Abstract
The stability of spore-forming soil bacteria is crucial for their effective use in agricultural biopreparations. This study evaluated the long-term survivability of selected strains (Paenibacillus amylolyticus, Priestia megaterium, Bacillus velezensis, Bacillus subtilis, and Bacillus licheniformis) with potential applications in biopreparations for crop residue decomposition. The effects of different storage and preservation conditions on vegetative cells and bacterial spores were studied over 12 months. Bacteria were stored at different temperatures (15 °C, 21 °C, 30 °C), pH levels (5, 9, and post-cultivation liquid pH), and osmotic pressures (2%, 5%, and 10% of carbamide, calcium chloride, and multicomponent fertilizer). Additionally, freeze-drying, spray-drying and freezing were performed using cryoprotectants (skimmed milk, trehalose, and glycerol). The results showed that bacterial stability depended on both the strain and storage conditions. Vegetative cells of P. amylolyticus and B. velezensis were most sensitive to temperatures of 30 °C, whereas the spores of most strains demonstrated high temperature resistance. The tested strains exhibited better survivability at pH 5 than pH 9. The addition of calcium chloride, carbamide, or multicomponent fertilizer proved beneficial for maintaining viability, especially increasing spore numbers. Trehalose and skimmed milk were the most effective cryoprotectants overall, though efficacy varied by strain and cell form. These findings provide insight into the optimal conditions for preserving the bacterial viability of spore-producing bacteria in bioformulations, which is crucial for maintaining their effectiveness in agricultural applications.
1. Introduction
Biopreparations, such as fungal, bacterial, bacterial/fungal–enzymatic, bacterio-fungal, and enzymatic formulations [1], have a wide range of applications, including as biopesticides, biofertilizers, biostimulants, and agents that stimulate biodegradation processes [1,2,3,4]. Biopreparations are increasingly recognized as vital components in sustainable agriculture, due to their ability to enhance soil health, improve crop yields, and reduce reliance on agrochemical inputs. Biopreparations contribute to the decomposition of crop residues, promoting nutrient cycling and improving soil fertility [5]. They have also been shown to positively affect physical properties such as porosity and temperature, which are critical for optimal plant growth [6]. Biopreparations are environmentally friendly, helping to restore soil vitality, balance energy use in agriculture, and reduce the chemical load on the environment—thus preventing soil acidification and promoting a healthier ecosystem, which is especially important in organic farming [7,8]. According to the strategic policy of the European Union (the European Green Deal including the “farm to fork” strategy), a radical reduction in the use of pesticides and synthetic fertilizer is necessary in order to achieve climate neutrality and ensure food security [9,10]. These regulations have also contributed to the growing interest in biopreparation-based solutions.
There are various challenges involved in the development and commercialization of microbial biopreparations, including regulatory barriers and the need for broader market acceptance. Producers must focus on creating high-quality products that meet farmers’ needs and address environmental as well as technological requirements [5]. Key determinants of product performance include storage conditions, their influence on microbial viability, and the choice of an appropriate formulation. Temperature plays a crucial role in the survivability of microorganisms during storage. While room temperature is more convenient and cheaper for distribution, studies indicate that storing biofertilizers at lower temperatures (e.g., 4 °C) generally enhances the survival rate of microbial inoculants. However, refrigerated storage is more energy consuming and logistically more demanding [11,12,13]. Bacterial spores are characterized by greater resistance to external factors, which may have a positive impact on storage time under less favourable conditions [14,15].
Biopreparations are commercially available in various forms, including liquid, freeze-dried, spray-dried, and granular products. Liquid formulations are the most widely available on the market, due to their relatively simple method of production and lower associated costs—primarily because they do not require stabilization steps such as freeze-drying [5]. However, preservation techniques such as freeze-drying or freezing are commonly used to improve long-term storage stability.
Freeze-drying (lyophilization) involves freezing, primary drying, and secondary drying stages, each of which is critical for maintaining the quality of the final product. Parameters such as temperature, pressure, and time must be carefully controlled [16]. Cryoprotectants are used during freeze-drying to ensure a high survival rate. Cryoprotectants play a key role in protecting cells and tissues from damage caused by freezing and thawing, by preventing the formation of large ice crystals and stabilizing cell structures. Cryoprotectants can be classified into two groups: those that penetrate the cell membrane, such as glycerol, ethylene glycol, and dimethyl sulfoxide, and those that remain extracellular, including glucose, sucrose, sorbitol, and other disaccharides [17,18,19,20]. Spray drying is an alternative method that involves three main steps: atomization of the liquid feed, dehydration, and collection of the powder [21]. Protective substances, such as skimmed milk or sugars, may also be added during spray drying to increase cell viability [22].
Although general information on microbial preservation methods is available, specific parameters for freeze-drying or spray drying are often proprietary and remain undisclosed by commercial producers. Even less information exists regarding the survival rates of microorganisms in post-cultivation liquid form, despite the prevalence of such formulations among market-available products [5].
The goal of this study was to evaluate the impact of different storage conditions on the survivability of Bacillus subtilis, Bacillus licheniformis, Bacillus velezensis, Peanibacillus amylolyticus, and Prestia megaterium. The chosen strains show potential to be used in biopreparations for agricultural purposes (B. licheniformis, B. subtilis, and B. velezensis as biocontrol agents; B. subtilis, B. licheniformis, B. velezensis, P. amylolyticus, and P. megaterium as plant growth biostimulants; B. subtilis, B. licheniformis, B. velezensis, P. amylolyticus, and P. megaterium as crop residue decomposition stimulants; B. licheniformis and B. subtilis as bioinsecticides) [23]. Therefore, knowing their survival rates is crucial for selecting the formulation of biopreparations as well as determining the shelf-life of the final bioproduct. This study particularly focuses on the long-term survivability of bacterial strains in the form of post-cultivation liquid. The results are of interest and importance for the development, optimization, and quality control of microbial formulations used in agriculture.
2. Materials and Methods
2.1. Microorganisms
The microorganisms used in this study are environmental isolates of Paenibacillus amylolyticus, Prestia megaterium, Bacillus velezensis, Bacillus subtilis, and Bacillus licheniformis that exhibit enzymatic ability to decompose post-harvest plant residues, as discussed in our previous study [23]. The nucleotide sequences of the strains were identified during the study and have been made available under the genome assembly accession numbers ASM4051364v1, ASM4051367v1, ASM4051368v1, ASM4051371v1, and ASM4051399v1.
2.2. Bacteria Cultivation
Biomass of the tested bacteria was cultivated using a medium composed of yeast extract (2 g/L), soy protein concentrate (5 g/L), calcium chloride dihydrate (0.5 g/L), glucose (10 g/L), sodium chloride (0.5 g/L), magnesium chloride hexahydrate (0.5 g/L), dipotassium phosphate (1.0 g/L), iron chloride (0.5 g/L), and manganese sulfate (0.5 g/L), dissolved in distilled water. The pH during the cultivation was adjusted using 15% (v/v) sodium hydroxide solution and 80% (v/v) lactic acid, as required. The prepared medium was sterilized in situ in a bioreactor (Obram, Olsztyn, Poland) at 121 °C for 20 min. The cultivation process employed aeration at a flow rate equivalent to 50% of the culture volume. For instance, a 100 L culture volume required an air flow of 50 L/min. The process was carried out at 33 °C for 48 h with mechanical agitation set at 90 RPM. Edible vegetable oil supplemented with 1% (v/v) soy phospholipids was used when necessary as an antifoaming agent.
