Abstract
This umbrella review summarized evidence from systematic reviews of randomized clinical trials regarding the efficacy of systemic and local premedication on anesthetic success in nonsurgical root canal treatment of teeth with symptomatic irreversible pulpitis. Searches were conducted in PubMed, Scopus, Web of Science, Cochrane Library, EMBASE, LILACS/BBO, and gray literature sources up to February 2025. Methodological quality was assessed using AMSTAR-2. The risk of bias was evaluated using the ROBIS tool. The Corrected Covered Area (CCA) was calculated to quantify the primary study overlap. Data regarding risk ratios and anesthetic success rates were synthesized qualitatively. Sixteen systematic reviews were included. The narrative synthesis suggests that oral NSAIDs (particularly ibuprofen > 400 mg and ketorolac 10–20 mg) and corticosteroids (dexamethasone) are associated with increased anesthetic success compared to placebo, with no significant difference between systemic and local administration. However, the reliability of these findings is impacted by the quality of the primary evidence: according to the appraisal, 13 reviews presented a high overall risk of bias/low methodological quality, while only three were classified as having low risk of bias. Furthermore, the CCA was 19.5%, indicating a high degree of redundancy among reviews. Consequently, while premedication appears effective, these conclusions must be interpreted with caution due to the substantial overlap and predominantly high risk of bias in the available literature.
1. Introduction
Effective pain control is essential in dental practice. The inferior alveolar nerve block (IANB) is routinely used to achieve anesthesia of mandibular teeth; however, its success rate is significantly reduced in cases of irreversible pulpitis. This reduction is primarily attributed to the inflammatory process, which alters nerve fiber physiology and limits the effectiveness of local anesthetics [1]. To address this limitation, different strategies have been investigated, including the use of systemic or local premedication. In this context, nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are promising for improving anesthetic success [2,3].
Systematic reviews and meta-analyses indicate that preoperative NSAIDs, including ibuprofen, diclofenac, and ketorolac, reduce nerve sensitivity by inhibiting prostaglandin synthesis. Ibuprofen at doses above 400 mg has shown particular effectiveness, increasing anesthetic success rates by up to 79% compared with placebo [4,5]. Moreover, corticosteroids, such as dexamethasone, have demonstrated significant benefits by reducing preoperative pain and improving pain control during endodontic treatment [6,7].
However, anesthetic failure is a multifactorial phenomenon influenced not only by local inflammatory processes but also by patient-related factors, including anxiety and central sensitization mechanisms. Consequently, a comprehensive evaluation of premedication strategies should extend beyond anti-inflammatory agents to encompass anxiolytics, opioids, and other pharmacological adjuvants. This broader perspective has often been overlooked in previous syntheses.
Although recent umbrella reviews have examined the role of premedication in irreversible pulpitis [5,8], these studies have largely focused on NSAIDs and the IANB technique, frequently including a limited number of systematic reviews. Importantly, prior syntheses did not consistently evaluate the risk of bias of the included reviews using the ROBIS tool, nor did they assess the degree of overlap of primary clinical trials through the Corrected Covered Area (CCA) metric. The absence of overlap analysis represents a relevant limitation in tertiary research, as it may lead to an overestimation of treatment effects due to the repeated counting of the same primary data.
Thus, this study conducted an umbrella review to analyze the available evidence on the efficacy of systemic and local premedication on the anesthetic success of teeth with irreversible pulpitis. Unlike previous overviews, this study explicitly assesses the quality and independence of the evidence using ROBIS and CCA metrics and synthesizes data across a broader pharmacological spectrum to guide clinical decision-making.
Premedication efficacy extends beyond NSAIDs and corticosteroids. Systematic reviews suggest that analgesic combinations, such as ibuprofen plus paracetamol, may provide additional benefits; however, the available evidence is limited by heterogeneous dosages and administration protocols, non-standardized pain assessment methods, and small sample sizes, which restrict the generalizability of the findings [9,10].
