Abstract
Background: Adolescence is characterized by rapid physical growth and neuromuscular reorganization, which may influence the development of postural control. Gender-specific differences in pubertal timing suggest that girls may achieve postural stability earlier than boys, but evidence remains inconsistent. This cross-sectional pilot study aimed to examine gender differences in static postural control among adolescents. Material and methods: A total of 59 students (28 females, 31 males; mean age 13.49 ± 0.97 years) from two schools in Bari, Italy, participated. Postural stability was assessed during bipedal and single-leg stance tasks under eyes-open and eyes-closed conditions using an inertial sensor placed at the lumbosacral region. The primary outcomes were sway path length and oscillation ellipse area. Results: Females demonstrated significantly shorter path length in eyes-open bipedal stance (p = 0.027, d = −0.51), as well as reduced ellipse area (p = 0.047, d = −0.44) and path length (p = 0.010, d = −0.62) in eyes-closed bipedal stance. No significant gender differences were observed in single-leg stance. Conclusions: These findings support the hypothesis that adolescent girls exhibit superior postural stability compared to boys, particularly under challenging sensory conditions. Such differences may reflect earlier maturational processes and suggest possible implications for motor development, injury prevention, and sports training.
1. Introduction
Adolescence represents one of the most dynamic periods of human development, characterized by rapid and profound changes in physical growth, body composition, and neuromuscular coordination [1,2]. This critical developmental window, spanning approximately from 10 to 19 years of age, encompasses the pubertal growth spurt, a period of accelerated linear growth that fundamentally alters body proportions and biomechanical characteristics. The timing and magnitude of these changes exhibit distinct gender-specific patterns, with girls typically experiencing their growth spurt approximately two years earlier than boys [3]. Understanding how these developmental differences influence postural control has important implications for adolescent motor development, injury prevention, and sports performance.
The adolescent growth spurt is perhaps most dramatically exemplified by the peak height velocity (PHV), defined as the period when individuals achieve their maximum rate of linear growth [4]. Research indicates that peak height velocity occurs at a mean age of 13.5 years and 11.5 years in boys and girls, respectively, with whole-year peak height velocity reaching 9.5 cm/y in boys and 8.3 cm/y in girls [5]. This temporal difference in maturation has cascading effects on various physiological systems, including those responsible for maintaining postural equilibrium. The rapid changes in stature, body mass distribution, and limb proportions that characterize this period create a dynamic environment where the postural control system must continuously adapt to evolving biomechanical constraints [6].
During the adolescent period, postural control, the ability to maintain the body’s center of mass within the base of support, undergoes significant reorganization [7]. This complex sensorimotor process integrates visual, vestibular, and proprioceptive inputs to generate appropriate motor responses that ensure stability during both quiet standing and dynamic activities [8]. The developmental trajectory of postural control during adolescence is particularly intriguing because it occurs against the backdrop of rapid anthropometric changes that potentially challenge system stability. Previous research has demonstrated that postural control continues to mature throughout childhood and adolescence, with adult-like performance typically achieved by the mid-to-late teenage years [9].
The intersection of gender-specific developmental patterns and postural control maturation has emerged as an area of considerable scientific interest. Emerging evidence suggests that the earlier onset of puberty in girls may confer advantages in terms of postural stability during the adolescent period. Recent findings provide novel evidence that postural control is superior in early adolescent girls than boys nine months prior to PHV, likely associated with an earlier maturation of muscle coordination. These findings challenge traditional assumptions about motor development and suggest that the temporal aspects of pubertal maturation may have previously underappreciated effects on neuromuscular function [10].
The mechanisms underlying potential gender differences in adolescent postural control are multifaceted and likely involve both structural and functional adaptations. From an anthropometric perspective, girls and boys follow distinct developmental trajectories during puberty [11]. Girls typically experience earlier stabilization of their growth parameters, potentially allowing for more rapid optimization of postural control strategies. Additionally, differences in body composition, limb proportions, and center of mass location between genders may create distinct biomechanical environments that influence postural stability [12]. The earlier completion of skeletal maturation in girls may result in reduced inertial instability during the critical period of postural system development.
Neuromotor factors may also contribute to observed gender differences in postural control. The coordination of multiple muscle groups required for effective postural control involves complex patterns of muscle activation and inhibition. Research suggests that girls may develop more efficient neuromuscular strategies earlier in adolescence, possibly related to their advanced maturational status. The integration of sensory information, particularly the weighting and reweighting of visual, vestibular, and proprioceptive inputs, may also follow gender-specific developmental patterns that influence postural performance [13].
