Abstract
In today’s world, environmental projects that contribute to the protection of water resources are needed due to the ongoing deterioration caused by the discharge of heavy metals, especially chromium. One way to investigate this problem is to use adsorbent biomasses, such as bacterial cellulose. This cellulose is increasingly popular due to its ability to chemisorb heavy metals present in water. Furthermore, the addition of iron chloride to this biomass improves its performance, creating more active sites and thus increasing its heavy metal adsorption capacity. Due to the promising results, pilot-scale research with physical models in fixed biomass columns has gained relevance, and adsorption isotherms could be used to adjust these models and optimize the design of these prototypes. For this reason, a project to treat water contaminated with Cr(VI) using bacterial cellulose and FeCl3 in a continuous system was created. Experiments were conducted with different concentrations, and treatment conditions were established based on the isotherms. Subsequently, elutions with EDTA were performed up to six times to allow biomass reuse in the continuous system with a bacterial cellulose column containing iron chloride. This achieved a total adsorption capacity of 626 mg/g, summing the six treatment cycles. The results provide practical parameters and evidence to support future studies to scale up and optimize Cr(VI) effluent treatment.
1. Introduction
Water is the most valuable resource on our planet, and its scarcity is increasing due to mismanagement by different business sectors. For example, effluents laden with heavy metals impact water resources due to the lack of effective and economical treatment systems. Chromium (VI) is a heavy metal that has had a detrimental effect on the wetlands and lagoons of the Bogotá savannah for many years. This is due to its ability to bioaccumulate in fish, macroinvertebrates and aquatic plants [1,2], generating a problem in the food chain that negatively impacts human health, since this heavy metal is highly carcinogenic [3,4]. Worldwide, numerous research projects are being carried out to identify effective treatment systems for industrial wastewater contaminated with heavy metals. One effective form of treatment is through the use of adsorbent biomasses, due to their ability to remove heavy metals through chemical adsorption processes [5,6]. The process in question is not a filtration process, but a chemisorption treatment of heavy metals present in water [7,8]; it is a wastewater treatment process that removes contaminants by forming strong chemical bonds based on the chemical reactivity and complementary functional groups of the biomass and the heavy metal, creating a stable complex [9,10]. An ideal adsorbent biomass is bacterial cellulose, a polymer industrially synthesized from yeast, red tea and kombucha [11,12,13]. It has the ability to chemically adsorb several heavy metals. The homogeneity of its biochemical structure (100% cellulose) translates into the presence of more uniform distribution of active groups, such as (OH). This improves the adsorption capacity [14,15,16,17]. Its high adsorption capacity is approximately 111 (mg/g) [18], without any type of chemical modification. In projects involving bacterial cellulose, global research on mitigating the effects of heavy metals in various water bodies has increased. The surface of this type of cellulose is two- and three-dimensional, which improves its porosity and specific surface area. Due to these conditions, bacterial cellulose possesses excellent contaminant adsorption capacity [19,20,21,22]. Furthermore, the high chemical reactivity of the exposed hydroxyl groups on bacterial cellulose ensures its ability to be modified by nanoparticles, e.g., iron chloride [23], in which case the potential of iron chloride as a biomass enhancer to increase the adsorption capacity in adsorbent biomasses has been investigated [24,25]. Upon entering the biomass, the reagent oxidizes the cellulose, increasing the number of hydroxyl groups available for heavy metal adsorption [26,27]. It has been determined that one of the most effective combinations of iron chloride and cellulose is one with a proportion of 10–20% of this reagent, with the aim of minimizing costs for future scale-up [28]. One way to optimize this process, making it more sustainable, is to incorporate elution and subsequent reuse processes, which increases treatment cycles [29]. An important agent is EDTA (ethylenediaminetetraacetic acid) [30]. The most effective method to determine the behavior in subsequent pilot-scale implementations is the use of fixed-column models in prototypes similar to those required in treatment plants, with the purpose of removing heavy metals from industrial effluents. These prototypes can be mathematically modeled to determine the optimal treatment conditions, such as initial and final concentrations and adsorption capacities [31]. One methodology to simulate and analyze the treatment of heavy metals is through isotherms. Heavy metal adsorption isotherms define the relationship between the amount of metal adsorbed on cellulose and the final concentration of heavy metals in the treated effluent; these mathematical models are used to describe the maximum adsorption capacity, mechanisms and properties of adsorbents in continuous systems [32,33]. Currently, the rivers and wetlands of the region adjacent to Bogotá remain polluted due to the absence of a cost-effective, efficient and, above all, effective treatment system for the removal of heavy metals, especially Cr(VI), from polluting effluents. It has been shown that bacterial cellulose has ideal removal characteristics, in addition to iron chloride, in these polluting effluents, but a continuous treatment system has not yet been created; therefore, the research project “Development of a treatment system based on bacterial cellulose combined with iron chloride for the removal of Cr(VI) in a continuous system” was initiated.
2. Materials and Methods
2.1. Production of Bacterial Cellulose
Bacterial cellulose was produced continuously for research purposes at the Fundación Universitaria Los Libertadores laboratories. The medium was heated in 5 L glass bioreactors with lids fitted with small holes. The first step was to prepare a culture medium containing 1.5 L of distilled water, red tea (4 g), glucose (4.5 g) and commercial yeast. Once the medium cooled, approximately 220 g of kombucha tea, a symbiotic culture of bacteria and yeast, was added. The medium was then transferred to an 18 L incubator (IO Xtemp Series Dual Incubator Oven, Hettich, Föhrenstr, Germany). In situ temperature, humidity and pH monitoring were performed every two days. After 12 days, a biofilm grew; this was removed and left to air dry. It was then ground to diameters of less than 0.211 mm using a knife mill and sieved through a 70-mesh sieve (standard U.S. sieve), achieving a yield of approximately 95%.
2.2. Adsorption Experiments
Cr(VI) stock solution: The 1000 mg/L stock solution was prepared with distilled water and potassium dichromate (K2Cr2O4). This stock solution was used to prepare the 400, 600 and 1000 mg/L Cr(VI) solutions. It was a synthetic solution that simulated water-contaminated effluent conditions. The column adsorption study was conducted at 24 °C. Samples were taken at each 500 mL treated volume interval and analyzed to determine the residual chromium concentration, obtaining 100-microliter samples.
2.3. Chromium Measurement
Through the diphenylcarbazide method, the amount of Cr(VI) residue was detailed. For this, 120 microliters of acetone (97% purity, w/v) and 80 microliters of diphenylcarbazide were prepared, which prepared 200 microliters of 0.5% diphenylcarbazide solution (97% purity). Subsequently, 900 microliters of phosphate buffer were prepared, with a purity of 90%. It was filtered and the pH was adjusted to 2 with (H3PO4). Finally, about 100 microliters of chromium were added to an Eppendorf tube, mixed with 200 microliters of diphenylcarbazide and the 900 microliters of phosphate buffer and transferred to an absorption cell, in which the absorbance at 540 nm was measured. The measurement uncertainty of the chromium adsorption study was 4%. Cr(VI) was measured using a spectrophotometer (Evolution 300, Thermo Fisher Scientific, Waltham, MA, USA), which measured and analyzed fluctuations in light absorption. For standardization purposes, analyses for the evaluation of Cr(VI) in water were performed using standard analysis parameters (Standard Methods for the Analysis of Water and Wastewater [34]).