2.3. Experimental Design
The post-cultivation liquid of the individual bacterial strains was aseptically dispensed into falcon tubes at a volume of 40 mL, then subjected to different temperatures (15 °C, 21 °C, and 30 °C), pH (5 and 9 corrected with 1 M HCl and 1 M NaOH (pH-meter FiveEasy Plus, METTLER TOLEDO, Zurich, Switzerland), and the pH of the post-cultivation liquid), as well as osmotic pressure (2%, 5%, and 10% of carbamide (Mocznik Pulrea 46N—nitrogen fertilizer, Zakłady Azotowe Puławy S.A., Pulawy, Poland), calcium chloride (Chempur, Piekary Śląskie, Poland), and a multicomponent fertilizer (Polifoska 6, Grupa Azoty S.A., Tarnow, Poland)). Figure 1 gives an overview of the process of sample preparation. Samples with different pH and osmotic pressure were stored at room temperature. Each variant was performed in triplicate. The numbers of vegetative cells and spores were determined over a period of 12 months (at months 0, 1, 2, 3, 4, 5, 6, 9, and 12).
Figure 1.
Overview of the process of sample preparation: (a) storage; (b) preservation. Created in BioRender. Rowińska, P. (2025) https://BioRender.com/2qwrk45 (accessed on 4 November 2025).
2.4. Preservation Techniques
Three methods of bacteria preservation were investigated: freeze-drying, freezing (−80 °C) and spray drying. The numbers of vegetative cells and spores were counted during storage for 12 months (enumeration at months 0, 2, 4, 6, and 12).
2.4.1. Freeze-Drying
Post-cultivation liquid was dispensed into falcon tubes for freeze-drying. The samples were centrifuged at 6000 RPM for 15 min at 4 °C (MPW-380R, MPW, Warsaw, Poland). The supernatant was decanted, leaving the biomass in the falcon tube. As a cryoprotectant, 20 mL of either a 10% solution of skimmed milk powder (BTL, Warsaw, Poland) or a 10% trehalose solution (Chempur, Piekary Slaskie, Poland) was added to the biomass. The prepared samples (2 mL) were transferred to sterilized freeze-drying vials and frozen at −80 °C (DW-86L338J, Haier Biomedical, Qingdao, China). The samples were then placed in a freeze dryer (2.5 L Triad Benchtop, FreeZone Triad, Labconco, Kansas City, MO, USA) and freeze dried at −15 °C for 24 h under a pressure of 0.15 mbar, followed by a secondary drying phase at 30 °C for 1 h. The freeze-dried samples were stored at room temperature (21 °C).
2.4.2. Freezing
For freezing, 40 mL of the sterile cryoprotectant was added to each Falcon tube after centrifugation. The cryoprotectants used were a 10% solution of skim milk powder (BTL, Poland), a 10% trehalose solution (Chempur, Poland), and a 20% glycerol solution (Chempur, Poland) combined with growth media in a 1:1 ratio. The samples were vortexed and dispensed into sterile Eppendorf tubes, each containing 2 mL of one sample. The tubes were then placed in a freezer (DW-86L338J, Haier Biomedical) at −80 °C. Post-culture liquid without cryoprotectants was frozen under the same conditions as a control.
2.4.3. Spray Drying
After the cultivation stage, 8% (mass/volume), maltodextrin and 2% (mass/volume) trehalose were added to the bioreactor and mixed with the entire culture volume. The resulting slurry was then transferred to a spray dryer (Lukro, Nove Mesto Nad Vahom, Slovakia) with a capacity of 70 kg of water evaporation per hour. Spray drying was performed using a high-pressure nozzle assembly with an inlet temperature of 180 °C and an outlet temperature of 69 °C. The resulting powders showed moisture contents of 1.8–2.6% and water activity under 0.2. The bacterial powders were stored at room temperature (21 °C).
2.5. Enumeration of Vegetative Cells and Spores
The number of vegetative cells was determined by the pour plate method using Tryptic Soy Agar (TSA, Merck-Millipore, Darmstadt, Germany). The plates were incubated at 30 °C for 24 h (CLW 750 Smart incubator, POL-EKO, Wodzislaw Slaski, Poland). The number of spores was determined in the same way, with the addition of heat shock before pour plate method. Heat shock was performed in a water bath (SUB Aqua Pro, Grant Instruments, Royston, England) for 10 min at 80 °C, after which the bacteria were cooled in an ice bath. The enumeration was carried out in three replicates.
2.6. Statistical Analysis
Statistically significant differences were determined using ANOVA and a post hoc Duncan’s test (p < 0.05) using RStudio, v. 2025.05.1 + 513 “Mariposa Orchid” Release (ab7c1bc795c7dcff8f26215b832a3649a19fc16c, 1 June 2025. Tests were conducted for each strain of vegetative cells and spores separately, to examine the effects of the tested conditions on their survivability.
3. Results
3.1. Storage Conditions
The post-cultivation liquid was stored under various conditions, with different pH, temperatures, and additives. In many of the variants, the number of bacteria fluctuated during storage (Figure 2 and Figure 3, Tables S1–S5), as decreasing and increasing values were noted in the same samples over time.
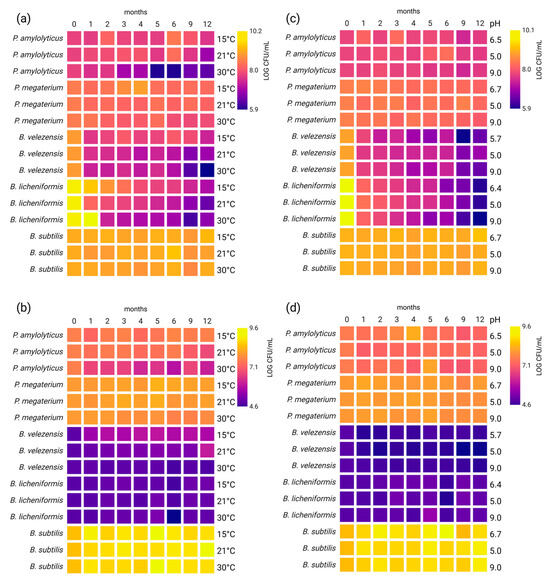
Figure 2.
Survivability of bacteria under different storage conditions: (a) temperature—vegetative cells, (b) temperature—spores, (c) pH—vegetative cells, (d) pH—spores. The heatmaps show changes in the number of viable cells (LOG CFU/mL) measured at each indicated time point (0, 1, 2, 3, 4, 5, 6, 9, and 12 months). The colour intensity reflects the CFU/mL value, with lighter colours corresponding to higher bacterial counts. Created in BioRender. Rowińska, P. (2025) https://BioRender.com/f69a7c3 (accessed on 4 November 2025).
