona and spark discharges delivered RONS and ions originating from the air, evaporated liquid, and electrodes to the liquid, resulting in an increase of the electric conductivity of the liquid in a similar way to what was reported elsewhere [18,24,42]. With the increase of the conductivity of treated DI water, occasional pulsed arc discharges appeared and finally, after 17–20 s of treatment, the discharge changed from repeating sparks to the stable AALCA.
Figure 3 shows the conductivity of 5 mL of DI water as a function of the duration of the treatment by AALCA. The conductivity of the water sample increased from about 0.1 µS/cm for pristine DI water to 190 µS/cm after treatment for 20 s by spark discharges, which enabled the generation of a low-current arc that was transferred to the surface of the DI water, which initially had low conductivity.
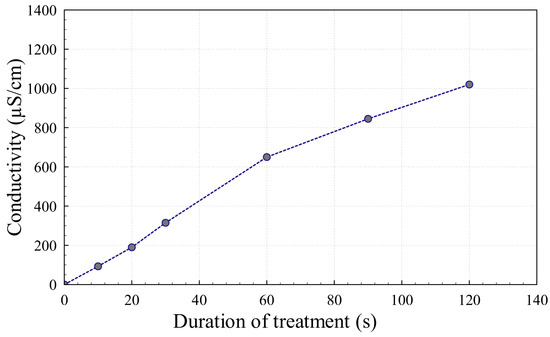
Figure 3.
Conductivity of deionized water (DI) water as a function of the duration of plasma treatment.
Moreover, the pH value gradually decreased from 6.76 for pristine DI water to 2.89 after treatment for 2 min, as shown in Figure 4, which is a considerably low value as compared with the PTW reported elsewhere [18,24,31,43]. The low pH value suggests a high concentration of RONS and possibly some ions (delivered from the electrodes by erosion) in the treated liquid, which could explain the increase of conductivity after the plasma treatment.

Figure 4.
pH value of DI and tap water as a function of the duration of plasma treatment.
The presented approach allows generation of a stable AALCA discharge that is transferred to the surface of a liquid, even if it has initially low electrical conductivity (such as DI water), without preparation and modification of the liquid. Variation of conductivity affected only the duration of the state of the repeating spark discharges (17–19 s for DI water with an initial conductivity of about 0.1 µS/cm and 0–2 s for tap water with an initial conductivity in the range of 170–250 µS/cm).
The main drawback of using AALCA is the heating and evaporation of the treated liquid, which is used as the cathode and interacts with high-energy ions and other charged species. Thermographic observations showed that the temperature of the 5 mL DI water sample treated by AALCA did not exceed 45 °C (see Figure 5a) after 1 min of treatment and 70 °C (see Figure 5b) after 5 min of treatment.
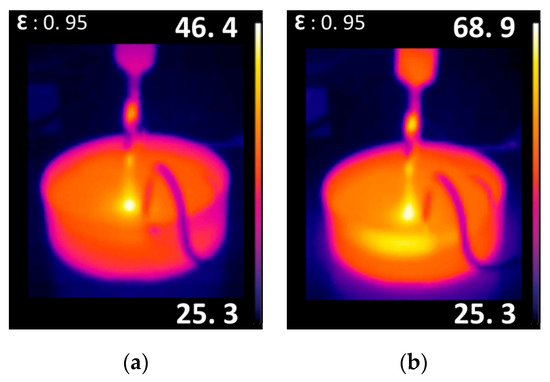
Figure 5.
Thermographic image of DI water treated with AALCA for 1 min (a) and 5 (b) min.
The relatively low temperature of the small volume of liquid treated with AALCA could be explained by local evaporation of the liquid. During treatment, the liquid was heated to the boiling temperature locally and evaporated when a bulk liquid was heated at a lower rate and remained below the boiling temperature. The higher temperature of the liquid in the area of contact with the plasma can be observed from thermographic images (Figure 5a,b).
It was confirmed that 1.2 mL of water was evaporated from the original 5 mL sample after 5 min of treatment. To ensure stable generation of AALCA even after partial evaporation of the liquid and change in the length of the discharge gap, 28 W of input power was used in all the experiments described below.
3.2. Species Generated in Gas Phase
Optical emission spectrometry (OES) in the wavelength region from 200 to 665 nm was used to investigate the main excited species generated by AALCA. OES spectra of AALCA is presented in Figure 6.
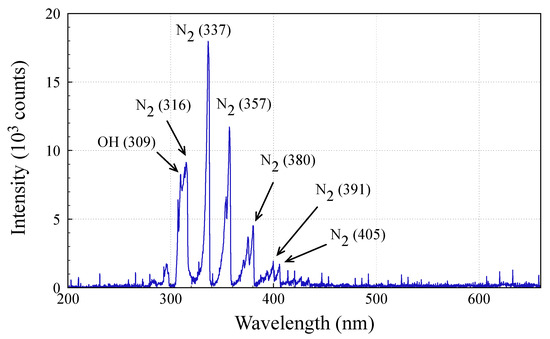
Figure 6.
