Plant Iridoids Affect Intraocular Pressure and Vascular Flow in the Rabbit Eye
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
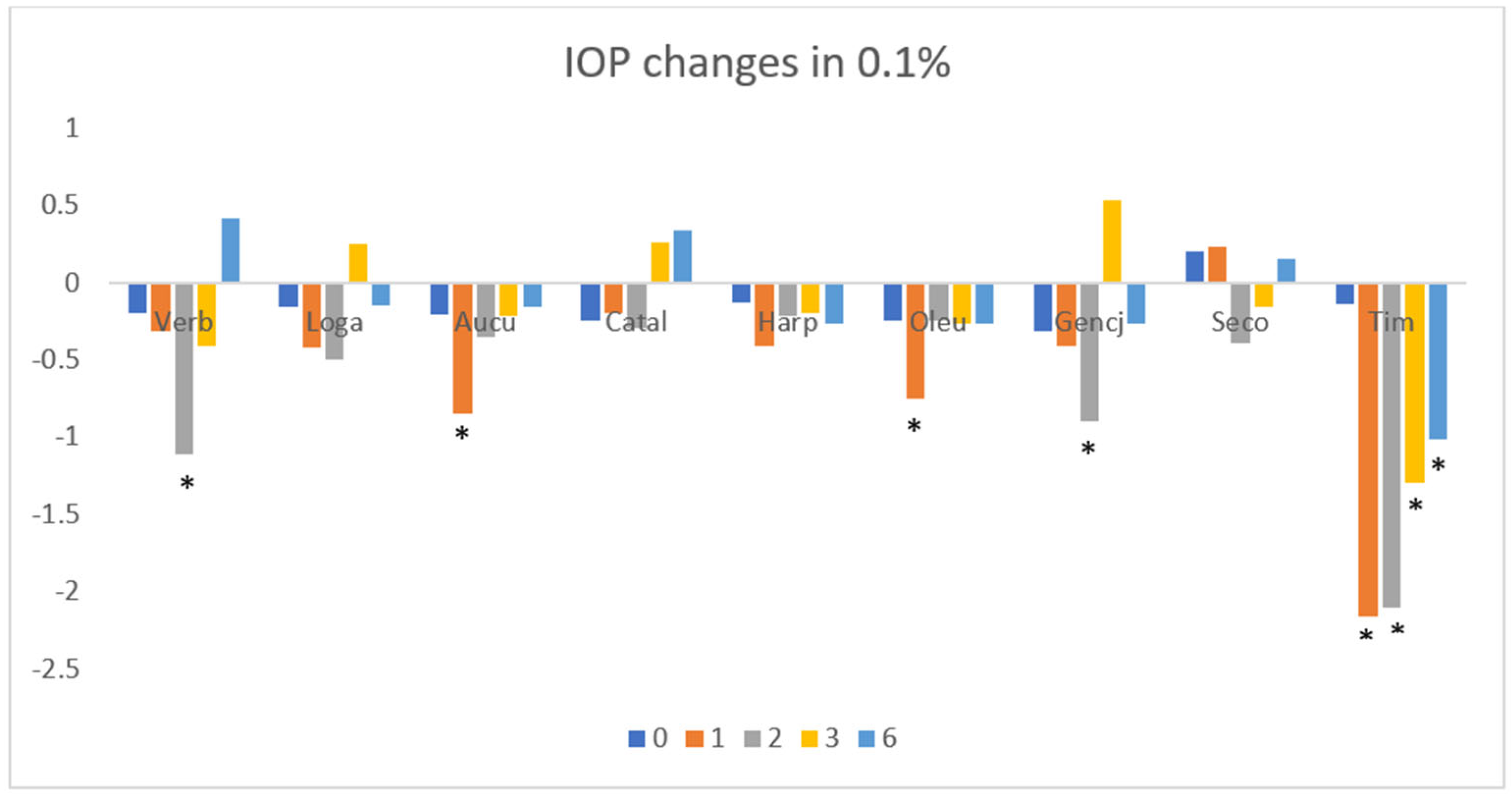
3.1. Intraocular Pressure
3.2. Blood Flow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IOP | intraocular pressure |
| VERB | verbenalin |
| LOGA | loganin |
| AUCU | aucubin |
| CATAL | catalpol |
| HARP | harpagoside |
| OLEU | oleuropein |
| GENCJ | gentiopicroside |
| SECO | secologanin |
| RGCs | retinal ganglion cells |
| POAG | primary open-angle glaucoma |
| RVO | Retinal vein obstruction |
| HPLC | High-Performance Liquid Chromatography |
| THC | Tetrahydrocannabinol |
| NTG | normal tension glaucoma |
| VEGF | vascular endothelial cell growth factor |
References
- Wang, L.; Meng, X.; Zhou, H.; Liu, Y.; Zhang, Y.; Liang, H.; Hou, G.; Kang, W.; Liu, Z. Iridoids and active ones in patrinia: A review. Heliyon 2023, 9, e16518. [Google Scholar] [CrossRef]
- Kouda, R.; Yakushiji, F. Recent advances in Iridoid chemistry: Biosynthesis and chemical synthesis. Chem.–Asian J. 2020, 15, 3771–3783. [Google Scholar] [CrossRef]
- Amle, V.S.; Rathod, D.A.; Keshamma, E.; Kumar, V.; Kumar, R.; Saha, P. Bioactive Herbal Medicine Use for Eye Sight: A Meta Analysis. J. Res. Appl. Sci. Biotechnol. 2022, 1, 42–50. [Google Scholar] [CrossRef]
- Paduch, R.; Woźniak, A.; Niedziela, P.; Rejdak, R. Assessment of eyebright (Euphrasia officinalis L.) extract activity in relation to human corneal cells using in vitro tests. Balk. Med. J. 2014, 31, 29–36. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, G. A review of plant species used to treat conjunctivitis. Phytother. Res. 2002, 16, 1–22. [Google Scholar] [CrossRef]
- Bielory, L.; Heimall, J. Review of complementary and alternative medicine in treatment of ocular allergies. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 395–399. [Google Scholar] [CrossRef]
- Kang, W.S.; Jung, E.; Kim, J. Aucuba japonica Extract and Aucubin Prevent Desiccating Stress-Induced Corneal Epithelial Cell Injury and Improve Tear Secretion in a Mouse Model of Dry Eye Disease. Molecules 2018, 23, 2599. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- He, S.; Stankowska, D.L.; Ellis, D.Z.; Krishnamoorthy, R.R.; Yorio, T. Targets of Neuroprotection in Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 85–106. [Google Scholar] [CrossRef]
- Schorr, E.M.; Rossi, K.C.; Stein, L.K.; Park, B.L.; Tuhrim, S.; Dhamoon, M.S. Characteristics and Outcomes of Retinal Artery Occlusion: Nationally Representative Data. Stroke 2020, 51, 800–807. [Google Scholar] [CrossRef]
- Youn, T.S.; Lavin, P.; Patrylo, M.; Schindler, J.; Kirshner, H.; Greer, D.M.; Schrag, M. Current treatment of central retinal artery occlusion: A national survey. J. Neurol. 2018, 265, 330–335. [Google Scholar] [CrossRef]