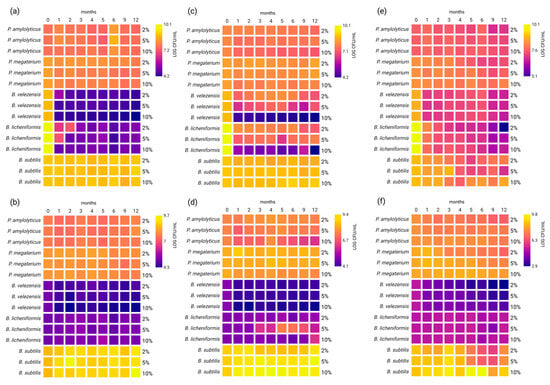
Figure 3.
Survivability of bacteria under different storage conditions of osmotic stress: (a) carbamide—vegetative cells; (b) carbamide—spores; (c) calcium chloride—vegetative cells; (d) calcium chloride—spores; (e) multicomponent fertilizer—vegetative cells; (f) multicomponent fertilizer—spores. The heatmaps show changes in the number of viable cells (LOG CFU/mL) measured at each indicated time point (0, 1, 2, 3, 4, 5, 6, 9, and 12 months). The colour intensity reflects the CFU/mL value, with lighter colours corresponding to higher bacterial counts. Created in BioRender. Rowińska, P. (2025) https://BioRender.com/6hmp3z3 (accessed on 4 November 2025).
The impact of storage conditions on vegetative cells and spores of P. amylolyticus, P. megaterium, B. velezensis, B. licheniformis, and B. subtilis was analyses individually for each strain.
Lowering the storage temperature generally resulted in higher survivability during long-term storage. This was particularly visible for P. amylolyticus after 12 months of storage. For this strain, 1.3 ± 0.03 LOG CFU/mL higher survivability of vegetative cells was observed at 15 °C compared to 30 °C, while at room temperature survivability was in between these rates (Figure 2a, Table S1a). The same pattern was seen in the survivability of spores (1.5 ± 0.02 LOG CFU/mL higher in 15 °C) (Figure 2b, Table S1b). Other tested strains also showed higher survivability at 15 °C or room temperature than at 30 °C during long-term storage. However, for both B. licheniformis and B. subtilis an increase in the number of vegetative cells was observed at 30 °C, in the first month of storage for B. licheniformis and in the sixth month for B. subtilis.
The strains were stored at pH 5, pH 9, and the pH of the post-cultivation liquid, which was different for each strain. Storage at pH 5 and the post-cultivation liquid pH resulted in the highest survivability. However, in contrast to the effects of temperature, there were many differences in survivability depending on strain. In the case of P. amylolyticus, decreases in the numbers of vegetative cells and spores were observed over the 12-month period, but with no statistically significant differences depending on pH (Figure 2c,d, Table S1a,b). Similarly, B. licheniformis demonstrated no statistically significant differences in terms of the survivability of vegetative cells at pH 5.0 and 6.4, with a decrease compared to month 0, while the number of spores increased with higher pH. The greatest increase was noted at pH 9.0 in the fifth month (an increase of 0.66 ± 0.08 LOG CFU/mL compared to 0 month) (Figure 2d, Table S2b). An increase in the number of spores was also noted for B. subtilis for all pH levels, although the highest survivability was observed at pH 5.0 and pH 9.0 (Figure 2b, Table S3b). In contrast, P. megaterium showed an increased number of spores at the pH of the post-cultivation liquid and a decrease in the survivability of vegetative cells at all tested pH levels, with the lowest decrease observed at pH 5.0 (0.30 ± 0.07 LOG CFU/mL) (Figure 2a,b, Table S4a,b). B. velezensis also showed a decrease in survivability at all tested pH levels, with the highest survivability of vegetative cells at pH 5.0 and of spores at the pH of the post-cultivation liquid (Figure 2a,b, Table S5a,b).
As can be seen in Figure 3, the impact of osmotic stress varied depending on the tested strains. All tested condition resulted in decreases in the number vegetative cells, with the exception of 5% carbamide and 5% CaCl2 for P. amylolyticus (Figure 3c, Table S1a), all tested concentrations of carbamide and 10% CaCl2 for B. subtilis (Figure 3c, Table S3a), and 2% and 5% CaCl2 for P. megaterium (Figure 3c, Table S4a). However, the size of the decrease varied depending on the bacteria strain. For example, decreases were noted for P. amylolyticus from 0.15 ± 0.07 LOG CFU/mL (10% carbamide) to 0.64 ± 0.04 LOG CFU/mL (2% multicomponent fertilizer) (Table S1a), whereas B. velezensis exhibited much higher values with decreases ranging from 1.87 ± 0.04 LOG CFU/mL (5% CaCl2) to 5.92 ± 0.02 LOG CFU/mL (10% CaCl2) (Table S5a). It is worth noticing that B. velezensis and B. licheniformis were the most sensitive to all carbamide concentrations, whereas the other strains did not exhibit such high differences between osmotic stressors. Major differences between the survivability of vegetative cells and spores were noted. Three of the five tested strains P. amylolyticus (Table S1b), B. licheniformis (Table S2b) and B. subtilis (Table S3b) exhibited increases in the number of spores when stored with the addition of carbamide, and two showed increases with the addition of CaCl2, namely B. licheniformis at all concentrations of CaCl2 (2%, 5% and 10%) (Table S2b) and P. megaterium at concentrations of 2% and 5% (Table S4b). Overall, the decreases in spore numbers were lower than the decreases in the numbers of vegetative cells, with the exception of B. velezensis, which showed sensitivity to multicomponent fertilizer. The number of spores of B. velezensis with the addition of multicomponent fertilizer decreased by between 1.57 ± 0.02 LOG CFU/mL and 2.44 ± 0.03 LOG CFU/mL (Table S2b).
It should be noted that the plate count method may underestimate total viable populations, as cells exposed to osmotic or thermal stress can enter a viable but non-culturable state.
3.2. Preservation Techniques
We studied the effects of two different cryoprotectants on the vegetative cells and spores after the freeze-drying process. The freeze-drying process showed distinct differences in microbial survivability depending on the cryoprotectant used. Both 10% skimmed milk and 10% trehalose demonstrated protective effects, though their efficacy varied significantly across different strains and cell types (Figure 4, Table S6). In the case of vegetative cells, 10% trehalose generally provided better protection than 10% skimmed milk, as was particularly evident for B. subtilis, B. velezensis, and P. amylolyticus trehalose-treated samples which maintained higher viability counts throughout the 12-month storage period. Trehalose-protected vegetative cells showed less dramatic decreases over time, with many measurements remaining in the same statistical groups for extended periods. However, skimmed milk was more effective in the case of P. megaterium. Moreover, there were no statistically significant differences between cryoprotectants for B. licheniformis. Spore survivability was less affected by cryoprotectant choice, though both protective agents helped maintain high CFU/mL.
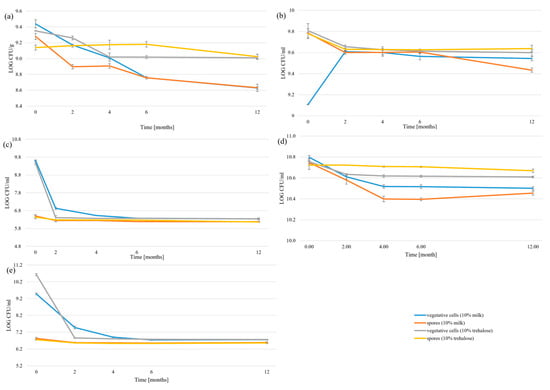
Figure 4.
