Abstract
Multiple sclerosis is a prevalent neurodegenerative disease that significantly affects gait and exercise capacity. The core system is involved in providing sufficient spinal stability for dealing with stability demands. People with multiple sclerosis exhibit reduced trunk stability, which may affect stability during tasks such as reaching, stepping, and unexpected perturbations. This systematic review aimed to evaluate the effects of core training on gait and exercise capacity in people with multiple sclerosis. A systematic review was conducted in the databases PubMed/Medline, Web of Science (WOS), ScienceDirect, CINAHL, Scopus, and Physiotherapy Evidence Database (PEDro). Randomized controlled trials up to January 2025 included the following PICO inclusion criteria: (Participants) adults with a multiple sclerosis diagnosis; (Intervention) a core stability training program (alone or combined with another intervention); (Comparison) compared to no intervention, placebo or any other intervention; (Outcomes) and including at least one outcome related to gait and/or exercise capacity. The search identified 781 records, and finally 12 studies were included in this review. Methodological quality and risk of bias were assessed using the PEDro scale (with 8 as the median score) and the Cochrane risk assessment tool (ROB2), showing in most cases some concerns, particularly regarding outcome selection. Most of the studies included reported significant improvements in gait assessed with the timed up and go, timed 25-foot walk, and six spot step tests. Also, the results of exercise capacity assessed with the 6 min and 2-min walk test significantly increased in most of the studies. The reviewed articles suggest that a core-based exercise program may be effective in improving gait and exercise capacity in people with multiple sclerosis. However, considering the heterogeneity of the interventions, results, population, and the high risk of bias of some trials, more research is needed to validate these preliminary results.
1. Introduction
Multiple sclerosis (MS) is a prevalent neurodegenerative disease characterized by the inflammation-mediated demyelination of axons throughout the central nervous system, resulting in a decline in various neurological functions [1,2]. Four clinical courses of MS have been identified: clinically isolated syndrome, relapsing-remitting MS, secondary progressive MS, and primary progressive MS [3]. Conventional treatments involve pharmacological strategies aimed at downregulating immune activation to halt disease progression, prevent relapses, or partially reverse disability. However, non-pharmacological interventions, such as physical exercise, have also proven beneficial in improving both physical and mental functional capacities in people with MS [4,5]. Physical exercise may include Pilates, yoga, aerobic training, resistance training, aquatic therapy, virtual reality training, or whole-body vibration training [5].
MS presents considerable heterogeneity in both symptom manifestation and clinical progression [6]. Fatigue, one of the most common symptoms, affects up to 80% of people with MS. It is typically perceived as a lack of physical and/or mental energy that interferes with daily activities and may be associated with reduced activity levels [4]. A recent study has shown that the cerebellum is involved in MS-related fatigue, including physical cognitive, and psychosocial domains, as well as in overall clinical disability [6]. Gait impairment and exercise capacity are also common among individuals with MS [7]. Gait is significantly affected at both self-selected and increased paces across all stages of the disease, with more pronounced deficits in advanced stages [8]. Common gait abnormalities include muscle weakness, spasticity, imbalance, and reduced walking speed [2,9]. Exercise capacity, defined as the maximum amount of physical exertion a person can sustain, is closely linked to cardiovascular and respiratory function [10]. A previous study reported that individuals with MS exhibited significantly higher heart rates during exercise and covered shorter distances compared to healthy controls. The reduced performance in the Six-Minute Walk Test (6MWT) was attributed to limitations in ADL, elevated resting heart rate, and fatigue [11].
Previous research has also shown a correlation with physical disability, as measured by the Expanded Disability Status Scale (EDSS), which evaluates neurological impairments and walking ability, even in individuals who are not severely disabled [12]. Preserving physical functions such as walking and activities of daily living (ADLs) is essential for the overall health and quality of life (QoL) of people with MS. Notably, postural instability during gait significantly impacts QoL [8]. Most individuals with MS exhibit postural control and gait abnormalities, even in the early stages of the disease [2,9]. It has been previously shown that people with MS demonstrate reduced trunk stability during seated arm movements when compared to healthy individuals, reinforcing the clinical observation that core stability is often impaired in this population [13]. Specifically, this population performs worse on tests assessing core muscle strength and endurance [14]. These deficits negatively impact responses to both self-initiated and unexpected disturbances, as well as muscle activation patterns, ultimately affecting the performance of daily activities. During reaching, stepping, or unexpected perturbations, the transverse abdominis muscle is activated before other trunk or limb muscles, highlighting its key role in core stabilization [15].
Ketelhut et al. [16] found that people with MS exhibit altered core muscle activation during walking, which may contribute to increased fatigability. A range of rehabilitative interventions has been proposed for core muscle training in individuals with neurological disorders, including dynamic neuromuscular stabilization, respiratory training, Pilates, Ai Chi, adapted sports, training on unstable surfaces, electrical stimulation, feedback-based training, and robotic therapy.
Although core stability training programs have been suggested as an effective method to improve postural balance in individuals with neurological conditions [15], no previous review has specifically examined the effects of core exercise on gait and exercise capacity in people with MS. Therefore, the objective of this study was to systematically review the impact of core training on gait and exercise capacity in people with MS.
2. Materials and Methods
2.1. Design
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17] (https://www.prisma-statement.org/ accessed on 14 August 2024). The review protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) with the reference number CRD42024589063.
