Abstract
The banana chip industry generates significant quantities of waste, including banana peels and used palm oil, which present both environmental and economic challenges. This study explored converting banana peel waste into porous adsorbents via chemical and thermal activation using sulfuric acid (S-BP) and 5% w/v acetic acid (A-BP) as activating agents. Characterization using field emission scanning electron microscopy (FESEM) and Brunauer–Emmett–Teller (BET) analysis revealed notable morphological distinctions and enhanced porosity. The BET surface areas of S-BP and A-BP were 338.959 m2/g and 201.722 m2/g, respectively, significantly higher than that of calcined banana peel (C-BP) at 3.202 m2/g. Despite the higher surface area of S-BP, A-BP, prepared under milder acetic acid conditions, was further investigated for adsorption studies. A-BP effectively reduced the free fatty acids (FFAs) in used palm oil from 3.108% to 1.69% within 30 min. Adsorption isotherms favored the Freundlich model (R2 = 0.9115), indicating multilayer adsorption behavior. The adsorption energy derived from the Dubinin–Radushkevich (D–R) model was determined to be 2.61 J/mol, indicating that the adsorption process primarily occurs through physisorption. This study highlights a sustainable approach to waste management and resource recovery, promoting circular economy principles in the banana chip industry.
1. Introduction
The banana chip industry is a major economic sector in regions with extensive banana cultivation, such as Sukhothai Province, Thailand. This industry generates substantial amounts of waste annually as by-products of its production processes. Among these, banana peels (BPs) constitute a significant fraction and are commonly discarded as landfill material or repurposed as low-value agricultural feed. However, due to their high organic content—including cellulose, hemicellulose, lignin, and various bioactive compounds—banana peels offer considerable potential for value-added applications [1,2]. By employing chemical and thermal modifications, these wastes can be transformed into porous adsorbents, effectively minimizing environmental impacts associated with disposal. This strategy not only mitigates waste management challenges but also supports the sustainable production of high-value materials from agricultural residues. These porous adsorbents have broad applications across various fields, such as water purification, oil spill remediation, and as supports for catalytic reactions [3,4,5,6].
The fabrication of porous adsorbents from banana peel waste involves several essential stages, including the pre-treatment of raw banana peels, calcination, chemical activation, and post-treatment procedures. These steps are carefully designed to enhance the adsorption properties of the material by substantially increasing its surface area and facilitating the formation of a well-defined porous structure. Conventional chemical activation techniques commonly employ potent chemical agents, such as sodium hydroxide (NaOH), potassium hydroxide (KOH), phosphoric acid (H3PO4), and sulfuric acid (H2SO4) [7,8]. Despite their effectiveness, these methods present considerable challenges, including safety hazards during handling, high production costs, and environmental issues due to the generation of hazardous waste. To overcome these challenges, acetic acid has been investigated as a sustainable chemical activation agent due to its widespread use in both household and industrial applications, offering a safe and cost-effective approach for the production of porous adsorbents. In the future, banana chip industries could use commercially available acetic acid to produce porous adsorbents on their own. This method offers a practical and eco-friendly solution for both small- and medium-sized enterprises (SMEs) and larger industries.
The production of banana chips in small- and medium-sized businesses often generates substantial quantities of used palm oil during the frying process, posing a common operational challenge. The recycling of used palm oil has the potential to lower costs, as it contains numerous polar molecules. Effective removal of these molecules through adsorption could address this issue. This process is important because the quality of used cooking oil is determined by key indicators of degradation, such as the free fatty acid content, the peroxide value, and the concentration of polar compounds.
Free fatty acids (FFAs) serve as critical indicators of oil hydrolysis and are widely monitored to assess oil quality. According to the Ministry of Public Health in Thailand, fresh oils with FFA levels exceeding 2% are generally deemed unsuitable for consumption. For used oils, it is advised that they be discarded once the FFA content reaches a threshold of 5% [9]. Peroxide values (PVs), which reflect the degree of lipid oxidation, are essential indicators for evaluating oil quality. For oils designated for food-grade applications, PVs must remain below 10 meq O2/kg to ensure compliance with safety and quality standards [10]. Polar compounds, including polymerized triglycerides, free fatty acids, and secondary oxidation products, are also regulated to preserve oil quality and safety. Specifically, the polar compound content in used cooking oil is typically restricted to a maximum of 25% for continued use in food frying [11]. Exceeding this limit renders the oil unsuitable for consumption and poses significant health risks.
In the adsorption process, activated carbon and other carbon-based materials are extensively utilized as adsorbents to mitigate degradation markers, owing to their high surface area, significant porosity, and robust capacity for adsorbing polar molecules [12,13,14,15,16,17,18,19].