Survivability of the freeze-drying process: (a) P. amylolyticus; (b) P. megaterium; (c) B. velezensis; (d) B. subtilis (e) B. licheniformis.
Survivability after spray-drying was compared to survivability after freeze-drying, as these methods are often used as alternatives. Survivability after spray drying showed higher stability than freeze-drying. In the case of vegetative cells, statistically significant decreases were noted for B. subtilis, B. licheniformis, and P. amylolyticus, with the highest decrease for P. amylolyticus after two months of storage (0.5 ± 0.02 LOG CFU/g). Spore numbers were even more stable, as only B. velezensis showed a decrease during the storage period (Figure 5).
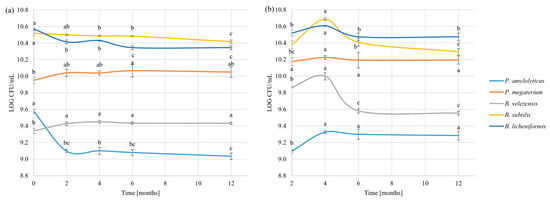
Figure 5.
Survivability after spray drying: (a) vegetative cells; (b) spores. Different letters indicate statistically different groups. Tests were performed independently for spores and vegetative cells of each strain (Duncan’s test (p < 0.05)).
We also studied the effects of two three cryoprotectants on the vegetative cells and spores during freezing, and compared the results for each strain to freezing without cryoprotectants. The results (Figure 6, Table S7) show dramatic differences between cryoprotectants. When no cryoprotectant was used, all strains experienced gradual but statistically significant decreases in viability over the 12-month period, with B. velezenis spores showing the greatest decrease (1.28 ± 0.02 LOG CFU/mL). Differences between the optimal cryoprotectants for the survivability of vegetative cells and spores were also observed. For vegetative cells of B. velezensis, the most effective cryoprotectant was skimmed milk, whereas for spores it was trehalose. Vegetative cells of B. licheniformis demonstrated higher survivability with skimmed milk, while P. megaterium vegetative cells and spores showed higher survivability with trehalose. Compared to the control, 20% glycerol was found to be an effective cryoprotectant, although it performed worse than skimmed milk and trehalose. All cryoprotectants performed equivalently in the case of B. subtilis, with no statistically significant differences between treatments throughout the storage period. Meanwhile, P. amylolyticus showed high survivability under control conditions, suggesting freezing resistance. These results show that the choice of optimal cryoprotectant depends on the strain. However, 10% trehalose and 10% skimmed milk were generally the most effective.
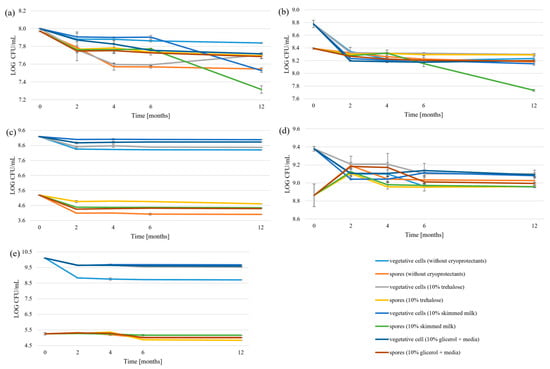
Figure 6.
Survivability of freezing: (a) P. amylolyticus, (b) P. megaterium, (c) B. velezensis, (d) B. subtilis, (e) B. licheniformis.
4. Discussion
This study demonstrated that the stability of biopreparations depends on the preparation, the strain type, and the storage conditions. The storage conditions are crucial for maintaining the viability and functionality of the microorganisms over time. The shelf life of the preparation depends on several factors, such as the type of bacteria, storage conditions, and the use of protective additives. Storage temperature is a critical factor determining the shelf life of liquid microbial biopreparations. As refrigeration is harder during the distribution process and storage by farmers, in this research we investigated lowering the temperature to 15 °C. This resulted in higher survivability for all strains, especially P. amyloliticus which showed sensitivity to high temperatures. These findings corelate with the literature data, as refrigeration (4 °C) has frequently been shown to slow down bacterial metabolism and maintain viability. For example, Mesorhizobium sp. and Pseudomonas sp. inoculants demonstrated better viability when stored under refrigerated conditions compared to room temperature [24]. However, there is a lack of data in the literature concerning the survivability of liquid biopreparations.
The tested strains exhibited better survivability at pH 5 and the pH of the post-cultivation liquid than at pH 9, except in the case of P. megaterium which exhibited high survivability at all tested pH levels. The literature data suggest that P. megaterium is stable at low pH. Under RNA-seq analysis, P. megaterium G18 exposed to acid stress (pH 4.5) showed differential expression of 207 genes, with 11 genes exhibiting increased transcription specifically at this lower pH. These genes were associated with processes such as maintenance of cell integrity, pH homeostasis, alternative energy generation, and modified metabolic pathways including the pentose phosphate pathway and fatty acid biosynthesis [25]. The higher survivability of B. subtilis at lower pH aligns with the literature, which shows that B. subtilis 168 can be cultivated and stored at different pH levels, specifically at pH 5.0, 7.0, and 8.5. Long-term adaptation to these pH conditions leads to significant changes in membrane lipid composition, affecting lipid order and dynamics [26]. Examining the survivability of microorganisms in different pH ranges is important not only for improving the shelf life of biopreparations for agricultural purposes by altering the pH, but also to ensure the survivability of microorganisms in soil, as natural soil pH falls within the range of 4–8, with the majority of agricultural soils ranging from 5.5 to 7.5 [27].
Our investigation of the survivability of bacteria under osmotic stress focused on substances often used in agricultural fertilizers. Our result show that the survivability of bacteria varies depending on the strain and stressor. Generally, vegetative cells were more sensitive than spores. The addition of CaCl2 decreased the number of vegetative cells of B. licheniformis and increased the number of spores. Similarly, P. megaterium showed an increase in the number of spores in the first months and no statistically significant differences between the beginning of storage and month 12 of storage. Calcium is known to increase the number of spores of Bacillus sp. [28,29], and our study confirmed that it can be used to increase the number of spores during the storage. The increased number of spores may be a result of calcium’s roles in signalling, influencing gene expression and cellular differentiation processes (including sporulation), together with its integral contribution to the spore cortex [30]. Moreover, the addition of calcium has been shown to significantly increase the expression of sporulation-related genes. For instance, in B. coagulans the presence of calcium carbonate, which provides calcium ions, led to a marked increase in the expression of kinC, kinE, and spo0A genes, which are essential for sporulation initiation [29].
The number of vegetative cells of B. subtilis decreased in trials with multicomponent fertilizer, but the number of spores increased with multicomponent fertilizer as well as carbamide. The impact of chemical multicomponent fertilizer or carbamide on bacteria has not been studied extensively and there is a lack of data on this subject. Our study suggests that it may also positively impact the number of spores, which may be attributed to the increased concentration of mineral salts and/or nitrogen necessary for spore formation, as well as to unfavourable conditions resulting from the increased concentration of osmotically active substances, which triggered sporulation.