Furthermore, hormonal influences during puberty may differentially affect neuromuscular development between genders. The distinct hormonal profiles associated with male and female puberty could influence muscle strength development, neural connectivity, and sensorimotor integration in ways that impact postural control [14]. Girls’ earlier exposure to pubertal hormones may facilitate earlier maturation of the neural circuits responsible for postural regulation.
The practical implications of understanding gender differences in adolescent postural control extend beyond basic developmental science. Adolescence is a period of increased participation in sports and physical activities, coinciding with elevated injury risk, particularly for certain types of lower extremity injuries [15]. If girls indeed demonstrate superior postural control during certain phases of adolescence, this information could inform targeted intervention strategies, training protocols, and injury prevention programs [11]. Additionally, understanding the normal developmental trajectory of postural control can help clinicians identify adolescents who may be experiencing atypical development and could benefit from targeted interventions.
The current body of literature, while growing, presents some conflicting findings regarding gender differences in adolescent postural control. Some studies have reported superior postural stability in girls, while others have found minimal gender differences or advantages for boys in specific conditions [16,17,18]. These discrepancies may be related to differences in study methodologies, the specific developmental phases examined, the postural tasks employed, and the metrics used to quantify postural performance. Evidence indicates that postural control is better in early adolescent girls than boys, with increased coactivation in ankle joint muscles related to better postural control [19,20], yet the generalizability and underlying mechanisms of these findings require further investigation.
The assessment of postural control in adolescents typically involves analysis of center of pressure (COP) movements during quiet standing tasks [21]. Key parameters include anteroposterior and mediolateral displacement ranges, mean velocity of COP movement, and various measures of sway area and frequency content. These measures provide insights into the strategies employed by the postural control system and the overall effectiveness of postural regulation. Differences in these parameters between genders could reflect distinct underlying control mechanisms or developmental timelines.
Despite increasing interest, the literature shows inconsistent evidence regarding gender-related differences in adolescent postural control; a recent work showed that males generally demonstrate better postural stability with eyes open, while females display reduced sway range and improved stability with eyes closed, indicating that males may rely more on vision for balance [22], while other studies showed inconsistent sex-related differences in balance performance [23]. Moreover, few studies have systematically examined these variations across sensory conditions [24,25,26]. In this context, the present study investigated static balance in a sample of adolescents from Southern Italy, focusing on gender-related differences. The analysis considers sway path length (PL) and oscillation ellipse area (EA) as primary indicators. We hypothesized that adolescent girls would demonstrate superior postural stability compared to boys, particularly under more challenging sensory conditions.
2. Materials and Methods
2.1. Participants
This cross-sectional pilot study initially recruited 69 volunteers. Participants were selected from two schools in Bari, Italy, with the assistance of physical education teachers who supported the project. After obtaining informed consent from parents, 59 students completed the testing phase. These were organized into two groups: 29 middle school pupils (15 boys, 14 girls; mean age 12.65 ± 0.55 years; average height 150.66 ± 8.25 cm; mean body mass 45.14 ± 9.72 kg), 30 fourth-grade pupils (16 boys, 14 girls; mean age 14.30 ± 0.47 years; height 160.67 ± 6.86 cm; body mass 53.47 ± 8.30 kg). All subjects were right-leg dominant, as determined by self-report and confirmed through the observation of habitual motor tasks (e.g., kicking a ball). The sample of 59 participants was primarily determined by practical considerations, including resource availability and recruitment feasibility as well as on previous similar studies [10,27].
Participants had to satisfy the following inclusion criteria: (a) fall within the targeted age range (11–15 years old); (b) be able to perform the balance tests; and (c) understand and comply with the testing instructions.
Participants were excluded if they had sustained injuries, experienced a concussion, or had any neurological conditions within the previous six months. Individuals who reported ongoing issues with joints, tendons, muscles, uncorrected visual impairments, diagnosed vestibular dysfunction, or use of medications that could affect balance were also not considered. Only trials in which participants could maintain standing posture without noticeable compensatory adjustments were analyzed.
All participants provided written informed consent prior to testing. The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Research Ethics Committee of the University of Bari (protocol code 0015637|16/02/23).
2.2. Testing and Procedures
Participants were instructed to maintain a parallel bipedal stance (feet shoulder-width apart, side by side), standing as still as possible on a designated area of the floor. Participants were free to decide their exact foot placement within the parallel stance, based on what felt most comfortable for maintaining stability. All trials were carried out using the same stance configuration.
In the first trial, participants were asked to fix their gaze on a cross located 5 m in front of them and to remain motionless for 30 s. This distance was used to provide a clear and stable visual reference point without excessively stimulating ocular convergence or accommodation that could interfere with postural control. Any visible compensatory adjustments, such as stepping, lifting a foot or toe, or removing a hand from the hip, resulted in the trial being discarded and repeated. In the single-leg (SL) stance, subjects were asked to balance on one leg while keeping their arms relaxed along the sides of the body. Each single-leg trial lasted 10 s, and both the dominant (DL) and the non-dominant leg (NL) were evaluated in separate trials.
