Abstract
Visuospatial memory plays a crucial role in everyday functioning. However, its assessment was less explored compared to other memory systems. Immersive virtual reality (iVR) devices can add valuable information about visuospatial memory providing more realistic and ecological environments and allowing for the recording of brain activity in real time. This present systematic review summarizes the current knowledge of brain dynamics during the execution of iVR-based visuospatial memory tasks. Nine articles were reviewed, employing visuospatial working memory, visuospatial memory recognition, and spatial memory tasks through commercial iVR devices such as HTC Vive or Oculus. Most studies measured brain activity using electroencephalography. The findings highlight different key aspects, such as the sensitivity of the prefrontal cortex under stressful conditions, the relationship between memory load and brain activity, the involvement of medial temporal lobe regions on spatial memory and its improvement using memory reactivation paradigms, the importance of some environmental characteristics (i.e., the space where the task is carried out), and the implication of the parietal cortex in processing allocentric information. These results enhance our understanding of brain activity during iVR-based visuospatial tasks and highlight iVR technologies as promising tools in cognitive neuroscience.
Keywords:
visuospatial memory; spatial memory; memory; virtual reality; assessment; fNIRS; EEG; systematic review 1. Introduction
Memory is crucial for the proper functioning of human cognition, being essential for the integration and execution of complex behaviors. Any disruption in the capability to encode, store, or retrieve information can lead to significant difficulties in carrying out goal-directed actions and adapting to environmental demands [1].
Visuospatial memory refers to the capacity to retain both visual (i.e., the physical characteristics of the objects and the colors) and spatial information (i.e., the position or movement within a given environment) [2,3]. Visuospatial memory is an essential component of numerous cognitive tasks, including navigation, mental rotation, mental representation of three-dimensional space, and motor planning, among others [4]. The impairment of this memory system is frequently observed in neurodegenerative processes, including the behavioral variant of frontotemporal dementia and Alzheimer’s disease [5]. It is also a feature of other neurological diseases, such as epilepsy [6]. Despite its importance, the number of available tasks for the assessment of visuospatial memory is comparatively limited in comparison to those for the assessment of other memory systems, such as verbal memory [7].
The assessment of visuospatial memory in neuropsychology is frequently conducted through the administration of paper-and-pencil tasks and computer-based tasks [8,9]. Examples of these tasks include the recognition of previously presented visual complex designs and the recall of complex geometric figures [7]. These visuospatial memory tasks are typically derived from neuropsychological standardized instruments, providing measures with high reliability and validity [10]. However, paper-and-pencil and computerized tests often demonstrate only moderate ecological validity—the extent to which test outcomes reflect real-world performance in everyday activities [10]. A potential explanation for this limitation is the discrepancy between the controlled conditions of clinical assessments and the complexity of real-life scenarios. In clinical settings, memory tasks are administered in quiet, structured environments with clear instructions, whereas in daily life, memory demands frequently occur alongside other cognitive challenges [11].
In this sense, the use of virtual reality (VR) systems has emerged as a promising solution to the problem of ecological validity [12]. VR technology enables the assessment of memory within immersive, naturalistic environments while preserving the safety and reproducibility of experimental conditions. Additionally, it provides researchers with enhanced control over experimental variables, ensuring both ecological relevance and methodological rigor [11]. Nevertheless, the potential of VR technology to enhance ecological validity varies significantly across different VR tools. The degree of immersion, defined as the extent to which an individual is isolated from the real world while engaging with the virtual environment, is a key factor influencing this variation. VR systems can be categorized as non-immersive (niVR) or immersive (iVR) systems, each offering distinct advantages and limitations in replicating real-world experiences [13]. For instance, stereoscopic features in VR environments have been shown to enhance immersion, but they have been demonstrated to negatively impact motor control, particularly in older adults [14]. This emphasized the importance of carefully adapting VR tools to the specific needs of different user populations and task demands, ensuring both effectiveness and usability. In niVR systems, the user interacts with the virtual environment via devices such as a mouse or joystick, with tasks typically displayed on a computer screen [13]. This setup allows for naturalistic 3D tasks viewed from a first-person perspective. However, external environmental distractions can potentially interfere with participants’ performance. This limitation is addressed by iVR systems, which employ head-mounted displays (HMDs) to create more realistic and engaging scenarios. Such devices serve to enhance the level of immersion experienced by users by isolating them from their physical surroundings, thereby providing a more controlled and immersive experimental environment [15]. Specifically, immersive systems often incorporate advanced features such as motion tracking, stereoscopic vision, and auditory feedback, which contribute to a heightened sense of presence and immersion [16,17,18]. For instance, systems that utilize precise motion tracking enable users to navigate and interact within a 3D space, fostering a more realistic experience [16,19]. Additionally, iVR can be enhanced through the integration of haptic feedback devices, which simulate touch and physical sensations, further bridging the gap between the virtual and real worlds [19,20]. Examples of commercially available iVR systems are the HTC Vive and Meta Quest. HTC Vive uses external base stations with Lighthouse technology for precision tracking, achieving sub-millimeter accuracy through infrared signals detected by the headset and controllers. It supports room-scale tracking, allowing for free movement within a defined space. High-resolution displays with up to a 120 Hz refresh rate ensure smooth visuals and reduce motion sickness. The Meta Quest is a standalone VR system designed to provide wireless VR experiences without requiring a PC or external sensors. Powered by the Qualcomm Snapdragon XR2 platform, it delivers high-quality visuals and responsive performance. Integrated cameras enable inside-out tracking, mapping the environment and user movements, enhancing portability, and eliminating the need for external tracking devices. The experience of immersion can also be implemented by mixed reality (MR) that merges virtual content with the real world in an interactive, immersive way [21]. The Microsoft HoloLens is an example of a MR device that blends holographic projections with the physical environment to create an interactive immersive experience.