2.4. Production of Iron-Modified Cellulose
Bacterial cellulose was mixed with iron chloride (Fe3 + [FeCl3 · 6H2O]) (98% purity) [28] to homogenize this reagent in the cellulose by dry weight. It must be homogenized by mixing 95% (w/w) of bacterial cellulose (57 g) and 10% of iron(III) chloride (3 g), creating a composite material of 60 g, creating the biomass (BCFe).
2.5. Experiments Conducted Within a Column
A system was built with three levels of cellulosic biomass fixed with iron chloride, each containing 20 g, with a diameter of 4.5 cm and a height of 7 cm. The columns had a funnel effect at the end of the treatment process to increase contact between the biomass and the fluid and to avoid vortex effects. The flow rate was 25 mL/min, regulated at the inlet and secured by dripping with downward flow. The biomass density was kept constant, as was the temperature, at 20 °C, and the pressure, at 1 bar. An additional compartment was used for final sampling. Adsorption evaluations were carried out at pH levels 6.5–7.5, favoring treatment with this type of cellulose and with Cr(VI) because they were similar to contaminated effluents [17]. Cr(VI) concentrations of 400, 600 and 1000 mg/L were evaluated. For statistical purposes, three tests were conducted per treatment, and the average of the data obtained was calculated. The estimated porosity of the bacterial cellulose material was 18% [16]. The development process for the pilot-scale systems is illustrated in Figure 1. Three experimental design parameters were used.
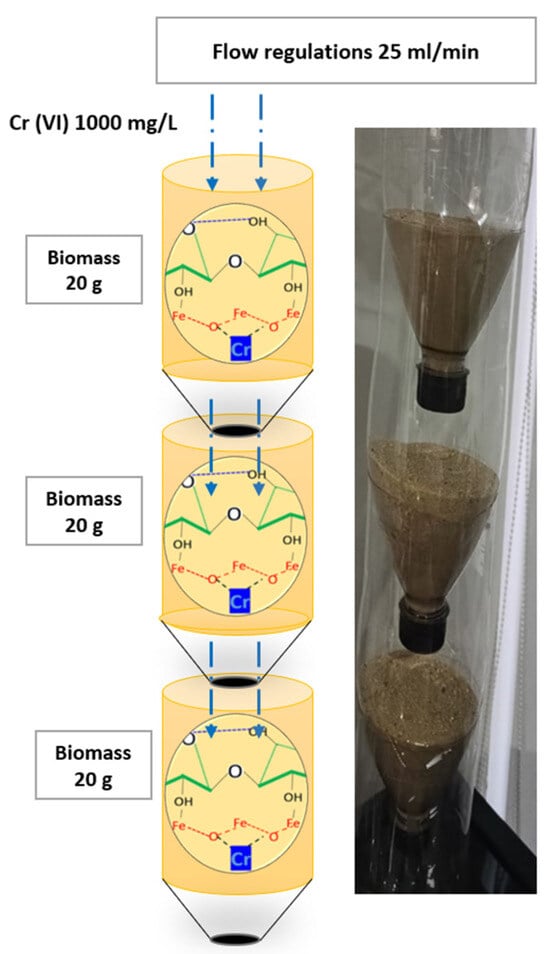
Figure 1.
Design of treatment a system pilot for the experiment with three levels of cellulose biomass.
Figure 1 shows that the capsules’ distribution has a funnel-like effect at the end of the biomasses. This facilitates flow, allowing for more direct contact between the Cr(VI)-laden solution and the biomass.
2.6. Desorption and Subsequent Adsorption Process
Elutions were used to facilitate solution reuse. Using the EDTANa2, (97% purity) reagent, elutions were performed when the biomass was saturated in order to reuse it in cycles where the biomass had high capacities. Similarly to the treatment, the solution was passed through the system in fixed columns, with an EDTA concentration of 500 mg/L, approximately 1 L of this solution. To remove excess EDTA, it was washed with 1 L of distilled water. In addition, after the elution processes, the amount of iron chloride was added to maintain the ratio of 95% BC biomass and 5% iron chloride.
2.7. Statistical Tests
All experiments were performed in triplicate, the average being the final result, where Student’s t-distribution was used. These results were analyzed using this method due to their representativeness in previous studies on heavy-metal-adsorbing biomasses [35].
2.8. The Isotherms Asociate
The adsorption isotherm models are obtained with the final adsorption capacities together with the final equilibrium concentrations of 200, 400, 600 and 1000 mg/L of Cr(VI). To obtain another point, the concentration of 200 mg/L Cr(VI) was added. The equilibrium of the isotherms were change <1% in Ce over 30 min.
2.8.1. Langmuir Isotherm
The isotherm model under consideration is of the Langmuir type. This model is the most suitable for representing the adsorption of heavy metals with adsorptive biomasses due to the multiple active sites on the surfaces of biomasses, which are energetically accessible. Furthermore, it is imperative that the adsorption be reversible for elution purposes, a condition which this isotherm fulfills. The maximum adsorption capacity is represented through the following equation, which is ideal for the scaling of these industrial wastewater treatment processes. The constant KL (L/mg) is a crucial parameter in the field, as it is instrumental in relating the cationic tuning between heavy metal and bacterial cellulose. Moreover, this parameter is utilized in the modeling of thermodynamic adsorption processes, which is a significant area of research in this field. It is imperative that adsorbent biomasses be modeled with this isotherm. This model is ideal for modeling, through this it assumes that the adsorption of heavy metals occurs in a totally homogeneous layer, with active sites such as (OH) fixed in this biomass [36].
In its linear form, the equation is as follows:
where B is the Langmuir parameter if there is a fit to the Langmuir isotherm. qm is the maximum capacity of adsorption.
2.8.2. Freundlich Isotherm
The Freundlich isotherm model posits the hypothesis that adsorption occurs on a heterogeneous surface, whereby the active sites possess differing adsorption energies [37]. This isotherm model is one of the most frequently employed to represent equilibrium data, particularly in the context of heterogeneous adsorbents. The underlying rationale for this selection is that the adsorption processes of these adsorbents are non-linear in nature, that is to say, they are not statistically deterministic but rather stochastic due to their inherent heterogeneity. This characteristic of heterogeneous adsorbents could be representative of the adsorbent medium itself [38]. The Freundlich Equation (3) is as follows:
where n is the Freundlich parameter, Kf is the parameters of the Freundlich and Cs is the concentration equilibrium.
