Dielectric Barrier Discharge as a Source of Microplasma for TiO2 Submicron Particle Deposition
Abstract
1. Introduction
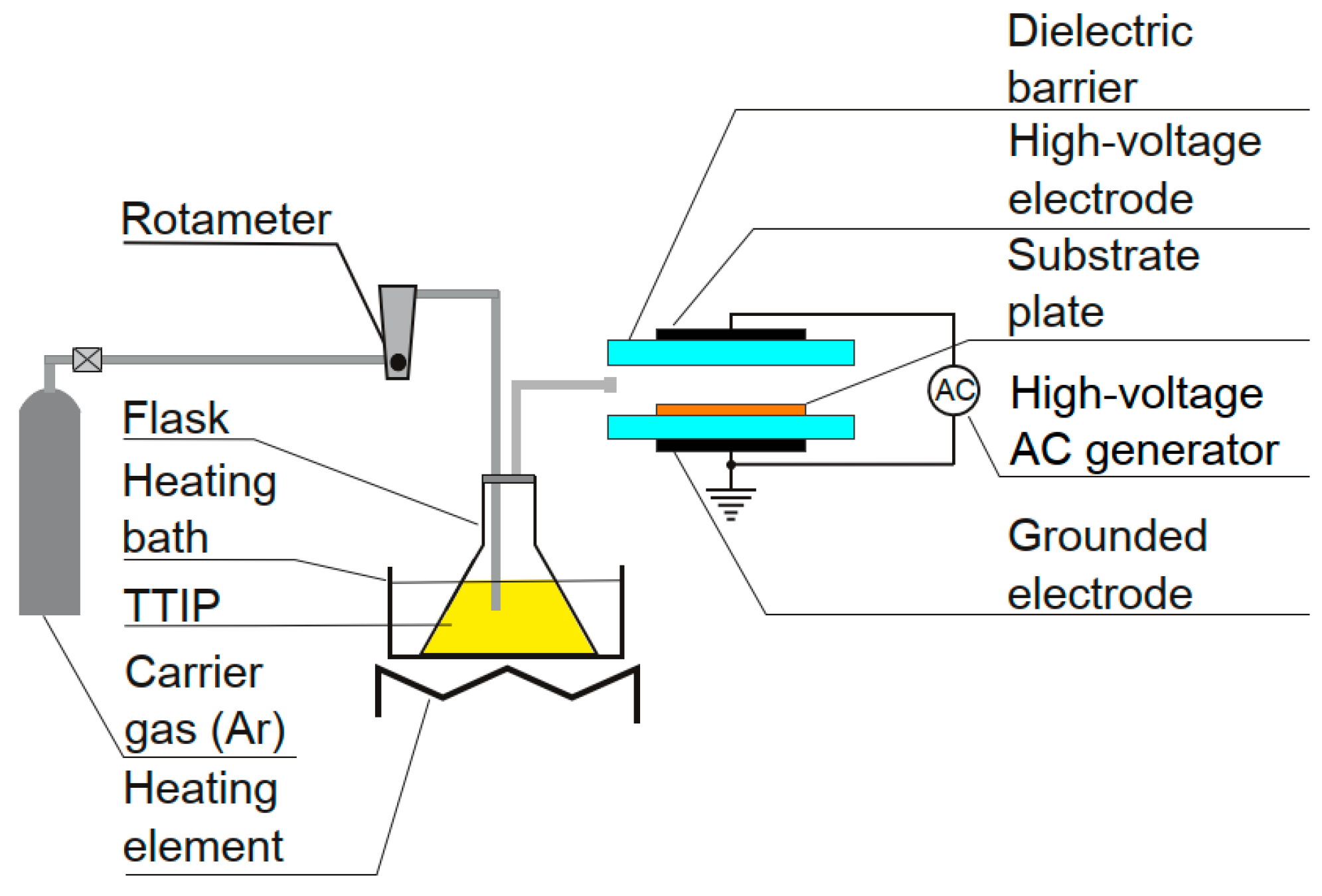
2. Materials and Methods
3. Results and Discussion
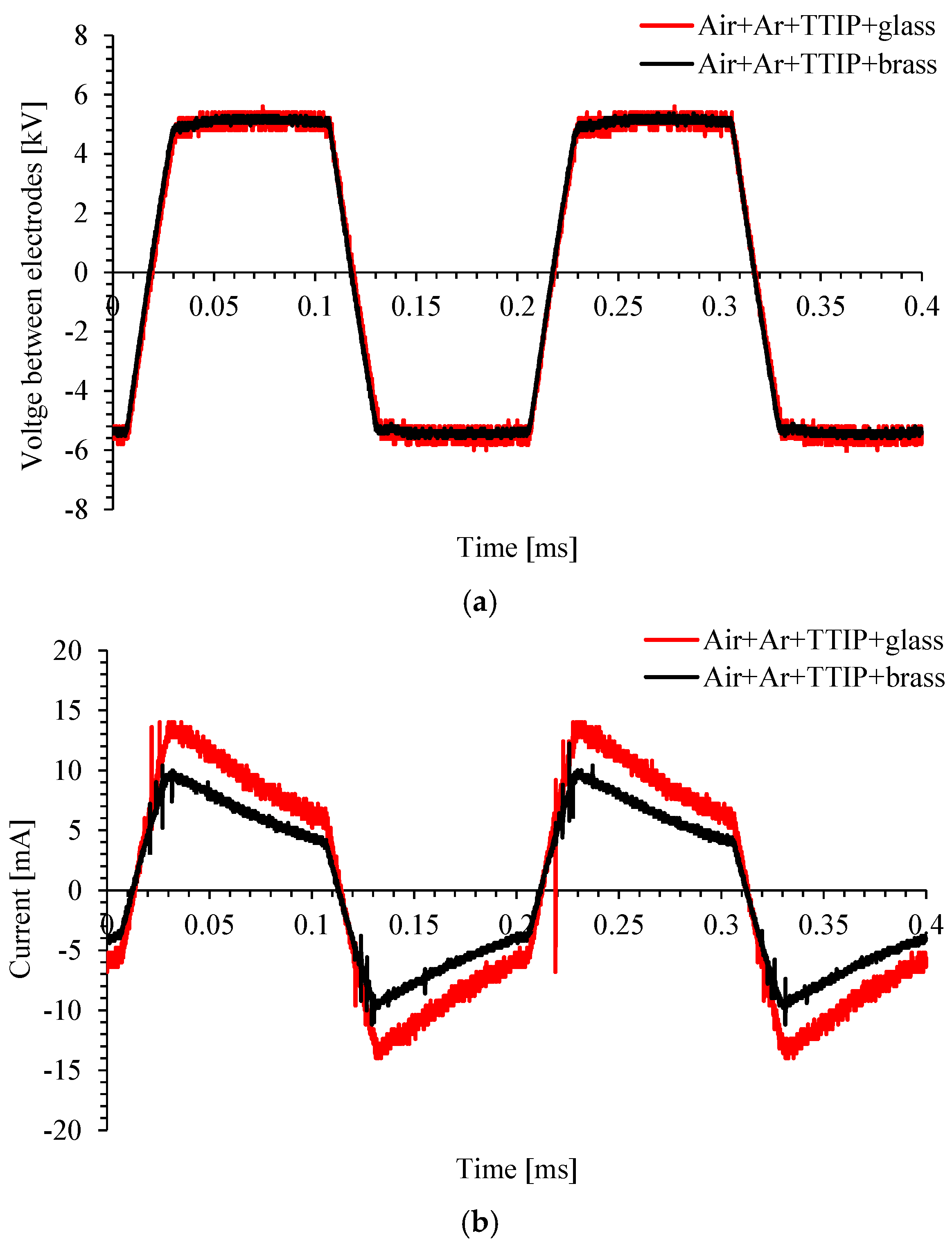
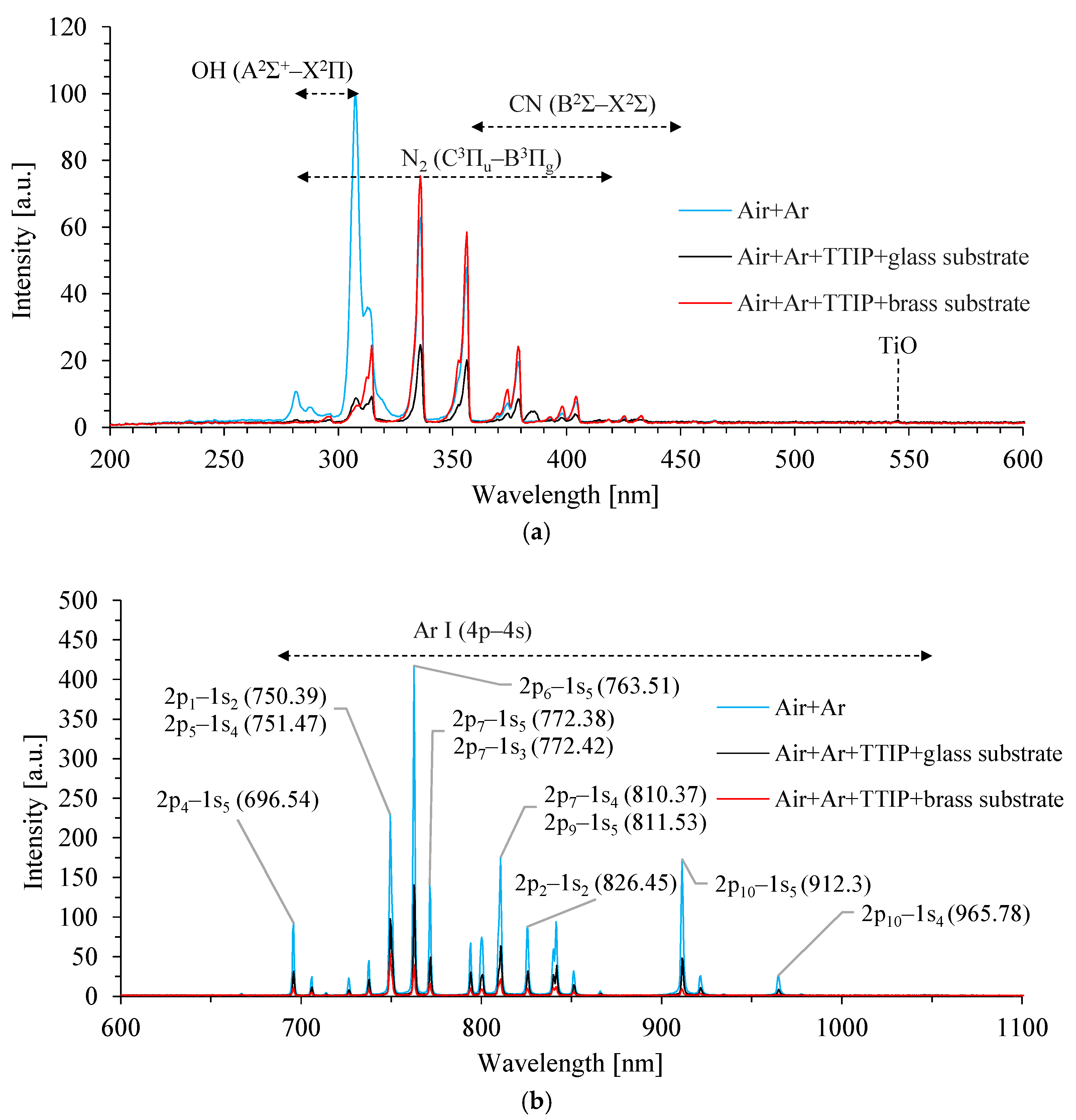
3.1. DBD Plasma Characterization
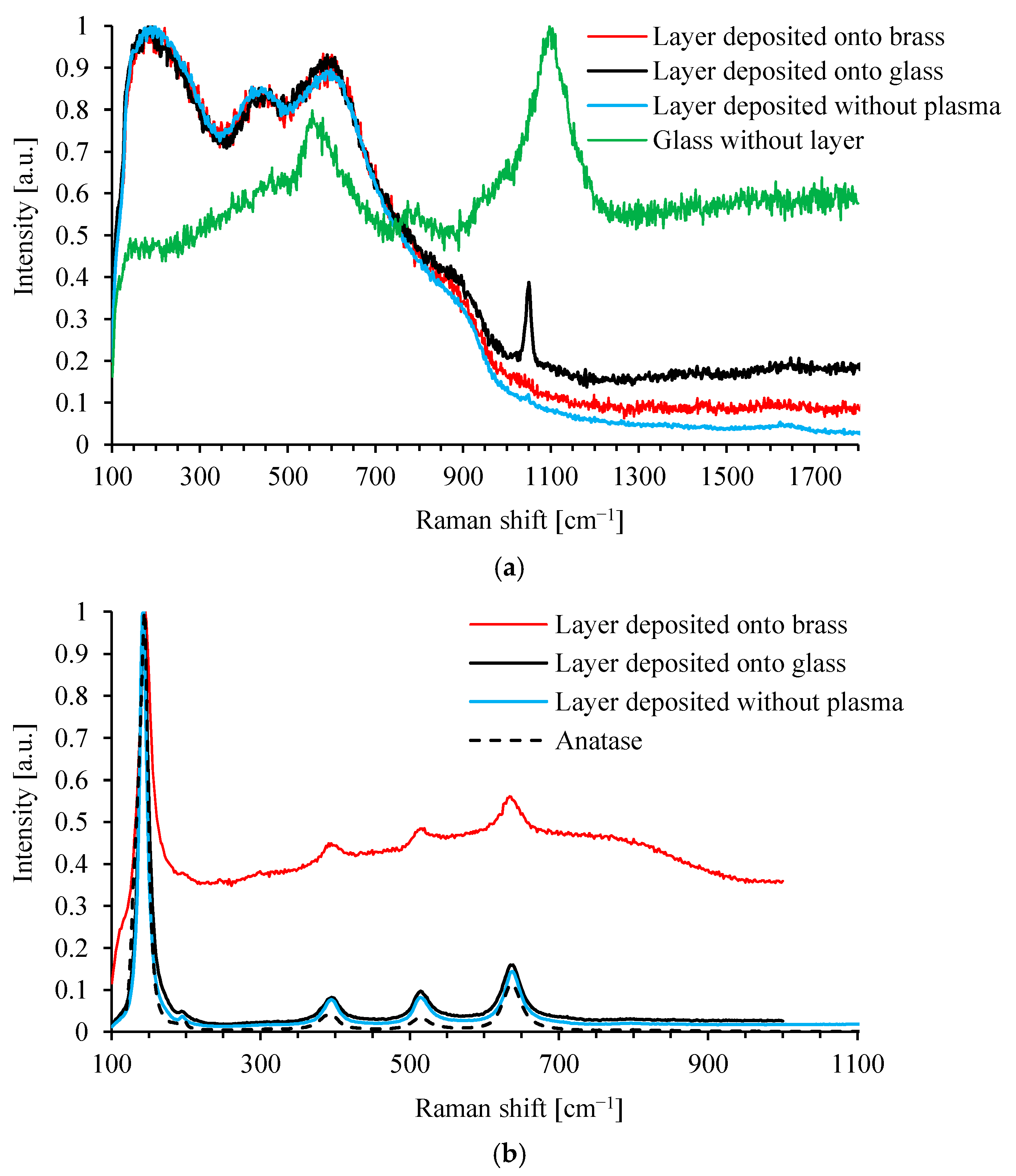
3.2. TiO2 Layer Properties
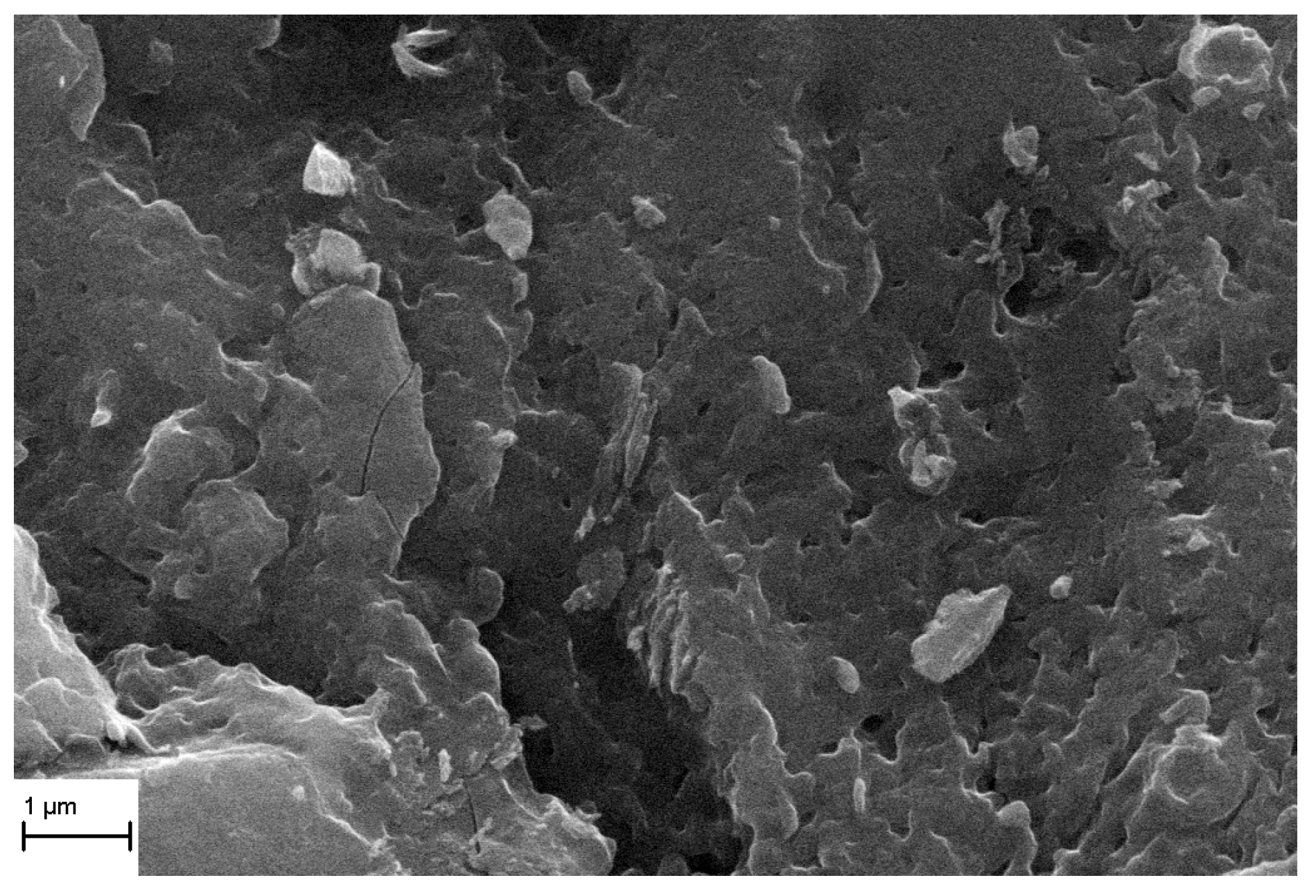

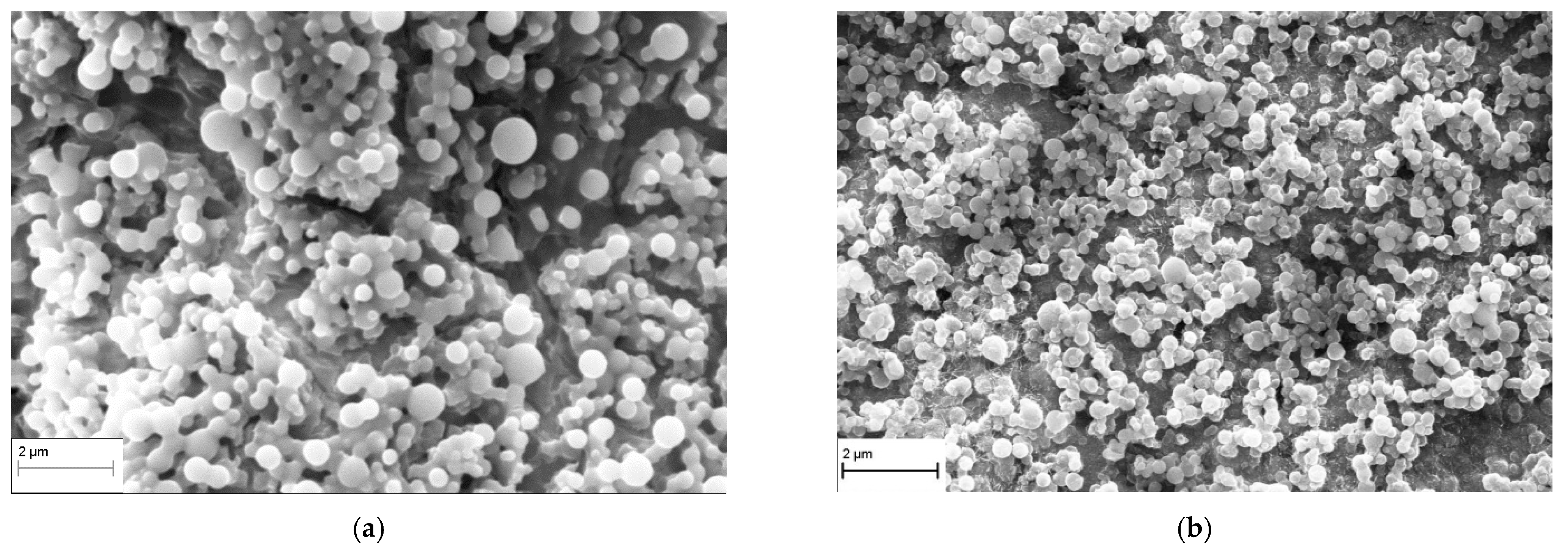
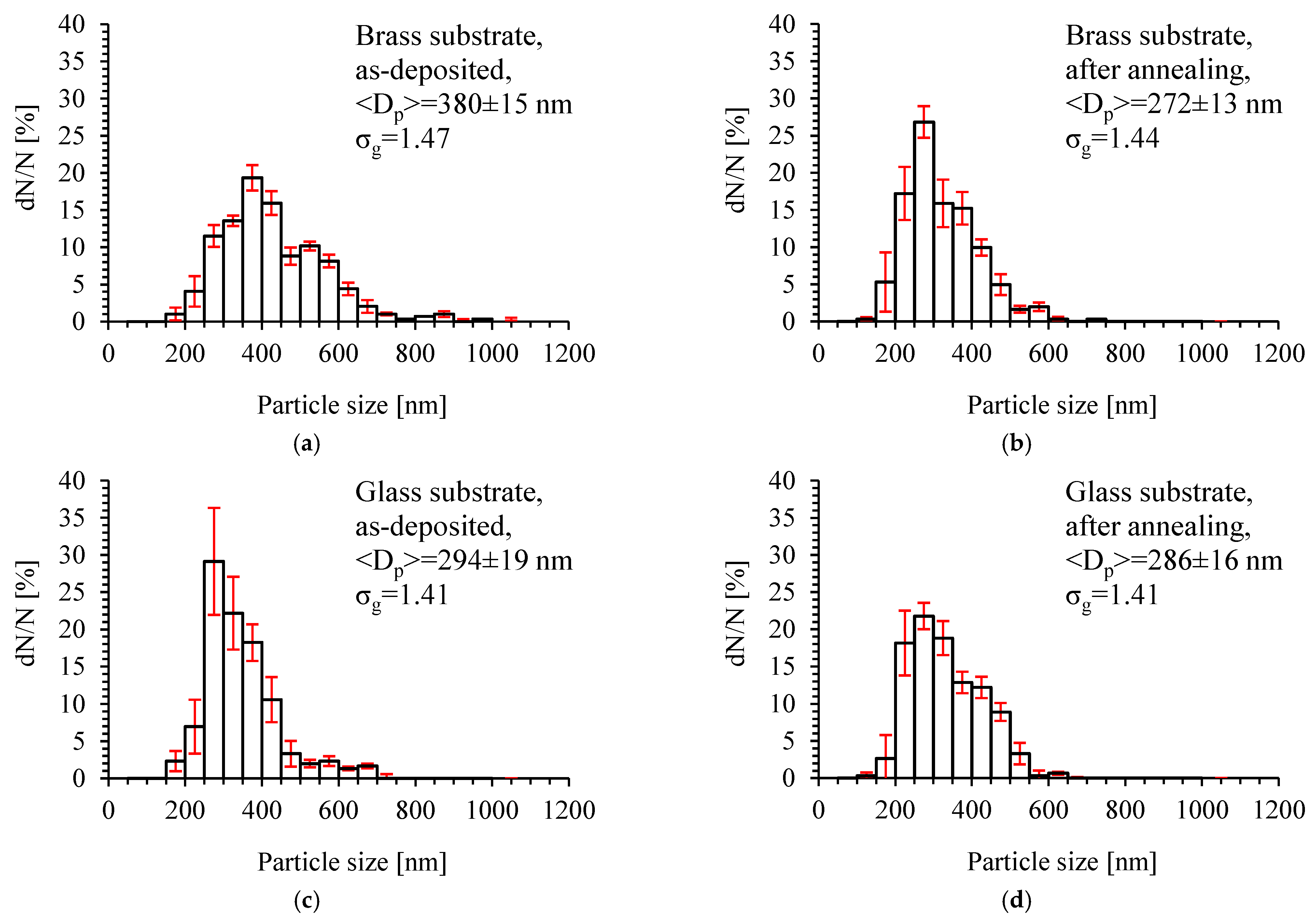
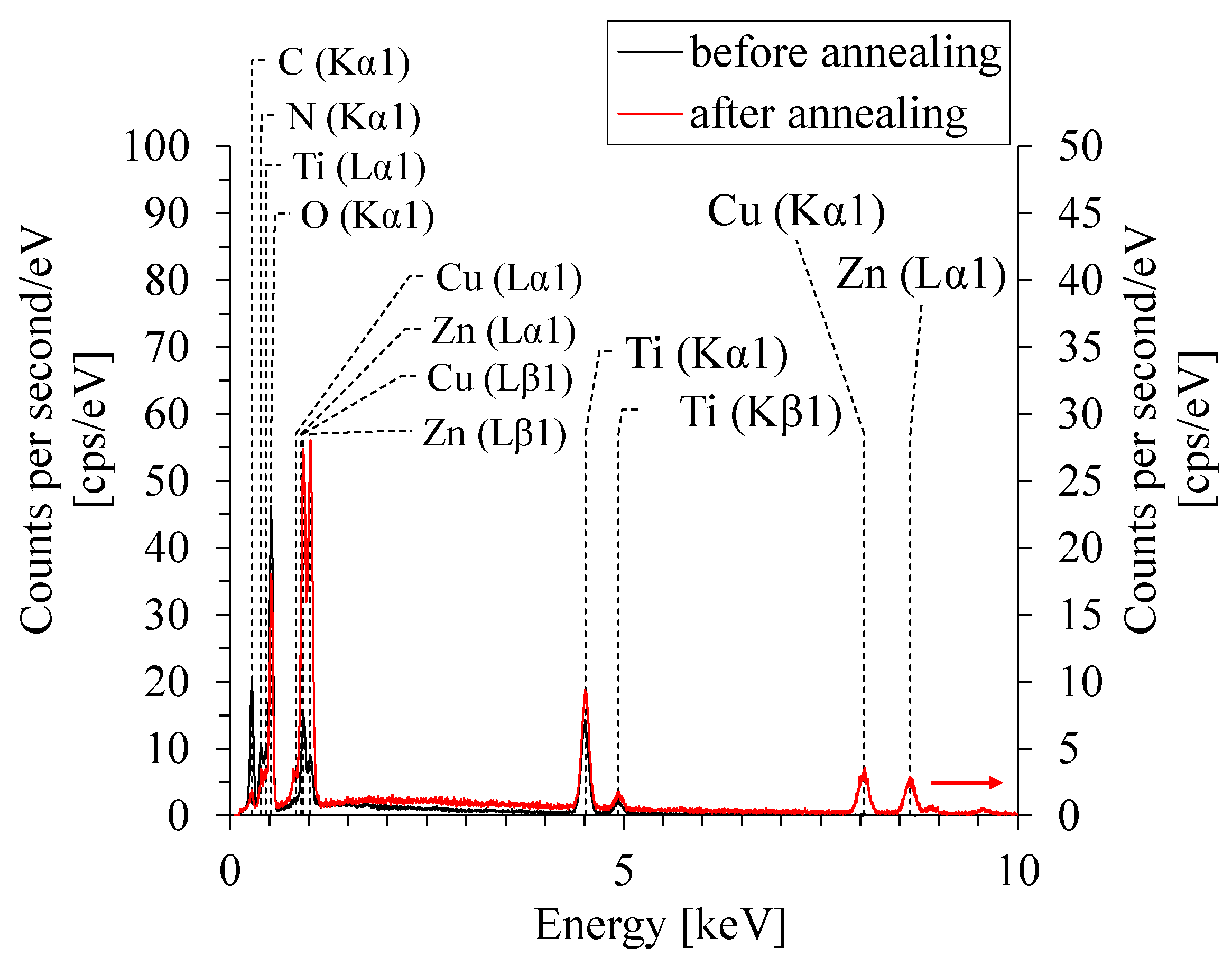
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBD | Dielectric Barrier Discharge |
| EDS | Energy-Dispersive Spectroscopy |
| TTIP | Titanium Tetraisopropoxide |
| MW | Microwave |
| RF | Radio Frequency |
References
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2022, 55, 373001. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low Temperature Plasma Science and Technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-Thermal Plasmas for Non-Catalytic and Catalytic VOC Abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef]
- Fridman, A.; Gutsol, A.; Cho, Y.I. Non-Thermal Atmospheric Pressure Plasma. In Advances in Heat Transfer; Elsevier: Amsterdam, The Netherlands, 2007; Volume 40, pp. 1–142. ISBN 978-0-12-373923-0. [Google Scholar]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric Pressure Plasma of Dielectric Barrier Discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Meyer, C.; Franzke, J.; Gurevich, E.L. Experimental Estimation of the Surface Charge Density in Micro Dielectric Barrier Discharges. J. Phys. D Appl. Phys. 2012, 45, 355205. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, M.; Ohnishi, N. Enhanced Electrohydrodynamic Force Generation in a Two-Stroke Cycle Dielectric-Barrier-Discharge Plasma Actuator. Appl. Phys. Lett. 2017, 110, 194101. [Google Scholar] [CrossRef]
- Ayan, H.; Fridman, G.; Gutsol, A.F.; Vasilets, V.N.; Fridman, A.; Friedman, G. Nanosecond-Pulsed Uniform Dielectric-Barrier Discharge. IEEE Trans. Plasma Sci. 2008, 36, 504–508. [Google Scholar] [CrossRef]
- Tao, S.; Kaihua, L.; Cheng, Z.; Ping, Y.; Shichang, Z.; Ruzheng, P. Experimental Study on Repetitive Unipolar Nanosecond-Pulse Dielectric Barrier Discharge in Air at Atmospheric Pressure. J. Phys. D Appl. Phys. 2008, 41, 215203. [Google Scholar] [CrossRef]