- Song, P.; Xu, Y.; Zha, M.; Zhang, Y.; Rudan, I. Global epidemiology of retinal vein occlusion: A systematic review and meta-analysis of prevalence, incidence, and risk factors. J. Glob. Health 2019, 9, 010427. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K.; Jose, J.A. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma–An open label study. Saudi J. Ophthalmol. 2015, 29, 197–200. [Google Scholar] [CrossRef]
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of cornelian cherry iridoid-polyphenolic fraction and Loganic acid to reduce intraocular pressure. Evid.-Based Complement. Altern. Med. 2015, 1, 939402. [Google Scholar] [CrossRef]
- Alasbahi, R.; Melzig, M. Forskolin and derivatives as tools for studying the role of cAMP. Die Pharm.-Int. J. Pharm. Sci. 2012, 67, 5–13. [Google Scholar]
- Wagh, V.; Patil, P.; Surana, S.; Wagh, K. Forskolin: Upcoming antiglaucoma molecule. J. Postgrad. Med. 2012, 58, 199–202. [Google Scholar] [CrossRef]
- Senapati, S.; Youssef, A.A.A.; Sweeney, C.; Cai, C.; Dudhipala, N.; Majumdar, S. Cannabidiol loaded topical ophthalmic nanoemulsion lowers intraocular pressure in normotensive Dutch-belted rabbits. Pharmaceutics 2022, 14, 2585. [Google Scholar] [CrossRef]
- Pinar-Sueiro, S.; Rodríguez-Puertas, R.; Vecino, E. Cannabinoid applications in glaucoma. Arch. Soc. Española Oftalmol. Engl. Ed. 2011, 86, 16–23. [Google Scholar] [CrossRef]
- Jay, W.; Green, K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch. Ophthalmol. 1983, 101, 591–593. [Google Scholar] [CrossRef]
- Green, K.; Symonds, C.M.; Oliver, N.W.; Elijah, R.D. Intraocular pressure following systemic administration of cannabinoids. Curr. Eye Res. 1982, 2, 247–253. [Google Scholar] [CrossRef]
- Miller, S.; Daily, L.; Leishman, E.; Bradshaw, H.; Straiker, A. Δ9-Tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5904–5911. [Google Scholar] [CrossRef]
- Adelli, G.R.; Bhagav, P.; Taskar, P.; Hingorani, T.; Pettaway, S.; Gul, W.; ElSohly, M.A.; Repka, M.A.; Majumdar, S. Development of a Δ9-tetrahydrocannabinol amino acid-dicarboxylate prodrug with improved ocular bioavailability. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2167–2179. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Tripathy, M.; Kapitonova, M.Y.; Kutty, M.K.; Smirnov, A.; Khalid, Z.; Ismail, N.M. Topical trans-resveratrol ameliorates steroid-induced anterior and posterior segment changes in rats. Exp. Eye Res. 2016, 143, 9–16. [Google Scholar] [CrossRef]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H. Topical curcumin nanocarriers are neuroprotective in eye disease. Sci. Rep. 2018, 8, 11066. [Google Scholar] [CrossRef]