Rest intervals of at least 90 s were provided between trials, during which participants could move freely around the room. The order of trials was kept constant, with the eyes-open (OE) condition always preceding the eyes-closed (CE) condition. This ensured that participants’ stability was confirmed before attempting the more demanding CE stance, thereby minimizing the risk of falls. Data collection took place during a physical education class under the supervision of the researchers and physical education teachers. All participants were able to understand the procedures and successfully completed every task, ensuring the reliability of the collected data.
Postural stability was assessed using an inertial measurement unit sensor (IMU) [28]. The IMU (Beyond Inertial, Motustech, Rome, Italy) (Figure 1) was placed over the lumbosacral region (between L4 and S1) using an adjustable elastic belt. This placement allowed accurate monitoring of body center of mass displacements during balance tasks (Figure 2). Previous studies have validated the use of an IMU placed at the lower lumbar region as a reliable proxy for estimating whole-body center of mass (CoM) motion and postural sway. One of them [29] demonstrated strong correlations between sway parameters derived from a lumbar-mounted IMU and those obtained from a force platform in both the mediolateral and anteroposterior directions during quiet standing, confirming that trunk acceleration could reflect CoM displacement. More recently, another study [30] reported that sway metrics derived from a single IMU positioned at L5 showed moderate-to-strong associations with force-plate measures across static and dynamic balance tasks, further supporting the validity of this approach.

Figure 1.
IMU sensor device (Beyond Inertial, Motustech, Rome, Italy).
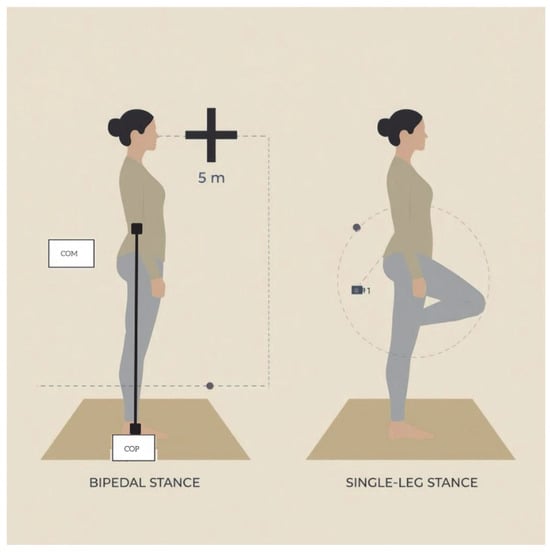
Figure 2.
Graphical representation of the experimental setup.
The sensor recorded two primary parameters: sway path length (PL), which measures the total length of the trajectory of postural sway, representing the extent and control of oscillatory movements, and oscillation ellipse area (EA), which measures the area of the ellipse enclosing sway dispersion, providing a measure of overall stability.
Before each measurement session, the IMU sensor was calibrated following standard procedure following the manufacturer’s instructions. In brief: (1) the device has an automatic self-calibration that allows a residual zero-offset estimation and verify stable accelerometer and gyroscope readings [31,32]; (2) the sensor’s distance from the ground was measured before each acquisition and entered into the software to allow the sensor to accurately calculate the postural oscillations. Data were collected at the device’s native sampling frequency and processed using the Beyond software (v.1.1.0.3) export and our custom analysis pipeline.
2.3. Statistical Analysis
All analyses were conducted using IBM SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA). Descriptive statistics (means and standard deviations) were calculated for all dependent variables separately for males and females. To exclude potential confounding effects of anthropometric characteristics, preliminary independent-samples t-tests were performed on age, height, body mass, and BMI to verify group comparability. The assumption of normality was assessed using the Shapiro–Wilk test. As all variables met this assumption, gender differences in postural control were examined with independent-samples t-tests. Effect sizes were calculated using Cohen’s d, interpreted as small (0.2), medium (0.5), or large (0.8) [33]. Statistical significance was set at p < 0.05.
3. Results
A total of 59 adolescents (28 females, 47.5%; 31 males, 52.5%) were included in the analysis. The mean age of participants was 13.49 years (SD = 0.97). Mean height was 155.75 cm (SD = 9.05), mean body mass was 49.37 kg (SD = 9.88), and mean BMI was 20.29 kg/m2 (SD = 3.49). No significant differences were found between males and females in age (t = −0.20, p = 0.840), height (t = −0.46, p = 0.651), body mass (t = 0.49, p = 0.629), or BMI (t = 0.81, p = 0.422). Descriptive statistics for postural control variables are presented in Table 1.