The neural substrates of visuospatial memory encompass multiple brain regions, including the dorsolateral prefrontal cortex (dlPFC) and the parietal cortex, which are involved in the processing of spatial information [22]. Studies also found that medial temporal lobe (MTL) regions, such as the hippocampus, participate in visuospatial tasks [23]. The study of brain activity during the performance of a visuospatial memory task with electrophysiological and functional neuroimaging techniques can provide valuable insights into the involvement of brain areas required during the task and offer a deeper understanding of this cognitive process. With the purpose of studying brain function and connectivity, a variety of techniques have been developed. These include neuroimaging methods, such as functional magnetic resonance imaging (fMRI); optical imaging methods, such as functional near-infrared spectroscopy (fNIRS); and electrophysiological techniques, including electroencephalography (EEG) and magnetoencephalography (MEG). EEG is an electrophysiological technique that measures the neural activity from the scalp potentials. It is a widely used research and clinical tool for monitoring brain function and detecting anomalies [24,25]. MEG is an additional electrophysiological technique based on the recording of magnetic fields through dense arrays of sensors [24]. Both techniques allow for the measurement of functional connectivity in the human brain with a high temporal resolution, although with a low spatial resolution [24]. On the other hand, fNIRS is an optical imaging technique that employs near-infrared light to measure cortical hemodynamics, that is, the relative changes in the concentration of oxygenated and deoxygenated hemoglobin secondary to neuronal activity [26,27].
Research has demonstrated the efficacy of integrating EEG and fNIRS with iVR, resulting in valuable insights into diverse cognitive, behavioral, and motor functions [28,29]. The integration of neuroimaging and neurophysiological techniques with iVR visuospatial tasks is critical for advancing our understanding of visuospatial memory because it enables the investigation of brain activity and behavior in ecologically valid yet highly controlled environments that preserve all spatial dimensions. Neuroimaging methods provide detailed insights into the neural circuits and dynamic brain processes underpinning visuospatial memory, while neurophysiological measures allow researchers to track the timing of visuospatial processes with high precision. The iVR tasks allow participants to engage in realistic, three-dimensional spatial navigation and memory scenarios that closely mimic real-world conditions. Consequently, when employed in conjunction, these tools serve to bridge the gap between laboratory-based experiments and real-world behavior, thereby enabling researchers to explore how neural mechanisms support the encoding, storage, and retrieval of spatial information in complex and realistic settings. This combination enhances the ecological validity of findings while maintaining the rigor of controlled experimental designs. Nevertheless, despite visuospatial memory being a critical cognitive function that is indispensable for daily activities, navigation, and planning, its assessment has frequently relied on traditional tasks that lack ecological validity. Indeed, as previously mentioned, the literature on the subject primarily evaluates visuospatial memory using traditional tools that fail to replicate real-world contexts. There are only a few studies that combine neuroimaging and neurophysiological techniques with iVR tasks to investigate the neural dynamics of visuospatial memory. Consequently, this review synthesizes the available evidence, focusing on studies that integrate these approaches. The aim is to address this crucial gap by exploring how iVR environments, when combined with methods for the assessment of brain function, enhance the ecological validity and precision of visuospatial memory assessment in neuroscience and neuropsychology. The proposed approach has the potential to offer more realistic insight into visuospatial memory processing.
This present review aims to provide a concise summary of the integration of iVR with neuroimaging or electrophysiological methods, including fNIRS, MEG, and EEG, in the assessment of visuospatial memory. Specifically, this review will examine how these tools contribute to understanding neural dynamics during iVR visuospatial memory tasks and evaluate the methodological advancements provided by this technological combination. For this purpose, a search through three different databases was carried out and only the studies that fit the inclusion criteria were included.
2. Materials and Methods
This present systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [30] and the Joanna Briggs Institute (JBI) critical appraisal tools [31]. The databases employed for the search were Web of Science (WoS), Scopus, and PubMed. The final search was carried out on 19 September 2024. The search was carried out using an algorithm that combined terms related to the assessment of visuospatial memory measured through iVR tools in experiments that employed the recording of brain activity during the task. The keywords employed were ((“spatial memory”) OR (“declarative memory”) OR (“episodic memory”) OR (“visual memory”) OR (“visuospatial memory”)) AND ((EEG) OR (electroencephalography) OR (MEG) OR (magnetoencephalography) OR (fNIRS) OR (“functional near infrared spectroscopy”)) AND ((“mixed reality”) OR (“virtual reality”) OR (virtual) OR (VR) OR (“360-degree”) OR (“360°”) OR (immersive) OR (panoramic)). There were no filters applied in PubMed, whereas, in WoS, the keywords were searched by topic with the filters “Article” and “Clinical trial”. In Scopus, the keywords were searched through title/abstract/keyword using the filter “Articles”. No restriction on date of publication in any database was established. Table 1 displays the keywords and filters applied for the three databases.

Table 1.
Keywords and filters applied for each database.
Selection Criteria
For inclusion in this systematic review, studies must:
- Include human adult samples.
- Report visuospatial memory outcomes.
- Record any neuronal activity measure through EEG, MEG, or fNIRS.
- Employ iVR technologies for the assessment of visuospatial memory.
Studies were excluded if they did not meet the inclusion criteria or if they were classified as review articles, brief communications, notes, theoretical articles, letters to the editor, or unpublished works that had not undergone peer review. Specifically, animal or pediatric samples were excluded, as were studies that did not provide specific outcomes related to visuospatial memory; studies that lacked a direct measurement of neuronal activity through EEG, MEG, or fNIRS; or those articles that employed technologies that were not iVR, such as non-immersive VR or traditional computer-based assessments.