B. Buczek et al. [12] reported that fresh vegetable oils are mostly non-polar. However, during frying, complex chemical reactions produce polar compounds. Studies have shown that treatment methods can effectively lower levels of free fatty acids, peroxide values, and polar compounds in used cooking oils. These treatments improve the quality of oil, allowing it to be reused for various purposes. The efficiency of activated carbon can be further improved by optimizing activation methods, such as chemical or physical activation, and selecting suitable raw materials for its production. Building upon the application of bio-based adsorbents, S. Miskah et al. [13] investigated the potential of activated carbon derived from agricultural waste, specifically durian peel, for the purification of used cooking oil. The study demonstrated that this adsorbent effectively reduced both the peroxide value and free fatty acid content of the oil, thereby improving its reusability. Notably, the performance was significantly enhanced using H2SO4-impregnated biomass, achieving optimal results after 150 min of contact time. This treatment reduced the free fatty acid content to 0.0637% and the peroxide value to 0.41 meq O2/kg. Natural adsorbents have shown considerable potential for oil purification, as demonstrated by R. Bostan et al. [14], who evaluated the performance of zeolite and eggshell in treating used sunflower and palm oils. Their adsorption studies, conducted with dosages varying from 5 to 30 g per 100 mL of oil, revealed that NaOH-activated zeolite and heat-treated eggshell exhibited superior adsorption capacities and enhanced surface areas. Furthermore, an increase in the adsorbent dosage and contact time effectively reduced acidity and peroxide values, underscoring the efficiency of natural adsorbents in improving oil quality. Building on the application of natural materials, W.D.P. Rengga et al. [15] investigated the use of banana-peel-derived activated carbon for the reduction of free fatty acids (FFAs) in used cooking oil. Their study emphasized the influence of activation temperatures (600 °C, 650 °C, and 700 °C) on the adsorption performance. Batch adsorption experiments conducted with powdered activated carbon (200 mesh) demonstrated that the adsorption process conformed closely to the Freundlich isotherm model (R2 = 0.97), suggesting effective physical adsorption. The maximum adsorption capacity of 27.404 mg/g was achieved at an activation temperature of 700 °C, underscoring the critical role of process optimization. The effectiveness of mineral-based adsorbents in regenerating used frying oils was further validated in a study by A. Miyagi et al. [16]. This investigation compared the performance of silica gel, magnesium oxide, activated clay, and aluminum hydroxide gel. Among these materials, silica gel demonstrated superior efficacy in enhancing oil quality, albeit with a relatively limited adsorption capacity. Analysis using the Langmuir adsorption model determined a maximum adsorption capacity of 219 mg/g for silica gel in the removal of total polar materials. Similarly, zeolites have been extensively studied for polar molecule removal. M.L.T.A. Putranti et al. [17] investigated the application of activated natural zeolite for the adsorption of free fatty acids (FFAs) from low-grade cooking oil. Zeolite activated with 0.75 M NaOH exhibited superior efficacy in FFA removal compared to HCl-activated zeolite, particularly after calcination. Adsorption equilibrium studies conducted at 25 °C, 40 °C, and 80 °C revealed that the NaOH-activated zeolite adhered to the Freundlich isotherm model, signifying multilayer adsorption and demonstrating consistent performance across varying conditions. Additionally, bio-based materials have been extensively modified to enhance their adsorption capabilities. N. H. Alias et al. [18] investigated the use of raw and modified Kapok-fiber-based adsorbents for the purification of used cooking oil. The unmodified Kapok fiber demonstrated an adsorption efficiency of 50.64%. However, modification via the incorporation of calcium oxide through an esterification process enhanced the material’s hydrophobicity, increasing the adsorption efficiency to 53.56%. In advanced methods, T.W. Chung et al. [19] explored the use of commercial resins (A26OH and IRA900Cl) for free fatty acid removal without alkali chemicals during food oil refining. The study showed that at 35 °C, A26OH removed up to 25.5% of free fatty acids, significantly outperforming IRA900Cl. Adsorption studies aligned with the Langmuir isotherm model, indicating a monolayer adsorption mechanism. The reviewed literature demonstrates the versatility of diverse adsorbents, such as activated carbon, natural materials, and advanced resins, in the effective removal of polar compounds from used cooking oil. These findings contribute to improving the quality of recovered used cooking oil for reuse and furthering the development of sustainable and efficient purification technologies.
Banana peel adsorbents are not only effective in purifying used oil but also demonstrate significant potential in various water treatment processes, including the removal of heavy metals, organic pollutants, and dyes. Their application in wastewater treatment has been widely studied, highlighting their efficiency in pollutant adsorption. Research by T. Bai et al. [20] showed that when banana peel hydrothermal carbon was modified with a KOH solution, it exhibited an adsorption capacity of 42.92 mg/g and a removal rate of 86.84% for Pb2+. Similarly, a study by D. Ramutshatsha-Makhwedzha et al. [21] demonstrated that modified banana peel effectively removed both Pb2+ and Cd2+, with an efficiency exceeding 99.9%. Further supporting the applicability of banana peel adsorbents in heavy metal removal, Z. Huang et al. [22] found that chemically activated banana peel biochar effectively removed Cr5+ from aqueous solutions, significantly lowering its concentration in wastewater. Beyond heavy metal removal, banana peel adsorbents have also been extensively studied for their ability to remove cationic dyes (basic dyes), which are widely used in the textile and pharmaceutical industries. Their high adsorption efficiency for these dyes is attributed to their negatively charged surface under neutral to slightly basic pH conditions. For instance, J. Yhon et al. [23] reported that banana peel powder achieved an adsorption efficiency of 62.5% for methylene blue at an optimized pH of 6. Similarly, M. Azhar-ul-Haq et al. [24] found that banana-peel-based activated carbon removed 93% of crystal violet from aqueous solutions within just 10 min of contact time. In addition to laboratory-scale experiments, banana peel adsorbents have also shown effectiveness in real industrial wastewater applications. M. Akter et al. [25] reported that the removal efficiency of dyes in actual textile effluent remained significant, indicating the practical potential of banana peel adsorbents for industrial dye removal. Furthermore, desorption studies revealed that about 95% of the adsorbent could be recovered through a simple acid–base treatment, suggesting its feasibility for regeneration and reuse in multiple adsorption cycles. These studies collectively highlight the potential of banana-peel-based adsorbents as a sustainable, cost-effective, and highly efficient material for wastewater treatment.
This study explores an innovative waste-to-resource approach aimed at contributing to sustainable development goals, addressing critical waste management issues, and advancing green technologies. The focus of this initiative is to transform banana peel waste generated by the banana chip industry into valuable porous adsorbents for the purification and recovery of used palm oil within the same industry, promoting a circular production system.