Table 1.
Anthropometric differences between the participants.
Under OE conditions, no significant gender difference was observed in the area of the EA (t = −1.32, p = 0.096, d = −0.34), while PL was significantly reduced in females compared to males (t = −1.97, p = 0.027, d = −0.51). Under CE conditions, females showed significantly reduced EA (t = −1.70, p = 0.047, d = −0.44) and PL (t = −2.38, p = 0.010, d = −0.62) compared to males (Table 2).

Table 2.
Postural control parameters during bipedal stance under OE and CE conditions.
Figure 3 shows no significant gender differences for bipedal stance under OE condition in the EA and reduced PL in females.
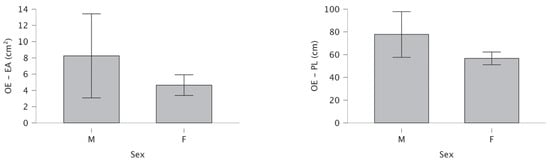
Figure 3.
Differences for open eyes ellipse area and path length.
Figure 4 shows significantly reduced EA and PL in females compared to males for bipedal stance under CE conditions.
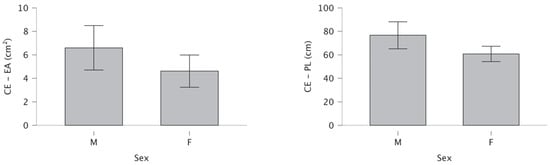
Figure 4.
Differences for closed-eyes ellipse area and path length.
For SL-stance, no significant differences were found between females and males in either the DL condition (t = −0.18, p = 0.430, d = −0.05) or the NL condition (t = −1.16, p = 0.125, d = −0.30) (Table 3).

Table 3.
Postural control parameters during SL stance on DL and NL conditions.
Figure 5 shows no significant gender differences for single-leg stance in the dominant and non-dominant leg.
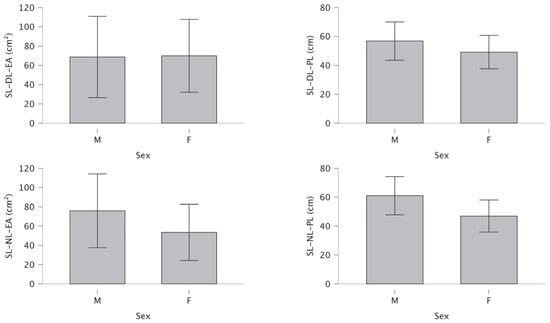
Figure 5.
Differences for dominant and non-dominant single-leg ellipse area and path length.
4. Discussion
Although adolescence represents a critical period of neuromuscular reorganization, few studies have examined how maturational timing and sensory integration interact to shape postural stability, presenting mixed results where some show female superiority and others find no difference, largely due to methodological heterogeneity and limited exploration across sensory conditions [22,23,24,25,26].
This work aimed to better characterize how gender and maturational stage influence postural control during adolescence, a phase when rapid growth and changing anthropometrics challenge the sensorimotor system, in a cohort of adolescents from Southern Italy.
Therefore, the present study investigated gender differences in postural control among adolescents, revealing significant advantages for females in both bipedal stance conditions. Our findings show that adolescent girls exhibited significantly shorter PL during OE conditions (d = −0.51) and both reduced EA (d = −0.44) and PL (d = −0.62) during CE conditions compared to boys. These results provide important insights into the developmental trajectory of postural control during adolescence and align with emerging evidence supporting superior postural stability in females during this critical developmental period.
Our findings of superior postural stability in adolescent girls are consistent with several recent investigations. Studies [20,34] previously demonstrated that girls have earlier postural control development and exhibit more adult-like balance compared to boys, while recent evidence shows that postural control is superior in early adolescent girls than boys 9 months prior to peak height velocity (PHV), likely associated with an earlier maturation of muscle coordination [18,35]. Similarly, a cross-sectional study [36] reported that girls have better balance variables and that girls had better postural stability than boys, demonstrating superior capability in integrating sensory inputs [37].
The magnitude of gender differences observed in our study (Cohen’s d: −0.44 to −0.62) suggests moderate to large effect sizes, indicating clinically meaningful differences between males and females. These effect sizes are comparable to those reported in a previous study [38], which found significant gender differences in COP parameters during quiet stance, with girls demonstrating reduced sway amplitude and velocity compared to boys of similar age.
Our results particularly align with other works [20,39], which reported that boys exhibited greater anteroposterior and mediolateral COP displacement than girls, with these differences becoming more pronounced in challenging postural tasks. This pattern suggests that gender differences in postural control may be task-dependent, with more complex balance challenges revealing greater disparities between males and females.