We also noted fluctuations in the number of bacteria over time during storage under the same conditions. Fluctuations in cell numbers may result from germination under unfavourable conditions, cell death, cryptic growth (when surviving bacteria utilize compounds released from dead cells during storage), and/or spontaneous germination of spores.
There is a possibility of the outgrowth of a spontaneous mutant better adapted to storage stress. There is a notable gap in comprehensive genetic analyses of mutation-driven adaptation under storage stress across most studies which leaves the place for further research, as there already have been reports regarding genetic adaptation to storage conditions for bacteria, e.g., Mycobacterium bovis [31], Yersinia pseudotuberculosis [32] and Escherichia coli [33]. We chose to focus on spore-forming strains, as spores usually show greater survivability under stress conditions than vegetative cells. Therefore, the survivability of non-spore forming bacteria in liquid form may be similar to that of the tested strains of vegetative cells.
Various preservation methods, such as freeze-drying (lyophilization) and spray-drying, can be used to extend the shelf life of bacterial biopreparations. In this study, samples treated with trehalose after freeze-drying tended to show higher bacterial survival rates over the 12-month storage period compared to those preserved with skimmed milk. However, no significant differences were detected between the cryoprotectants in the cases of B. licheniformis vegetative cells and spores or P. megaterium spores. B. subtilis exhibited the highest stability, similar to results obtained by other authors, although in our study P. megaterium demonstrated higher stability than they reported [34]. Freeze-drying with appropriate cryoprotectants, such as skimmed milk or trehalose, has been found to be effective at maintaining the viability of strains [35,36]. Cryoprotectants differ in effectiveness, which may depend on the bacteria strain and their mechanisms of actions. Trehalose stabilizes proteins by slowing down the dynamics of hydration water, which is crucial for maintaining protein structure during freezing. This is achieved by modifying the relaxation dynamics of the water molecules surrounding proteins, thereby reducing structural fluctuations and preventing denaturation [37] and reducing ice crystal formation, which is a major cause of cellular damage during freezing. This is achieved by altering the water structure and lowering the freezing point, thus protecting cells and tissues from ice-induced damage [38,39]. Milk’s cryoprotective properties are largely attributed to its protein and fat content, which help stabilize cell membranes and proteins during freezing. The proteins in milk can bind to ice crystals, inhibiting their growth and reducing cellular damage [39]. Milk combined with other cryoprotectants such as sucrose, trehalose, or sodium glutamate can contribute to the higher survivability of cells [40,41]. Bacillus species are generally classified as either resistant or moderately resistant to freeze-drying, with survival rates often exceeding 70% even after long-term storage [19]. However, depending on the cryoprotectant used, storage time, and temperature, lower values such as 63.72 ± 14.47% [42] or decreases of 1.62 LOG CFU/g [43] have been reported.
The spores of all the tested bacteria exhibited higher survival rates than the vegetative cells. The increased resistance of spores to freeze-drying is attributed to their unique structural features and metabolic inactivity. Vegetative cells, which are actively growing and metabolizing, are more susceptible to damage during the freeze-drying process [44]. Spray drying, which can be considered as an alternative to freeze-drying, did not result in high losses of living cells. In this preservation method, both the spore-forming capacity of the bacteria and the selected process parameters can significantly influence survival. It has been reported previously that B. subtilis CPA-8, due to its endospore production, exhibits significant heat resistance and a higher survival rate than bacteria that do not produce spores [45]. Freeze-drying is a well-known method for preserving spore-forming bacteria, such as Bacillus and Clostridium, which are able to withstand desiccation. This method ensures long-term viability of bacterial spores, which are preserved in a dormant state [46,47]. It is commonly used for fungi that produce many spores [47,48]. However, it may also be used for the storage of non-spore forming bacteria. The choice of protectant is crucial for the survival of bacteria during freeze-drying [49].
The viability of bacteria during spray drying is significantly affected by the drying conditions, such as inlet and outlet temperatures, which can cause thermal and mechanical stress. Lower temperatures and optimized drying conditions can help mitigate these effects [50,51].
A temperature of −80 °C has been suggested as optimal for long term storage [52,53]. Our results confirm that survival rates can remain stable for up to 12 months with an appropriate cryoprotectant. Similarly to freeze-drying, spores exhibited higher survival rates than vegetative spores, except for B. velezensis. Glycerol mixed with medium acted as an effective cryoprotectant, which is in conformity with literature data [52,54]. However, solutions of trehalose and skimmed milk were more effective with the tested strains. The appropriate choice of cryoprotectant depends on the bacterial strain and influence survival rates. This has been the subject of extensive research. For instance, one study demonstrated that B. subtilis preserved in 10% glycerol or 10% skimmed milk exhibited high viability after 60 days of storage at −80 °C [55]. Other compatible solutes for freezing include glycine betaine, which further improves the cold stress tolerance of B. subtilis, allowing it to proliferate even at low temperatures [56]. Apart from commonly used cryoprotectants, Tween 80 has been identified as an effective cryoprotectant for B. licheniformis, not only protecting cells during freezing but also facilitating faster recovery post-thawing [57]. The production of biopreparations requires storage of pure cultures of microorganisms. This study investigated freezing as a method for long-term storage, as freezing ensures that bacteria remain viable and free from contamination or genetic drift, which is supported by literature data [32,58,59]. Freezing at low temperatures is commonly used to preserve various types of microorganisms during storage and avoid genetic drift. Freezing at ultra-low temperatures, such as in liquid nitrogen or at −80 °C, is a widely used and highly effective method for preserving fungi that produce spores, such as Aspergillus and Penicillium [48,60]. Cryoprotective agents such as glycerol or DMSO are often used to prevent cell damage during freezing and thawing. Non-spore-forming bacteria, such as Pseudomonas aeruginosa, can also be stored at −80 °C with cryoprotective agents [47].
The survival rate of P. amylolyticus spores after spray drying and B. subtilis spores after freezing and exceeded 100%. The increased number of spores after the process may be a result of the addition of cryoprotectants, which resulted in larger numbers of spores than in the initial sample being revied [44] and/or sporulation as a result of extreme conditions, as a result of the increased expression of genes associated with sporulation during drying [61]. Moreover, before preservation the fraction of cells may still be in a vegetative state or in mid-sporulation. During the early stages of drying or sample preparation, sporulation may continue or finish in some cells, especially if there are triggers (e.g., nutrient starvation, moisture loss). This aspect needs further investigation in order to understand the mechanisms that could help optimize protocols for higher spore survival rates.
5. Conclusions
This research provides new practical knowledge in the field of biotechnology for the production of microbiological preparations. Bacterial survivability was highly dependent on both species-specific traits and storage conditions. Temperature had the most significant effect on viability, with a notable decline in the number of vegetative cells at temperatures of 30 °C, particularly in B. velezensis and P. amylolyticus. Lower temperatures (15 °C and 21 °C) better preserved microbial populations, with some strains maintaining or increasing spore counts over 12 months. The impact of pH and osmotic pressure varied between strains. Moderate concentrations of calcium chloride and carbamide benefited P. megaterium and B. licheniformis. B. subtilis and B. licheniformis showed the highest resilience. B. licheniformis spores demonstrated remarkable stability under all conditions (with the addition of calcium chloride, carbamide, or multicomponent fertilizer).