Despite promising results, anesthetic failure in teeth with irreversible pulpitis remains a challenge. Premedication with NSAIDs or corticosteroids may offer improvements, but no consensus exists on the most effective agent or dosage to increase anesthetic success rates [2,10]. This umbrella review synthesized the available evidence on the effects of local and systemic premedication on anesthetic success in nonsurgical root canal treatment of teeth with irreversible pulpitis, aiming to guide clinical decisions.
2. Materials and Methods
2.1. Protocol and Registration
This umbrella review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11] (Table S1) and the methodological framework proposed by Thomson et al. [12]. The quality and risk of bias of the included systematic reviews were assessed using AMSTAR-2 [13] and ROBIS [14] tools, respectively. The protocol was prospectively registered in the Open Science Framework (Center for Open Science, Charlottesville, VA, USA) (https://osf.io/3kcz2, accessed on 10 February 2025).
2.2. Review Question
The research question was formulated based on the PICO strategy (Table 1): “Which systemic or local premedication compared to placebo or other regimens improves anesthetic efficacy in patients with symptomatic irreversible pulpitis?”

Table 1.
Description of the PICO strategy components.
Although NSAIDs and corticosteroids are the primary agents of interest, this review adopted a broad pharmacological scope, including opioids, anxiolytics, and other adjuvants to comprehensively capture all potential strategies for managing the multifactorial nature of endodontic pain, which includes central sensitization and anxiety components. Systemic premedication was defined as oral or parenteral administration, and local premedication as intraligamentary or submucosal injection.
2.3. Search Strategy
The search strategy was applied across multiple bibliographic and citation databases, as well as gray literature sources, to identify all relevant systematic reviews published up to February 2025, without restriction on language or publication date.
Study screening, selection, data extraction, and risk of bias (or methodological quality) assessment were conducted independently by two reviewers (or by independent reviewers in duplicate), with disagreements resolved by consensus or by a third reviewer (or arbitrator).
The search strategy, which combined Medical Subject Headings (MeSH), Boolean operators (AND and OR), and proximity operators, was structured for MEDLINE (PubMed) and then adapted to each database (Table 2). The reference lists of eligible articles were manually searched to retrieve systematic reviews not identified electronically.

Table 2.
Detailed search strategies, keywords (MeSH/DeCS), and Boolean operators applied in electronic databases regarding the influence of premedication on anesthetic success in irreversible pulpitis.
2.4. Eligibility Criteria
Systematic reviews of clinical trials, with or without a meta-analysis, evaluating the efficacy of local or systemic premedication on the anesthetic success of teeth with irreversible pulpitis were selected. The exclusion criteria comprised observational studies, narrative reviews, case reports, animal studies, in vitro investigations, letters to the editor, and short communications. Crucially, regarding study design, we included only systematic reviews that exclusively synthesized randomized clinical trials (RCTs). Systematic reviews that mixed RCTs with observational studies were included only if data from RCTs could be extracted separately; otherwise, they were excluded to maintain a high level of evidence.
2.5. Selection of Studies
Records retrieved from each database were initially transferred to EndNote Web™ (Clarivate Analytics, Philadelphia, PA, USA). Following automatic de-duplication, any remaining duplicates were identified and eliminated manually. References sourced from OATD and ProQuest were organized in Microsoft Word™ 2019 (Microsoft Corp., Redmond, WA, USA) for manual duplicate removal. The resulting dataset was then uploaded to Rayyan QCRI (Qatar Computing Research Institute, Doha, Qatar) [15]. Finally, a manual screening of the reference lists of included studies was conducted, adhering to the same eligibility criteria established for electronic search.
2.6. Data Extraction
The extracted data covered three main domains: study characteristics (authors, year, country, journal, databases, and objectives), methodological details (number of included primary studies, population, age range, tooth type, diagnosis, anesthetic protocols, and risk of bias assessment tools), and outcomes (success evaluation methods, meta-analysis results, certainty of evidence, and conclusions).
2.7. Methodological Quality (AMSTAR-2)
The methodological quality of the included reviews was appraised using the AMSTAR-2 instrument [13]. This tool comprises a 16-item online checklist (https://amstar.ca/Amstar_Checklist.php (accessed on 5 July 2025) that evaluates both critical and non-critical domains to determine the overall confidence in the results, ranging from critically low to high [13]. Assessment involved rating each item as yes, partially yes, or no, based on the extent of compliance within each study.