The superior postural control observed in adolescent girls in our study can be understood within the broader context of gender-specific developmental trajectories. A systematic review [11] suggested that the earlier onset of puberty in girls may confer advantages in terms of postural stability during adolescence. Our findings support this hypothesis, as the participants in our study (mean age 13.49 years) fall within the typical period when girls experience more advanced pubertal development compared to boys.
The hormonal changes associated with puberty trigger not only physical but also behavioral and neuromotor adaptations that may influence postural control development. The earlier hormonal changes experienced by girls may facilitate earlier maturation of the neural circuits responsible for postural regulation, potentially explaining the superior performance observed in our female participants.
Furthermore, the stabilization of growth parameters that typically occurs earlier in girls may allow for more rapid optimization of postural control strategies. It has been proposed [40,41] that the completion of major anthropometric changes provides a more stable biomechanical environment for the development of efficient postural control mechanisms. Our results suggest that this stabilization may confer measurable advantages in postural stability metrics.
A particularly noteworthy finding in our study was that gender differences became more pronounced during CE conditions, where both EA and PL showed significant differences, compared to OE conditions, where only PL differed significantly. This pattern suggests different sensorimotor integration strategies between genders, with implications for understanding the underlying mechanisms of postural control development.
Similar findings were reported by previous studies [17,42], noting that while girls had better postural stability than boys, they were more affected by altered sensory input information, suggesting that girls are more capable of integrating their sensory inputs, whereas boys treat each sensory input somewhat separately and rely more on somatosensory feedback. This differential reliance on visual versus proprioceptive information may explain why gender differences were more pronounced in our CE condition.
Different studies [43,44] suggest that the development of postural control involves the progressive integration of multiple sensory systems, with visual input playing a crucial role during childhood and adolescence. Our findings suggest that girls may achieve more efficient sensorimotor integration earlier in development, potentially due to advanced neural maturation associated with earlier pubertal onset. In the present study, the main postural metrics, sway PL and oscillation EA, were used as quantitative indicators of the effectiveness and efficiency of the postural control system. Shorter PL and smaller EA values reflect a reduced amplitude and velocity of center-of-mass oscillations, which indicate that the sensory and motor subsystems are interacting with less corrective activity to maintain equilibrium. Thus, rather than implying that one specific muscular synergy alone accounts for stability, these metrics capture the global outcome of multisensory and neuromuscular integration.
The mechanisms underlying the superior postural control observed in girls may involve differences in neuromuscular coordination strategies. Recent research [20] reported that adolescent girls exhibit greater tibialis anterior–medial gastrocnemius coactivation, a pattern that has been associated with enhanced ankle joint stability and reduced postural sway. However, this local mechanism represents only one element within the broader and highly integrated system governing postural control. Effective balance maintenance depends not only on distal ankle strategies but also on the coordinated involvement of hip and trunk musculature, the dynamic reweighting of visual, vestibular, and proprioceptive information, and adaptive neural processes at the central level. Consequently, the shorter sway PL and smaller oscillation EA observed in girls in the present study should be interpreted as global indicators of more effective multisystem coordination rather than as evidence of a single muscular mechanism. These findings likely reflect an earlier maturation of sensorimotor integration pathways and postural strategy optimization rather than isolated changes in ankle muscle activity.
Additionally, it was reported [45] that gender differences in postural control may be related to differences in muscle strength and activation patterns, with girls developing more efficient neuromuscular strategies during adolescence. The shorter PL and reduced EA observed in our female participants may reflect these more mature activation patterns.
Interestingly, our study found no significant gender differences in SL balance performance, contrasting with the clear advantages observed in bipedal stance conditions. This finding is consistent with some previous research [46] suggesting that gender differences in postural control may be task-specific and dependent on the complexity and demands of the balance challenge and noting that while gender differences were evident in quiet bipedal stance, these differences were less pronounced or absent in single-leg balance tasks. This may reflect the fact that SL balance represents a more challenging task that requires maximum effort from all participants, potentially minimizing the impact of subtle developmental differences.
Our findings suggest that the superior postural control observed in adolescent girls may be associated with distinct injury risk profiles between genders. Previous studies have reported that impaired postural stability and altered neuromuscular control are linked to a higher incidence of lower limb injuries, including anterior cruciate ligament sprains and ankle instability, particularly in adolescent athletes [11,47]. Although these studies indicate that poorer postural control is associated with increased injury susceptibility, such associations should not be interpreted as evidence of a direct causal relationship. The present results, therefore, may reflect maturational or sensorimotor differences that influence movement strategies and joint stabilization capacity, rather than direct protection from specific injuries. In this context, the more stable postural patterns observed in girls could contribute to a different balance between stability and movement adaptability, with potential implications for sport participation and injury prevention programs.