Cryoprotectants significantly enhanced survival after freeze-drying and freezing. Trehalose and skimmed milk were the most effective cryoprotectants, although performance varied by strain and cell type. Spore forms showed better preservation than vegetative cells, confirming their advantages for long-term formulations.
Our results underscore the importance of maintaining the viability of spore-forming bacteria in bioformulations and thus their effectiveness in agricultural applications. This study can be used as a reference for industrial biotechnologists when developing production strategies for new biopreparations and determining their shelf life. Future studies should employ multifactorial experimental designs to optimize storage protocols that maintain bacterial viability in bioformulations and identify conditions that balance survivability with industry requirements and cost (e.g., transport, storage in shops and on farms). In addition, longer studies monitoring the shelf life of biopreparations in various forms is recommended. During monitoring implementing corroborative viability or molecular assays would allow to monitor also viable but non-culturable cells number. The selection of cryoprotectants may be broadened to explore different concentrations of preferred agents and combinations thereof. It is also worth noting that water activity likely changed during storage due to the consumption of nutrients and chemical compounds in the medium, as well as the production of metabolites by the tested microorganisms. We recognize that this topic could offer an interesting direction for future research which may be helpful in explaining the mechanistic model of osmotic stress in bacteria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152211856/s1, Table S1: Survivability of Peanibacillus amylolyticus under different storage conditions. Different letters describe groups statistically different (ANOVA and Duncan’s test (p < 0.05)), test was individually for temperatures, pH and osmotic stressors; Table S2: Survivability of Bacillus licheniformis under different storage conditions. Different letters describe groups statistically different (ANOVA and Duncan’s test (p < 0.05)), test was individually for temperatures, pH and osmotic stressors; Table S3: Survivability of Bacillus subtilis under different storage conditions. Different letters describe groups statistically different (ANOVA and Duncan’s test (p < 0.05)), test was individually for temperatures, pH and osmotic stressors; Table S4: Survivability of Prestia megaterium under different storage conditions. Different letters describe groups statistically different (ANOVA and Duncan’s test (p < 0.05)), test was individually for temperatures, pH and osmotic stressors; Table S5: Survivability of Bacillus velezensis under different storage conditions. Different letters describe groups statistically different (ANOVA and Duncan’s test (p < 0.05)), test was individually for temperatures, pH and osmotic stressors; Table S6: Survivability after freeze-drying [LOG CFU/g]. Different letters indicate statistically different groups. Tests were performed independently for spores and vegetative cells of each strain (Duncan’s test p < 0.05)); Table S7: Survivability after freezing [LOG CFU/mL]. Different letters indicate statistically different groups. Tests were performed independently for spores and vegetative cells of each strain (Duncan’s test (p < 0.05)).
Author Contributions
Conceptualization, P.R., B.G. and J.S.; methodology, P.R., B.G., S.P. and J.S.; validation, P.R. and J.S.; formal analysis, P.R.; investigation, P.R., M.W. and S.P.; resources, J.S., B.G. and S.P.; data curation, P.R.; writing—original draft preparation, P.R., J.S., B.G., M.W. and S.P.; writing—review and editing, P.R. and J.S.; visualization, P.R. and M.W.; supervision, J.S.; project administration, J.S.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Agency for Restructuring and Modernisation of Agriculture, Poland [grant number 00077.DDD.6509.000167.2022.05].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available within the paper and its Supplementary Information.
Acknowledgments
This article was completed while the first author was a Doctoral Candidate of the Interdisciplinary Doctoral School at Lodz University of Technology, Poland. Graphical abstract, Figure 1, Figure 2 and Figure 3 were created in https://BioRender.com (accessed on 4 November 2025).
Conflicts of Interest
Author Szymon Powałowski was employed by JHJ Sp. z o.o. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFU | Colony Forming Unit |
References
- Toader, G.; Chiurciu, V.; Filip, V.; Burnichi, F.; Toader, E.V.; Enea, C.; Trifan, D.; Rîșnoveanu, L. Bacterial biopreparations—A “green revolution” for agriculture. Res. J. Agric. Sci. 2020, 52, 198–205. [Google Scholar]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Marwal, A.; Srivastava, A.K.; Gaur, R.K. Plant viruses as biopesticides. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–194. [Google Scholar] [CrossRef]
- Narwade, J.D.; Odaneth, A.A.; Lele, S.S. Solid-state fermentation in an earthen vessel: Trichoderma viride spore-based biopesticide production using corn cobs. Fungal Biol. 2023, 127, 1146–1156. [Google Scholar] [CrossRef]
- Rowińska, P.; Gutarowska, B.; Janas, R.; Szulc, J. Biopreparations for the decomposition of crop residues. Microb. Biotechnol. 2024, 17, e14534. [Google Scholar] [CrossRef]
- Buragienė, S.; Šarauskis, E.; Adamavičienė, A.; Romaneckas, K.; Lekavičienė, K.; Rimkuvienė, D.; Naujokienė, V. The effect of different biopreparations on soil physical properties and CO2 emissions when growing winter wheat and oilseed rape. SOIL 2023, 9, 593–608. [Google Scholar] [CrossRef]
- Toader, E.V.; Toader, G.; Trifan, D.; Lungu, E.; Ghiorghe, A.I. Innovative Ecological Technologies for Soil Restoration: Bacterial Biopreparations. In Proceedings of the International Conference Competitiveness of Agro-Food and Environmental Economy, Bucharest, Romania, 18 October 2022. [Google Scholar] [CrossRef]
- Khomenko, T.; Tonkha, O.; Hordiienko, L.; Pikovska, O. Impact of biopreparations on the phytopathological state of potato plants. Plant Soil Sci. 2024, 15, 20–29. [Google Scholar] [CrossRef]
- European Commission COM(2020)380. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vl8tqb8jwtyy (accessed on 14 July 2024).
- European Commission COM(2020)381. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vl8tofp7dtuc (accessed on 14 July 2024).