2.8. Risk of Bias (ROBIS)
The risk of bias in the included systematic reviews was evaluated independently by two authors (M.V.B.D and R.S.F.S.F.) using the ROBIS tool [14], with a third author (L.R.P.) consulted to resolve any disagreements. The ROBIS assessment involves 21 signaling questions across four domains covering eligibility criteria, study identification and selection, data collection and appraisal, and synthesis of findings. Responses were graded as yes (Y), probably yes (PY), probably not (PN), no (N), or no information (NI). Yes denotes low concern, while no information indicates insufficient data. Regarding the overall domain rating, a low risk of bias was assigned if all answers were “yes” or “probably yes.” Conversely, a domain was classified as high risk if any question received a “no” or “probably not” rating. The risk was considered uncertain if questions were marked “no information” but had the potential to be “yes” or “probably yes” [14].
2.9. Assessment of Overlap Among Primary Studies
To quantify the extent of duplication across the included systematic reviews, we employed the Corrected Covered Area (CCA), a validated metric designed to measure the overlap of primary studies [16]. The calculation incorporates the total number of included publications, the count of unique primary studies, and the total number of systematic reviews analyzed. We interpreted CCA values as follows: 0–5% represented slight overlap, 6–10% represented moderate overlap, 11–15% high overlap, and >15% very high overlap [16]. High CCA scores indicate significant redundancy, requiring cautious interpretation of synthesized results to avoid overestimating treatment effects.
2.10. Data Synthesis and Analysis
Given the expected heterogeneity in drug protocols and outcome definitions across the included reviews, a quantitative re-meta-analysis (pooling data from multiple reviews) was not deemed appropriate due to the high risk of statistical redundancy. Instead, a narrative synthesis was conducted. Data were structured and grouped primarily by pharmacological class (NSAIDs, corticosteroids, others) and secondarily by route of administration (systemic vs. local). The synthesis prioritized findings from reviews with higher methodological quality (AMSTAR-2) and lower risk of bias (ROBIS).
3. Results
3.1. Selection of Studies
During the first phase of study selection, 531 potentially relevant records were identified in all electronic searches, including the gray literature. After removing the duplicates, 329 records were analyzed by title and abstract. Eighteen studies met the eligibility criteria and were included in the full-text analysis. Three studies were excluded after the full-text reading for the following reasons: they evaluated the adverse effects of tramadol only as a local anesthetic agent [17] and consisted of short communications [9,18]. Only one study was identified through citation searches, totaling 16 systematic reviews included in this umbrella review. Figure 1 shows the study selection process in accordance with the PRISMA statement.
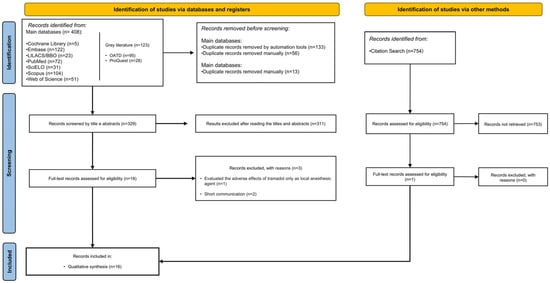
Figure 1.
PRISMA flowchart of the search results and study selection process.
3.2. Characteristics of Included Reviews
As detailed in Table 3, the 16 included systematic reviews reflect a global interest in this topic, with a notable increase in publications over the last decade. While the scope varied (with the number of included primary trials ranging from 4 to 62 per review), most reviews searched multiple databases, though coverage of gray literature remained limited.

Table 3.
Summary of the main characteristics of the included studies.
Regarding the clinical and methodological characteristics summarized in Table 4, there was clear homogeneity in the target population: studies focused almost exclusively on mandibular teeth with symptomatic irreversible pulpitis undergoing IANB. In terms of pharmacological interventions, a wide diversity of protocols was observed. NSAIDs and non-opioid analgesics were the most investigated classes, featured in nearly all reviews, followed by corticosteroids and opioids. Systemic administration (oral) was the standard comparator, although several reviews also synthesized data on local administration routes (intraligamentary, submucosal). Lidocaine remains the standard anesthetic agent across the primary literature, with articaine appearing as a frequent alternative in more recent reviews.