From a clinical perspective, understanding normal developmental patterns of postural control can help healthcare providers identify adolescents who may be experiencing atypical development. The reference values and gender differences reported in our study may inform screening protocols and intervention strategies for adolescents with balance-related concerns.
5. Limitations and Future Directions
Several limitations should be considered when interpreting our findings. First, the cross-sectional design limits our ability to track developmental changes over time. Longitudinal studies following individuals through the pubertal transition would provide more definitive insights into the temporal aspects of postural control development.
Future research should consider incorporating more diverse assessment protocols, as subtle changes in postural control in adolescents could be better assessed based on the results of combined static and dynamic tests. Additionally, the inclusion of pubertal maturation assessments would allow for more precise characterization of the relationship between biological development and postural control performance.
The use of inertial sensors, while providing valuable insights into overall postural stability, may not capture the full complexity of postural control strategies. Future studies incorporating force platform analysis, electromyography, and kinematic assessments would provide a more comprehensive understanding of the underlying mechanisms.
Finally, the small sample size and the geographical setting could limit the generalization and make the results preliminary.
6. Conclusions
Our study’s results demonstrated superior postural control in girls under challenging standing sensory conditions. These findings support the hypothesis that earlier pubertal maturation in girls confers advantages in terms of postural stability development. The moderate to large effect sizes observed suggest clinically meaningful differences that may have important implications for motor development, injury prevention, and sports performance. Future longitudinal research incorporating comprehensive developmental assessments will be crucial for fully understanding the mechanisms underlying these gender differences and their long-term implications for motor function and health.
Author Contributions
Conceptualization, L.P., A.P., I.P. and F.F.; methodology, L.P., G.G. and L.R.; software, G.G., I.P. and A.P.; validation, L.P., L.R. and A.P.; formal analysis, L.P., I.P. and A.P.; investigation, L.R. and G.G.; resources, I.P., A.P., G.G. and L.R.; data curation, I.P., A.P. and L.P.; writing—original draft preparation, L.P., A.P., I.P. and F.F.; writing—review and editing, L.P., G.G., F.F., S.C. and L.R.; visualization, S.C. and G.G.; supervision, G.G. and S.C.; project administration, S.C. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Bari University (protocol code 0015637|16/02/23).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mastorci, F.; Lazzeri, M.F.L.; Vassalle, C.; Pingitore, A. The Transition from Childhood to Adolescence: Between Health and Vulnerability. Children 2024, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.; Burnett, S.; Dahl, R.E. The Role of Puberty in the Developing Adolescent Brain. Hum. Brain Mapp. 2010, 31, 926–933. [Google Scholar] [CrossRef]
- Rogol, A.D.; Roemmich, J.N.; Clark, P.A. Growth at Puberty. J. Adolesc. Health 2002, 31, 192–200. [Google Scholar] [CrossRef]
- Cole, T.J.; Cortina-Borja, M.; Sandhu, J.; Kelly, F.P.; Pan, H. Nonlinear Growth Generates Age Changes in the Moments of the Frequency Distribution: The Example of Height in Puberty. Biostatistics 2008, 9, 159–171. [Google Scholar] [CrossRef]
- Iuliano-Burns, S.; Mirwald, R.L.; Bailey, D.A. Timing and Magnitude of Peak Height Velocity and Peak Tissue Velocities for Early, Average, and Late Maturing Boys and Girls. Am. J. Hum. Biol. 2001, 13, 1–8. [Google Scholar] [CrossRef]