The adsorbents in this work were divided into three sample categories for comparison: untreated banana peel waste (C-BP), banana peel waste impregnated with H2SO4 (S-BP), and banana peel waste impregnated with 5% w/v commercial acetic acid (A-BP). The differences in the size, morphology, and surface properties of these three prepared samples have been thoroughly analyzed. Moreover, the efficiency of the adsorbents in the removal of free fatty acids (FFAs) has been extensively investigated, with evaluations typically based on adsorption isotherm analyses conducted under ambient conditions.
2. Materials and Methods
Waste banana peels and used palm oil were collected from Nong Tum Subdistrict in Kong Krailat District, Sukhothai Province, Thailand. Sulfuric acid (97% H2SO4) was procured from Merck, Darmstadt, Germany, while food-grade acetic acid (5% v/v) was purchased from an online store in Thailand. All chemicals were used as received without further purification.
2.1. Preparation of Banana Peel (BP) Adsorbent
The raw banana peels were cut into small pieces and thoroughly washed to remove dust and impurities. The cleaned peels were then dried using a temperature-controlled drying oven set at 120 °C for 12 h. The dried banana peel was calcined at 600 °C in a hot air oven for a duration of 2 h. After calcination, the material was stored in a desiccator to prevent moisture absorption. This prepared material was designated as C-BP.
In the chemical activation process, 50.00 g of thoroughly dried banana peel was mixed with 500 mL of a 28% w/w concentrated H2SO4 solution, designated as S-BP. For comparison, an equal amount of dried banana peel was soaked in a 5% w/v vinegar solution for 24 h, referred to as A-BP. During the soaking process, the acidic solution was absorbed by the banana peel, penetrating its surface and initiating interactions with the material.
Subsequently, both S-BP and A-BP, the soaked banana peel samples, were subjected to high-temperature activation at 600 °C for a duration of 2 h. During this stage, the absorbed acid decomposed into volatile gases, which escaped, thereby creating a network of pores within the material. This templated pore structure significantly enhanced the porosity of the activated material, making it highly suitable for applications that require a large surface area and efficient adsorption capabilities.
2.2. Characterization
Prior to conducting the adsorption experiment, the surface morphology of the three different adsorbent samples was analyzed to identify the sample exhibiting the highest porosity and surface area. The selected sample was employed in adsorption experiments, with its morphology characterized using field emission scanning electron microscopy (FESEM) on a Thermo Scientific Apreo system (Hillsboro, OR, USA). Nitrogen adsorption–desorption isotherms were utilized to determine the surface area, total pore volume, and average pore diameter of the activated carbon samples, employing a Micromeritics TriStar II 3020 analyzer (Norcross, GA, USA). The specific surface area of the prepared samples was calculated using the Brunauer–Emmett–Teller (BET) theory. The functional groups in the sample were identified using Fourier-transform infrared spectroscopy (FTIR) with a Perkin Elmer Spectrum GX instrument (Waltham, MA, USA). All instrumental analyses, including FESEM, BET, and FTIR, were performed at the Science Laboratory Center, Faculty of Science, Naresuan University, Phitsanulok, Thailand. Additionally, the heavy metal ion content in the adsorbent sample was analyzed using inductively coupled plasma optical emission spectroscopy (ICP-OES) following EPA Method 3052, with the analysis performed at Central Lab Thai, Chiang Mai, Thailand.
2.3. Adsorption Experiment
An adsorption experiment was conducted by preparing initial concentrations of used oil (Co) at 50%, 25%, 12.5%, and 6.25% v/v through dilution with hexane at room temperature. Additionally, undiluted used oil (100% v/v) was included as a control for comparison. Subsequently, 50 mL of each used oil concentration was mixed with 1 g of A-BP adsorbent under ambient conditions (308.15 K). The reaction was conducted as a batch experiment with continuous stirring for 60 min to achieve equilibrium. At 10 min intervals, aliquots of the suspension were filtered using Whatman No. 1 filter paper. The resulting supernatant was analyzed to determine the percentage of free fatty acids (%FFA) in the used oil via titration with a standardized base solution. This calculation assumes that oleic acid is the predominant free fatty acid present in the oil. The result reflects the extent of hydrolysis or degradation occurring in the used oil.
After the adsorption process was completed, a 5 g sample of the used oil solution was dissolved in a solvent mixture consisting of 25 mL ethanol and 25 mL diethyl ether. Phenolphthalein (1 mL) was introduced as an indicator into the solution, followed by titration with a standardized 0.5 M potassium hydroxide (KOH) solution until the appearance of a stable pink endpoint. The free fatty acid content was quantified based on the oleic acid equivalent, as described in Equation (1) [26,27].
where VKOH represents the volume of the KOH solution used (titrant), CKOH denotes the concentration of the KOH solution (0.5 M), MWoleic acid is the molecular weight of oleic acid (282 g/mol), and W indicates the weight of the used oil (5 g).
2.4. Determination of Adsorption Equilibrium and Modeling of Adsorption Isotherms
Prior to the adsorption test, used oil at concentrations (Co) of 100%, 50%, 25%, 12.5%, and 6.25% v/v was titrated with a KOH solution to determine the concentration of polar molecules (oleic acid) in the used oil. The concentration of Co was subsequently converted to mg/L, and the data are presented in Table 1.

Table 1.
Initial concentrations of used oil (Co) and the number of polar molecules of oleic acid in 5 g of used oil.
At different time intervals before reaching equilibrium, the purified used oil samples were titrated with KOH to measure the concentration of polar molecules in the used oil, denoted as Ct (mol/L), which was then converted into mg/L. The adsorption process proceeded until the equilibrium adsorption capacity (Ce) was achieved, which occurred at an equilibrium time of 30 min. The equilibrium concentration of used oil adsorbed onto the solid adsorbent (Qₑ, mg/g) was determined by calculating the difference between the initial concentration of used oil (Co) and its concentration at equilibrium time (Ce) using Equation (2).