Table 4.
Summary of the methodological characteristics, clinical features, anesthetic protocols, and quality assessment tools of the included systematic reviews.
3.3. Synthesis of Main Findings Regarding the Efficacy of NSAIDs and Corticosteroids
Fourteen reviews with meta-analyses consistently indicated that premedication with NSAIDs (particularly ibuprofen > 400 mg and ketorolac 10–20 mg) significantly increased the likelihood of anesthetic success compared to placebo. Corticosteroids (dexamethasone) also showed significant efficacy, particularly in reducing post-operative pain and increasing success rates in symptomatic teeth. Route of Administration: Direct comparisons between systemic (oral) and local (intraligamentary/submucosal) administration routes were inconclusive, with most reviews finding no statistically significant difference in efficacy between the two approaches. Definition of Success: It is critical to note that the definition of “anesthetic success” varied substantially across reviews, ranging from “no pain on access” (Visual Analog Scale = 0) to “mild pain” (VAS < 54 mm) or simply the absence of supplemental anesthesia. This heterogeneity precludes a unified pooled estimate of effect size. Certainty of Evidence: While findings generally favor premedication, the certainty of evidence (GRADE) re-ported by the reviews varied widely, from very low to high. Notably, reviews showing the largest effect sizes often included primary studies with higher risks of bias (Table 5).

Table 5.
Findings of the included studies.
3.4. Methodological Quality (AMSTAR-2)
Methodological quality was critically low for most eligible studies, and only two had a high quality [8,23] (Table 6). The critical items with the highest failure rates were: Q2-lack of a pre-registered review protocol (9 of 16 studies); Q7-absence of a list of studies excluded after full-text analysis, with justifications (9 of 16 studies); Q4-absence of a comprehensive search strategy (search restricted to unjustified English studies or a few databases) (5 of 16 studies). The key failures in non-critical domains included the lack of description of funding for eligible studies (16 of 16 studies) and the lack of assessment methods for the impact of the risk of bias on the quantitative synthesis (6 of 16 studies). Critically, high-influence reviews (those with the largest meta-analyses) frequently failed in the domain of “comprehensive search strategy” and “investigation of publication bias,” which directly impacts the reliability of the summarized effect sizes.

Table 6.
Quality assessment of the studies using AMSTAR-2.
3.5. Risk of Bias (ROBIS)
Most systematic reviews had a high risk of bias, and only three had a low risk of bias [3,8,23] (Figure 2). In the “eligibility criteria” domain, studies that did not offer or have a pre-published protocol were considered to have a high risk of bias. In the “identification and selection of studies” domain, articles with inadequate search strategies or additional tools to minimize the risk of selection bias of eligible studies were deemed high risk. The “data collection and evaluation” domain had an uncertain risk, as the studies did not provide information on methods to reduce biases during these steps. Finally, in the “syntheses and conclusions” domain, the risk of bias was considered high when studies failed to apply methods to assess the impact of individual study bias on the quantitative analyses and did not address this potential effect in their discussion.
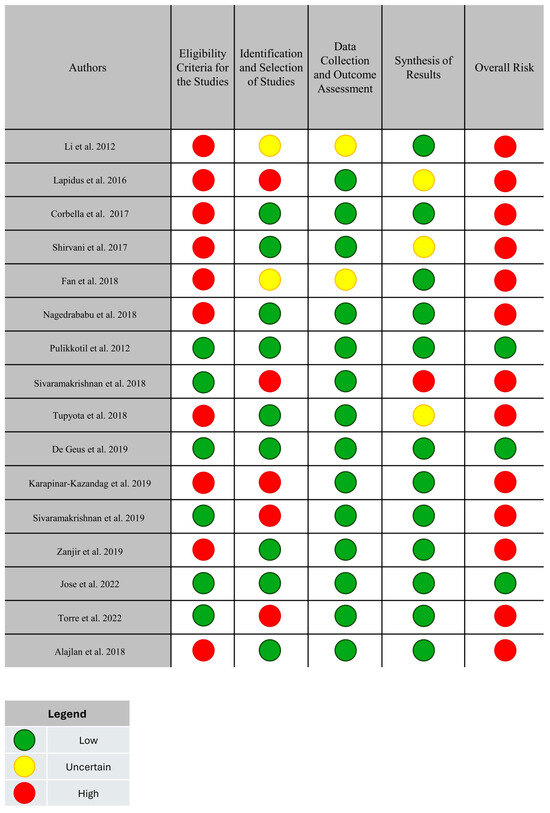
Figure 2.