- Akter, T.; Shah, S.T.; Mamun, M.A.A.; Bari, M.L.; Begum, S.; Rahman, N.; Miah, M.I. Costeffective formulation of bio-fertilizer using agricultural residues as carriers and determination of shelflife of bio-fertilizer inoculants. Dhaka Univ. J. Biol. Sci. 2023, 32, 189–199. [Google Scholar] [CrossRef]
- Thirumal, G. Effects of Irradiated Carriers, Storage Temperatures, on Rhizobium Bioinoculant at Different Intervals. Int. J. Pure Appl. Biosci. 2017, 5, 1240–1246. [Google Scholar] [CrossRef]
- Thirumal, G. Evaluate the Shelf Life of Irradiated Carrier Based Pseudomonas Biofertilizer Stored at Different Temperatures at Different Intervals. Int. J. Pure Appl. Biosci. 2017, 5, 2158–2164. [Google Scholar] [CrossRef]
- Tian, Z.; Hou, L.; Hu, M.; Gao, Y.; Li, D.; Fan, B.; Wang, F.; Li, S. Optimization of Sporulation Conditions for Bacillus subtilis BSNK-5. Processes 2022, 10, 1133. [Google Scholar] [CrossRef]
- Kamoun, F.; Weekers, F.; Ayed, R.B.; Mechri, S.; Jabeur, F.; Thonart, P.; Jaouadi, B. Multiple linear regression models to simulate spore yields of Bacillus amyloliquefaciens BS13 through optimization of medium composition. Biotechnol. Appl. Biochem. 2022, 69, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Poymanov, V.V.; Nazarov, S.A.; Toroptsev, V.V.; Ustinov, N.Y.; Kozyrenko, E.V.; Krylov, K.V. Study of freeze-drying process of bacterial concentrates in the apparatus using thermoelectric modules. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 072027. [Google Scholar] [CrossRef]
- Niwińska, A. Przegląd metod kriokonserwacji pod kątem techniki witrifikacyjnej. Życie Weter. 2016, 91, 505–507. [Google Scholar]
- Li, X.M.; Che, L.H.; Wu, Y.; Li, C.; Xu, B.C. An effective strategy for improving the freeze-drying survival rate of Lactobacillus curvatus and its potential protective mechanism. Food Biosci. 2024, 58, 103794. [Google Scholar] [CrossRef]
- Bogdan-Golubi, N.; Slanina, V. The viability of bacillus, pseudomonas and lactic acid bacteria strains after 15 years of storage. In Proceedings of the 5th International Scientific Conference on Microbial Biotechnology, Chisinau, Moldova, 12–13 October 2022; Institute of Microbiology and Biotechnology: Chisinau, Moldova, 2022. [Google Scholar] [CrossRef]
- Farfan Pajuelo, D.G.; Carpio Mamani, M.; Maraza Choque, G.J.; Chachaque Callo, D.M.; Cáceda Quiroz, C.J. Effect of Lyoprotective Agents on the Preservation of Survival of a Bacillus cereus Strain PBG in the Freeze-Drying Process. Microorganisms 2023, 11, 2705. [Google Scholar] [CrossRef]
- Donthi, M.R.; Butreddy, A.; Saha, R.N.; Kesharwani, P.; Dubey, S.K. Leveraging spray drying technique for advancing biologic product development—A mini review. Health Sci. Rev. 2024, 10, 100142. [Google Scholar] [CrossRef]
- Kakuda, L.; Jaramillo, Y.; Niño-Arias, F.C.; Souza, M.F.; Conceição, E.C.; Alves, V.F.; Almeida, O.G.G.; De Martinis, E.C.P.; Oliveira, W.P. Process Development for the Spray-Drying of Probiotic Bacteria and Evaluation of the Product Quality. J. Vis. Exp. 2023, 194, e65192. [Google Scholar] [CrossRef]
- Rowińska, P.; Sypka, M.; Białkowska, A.M.; Stryjek, M.; Nowak, A.; Janas, R.; Gutarowska, B.; Szulc, J. Evaluating Soil Bacteria for the Development of New Biopreparations with Agricultural Applications. Appl. Sci. 2025, 15, 6400. [Google Scholar] [CrossRef]
- Sahai, P.; Chandra, R. Shelf life of liquid and carrier based Mesorhizobium sp. and Pseudomonas sp. inoculants under different storage conditions. J. Food Legumes 2009, 22, 280–282. [Google Scholar] [CrossRef]
- Goswami, G.; Panda, D.; Samanta, R.; Boro, R.C.; Modi, M.K.; Bujarbaruah, K.M.; Barooah, M. Bacillus megaterium adapts to acid stress condition through a network of genes: Insight from a genome-wide transcriptome analysis. Sci. Rep. 2018, 8, 16105. [Google Scholar] [CrossRef]
- Petrackova, D.; Vecer, J.; Svobodova, J.; Herman, P. Long-Term Adaptation of Bacillus subtilis 168 to Extreme pH Affects Chemical and Physical Properties of the Cellular Membrane. J. Membr. Biol. 2010, 233, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.; Dhamu, V.N.; Paul, A.; Muthukumar, S.; Prasad, S. An Interfacial Label-Free Electrochemical Approach to in-Situ Soil pH Monitoring. ECS Meet. Abstr. 2022, MA2022-02, 2275. [Google Scholar] [CrossRef]
- Sinnelä, M.T.; Park, Y.K.; Lee, J.H.; Jeong, K.C.; Kim, Y.W.; Hwang, H.J.; Mah, J.H. Effects of Calcium and Manganese on Sporulation of Bacillus Species Involved in Food Poisoning and Spoilage. Foods 2019, 8, 119. [Google Scholar] [CrossRef]
- Tian, J.; Wu, Y.; Li, C.; Zhou, Y.; Zhou, Z.; Gu, S. Effect of CaCO3 on Sporulation of Bacillus coagulans CGMCC 9951. Food Sci. 2020, 41, 113–119. [Google Scholar] [CrossRef]
- Domínguez, D.C. Calcium Signaling in Prokaryotes. In Calcium and Signal Transduction; InTech: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Tkachenko, O.; Kozak, N.; Bilan, M.; Hlebeniuk, V.; Alekseeva, N.; Kovaleva, L.; Nedosekov, V.; Galatiuk, O. The Effect of Long-Term Storage on Mycobacterium bovis. Pol. J. Microbiol. 2021, 70, 327–337. [Google Scholar] [CrossRef]
- Somova, L.M.; Timchenko, N.F.; Lyapun, I.N.; Drobot, E.I.; Matosova, E.V.; Bynina, M.P. Ultrastructural Changes of Bacteria in Static Cultures of Yersinia pseudotuberculosis under Long Storage under Conditions of Low Temperature. Bull. Exp. Biol. Med. 2020, 170, 223–225. [Google Scholar] [CrossRef]
- Ratib, N.R.; Seidl, F.; Ehrenreich, I.M.; Finkel, S.E. Evolution in Long-Term Stationary-Phase Batch Culture: Emergence of Divergent Escherichia coli Lineages over 1200 Days. MBio 2021, 12, e03337-20. [Google Scholar] [CrossRef]
- Borowski, S.; Matusiak, K.; Powałowski, S.; Pielech-Przybylska, K.; Makowski, K.; Nowak, A.; Rosowski, M.; Komorowski, P.; Gutarowska, B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeterior. Biodegrad. 2017, 119, 299–308. [Google Scholar] [CrossRef]
- Boontun, C.; Vatanyoopaisarn, S.; Phalakornkule, C.; Domrongpokkaphan, V.; Thitisak, P.; Thaveetheptaikul, P.; Bamrungchue, N. Influence of protectant for encapsulation by freeze-drying and spray-drying techniques, and packaging environments on the stability of the probiotic Bifidobacterium animalis subsp. lactis strain KMP-H9-01 during storage. Dry. Technol. 2024, 42, 762–774. [Google Scholar] [CrossRef]