2.8.3. Dubinin–Radushkevichisotherm
Due to its ideal representation of treatment, it has been used in models of zeolite and activated carbon adsorbent media [2]. The more heterogeneous the metal adsorbing substrate, the better the process. Due to this, and with the incorporation of ferric chloride, this model is not ideal. G is a constant related to the sorption energy, and β is the Polanyi potential. The maximum saturation capacity q is used to validate the capacities obtained in the Langmuir isotherm.
where qm is the maximum adsorption capacity, β is the activity coefficient related to the mean free adsorption energy (mol2/kJ2) and G is the Polanyi potential (kJ2/mol2) which is evaluated as follows:
where R is the universal gas constant (J/mol K). Substituting the equilibrium equations of all isotherms, the normalized overall mass balance of these isotherms is defined as follows:
2.8.4. Temkin Isotherm Model
This isotherm model represents heterogeneous adsorbent models and is ideal for modeling processes involving temperature and pH changes. However, it has been used successfully to describe models of adsorbing biomasses. The heat of adsorption is vital to determining the functions of this isotherm [39,40].
where Kt is the Temkin isotherm constant (L/mg); bT is the constant related to the heat of adsorption; T, the absolute temperature (K); R, the universal gas constant (8314 J/mol. K) and B, the constant related to the heat of adsorption (J/mol).
2.8.5. SIPS Isotherm Model
This model combines the Langmuir and Freundlich isotherms. At low concentrations, the model fits the Freundlich isotherm even more closely, while at high levels, it anticipates monolayer contaminant adsorption capacities, which is characteristic of the Langmuir relationship. Due to these saturation conditions with high concentrations of 1000 mg/L of Cr(VI), this biomass exhibited behaviors based on the Langmuir isotherm, as identified by the SIPS parameter [41,42]. For the calculation, n was determined to be homogeneous when it was equal to 1, which resembles a monolayer process and is very similar to the Langmuir isotherm.
where Ks is the Sips parameter, and n is a measure of surface heterogeneity.
2.8.6. Toth Isotherm Model
This is an improved model of the Langmuir isotherm, due to modeling considerations. It is an asymmetric Gaussian, in which most sites have adsorption energies lower than the mean or peak value. It is an isotherm that helps validate and calibrate the Langmuir isotherm parameters [43].
where Kt is the Toth parameter, and m is a measure of surface heterogeneity
2.8.7. Redlich–Peterson Isotherm Model
Unlike the SIPS isotherm, this isotherm approximates the Freundlich relation at high contaminant concentrations, since the exponent β tends towards zero. Low concentrations cause this model to approach the ideal Langmuir condition, where β values are close to unity [44].
where Kr is the Redlich–Peterson parameter, and β is a measure of surface heterogeneity.
2.8.8. Khan Isotherm Model
The Khan model is a generalization of the Langmuir and Freundlich models used for the adsorption of adsorbates in pure solutions. This three-parameter isotherm model is designed for both multicomponent and single-component adsorption processes. With relatively high correlation coefficients, this model can accurately determine adsorption peaks [45].
where Kk is the Khan parameter, and A is a measure of surface heterogeneity.
2.9. Biomass Characterization
ATR-FTIR: Bacterial cellulose biomasses were analyzed using Fourier transform infrared spectroscopy (79 Jasco FTIR 430) (Tokyo, Japan) in the spectral range of 4000 to 400 cm−1, a 4 cm−1 resolution, and a scan speed of 2 mm s−1. The KBr pellet method was also performed at room temperature, eliminating the spectral background.
SEM and EDS Analysis: Bacterial cellulose, along with Cr(VI) analysis, was characterized using SEM and EDS using a TESCAN FE-MEB LYRA3 focused ion beam scanning electron microscope (FEM-SEM LYRA3) (Brno, Czech Republic). Samples were also analyzed using an integrated energy-dispersive X-ray spectroscopy (EDS) microanalysis system.
3. Results
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
As illustrated in Figure 2, the addition of iron chloride to bacterial cellulose results in the characteristic spectra of the material. Following this, the adsorption of Cr(VI) into the biochemical structure of the cellulose is evident. The hydroxyl (OH) groups present within the 3400 cm−1 band are instrumental in the process of removing Cr(VI) ions.
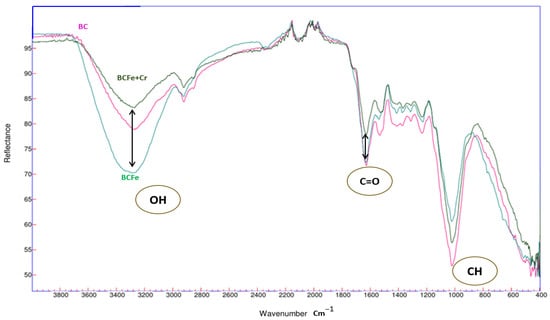
Figure 2.
FTIR characterizations of BCFe.
The incorporation of iron chloride into the BC biomass has been shown to induce stretching of the hydroxyl group (OH), resulting in the formation of FeOOH groups [24]. This modification of the cellulose has been demonstrated. It has also been observed that the band of the (CH) group underwent a stretching phenomenon at 1032. This transformation in the biomass ostensibly favors the cation exchange process with heavy metals among the Cr(VI) [23]. Following the experimental Cr(VI) adsorption process, significant alterations in its stretching levels were observed during FTIR tests. A significant alteration in the hydroxyl group (OH) was discerned following the chemisorption process of the (OH) group [36]. A proportion of the hydroxyl groups were forfeited during the characterization process due to the presence of Cr(VI) within the biomass, which was predominantly accountable for the adsorption of this heavy metal. This is attributable to the pronounced vibrations of the O-Cr.
3.2. SEM and EDS Analysis
Figure 3 shows some characteristic images of the bacterial cellulose used in this research, with representations obtained through SEM and EDS analysis.
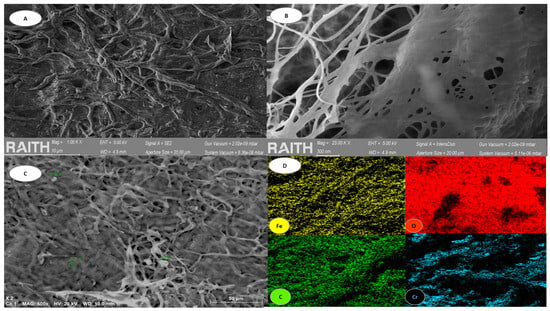
Figure 3.
BC analysis. (A) shows SEM morphology at 10 µm. (B) shows SEM morphology at 300 µm. (C) shows SEM morphology at 50 µm. (D) shows EDS mapping that revealed distribution of elements in the biomass.
Uniform filaments and fibrillation can be observed due to their homogeneity, creating three-dimensional structures and reaching nanofiber levels, which facilitates heavy metal adsorption processes [46,47] (Figure 3A–C). Furthermore, after the addition of iron chloride and subsequent Cr(VI) chemisorption, a sample of this biomass was obtained at its maximum Cr(VI) concentration, which was used during the experiment to perform an EDS mapping, which revealed the distribution of the elements oxygen (O), carbon (C), iron (Fe) and chromium (Cr) along the three-dimensional structure (Figure 3C). In addition to the previous figure, Figure 4 is presented, showing the different elements of the sample after iron adhesion and Cr(VI) incorporation.
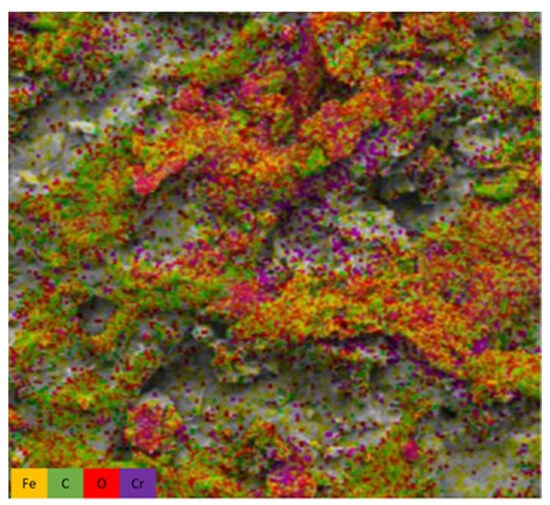
Figure 4.