Individual risk of bias in the systematic reviews using the ROBIS tool. The green circles indicate a low risk of bias. The red circles indicate a high risk of bias. The yellow circles indicate an uncertain risk of bias.
3.6. Overlap Analysis
To assess redundancy among the included systematic reviews, the Corrected Covered Area (CCA) was calculated, as proposed by Pieper et al. This metric quantifies the degree of overlap of primary studies across reviews by considering the total number of included studies, the number of unique primary studies, and the number of systematic reviews. In the present overview, a total of 338 study occurrences were identified across 16 systematic reviews, corresponding to 104 unique primary studies. Based on these values, the CCA was estimated at 19.5%, indicating a very high degree of overlap among the included reviews. This finding suggests that a substantial proportion of the systematic reviews relied on largely overlapping clinical evidence rather than independent primary datasets. Figure 3 illustrates this overlap graphically using the Jaccard similarity index, in which darker cells represent a higher degree of shared primary studies between reviews, highlighting the pronounced redundancy observed [7,22] (J = 0.98).
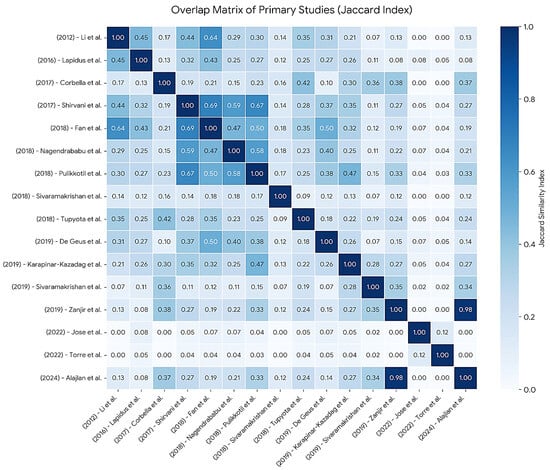
Figure 3.
Heatmap of the Jaccard Similarity Index matrix displaying pairwise overlap of primary studies among the 16 included systematic reviews. The values within the cells represent the Jaccard coefficient (J), where 0.00 indicates no shared studies and 1.00 indicates identical sets of included studies. The color gradient reflects the intensity of overlap, with darker shades denoting higher redundancy between the corresponding reviews. The diagonal line represents the self-comparison of each review (J = 1.00).
4. Discussion
The management of pain in patients with irreversible pulpitis presents a significant clinical challenge, as inflammation in the mandibular molars often impedes the efficacy of the inferior alveolar nerve block (IANB). This comprehensive review synthesized evidence from 16 systematic reviews to evaluate the impact of oral premedication on anesthetic success. The consolidated data suggests that premedication with anti-inflammatory agents, specifically NSAIDs and corticosteroids, may enhance IANB success rates compared to placebo, although the overall quality of evidence ranges from critically low to high, warranting a cautious interpretation of these findings.
4.1. Efficacy of NSAIDs
Synthesized data from multiple reviews indicates that NSAIDs are the most extensively studied class of premedication [3,4,8,21,24,25,26]. The mechanism of action, which involves the inhibition of prostaglandin synthesis, appears biologically plausible for countering the inflammatory mediators that sensitize nociceptors in irreversible pulpitis. However, the synthesis of results reveals a clear dose-dependency. While some reviews noted inconsistent results with lower doses [24], there is a convergence of evidence suggesting that ibuprofen is effective primarily at doses exceeding 400 mg (e.g., 600–800 mg) [4,25]. Similarly, ketorolac (10 mg) and diclofenac (50 mg) showed potential superiority over lower doses of ibuprofen [8]. This suggests that for NSAIDs to be clinically beneficial in this context, therapeutic dosing must be sufficient to counteract the intense inflammatory environment of the pulp.