Optical emission spectrometry (OES) spectra of AALCA.
Owing to the high concentration of nitrogen in the air, the observed spectrum was dominated by N2 (C→B) emissions as a result of many excitation processes in the plasma. As shown in Figure 6, the optical emissions from the N2 second positive system (C3Π → B3Π) at 316, 337, 357, 380, and 405 nm; the N2 + first negative system (B2Σu + → X2Σg +) at 391.4 nm; and the OH emissions from the transition of A2Σ+ → X2Π at 309 nm were clearly observed for AALCA [44]. Evaporation of the liquid discussed above could be the source of OH radicals, which could be produced by electron impact dissociation of water molecules [14]. Despite the presence of hydrogen from evaporated water, hydrogen emission lines (e.g., Hα and Hβ) were not observed in the spectra.
The observed OES spectrum that was dominated by N2 emissions was similar to emission spectra observed in other types of air plasmas transferred to the surface of water reported elsewhere [18,24]. The OES results indicated the presence of excited OH radicals and atomic nitrogen in the AALCA which could be delivered to the liquid from the gas phase and converted to other RONS in the solution, such as hydrogen peroxide (H2O2), nitrite (NO2−), and nitrate (NO3−).
3.3. Generation of RONS in Water
To check the production efficiency of RONS in the liquids treated with AALCA and compare it to commercially available plasma sources, samples of 5 mL DI water were treated with AALCA, NEAPPJ, and NEAPRS. The sample was treated with AALCA at an input power of 28 W for 30 and 60 s. NEAPPJ was operated using He gas with a flow rate of 3 slm and a distance from the nozzle of the plasma jet to the surface of the liquid of 20 mm, ensuring contact of plasma with liquid and the effective delivery of RONS. NEAPRS was operated with a mixture of Ar (4.75 slm), N2 (200 sccm), and O2 (50 sccm) at a total flow rate of 5 slm recommended by the manufacturer. The ratio of N2 to O2 was chosen to be similar to the ratio in air for reliable comparison to the plasma operated in ambient air. Estimated values of RONS concentrations are summarized in Figure 7.
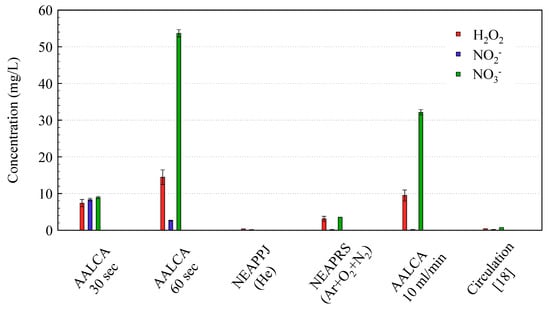
Figure 7.
Comparison of concentrations of reactive oxygen and nitrogen species (RONS) in plasma-treated water (PTW) produced using treatment of 5 mL of DI water with AALCA, nonequilibrium atmospheric pressure radical source (NEAPRS), and nonequilibrium atmospheric pressure plasma jets (NEAPPJ); treatment of 10 mL/min flow of DI water with AALCA in the present work; and air plasma treatment of circulating liquid reported elsewhere [18].
It was found that the concentrations of H2O2, NO2−, and NO3− were 7.4, 8.3, and 8.9 mg/L, respectively, after 30 s of irradiation by AALCA; moreover, the concentrations of H2O2 and NO3− further increased to 14.5 and 53.7 mg/L, respectively, when the concentration of NO2− decreased to 2.7 mg/L with an increase of the treatment duration to 60 s. Owing to the longer treatment duration, NO2− might convert to more stable NO3−, resulting in a change of concentration ratios. The observed concentrations were two orders of magnitude higher than those of 5 mL DI water treated for 1 min with commercially available NEAPPJ operated with He (0.37 mg/L of H2O2, 0.18 mg/L of NO2−, and 0.06 mg/L of NO3−). Additionally, considerably small concentrations of RONS in water treated using NEAPPJ (up to 890 µg/L of H2O2, up to 180 µg/L of NO2−, and up to 410 µg/L of NO3−) [20,21,22,45,46] and custom plasma jets operated with air or addition of air (up to 9.3 mg/L of H2O2, up to 4 mg/L of NO2−, and up to 5.5 mg/L of NO3−) [14,28,47,48] were reported elsewhere, confirming the high efficiency of the developed AALCA as compared with commonly used nonequilibrium plasma jets.