In this case, V represents the volume of the titration solution (0.05 L of adsorbate solution), while W denotes the mass of the adsorbent, fixed at 1 g.
The interaction between the adsorbate and the adsorbent was evaluated through equilibrium analysis of the adsorption process, employing the Ce and Qe parameters. The isothermal study of the adsorption process was conducted using the classical Langmuir and Freundlich models [28,29,30,31], which describe monolayer and multilayer adsorption, respectively. The linearized expressions of these models are provided in Equations (3) and (4).
In the Langmuir isotherm model, Qm (mg/g) denotes the maximum adsorption capacity, which is derived from the slope of the linearized plot. This parameter represents the theoretical maximum amount of adsorbate that can be adsorbed onto the surface of the adsorbent. The parameters KL (L/mg) and KF (mg/g) are the Langmuir and Freundlich constants, respectively, related to the affinity of the adsorption sites. In the Freundlich equation, the parameter n represents the adsorption intensity, which serves as an indicator of the favorability of the adsorption process. A slope value of 1/n < 0.5 suggests that the process is unfavorable for multilayer adsorption.
To provide a more comprehensive analysis, additional adsorption isotherm models, including the Dubinin–Radushkevich (D–R) isotherm, have been incorporated into this study, alongside the Langmuir and Freundlich models.
The Dubinin–Radushkevich (D–R) isotherm was employed to elucidate the adsorption mechanism on heterogeneous surfaces. This model facilitates the determination of the adsorption nature, distinguishing between physisorption and chemisorption, by evaluating the adsorption energy [32]. The mathematical expression of this isotherm is represented in Equation (5):
In this study, Qm denotes the maximum sorption capacity, while β represents the Dubinin–Radushkevich (D–R) constant. The Polanyi potential (ε) is mathematically related to the equilibrium concentration (Ce) through Equation (6):
where R represents the universal gas constant (8.314 J/mol K), and T denotes the adsorption temperature (308.15 K).
In addition, the D–R constant (β) was further utilized to determine the adsorption energy (E), providing additional insights into the adsorption mechanism governing this process, as described in Equation (7) [33].
2.5. Adsorption Kinetic Models
Adsorption kinetic models are utilized to elucidate the underlying mechanisms and characteristics of the adsorption process. In this study, pseudo-first-order and pseudo-second-order kinetic models were employed to analyze the experimental data [34,35]. The linearized expressions for these models are presented in Equations (8) and (9) below:
- -
- Pseudo-first-order kinetic model:
- -
- Pseudo-second-order kinetic model:
3. Results and Discussion
FESEM analysis was performed on the three samples: C-BP (calcined banana peel), S-BP (H2SO4-treated banana peel), and A-BP (acetic-acid-treated banana peel). This analysis reveals important details about their surface morphology and porous structure. As illustrated in Figure 1a,b, the surface morphology of the C-BP sample prepared without chemical activation exhibited a dense and fibrous structure that closely resembled its natural form. This characteristic results in low porosity, thereby reducing its effectiveness for adsorption applications. The S-BP sample underwent significant changes due to the strong acid treatment. This treatment broke down the dense lignocellulosic structure, creating a rough surface with visible layers and sharp edges, as shown in Figure 1c,d. The effect of the sulfuric acid was intensified during calcination, which removed organic material and exposed the inorganic phases. As a result, the surface developed many voids and pores, increasing its porosity and adsorption capacity compared to the untreated sample.
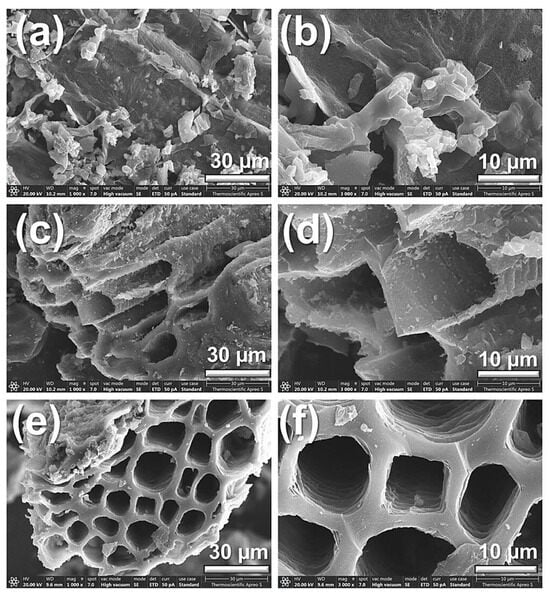
Figure 1.
FESEM images of (a,b) C-BP, (c,d) S-BP, and (e,f) A-BP captured at magnifications of 1000× and 3000×, respectively.
This study aimed to employ mild conditions and straightforward chemical treatments. To achieve this, the banana peel was activated using acetic acid from commercial vinegar (5% concentration). The FESEM images of the A-BP sample, presented in Figure 1e,f, demonstrate a distinct honeycomb-like structure characterized by large, well-defined pores approximately 10–20 μm in size. The surface morphology appears more organized and less fragmented compared to that of S-BP, indicative of the gentler impact of the acetic acid treatment. As a weak acid, acetic acid facilitates a controlled interaction with the lignocellulosic components of banana peel, including cellulose, hemicellulose, and lignin [36]. This interaction preserves the natural framework of the material, yielding a uniform and interconnected porous structure while minimizing excessive degradation. The resulting chemical modification effectively enhances the porosity and stability of A-BP, making it a viable material for adsorption applications.
The untreated C-BP sample demonstrated the lowest BET surface area (3.202 m2/g) and pore volume (0.008 cm3/g), as detailed in Table 2. This finding aligns with the dense and compact morphology observed in the FESEM images. Consequently, the material exhibited limited porosity and a reduced surface area, rendering C-BP unsuitable for applications requiring high adsorption capacities.