4.2. Corticosteroids and Opioids
Corticosteroids, particularly dexamethasone, emerged in several reviews as a highly effective intervention for increasing anesthetic success [6,7,8]. Comparisons indicated that dexamethasone might outperform other premedications, including NSAIDs and tramadol, in achieving successful anesthesia [8]. Notably, the route of administration (systemic vs. local) did not appear to significantly alter efficacy, suggesting that the anti-inflammatory mechanism itself is the primary driver of success rather than the delivery method [6]. Opioids, such as tramadol or combinations of paracetamol/opioids, also demonstrated benefits compared to placebo [18,22], though they are less frequently recommended as a first-line option due to their side effect profile compared to NSAIDs/corticosteroids.
4.3. Sedatives and Anxiolytics
Although anxiety is a known factor in pain perception, the evidence supporting the use of benzodiazepines (e.g., triazolam, alprazolam) solely to increase the success of the anesthetic block is weak and inconclusive [25,28]. While these agents are valuable for behavioral management and anxiety reduction, current systematic reviews do not support their routine use specifically for enhancing the physiological mechanism of IANB in irreversible pulpitis.
4.4. Quality of Evidence and Risk of Bias
A critical finding of this overview is the fragility of the existing evidence base. The majority of included systematic reviews (62.5%) were classified as having “critically low” methodological quality according to AMSTAR 2, primarily due to the lack of pre-registered protocols and insufficient justification for excluded studies. Furthermore, the ROBIS analysis highlighted great concerns regarding eligibility criteria in many reviews. Consequently, while the direction of effect favors premedication, the “assertive” conclusions found in some individual reviews must be tempered. The high risk of bias in primary studies, often due to poor randomization or allocation concealment [4], means that current estimates of effect size may be inflated.
4.5. Limitations
This overview presents specific limitations that require context. First, the limitations intrinsic to the primary systematic reviews, such as high heterogeneity in drug dosages, timing of administration, anesthetic techniques, outcome definitions, subjective pain assessment scales, and small sample sizes, are inevitably reflected in this synthesis. However, by aggregating these findings, this overview highlights the critical need for standardization in future trials.
Second, a significant challenge in umbrella reviews is the potential for overlapping primary studies, which can inflate the perceived weight of evidence. Unlike previous works, we formally addressed this by calculating the Corrected Covered Area (CCA). The resultant very high overlap (19.5%), driven by redundancy in key reviews such as [7,22], indicates that current systematic reviews are heavily derived from the same core set of clinical trials. By quantifying this, our study offers a more transparent interpretation of the cumulative evidence than previous qualitative summaries.
Finally, while this work complements other recent overviews [5,8], it distinguishes itself by applying rigorous methodological tools (ROBIS) and providing a quantitative assessment of primary study redundancy (CCA matrix). Although publication bias was not formally assessed, the inclusion of critically low-quality reviews suggests that “positive” findings may be overrepresented, a factor we have mitigated by recommending a cautious interpretation of the results.
5. Conclusions
Current evidence supports the efficacy of systemic premedication with NSAIDs (particularly ibuprofen > 400 mg and ketorolac 10–20 mg) and corticosteroids (dexamethasone 0.5 mg) in improving inferior alveolar nerve block success rates in irreversible pulpitis. Similarly, local administration of corticosteroids (dexamethasone 4 mg/mL) appears to potentiate anesthesia by reducing inflammation. However, these findings must be interpreted with caution due to the predominantly low methodological quality of the included reviews and the substantial overlap identified among the included studies (CCA = 19.5%). This high redundancy suggests that the perceived consensus is largely driven by the repeated analysis of a recurrent set of primary studies rather than a breadth of independent evidence. Consequently, while these protocols are promising, the combination of data overlap and methodological heterogeneity precludes the establishment of a definitive “gold standard.” Future research should shift focus from conducting further systematic reviews to producing well-designed, standardized clinical trials to generate new, independent data. Furthermore, greater integration of patient-related factors, such as anxiety, variability in pain perception, and operator-dependent influences, would provide a more comprehensive clinical perspective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app16010383/s1, Table S1: Checklist from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses–Extension for Scoping Reviews (PRISMA-ScR).