2.2. Search Strategy
Six databases were systematically searched from their inception to January 2025: CINAHL, PubMed, Scopus, Web of Science, ScienceDirect, and PEDro. The following search terms were used: (“Multiple Sclerosis” [MeSH] OR “multiple sclerosis” OR “disseminated sclerosis”) AND (“Core Stability” [MeSH] OR “core” OR “trunk” OR “lumbopelvic hip complex” OR “Pelvic Floor” [MeSH] OR “pelvic floor” OR “diaphragm” OR “Abdominal Muscles” [MeSH] OR “abdomin*” OR “low back” OR “multifid*” OR “lumbar” OR “quadratus lumborum” OR “erector spinae” OR “external oblique” OR “internal oblique”) AND (“Resistance Training” [MeSH] OR “Endurance Training” [MeSH] OR “train*” OR “Exercise” [MeSH] OR “Exercise Therapy” [Mesh] OR “Exercise Movement Techniques” [MeSH] OR “exercis*” OR “Pilates” OR “strength*” OR “stabili*” OR “intervention” OR “Rehabilitation” [MeSH] OR “rehabilitation”) AND (“gait” OR “exercise capacity” OR “walking” OR “cardiorespiratory outcome” OR “locomotion”). The complete search strategy is detailed in Appendix A.
The reference lists of all included and cited studies were manually searched to minimize publication bias and include relevant gray literature. This strategy aimed to identify those studies that were not captured by the database search.
2.3. Study Selection
The PICOS criteria (Participants, Interventions, Comparisons, Outcomes, and Study design) were applied to define the research question and determine study eligibility: (1) Participants: Adults with MS, aged 18 or older; (2) Interventions: Core training-based interventions alone or combined with other interventions, being the core training the primary focus; (3) Comparisons: No intervention, placebo, or other interventions that did not include core training; (4) Outcomes: Measures of gait (e.g., timed up and go or 10-m walk test) or exercise capacity (e.g., 6MWT); (5) Study design: Randomized controlled trials. Studies published in English, Spanish, and French were included.
A total of 781 records were identified. After removing duplicates using EndNote bibliographic management software (n = 136), 645 titles and abstracts were screened, and 614 were excluded for not meeting the inclusion criteria. Then, the full text of 31 articles was reviewed, and 12 articles met all the eligibility criteria and were included in the final analysis. Two reviewers (J.D.R.-M. and M.R.-M.) independently conducted the search and study selection. Disagreements were resolved by consulting a third reviewer (I.C.-M.).
2.4. Data Extraction
The following data were extracted from each included study: author (year), participant characteristics, MS phenotype, sample size, age (years), and gender distribution (F/M). Information about the intervention was also collected, including duration, volume/intensity, frequency (sessions/week), program length (weeks), measured outcomes, and main results.
Data extraction was carried out independently by two reviewers (J.D.R.-M. and M.R.-M.). Any discrepancies were resolved with the assistance of a third reviewer (I.C.-M).
2.5. Methodological Quality of Included Studies
The methodological quality of the included randomized controlled trials was assessed using the PEDro scale, which includes the following items: 1. eligibility criteria specified, 2. Random allocation of participants, 3. Concealed allocation, 4. Baseline comparability between groups, 5. Blinding of participants, 6. Blinding of therapists, 7. Blinding of assessors, 8. Outcome measures obtained from at least 85% of participants, 9. Intention-to-treat analysis, 10. Between-group statistical comparisons, 11. Reporting of point estimates and variability for at least one key outcome. The suggested cut-off points for the PEDro scale were as follows: excellent (9–11), good (6–8), fair (4–5), and poor (<3) [18]. Methodological quality was assessed independently by two reviewers (J.D.R.-M. and M.R.-M.), and disagreements were resolved through consultation with a third reviewer (I.C.-M.).
2.6. Risk of Bias of Included Studies
The Revised Cochrane risk of bias tool (RoB-2) was used to assess the risk of bias in the randomized controlled trials included. This tool evaluates five domains: bias arising from the randomization process, due to deviations from the intended interventions, to missing outcome data, in the measurement of the outcome, and the selection of the reported result. Each domain was rated as “high risk of bias”, “low risk of bias,” or “some concerns” [19]. Two reviewers (J.D.R.-M. and M.R.-M.) assessed the methodological quality and risk of bias independently, and in case of disagreement, a third reviewer was consulted (I.C.-M).
3. Results
3.1. Search Selection
The search initially yielded a total of 781 records. After removing duplicates (n = 136), 645 records were analyzed. After screening by title and abstract, 614 manuscripts were excluded. Finally, 19 full-text articles were excluded after further screening, resulting in a total of 12 studies being included in this review. No additional studies were identified through other sources. A summary of the literature selection process is presented in Figure 1.
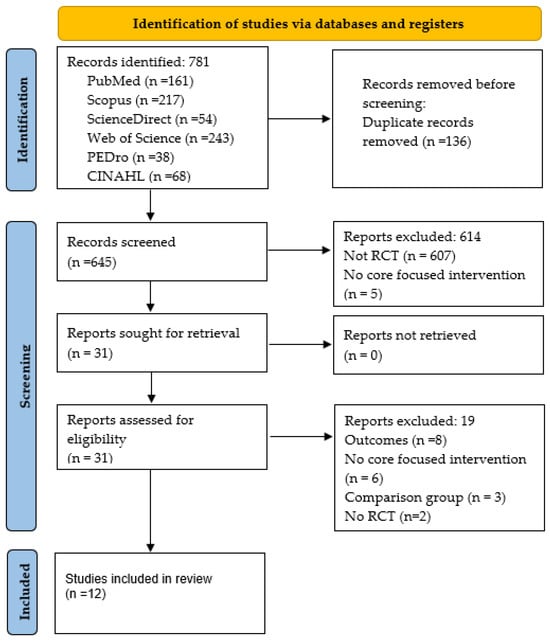
Figure 1.