- Rusek, W.; Baran, J.; Leszczak, J.; Adamczyk, M.; Baran, R.; Weres, A.; Inglot, G.; Czenczek-Lewandowska, E.; Pop, T. Changes in Children’s Body Composition and Posture during Puberty Growth. Children 2021, 8, 288. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Fredyk, A. Maturation of the Postural Control in Adolescent Girls: A 3-Year Follow-up Study. Gait Posture 2021, 83, 300–305. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensorimotor Integration in Human Postural Control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, A.W.; Armitano-Lago, C.N.; Cone, B.L.; Bonnette, S.; Rhea, C.K.; Cummins-Sebree, S.; Riley, M.A. Postural Control Development from Late Childhood through Young Adulthood. Gait Posture 2021, 86, 169–173. [Google Scholar] [CrossRef]
- Dorneles, P.P.; Pranke, G.I.; Mota, C.B. Comparação Do Equilíbrio Postural Entre Adolescentes Do Sexo Feminino e Masculino. Fisioter. Pesqui. 2013, 20, 210–214. [Google Scholar] [CrossRef]
- Holden, S.; Boreham, C.; Delahunt, E. Sex Differences in Landing Biomechanics and Postural Stability During Adolescence: A Systematic Review with Meta-Analyses. Sports Med. 2016, 46, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Stattin, H.; Magnusson, D. Pubertal Maturation in Female Development, 1st ed.; Routledge: New York, NY, USA, 2018; ISBN 978-1-315-78901-9. [Google Scholar]
- Ludwig, O.; Kelm, J.; Hammes, A.; Schmitt, E.; Fröhlich, M. Neuromuscular Performance of Balance and Posture Control in Childhood and Adolescence. Heliyon 2020, 6, e04541. [Google Scholar] [CrossRef]
- De Almeida-Neto, P.F.; Silva Dantas, P.M.; Pinto, V.C.M.; Cesário, T.D.M.; Ribeiro Campos, N.M.; Santana, E.E.; De Matos, D.G.; Aidar, F.J.; Tinoco Cabral, B.G.D.A. Biological Maturation and Hormonal Markers, Relationship to Neuromotor Performance in Female Children. Int. J. Environ. Res. Public Health 2020, 17, 3277. [Google Scholar] [CrossRef]
- Costa E Silva, L.; Fragoso, M.I.; Teles, J. Physical Activity–Related Injury Profile in Children and Adolescents According to Their Age, Maturation, and Level of Sports Participation. Sports Health 2017, 9, 118–125. [Google Scholar] [CrossRef]
- Jastrzębska, A.D. Gender Differences in Postural Stability among 13-Year-Old Alpine Skiers. Int. J. Environ. Res. Public Health 2020, 17, 3859. [Google Scholar] [CrossRef]
- Smith, A.; Ulmer, F.; Wong, D. Gender Differences in Postural Stability Among Children. J. Hum. Kinet. 2012, 33, 25–32. [Google Scholar] [CrossRef]
- Neves, J.C.D.J.; Souza, A.K.V.; Fujisawa, D.S. Is Postural Control Different in Boys and Girls? Comparison Between Sex. Fisioter. Pesqui. 2020, 27, 385–391. [Google Scholar] [CrossRef]
- García-Liñeira, J.; Leirós-Rodríguez, R.; Romo-Pérez, V.; García-Soidán, J.L. Sex Differences in Postural Control under Unstable Conditions in Schoolchildren with Accelerometric Assessment. Gait Posture 2021, 87, 81–86. [Google Scholar] [CrossRef]
- Paschaleri, Z.; Arabatzi, F.; Christou, E.A. Postural Control in Adolescent Boys and Girls before the Age of Peak Height Velocity: Effects of Task Difficulty. Gait Posture 2022, 92, 461–466. [Google Scholar] [CrossRef]
- Palmieri, R.M.; Ingersoll, C.D.; Stone, M.B.; Krause, B.A. Center-of-Pressure Parameters Used in the Assessment of Postural Control. J. Sport Rehabil. 2002, 11, 51–66. [Google Scholar] [CrossRef]
- Osama, M. Gender-Based Differences in Postural Stability, Sensory Integration of Balance and Fall Risk between Healthy Young Male and Female Adults: The GENAB Study. J. Back Musculoskelet. Rehabil. 2025, 38, 829–837. [Google Scholar] [CrossRef]
- Schedler, S.; Kiss, R.; Muehlbauer, T. Age and Sex Differences in Human Balance Performance from 6-18 Years of Age: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0214434. [Google Scholar] [CrossRef]
- Steindl, R.; Kunz, K.; Schrott-Fischer, A.; Scholtz, A. Effect of Age and Sex on Maturation of Sensory Systems and Balance Control. Dev. Med. Child Neurol. 2006, 48, 477. [Google Scholar] [CrossRef]
- Goble, D.J.; Barnes, K.; Lang, J.I.; Kapur, S.; Rosiek, S.K.; Haworth, J.L. Developmental Normative Data for the Balance Tracking System Modified Clinical Test of Sensory Integration and Balance Protocol. J. Exp. Child Psychol. 2025, 252, 106146. [Google Scholar] [CrossRef]