- Yehia, A. Characteristics of the Dielectric Barrier Corona Discharges. AIP Adv. 2019, 9, 045214. [Google Scholar] [CrossRef]
- Pal, U.N.; Sharma, A.K.; Soni, J.S.; Kr, S.; Khatun, H.; Kumar, M.; Meena, B.L.; Tyagi, M.S.; Lee, B.-J.; Iberler, M.; et al. Electrical Modelling Approach for Discharge Analysis of a Coaxial DBD Tube Filled with Argon. J. Phys. D Appl. Phys. 2009, 42, 045213. [Google Scholar] [CrossRef][Green Version]
- Rahimpour, M.; Taghvaei, H.; Rahimpour, M.R. Degradation of Crystal Violet in Water Solution Using Post Discharge DBD Plasma Treatment: Factorial Design Experiment and Modeling. Chemosphere 2019, 232, 213–223. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, B.; Liu, C. Characterization of ZnO Nanotube Fabricated by the Plasma Decomposition of Zn(OH)2 Via Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2012, 32, 201–209. [Google Scholar] [CrossRef]
- Yu, S.J.; Chang, M.B. Oxidative Conversion of PFC via Plasma Processing with Dielectric Barrier Discharges. Plasma Chem. Plasma Process. 2001, 21, 311–327. [Google Scholar] [CrossRef]
- Tański, M.; Kocik, M.; Mizeraczyk, J. Liquid Pumping by Miniature Electrohydrodynamic Pump Driven by DC Voltage. In Proceedings of the 2011 IEEE International Conference on Dielectric Liquids, Trondheim, Norway, 26–30 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–3. [Google Scholar]
- Baroch, P.; Saito, N.; Takai, O. Special Type of Plasma Dielectric Barrier Discharge Reactor for Direct Ozonization of Water and Degradation of Organic Pollution. J. Phys. D Appl. Phys. 2008, 41, 085207. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Miessner, H.; Mahyar, A.; Mueller, S.; Kalass, D.; Moeller, D.; Omer, K.M. Removal of Dichloroacetic Acid from Aqueous Solution Using Non-Thermal Plasma Generated by Dielectric Barrier Discharge and Nano-Pulse Corona Discharge. Sep. Purif. Technol. 2019, 216, 51–57. [Google Scholar] [CrossRef]
- Leon-Garzon, A.R.; Dotelli, G.; Tommasini, M.; Bianchi, C.L.; Pirola, C.; Villa, A.; Lucotti, A.; Sacchi, B.; Barbieri, L. Experimental Characterization of Polymer Surfaces Subject to Corona Discharges in Controlled Atmospheres. Polymers 2019, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Nejman, A.; Kamińska, I.; Cieślak, M. Influence of Corona Discharge on the Surface and Thermal Properties of Aramid Fabrics. Plasma Process. Polym. 2019, 16, 1800194. [Google Scholar] [CrossRef]