Table 2.
The BET surface area and pore volume of the synthesized adsorbents.
To further investigate the surface properties, N2 adsorption–desorption isotherms for the C-BP sample were generated and are shown in Figure 2a. The isotherm exhibited a profile typical of an IUPAC Type II isotherm [37], commonly associated with non-porous materials. The BJH pore size distribution plot presented in Figure 2c indicates an absence of well-defined pores, distinguishing it from the other samples analyzed in this study.
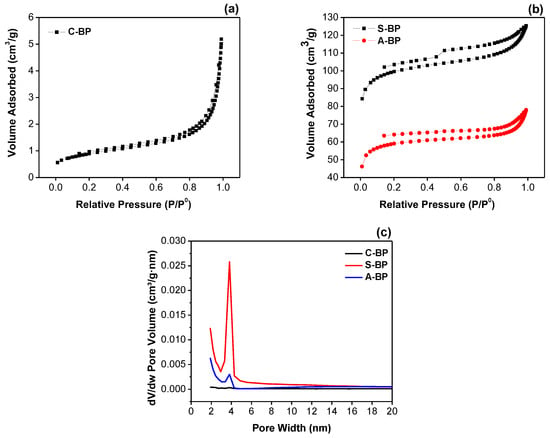
Figure 2.
N2 adsorption–desorption isotherm and BJH pore size distribution.
In contrast, the sulfuric-acid-treated banana peel (S-BP) exhibited the highest BET surface area (338.959 m2/g) and pore volume (0.059 cm3/g) among the analyzed samples. This result is consistent with the highly porous and fragmented morphology observed in the FESEM images. Similarly, the banana peel treated with acetic acid (A-BP) showed a notable enhancement in the surface area (201.722 m2/g) and pore volume (0.041 cm3/g). As shown in Figure 2b, the adsorption–desorption isotherms of the S-BP and A-BP samples exhibited hysteresis loops typical of a Type IV isotherm with H3 slit-shaped pores [37,38]. These loops indicate the presence of significant mesopores and macropores, which is consistent with the FESEM observations revealing macropores in the range of 10–20 µm (>0.05 µm) [39]. Additionally, the BJH pore size distribution, depicted in Figure 2c, confirms an average total pore diameter within the mesopore range (4–5 nm).
The primary aim of this study is to explore potential applications in the food industry, with a particular focus on the banana chip sector. Consequently, sulfuric acid (H2SO4) was used solely for comparative purposes to highlight that acetic acid, a milder and food-safe alternative, can also effectively serve for activation. The results demonstrate that 5% acetic acid, commonly employed in food processing, significantly enhances the porosity of banana peel. For the final selection, this study primarily focuses on samples activated with acetic acid (A-BP) to ensure compatibility with edible palm oil.
The data in Table 3 provide critical insights into the adsorption equilibrium characteristics for the removal of free fatty acids (%FFA) from used palm oil after adsorption, presenting the equilibrium concentration (Ce), adsorbed quantity (Qe), and related parameters after 30 min of adsorption equilibrium time. The free fatty acid (FFA) content in the used palm oil prior to adsorption was determined to be 3.108%, indicating substantial degradation of the oil or prolonged usage (as FFA levels exceeding 2% are generally deemed unsuitable for consumption). At the highest initial concentration prior to dilution (Co = 100% v/v, 3631 mg/L), the FFA content decreased to 1.69%, representing a substantial reduction of approximately 54.38% compared to the initial FFA content.

Table 3.
Concentration at equilibrium (Ce and Qe) and percentage of free fatty acids (%FFA) in used oil after 30 min of adsorption equilibrium (adsorption onto A-BP).
This reduction highlights the efficiency of the adsorbent in removing free fatty acids, achieved within a 30 min adsorption equilibrium period. The remaining diluted concentrations listed in Table 3 were used to evaluate the adsorption isotherms, facilitating the analysis of adsorption behavior of the Langmuir and Freundlich isotherm models, as illustrated in Figure 3a,b, respectively. The isotherm parameters obtained from both models are summarized in Table 4.
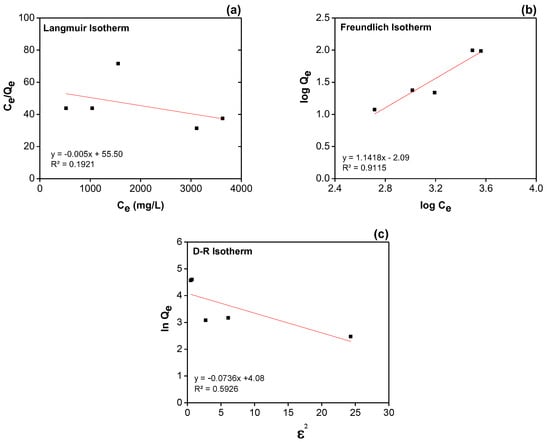
Figure 3.
Comparison of linearized isotherm models: (a) Langmuir, (b) Freundlich, and (c) Dubinin–Radushkevich (D–R) isotherms.

Table 4.
Langmuir, Freundlich, and D–R parameters.
The correlation coefficient (R2 = 0.9115) of the linear Freundlich model demonstrates a superior fit to the adsorption data compared to the linear Langmuir model (R2 = 0.1921). Furthermore, the maximum monolayer adsorption capacity (Qm), calculated from the slope of the Langmuir plot, and the KL parameter, obtained from its y-intercept, were found to exhibit negative values. These results suggest that the Langmuir monolayer adsorption model was not suitable for describing the adsorption behavior observed in this experiment. Moreover, the Freundlich parameter (1/n), referring to the intensity of adsorption with a value of 1.1418 (greater than 0.5), supports the conclusion that the Freundlich model provided a better representation of the multilayer adsorption process [41]. These findings suggest that the adsorption behavior of polar molecules in used oil on the heterogeneous surface of A-BP involves multilayer adsorption. This implies that higher concentrations of used oil can adsorb layer by layer without limitation to a monolayer.