Figure 1 shows a flowchart of the study selection. Only studies that employed iVR tools for the assessment of visuospatial memory while simultaneously recording neuronal activity were included in the review. After removing duplicated articles and completing an initial screening by title and abstract, a total of 64 articles were selected for a full-text screening. At this stage, we excluded those articles that employed niVR devices (Reason 1 of the flowchart), articles that did not report spatial memory outcomes (Reason 2), articles that lacked neurophysiological records related to spatial memory performance (Reason 3), and articles with no accessible full text (Reason 4).
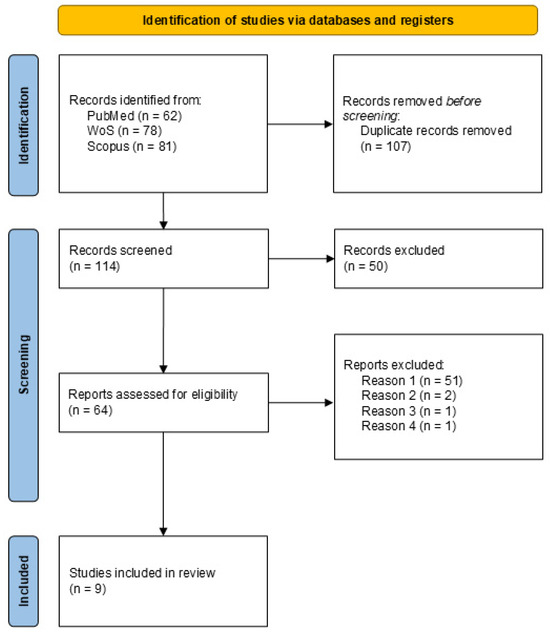
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews that included searches of databases and registers only.
3. Results
A total of nine articles were included in this present systematic review. Table 2 shows an overview of the sample characteristics, main objectives, and visuospatial memory task description, as well as the main behavioral outcomes for each study. Table 3 provides a summary of the technical aspects, including the iVR device employed, the neurophysiological tool used for the assessment of brain activity, and the main brain results obtained.

Table 2.
Sociodemographic and neuropsychological description of the reviewed studies.

Table 3.
Technical and neurophysiological overview of the reviewed studies.
3.1. Study Characteristics
3.1.1. Methodology of the Studies
In three studies (33.33%), experimental designs were employed wherein participants were divided into different experimental conditions [32,37,39]. Conversely, a cross-sectional design was utilized in six studies, wherein all participants completed all experimental conditions at a single point in time [1,33,34,35,36,38]. The method of recruitment of participants was not specified in the majority of the reviewed studies [1,34,35,36,37,38,39]. The investigation undertaken by Abujelala et al. [32] involved the participation of firefighters recruited from a local fire station. Conversely, the research carried out by Darfler et al. [33] involved the utilization of a more expansive recruitment strategy, encompassing word-of-mouth methods and announcements disseminated through departmental email lists.
3.1.2. Sample Characteristics
Eight of the nine studies reviewed (88.89%) assessed visuospatial memory in healthy adult samples [32,33,34,35,36,37,38,39], and only the study by Maoz et al. [1] explored visuospatial memory function in epilepsy patients. Sample sizes ranged from six [1] to forty-one [39], with 22.22% of the studies employing only male participants [32,39].
3.1.3. Memory Assessment
Visuospatial memory tasks varied across studies. Two studies (22.22%) applied a visuospatial working memory task that centered on the reproduction of a previously seen sequence [32,33]. Two of the reviewed studies (22.22%) explored visuospatial working memory with tasks requiring the maintenance and manipulation of visuospatial information without retention intervals in an updating paradigm [35,36]. Three studies (33.33%) employed recognition visuospatial memory tasks [33,34,38], either four options [33] or yes/no [34,38] forced decision tasks, in which some features of the objects (i.e., orientation, color, location) could change. Spatial memory was also assessed in three studies (33.33%) by observing a map at the beginning of the task and then navigating to a specific location [37], deciding the origin between two possible routes [39], or recalling the location of objects [1].
3.1.4. Type of iVR Device Employed for Memory Assessment
In the studies reviewed, the devices employed include the HTC Vive [34,36,39], the Oculus [1,33,37], the Pico Neo [1], and the Microsoft HoloLens [35] (see Table 3).
The HTC Vive, used in 33.33% of the studies [34,36,39], is renowned for its high degree of immersion, utilizing external base stations and Lighthouse technology for position tracking and comprehensive room-scale navigation. The system facilitates a room-scale tracking area, enabling users to navigate freely inside a space, such as a street [39].
Other reviewed studies (33.33%) employed the Oculus, designed by Meta Platforms with the primary objective of enabling developers to create VR content and experiences [1,33,37]. This iVR device allows for the recreation of outdoor spaces, such as a large city with different buildings [37], as well as indoor spaces, such as a seminar classroom with a projector screen [33] or a room containing different furniture and colored halos [1]. The Pico Neo, developed by Pico Immersive Pte. Ltd. (Singapore), was developed with the same purpose as the Oculus, and the study by Maoz et al. [1] used both iVR devices indistinctly.
Two studies (22.22%) did not specify the iVR device employed to assess visuospatial memory [32,38]. The study by Abujelala et al. [32] simulated a chemical power plant with multiple valves, whereas the study by Snider et al. [38] employed a room that contained objects that rested on shelves, tables, and the floor.
The HoloLens is used in the study by Lee and Kim [35] to blend digital content with physical surfaces. This MR device was used to modify the wall color, material, and ceiling height of a real room.
3.2. Main Results Obtained
The reviewed studies explored the importance of behavioral aspects that affect visuospatial memory when performing iVR-based tasks, including the impact of stress and environmental and stimuli characteristics, as well as the effects of memory reactivation and learning. Additionally, neural activity correlates of visuospatial memory from the iVR-based visuospatial memory assessment were recorded and identified. Table 4 provides a summary of the main results obtained in the reviewed articles.

Table 4.
Main results of the reviewed studies.