Considering the low power consumption of conventional NEAPPJ compared with AALCA, a more reliable comparison is to NEAPRS, which has a power consumption of about 50 W, which is comparable to AALCA. After treatment of 5 mL of DI water with NEAPRS for 1 min, the concentrations of H2O2, NO2−, and NO3− were 3.1, 0.2, and 3.6 mg/L, respectively, which was one order of magnitude lower than the 5 mL of DI water treated with AALCA for 1 min.
3.4. Inactivation of E. coli
There are numerous reports about cancer therapy, disinfection, and inactivation of fungi using PTW, and the high concentration of RONS in PTW produced by AALCA looks promising for many such applications. To confirm the bactericidal effect of direct treatment using AALCA, a suspension containing E. coli in a concentration of 107 mL−1 was treated with plasma. The number of surviving E. coli was estimated using the colony counting method and the results are presented in Figure 8.
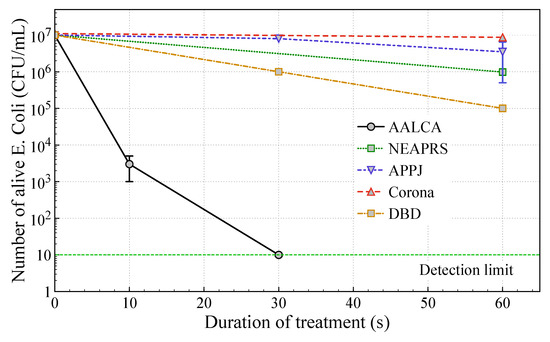
Figure 8.
Number of surviving E. coli after treatment by AALCA, NEAPRS, NEAPPJ [43], dielectric barrier discharges (DBD) [49] or corona [50], discharges for various time of irradiation.
It was found that the number of surviving E. coli was about 2 × 103 mL−1 after direct plasma treatment for 10 s and decreased to the colony counting method detection limit of 10 mL 1 after 30 s, showing a 6-log reduction in the concentration of bacteria. As shown in Figure 8, the bactericidal effect of treatment with AALCA was significantly higher than the bactericidal effect of treatment with NEAPRS and APPJ, corona, or DBD discharges reported elsewhere [9,43,49,50,51,52,53,54,55,56]. It was confirmed by thermographic observation that the suspension was heated by irradiation to temperatures up to 38 °C, which is below the temperature harmful for E. coli reported elsewhere, suggesting that heating of the sample was not involved in the sterilization process [57]. On the other hand, the low pH value of about 3.4 after treatment of DI water by AALCA for 30 s could be effective for inactivation of bacteria [43]. To investigate the effect of temperature and pH on killing the bacteria, E. coli was suspended for 30 s in DI water heated to 40 °C (to ensure that the temperature of the suspension was higher than the temperature of the sample treated by AALCA for 30 s), citrate-phosphate buffer solution with a pH of 3.3 (similar to the pH observed for DI water treated by AALCA for 30 s), and citrate-phosphate buffer solution with a pH of 3.3 heated to 40 °C. Then, the number of surviving bacteria was estimated using the colony counting method. It was found that the concentration of bacteria did not change after suspension for 30 s in DI water heated to 40 °C or citrate-phosphate buffer solution with a pH of 3.3; however, a 1-log reduction in the concentration of E. coli was observed in the case of suspension in the citrate-phosphate buffer solution with a pH of 3.3 that was heated to 40 °C, indicating that the combination of heating the sample and reducing the pH value during AALCA treatment could contribute to observed bactericidal effect.
On the other hand, the high concentration of RONS in the DI water treated by AALCA (Figure 7) could be the origin of the observed bactericidal effect. To investigate the effect of H2O2, NO2−, and NO3− on sterilization of the bacteria, a solution containing 7 mg/L of H2O2, 8 mg/L of NO2−, and 9 mg/L of NO3− (similar to the concentrations observed after treatment by AALCA for 30 s) was prepared using chemical reagents, and E. coli was suspended for 30 s in the prepared solution. It was found by the colony counting method that the concentration of E. coli did not change after suspension for 30 s in the solution containing H2O2, NO2−, and NO3−, indicating that the high concentration of long-living RONS did not contribute to the strong bactericidal effect observed after 30 s of treatment. However, in the case of direct treatment with AALCA, not only long-living RONS could be involved in the bactericidal effect but also short-living RONS (e.g., ONOO− and HOO●), the strong electric field at the stage of corona and repeating spark discharges, and ultraviolet emissions, as it was reported elsewhere [18,41,58,59].