- Hirooka, K.; Tokuda, M.; Miyamoto, O.; Itano, T.; Baba, T.; Shiraga, F. The Ginkgo biloba extract (EGb 761) provides a neuroprotective effect on retinal ganglion cells in a rat model of chronic glaucoma. Curr. Eye Res. 2004, 28, 153–157. [Google Scholar] [CrossRef]
- Dorairaj, S.; Ritch, R.; Liebmann, J.M. Visual improvement in a patient taking ginkgo biloba extract: A case study. Explore 2007, 3, 391–395. [Google Scholar] [CrossRef]
- Guo, X.; Kong, X.; Huang, R.; Jin, L.; Ding, X.; He, M.; Liu, X.; Patel, M.C.; Congdon, N.G. Effect of Ginkgo biloba on visual field and contrast sensitivity in Chinese patients with normal tension glaucoma: A randomized, crossover clinical trial. Investig. Ophthalmol. Vis. Sci. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Hsu, J.-H.; Fu, H.-I.; Chen, C.-C.; Tung, K.-C. Lowering the intraocular pressure in rats and rabbits by cordyceps cicadae extract and its active compounds. Molecules 2022, 27, 707. [Google Scholar] [CrossRef]
- Manabe, K.; Kaidzu, S.; Tsutsui, A.; Mochiji, M.; Matsuoka, Y.; Takagi, Y.; Miyamoto, E.; Tanito, M. Effects of French maritime pine bark/bilberry fruit extracts on intraocular pressure for primary open-angle glaucoma. J. Clin. Biochem. Nutr. 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zheng, C.Z.; Hao, X.P.; Zhang, D.J.; Mao, A.W.; Yuan, P. Catalpol ameliorates diabetic atherosclerosis in diabetic rabbits. Am. J. Transl. Res. 2016, 8, 4278–4288. [Google Scholar]
- Zhang, Y.P.; Pan, C.S.; Yan, L.; Liu, Y.Y.; Hu, B.H.; Chang, X.; Li, Q.; Huang, D.D.; Sun, H.Y.; Fu, G.; et al. Catalpol restores LPS-elicited rat microcirculation disorder by regulation of a network of signaling involving inhibition of TLR-4 and SRC. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G1091–G1104. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Q.; Shao, S.; Chen, Y.; Chen, W.; Xu, X. Lyophilized Powder of Catalpol and Puerarin Protected Cerebral Vessels from Ischemia by Its Anti-apoptosis on Endothelial Cells. Int. J. Biol. Sci. 2017, 13, 327–338. [Google Scholar] [CrossRef]
- Dong, W.; Xian, Y.; Yuan, W.; Huifeng, Z.; Tao, W.; Zhiqiang, L.; Shan, F.; Ya, F.; Hongli, W.; Jinghuan, W.; et al. Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats’ stroke model. J. Ethnopharmacol. 2016, 191, 169–179. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, J.; Zha, Z.; Zhao, H.; Xue, B.; Jin, L.; Wang, L. Effect and Mechanism of Catalpol on Remyelination via Regulation of the NOTCH1 Signaling Pathway. Front. Pharmacol. 2021, 12, 628209. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Shi, M.; Xie, L.; Deng, M.; Chen, H.; Li, X. Therapeutic potential of catalpol and geniposide in Alzheimer’s and Parkinson’s diseases: A snapshot of their underlying mechanisms. Brain Res. Bull. 2021, 174, 281–295. [Google Scholar] [CrossRef]