3.2.1. Stress Impact
Two of the reviewed studies (22.22%) wanted to explore the effect of stress on visuospatial memory performance [32,33]. The study by Abujelala et al. [32] employed machine learning (ML) methodology and found that the Random Forest (RF) algorithm obtained the best results when trained and tested under similar conditions, whether stressful or non-stressful. These results were obtained in a visuospatial working memory task where participants had to reproduce a sequence [32]. Another study explored the effect of perceived observation in a virtual environment, which can be considered a stressful condition [33]. The results did not reveal differences between perceived and non-perceived observation conditions on accuracy in two classical visual short-term memory tasks performed through immersive virtual environments, the Benton Visual Retention Test (BVRT) and the Visual Pattern Test (VPT) [33]. However, participants spent more time completing the BVRT compared to the VPT in the perceived observation condition [31].
3.2.2. Impact of Stimuli and Environmental Features
Some studies (22.22%) aimed to explore the impact of the stimuli’s eccentricity on memory performance [34,39]. The results showed lower visuospatial memory performances at higher eccentricities compared to performances with smaller eccentricities, with no differences between smaller eccentricities [34]. Moreover, these differences were observed independently of whether the memory load of the task was low or high [34]. The study by Yang et al. [39] explored the relationship between eccentricity, frames of reference, and navigation displacement in a spatial memory task. The results found that with a low eccentricity, there is a better performance with an egocentric frame of reference, independently of whether the navigation was active or passive [39]. In contrast, with high eccentricity, no differences between frames of reference were found, neither in active nor passive navigation [39].
Also, 22.22% of the reviewed studies explored other stimuli features: the number of items to encode, maintain, and recall (i.e., memory load) as well as the size of items [34,36]. Memory load was explored in the study by Klotzsche et al. [34], finding better visuospatial memory performances in lower memory loads. Regarding the size of the items, there was no effect of item size on performance in a visuospatial working memory task [36].
The influence of environmental characteristics was studied on visuospatial working memory tasks, finding that neutral spaces and personally designed spaces enhanced the performance more than spaces generally preferred or non-preferred for the general population [35]. Furthermore, the study by Redlinger et al. [36] explored if the addition of a background image could influence visuospatial working memory performance, compared to a black background, showing no differences between the two conditions.
3.2.3. Learning and Memory Reactivation Effects
Learning across trials was a phenomenon observed in 22.22% of the reviewed studies [1,38]. Specifically, there were observed significant visuospatial memory performance improvements over the trials in both healthy and epilepsy samples [1,38]. Other variables related to motor behavior, such as time spent walking, distance traveled, and average walking speed in the task, were similar across blocks of trials in healthy adults [38]. In epilepsy patients, the distance error in the relocation of objects had a significant reduction over the blocks [1].
Additionally, the study by Shimizu et al. [37] developed an EEG closed-loop targeted memory reactivation (CL-TMR) in order to reactivate the newly learned spatial information during sleep, and they observed that participants who were memory-reactivated obtained significant improvements in spatial memory compared to those who were not reactivated.
3.2.4. Neural Activity Correlates
The studies included in this review investigated the brain activity associated with visuospatial memory tasks administered through iVR by using EGG [33,34,35,36,37,38,39], intracranial EEG (iEEG) [1], and fNIRS [32].
Albujelala et al. [32] observed distinct brain activation patterns measured by fNIRS during the encoding and retrieval phases of an episodic memory task, which were effectively used by ML algorithms to categorize these stages. The RF model, trained and evaluated under consistent conditions, achieved an F1 score of 0.844 and an accuracy of 79.10%. However, when assessed under different conditions, its performance dropped, with an F1 score of 0.723 and an accuracy of 60.61%. Additionally, key brain regions, including the right dlPFC, medial prefrontal cortex (mPFC), and left dlPFC, were identified as essential for differentiating between encoding and retrieval, aiding attentional inhibition and working memory encoding.
Concerning the study by Darfler et al. [33] using EEG, notable alterations in alpha, beta, and theta oscillations in the occipital cortex (OC) and the dlPFC were assessed during visual stimulus and test-response intervals. They found that the presence of a “virtual observer” suppressed theta-band power during the storage and retrieval of visual information, particularly in the BVRT. This suggests that the observer may increase mental workload, affecting attentional concentration and task performance. During the stimulus phase, the dlPFC showed prolonged alpha-band activity (8–12 Hz) in both conditions, indicating its role in processing visual inputs. Theta-band activity increased about 1200 ms post-stimulus under the observation condition. Additionally, beta-band activity (12.5–30 Hz) was higher in the no-observation condition after 1600 ms.
Klotzsche et al. [34] examined brain activity with EEG related to visuospatial short-term memory and observed changes in contralateral delay activity (CDA) amplitude, which was significantly affected by memory load at smaller eccentricities (4 and 9 degrees of visual angle). However, no such effect was seen at the largest eccentricity (14 degrees), suggesting spatial limitations in processing memory load. Additionally, lateralized alpha power during the retention phase was observed. The study further found that increased memory load impaired performance, with the CDA being more sensitive to memory load than alpha lateralization.
Yang et al. [39] focused on brain activity related to allocentric and egocentric spatial reference frames. During allocentric navigation, there was alpha desynchronization in the parietal cortex (PC), while egocentric navigation showed no significant alpha changes. Both strategies led to beta and low gamma power increases in the OC, with egocentric navigation showing stronger enhancements. A positive correlation in beta activity was observed between the PC and other cortical areas (occipital, frontal, central) during active navigation for the allocentric group and during passive navigation for the egocentric group.
Lee and Kim [35] investigated the effect of various interior space designs on brain activity. A notable observation was that mental workload oscillated significantly according to the design of the indoor environment. Individuals encountered a greater cognitive load in the least favored environment. Conversely, the favored setting correlated with reduced mental load. The study indicated that executive function and working memory were significantly influenced by the spatial design. The examination of EEG data via one-way RM ANOVA indicated substantial variations in mental stress levels linked to alterations in indoor space design.