As shown in Figure 3c, the equilibrium data for polar molecule adsorption onto A-BP exhibited a superior fit to the Dubinin–Radushkevich (D–R) isotherm model compared to the Langmuir model, with an R2 value of 0.5926. The adsorption capacity determined from the D–R model was 59.15 mg/g. Notably, the Langmuir model was not utilized for capacity estimation due to the occurrence of a negative slope.
To determine the predominant adsorption mechanism—either chemisorption or physisorption—the adsorption energy (E) was estimated using the Dubinin–Radushkevich (D–R) equation. Specifically, the adsorption energy was calculated based on the D–R constant (β) using the relationship .
Adsorption energy values typically range between 8 and 16 kJ/mol for chemisorption processes, whereas values below 8 kJ/mol indicate that physisorption is the predominant mechanism [33,41]. In the present study, the adsorption energy (E) was determined to be 2.61 kJ/mol, suggesting that the uptake of polar molecules from used oil by A-BP primarily occurred via a surface physisorption process. The adsorption process is predominantly influenced by weak intermolecular interactions involving functional groups such as hydroxyl (–OH) and carboxyl (–COOH) on A-BP. These functional groups facilitate hydrogen bonding with polar molecules present in the used oil. This observation is further supported by the functional group analysis of the A-BP sample, as illustrated in Figure 4.
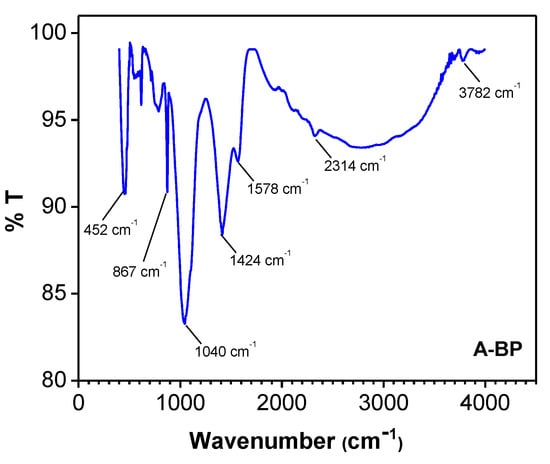
Figure 4.
FTIR of A-BP.
The FTIR spectrum exhibits a peak at 3782 cm−1, corresponding to O–H stretching vibrations and indicative of hydroxyl (–OH) groups commonly associated with cellulose, hemicellulose, and lignin in banana peel. The absorption band observed at 1578 cm−1 is likely attributed to C=O stretching vibrations from carboxyl (–COOH) groups or the asymmetric stretching of carboxylate (COO−), which may have formed due to the acetic acid treatment. Furthermore, the absorption bands around 1424 cm−1 can be ascribed to O–H bending in carboxyl (–COOH) groups or symmetric COO− stretching, further confirming the presence of carboxyl functionalities. The band detected at approximately 1040 cm−1 is associated with C–O stretching, which may correspond to either carboxyl (–COOH) or ester (–COOR) groups, potentially resulting from esterification reactions between acetic acid and the organic components of the banana peel.
Additionally, weak absorption bands detected at 2314 cm−1 are assigned to C–H stretching modes, suggesting the presence of aliphatic structures in the banana peel. The signals appearing at 452 cm−1 and 867 cm−1 can be attributed to metal–oxygen (M–O) stretching vibrations, indicating the presence of trace metal oxides, such as Fe–O, Ca–O, or Mn–O in the banana peel.
The application of two kinetic models within a heterogeneous system is presented for the 100% v/v concentration of used palm oil in Figure 5. The corresponding parameters are provided in Table 5. The results show that the plot in Figure 5a for the pseudo-first-order kinetic model does not exhibit linearity over the entire time range, whereas the pseudo-second-order model provides a better fit, as shown in Figure 5b. This indicates that the reaction was influenced by both the used oil adsorbate and the solid adsorbent (A-BP). The adsorbent not only functioned as a catalyst but also acted as one of the reactants due to the active sites and porosity present on its surface.
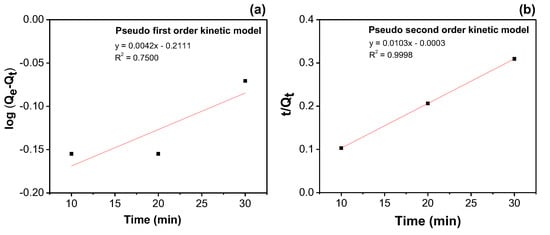
Figure 5.
(a) Pseudo-first-order kinetic plot and (b) pseudo-second-order kinetic plot for the degradation of used oil at a concentration of 100% v/v.

Table 5.
The reaction rates and the corresponding constants for the used oil concentration of 100% v/v.
To enhance the reliability of this study, additional experiments were conducted to evaluate the presence of residual toxins in the A-BP adsorbent. These tests aimed to confirm its safety for use as an adsorbent in food applications, particularly in frying oil.
As shown in Table 6, the analysis revealed the presence of lead (Pb) in A-CP at a concentration of 0.34 mg/kg, while other heavy metals, including arsenic (As), cadmium (Cd), and mercury (Hg), were undetectable. The detection of Pb in the sample is attributed to contamination from the storage container used for banana peels. However, the detected value remained below the standard limit of 1 mg/kg, as established by the Food and Drug Administration, Ministry of Public Health. Lead (Pb) has also been found in food products such as seasoning powder (0.37 mg/kg), fish (0.31 mg/kg), and pork (0.64 mg/kg). Therefore, the Pb level in A-BP is not hazardous and is lower than the concentrations commonly found in daily food items.