- Gao, X.; Xu, J.; Liu, H. Protective effects of catalpol on mitochondria of hepatocytes in cholestatic liver injury. Mol. Med. Rep. 2020, 22, 2424–2432. [Google Scholar] [CrossRef]
- Kaeidi, A.; Sahamsizadeh, A.; Allahtavakoli, M.; Fatemi, I.; Rahmani, M.; Hakimizadeh, E.; Hassanshahi, J. The effect of oleuropein on unilateral ureteral obstruction induced-kidney injury in rats: The role of oxidative stress, inflammation and apoptosis. Mol. Biol. Rep. 2020, 47, 1371–1379. [Google Scholar] [CrossRef]
- Murata, K.; Abe, Y.; Futamura-Masuda, M.; Uwaya, A.; Isami, F.; Deng, S.; Matsuda, H. Effect of Morinda citrifolia fruit extract and its iridoid glycosides on blood fluidity. J. Nat. Med. 2014, 68, 498–504. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Zhang, L.; Li, C.; Coffie, J.W.; Geng, X.; Qiu, L.; You, X.; Fang, Z.; Song, M.; et al. Aucubin promotes angiogenesis via estrogen receptor beta in a mouse model of hindlimb ischemia. J. Steroid Biochem. Mol. Biol. 2017, 172, 149–159. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, X.; Zhang, Q.; Wang, Q.; She, C.; Li, Z. Aucubin protects against retinal ganglion cell injury in diabetic rats via inhibition of the p38MAPK pathway. Am. J. Transl. Res. 2023, 15, 1007–1016. [Google Scholar]
- Park, S.-B.; Jung, W.K.; Yu, H.-Y.; Kim, Y.H.; Kim, J. Effect of Aucubin-Containing Eye Drops on Tear Hyposecretion and Lacrimal Gland Damage Induced by Urban Particulate Matter in Rats. Molecules 2022, 27, 2926. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, M.; Wang, T.; Zhang, H.; Li, S.; Guo, Y. Picroside II protects the blood-brain barrier by inhibiting the oxidative signaling pathway in cerebral ischemia-reperfusion injury. PLoS ONE 2017, 12, e0174414. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, L.; Guo, Y.; Zhang, M.; Pei, H. Picroside II Inhibits Neuronal Apoptosis and Improves the Morphology and Structure of Brain Tissue following Cerebral Ischemic Injury in Rats. PLoS ONE 2015, 10, e0124099. [Google Scholar] [CrossRef]
- Wei, H.J.; Yang, H.H.; Chen, C.H.; Lin, W.W.; Chen, S.C.; Lai, P.H.; Chang, Y.; Sung, H.W. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J. Control. Release Off. J. Control. Release Soc. 2007, 120, 27–34. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Chen, J.; Lu, P.; Chen, C.; Fu, M.; Fang, J.; Gao, J.; Zhu, L.; Liang, R.; et al. An effective solution to discover synergistic drugs for anti-cerebral ischemia from traditional Chinese medicinal formulae. PLoS ONE 2013, 8, e78902. [Google Scholar] [CrossRef]