Regarding the exploration and navigation of spatial environments, Snider et al. [38] studied brain activity with EEG during free exploration of a virtual world and found that the PC generated significant spatial maps, particularly in the low-frequency range of 2–8 Hz. The researchers also measured spatial displacement autocorrelation (STAcc), which reflects the relationship between brain activity and spatial locations. STAcc was found to be significant and persistent across multiple trials, indicating that the brain maintains a stable representation of spatial information over time. A key finding was the direct correlation between the strength of the spatial maps created during exploration and participants’ ability to recall object positions the next day. Maoz et al. [1] investigated MTL theta band oscillations and their role in spatial navigation with iEEG. They found that MTL theta activity is influenced by successful memory retrieval and geographical locations. Redlinger et al. [36] examined theta rhythm activity with EEG, which was analyzed in response to visual game-like elements. They observed significant fluctuations in the theta band at the Oz electrode site. However, the frontal theta rhythm at Fz showed no significant changes. The study also found that alpha rhythm variations were not significant. Notable changes in the beta rhythm were observed, but these did not correlate with task performance.
Additionally, Shimizu et al. [37] investigated CL-TMR during sleep, focusing on its effects on cerebral activity related to spatial navigation tasks. A key finding was the increase in fast spindle activity (12–15 Hz) in the frontal regions, particularly on Day 2. The study also found a positive correlation between fast spindle energy at frontal electrode sites and navigation performance. Participants with higher fast spindle activity showed improved navigation efficiency after the sleep intervention.
4. Discussion
The studies included in this review provide valuable insights into the brain activity associated with visuospatial memory tasks administered through iVR, mainly utilizing EEG to explore the underlying neural mechanisms. The collective findings of these studies highlight the interrelationship between brain oscillations, spatial navigation, visuospatial memory, and environmental factors. Furthermore, they highlight the contribution of both VR technology and neuroscientific tools in enhancing our understanding of cognitive processes.
Most of the reviewed studies assessed visuospatial memory performance through the use of iVR devices and examined brain functioning in healthy samples, thereby providing valuable insights into these processes in non-clinical contexts. Nevertheless, it is also essential to investigate the neural correlates when individuals with clinical conditions (e.g., epilepsy, neurodegenerative diseases) perform visuospatial iVR-based tasks. The feasibility and usability of iVR devices for visuospatial memory assessment have been previously explored in neurological patients [40]. However, there is a lack of information regarding the neural correlates associated with the performance of these tasks.
The results showed the efficacy of ML methods in differentiating between encoding and retrieval processes when participants were subjected to comparable stressors during training and testing phases [32]. This finding offers valuable insights into the influence of stress on visuospatial working memory. The impact of stress has also been demonstrated in prior studies that employed alternative methodologies, such as computerized tasks, to assess working memory [41]. Furthermore, evidence indicates that brain dynamics, particularly in the dlPFC and in the mPFC, are vulnerable to stressful conditions [32,33]. These findings corroborate those of previous studies that have emphasized the pivotal role of the dlPFC in working memory and its heightened sensitivity to stress compared to other brain regions [42].
The investigation of environmental characteristics on visuospatial updating tasks demonstrated enhanced performance and reduced mental load in personally designed spaces compared to spaces that were generally less preferred [35], providing evidence for the influence of context on working memory. Although the relevance of indoor spaces’ design has been previously reported [43], the use of novel and more immersive technologies provides valuable insights into the functioning of visuospatial working memory and its associated brain dynamics in more ecological contexts. The influence of other contextual factors, such as the addition of a background image, the presence of visual distracters, and the size of items in a visuospatial updating task, has been found to be insignificant in terms of their ability to influence performance [36]. Complementing this, the absence of notable alpha rhythm alterations may indicate that the visual elements do not exert a discernible influence on this aspect of brain activity. However, alterations in the beta rhythm were observed yet not correlated with the outcomes of the visuospatial working memory task [36]. The analysis revealed OC activity and no significant changes in the frontal theta rhythm, which is typically linked to concentration and task engagement. This suggests that the EEG modifications are indicative of perceptual-level cognitive processes rather than reflecting the expected concentration indicators [36].
Another context variable that was explored was the eccentricity of the stimulus. The findings indicated that visuospatial memory performance was inferior at higher eccentricities in comparison to those at smaller ones [34]. Nevertheless, the replication of these findings in a perceptual task indicates that the memory decline at higher eccentricities can be partially attributed to perceptual processes [34]. Additionally, eccentricity influenced the impact of memory load on CDA amplitude but only at lower eccentricities. At higher memory demands, CDA amplitude increased [34]. This result reflects a strong link between memory load and brain activity, in consonance with previous studies [44]. The absence of notable effects of memory load at larger eccentricities can be attributed to spatial limitations in the processing of memory load. Another component of the EEG signal, the power of oscillations in the alpha frequency range, was also explored in visuospatial memory. This revealed a pronounced lateralization of alpha power during the retention phase of the task, with a reduction in power in contralateral compared to ipsilateral signals [34]. This finding aligns with the results of previous research [45]. However, no memory load effects on alpha lateralization were observed, suggesting that the spatial focus of attention, linked to the pattern of alpha power, may operate independently of memory load. This highlights the complex interaction between attention and memory. Interestingly, the authors of the study employed multivariate approaches and observed that alpha power could serve as a decoding mechanism for the level of memory load [34]. This indicates that memory load can be predicted from EEG signals and that multivariate approaches can be useful to detect this information [34].