- Žigon, J.; Petrič, M.; Dahle, S. Dielectric Barrier Discharge (DBD) Plasma Pretreatment of Lignocellulosic Materials in Air at Atmospheric Pressure for Their Improved Wettability: A Literature Review. Holzforschung 2018, 72, 979–991. [Google Scholar] [CrossRef]
- Krupa, A.; Sobczyk, A.T.; Jaworek, A. Surface Properties of Plasma-Modified Poly (Vinylidene Fluoride) and Poly (Vinyl Chloride) Nanofibres. Fibres Text. East. Eur. 2014, 22, 35–39. [Google Scholar]
- Rajasekaran, P.; Mertmann, P.; Bibinov, N.; Wandke, D.; Viöl, W.; Awakowicz, P. Filamentary and Homogeneous Modes of Dielectric Barrier Discharge (DBD) in Air: Investigation through Plasma Characterization and Simulation of Surface Irradiation. Plasma Process. Polym. 2010, 7, 665–675. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, J.; Xie, X.; Qiu, Y.; Kuffel, E. Experimental Study on the Transition of the Discharge Modes in Air Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2009, 42, 085203. [Google Scholar] [CrossRef]
- Tański, M.; Reza, A.; Przytuła, D.; Garasz, K. Ozone Generation by Surface Dielectric Barrier Discharge. Appl. Sci. 2023, 13, 7001. [Google Scholar] [CrossRef]
- Gouri, R.; Zouzou, N.; Tilmatine, A.; Moreau, E.; Dascalescu, L. Collection Efficiency of Submicrometre Particles Using Single and Double DBD in a Wire-to-Square Tube ESP. J. Phys. D: Appl. Phys. 2011, 44, 495201. [Google Scholar] [CrossRef]
- Nadjem, A.; Kachi, M.; Bekkara, F.; Medles, K.; Zeghloul, T.; Dascalescu, L. Triboelectrification of Granular Insulating Materials as Affected by Dielectric Barrier Discharge (DBD) Treatment. J. Electrostat. 2017, 86, 18–23. [Google Scholar] [CrossRef]
- Jodzis, S.; Patkowski, W. Macrokinetic Study on Ozone Boundary Concentration. Effect of Temperature. J. Electrostat. 2017, 85, 43–51. [Google Scholar] [CrossRef]
- Ono, R.; Oda, T. Ozone Production Process in Pulsed Positive Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2007, 40, 176–182. [Google Scholar] [CrossRef]
- Williamson, J.M.; Trump, D.D.; Bletzinger, P.; Ganguly, B.N. Comparison of High-Voltage Ac and Pulsed Operation of a Surface Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2006, 39, 4400–4406. [Google Scholar] [CrossRef]