Flow diagram.
3.2. Characteristics of the Included Studies and Participants
The characteristics of the studies included in this review are shown in Table 1. The studies are organized chronologically, from the earliest (2016) to the most recent (2023). Three studies were conducted in Turkey [20,21,22], four in Iran [23,24,25,26], and one each in Sweden [27], Denmark [28], Norway [29], Canada [30], and the United Kingdom [31]. In total, the studies included 639 participants, with a mean age of 42.08 years. The most common MS phenotypes were relapsing-remitting and primary progressive MS. The mean time since disease onset varied across studies, ranging from 1 year to over 20 years.

Table 1.
Characteristics of included studies.
3.3. Characteristics of Interventions
The main results and characteristics of the interventions are shown in Table 2.

Table 2.
Characteristics of interventions.
All interventions focused on teaching the proper activation of the transverse abdominis [20,21,22,23,24,25,26,27,28,29,30,31]. Once achieved, participants progressed to more complex tasks requiring greater postural stability. Most studies incorporated resistance bands, exercise balls, or other equipment to facilitate progression. One study used the CoreAlign device [30], while another utilized the suspension system such as TRX [26]. Participants in the control groups were generally instructed to maintain their usual physical activity levels. Session duration ranged from 30 to 90 min, with frequencies varying from one to three times per week. The total intervention periods ranged from 4 to 12 weeks.
All studies conducted both pre- and post-intervention assessments, while two studies also included follow-up measurements. Follow-up assessments were conducted at 16 weeks in one study [31], and at 18 and 30 weeks in another [29].
Five studies used the Timed Up and Go (TUG) Test [21,24,25,27,30]; of these, two [24,30] reported significant between-group improvements, two [21,25] showed significant within-group improvements [21,25], and one [27] found no differences. Five studies used the Multiple Sclerosis Walking Scale (MSWS) [22,27,28,29,31]; three [27,28,29] reported a significant between-group decrease, while two [22,31] found no significant changes. The 6MWT was used in five studies [22,23,25,28,30]; four [22,23,25,30] reported significant between-group improvements, and one [28] found no differences. Three studies used the 10-Meter Walk Test (10MWT) [23,29,31]; two [23,29] showed significant between-group improvements, and one [31] found no differences. Two studies utilized the 2-Minute Walk Test (2MWT) [20,29]; both showed significant between-group improvements. The Timed 25-Foot Walk Test (T25FWT) [22,26,28] was employed in three studies; one [28] showed significant between-group improvements, one [26] reported significant within-group changes, and one [22] found no changes. Finally, one study [28] used the Six-Spot Step Test (SSST), reporting significant between-group improvement. Overall, the included studies show improvements in gait performance and exercise capacity.
3.4. Methodological Quality and Risk of Bias
Methodological and risk of bias assessments are included in Table 3 and Figure 2. The included studies scored between 7 and 9 on the PEDro scale, with four studies scoring 9, six scoring 8, and two studies scoring 7. None of the studies met the criterion for therapist blinding. According to the Cochrane risk of bias assessment, most studies raised some concerns, particularly regarding outcome selection, since many did not predefine which variables would be evaluated. Three studies [21,22,26] showed a high risk of bias related to the randomization process, as they did not provide any information suggesting that allocation was concealed. Another study [23] was considered to have a high risk of bias due to missing outcome data, as no information was provided regarding the extent or handling of the missing data.

Table 3.
PEDro scale.
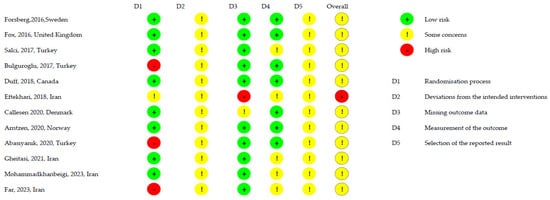
Figure 2.
Cochrane risk assessment tool [20,21,22,23,24,25,26,27,28,29,30,31].
4. Discussion
This systematic review aimed to evaluate the effects of core-focused interventions on gait and exercise capacity in people with MS. Most of the studies reported significant improvements in gait, assessed with the TUG, T25FW, and SSST. Exercise capacity, measured using the 6MWT and 2MWT, also significantly improved in the majority of the studies.
4.1. Multiple Sclerosis and Gait
This systematic review shows that gait improved in most studies following a core training program. People with MS commonly exhibit impaired gait patterns compared to healthy controls. Walking is considered one of the highest priorities for people with MS, as approximately 90% experience gait disturbances, which negatively impact their QoL [32,33]. One of the prerequisites for normal gait is adequate postural control [33].
The transverse abdominis plays a fundamental role in functional performance, particularly in trunk stabilization, which is essential for postural control [34]. Trunk instability is a significant contributor to motor control impairment and is a common symptom in people with MS [35]. Therefore, a rehabilitation program focused on core training could be beneficial for this population. Furthermore, previous studies have also shown the positive effects of core training on trunk stability and gait in other neurological populations, including post-stroke people [36,37] and individuals with cerebral palsy [38].