- Kavak, A.E.; Zent, İ.; Özdemir, A.; Dertli, E. Optimization of cryoprotectant formulation to enhance the viability of Lactiplantibacillus plantarum NBC99 isolated from human origin. Prep. Biochem. Biotechnol. 2024, 54, 958–966. [Google Scholar] [CrossRef]
- Camisasca, G.; De Marzio, M.; Gallo, P. Effect of trehalose on protein cryoprotection: Insights into the mechanism of slowing down of hydration water. J. Chem. Phys. 2020, 153, 224503. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Liu, F.; Xie, J.; Zhu, Q.; Tan, S. Trehalose in Biomedical Cryopreservation–Properties, Mechanisms, Delivery Methods, Applications, Benefits, and Problems. ACS Biomater. Sci. Eng. 2023, 9, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Zhang, M.; Sun, Y.; Bhandari, B.; Fan, D. Effects of cryoprotectants on Nostoc sphaeroides superchilled at low temperature (−3.0 °C) and their action mechanisms. J. Food Process Eng. 2020, 43, e13488. [Google Scholar] [CrossRef]
- Di, L.; Ma, W.; Kang, W.; Huang, Y.; Wu, Z.; Yin, B.; Yang, R.; Liu, X.; Pan, L.; Wang, J.; et al. Synergistic combination of cryoprotectants for high freeze-dried survival rate and viable cell counts of Streptococcus thermophilus. Dry. Technol. 2023, 41, 1444–1453. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Kang, W.; Han, Y.; Yin, B.; Yang, R.; Tang, R.; Pan, L.; Wang, J.; Li, W.; et al. Synergistic combination of cryoprotectants improved freeze-dried survival rate and viable counts of Lactiplantibacillus plantarum. Int. J. Dairy Technol. 2024, 77, 348–357. [Google Scholar] [CrossRef]
- Mahidsanan, T.; Gasaluck, P.; Eumkeb, G. A novel soybean flour as a cryoprotectant in freeze-dried Bacillus subtilis SB-MYP-1. LWT 2017, 77, 152–159. [Google Scholar] [CrossRef]
- Phoane, K.V.; Tjoa, E.; Joon, S.; Karmawan, L.U.; Moehario, L.H. Effect of Temperature and Preservation Period on the Viability of Lyophilized Bacillus subtilis. Indo Glob. J. Pharm. Sci. 2022, 12, 153–155. [Google Scholar] [CrossRef]
- Morgan, C.; Vesey, G. Freeze-Drying of Microorganisms. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Oxford, UK, 2009; pp. 162–173. [Google Scholar] [CrossRef]
- Yánez-Mendizabal, V.; Viñas, I.; Usall, J.; Cañamás, T.; Teixidó, N. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735. [Google Scholar] [CrossRef]
- Hebishy, E.; Yerlikaya, O.; Mahony, J.; Akpinar, A.; Saygili, D. Microbiological aspects and challenges of whey powders—I thermoduric, thermophilic and spore-forming bacteria. Int. J. Dairy Technol. 2023, 76, 779–800. [Google Scholar] [CrossRef]
- She, R.C.; Petti, C.A. Procedures for the Storage of Microorganisms. In Manual of Clinical Microbiology, 11th ed.; Jorgensen, J.H., Pfaller, M.A., Carroll, K.C., Funke, G., Landry, M.L., Richter, S.S., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 161–168. [Google Scholar] [CrossRef]
- Shin, M.S.; Hong, S.B. Maintenance of Filamentous Fungi in Korean Agricultural Culture Collection (KACC). Korean J. Mycol. 2014, 42, 97–103. [Google Scholar] [CrossRef][Green Version]
- Peiren, J.; Buyse, J.; De Vos, P.; Lang, E.; Clermont, D.; Hamon, S.; Bégaud, E.; Bizet, C.; Pascual, J.; Ruvira, M.A.; et al. Improving survival and storage stability of bacteria recalcitrant to freeze-drying: A coordinated study by European culture collections. Appl. Microbiol. Biotechnol. 2015, 99, 3559–3571. [Google Scholar] [CrossRef]
- Hanidah, I.I.; Kirana, A.I.; Nurhadi, B.; Sumanti, D.M. The Functionality of Probiotic Bacteria Microencapsulation by Spray Drying: A Literature Review. Ind. J. Teknol. Dan Manaj. Agroind. 2021, 10, 274–282. [Google Scholar] [CrossRef]
- Moreira, M.T.C.; Martins, E.; Perrone, Í.T.; de Freitas, R.; Queiroz, L.S.; de Carvalho, A.F. Challenges associated with spray drying of lactic acid bacteria: Understanding cell viability loss. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3267–3283. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, K.; Chrungoo, N.K.; Joshi, S.R. Cryopreservation Design for Bacterial Cell: A Non-Conventional Gizmatic Approach. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 811–820. [Google Scholar] [CrossRef]
- Gorman, R.; Adley, C.C. An evaluation of five preservation techniques and conventional freezing temperatures of −20 °C and −85 °C for long-term preservation of Campylobacter jejuni. Lett. Appl. Microbiol. 2004, 38, 306–310. [Google Scholar] [CrossRef]
- Sunarno; Nursofiah, S.; Hartoyo, Y.; Amalia, N.; Febrianti, T.; Febriyana, D.; Saraswati, R.D.; Puspandari, N.; Sariadji, K.; Khariri; et al. Long-term Storage of Bacterial Isolates by Using Tryptic Soy Broth with 15% Glycerol in The Deep Freezer (−70 to −80 °C). IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012070. [Google Scholar] [CrossRef]
- Susilawati, L.; Purnomo, E.S. Viabilitas Sel Bakteri Dengan Cryoprotectant Agents Berbeda (Sebagai Acuan Dalam Preservasi Culture Collection di Laboratorium Mikrobiologi). Biog. J. Ilm. Biol. 2016, 4, 34–40. [Google Scholar] [CrossRef]
- Hoffmann, T.; Bremer, E. Protection of Bacillus subtilis against Cold Stress via Compatible-Solute Acquisition. J. Bacteriol. 2011, 193, 1552–1562. [Google Scholar] [CrossRef]
- Hancocks, N.H.; Thomas, C.R.; Stocks, S.M.; Hewitt, C.J. An investigation into the preservation of microbial cell banks for α-amylase production during 5 l fed-batch Bacillus licheniformis fermentations. Biotechnol. Lett. 2010, 32, 1405–1412. [Google Scholar] [CrossRef][Green Version]
- Rego, A.V.P.L.M.; Silva, M.S.; Conceição, R.S.; Machado, B.A.S. Four Different Cryoprotectors in Preservation of Staphylococcus aureus. J. Bioeng. Technol. Health 2023, 6, 121–123. [Google Scholar] [CrossRef]
- Tedeschi, R.; De Paoli, P. Collection and Preservation of Frozen Microorganisms. In Methods in Molecular Biology; Springer: Totowa, NJ, USA, 2011; Volume 675, pp. 313–326. [Google Scholar] [CrossRef]
- Łukaszewska-Skrzypniak, N.; Sadowska, K.; Stępniewska-Jarosz, S.; Zenelt, W.; Borodynko-Filas, N. The review of fungi and omycota long-term storage methods. Prog. Plant Prot. 2022, 62, 181–189. [Google Scholar] [CrossRef]
- Li, C.; Zhao, K.; Ma, L.; Zhao, J.; Zhao, Z.M. Effects of drying strategies on sporulation and titer of microbial ecological agents with Bacillus subtilis. Front. Nutr. 2022, 9, 1025248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).