- Avramidis, G.; Stüwe, B.; Wascher, R.; Bellmann, M.; Wieneke, S.; Von Tiedemann, A.; Viöl, W. Fungicidal Effects of an Atmospheric Pressure Gas Discharge and Degradation Mechanisms. Surf. Coat. Technol. 2010, 205, S405–S408. [Google Scholar] [CrossRef]
- Usta, Y.H.; Çukur, E.; Yıldırım, Ç.; Ercan, U.K. Design of a Portable, Battery-Powered Non-Thermal Atmospheric Plasma Device and Characterization of Its Antibacterial Efficacies. J. Electrostat. 2019, 99, 1–8. [Google Scholar] [CrossRef]
- Kim, M.C.; Klages, C.-P. One-Step Process to Deposit a Soft Super-Hydrophobic Film by Filamentary Dielectric Barrier Discharge-Assisted CVD Using HMCTSO as a Precursor. Surf. Coat. Technol. 2009, 204, 428–432. [Google Scholar] [CrossRef]
- Martens, U.; Thejaswini, H.C.; Majumdar, A.; Hippler, R. Deposition of Amorphous Hydrogenated Carbon Nitride Films with a Dielectric Barrier Discharge. Plasma Process. Polym. 2012, 9, 647–651. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Ishijima, T.; Seto, T. Humidity Effects on Surface Dielectric Barrier Discharge for Gaseous Naphthalene Decomposition. Phys. Phys. Phys. of Plasmas 2018, 25, 043512. [Google Scholar] [CrossRef]
- Ye, Z.L.; Shen, Y.; Xi, R.Z.; Hou, H.Q. Destruction of Benzene in an Air Stream by the Outer Combined Plasma Photolysis Method. J. Phys. D Appl. Phys. 2008, 41, 025201. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jo, J.-O. Degradation of Organic Contaminant by Using Dielectric Barrier Discharge Reactor Immersed in Wastewater. IEEE Trans. Plasma Sci. 2006, 34, 2624–2629. [Google Scholar] [CrossRef]
- Shibata, T.; Nishiyama, H. Water Treatment by Dielectric Barrier Discharge Tube with Vapor Flow. Int. J. Plasma Environ. Sci. Technol. 2017, 11, 112–117. [Google Scholar]
- Massines, F.; Gouda, G.; Gherardi, N.; Duran, M.; Croquesel, E. The Role of Dielectric Barrier Discharge Atmosphere and Physics on Polypropylene Surface Treatment. Plasma Process. Polym. 2001, 6, 35–49. [Google Scholar] [CrossRef]
- Zaleska, A.; Hanel, A.; Nischk, M. Photocatalytic Air Purification. Recent Pat. Eng. 2010, 4, 200–216. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Buabeng, F.P.; Mwabora, J.M.; Amaniampong, P.N.; Agbe, H.; Nyankson, E.; Obada, D.O.; Asiedu, N.Y. The Effect of Titanium Dioxide Synthesis Technique and Its Photocatalytic Degradation of Organic Dye Pollutants. Heliyon 2018, 4, e00681. [Google Scholar] [CrossRef]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-Light-Activated TiO2-Based Photocatalysts for the Inactivation of Pathogenic Bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Gosavi, S.; Tabei, R.; Roy, N.; Latthe, S.S.; Hunge, Y.M.; Suzuki, N.; Kondo, T.; Yuasa, M.; Teshima, K.; Fujishima, A.; et al. Low Temperature Deposition of TiO2 Thin Films through Atmospheric Pressure Plasma Jet Processing. Catalysts 2021, 11, 91. [Google Scholar] [CrossRef]
- Yang, W.; Wolden, C.A. Plasma-Enhanced Chemical Vapor Deposition of TiO2 Thin Films for Dielectric Applications. Thin Solid Films 2006, 515, 1708–1713. [Google Scholar] [CrossRef]
- Aita, T.; Ogawa, K.; Saito, Y.; Sumiyoshi, Y.; Higuchi, T.; Sato, S. Microstructures of SiO2 and TiOX Films Deposited by Atmospheric Pressure Inductively Coupled Micro-Plasma Jet. Surf. Coat. Technol. 2010, 205, 861–866. [Google Scholar] [CrossRef]
- Yamauchi, S.; Suzuki, H.; Akutsu, R. Plasma-Assisted Chemical Vapor Deposition of Titanium Oxide Layer at Room-Temperature. J. Cryst. Process Technol. 2014, 4, 20–26. [Google Scholar] [CrossRef]
- Gazal, Y.; Chazelas, C.; Dublanche-Tixier, C.; Tristant, P. Contribution of Optical Emission Spectroscopy Measurements to the Understanding of TiO2 Growth by Chemical Vapor Deposition Using an Atmospheric-Pressure Plasma Torch. J. Appl. Phys. 2017, 121, 123301. [Google Scholar] [CrossRef]
- Collette, S.; Hubert, J.; Batan, A.; Baert, K.; Raes, M.; Vandendael, I.; Daniel, A.; Archambeau, C.; Terryn, H.; Reniers, F. Photocatalytic TiO2 Thin Films Synthesized by the Post-Discharge of an RF Atmospheric Plasma Torch. Surf. Coat. Technol. 2016, 289, 172–178. [Google Scholar] [CrossRef]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra, 4th ed.; Chapman and Hall: London, NY, USA, 1976; ISBN 0-470-15164-1. [Google Scholar]
- Czech, T.; Sobczyk, A.T.; Jaworek, A. Light Emission Spectra of Molecules in Negative and Positive Back Discharges in Nitrogen with Carbon Dioxide Mixture at Atmospheric Pressure. Eur. Phys. J. D 2015, 69, 223. [Google Scholar] [CrossRef][Green Version]
- Boffard, J.B.; Piech, G.A.; Gehrke, M.F.; Anderson, L.W.; Lin, C.C. Measurement of Electron-Impact Excitation Cross Sections out of Metastable Levels of Argon and Comparison with Ground-State Excitation. Phys. Rev. A 1999, 59, 2749–2763. [Google Scholar] [CrossRef]