4.2. Core-Focused Training
The included studies implemented motor control exercises focusing on core activation. These exercises aimed to improve the coordination and timing of deep trunk muscles, such as the transverse abdominis. Progression in the programs usually began once the voluntary contraction of the transverse abdominis was achieved. Then, more demanding positions, such as seated, standing, or suspended, were used. A lack of coordination during muscle contraction may lead to reduced movement efficiency and compensatory patterns. Therefore, the literature recommends starting to teach the neutral pelvic position and the isolated activation of the transverse abdominis [39]. Once this initial goal is achieved, the difficulty of the exercises is progressively increased by incorporating more complex movements and combining them with other types of training [39]. Additional progression strategies, such as using elastic bands and exercise balls, were also employed to increase difficulty. In later stages, dual task exercises (e.g., carrying trays while maintaining transverse abdominis activation) were introduced. This final stage aligns with recent research highlighting the importance of incorporating cognitive-motor tasks into conventional training to reduce interference and enhance overall rehabilitation outcomes [40].
4.3. Core Training for Gait and Exercise Capacity
Gait and exercise capacity were assessed using various tools. Compared to the control group, most studies reported significant improvements in the experimental group for both outcomes.
Gait was assessed using different tests. The T25FW test reported improvements in two out of three studies, although only one reported a difference between groups. This test is considered the gold standard for assessing ambulation in people with MS [41]. The most frequently used test was the TUG, which reported improvements in four out of five studies; however, only two reported significant differences between groups. The TUG test is a reliable and widely used tool for assessing gait in people with MS [41,42]. The MSWS was the second most commonly used tool for evaluating gait, showing improvements in three out of five studies. As a patient-reported outcome, the MSWS is a highly recommended scale because it shows the patient’s perspective, does not require clinical resources, and has been shown to correlate the distance walked in the 6MWT [43]. The 10MTW showed improvements in two of the three articles in which it was used. It is widely used to assess gait speed in MS [44], as well as in other pathologies, such as stroke [45]. Additionally, the SSST was used in one study, which reported significant improvement. This test has shown reliability in the stroke [46,47] and MS populations [48].
The differences observed in the SSST, 6MWT, and 2MWT were clinically significant, exceeding the minimal clinically important difference (MCID) [49,50,51]. For the 25FWT and 10MWT, clinical significance varied, as not all the studies surpassed the MCID threshold [52,53]. Although statistically significant differences were observed for the TUG and MSWS, these did not meet the MCID, thus limiting clinical relevance [54,55].
There is a consensus that exercise-based interventions are beneficial in people with MS [55,56,57,58,59,60,61]. Despite the well-established benefits of physical exercise on functional performance in this population, it remains unclear whether one type of exercise is superior to others. One study attempted to determine whether differences exist among various exercise modalities but found no significant differences [62]. A recent systematic review and meta-analysis assessing the effects of core stability training on balance in people with MS concluded that six to ten weeks of training is an effective therapeutic strategy for balance improvement [15]. However, to our knowledge, this is the first systematic review to specifically evaluate the effects of core-focused interventions on gait and exercise capacity in people with MS. Previous systematic reviews [57,58,59,60,61,62] highlighted similar concerns, including the need for high-quality randomized controlled trials, standardized measurement tools to enhance research quality, and detailed descriptions of interventions to ensure replicability.
The studies included in this review consistently emphasize the need for further research with larger sample sizes and well-defined parameters for intensity, duration, and frequency to facilitate the development of clinical practice guidelines and validate the efficacy of interventions.
4.4. Limitations
This systematic review presents several limitations. It only included studies published in English, Spanish, and French, potentially excluding relevant articles in other languages. Our systematic review includes studies focused on core training alone or in combination with other therapies. This may have introduced bias or confounding factors. While the methodological quality of the studies included was generally good, some limitations were observed. A primary limitation was the lack of blinding among professionals conducting the interventions. However, blinding therapists is impractical, as they must guide and instruct the people throughout the intervention. Another methodological limitation observed was that people could potentially guess if they were not assigned to the intervention group. The high risk of bias observed in some trials makes it necessary to interpret the results with caution.
Future research should consider assessing gait in three categories: short-distance, long-distance, and self-reported. Exercise capacity should be widely explored in further studies to increase the evidence regarding the effects of this type of intervention on exercise capacity in people with MS. In addition, given the existing heterogeneity, future studies should implement standardized intervention protocols and develop more rigorous, high-quality designs to validate these preliminary findings.
5. Conclusions
The studies reviewed suggest that core-based exercise programs may effectively improve gait and exercise capacity in people with MS. However, we should be cautious considering heterogeneity in the assessment tools, the characteristics of the population, and intervention designs, and the high risk of bias of some trials. Core training programs for this population should focus on motor control and core stability, with an emphasis on the activation of the transverse abdominis and individualized progression. More research, including large-scale multicenter randomized controlled trials with pre-registered protocols, is needed to fully investigate the effectiveness of this intervention in people with MS. Additionally, applying consistent intervention models across multiple studies would allow for more rigorous evaluation and a comparison of the results.