Showing all the characteristic elements of the BCFe sample.
Different-colored dots can be seen representing the elements in the samples: yellow dots for iron, red for oxygen, green for carbon and purple dots for Cr(VI) deposits. Table 1 shows the physicochemical characterization of the elements in the BCFe sample after adsorption processes of 1000 mg/L of Cr(VI).

Table 1.
Physicochemical characterization of the BCFe sample.
Figure 5 shows the different elements in the sample after iron adhesion and Cr(VI) incorporation.
Figure 5.
Representation of cellulose bacterial with iron.
As demonstrated in Figure 5, a synopsis of the physicochemical characteristics of bacterial cellulose biomass in conjunction with iron chloride is provided. The utilization of iron chloride (Fe(III)) in conjunction with cellulose has been the subject of numerous investigations, with the objective of enhancing the adsorption capacity of heavy metals [24]. Iron (III) is an element that, in the presence of water, oxidizes cellulose, forming iron hydroxides (FeOOH); these oxidation processes are imperative in the elimination of contaminants, such as heavy metals [48,49]. In the present study, Figure 6 shows the observed removal process of Cr(VI) from effluents by bacterial cellulose biomass with iron. Similar ion-exchange and complexation patterns between H+ ions from the biomass and oxygen-containing metal species were observed for Cu2+, Ni2+, Co2+ and Fe3+ [50,51,52].
Figure 6.
Representation of cellulose bacterial with iron and adsorption of Cr(VI).
3.3. Result of the Cr(VI) Adsorption-Process Column Experiments
Figure 7 shows a schematic of the configuration of the process used for the removal of Cr(VI), using fixed-bed columns in series. The yields are with an initial concentration of 400, 600 and 1000 mg/L. The final result was obtained as an average using Student’s t-distribution.
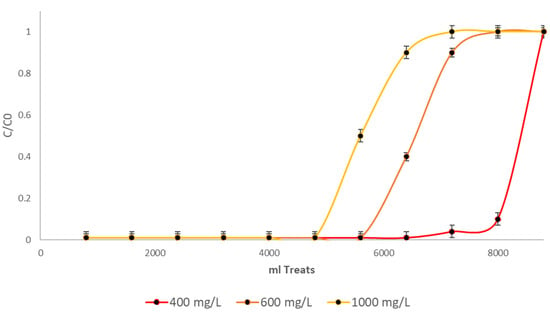
Figure 7.
Treatment with the BCFe biomass in three experiments, showing the arithmetic mean of n = 3, with error bars and standard deviations.
As illustrated in Figure 7, Cr(VI) removal rates varied with three different initial chromium concentrations. The concentrations of the substance investigated were 400 mg/L, 600 mg/L and 1000 mg/L. It is important to note that all the aforementioned processes were performed in triplicate and were based on the arithmetic mean. Final concentrations were consistently below 0.1 mg/L, indicating effective chromium removal from the water. Consequently, under all three initial conditions, the curves did not develop until biomass saturation, at which point samples above 500 mL were collected at the column outlet.
It is evident that optimal performances were obtained with initial concentrations of 400 mg/L, with an approximate volume of 8 L of contaminated water treated. With concentrations of 600 mg/L, approximately 6 L of water was treated, and with initial concentrations of 1000 mg/L, around 5 L of water was treated. The results derived from the treatment volume are optimal, since this system emulates a fixed-column process for the treatment of effluents contaminated with this heavy metal. In experimental processes with bacterial cellulose without any modification and using parameters similar to those of the present investigation, it was shown that this biomass treats around 4.5 L of water with initial concentrations of 1000 mg/L [18], concluding that, by means of the iron chloride reagent, the treatment volume increases around 18%. The use of cellulose xanthate [53] and cellulose with sodium tripolyphosphate (TPP) [54] has also been reported, where volumes of 4 L and 5 L were treated, respectively. Furthermore, the combination of cellulose with EDTA is not exclusively for elutions; an excellent treatment with EDTA and bacterial cellulose has been documented to remove Cr(VI) [55], treating around 5 L of water with initial concentrations of 1000 mg/L. In addition, the application of TiO2 modifications has been explored, treating volumes of 5 to 6 L [56]. By means of iron modifications, the cellulose biomass of E. crassipes also increased its adsorption capacity by 20% [27] compared to biomass without this reagent. It has also been shown that, in studies in fixed columns and with similar flow rates, this reagent optimized the Cr(VI) removal process [26].
3.4. Isotherm Studies
Different types of isotherms were studied to establish the dynamics and distribution of Cr(VI) on the new adsorbent biomass model. Various studies have determined that bacterial cellulose is a homogeneous monolayer [57,58] due to its almost perfect glucose distribution, forming three-dimensional networks of this monosaccharide. However, when iron chloride is present in its biochemical structure, it is best to determine the isothermal distribution under which it adapts to this process. Generally, the distribution of contaminants, especially heavy metals, is governed by quasi-probabilistic distributions, in which a homogeneous layer is present, resulting in a Gaussian-type distribution. Figure 8 shows the relationship between the adsorption capacity q(mg/g) vs. the equilibrium concentration, along with other isotherm curves. These curves show the behavior and the experimental data fit some, but not others.
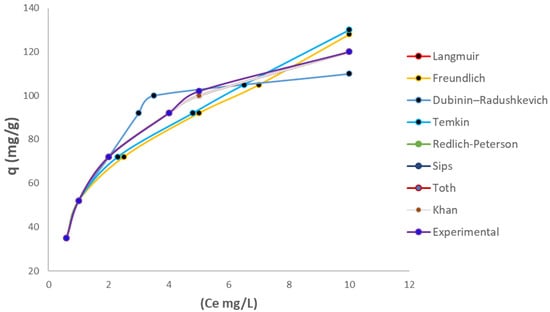
Figure 8.
Representation of the isotherms in comparison with the experimental data.
Based on the results obtained in this investigation and the analysis of R2 and the values of the statistical indices, among the two-parameter isotherm models, the Langmuir model provides a good fit for the Cr(VI) ion. It can be observed that the Langmuir model is very similar to the experimental results. Table 2 shows all the fits and parameters of each isotherm. The correlation coefficients (R2) show that, in theory, all models could be used.

Table 2.
Parameters of each isotherm.