On the other hand, a long-term bactericidal effect produced by long-living RONS in PTW was reported elsewhere, which suggests a strong long-term bactericidal effect of PTW produced by AALCA owing to its high concentration of RONS [31,32,43]. To investigate the bactericidal effect of PTW, 5 mL of DI water was treated with AALCA for 1 min at an input power of 28 W. Then, 0.3 mL of the E. coli suspension was mixed with 2.7 mL of the produced PTW and incubated for various times. After incubation, the number of surviving bacteria was estimated using the colony counting method. The estimated values of E. coli after various durations of incubation are presented in Figure 9. It could be clearly observed that the concentration of E. coli gradually decreased as the incubation time increased after suspension of E. coli in DI water treated with AALCA and reached the detection limit of 10 CFU/mL at 60 min, showing a 6-log reduction in the concentration of bacteria. Considering that the concentration of E. coli did not change in the control sample of E. coli suspended in pristine DI water even after 24 h of incubation, it could be concluded that the DI water treated with AALCA had a strong bactericidal effect that originated from the high concentration of RONS and the low pH level [31,32,43].
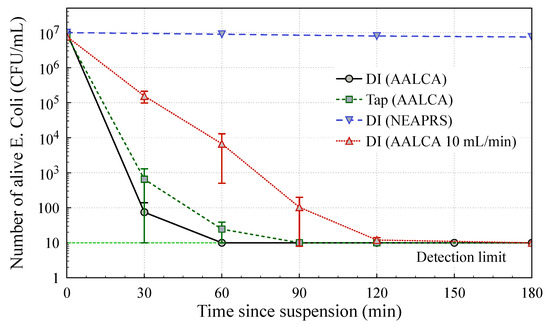
Figure 9.
Dependency of the number of surviving bacteria on the duration of incubation after mixture of E. coli suspension with DI or tap water treated with AALCA, DI water treated with NEAPRS, or DI water treated with AALCA at a 10 mL/min flow.
Treatment of liquid using AALCA could be performed irrespective of its electrical conductivity; therefore, further reduction of production costs is possible by using tap or spring water. Tap water was treated with AALCA under the same conditions and the bactericidal effect of plasma-treated tap water was investigated in the same way as for DI water. It can be clearly observed in Figure 9 that tap water treated by AALCA also had a bactericidal effect, and the concentration of bacteria reached the detection limit of 10 CFU/mL after 90 min, showing a 6-log reduction in the concentration of bacteria. Considering that the concentration of bacteria in the control sample of E. coli suspended in pristine tap water did not change after 90 min of incubation and reduced by 1 log only after 24 h of incubation, it can be concluded that, like with DI water, tap water treated with AALCA has a strong bactericidal effect that originates mostly from the high concentration of RONS. The difference in the bactericidal effect can be explained by the complex composition of tap water. Some components of tap water may interfere with chemical reactions in PTW and reduce the amount of species responsible for the bactericidal effect. Additionally, the pH value of the tap water was higher than that of DI water (Figure 4), which may have reduced the bactericidal efficacy of tap water treated with AALCA as compared with DI water [33,43].
The observed bactericidal effect of both DI and tap water, together with its low production cost, makes PTW produced by AALCA appear to be promising as a low-cost disinfectant which could be used in biomedical applications. However, the composition and properties of tap water can vary strongly depending on the area of use. Therefore, more comprehensive investigations are needed for the practical use of plasma-treated tap water in various locations.
3.5. Treatment of Flowing and Circulating Liquid
The evaporation of liquid reported above might be a problem for AALCA treatment; however, considering the high efficiency of RONS production and the moderate temperature of the liquid treated using the proposed method, AALCA treatment looks promising for practical PTW use. A possible approach to reduce the production cost of PTW and ameliorate the problems associated with heating and evaporation of the liquid is treatment by continuous flow of liquid. Treatment of a flow of DI water was performed using the same setup and two peristatic pumps, as shown in Figure 10. Pristine DI water was introduced to a dish at a flow rate of 10.25 mL/min using the first pump and PTW was evacuated from the dish at a flow rate of 10 mL/min using the second pump. The flow allowed for reducing the temperature of the liquid during treatment. It was found by thermographic measurements (Figure 10) that the temperature of the treated water was about 45 °C in the area of contact with plasma after 5 min of operation, which was significantly lower than the case of the 5 mL sample treatment. Figure 10 shows a thermographic image of plastic tubes with water on the input and output of the plasma treatment device. The temperature of the water increased from about 25 °C to about 30 °C during the treatment (similar results were confirmed by measurements using a thermocouple). The use of flow allows for a temperature of the produced PTW below 37 °C, which is safe for the human body and could be used in medical applications [47,60]. Additionally, the difference in the flow rate allowed for compensation of the evaporation of the liquid and retention of the same level of liquid inside the dish, ensuring the same discharge gap and parameters of the plasma during treatment.

Figure 10.
Schematic of experimental setup for treatment of continuous flow of liquid, thermographic image of flowing DI water treated with AALCA (right, bottom), and comparison of water temperatures in tubes before and after treatment (right, top).