- Sobczyk, A.T.; Jaworek, A. Carbon Microstructures Synthesis in Low Temperature Plasma Generated by Microdischarges. Appl. Sci. 2021, 11, 5845. [Google Scholar] [CrossRef]
- Rincón, R.; Muñoz, J.; Sáez, M.; Calzada, M.D. Spectroscopic Characterization of Atmospheric Pressure Argon Plasmas Sustained with the Torche à Injection Axiale Sur Guide d’Ondes. Spectrochim. Acta Part B At. Spectrosc. 2013, 81, 26–35. [Google Scholar] [CrossRef]
- Kułakowska-Pawlak, B.; Żyrnicki, W. Characterization of a d.c. Titanium Tetraisopropoxide/Plasma Using Emission Spectroscopy. Thin Solid Films 1995, 266, 8–13. [Google Scholar] [CrossRef]
- Jamróz, P.; Żyrnicki, W.; Pohl, P. The Effect of a Miniature Argon Flow Rate on the Spectral Characteristics of a Direct Current Atmospheric Pressure Glow Micro-Discharge between an Argon Microjet and a Small Sized Flowing Liquid Cathode. Spectrochim. Acta Part B At. Spectrosc. 2012, 73, 26–34. [Google Scholar] [CrossRef]
- Ershov, K.S.; Kochubei, S.A.; Kiselev, V.G.; Baklanov, A.V. Decomposition Pathways of Titanium Isopropoxide Ti(Oi Pr)4: New Insights from UV-Photodissociation Experiments and Quantum Chemical Calculations. J. Phys. Chem. A 2018, 122, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Chan, C.K.; Porter, J.F.; Guo, W. Micro-Raman Spectroscopic Characterization of Nanosized TiO2 Powders Prepared by Vapor Hydrolysis. J. Mater. Res. 1998, 13, 2602–2609. [Google Scholar] [CrossRef]
- Di, L.-B.; Li, X.-S.; Shi, C.; Xu, Y.; Zhao, D.-Z.; Zhu, A.-M. Atmospheric-Pressure Plasma CVD of TiO2 Photocatalytic Films Using Surface Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2009, 42, 032001. [Google Scholar] [CrossRef]
- Nogueira, A.E.; Ribeiro, L.S.; Gorup, L.F.; Silva, G.T.S.T.; Silva, F.F.B.; Ribeiro, C.; Camargo, E.R. New Approach of the Oxidant Peroxo Method (OPM) Route to Obtain Ti(OH)4 Nanoparticles with High Photocatalytic Activity under Visible Radiation. Int. J. Photoenergy 2018, 2018, 6098302. [Google Scholar] [CrossRef]
- Shen, M.; Wang, M.; Wang, Q.; Tian, J.; Zhang, L.; Wang, L.; Shi, J. A Ti-OH Bond Breaking Route for Creating Oxygen Vacancy in Titania towards Efficient CO2 Photoreduction. Chem. Eng. J. 2021, 425, 131513. [Google Scholar] [CrossRef]