The frame of reference employed during a spatial memory task has been demonstrated to affect performance, with evidence suggesting that higher general spatial navigation abilities are exhibited when using an allocentric frame of reference than when using an egocentric frame of reference [39]. Moreover, the implication of PC appears to be pivotal for the processing of allocentric information and for the facilitation of transitions between egocentric and allocentric frames. A recent iEEG study observed that the maximum number of allocentric-selective channels were located in the PC when participants were performing a niVR-based spatial judgment task, supporting these results [46]. Furthermore, the associations between PC and frontal, occipital, and central cortical areas suggest a shared processing across these brain regions, with previous reviews showing that both the allocentric and egocentric frames of reference produce activations in different brain cortices, including the OC, the superior temporal cortex, and the PC, as well as the frontal cortex [47]. Additionally, the role of PC in generating spatial maps in a low-frequency range [38] suggests the link between spatial navigation and memory formation with these brain rhythms. Moreover, the discovery that the strength of the spatial maps generated correlated with visuospatial memory performance lends further support to the notion that freely moving during encoding is crucial for obtaining more accurate mental representations, which in turn leads to enhanced visuospatial memory.
The role of MTL in spatial memory was also observed in treated epilepsy patients, with the increase of MTL theta band power during spatial memory recall [1]. Moreover, MTL theta oscillations were modulated by the spatial position of objects depending on environmental context and task demands. Taken together, these results suggest a relationship between MTL theta oscillations and spatial memory as well as spatial context-related information. This conclusion is supported by a recent meta-analysis of functional magnetic resonance imaging (fMRI) studies examining the neural substrates of spatial memory encoding and retrieval. The analysis identified activations along various MTL structures [48]. In addition, it is important to highlight that the possibility of measuring MTL neural activity in a free-movement and iVR-based task provides more ecological results and allows for better experimental control of the variables.
Learning effects were observed in iVR-based visuospatial memory and spatial navigation tasks in both clinical and healthy samples [1,38]. Previous research that assessed visuospatial memory employing a niVR device found that although healthy participants exhibited more pronounced learning effects than epilepsy patients, both groups demonstrated evidence of learning progress [49].
Spatial memory and navigation can be improved using a memory reactivation paradigm, such as the CL-TMR [37]. In fact, CL-TMR increases fast spindle activity in the frontal cortex, suggesting a brain activity enhancement during sleep and aiding memory consolidation. Complementing this, a recent study found that memory reactivation during sleep induced an increase in theta and spindle oscillations, which were related to memory processing [50]. Related to spatial navigation, the fact that higher fast spindle activity was linked to more spatial navigation efficiency in the CLM-TMR condition suggests that cerebral activity during sleep can also facilitate spatial navigation abilities.
The integration of iVR with neuroimaging and neurophysiological technologies, as demonstrated in the reviewed studies, enhances the ecological validity of visuospatial memory assessment by offering controlled environments while assessing brain function. The results of this review highlight the contribution of iVR in combination with EEG and fNIRS in elucidating the neural substrates of visuospatial memory processes, including encoding, retrieval, and navigation. These methods have revealed robust associations between brain activity in key regions such as the prefrontal cortex, MTL, and PC with task performance, emphasizing the neural correlates of memory under diverse contextual conditions. Stress, environmental design, and stimuli properties exert a substantial influence on performance and brain dynamics. The findings also highlight the need for future research that extends these approaches to clinical cohorts and integrates real-world task demands. This will allow for further validation and refinement of the role of iVR in cognitive neuroscience and neuropsychology.
It is important to acknowledge the limitations of this present systematic review, which may influence the interpretation of the findings. There is a potential risk of omitting relevant studies that were not included in the databases analyzed, which could result in an incomplete representation of the existing research landscape. Achieving consistency among the findings of the limited number of studies included presents a challenge, primarily due to the heterogeneity of their objectives, methodologies, and contexts. These variations can complicate the synthesis of the results and may limit the generalizability of the conclusions. Nevertheless, this review offers an initial framework to understand the brain dynamics involved in iVR-based visuospatial memory tasks, providing a meaningful contribution to this new field by elucidating the neural substrates of visuospatial memory as assessed through iVR-based tasks, providing valuable insights into this cognitive process within more ecologically valid and realistic environments. Furthermore, it provides a critical baseline for researchers entering this field, highlighting prevailing methodologies and emergent trends. This, in turn, provides a foundation for the conceptualization of future studies and the refinement of research methodologies.
5. Conclusions
This review summarizes the brain activity results during spatial tasks implemented with iVR devices, such as HTC Vive or Oculus. From the impact of stress and environmental factors on cognitive performance and their brain dynamics [32,33,34,35,36] to the function of diverse brain rhythms in encoding, retrieval, and navigation [1,37,38,39], these findings offer a more profound comprehension of the neural mechanisms underlying spatial information processing combining mainly iVR and EEG methods. These methodologies offer a promising direction for future research in cognitive neuroscience and its applications, including navigation training, memory enhancement, and environmental design.