The treated water was collected from the output of the second pump and concentrations of RONS in the collected PTW were estimated using HPLC and ion chromatography. Estimated concentrations of H2O2, NO2−, and NO3− in the produced PTW were 9.6, 0.2, and 32.2 mg/L, respectively, as shown in Figure 7. The pH value of the produced PTW was about 3.25. The production rate of RONS was comparable to the treatment of stationary samples of 5 mL. Despite the reduced efficiency of production of NO2−, the total amount of RONS in the case of the flow of water was still two orders of magnitude higher than the amounts observed for the case of conventional NEAPPJ or DBD [14,20,32,43]. Zhou et al. reported PTW production by cold plasma treatment at ambient air of 50 mL of circulating liquid at a flow rate of 200 mL/min [18]. It is difficult to perform a direct comparison of results considering the difference in the flow ratio of the liquid and the experimental setup. It would take 5 min to produce 50 mL of PTW using AALCA with a 10 mL/min flow of the water used in the present work; therefore, the results of the present work were compared to the results reported by Zhou et al. for 5 min of treatment (0.4 of H2O2, 0.2 of NO2−, and 0.75 of NO3−) [18]. It can be clearly observed in Figure 7 that treatment with AALCA is more effective than the method reported by Zhou et al., and treatment of a liquid flow can be applied to produce a large volume of PTW. However, the concentration of NO2− in the produced PTW was lower than that of the fast treatment of stationary liquids with small volumes, and the ratio of concentrations of NO2− and NO3− depends on the flow rate, duration of treatment, and discharge parameters.
To investigate the bactericidal effect of treatment of flowing liquid using AALCA, an E. coli suspension with a concentration of bacteria of 107 mL−1 was treated with AALCA at an input power of 28 W and a flow rate of 10 mL/min. It was found by the colony counting method that the concentration of bacteria decreased to the detection limit of 10 CFU/mL after treatment by AALCA at a flow rate of 10 mL/min, showing a 6-log reduction in the concentration of bacteria. This result demonstrates that treatment of a continuous flow of liquid by AALCA is applicable to disinfection of larger volumes of liquids.
To investigate the bactericidal effect of PTW produced by treatment of flowing liquid using AALCA, DI water was treated with AALCA at an input power of 28 W and a flow rate of 10 mL/min and collected in a clean vial. Then, 0.3 mL of the E. coli suspension was mixed with 2.7 mL of collected PTW and incubated for various times. After incubation, the number of surviving bacteria was estimated using the colony counting method. The estimated values of E. coli after various durations of incubation are presented in Figure 9. It can be clearly observed that the concentration of bacteria gradually decreased as the incubation time increased after suspension of E. coli to produce PTW, and it reached the detection limit of 10 CFU/mL at 180 min, showing a 6-log reduction in the concentration of bacteria. Considering that the concentration of E. coli did not change in the control sample of E. coli suspended in pristine DI water, it can be concluded that PTW produced by treatment of a continuous flow of liquid with AALCA has a strong bactericidal effect originating from the high concentration of RONS. The difference in bactericidal effect observed for the 5 mL sample and the flowing DI water can be explained by the difference in concentrations of RONS and the higher pH. In the case of flowing DI water, the concentration of NO2− was about 10 times lower and the pH was higher than the 5 mL of DI water treated by AALCA. Moreover, in the case of a flowing liquid, the concentrations of short-living reactive species could be different, resulting in a reduced bactericidal effect.
We found in this work that AALCA is effective at generating RONS and features low production and operation costs, which helps to overcome a number of problems in existing systems for plasma treatment of liquids. However, a major drawback of AALCA is the heating and evaporation of the liquid. We demonstrated that these problems can be solved by treatment of a continuous flow of liquid, which avoids changes in the discharge gap by evaporation and achieves a temperature safe for the human body that is below 37 °C after treatment [60]. The low temperature and high concentration of RONS in PTW produced by treatment of flowing liquid looks promising for biomedical and agricultural applications. Furthermore, production of PTW using a continuous flow of liquid could be easily scaled to large volumes, which looks promising for water disinfection and purification, as well as other applications requiring low-cost treatment of large volumes of liquid. Treatment of a liquid flow using AALCA allows for the low-cost production of PTW and solves several problems associated with using a stationary solution, however, further tuning of the discharge conditions and development of a custom pumping system is necessary for precise control of RONS concentrations in the produced PTW.
Additionally, the stable generation of AALCA was confirmed using liquids with various conductivities, such as DI water, tap water, ethanol, propylene glycol, phosphate buffer, phosphate saline buffer, and phosphate buffer containing amino acids. The possibility of generating such a type of plasma on the surface of a liquid irrespective of the electrical conductivity could be essential for various biomedical and agricultural applications, where buffer solutions with various conductivities are commonly used to control the pH level.
Moreover, the possibility of treating liquid precursors of various conductivities by AALCA looks promising for the synthesis of nanoparticles and nanocarbons [1,8,10,11].