- Fictorie, C.P.; Evans, J.F.; Gladfelter, W.L. Kinetic and Mechanistic Study of the Chemical Vapor Deposition of Titanium Dioxide Thin Films Using Tetrakis-(Isopropoxo)-Titanium(IV). J. Vac. Sci. Technol. A Vac. Surf. Film. 1994, 12, 1108–1113. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.; Kangasluoma, J.; Attoui, M.; Junninen, H.; Kulmala, M.; Petäjä, T.; Biswas, P. Cluster Formation Mechanisms of Titanium Dioxide during Combustion Synthesis: Observation with an APi-TOF. Aerosol Sci. Technol. 2017, 51, 1071–1081. [Google Scholar] [CrossRef]
- Buerger, P.; Nurkowski, D.; Akroyd, J.; Kraft, M. A Kinetic Mechanism for the Thermal Decomposition of Titanium Tetraisopropoxide. Proc. Combust. Inst. 2017, 36, 1019–1027. [Google Scholar] [CrossRef]
- Bjarnason, E.H.; Ómarsson, B.; Engmann, S.; Ómarsson, F.H.; Ingólfsson, O. Dissociative Electron Attachment to Titatinum Tetrachloride and Titanium Tetraisopropoxide. Eur. Phys. J. D 2014, 68, 121. [Google Scholar] [CrossRef]
- Baszczuk, A.; Jasiorski, M.; Winnicki, M. Low-Temperature Transformation of Amorphous Sol–Gel TiO2 Powder to Anatase During Cold Spray Deposition. J. Therm. Spray Technol. 2018, 27, 1551–1562. [Google Scholar] [CrossRef]
| Wavelength [nm] | Transition (Upper Level–Lower Level) | Upper-Level Energy–Lower-Level Energy [eV] | |
|---|---|---|---|
| Paschen Notation | Racah Notation | ||
| 667.73 | 2p1–1s4 | 4p’[1/2]0–4s[3/2]1 | 13.48–11.62 |
| 696.54 | 2p2–1s5 | 4p’[1/2]1–4s[3/2]2 | 13.33–11.55 |
| 706.72 | 2p3–1s5 | 4p’[3/2]2–4s[3/2]2 | 13.30–11.55 |
| 714.70 | 2p4–1s5 | 4p’[3/2]1–4s[3/2]2 | 13.28–11.55 |
| 727.29 | 2p2–1s4 | 4p’[1/2]1–4s[3/2]1 | 13.33–11.62 |
| 738.40 | 2p3–1s4 | 4p’[3/2]2–4s[3/2]1 | 13.30–11.62 |
| 750.39 | 2p1–1s2 | 4p’[1/2]0–4s’[1/2]1 | 13.48–11.83 |
| 751.47 | 2p5–1s4 | 4p[1/2]0–4s[3/2]2 | 13.27–11.62 |
| 763.51 | 2p6–1s5 | 4p[3/2]2–4s[3/2]2 | 13.17–11.55 |
| 772.38 | 2p7–1s5 | 4p’[1/2]1–4s[3/2]2 | 13.33–11.55 |
| 772.42 | 2p7–1s3 | 4p’[1/2]1–4s’[1/2]0 | 13.33–11.72 |
| 794.82 | 2p4–1s3 | 4p’[3/2]1–4s’[1/2]0 | 13.28–11.72 |
| 800.62 | 2p6–1s4 | 4p[3/2]2–4s[3/2]2 | 13.17–11.62 |
| 801.48 | 2p8–1s5 | 4p[5/2]2–4s[3/2]2 | 13.09–11.55 |
| 810.37 | 2p7–1s4 | 4p[3/2]1–4s[3/2]2 | 13.15–11.62 |
| 811.53 | 2p9–1s5 | 4p[5/2]3–4s[3/2]2 | 13.08–11.55 |
| 826.45 | 2p2–1s2 | 4p’[1/2]1–4s’[1/2]1 | 13.33–11.83 |
| 840.82 | 2p3–1s2 | 4p’[3/2]2–4s’[1/2]1 | 13.30–11.83 |