Author Contributions
Conceptualization, I.C.-M. and M.C.V.; methodology, J.D.R.-M., P.R.-C. and M.R.-M.; software, J.M.-N.; validation, M.C.V., formal analysis, J.D.R.-M. and M.R.-M.; investigation, I.C.-M.; resources, J.M.-N.; data curation, J.D.R.-M. and P.R.-C.; writing—original draft preparation, J.D.R.-M.; writing—review and editing, I.C.-M. and J.D.R.-M.; visualization, J.M.-N.; supervision, I.C.-M.; project administration, M.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MS | Multiple Sclerosis |
| TUG | Timed Up and Go |
| MSWS | Multiple Sclerosis Walking Scale |
| 6MWT | 6-Minute Walking Test |
| 10MWT | 10-Meter Walk Test |
| 2MWT | 2-Minute Walk Test |
| T25FWT | Timed 25-Foot Walk Test |
| SSST | Six-Spot Step Test |
| QoL | Quality of Life |
Appendix A

Table A1.
Search strategy studies.
Table A1.
Search strategy studies.
| Database | Cinahl |
|---|---|
| Date | 3 September 2024 |
| Strategy | #1 AND #2 AND #3 AND #4 |
| #1 | AB (“Multiple Sclerosis” [MeSH] OR “multiple sclerosis” OR “disseminated sclerosis”) |
| #2 | AB (“Core Stability” [MeSH] OR “core” OR “trunk” OR “lumbopelvic hip complex” OR “Pelvic Floor” [MeSH] OR “pelvic floor” OR “diaphragm” OR “Abdominal Muscles” [MeSH] OR “abdomin*” OR “low back” OR “multifid*” OR “lumbar” OR “quadratus lumborum” OR “erector spinae” OR “external oblique” OR “internal oblique”) |
| #3 | AB (“Resistance Training” [MeSH] OR “Endurance Training” [MeSH] OR “train*” OR “Exercise” [MeSH] OR “Exercise Therapy” [Mesh] OR “Exercise Movement Techniques” [MeSH] OR “exercis*” OR “Pilates” OR “strength*” OR “stabili*” OR “intervention” OR “Rehabilitation” [MeSH] OR “rehabilitation”) |
| #4 | AB (“gait” OR “exercise capacity” OR “walking” OR “cardiorespiratory outcome” OR “locomotion”) |
| Database | Medline (via Pubmed) |
| Date | 3 September 2024 |
| Strategy | #1 AND #2 AND #3 AND #4 |
| #1 | AB (“Multiple Sclerosis” [MeSH] OR “multiple sclerosis” OR “disseminated sclerosis”) |
| #2 | AB (“Core Stability” [MeSH] OR “core” OR “trunk” OR “lumbopelvic hip complex” OR “Pelvic Floor” [MeSH] OR “pelvic floor” OR “diaphragm” OR “Abdominal Muscles” [MeSH] OR “abdomin*” OR “low back” OR “multifid*” OR “lumbar” OR “quadratus lumborum” OR “erector spinae” OR “external oblique” OR “internal oblique”) |
| #3 | AB (“Resistance Training” [MeSH] OR “Endurance Training” [MeSH] OR “train*” OR “Exercise” [MeSH] OR “Exercise Therapy” [Mesh] OR “Exercise Movement Techniques” [MeSH] OR “exercis*” OR “Pilates” OR “strength*” OR “stabili*” OR “intervention” OR “Rehabilitation” [MeSH] OR “rehabilitation”) |
| #4 | AB (“gait” OR “exercise capacity” OR “walking” OR “cardiorespiratory outcome” OR “locomotion”) |
| Database | Scopus |
| Date | 3 September 2024 |
| Strategy | #1 AND #2 AND #3 AND #4 |
| #1 | AB (“Multiple Sclerosis” [MeSH] OR “multiple sclerosis” OR “disseminated sclerosis”) |
| #2 | AB (“Core Stability” OR “core” OR “trunk” OR “lumbopelvic hip complex” OR “Pelvic Floor” OR “pelvic floor” OR “diaphragm” OR “Abdominal Muscles” OR “abdominal” OR “low back” OR “multifidos” OR “lumbar” OR “quadratus lumborum” OR “erector spinae” OR “external oblique” OR “internal oblique”) |
| #3 | AB “Resistance Training” OR “Endurance Training” OR “training” OR “Exercise” OR “Exercise Therapy” OR “Exercise Movement Techniques” OR “exercise” OR “Pilates” OR “strength” OR “stability” OR “intervention” OR “Rehabilitation” OR “rehabilitation”) |
| #4 | AB (“gait” OR “exercise capacity” OR “walking” OR “cardiorespiratory outcome” OR “locomotion”) |
| Database | Web of Science |
| Date | 3 September 2024 |
| Strategy | #1 AND #2 AND #3 AND #4 |
| #1 | AB (“Multiple Sclerosis” [MeSH] OR “multiple sclerosis” OR “disseminated sclerosis”) |
| #2 | AB (“Core Stability” [MeSH] OR “core” OR “trunk” OR “lumbopelvic hip complex” OR “Pelvic Floor” [MeSH] OR “pelvic floor” OR “diaphragm” OR “Abdominal Muscles” [MeSH] OR “abdomin*” OR “low back” OR “multifid*” OR “lumbar” OR “quadratus lumborum” OR “erector spinae” OR “external oblique” OR “internal oblique”) |
| #3 | AB (“Resistance Training” [MeSH] OR “Endurance Training” [MeSH] OR “train*” OR “Exercise” [MeSH] OR “Exercise Therapy” [Mesh] OR “Exercise Movement Techniques” [MeSH] OR “exercis*” OR “Pilates” OR “strength*” OR “stabili*” OR “intervention” OR “Rehabilitation” [MeSH] OR “rehabilitation”) |
| #4 | AB (“gait” OR “exercise capacity” OR “walking” OR “cardiorespiratory outcome” OR “locomotion”) |
| Database | ScienceDirect |
| Date | 3 September 2024 |
| Strategy | #1 AND #2 AND #3 AND #4 |
| #1 | AB (“Multiple Sclerosis” [mesh]) |
| #2 | AB (“core” OR “trunk”) |