4. Conclusions
In this work, we developed a compact tool for irradiation of liquids by a low-current arc at ambient air. It was possible to generate a stable discharge, even when using liquids that had low electrical conductivity, as a sequence of corona, repeating spark, and low-current arc discharges. Moreover, the possibility of treating a continuous flow of liquid was confirmed. It was found that the concentration of RONS after treatment using an ambient air low-current arc was two orders of magnitude higher than that of PTW produced using conventional APPJs or DBD plasmas under similar treatment conditions. The strong bactericidal effect of treatment with AALCA and the produced PTW was confirmed by survival tests of E. coli. Additionally, we demonstrated a method to overcome the overheating problem and increase the volume of produced PTW by treatment of a continuous flow of liquid. This work is a step towards the practical use of plasma-treated liquids in biomedical and agricultural applications.
Author Contributions
Experiments, investigation, and writing—review and editing, V.G.; survival test of E. coli, N.I.; resources, M.H. (Masaru Hori); funding acquisition, M.H. (Mineo Hiramatsu); validation and supervision, M.I.
Funding
This research was funded by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, grant number S1511021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hagino, T.; Kondo, H.; Ishikawa, K.; Kano, H.; Sekine, M.; Hori, M. Ultrahigh-speed synthesis of nanographene using alcohol in-liquid plasma. Appl. Phys. Express 2012, 5, 035101. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Cheng, X.; Gjika, E.; Keidar, M. Effects of cold atmospheric plasma generated in deionized water in cell cancer therapy. Plasma Process. Polym. 2016, 13, 1151–1156. [Google Scholar] [CrossRef]
- Gamaleev, V.; Iwata, N.; Oh, J.S.; Hiramatsu, M.; Ito, M. Development of an Ambient Air Flow Rotating Arc Jet for Low-Temperature Treatment. IEEE Access 2019, 7, 93100–93107. [Google Scholar] [CrossRef]
- Iwata, N.; Gamaleev, V.; Hashizume, H.; Oh, J.; Ohta, T.; Ishikawa, K.; Hori, M.; Ito, M. Simultaneous achievement of antimicrobial property and plant growth promotion using plasma-activated benzoic compound solution. Plasma Process. Polym. 2019, e1900023. [Google Scholar] [CrossRef]
- Zhang, J.; Kwon, T.; Kim, S.; Jeong, D. Plasma farming: Non-Thermal dielectric barrier discharge plasma technology for improving the growth of soybean sprouts and chickens. Plasma 2018, 1, 285–296. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, J.; Fang, J. In vitro studies of the antimicrobial effect of non-thermal plasma-activated water as a novel mouthwash. Eur. J. Oral Sci. 2017, 125, 463–470. [Google Scholar] [CrossRef]
- Amano, T.; Kondo, H.; Ishikawa, K.; Tsutsumi, T.; Takeda, K.; Hiramatsu, M.; Sekine, M.; Hori, M. Rapid growth of micron-sized graphene flakes using in-liquid plasma employing iron phthalocyanine-added ethanol. Appl. Phys. Express 2018, 11, 015102. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef]
- Saito, N.; Bratescu, M.A.; Hashimi, K. Solution plasma: A new reaction field for nanomaterials synthesis. Jpn. J. Appl. Phys. 2018, 57, 0102A4. [Google Scholar] [CrossRef]
- Gamaleev, V.; Kajikawa, K.; Takeda, K.; Hiramatsu, M. Investigation of nanographene produced by in-liquid plasma for development of highly durable polymer electrolyte fuel cells. C 2018, 4, 65. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, e1700073. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, R.; Bazaka, K.; Liu, Y.; Zhou, R.; Chen, G.; Chen, Z.; Qinghuo, L.; Yang, S.; Ostrikov, K.K. Quantification of plasma produced OH radical density for water sterilization. Plasma Process. Polym. 2018, 15, e1700241. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Cheng, C.; Xu, Z.; Xia, W. Generation of reactive species in atmospheric pressure dielectric barrier discharge with liquid water. Plasma Sci. Technol. 2018, 20, 44009. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, W.; Cheng, Y.; Lin, S.; Lim, T.M.; Xiong, J. Generation of reactive species by gas-phase dielectric barrier discharges. Ind. Eng. Chem. Res. 2011, 50, 9839–9846. [Google Scholar] [CrossRef]