| 842.46 | 2p8–1s4 | 4p[5/2]2–4s[3/2]1 | 13.09–11.62 |
| 852.14 | 2p4–1s2 | 4p’[3/2]1–4s’[1/2]1 | 13.28–11.83 |
| 866.79 | 2p7–1s3 | 4p[3/2]1–4s’[1/2]0 | 13.15–11.72 |
| 912.30 | 2p10–1s5 | 4p[1/2]1–4s[3/2]2 | 12.91–11.55 |
| 965.78 | 2p10–1s4 | 4p[1/2]1–4s[3/2]1 | 12.91–11.62 |
| 978.45 | 2p8–1s2 | 4p[5/2]2–4s’[1/2]1 | 13.09–11.83 |
| 1047.0 | 2p10–1s3 | 4p[1/2]1–4s’[1/2]0 | 12.91–11.72 |
 Layer of cast TTIP on glass substrate Layer of cast TTIP on glass substrate | Element | Before annealing [norm. wt.%] | After annealing [norm. wt.%] |
| Carbon | 3.52 | 2.73 | |
| Oxygen | 45.45 | 47.73 | |
| Silicon | 0 | 0.35 | |
| Titanium | 51.03 | 49.19 | |
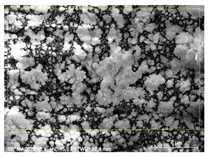 Layer deposited on glass substrate in DBD Layer deposited on glass substrate in DBD | Element | Before annealing [norm. wt.%] | After annealing [norm. wt.%] |
| Carbon | 3.15 | 2.60 | |
| Oxygen | 43.33 | 47.35 | |
| Sodium | 2.69 | 1.46 | |
| Magnesium | 0.83 | 0.22 | |
| Silicon | 14.91 | 4.15 | |
| Potassium | 0.25 | 0.12 | |
| Calcium | 1.61 | 0.94 | |
| Titanium | 33.24 | 40.99 | |
| Palladium | 0 | 0.44 | |
| Gold | 0 | 1.73 | |
 Layer deposited on brass substrate in DBD Layer deposited on brass substrate in DBD | Element | Before annealing [norm. wt.%] | After annealing [norm. wt.%] |
| Carbon | 9.72 | 3.51 | |
| Oxygen | 33.79 | 19.21 | |
| Titanium | 31.30 | 12.71 | |
| Copper | 12.34 | 31.62 | |
| Zinc | 12.86 | 32.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczyk, A.T.; Jaworek, A. Dielectric Barrier Discharge as a Source of Microplasma for TiO2 Submicron Particle Deposition. Appl. Sci. 2025, 15, 11474. https://doi.org/10.3390/app152111474
Sobczyk AT, Jaworek A. Dielectric Barrier Discharge as a Source of Microplasma for TiO2 Submicron Particle Deposition. Applied Sciences. 2025; 15(21):11474. https://doi.org/10.3390/app152111474
Chicago/Turabian StyleSobczyk, Arkadiusz Tomasz, and Anatol Jaworek. 2025. "Dielectric Barrier Discharge as a Source of Microplasma for TiO2 Submicron Particle Deposition" Applied Sciences 15, no. 21: 11474. https://doi.org/10.3390/app152111474
APA StyleSobczyk, A. T., & Jaworek, A. (2025). Dielectric Barrier Discharge as a Source of Microplasma for TiO2 Submicron Particle Deposition. Applied Sciences, 15(21), 11474. https://doi.org/10.3390/app152111474