It has been demonstrated that the energy distribution for adsorption sites on bacterial cellulose is predominantly uniform and Gaussian, as opposed to exponential, as evidenced in [2,59]. In experiments with bacterial cellulose without any chemical agent, a fit with the Langmuir isotherm was also observed and a maximum capacity of 111 mg/g was obtained [18]. Moreover, experimental findings confirmed that the three-parameter isotherm models employed exhibited an optimal alignment with experimental outcomes, as evidenced by elevated regression coefficients and minimal values for statistical parameters. This result suggests that the adsorption of Cr(VI) on BCFe adsorbent provides homogeneous monolayer coverage and that the Langmuir isotherm is the most appropriate model to describe this process [60,61]. It was observed that the Sips, Toth, Redlich–Peterson and Khan isotherms obtained similar adsorption capacities and also demonstrated a high degree of compatibility with the experimental designs. The Temkin isotherm model is more appropriate for heterogeneous processes, whereas this biomass is more indicative of homogeneous isotherms. It is evident that the SIPS isotherm has an n coefficient of 1, thus indicating that the process is homogeneous and monolayer in nature. In this instance, the Redlich–Peterson model proved to be a more suitable option for the Frendlinch isotherm, given its elevated concentrations. The Toth model was demonstrated to facilitate the calibration of the adsorption capacity exhibited by the Langmuir isotherm, a phenomenon in which the outcomes were to be analogous.
3.5. Desorption–Elution and Reuse
Reuse and elutions are fundamental parameters for the scaling and viability of adsorbent biomasses. Elutions were carried out with EDTA based on the same treatment processes, and interesting results were achieved. The adsorption capacities achieved a significant increase at the end of the cycles. In the first cycle, the adsorption capacities were practically equal to those of cycle (0), which shows that the Cr (VI) removal process was not affected, demonstrating that EDTA is an excellent chelating agent [62]. Subsequently, the adsorption capacities gradually decreased due to the possible wear of bacterial cellulose. Figure 9 shows the cycles and the total capacities of adsorption.
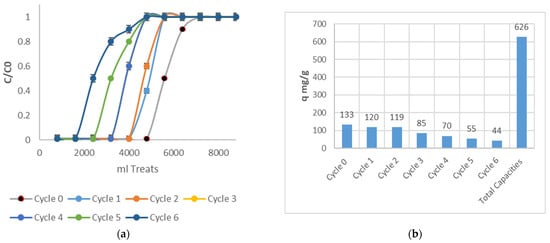
Figure 9.
Adsorption capacities in different cycles’ adsorption processes. (a) shows the different cycles and (b) shows the adsorption capacity of each cycle, along with a summation of all adsorption capacities.
According to Figure 9a,b, sustainable performance is evident for applications in six different biomass reuse cycles, showing the different adsorption capacities for each cycle. Summing all these adsorption capacities gives a cumulative total of 626 mg/g, demonstrating that this process is of vital importance, increasing the economic and environmental sustainability of Cr(VI) treatment, which is suitable for future scale-up processes. After the elution and reuse processes, isotherm tests were performed to validate whether the biomass had undergone changes in its biochemical structure. An isotherm analysis was performed on the eluted and reused biomass after six treatment cycles and, along with equilibrium and adsorption capacity data, was used to establish the adsorption behavior of this new biomass. Figure 10 shows the isotherm process before Cycle 6 of the treatment.
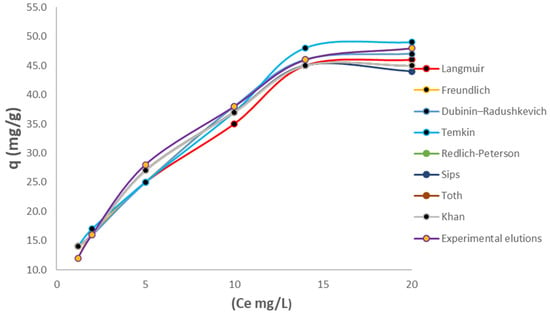
Figure 10.
Isotherm process before Cycle 6 of treatment.
The biomass no longer has the homogeneous monolayer it had before the elution and reuse processes due to the wear caused by the addition of EDTA and subsequent reuse. At this point, it was oriented more toward the Freundlich isotherm, although it also conformed to the Langmuir isotherm, as can be seen in Table 3.

Table 3.
Parameters of each isotherm before Cycle 6 of treatment.
Before regeneration, the BCFe adsorption isotherm followed a Langmuir model (R2 ≈ 0.99), indicating homogeneous monolayer adsorption. After six cycles, the data were better fit by a Freundlich model (R2 ≈ 0.95 vs. 0.94 for Langmuir). The fit was necessary because of the observed decrease in adsorption capacities at lower concentrations. The Freundlich isotherm model hypothesizes that Cr(VI) adsorption in Cycle 6 occurs on a heterogeneous surface, meaning that the active sites on the biomass have been modified as a consequence of continuous adsorption elutions [63]. The Temkin isotherm model also represented an important fit due to the increased heterogeneity exhibited by the biomass. The SIPS isotherm had a coefficient n of less than 1, indicating a heterogeneous process and therefore demonstrating a fit with the Freundlich model. In this case, the Redlich–Peterson model showed a stronger correlation with the Langmuir isotherm, which was attributable to its lower final concentrations.
In order to ascertain the extent of the impact of EDTA, its elutions and subsequent treatments, the biomass was characterized in Figure 11 using SEM and EDS analysis.
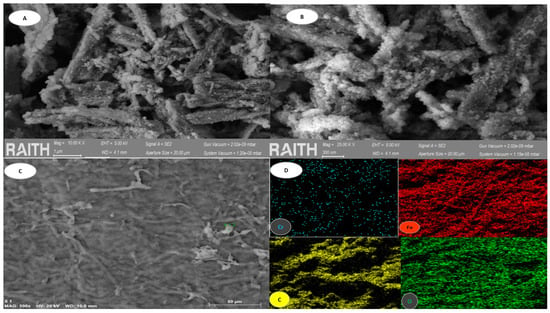
Figure 11.
BCFe in the Cycle 6 analysis. (A) shows SEM morphology at 1 µm. (B) shows SEM morphology at 300 µm. (C) shows SEM morphology at 50 µm. (D) is an image of particles of adsorbent obtained from BCFe.
A comparison of the images in Figure 11 (see Figure 11A–C) reveals the presence of some characteristic deterioration following elution and subsequent reuse. Evidence of porosities and poor homogeneity at the nanofiber level is apparent, and these characteristics are no longer evident after the reuse and recycling process. As illustrated in Figure 11D, electron probe microanalysis was conducted, yielding a distribution of the elements oxygen (O), carbon (C), iron (Fe) and chromium (Cr) throughout the three-dimensional structure. Table 4 illustrates the physicochemical characterization of the elements in the BCFe sample following the adsorption processes of 1000 mg/L of Cr(VI) after six cycles of treatments.

Table 4.
The following investigation is concerned with the physicochemical characterization of the BCFe sample.
EDS evaluation shows the characterization of the BCFe biomass after 6 cycles of EDTA elutions and subsequent Cr(VI) adsorption treatment. Compared to Table 1, there is a decrease in the amount of iron in the sample. Also, a significant reduction in Cr(VI) can be seen due to the loss of biomass adsorption processes due to outgassing caused by the elutions. Due to the composition of EDTA, C10H16N2O8, there was a presence of nitrogen and an increase in the characterization of the carbon and oxygen sample. Figure 12 shows a representation of the EDTA elution process.