| #3 | AB (“Resistance Training” [mesh] OR “Endurance Training” [mesh] “Exercise” [mesh]) |
| #4 | AB (“gait” OR “exercise capacity” OR “walking) |
| Database | PEDro |
| Date | 3 September 2024 |
| Strategy | #1 AND #2 + #3 AND #4 |
| #1 | (“Multiple Sclerosis”) |
| #2 | (“core”) |
| #3 | (“Multiple Sclerosis”) |
| #4 | (“trunk”) |
References
- Geurts, J.J.; Barkhof, F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008, 7, 841–851. [Google Scholar] [CrossRef]
- Cameron, M.H.; Wagner, J.M. Gait abnormalities in multiple sclerosis: Pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 2011, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Oliva Ramirez, A.; Keenan, A.; Kalau, O.; Worthington, E.; Cohen, L.; Singh, S. Prevalence and burden of multiple sclerosis-related fatigue: A systematic literature review. BMC Neurol. 2021, 21, 468. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Sanchez-López, M.; Cavero-Redondo, I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-Analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022, 103, 970–987. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Different Exercise Therapies on Balance Function and Functional Walking Ability in Multiple Sclerosis Disease Patients-A Network Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 7175. [Google Scholar] [CrossRef]
- Manocchio, N.; Argento, O.; Bossa, M.; Spanò, B.; Pellicciari, L.; Foti, C.; Nocentini, U. The Role of the Cerebellum in Multiple Sclerosis-Related Fatigue and Disability. J. Clin. Med. 2025, 14, 2840. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Lucchinetti, C.F.; Noseworthy, J.H. Multiple sclerosis: Current pathophysiological concepts. Lab. Investig. 2001, 81, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Menascu, S.; Vinogradsky, A.; Baransi, H.; Kalron, A. Range of motion abnormalities in the lower limb joints during gait in youth with multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105417. [Google Scholar] [CrossRef]
- Goldstein, R.E. Chapter 8. Exercise capacity. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Savci, S.; Inal-Ince, D.; Arikan, H.; Guclu-Gunduz, A.; Cetisli-Korkmaz, N.; Armutlu, K.; Karabudak, R. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil. Rehabil. 2005, 27, 1365–1371. [Google Scholar] [CrossRef]
- Romberg, A.; Virtanen, A.; Aunola, S.; Karppi, S.L.; Karanko, H.; Ruutiainen, J. Exercise capacity, disability and leisure physical activity of subjects with multiple sclerosis. Mult. Scler. 2004, 10, 212–218. [Google Scholar] [CrossRef]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. Handb. Clin. Neurol. 2018, 159, 237–250. [Google Scholar] [PubMed]
- Yoosefinejad, A.K.; Motealleh, A.; Khademi, S.; Hosseini, S.F. Lower Endurance and Strength of Core Muscles in Patients with Multiple Sclerosis. Int. J. MS Care 2017, 19, 100–104. [Google Scholar] [CrossRef]
- Choobsaz, H.; Sangtarash, F.; Javaherian, M.; Hadizadeh, M. Investigating the effects of core stability training on balance and gait in people with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2024, 87, 105686. [Google Scholar] [CrossRef]
- Ketelhut, N.B.; Kindred, J.H.; Manago, M.M.; Hebert, J.R.; Rudroff, T. Core muscle characteristics during walking of patients with multiple sclerosis. J. Rehabil. Res. Dev. 2015, 52, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Salcı, Y.; Fil, A.; Armutlu, K.; Yildiz, F.G.; Kurne, A.; Aksoy, S.; Nurlu, G.; Karabudak, R. Effects of different exercise modalities on ataxia in multiple sclerosis patients: A randomized controlled study. Disabil. Rehabil. 2017, 39, 2626–2632. [Google Scholar] [CrossRef]
- Bulguroglu, I.; Guclu-Gunduz, A.; Yazici, G.; Ozkul, C.; Irkec, C.; Nazliel, B.; Batur-Caglayan, H.Z. The effects of Mat Pilates and Reformer Pilates in patients with Multiple Sclerosis: A randomized controlled study. NeuroRehabilitation 2017, 41, 413–422. [Google Scholar] [CrossRef]
- Abasıyanık, Z.; Ertekin, Ö.; Kahraman, T.; Yigit, P.; Özakbaş, S. The effects of Clinical Pilates training on walking, balance, fall risk, respiratory, and cognitive functions in persons with multiple sclerosis: A randomized controlled trial. Explore 2020, 16, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, E.; Etemadifar, M. Impact of Clinical Mat Pilates on Body Composition and Functional Indices in Female Patients With Multiple Sclerosis. Crescent J. Med. Biol. Sci. 2018, 5, 297–305. [Google Scholar]
- Gheitasi, M.; Bayattork, M.; Andersen, L.L.; Imani, S.; Daneshfar, A. Effect of twelve weeks pilates training on functional balance of male patients with multiple sclerosis: Randomized controlled trial. J. Bodyw. Mov. Ther. 2021, 25, 41–45. [Google Scholar] [CrossRef]