- Xu, H.; Liu, D.; Wang, W.; Liu, Z.; Guo, L.; Rong, M.; Kong, M.G. Investigation on the RONS and bactericidal effects induced by He + O2 cold plasma jets: In open air and in an airtight chamber. Phys. Plasmas 2018, 25, 113506. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Oh, J.S.; Kakuta, M.; Furuta, H.; Akatsuka, H.; Hatta, A. Effect of plasma jet diameter on the efficiency of reactive oxygen and nitrogen species generation in water. Jpn. J. Appl. Phys. 2016, 55, 06HD01. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef]
- Ogawa, K.; Oh, J.; Gaur, N.; Hong, S.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D.; Ito, M.; Szili, E.J. Modulating the concentrations of reactive oxygen and nitrogen species and oxygen in water with helium and argon gas and plasma jets. Jpn. J. Appl. Phys. 2019, 58, SAAB01. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.J.; Joh, H.M.; Chung, T.H. Characterization of an atmospheric pressure plasma jet array and its application to cancer cell treatment using plasma activated medium. Phys. Plasmas 2018, 25, 073505. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhuang, J.; Zong, Z.; Zhang, X.; Liu, D.; Bazaka, K.; Ostrikov, K. Interaction of atmospheric-pressure air microplasmas with amino acids as fundamental processes in aqueous solution. PLoS ONE 2016, 11, e0155584. [Google Scholar] [CrossRef]
- Schmidt, M.; Hahn, V.; Altrock, B.; Gerling, T.; Gerber, I.C.; Weltmann, K.; Woedtke, T. Von Plasma-Activation of Larger Liquid Volumes by an Inductively-Limited Discharge for Antimicrobial Purposes. Appl. Sci. 2019, 9, 2150. [Google Scholar] [CrossRef]
- Gharagozalian, M.; Dorranian, D.; Ghoranneviss, M. Water treatment by the AC gliding arc air plasma. J. Theor. Appl. Phys. 2017, 11, 171–180. [Google Scholar] [CrossRef]
- Starek, A.; Pawłat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 2019, 9, 8407. [Google Scholar] [CrossRef]
- Hasan, M.I.; Walsh, J.L. The generation and transport of reactive nitrogen species from a low temperature atmospheric pressure air plasma source. Phys. Chem. Chem. Phys. 2018, 20, 28499–28510. [Google Scholar] [CrossRef]
- Joh, H.M.; Choi, J.Y.; Kim, S.J.; Chung, T.H. Effects of the pulse width on the reactive species production and DNA damage in cancer cells exposed to atmospheric pressure microsecond-pulsed helium plasma jets. AIP Adv. 2019, 26, 053509. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical application of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef]
- Lo, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Iwata, N.; Gamaleev, V.; Oh, J.S.; Ohta, T.; Hori, M.; Ito, M. Investigation on the long-term bactericidal effect and chemical composition of radical-activated water. Plasma Process. Polym. 2019, e1900055. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Kim, H.; Wright, K.C.; Hwang, I.; Lee, D.; Rabinovich, A.; Fridman, A.; Cho, Y.I. Effects of H2O2 and low pH produced by gliding Arc discharge on the inactivation of escherichia Coli in Water. Plasma Med. 2011, 1, 295–307. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Yan, J.; Li, X.; Wang, W.; Tu, X. Warm plasma activation of CO2 in a rotating gliding arc discharge reactor. J. CO2 Util. 2018, 27, 472–479. [Google Scholar] [CrossRef]
- Gamaleev, V.; Furuta, H.; Hatta, A. Detection of metal contaminants in seawater by spectral analysis of microarc discharge. Jpn. J. Appl. Phys. 2018, 57, 0102B8. [Google Scholar] [CrossRef]
- Gamaleev, V.; Furuta, H.; Hatta, A. Atomic emission spectroscopy of microarc discharge in sea water for on-site detection of metals. IEEE Trans. Plasma Sci. 2019, 47, 1841–1850. [Google Scholar] [CrossRef]
- Gamaleev, V.; Furuta, H.; Hatta, A. Generation of micro-arc discharge plasma in highly pressurized seawater. Appl. Phys. Lett. 2018, 113, 214102. [Google Scholar] [CrossRef]
- Chanan, N.; Kusumandari; Saraswati, T.E. Water Treatment Using Plasma Discharge with Variation of Electrode Materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012025. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ikehara, S.; Takei, H.; Akimoto, Y.; Sakakita, H.; Ishikawa, K.; Ueda, M.; Ikeda, J.; Yamagishi, M.; Kim, J.; et al. Red blood cell coagulation induced by low-temperature plasma treatment. Arch. Biochem. Biophys. 2016, 605, 95–101. [Google Scholar] [CrossRef]
- Iseki, S.; Hashizume, H.; Jia, F.; Takeda, K.; Ishikawa, K.; Ohta, T.; Ito, M.; Hori, M. Inactivation of Penicillium digitatum Spores by a High-Density Ground-State Atomic Oxygen-Radical Source Employing an Atmospheric-Pressure Plasma. Appl. Phys. Express 2011, 4, 116201. [Google Scholar] [CrossRef]