Author Contributions
M.V.d.B.D.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft. L.R.P. and R.S.F.d.S.-F.: investigation, formal analysis, methodology, visualization, writing—original draft. G.R.A., R.B.d.B.-J., and J.M.d.C.R.: resources, visualization, writing—review and editing. F.d.S.M.: project administration, conceptualization, resources, supervision, visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brazil (CAPES)-Finance Code 001. We are thankful for the support of Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil (CNPq) and of Fundação de Amparo à Pesquisa do Estado de Minas Gerais-Brazil (FAPEMIG).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sivaramakrishnan, G.; Sridharan, K. Oral ketorolac with inferior alveolar nerve block for irreversible pulpitis: A systematic review and meta-analysis. Open Dent. J. 2018, 12, 340–346. [Google Scholar] [CrossRef]
- Parirokh, M.; Abbott, P.V. Present status and future directions—Mechanisms and management of local anaesthetic failures. Int. Endod. J. 2022, 55, 951–994. [Google Scholar] [CrossRef]
- de Geus, J.L.; Wambier, L.M.; Boing, T.F.; Loguercio, A.D.; Reis, A. Effect of ibuprofen on the efficacy of inferior alveolar nerve block in patients with irreversible pulpitis: A meta-analysis. Aust. Endod. J. 2019, 45, 246–258. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Pulikkotil, S.J.; Veettil, S.K.; Teerawattanapong, N.; Setzer, F.C. Effect of nonsteroidal anti-inflammatory drug as an oral premedication on the anaesthetic success of inferior alveolar nerve block in treatment of irreversible pulpitis: A systematic review with meta-analysis and trial sequential analysis. J. Endod. 2018, 44, 914–922.e2. [Google Scholar] [CrossRef] [PubMed]
- Khademi, A.; Iranmanesh, P.; Mosayebi, N.; Heydari, M.; Bagherieh, S. Effect of premedication on the success of inferior alveolar nerve block in patients diagnosed with irreversible pulpitis: An umbrella review. Dent. Res. J. 2023, 20, 75. [Google Scholar] [CrossRef]
- Franco-de la Torre, L.; Gómez-Sánchez, E.; Serafín-Higuera, N.A.; Alonso-Castro, Á.J.; López-Verdín, S.; Molina-Frechero, N.; Granados-Soto, V.; Isiordia-Espinoza, M.A. Dexamethasone increases the anaesthetic success in patients with symptomatic irreversible pulpitis: A meta-analysis. Pharmaceuticals 2022, 15, 878. [Google Scholar] [CrossRef]
- Alajlan, N.; Carrasco-Labra, A.; Karabucak, B.; Lee, S.M. Systemic corticosteroid uses in endodontics—Part 2: Enhancing the success of local anesthesia. J. Endod. 2024, 50, 899–906. [Google Scholar] [CrossRef]
- Só, G.B.; Silva, I.A.; Weissheimer, T.; Lenzi, T.L.; Só, M.V.R.; da Rosa, R.A. Do NSAIDs used prior to standard inferior alveolar nerve blocks improve the analgesia of mandibular molars with irreversible pulpitis? An umbrella review. Clin. Oral. Investig. 2023, 27, 1885–1897. [Google Scholar] [CrossRef]
- Wong, Y.J. Does oral nonsteroidal anti-inflammatory drug (NSAID) premedication in patients with irreversible pulpitis increase the success rate of inferior alveolar nerve block? Evid. Based Dent. 2019, 20, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.J.; Nagendrababu, V.; Veettil, S.K.; Jinatongthai, P.; Setzer, F.C. Effect of oral premedication on the anaesthetic efficacy of inferior alveolar nerve block in patients with irreversible pulpitis: A systematic review and network meta-analysis of randomized controlled trials. Int. Endod. J. 2018, 51, 989–1004. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; Chou, R.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Thomson, K.; Bambra, C.; McNamara, C.; Huijts, T.; Todd, A. The effects of public health policies on population health and health inequalities in European welfare states: Protocol for an umbrella review. Syst. Rev. 2016, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; ROBIS Group. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef]