- Goble, D.J.; Brown, E.C.; Marks, C.R.C.; Baweja, H.S. Expanded Normative Data for the Balance Tracking System Modified Clinical Test of Sensory Integration and Balance Protocol. Med. Devices Sens. 2020, 3, e10084. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Smits, L.; Kotz, D.; Budé, L.; Spigt, M.; Serroyen, J.; Crutzen, R. A Simple Formula for the Calculation of Sample Size in Pilot Studies. J. Clin. Epidemiol. 2015, 68, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.; Lech, G.; Witkowski, K.; Kubacki, R.; Piepiora, P. Evaluation of Measurement Reliability for Selected Indices of Postural Stability Based on Data from the GYKO Inertial Sensor System. Biol. Sport 2024, 41, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ekvall Hansson, E.; Tornberg, Å. Coherence and Reliability of a Wearable Inertial Measurement Unit for Measuring Postural Sway. BMC Res. Notes 2019, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Bisi, M.C.; Stagni, R. Is IMU- Alternative to GRF-Based Posturography? A Comparative Assessment on Young Healthy Adults. J. Biomech. 2025, 185, 112687. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Bao, B.; Zhang, S.; Yang, L.; Yang, H.; Guo, K.; Meng, D. Balance Evaluation System Using Wearable IMU Sensing. Meas. Control 2025, 58, 281–292. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Mecheri, H.; Larue, C.; Plamondon, A. Validation of Inertial Measurement Units with an Optoelectronic System for Whole-Body Motion Analysis. Med. Biol. Eng. Comput. 2017, 55, 609–619. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Shams, A.; Vameghi, R.; Shamsipour Dehkordi, P.; Allafan, N.; Bayati, M. The Development of Postural Control among Children: Repeatability and Normative Data for Computerized Dynamic Posturography System. Gait Posture 2020, 78, 40–47. [Google Scholar] [CrossRef]
- García, J.M.Y.; Mora-Custodio, R.; Ribas-Serna, J.; González-Badillo, J.J.; Rodríguez-Rosell, D. Movement Velocity as a Determinant of Actual Intensity in Resistance Exercise. Int. J. Sports Med. 2022, 43, 1033–1042. [Google Scholar] [CrossRef]
- Azevedo, N.; Ribeiro, J.C.; Machado, L. Balance and Posture in Children and Adolescents: A Cross-Sectional Study. Sensors 2022, 22, 4973. [Google Scholar] [CrossRef]
- Nolan, L.; Grigorenko, A.; Thorstensson, A. Balance Control: Sex and Age Differences in 9- to 16-Year-Olds. Dev. Med. Child Neurol. 2005, 47, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Fling, B.W.; Lee, H.; Peterson, D.S. The Assessment of Center of Mass and Center of Pressure during Quiet Stance: Current Applications and Future Directions. J. Biomech. 2021, 123, 110485. [Google Scholar] [CrossRef] [PubMed]
- Paschaleri, Z.; Kannas, T.M.; Chalatzoglidis, G.; Arabatzi, F. Maturity Affects Static Balance in Early Adolescent Boys and Girls Associated with Achilles Tendon Stiffness. Saj 2024, 12, 120–128. [Google Scholar] [CrossRef]
- Schmuckler, M.A. Quantifying the Weights of Sensory Influences on Postural Control across Development. Braz. J. Mot. Behav. 2024, 18, e430. [Google Scholar] [CrossRef]
- Cheung, T.C.K.; Schmuckler, M.A. Multisensory and Biomechanical Influences on Postural Control in Children. J. Exp. Child Psychol. 2024, 238, 105796. [Google Scholar] [CrossRef]
- Sá, C.D.S.C.D.; Boffino, C.C.; Ramos, R.T.; Tanaka, C. Development of Postural Control and Maturation of Sensory Systems in Children of Different Ages a Cross-Sectional Study. Braz. J. Phys. Ther. 2018, 22, 70–76. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Iwasaki, Y. Developmental Perspective of Sensory Organization on Postural Control. Brain Dev. 1995, 17, 111–113. [Google Scholar] [CrossRef]
- Sinno, S.; Dumas, G.; Mallinson, A.; Najem, F.; Abouchacra, K.S.; Nashner, L.; Perrin, P. Changes in the Sensory Weighting Strategies in Balance Control Throughout Maturation in Children. J. Am. Acad. Audiol. 2021, 32, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L. Narrative Review of Sex Differences in Muscle Strength, Endurance, Activation, Size, Fiber Type, and Strength Training Participation Rates, Preferences, Motivations, Injuries, and Neuromuscular Adaptations. J. Strength Cond. Res. 2023, 37, 494–536. [Google Scholar] [CrossRef] [PubMed]
- Nolff, M.R.; Conner, N.O.; Haworth, J.L.; Goble, D.J. Lower Limb Asymmetry Evaluation Using the Balance Tracking System (BTrackS) Single Leg Stance Protocol. J. Mot. Behav. 2023, 55, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Shiyko, M.P.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Biomechanical Deficit Profiles Associated with ACL Injury Risk in Female Athletes. Med. Sci. Sports Exerc. 2016, 48, 107–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).