Author Contributions
Conceptualization, T.L., D.P. and M.M.; methodology, T.L., D.P., C.Z. and M.M.; formal analysis, T.L., D.P. and C.Z.; investigation, T.L., D.P. and C.Z.; resources, T.L., D.P., C.Z. and M.M.; data curation, T.L., D.P. and C.Z.; writing—original draft preparation, T.L. and D.P.; writing—review and editing, C.Z. and M.M.; visualization, T.L. and D.P.; supervision, M.M.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the Programa “Severo Ochoa” de la Consejería de Ciencia, Empresas, Formación y Empleo del Principado de Asturias [BP22-005] to T.L.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maoz, S.L.L.; Stangl, M.; Topalovic, U.; Batista, D.; Hiller, S.; Aghajan, Z.M.; Knowlton, B.; Stern, J.; Langevin, J.P.; Fried, I.; et al. Dynamic neural representations of memory and space during human ambulatory navigation. Nat. Commun. 2023, 14, 6643. [Google Scholar] [CrossRef] [PubMed]
- Vicari, S.; Bellucci, S.; Carlesimo, G.A. Visual and spatial long-term memory: Differential pattern of impairments in Williams and Down syndromes. Dev. Med. Child Neurol. 2005, 47, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dehn, M.J. An integrated model of working memory. In Working Memory and Academic Learning: Assessment and Intervention, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 58–59. [Google Scholar]
- Dehn, M.J. Working memory development and related cognitive processes. In Working Memory and Academic Learning: Assessment and Intervention, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 80. [Google Scholar]
- Foxe, D.; Irish, M.; Carrick, J.; Cheung, S.C.; Teng, H.; Burrell, J.R.; Kessels, R.P.C.; Piguet, O. Visuospatial working memory in behavioural variant frontotemporal dementia: A comparative analysis with Alzheimer’s disease using the box task. J. Neurol. 2024, 271, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Frisch, C.; Helmstaedter, C. Object location performance: Detection of functional impairment in right temporal lobe epilepsy. Epilepsy Behav. 2014, 35, 28–33. [Google Scholar] [CrossRef]
- Diaz-Orueta, U.; Rogers, B.M.; Blanco-Campal, A.; Burke, T. The challenge of neuropsychological assessment of visual/visuo-spatial memory: A critical, historical review, and lessons for the present and future. Front. Psychol. 2022, 13, 962025. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Brunetti, R.; Del Gatto, C.; Delogu, F. eCorsi: Implementation and testing of the Corsi block-tapping task for digital tablets. Front. Psychol. 2014, 5, 939. [Google Scholar] [CrossRef]
- Neguț, A.; Matu, S.A.; Sava, F.A.; David, D. Virtual reality measures in neuropsychological assessment: A meta-analytic review. Clin. Neuropsychol. 2016, 30, 165–184. [Google Scholar] [CrossRef]
- Mancuso, V.; Sarcinella, E.D.; Bruni, F.; Arlati, S.; Di Santo, S.G.; Cavallo, M.; Cipresso, P.; Pedroli, E. Systematic review of memory assessment in virtual reality: Evaluating convergent and divergent validity with traditional neuropsychological measures. Front. Hum. Neurosci. 2024, 18, 1380575. [Google Scholar] [CrossRef]
- Alcañiz, M.; Parra, E.; Chicchi Giglioli, I.A. Virtual reality as an emerging methodology for leadership assessment and training. Front. Psychol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Bevilacqua, R.; Maranesi, E.; Riccardi, G.R.; Donna, V.D.; Pelliccioni, P.; Luzi, R.; Lattanzio, F.; Pelliccioni, G. Non-immersive virtual reality for rehabilitation of the older people: A systematic review into efficacy and effectiveness. J. Clin. Med. 2019, 8, 1882. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Lee, J.; Kim, J. Stereoscopic objects affect reaching performance in virtual reality environments: Influence of age on motor control. Front. Virtual Real. 2024, 5, 1475482. [Google Scholar] [CrossRef]
- Choi, J.W.; Kwon, H.; Choi, J.; Kaongoen, N.; Hwang, C.; Kim, M.; Kim, B.H.; Jo, S. Neural applications using immersive virtual reality: A review on EEG studies. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.; Röthke, K.; Siegfried, N.; Benlian, A. Lose yourself in VR: Exploring the effects of virtual reality on individuals’ immersion. In Proceedings of the 54th Annual Hawaii International Conference on System Sciences, Maui, HI, USA, 7–10 January 2020; pp. 1510–1519. [Google Scholar] [CrossRef]
- Parong, J.; Mayer, R.E. Cognitive and affective processes for learning science in immersive virtual reality. J. Comput. Assist. Learn. 2020, 37, 226–241. [Google Scholar] [CrossRef]
- Li, J. A virtual reality memory scene designed with memory reproduction as the concept. Appl. Comput. Eng. 2023, 21, 99–107. [Google Scholar] [CrossRef]
- Wang, L. The immersive and interactive experience in virtual reality games and films. Lect. Notes Educ. Psychol. Public Media 2023, 5, 491–500. [Google Scholar] [CrossRef]
- Peng, X. Research and recommendations on user immersion in virtual reality (VR) technology based on data mining. Appl. Comput. Eng. 2024, 52, 285–291. [Google Scholar] [CrossRef]
- Onime, C.; Uhomoibhi, J.; Wang, H. A Mixed-Reality environment for personalised and collaborative learning in science and engineering. In Teaching and Learning in a Digital World. ICL 2017; Springer: Cham, Switzerland, 2018; pp. 567–578. [Google Scholar] [CrossRef]
- Murphy, P.N.; Bruno, R.; Ryland, I.; Wareing, M.; Fisk, J.E.; Montgomery, C.; Hilton, J. The effects of ‘ecstasy’ (MDMA) on visuospatial memory performance: Findings from a systematic review with meta-analyses. Hum. Psychopharmacol. 2012, 27, 113–138. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; Piras, F.; Assogna, F.; Pontieri, F.E.; Caltagirone, C.; Spalletta, G. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology 2012, 78, 1939–1945. [Google Scholar] [CrossRef]
- Ahmad, R.F.; Malik, A.S.; Kamel, N.; Reza, F.; Abdullah, J.M. Simultaneous EEG-fMRI for working memory of the human brain. Australas Phys. Eng. Sci. Med. 2016, 39, 363–378. [Google Scholar] [CrossRef]
- Perpetuini, D.; Chiarelli, A.M.; Filippini, C.; Cardone, D.; Croce, P.; Rotunno, L.; Anzoletti, N.; Zito, M.; Zappasodi, F.; Merla, A. Working memory decline in Alzheimer’s disease is detected by complexity analysis of multimodal EEG-fNIRS. Entropy 2020, 22, 1380. [Google Scholar] [CrossRef] [PubMed]
- Llana, T.; Fernandez-Baizan, C.; Mendez-Lopez, M.; Fidalgo, C.; Mendez, M. Functional near-infrared spectroscopy in the neuropsychological assessment of spatial memory: A systematic review. Acta Psychol. 2022, 224, 103525. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Bucco, R.; Zito, M.; Merla, A. Study of memory deficit in Alzheimer’s disease by means of complexity analysis of fNIRS signal. Neurophotonics 2018, 5, 011010. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, C.; Herff, C.; Sato, T.; Rechowicz, K.; Yamani, Y.; Krusienski, D.J. Estimating cognitive workload in an interactive virtual reality environment using EEG. Front. Hum. Neurosci. 2019, 13, 401. [Google Scholar] [CrossRef]
- Peng, K.; Moussavi, Z.; Karunakaran, K.D.; Borsook, D.; Lesage, F.; Nguyen, D.K. iVR-fNIRS: Studying brain functions in a fully immersive virtual environment. Neurophotonics 2024, 11, 020601. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aromataris, E.; Lockwood, C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis. 2024. Available online: https://synthesismanual.jbi.global (accessed on 30 October 2024).