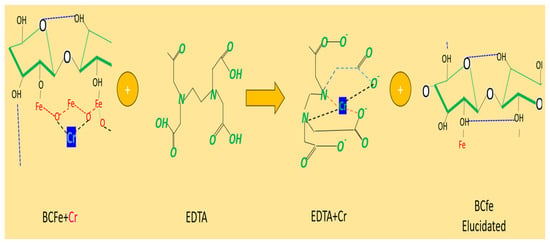
Figure 12.
Representation of the EDTA elution process.
Elution and reuse experiments demonstrate the remarkable resilience of repeated elution with EDTA, thus proving that this material is indeed susceptible to repeated reuse [64,65]. The reuse and continuous recycling of biomass is imperative in the large-scale construction of treatment systems for the improvement of water quality [66,67]. Bacterial cellulose is particularly well-suited to this purpose due to its high resistance to this chemical mechanism [68]. In heavy metal treatment systems, the chelating agent EDTA is employed for the purpose of recovering biomass and subsequently reusing it on a continuous basis. This approach is particularly well-suited to processes aimed at enhancing adsorption capacities. In addition to its high elution capacity, it is considerably economical and non-toxic to the environment [69]. Through the analysis of isotherm and SEM imaging, it can be determined that the transition from biomass homogeneity, conforming to the Langmuir isotherm, to a heterogeneous biomass, conforming to the Freundlich isotherm, is indicative of wear after elutions and subsequent treatments. This indicator is reflected in the low capacities and also in the lack of uniformity of the data obtained and is an important indicator when modeling and designing treatment systems based on adsorbent biomasses in continuous systems.
4. Conclusions
A treatment system for water contaminated with Cr(VI) was designed and built in a continuous fixed-column system. This system incorporates bacterial cellulose biomass reinforced with iron chloride. A total capacity of 626 mg/g was achieved through six elution, reuse and treatment cycles for the removal of Cr(VI). Bacterial cellulose is characterized by being a biomass with a homogeneous structure. In the presence of iron chloride, it establishes monolayer conditions, thus demonstrating its conformity to the Langmuir isotherm. However, after the elution and reuse processes, the biomass underwent a transition to heterogeneous conditions, showing a better fit to the Freundlich isotherm. This information provides design data that could inform the scale-up of Cr(VI) remediation systems. The excellent removal and reusability of this material suggest that a treatment system based on bacterial cellulose biomass reinforced with iron chloride could be used for the construction of an industrial-scale treatment system.
Funding
The university Los Libertadores is the company that contributed to development of this article and related processes through the research macroproject “Development of isothermal models in biomass pollutant adsorption processes” on adsorbent biomasses with code ING-07–25.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Castellanos, H.G.; Aryanfar, Y.; Mohtaram, S.; Keçebaş, A.; Karaca-Dolgun, G.; Ahmad, S.; Asiri, A.N.M.; Islam, S. The efficacy of nano-cellulose-based composites in heavy metal removal from wastewater: A comprehensive review. J. Chem. Technol. Biotechnol. 2025, 100, 291–312. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical review of bioadsorption on modified cellulose and removal of divalent heavy metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Xie, Y.-X.; Wang, L.; Zhou, Z.-H.; Liu, W.-J.; Wang, W.; Yang, J.-H.; He, M.-L.; Qiu, J.-G.; Jiang, B.-H. m6A RNA methyltransferase METTL16 induces Cr (VI) carcinogenesis and lung cancer development through glutamine biosynthesis and GLUL expression. J. Hazard. Mater. 2024, 480, 136093. [Google Scholar] [CrossRef] [PubMed]
- Mahiout, S.; Kiilunen, M.; Vermeire, T.; Viegas, S.; Woutersen, M.; Santonen, T. Occupational exposure to Cr (VI) in Finland in 1980–2016 and related lung cancer risk assessment. Regul. Toxicol. Pharmacol. 2022, 136, 105276. [Google Scholar] [CrossRef]
- Sweef, O.; Yang, C.; Wang, Z. The oncogenic and tumor suppressive long non-coding RNA–microRNA–messenger RNA regulatory axes identified by analyzing multiple platform omics data from cr (VI)-transformed cells and their implications in lung cancer. Biomedicines 2022, 10, 2334. [Google Scholar] [CrossRef]
- Jiang, Z.; Ho, S.H.; Wang, X.; Li, Y.; Wang, C. Application of biodegradable cellulose-based biomass materials in wastewater treatment. Environ. Pollut. 2021, 290, 118087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.D.; Wang, Y.; Zou, X.H.; Yin, W.M.; Wang, X.Y.; Guo, Y.R.; Pan, Q.J. Fabrication of cellulose@ Mg (OH) 2 composite filter via interfacial bonding and its trapping effect for heavy metal ions. Chem. Eng. J. 2021, 426, 130812. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose hydrogel and its derivatives: A review of application in heavy metal adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Putri, R.E.D.; Lestari, D.I.; Sari, D.A.; Putri, Z.P.; Maliki, S. Potential of Biomass Raw Material for Biochar Production: A Review: POTENSI Bahan Baku Biomassa Untuk Produksi Biochar: Tinjauan. Chem. Eng. J. Storage 2025, 5, 528–553. [Google Scholar] [CrossRef]
- El Mahdaoui, A.; Radi, S.; Elidrissi, A.; Faustino, M.A.F.; Neves, M.G.P.; Moura, N.M. Progress in the modification of cellulose-based adsorbents for the removal of toxic heavy metal ions. J. Environ. Chem. Eng. 2024, 12, 113870. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, Y.; Shang, Q.; Yang, X.; Wang, D.; Liao, G. Fabrication of multifunctional biomass-based aerogel with 3D hierarchical porous structure from waste reed for the synergetic adsorption of dyes and heavy metal ions. Chem. Eng. J. 2023, 451, 138934. [Google Scholar] [CrossRef]
- Patra, N.; Ramesh, P.; Țălu, Ș. Advancements in Cellulose-Based Materials for CO2 Capture and Conversion. Polymers 2025, 17, 848. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, M.; Zhang, X.; Zhang, H.; Wang, G.; Zhang, Y. Hierarchically porous poly (amidoxime)/bacterial cellulose composite aerogel for highly efficient scavenging of heavy metals. J. Colloid Interface Sci. 2021, 600, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Dong, J.-J.; Azi, F.; Feng, X.; Ge, Z.-W.; Yang, S.; Sun, Y.-X.; Guan, X.-Q.; Dong, M.-S. Mechanism of Cr (VI) removal by polyphenols-rich bacterial cellulose gel produced from fermented wine pomace. NPJ Clean Water 2024, 7, 21. [Google Scholar] [CrossRef]