- Lapi, D.; Di Maro, M.; Mastantuono, T.; Battiloro, L.; Sabatino, L.; Muscariello, E.; Colantuoni, A. Effects of oleuropein and pinoresinol on microvascular damage induced by hypoperfusion and reperfusion in rat pial circulation. Microcirculation 2015, 22, 79–90. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J.; Gong, C.; Wang, B.; Zhou, P.; Zhou, S.; Yao, X. Gentiopicroside Ameliorates Diabetic Peripheral Neuropathy by Modulating PPAR-Γ/AMPK/ACC Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 585–596. [Google Scholar] [CrossRef]
- Duan, F.X.; Shi, Y.J.; Chen, J.; Song, X.; Shen, L.; Qi, Q.; Ding, S.Q.; Wang, Q.Y.; Wang, R.; Lü, H.Z.; et al. The neuroprotective role of morroniside against spinal cord injury in female rats. Neurochem. Int. 2021, 148, 105105. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef]
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Lee, S.O.; Han, S.M.; Maeng, S.; Park, J.H. Loganin enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Physiol. Behav. 2017, 17, 243–248. [Google Scholar] [CrossRef]
- Fan, N.; Wang, P.; Tang, L.; Liu, X. Ocular Blood Flow and Normal Tension Glaucoma. BioMed Res. Int. 2015, 2015, 308505. [Google Scholar] [CrossRef]
- Abegão Pinto, L.; Willekens, K.; Van Keer, K.; Shibesh, A. Ocular blood flow in glaucoma—The Leuven Eye Study. Acta Ophthalmol. 2016, 94, 592–598. [Google Scholar] [CrossRef]
- Naik, S.; Pandey, A.; Lewis, S.A.; Rao, B.S.S.; Mutalik, S. Neuroprotection: A versatile approach to combat glaucoma. Eur. J. Pharmacol. 2020, 881, 173208. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Sena, D.F.; Lindsley, K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2017, 1, Cd006539. [Google Scholar] [CrossRef]
- Ganguly, S.; Wulff, D.; Phan, C.-M.; Jones, L.W.; Tang, X.S. Injectable and 3D Extrusion Printable Hydrophilic Silicone-Based Hydrogels for Controlled Ocular Delivery of Ophthalmic Drugs. ACS Appl. Bio Mater. 2024, 7, 6286–6296. [Google Scholar] [CrossRef]
- Su, W.; Wang, R.; Qian, C.; Li, X.; Tong, Q.; Jiao, T. Research progress review of preparation and applications of fluorescent hydrogels. J. Chem. 2020, 2020, 8246429. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumny, D.; Sozański, T.; Szeląg, A.; Szumny, A. Plant Iridoids Affect Intraocular Pressure and Vascular Flow in the Rabbit Eye. Appl. Sci. 2025, 15, 5055. https://doi.org/10.3390/app15095055
Szumny D, Sozański T, Szeląg A, Szumny A. Plant Iridoids Affect Intraocular Pressure and Vascular Flow in the Rabbit Eye. Applied Sciences. 2025; 15(9):5055. https://doi.org/10.3390/app15095055
Chicago/Turabian StyleSzumny, Dorota, Tomasz Sozański, Adam Szeląg, and Antoni Szumny. 2025. "Plant Iridoids Affect Intraocular Pressure and Vascular Flow in the Rabbit Eye" Applied Sciences 15, no. 9: 5055. https://doi.org/10.3390/app15095055
APA StyleSzumny, D., Sozański, T., Szeląg, A., & Szumny, A. (2025). Plant Iridoids Affect Intraocular Pressure and Vascular Flow in the Rabbit Eye. Applied Sciences, 15(9), 5055. https://doi.org/10.3390/app15095055