- Mane, R.J.; Choi, J.J.E.; Sharpe-Davidson, W.F. Tramadol as a local anaesthetic agent in dentistry: A systematic review of local and systemic adverse effects. Saudi Dent. J. 2021, 33, 842–852. [Google Scholar] [CrossRef]
- Grant, R.; Brown, T.; Young, L.; Lamont, T. How can local anaesthesia be improved in the management of irreversible pulpitis? Evid. Based Dent. 2021, 22, 26–27. [Google Scholar] [CrossRef]
- Shirvani, A.; Shamszadeh, S.; Eghbal, M.J.; Marvasti, L.A.; Asgary, S. Effect of preoperative oral analgesics on pulpal anesthesia in patients with irreversible pulpitis: A systematic review and meta-analysis. Clin. Oral. Investig. 2017, 21, 43–52. [Google Scholar] [CrossRef]
- Corbella, S.; Taschieri, S.; Mannocci, F.; Rosen, E.; Tsesis, I.; Del Fabbro, M. Inferior alveolar nerve block for the treatment of teeth presenting with irreversible pulpitis: A systematic review and meta-analysis. Quintessence Int. 2017, 48, 69–77. [Google Scholar] [CrossRef]
- Sivaramakrishnan, G.; Alsobaiei, M.; Sridharan, K. Interventions for anaesthetic success in symptomatic irreversible pulpitis: A network meta-analysis of randomized controlled trials. J. Dent. Anesth. Pain Med. 2019, 19, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Zanjir, M.; Lighvan, N.L.; Yarascavitch, C.; Beyene, J.; Shah, P.S.; Azarpazhooh, A. Efficacy and safety of pulpal anesthesia strategies during endodontic treatment of permanent mandibular molars with symptomatic irreversible pulpitis: A systematic review and network meta-analysis. J. Endod. 2019, 45, 1435–1446.e10. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Teja, K.V.; Palanivelu, A.; Khandelwal, A.; Siddique, R. Analgesic efficacy of corticosteroids and nonsteroidal anti-inflammatory drugs through oral route in the reduction of postendodontic pain: A systematic review. J. Conserv. Dent. 2022, 25, 9–19. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Ma, X.; Li, L.; Shi, Z. Preoperative oral nonsteroidal anti-inflammatory drugs for the success of the inferior alveolar nerve block in irreversible pulpitis treatment: A systematic review and meta-analysis based on randomized controlled trials. Quintessence Int. 2012, 43, 209–219. Available online: https://www.quintessence-publishing.com/gbr/en/article/840495 (accessed on 9 November 2025).
- Lapidus, D.; Goldberg, J.; Hobbs, E.H.; Ram, S.; Clark, G.T.; Enciso, R. Effect of premedication to provide analgesia as a supplement to inferior alveolar nerve block in patients with irreversible pulpitis. J. Am. Dent. Assoc. 2016, 147, 427–437. [Google Scholar] [CrossRef]
- Fan, D.; Pan, J.; Cao, Y.; Liu, W.; Li, C.; Liu, B. Pre-emptive use of nonsteroidal anti-inflammatory drugs for a successful inferior alveolar nerve block in patients with irreversible pulpitis: A systematic review and network meta-analysis. Int. J. Clin. Exp. Med. 2018, 11, 11567–11577. Available online: http://www.ijcem.com (accessed on 9 November 2025).
- Tupyota, P.; Chailertvanitkul, P.; Laopaiboon, M.; Ngamjarus, C.; Abbott, P.V.; Krisanaprakornkit, S. Supplementary techniques for pain control during root canal treatment of lower posterior teeth with irreversible pulpitis: A systematic review and meta-analysis. Aust. Endod. J. 2018, 44, 14–25. [Google Scholar] [CrossRef]
- Karapinar-Kazandag, M.; Tanalp, J.; Ersev, H. Effect of premedication on the success of inferior alveolar nerve block in patients with irreversible pulpitis: A systematic review of the literature. Biomed. Res. Int. 2019, 2019, 6587429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.