- Tran, G.T.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Bacterial cellulose and composites for the treatment of water pollution: A review. Environ. Chem. Lett. 2025, 23, 707–732. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B.; Lozano, A.M. Design of Biomass Adsorbents Based on Bacterial Cellulose and E. crassipes for the Removal of Cr (VI). Polymers 2025, 17, 1712. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Roy, J.S.; Fréchette, J.; Morency, S.; Gomes, O.P.; Dumée, L.F.; Greener, J.; Messaddeq, Y. Removal of cadmium and chromium heavy metals from aqueous medium using composite bacterial cellulose membrane. Chem. Eng. J 2024, 490, 151665. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Castro, Y.P. Development of a composite material between bacterial cellulose and E crassipes, for the treatment of water contaminated by chromium (VI). Int. J. Environ. Sci. Technol. 2022, 19, 6285–6298. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Wan, C.; Zhang, Z.; Cao, W.; Wang, G.; Wu, Y. A comprehensive review of cellulose nanomaterials for adsorption of wastewater pollutants: Focus on dye and heavy metal Cr adsorption and oil/water separation. Collagen Leather 2024, 6, 35. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Bioremoval of heavy metals by bacterial biomass. Environ. Monit. Assess. 2015, 187, 4173. [Google Scholar] [CrossRef]
- Leng, L.; Zheng, H.; Shen, T.; Wu, Z.; Xiong, T.; Liu, S.; Cao, J.; Peng, H.; Zhan, H.; Li, H. Engineering Biochar from biomass pyrolysis for effective adsorption of heavy metal: An innovative machine learning approach. Sep. Purif. Technol. 2025, 361, 131592. [Google Scholar] [CrossRef]
- Kaleem, M.; Minhas, L.A.; Hashmi, M.Z.; Farooqi, H.M.U.; Waqar, R.; Kamal, K.; Aljaluod, R.S.; Alarjani, K.M.; Mumtaz, A.S. Biogenic synthesis of iron oxide nanoparticles and experimental modeling studies on the removal of heavy metals from wastewater. J. Saudi Chem. Soc. 2024, 28, 101777. [Google Scholar] [CrossRef]
- Gu, J.; Yi, W.; Liu, X.; Ru, Y.; Tan, L.; Liu, T. Synthesis of highly porous ferric hydroxide-bacterial cellulose nanocomposites via in-situ mineralization for efficient glyphosate removal. Cellulose 2024, 31, 8041–8053. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Wei, W.; Wang, S.; Xu, T.; Xiao, H.; Dai, H.; Zhou, X.; Bian, H. In-situ deposition of β-FeOOH nanoparticles on commercially available filter paper for fast and efficient removal of antibiotic. Adv. Compos. Hybrid Mater. 2025, 8, 196. [Google Scholar]
- Cheng, R.; Chen, Y.; Kang, M.; Jiang, P.; Shi, L.; Zheng, J.; Zheng, X.; Wang, J. Anchoring nanoscale zero-valent iron within bacterial cellulose particles for boosting efficient adsorption of Co (II) and Sr (II) from seawater: Dual system and varying adsorption mechanisms. J. Environ. Sci. 2025, 154, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Carreno Sayago, U.F. Design, scaling, and development of biofilters with e crassipes for treatment of water contaminated with Cr (VI). Water 2021, 13, 1317. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H.; Na, Z.; Lin, K. A novel biodegradable arsenic adsorbent by immobilization of iron oxyhydroxide (FeOOH) on the root powder of long-root Eichhornia crassipes. Chemosphere 2018, 192, 258–266. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, V.B.; Lozano, A.M. Development of a Treatment System of Water with Cr (VI) Through Models Using E. crassipes Biomass with Iron Chloride. Toxics 2025, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Juturu, R.; Murty, V.R.; Selvaraj, R. Efficient adsorption of Cr (VI) onto hematite nanoparticles: ANN, ANFIS modelling, isotherm, kinetic, thermodynamic studies and mechanistic insights. Chemosphere 2024, 349, 140731. [Google Scholar] [CrossRef]
- Khan, A.A.; Naqvi, S.R.; Ali, I.; Arshad, M.; AlMohamadi, H.; Sikandar, U. Algal-derived biochar as an efficient adsorbent for removal of Cr (VI) in textile industry wastewater: Non-linear isotherm, kinetics and ANN studies. Chemosphere 2023, 316, 137826. [Google Scholar] [CrossRef]
- Nizam, T.; Krishnan, K.A.; Joseph, A.; Krishnan, R.R. Isotherm, kinetic and thermodynamic modelling of liquid phase adsorption of the heavy metal ions Zn (II), Pb (II) and Cr (VI) onto MgFe2O4 nanoparticles. Groundw. Sustain. Dev. 2024, 25, 101120. [Google Scholar] [CrossRef]
- Areti, H.A.; Jabesa, A.; Daba, B.J.; Jibril, D. Response surface method based parametric optimization of Cr (VI) removal from tannery wastewater using a mixed banana peel and corn cob activated carbon: Kinetic and isotherm modeling studies. J. Water Process Eng. 2024, 59, 104977. [Google Scholar] [CrossRef]
- Kumar, M.; Pakshirajan, K. Heavy metal removal and recovery as nanoparticles from multicomponent system at low pH condition by biogenic sulfide precipitation using anaerobic sulfate reducing biomass. Environ. Qual. Manag. 2022, 32, 253–264. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation; Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. (Eds.) Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Ojembarrena, F.D.B.; García, S.; Merayo, N.; Blanco, A.; Negro, C. Ni (II) and Pb (II) removal using bacterial cellulose membranes. Polymers 2023, 15, 3684. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Rojo, Ú.M.; Cerrutti, P.; Foresti, M.L.; Errea, M.I. Carboxymethylated bacterial cellulose: An environmentally friendly adsorbent for lead removal from water. J. Environ. Chem. Eng. 2018, 6, 6844–6852. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Farnane, M.; Machrouhi, A.; Elhalil, A.; Barka, N. Adsorptive removal of heavy metals from aqueous solution using chemically activated Diplotaxis Harra biomass. Surf. Interfaces 2016, 4, 84–94. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Kurniawan, A.; Ki, O.L.; Ismadji, S. Incorporation of selectivity factor in modeling binary component adsorption isotherms for heavy metals-biomass system. Chem. Eng. J. 2013, 219, 137–148. [Google Scholar] [CrossRef]
- Min, L.U.; Zhang, Y.M. Thermodynamics and kinetics of adsorption for heavy metal ions from aqueous solutions onto surface amino-bacterial cellulose. Trans. Nonferrous Met. Soc. China 2014, 24, 1912–1917. [Google Scholar] [CrossRef]
- Mohammed, A.H.; Shartooh, S.M.; Trigui, M. Biosorption and Isotherm Modeling of Heavy Metals Using Phragmites australis. Sustainability 2025, 17, 5366. [Google Scholar] [CrossRef]