Table 6.
Heavy metal contents detected in the adsorbent prepared from raw banana peels.
4. Conclusions
This study presents a sustainable approach to waste management and resource recovery in the banana chip industry by converting banana peel waste into porous adsorbents for purifying used palm oil. Through chemical and thermal activation, the research demonstrates the effectiveness of 5% w/v acetic acid as an environmentally friendly alternative to conventional strong activators, such as sulfuric acid. This mild activation process is particularly advantageous for small- and medium-sized enterprises (SMEs) aiming to implement sustainable practices. The adsorption experiments utilizing used palm oil demonstrated that A-BP effectively reduced the free fatty acid (FFA) content from 3.108% to 1.69%, corresponding to a reduction of approximately 54.38%. This significant decrease underscores the potential of A-BP for industrial applications in FFA removal. Based on the adsorption test results, the adsorption mechanism primarily follows a physisorption process, in which the adsorption sites are occupied by multiple layers of adsorbate.
These findings emphasize the potential of utilizing agricultural waste in valorization processes to advance a circular economy, thereby contributing to environmental sustainability and driving innovation in waste-to-resource conversion. Beyond their application in used oil purification, banana-peel-derived adsorbents have demonstrated remarkable efficacy in wastewater treatment, facilitating the removal of dyes, heavy metals, and organic pollutants from industrial effluents through bio-based adsorption processes.
Author Contributions
Conceptualization, P.J. and D.C.; methodology, P.J. and P.T.; validation, P.J.; formal analysis, P.J., P.T. and D.C.; investigation, P.J., P.T. and D.C.; resources, W.K. and A.N.; writing—original draft preparation, D.C.; writing—review and editing, D.C.; supervision, W.K. and A.N.; funding acquisition, W.K. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Naresuan University (NU) and the National Science, Research and Innovation Fund (NSRF). Grant No. R2568B031.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zaini, H.M.; Saallah, S.; Roslan, J.; Sulaiman, N.S.; Munsu, E.; Wahab, N.A.; Pindi, W. Banana Biomass Waste: A Prospective Nanocellulose Source and Its Potential Application in Food Industry—A Review. Heliyon 2023, 9, e18734. [Google Scholar] [CrossRef]
- Pereira, M.A.F.; Cesca, K.; Poletto, P.; de Oliveira, D. New Perspectives for Banana Peel Polysaccharides and Their Conversion to Oligosaccharides. Food Res. Int. 2021, 149, 110706. [Google Scholar] [CrossRef]
- Urgel, J.J.D.T.; Briones, J.M.A.; Diaz, E.B.; Dimaculangan, K.M.N.; Rangel, K.L.; Lopez, E.C.R. Removal of diesel oil from water using biochar derived from waste banana peels as adsorbent. Carbon Res. 2024, 3, 13. [Google Scholar] [CrossRef]
- Ye, T.; Li, M.; Li, X.; Chen, T.; Su, Z. Study on the performance of modified banana leaf fiber in removing oil spill from seawater. Water Air Soil Pollut. 2023, 234, 239. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Al-Sareji, O.J.; Grmasha, R.A.; Meiczinger, M.; Al-Juboori, R.A.; Somogyi, V.; Hashim, K.S. A sustainable banana peel activated carbon for removing pharmaceutical pollutants from different waters: Production, characterization, and application. Materials 2024, 17, 1032. [Google Scholar] [CrossRef] [PubMed]
- Goel, C.; Mohan, S.; Dinesha, P.; Rosen, M.A. CO2 adsorption by KOH-activated hydrochar derived from banana peel waste. Chem. Pap. 2024, 78, 3845–3856. [Google Scholar] [CrossRef]
- Bakar, N.A.; Othman, N.; Yunus, Z.M.; Altowayti, W.A.H.; Al-Gheethi, A.; Asharuddin, S.M.; Tahir, M.; Fitriani, N.; Mohd-Salleh, S.N.A. Nipah (Musa acuminata balbisiana) banana peel as a lignocellulosic precursor for activated carbon: Characterization study after carbonization process with phosphoric acid impregnated activated carbon. Biomass Convers. Biorefinery 2021, 13, 11085–11098. [Google Scholar] [CrossRef]
- Tan, B.A.; Nair, A.; Zakaria, M.I.S.; Low, J.Y.S.; Kua, S.F.; Koo, K.L.; Wong, Y.C.; Neoh, B.K.; Lim, C.M.; Appleton, D.R. Free fatty acid formation points in palm oil processing and the impact on oil quality. Agriculture 2023, 13, 957. [Google Scholar] [CrossRef]
- Bustani, S.; Soni, S. Review on the impact of peroxide value from edible oil: Indian perspective. J. Surv. Fish. Sci. 2023, 10, 26–33. [Google Scholar]
- Chen, W.-A.; Chiu, C.P.; Cheng, W.-C.; Hsu, C.-K.; Kuo, M.-I. Total polar compounds and acid values of repeatedly used frying oils measured by standard and rapid methods. J. Food Drug Anal. 2013, 21, 58–65. [Google Scholar]
- Buczek, B.; Chwiałkowski, W. Purification of the used palm oil by adsorption. Pol. J. Chem. Technol. 2008, 10, 19–21. [Google Scholar] [CrossRef]
- Miskah, S.; Aprianti, T.; Agustien, M.; Utama, Y.; Said, M. Purification of used cooking oil using activated carbon adsorbent from durian peel. IOP Conf. Ser. Earth Environ. Sci. 2019, 396, 012003. [Google Scholar] [CrossRef]