- Mohammadkhanbeigi, S.; Tabrizi, Y.; Nabavi, S.; Minoonejad, H. The Comparable Effect of tDCS and Core Exercises on Balance and Mobility in Patients With Multiple Sclerosis. Iran. Rehabil. J. 2023, 20, 569–578. [Google Scholar] [CrossRef]
- Far, S.S.; Amiri, B.; Sahebozamani, M.; Ebrahimi, H.A.; Zemková, E. The effect of multi-function swing suspension training on upper and lower extremities function and quality of life in multiple sclerosis women with different disability status. Mult. Scler. Relat. Disord. 2023, 80, 105113. [Google Scholar] [CrossRef]
- Forsberg, A.; von Koch, L.; Nilsagård, Y. Effects on Balance and Walking with the CoDuSe Balance Exercise Program in People with Multiple Sclerosis: A Multicenter Randomized Controlled Trial. Mult. Scler. Int. 2016, 2016, 7076265. [Google Scholar] [CrossRef]
- Callesen, J.; Cattaneo, D.; Brincks, J.; Kjeldgaard Jørgensen, M.L.; Dalgas, U. How do resistance training and balance and motor control training affect gait performance and fatigue impact in people with multiple sclerosis? A randomized controlled multi-center study. Mult. Scler. 2020, 26, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, E.C.; Straume, B.; Odeh, F.; Feys, P.; Normann, B. Group-based, individualized, comprehensive core stability and balance intervention provides immediate and long-term improvements in walking in individuals with multiple sclerosis: A randomized controlled trial. Physiother. Res. Int. 2020, 25, e1798. [Google Scholar] [CrossRef]
- Duff, W.R.D.; Andrushko, J.W.; Renshaw, D.W.; Chilibeck, P.D.; Farthing, J.P.; Danielson, J.; Evans, C.D. Impact of Pilates Exercise in Multiple Sclerosis: A Randomized Controlled Trial. Int. J. MS Care 2018, 20, 92–100. [Google Scholar] [CrossRef]
- Fox, E.E.; Hough, A.D.; Creanor, S.; Gear, M.; Freeman, J.A. Effects of Pilates-Based Core Stability Training in Ambulant People With Multiple Sclerosis: Multicenter, Assessor-Blinded, Randomized Controlled Trial. Phys. Ther. 2016, 96, 1170–1178. [Google Scholar] [CrossRef]
- Abasıyanık, Z.; Kahraman, T.; Veldkamp, R.; Ertekin, Ö.; Kalron, A.; Feys, P. Changes in Gait Characteristics During and Immediately After the 6-Minute Walk Test in Persons With Multiple Sclerosis: A Systematic Review. Phys. Ther. 2022, 102, pzac036. [Google Scholar] [CrossRef] [PubMed]
- González del Rio, M.; Merchan Ruiz, M. Escala de Movilidad de 12 ítems para esclerosis múltiple: Análisis Mediante Diagnósticos de Enfermería. Rev. Científica Soc. Española Enfermería Neurológica 2020, 51, 23–26. [Google Scholar] [CrossRef]
- Hoffman, J.; Gabel, P. Expanding Panjabi’s stability model to express movement: A theoretical model. Med. Hypotheses 2013, 80, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Farid, R.; Norasteh, A.A.; Hatamian, H. The Effect of Core Stability Exercise Program on the Balance of Patients with Multiple Sclerosis. Casp. J. Neurol. Sci. 2016, 2, 9–17. [Google Scholar] [CrossRef]
- Van Criekinge, T.; Truijen, S.; Schröder, J.; Maebe, Z.; Blanckaert, K.; van der Waal, C.; Vink, M.; Saeys, W. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of Core Stability Training on Trunk Function, Standing Balance, and Mobility in Stroke Patients. Neurorehabilit. Neural Repair 2017, 31, 240–249. [Google Scholar] [CrossRef]
- Al-Nemr, A.; Kora, A.N. Effect of core stabilization versus rebound therapy on balance in children with cerebral palsy. Acta Neurol. Belg. 2024, 124, 843–851. [Google Scholar] [CrossRef]
- Akuthota, V.; Ferreiro, A.; Moore, T.; Fredericson, M. Core stability exercise principles. Curr. Sports Med. Rep. 2008, 7, 39–44. [Google Scholar] [CrossRef]
- Tramontano, M.; Argento, O.; Manocchio, N.; Piacentini, C.; Orejel Bustos, A.S.; De Angelis, S.; Bossa, M.; Nocentini, U. Dynamic Cognitive-Motor Training versus Cognitive Computer-Based Training in People with Multiple Sclerosis: A Preliminary Randomized Controlled Trial with 2-Month Follow-Up. J. Clin. Med. 2024, 13, 2664. [Google Scholar] [CrossRef]
- Bennett, S.E.; Bromley, L.E.; Fisher, N.M.; Tomita, M.R.; Niewczyk, P. Validity and Reliability of Four Clinical Gait Measures in Patients with Multiple Sclerosis. Int. J. MS Care 2017, 19, 247–252. [Google Scholar] [CrossRef]
- Valet, M.; Lejeune, T.; Devis, M.; van Pesch, V.; El Sankari, S.; Stoquart, G. Timed Up-and-Go and 2-Minute Walk Test in patients with multiple sclerosis with mild disability: Reliability, responsiveness and link with perceived fatigue. Eur. J. Phys. Rehabil. Med. 2019, 55, 450–455. [Google Scholar] [CrossRef]