- Abujelala, M.; Karthikeyan, R.; Tyagi, O.; Du, J.; Mehta, R.K. Brain activity-based metrics for assessing learning states in VR under stress among firefighters: An explorative machine learning approach in neuroergonomics. Brain Sci. 2021, 11, 885. [Google Scholar] [CrossRef]
- Darfler, M.; Cruz-Garza, J.G.; Kalantari, S. An EEG-based investigation of the effect of perceived observation on visual memory in virtual environments. Brain Sci. 2022, 12, 269. [Google Scholar] [CrossRef]
- Klotzsche, F.; Gaebler, M.; Villringer, A.; Sommer, W.; Nikulin, V.; Ohl, S. Visual short-term memory-related EEG components in a virtual reality setup. Psychophysiology 2023, 60, e14378. [Google Scholar] [CrossRef]
- Lee, K.T.; Kim, J.H. Relationship between psycho-physiological indicators and task performance under various indoor space designs for telecommuting environment by introducing mixed-reality. Sci. Rep. 2024, 14, 1977. [Google Scholar] [CrossRef]
- Redlinger, E.; Glas, B.; Rong, Y. Impact of visual game-like features on cognitive performance in a virtual reality working memory task: Within-subjects experiment. JMIR Serious Games 2022, 10, e35295. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.E.; Connolly, P.M.; Cellini, N.; Armstrong, D.M.; Hernandez, L.T.; Estrada, R.; Aguilar, M.; Weisend, M.P.; Mednick, S.C.; Simons, S.B. Closed-loop targeted memory reactivation during sleep improves spatial navigation. Front. Hum. Neurosci. 2018, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.; Plank, M.; Lynch, G.; Halgren, E.; Poizner, H. Human cortical θ during free exploration encodes space and predicts subsequent memory. J. Neurosci. 2013, 33, 15056–15068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.S.; Liu, J.; Singh, A.K.; Huang, K.C.; Lin, C.T. Brain dynamics of spatial reference frame proclivity in active navigation. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1701–1710. [Google Scholar] [CrossRef]
- Belger, J.; Blume, M.; Akbal, M.; Chojecki, P.; de Mooij, J.; Gaebler, M.; Klotzsche, F.; Krohn, S.; Lafci, M.T.; Quinque, E.; et al. The immersive virtual memory task: Assessing object-location memory in neurological patients using immersive virtual reality. Neuropsychol. Rehabil. 2024, 34, 870–898. [Google Scholar] [CrossRef]
- Oei, N.Y.; Everaerd, W.T.; Elzinga, B.M.; van Well, S.; Bermond, B. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress 2006, 9, 133–141. [Google Scholar] [CrossRef]
- Bogdanov, M.; Schwabe, L. Transcranial stimulation of the dorsolateral prefrontal cortex prevents stress-induced working memory deficits. J. Neurosci. 2016, 36, 1429–1437. [Google Scholar] [CrossRef]
- Lee, K.-T.; Im, J.-B.; Park, S.-J.; Kim, J.-H. Conceptual framework to support personalized indoor space design decision-making: A systematic literature review. Buildings 2022, 12, 716. [Google Scholar] [CrossRef]
- Tuladhar, A.M.; ter Huurne, N.; Schoffelen, J.M.; Maris, E.; Oostenveld, R.; Jensen, O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum. Brain Mapp. 2007, 28, 785–792. [Google Scholar] [CrossRef]
- Adam, K.C.S.; Robison, M.K.; Vogel, E.K. Contralateral delay activity tracks fluctuations in working memory performance. J. Cogn. Neurosci. 2018, 30, 1229–1240. [Google Scholar] [CrossRef]
- Moraresku, S.; Hammer, J.; Janca, R.; Jezdik, P.; Kalina, A.; Marusic, P.; Vlcek, K. Timing of allocentric and egocentric spatial processing in human intracranial EEG. Brain Topogr. 2023, 36, 870–889. [Google Scholar] [CrossRef] [PubMed]
- Moraresku, S.; Vlcek, K. The use of egocentric and allocentric reference frames in static and dynamic conditions in humans. Physiol. Res. 2020, 69, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.A.; Fritch, H.A.; Slotnick, S.D. Spatial memory encoding is associated with the anterior and posterior hippocampus: An fMRI activation likelihood estimation meta-analysis. Hippocampus 2024, 34, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Eggert, T.; Nguyen, P.V.; Ernst, K.; Loosli, S.V.; Straube, A. A new test to detect impairments of sequential visuospatial memory due to lesions of the temporal lobe. PLoS ONE 2022, 17, e0272365. [Google Scholar] [CrossRef]
- Guttesen, A.Á.V.; Denis, D.; Gaskell, M.G.; Cairney, S.A. Delineating memory reactivation in sleep with verbal and non-verbal retrieval cues. Cereb. Cortex. 2024, 34, bhae183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).