- Zhu, C.; Chu, Z.; Ni, C.; Chen, Y.; Chen, Z.; Yang, Z. Robust functionalized cellulose-based porous composite for efficient capture and ultra-fast desorption of aqueous heavy metal pollution. Carbohydr. Polym. 2024, 324, 121513. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Shao, G.; Qin, B.; Wang, Y.; Wang, T.; Liu, Z.; Fu, Y. Supramolecular cellulose-based heavy metal adsorbent for efficient and accurate removal of cobalt (II) for water treatment. React. Funct. Polym. 2024, 194, 105759. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Yasin, S.A.; Abdullah, S.M.; Thalji, M.R.; Aziz, F.; Assiri, M.A.; Chong, K.F.; Ali, G.A.M.; Bakr, Z.H. Removal of Cr (VI) using thiol-modified cellulose nanostructure for water sustainability: Detailed adsorption study. Biomass Convers. Biorefinery 2025, 15, 10791–10807. [Google Scholar] [CrossRef]
- Rastgar, S.; Rezaei, H.; Yousefi, H. Study of kinetics, isotherms and thermodynamics of lead adsorption from aqueous solutions using Lignocellulose Nano-fibers (LCNFs). Environ. Resour. Res. 2020, 8, 64–82. [Google Scholar]
- Jamshaid, A.; Hamid, A.; Muhammad, N.; Naseer, A.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Shah, N.S. Cellulose-based materials for the removal of heavy metals from wastewater–an overview. ChemBioEng Rev. 2017, 4, 240–256. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Westholm, L.J.; Lou, S.; Sainio, T.; Sillanpää, M. Adsorption of Ni2+, Cd2+, PO43− and NO3− from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite. Chem. Eng. J. 2014, 252, 64–74. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Zhou, T.; Wu, Q.; Liu, C.; Li, S.; Feng, Q.; Wang, H.; Li, Z. Environmentally friendly bacterial cellulose hydrogel-derived aerogel and membrane for efficient water purification. J. Environ. Chem. Eng. 2025, 13, 116023. [Google Scholar] [CrossRef]
- Younas, M.; Ali, J.; Hou, J.; Chen, Y.; Juan, L.; Bonilla-Petriciolet, A.; Shaoya, M. Bacterial cellulose modified with thiomolybdate sulfides: An outstanding eco-friendly adsorbent for the removal of multiple heavy metals from aqueous medium. Sep. Purif. Technol. 2025, 374, 133656. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef]
- Huang, X.; Zhan, X.; Wen, C.; Xu, F.; Luo, L. Amino-functionalized magnetic bacterial cellulose/activated carbon composite for Pb2+ and methyl orange sorption from aqueous solution. J. Mater. Sci. Technol. 2018, 34, 855–863. [Google Scholar] [CrossRef]
- Wei, Y.; Fang, Z.; Zheng, L.; Tsang, E.P. Nanopartículas de hierro biosintetizada en extractos acuosos de Eichhornia crassipes y su mecanismo en la remoción de chrome hexavalente. Superf. Appl. Sci. 2017, 399, 322–329. [Google Scholar] [CrossRef]
- de Paiva, G.M.; Palladino, F.; Nucci, E.R.; Machado, A.R.T.; Rosa, C.A.; Santos, I.J.B. Bacterial nanocellulose produced as a by-product of the brewing industry and used as an adsorbent for synthetic solutions of Co (II), Cu (II), Ni (II) AND Fe (III). J. Polym. Environ. 2024, 32, 6803–6819. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, B.V. Development of a treatment for water contaminated with Cr (VI) using cellulose xanthogenate from E. crassipes on a pilot scale. Sci. Rep. 2023, 13, 1970. [Google Scholar] [CrossRef] [PubMed]
- Sayago, U.F.C.; Ballesteros, V.A.B. The design of a process for adsorbing and eluting chromium (VI) using fixed-bed columns of E. crassipes with sodium tripolyphosphate (TPP). Water 2024, 16, 952. [Google Scholar] [CrossRef]
- Shi, Y.; Feng, J.; Zhang, Z.; Cao, N.; Li, J.; Li, H.; Li, L.; Hua, Q.; Ma, Q.; Zhang, K. Simultaneous removal of Cr (VI) anions and metal cations by EDTA-crosslinking-chitosan/polypyrrole composites. Sep. Purif. Technol. 2023, 327, 124926. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Ballesteros, B.V. Development of a wastewater treatment system contaminated with Cr (VI) through vegetable biomass modified with TiO2. Int. J. Environ. Sci. Technol. 2025, 22, 6521–6534. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2009, 161, 1355–1359. [Google Scholar] [CrossRef]
- Nguyen, T.A. Revolutionizing Lead (II) Ion Removal from Water: Eco-Friendly Composite Film with Graphene Oxide and Bacterial Cellulose. Trends Sci. 2025, 22, 9589. [Google Scholar] [CrossRef]
- Abiodun, O.-A.O.; Oluwaseun, O.; Oladayo, O.K.; Abayomi, O.; George, A.A.; Opatola, E.; Orah, R.F.; Isukuru, E.J.; Ede, I.C.; Oluwayomi, O.T.; et al. Remediation of heavy metals using biomass-based adsorbents: Adsorption kinetics and isotherm models. Clean Technol. 2023, 5, 934–960. [Google Scholar] [CrossRef]
- Gürkan, E.H.; İlyas, B.; Tibet, Y. Adsorption performance of heavy metal ions from aqueous solutions by a waste biomass based hydrogel: Comparison of isotherm and kinetic models. Int. J. Environ. Anal. Chem. 2023, 103, 1343–1360. [Google Scholar] [CrossRef]
- Du, P.; Xu, L.; Ke, Z.; Liu, J.; Wang, T.; Chen, S.; Mei, M.; Li, J.; Zhu, S. A highly efficient biomass-based adsorbent fabricated by graft copolymerization: Kinetics, isotherms, mechanism and coadsorption investigations for cationic dye and heavy metal. J. Colloid Interface Sci. 2022, 616, 12–22. [Google Scholar] [CrossRef]
- Lin, Z.; Li, L.; Song, K.; Yang, B.; Zhou, G.; Zhang, G.; Teng, J.; Wang, E.; Liu, X.; Ling, F.; et al. Boronic acid-modified bacterial cellulose microspheres as packing materials for enveloped virus removal. Sci. Total Environ. 2023, 859, 160341. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Sasa, R.; Kinoshita, N.; Kishimoto, R.; Kono, H. Nano-Fibrillated Bacterial Cellulose Nanofiber Surface Modification with EDTA for the Effective Removal of Heavy Metal Ions in Aqueous Solutions. Materials 2025, 18, 374. [Google Scholar] [CrossRef] [PubMed]
- Alsaeedi, H.; Ahmad, H.; Altowairqi, M.F.; Alhamed, A.A.; Alsalme, A. Covalently Functionalized Cellulose Nanoparticles for Simultaneous Enrichment of Pb (II), Cd (II) and Cu (II) Ions. Polymers 2023, 15, 532. [Google Scholar] [CrossRef]
- Guo, J.; Tian, H.; He, J. Integration of CuS nanoparticles and cellulose fibers towards fast, selective and efficient capture and separation of mercury ions. Chem. Eng. J. 2021, 408, 127336. [Google Scholar] [CrossRef]
- Yi, C.; Niu, H.-Y.; Sui, L.; Zhu, J.-J.; Tian, Y.; Niu, C.-G.; Chen, Z.-L.; Wei, H.; Huang, D.-W. A low-cost bio-based cellulose composite hydrogel with cross-linked structures for efficient capture of heavy metal ions. Sep. Purif. Technol. 2025, 358, 130213. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, L.; You, N.; Ding, B.; Fan, H. Chelating Cellulose for Removal of Heavy Metals. Korean J. Chem. Eng. 2024, 41, 2729–2739. [Google Scholar] [CrossRef]
- Aljohani, M.S.; Alnoman, R.B.; Alharbi, H.Y.; Al-Anazia, M.; Monier, M. Designing of a cellulose-based ion-imprinted biosorbent for selective removal of lead (II) from aqueous solutions. Int. J. Biol. Macromol. 2024, 259, 129145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).