- Bostan, R.; Glevitzky, M.; Varvara, S.; Dumitrel, G.-A.; Rusu, G.I.; Popa, M.; Glevitzky, I.; Vică, M.L. Utilization of natural adsorbents in the purification of used sunflower and palm cooking oils. Appl. Sci. 2024, 14, 4417. [Google Scholar] [CrossRef]
- Rengga, W.D.P.; Seubsai, A.; Roddecha, S.; Yudistira, A.; Wiharto, A.D. Isotherm adsorption of free fatty acid in waste cooking oil using activated carbon from banana peel as a bio-adsorbent. J. Phys. Conf. Ser. 2021, 1918, 032008. [Google Scholar] [CrossRef]
- Miyagi, A.; Nakajima, M. Regeneration of used frying oils using adsorption processing. J. Am. Oil Chem. Soc. 2003, 80, 91. [Google Scholar] [CrossRef]
- Putranti, M.L.T.A.; Wirawan, S.K.; Bendiyasa, I.M. Adsorption of free fatty acid (FFA) in low-grade cooking oil using activated natural zeolite as adsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012085. [Google Scholar] [CrossRef]
- Alias, N.H.; Hasan, S.I.Z. Adsorption of used cooking oil (UCO) using raw and modified kapok fiber through esterification. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012028. [Google Scholar] [CrossRef]
- Chung, T.-W.; Wu, Y.-L.; Hsu, S.-H. Removal of free fatty acid from plant oil by the adsorption process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 362, 012019. [Google Scholar] [CrossRef]
- Bai, T.; Zhao, J.; Tian, L.; Zhang, L.; Jin, Z. The Adsorption of Pb(II) from Aqueous Solution Using KOH-Modified Banana Peel Hydrothermal Carbon: Adsorption Properties and Mechanistic Studies. Materials 2024, 17, 311. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials 2022, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Campbell, R.; Mangwandi, C. Kinetics and Thermodynamics Study on Removal of Cr(VI) from Aqueous Solutions Using Acid-Modified Banana Peel (ABP) Adsorbents. Molecules 2024, 29, 990. [Google Scholar] [CrossRef]
- Yhon, J.; Mendza, J.; Osorio, E.; Domínguez, M.P. Continuous Removal of Dyes from Wastewater Using Banana-Peel Bioadsorbent: A Low-Cost Alternative for Wastewater Treatment. Sustainability 2023, 15, 9870. [Google Scholar] [CrossRef]
- Azhar-ul-Haq, M.; Javed, T.; Abid, M.A.; Masood, H.T.; Muslim, N. Adsorptive removal of hazardous crystal violet dye onto banana peel powder: Equilibrium, kinetic and thermodynamic studies. J. Dispers. Sci. Technol. 2022, 45, 475–490. [Google Scholar] [CrossRef]
- Akter, M.; Rahman, F.B.A.; Abedin, M.Z.; Kabir, S.M.F. Adsorption Characteristics of Banana Peel in the Removal of Dyes from Textile Effluent. Textiles 2021, 1, 361–375. [Google Scholar] [CrossRef]
- Purwasasmita, M.; Nabu, E.; Khoiruddin, K.; Wenten, I.G. Non-dispersive chemical deacidification of crude palm oil in hollow fiber membrane contactor. J. Eng. Technol. Sci. 2015, 47, 426–446. [Google Scholar] [CrossRef]
- Ke, P.J.; Woyewoda, A.D. A titrimetric method for determination of free fatty acids in tissues and lipids with ternary solvents and m-cresol purple indicator. Anal. Chim. Acta 1978, 99, 387–391. [Google Scholar] [CrossRef]
- Reed, B.E.; Matsumoto, M.R. Modeling cadmium adsorption by activated carbon using the Langmuir and Freundlich isotherm expressions. Sep. Sci. Technol. 1993, 28, 2179–2195. [Google Scholar] [CrossRef]
- Palle, K.; Vunguturi, S.; Rao, K.S.; Gayatri, S.N.; Babu, P.R.; Ali, M.; Kola, R. Comparative study of adsorption isotherms on activated carbons synthesized from rice husk towards carbon dioxide adsorption. Chem. Pap. 2022, 76, 7525–7534. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic Parameters of Liquid–Phase Adsorption Process Calculated from Different Equilibrium Constants Related to Adsorption Isotherms: A Comparison Study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. An Overview of Dyes Removal via Activated Carbon Adsorption Process. Desalination Water Treat. 2011, 19, 255–274. [Google Scholar] [CrossRef]
- Dada, A.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, J.; He, F.; Tang, J. Effective phosphate adsorption by Zr/Al-pillared montmorillonite: Insight into equilibrium, kinetics and thermodynamics. Appl. Clay Sci. 2015, 104, 252–260. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Mohd Jamil, N.A.; Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Isolation of Cellulose Nanocrystals from Banana Peel Using One-Pot Microwave and Mild Oxidative Hydrolysis System. Nanomaterials 2022, 12, 3537. [Google Scholar] [CrossRef]
- AlOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore structure and fractal characteristics of different shale lithofacies in the Dalong formation in the western area of the lower Yangtze platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, C.; Kou, L.; Wang, R.; Wang, Y.; Li, R. Hierarchical porous polystyrene-based activated carbon spheres for CO2 capture. Environ. Sci. Pollut. Res. 2022, 29, 13098–13113. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.; Heller, W. The adsorption of cis- and trans-azobenzene. J. Am. Chem. Soc. 1939, 61, 2228–2230. [Google Scholar] [CrossRef]
- Batool, F.; Akbar, J.; Iqbal, S.; Noreen, S.; Bukhari, S.N.A. Study of Isothermal, Kinetic, and Thermodynamic Parameters for Adsorption of Cadmium: An Overview of Linear and Nonlinear Approach and Error Analysis. Bioinorg. Chem. Appl. 2018, 3, 3463724. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).