- Bruggeman, P.; Ribel, E.; Maslani, A.; Degroote, J.; Malesevic, A.; Rego, R.; Vierendeels, J.; Leys, C. Characteristics of atmospheric pressure air discharges with a liquid cathode and a metal anode. Plasma Sources Sci. Technol. 2008, 17, 025012. [Google Scholar] [CrossRef]
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application. Plasma Process. Polym. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Bibinov, N.K.; Fateev, A.A.; Wiesemann, K. On the influence of metastable reactions on rotational temperatures in dielectric barrier discharges in He-N2mixtures. J. Phys. D Appl. Phys. 2001, 34, 1819–1826. [Google Scholar] [CrossRef]
- Oh, J.; Szili, E.J.; Ogawa, K.; Short, R.D.; Ito, M.; Furuta, H.; Hatta, A. UV-vis spectroscopy study of plasma-activated water: Dependence of the chemical composition on plasma exposure time and treatment distance. Jpn. J. Appl. Phys. 2018, 57, 0102B9. [Google Scholar] [CrossRef]
- Oh, J.-S.; Szili, E.J.; Gaur, N.; Hong, S.-H.; Furuta, H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D. How to assess the plasma delivery of RONS into tissue fluid and tissue. J. Phys. D Appl. Phys. 2016, 49, 304005. [Google Scholar] [CrossRef]
- Xaubet, M.; Baudler, J.S.; Gerling, T.; Giuliani, L.; Minotti, F.; Grondona, D.; Von Woedtke, T.; Weltmann, K.D. Design optimization of an air atmospheric pressure plasma-jet device intended for medical use. Plasma Process. Polym. 2018, 15, e1700211. [Google Scholar] [CrossRef]
- Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Tarabová, B.; Kovaľová, Z.; Kučerová, K.; Machala, Z.; Janda, M.; Hensel, K. Evaluation of Oxidative Species in Gaseous and Liquid Phase Generated by Mini-Gliding Arc Discharge. Plasma Chem. Plasma Process. 2019, 39, 627–642. [Google Scholar] [CrossRef]
- Hong, E.J.; Shin, J.H.; Ji, S.H.; Ahn, J.H.; Kim, Y.J.; Choi, E.H.; Ki, S.H. Inactivation of Escherichia coli and Staphylococcus aureus on contaminated perilla leaves by Dielectric Barrier Discharge (DBD) plasma treatment. Arch. Biochem. Biophys. 2018, 643, 32–41. [Google Scholar] [CrossRef]
- Xu, Z.; Cheng, C.; Shen, J.; Lan, Y.; Hu, S.; Han, W.; Chu, P.K. In vitro antimicrobial effects and mechanisms of direct current air-liquid discharge plasma on planktonic Staphylococcus aureus and Escherichia coli in liquids. Bioelectrochemistry 2018, 121, 125–134. [Google Scholar] [CrossRef]
- Machala, L.; Tarabova, B.; Sersenova, J.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species. J. Phys. D Appl. Phys. 2019, 52, 034002. [Google Scholar] [CrossRef]
- Dors, M.; Metel, E.; Mizeraczyk, J.; Marotta, E. Pulsed corona discharge in water for coli bacteria inactivation. Int. J. Plasma Environ. Sci. Technol. 2008, 2, 34–37. [Google Scholar] [CrossRef]
- Du, C.; Tang, J.; Mo, J.; Ma, D.; Wang, J.; Wang, K.; Zeng, Y. Decontamination of bacteria by gas-liquid gliding arc discharge: Application to escherichia coli. IEEE Trans. Plasma Sci. 2014, 42, 2221–2228. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Satoh, K.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Fouracre, R.A. Pulsed-plasma disinfection of water containing Escherichia coli. Jpn. J. Appl. Phys. 2007, 46, 1137–1141. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Khandan, M.; Aria, S.H. Deactivation of Escherichia coli with different volumes in drinking water using cold atmospheric plasma. Contrib. Plasma Phys. 2019, e201800106. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Thomas, D.J.I.; Avery, S.M. Thermal death of Escherichia coli O157: H7 in cattle feeds. Lett. Appl. Microbiol. 2007, 44, 357–363. [Google Scholar] [CrossRef]
- Vermeulen, N.; Keeler, W.J.; Nandakumar, K.; Leung, K.T. The bactericidal effect of ultraviolet and visible light on Escherichia coli. Biotechnol. Bioeng. 2008, 99, 550–556. [Google Scholar] [CrossRef]
- Machado, L.F.; Pereira, R.N.; Martins, R.C.; Teixeira, J.A.; Vicente, A.A. Moderate electric fields can inactivate Escherichia coli at room temperature. J. Food Eng. 2010, 96, 520–527. [Google Scholar] [CrossRef]
- DIN EN 60601-1-10:2016–04 Medical Electrical Equipment—Part 1: General Requirements for Basic Safety and Essential Performance; Beuth Verlag: Berlin, Germany, 2016.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).