- Chorschew, A.; Kesgin, F.; Bellmann-Strobl, J.; Flachenecker, P.; Schiffmann, I.; Rosenthal, F.; Althoff, P.; Drebinger, D.; Arsenova, R.; Rasche, L.; et al. Translation and validation of the multiple sclerosis walking scale 12 for the German population—The MSWS-12/D. Health Qual. Life Outcomes 2023, 21, 110. [Google Scholar] [CrossRef]
- Feys, P.; Bibby, B.; Romberg, A.; Santoyo, C.; Gebara, B.; de Noordhout, B.M.; Knuts, K.; Bethoux, F.; Skjerbæk, A.; Jensen, E.; et al. Within-day variability on short and long walking tests in persons with multiple sclerosis. J. Neurol. Sci. 2014, 338, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.S.P.; Lau, F.W.K.; Chan, G.K.L.; Sykes, K. The validity and reliability of a 6-Metre Timed Walk for the functional assessment of patients with stroke. Physiother. Theory Pract. 2010, 26, 251–255. [Google Scholar] [CrossRef]
- Arvidsson Lindvall, M.; Anderzén-Carlsson, A.; Appelros, P.; Forsberg, A. Validity and test-retest reliability of the six-spot step test in persons after stroke. Physiother. Theory Pract. 2020, 36, 211–218. [Google Scholar] [CrossRef]
- Liu, T.W.; Ng, S.S.; Cheung, K.Y.; Cheung, M.Y.; Hung, R.N.; Lam, M.F.; Wong, A.T.; Lai, C.Y.; Tse, M.M. Reliability and validity of Six-Spot Step Test (SSST) in stroke survivors. Eur. J. Phys. Rehabil. Med. 2021, 57, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Motl, R.W.; Sosnoff, J.J.; Pula, J.H. Further validation of the Six-Spot Step Test as a measure of ambulation in multiple sclerosis. Gait Posture 2015, 41, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Pommerich, U.M.; Brincks, J.; Skjerbæk, A.G.; Dalgas, U. The subjective minimal important change for the Six Spot Step Test in people with multiple sclerosis—The Danish MS Hospitals Rehabilitation study. Acta Neurol. Belg. 2022, 122, 893–901. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Bowman, T.; Mestanza Mattos, F.G.; Allera Longo, C.; Bocini, S.; Gennuso, M.; Marazzini, F.; Giuseppe Materazzi, F.; Pelosin, E.; Putzolu, M.; Salvalaggio, S.; et al. The minimally clinically important difference in the 2-minute walk test for people in the subacute phase after a stroke. Top. Stroke Rehabil. 2024, 11, 1–9. [Google Scholar] [CrossRef]
- Coleman, C.I.; Sobieraj, D.M.; Marinucci, L.N. Minimally important clinical difference of the Timed 25-Foot Walk Test: Results from a randomized controlled trial in patients with multiple sclerosis. Curr. Med. Res. Opin. 2012, 28, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Kamimoto, T.; Sakai, K.; Yamada, M.; Kawakami, M. Estimation of minimal detectable change in the 10-meter walking test for patients with stroke: A study stratified by gait speed. Front. Neurol. 2023, 14, 1219505. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, O.P.; Stienen, M.N.; Corniola, M.V.; Joswig, H.; Schaller, K.; Hildebrandt, G.; Smoll, N.R. Assessment of the Minimum Clinically Important Difference in the Timed Up and Go Test After Surgery for Lumbar Degenerative Disc Disease. Neurosurgery 2017, 80, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.; McNeill, M.; Hobart, J.; Wyrwich, K.W.; Poon, J.L.; Auguste, P.; Zhong, J.; Elkins, J. Identifying an important change estimate for the Multiple Sclerosis Walking Scale-12 (MSWS-12v1) for interpreting clinical trial results. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 2055217315596993. [Google Scholar] [CrossRef]
- Du, L.; Xi, H.; Zhang, S.; Zhou, Y.; Tao, X.; Lv, Y.; Hou, X.; Yu, L. Effects of exercise in people with multiple sclerosis: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1387658. [Google Scholar] [CrossRef]
- Reynolds, E.R.; Ashbaugh, A.D.; Hockenberry, B.J.; McGrew, C.A. Multiple Sclerosis and Exercise: A Literature Review. Curr. Sports Med. Rep. 2018, 17, 31–35. [Google Scholar] [CrossRef]
- Heine, M.; van de Port, I.; Rietberg, M.B.; van Wegen, E.E.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015, 11, CD009956. [Google Scholar] [CrossRef]
- Paltamaa, J.; Sjögren, T.; Peurala, S.H.; Heinonen, A. Effects of physiotherapy interventions on balance in multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2012, 44, 811–823. [Google Scholar] [CrossRef]
- Kjølhede, T.; Vissing, K.; Dalgas, U. Multiple sclerosis and progressive resistance training: A systematic review. Mult. Scler. 2012, 18, 1215–1228. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Kerling, A.; Keweloh, K.; Tegtbur, U.; Kück, M.; Grams, L.; Horstmann, H.; Windhagen, A. Effects of a Short Physical Exercise Intervention on Patients with Multiple Sclerosis (MS). Int. J. Mol. Sci. 2015, 16, 15761–15775. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).