Abstract
In 2015, the global community set 17 Sustainable Development Goals (SDGs), with the second goal aiming to end hunger by 2030. In sustainable agriculture, seed treatment plays a crucial role and cold plasma (CP) has emerged as a promising, eco-friendly technology for improving seed performance. This review highlights CP as an innovative seed treatment method with significant potential to enhance seed vigor, germination, and crop yield, particularly under stress conditions such as drought, salinity, and biotic challenges. CP works by generating reactive oxygen and nitrogen species (RONS), which modulate key biochemical and physiological responses in seeds. These responses include improvements in water uptake, enhanced germination rates, and better stress tolerance. Moreover, CP exhibits strong antimicrobial properties, making it a chemical-free alternative for seed decontamination. Despite these benefits, the application of CP in large-scale agriculture faces several challenges. Also, this review critically examines the limitations of CP treatment, including the lack of standardized protocols and insufficient field validation. Additionally, it compares CP treatment with conventional chemical and microbial methods, offering insights into its potential advantages and remaining obstacles. This emerging technology holds promise for enhancing crop productivity while minimizing environmental impact, but further research and validation are essential for its broader adoption in sustainable agricultural practices.
1. Introduction
The challenge of feeding a global population projected to reach 10 billion by 2050 has driven the intensification of agriculture [1]. However, the heavy reliance on conventional practices (such as chemical fertilizers, pesticides, and excessive water use) has resulted in severe environmental degradation [2]. Consequently, there is an urgent need for solutions towards sustainable agriculture, focusing on innovative technologies that can boost crop yields and reduce negative environmental impacts [3]. In fact, seeds are fundamental structures that play a crucial role in both the plant life cycle and agricultural production systems, as their quality directly affects germination potential, seedling vigor, and the final yield and quality of the crops [4]. To enhance seed performance and protect against pathogens, traditional methods often involve chemical treatments. Chemical seed treatments offer benefits such as enhanced emergence, protection against seedborne and soilborne pests and pathogens, improved crop vigor and uniformity, reduced storage losses, and compliance with phytosanitary regulations [5,6,7]. The increasing use of agrochemicals, while boosting agricultural productivity, poses growing concerns for the health of living organisms and the environment, making it a significant issue in the agricultural sector. These chemical agents pose risks to non-target organisms, can accumulate in the ecosystem, and contribute to the development of resistant pathogen strains [8]. This has boosted ongoing research into sustainable and environmentally friendly alternatives for seed treatment.
In this context, cold plasma (CP), also known as non-thermal technology, is emerging as a novel and promising tool in agricultural technology [9,10,11,12]. Plasma, often described as the fourth state of matter, is created when enough energy (in any form) is applied to a gaseous medium (air, helium, argon, etc.) [6]. Plasma is a partially ionized gas composed of a rich mixture of ions, electrons, free radicals, excited atoms, and molecules, as well as UV radiation [7,13]. It is generated under atmospheric pressure conditions using low-energy electrical discharges and it operates at near-room temperatures, making it suitable for various agricultural applications [14]. The application of CP to plant materials, particularly seeds, is a non-thermal, water-less, and chemical-free process that has demonstrated a remarkable ability to modify seed properties and stimulate plant growth [15]. It is routinely generated via dielectric barrier discharge, plasma jets, corona discharge, RF-driven micro-hollow cathode discharges, gliding arc, and microwave sources [10,11,16].
Initial studies have demonstrated that the antimicrobial properties of CP offer a viable alternative to seed surface sterilization, effectively inactivating pathogenic fungi and bacteria without the use of chemical fungicides [12,17,18,19,20,21]. Also, CP seed treatment is effective in breaking seed dormancy, enhancing germination rates, and promoting seedling development in a diverse range of crop species, including alfalfa, barley, wheat, maize, soybean, and tomato [15,22,23,24,25,26,27,28,29,30]. The likely mechanisms for these beneficial effects are multifaceted, involving physical changes to the seed coat, such as surface etching and increased wettability, as well as the induction of biochemical responses within the seed through the action of reactive oxygen and nitrogen species (RONS) [16,18].
Many studies underline the potential of CP to enhance seed germination and seedling vigor, but results are occasionally inconsistent, with some reporting neutral or even negative effects. These discrepancies likely arise from differences in experimental conditions, including the type of plasma device used (e.g., dielectric barrier discharge vs. atmospheric pressure plasma jet), gas composition, treatment duration, and applied power. In addition, intrinsic seed characteristics such as coat thickness, moisture content, and species-specific physiology strongly influence responses to plasma exposure. Consequently, such divergences in findings can largely be due to the absence of standardized experimental protocols [22]. Recognizing these factors is critical for interpreting existing results and developing standardized treatment protocols that ensure reproducibility and comparability across studies. For example, when polyethylene glycol (PEG) is used to simulate drought stress in germination tests after plasma treatment, synergistic or antagonistic effects between plasma and PEG may complicate the interpretation of results. Previous studies have shown that while plasma exposure often improves seed performance under optimal conditions, its interaction with osmotic stress can sometimes modify the pattern of seed responses [31,32]. The interpretation of these findings remains difficult, most likely due to a possible synergistic interaction between plasma exposure and PEG-induced osmotic stress.
Although several review articles have summarized cold plasma (CP) applications, most of them have concentrated on food preservation, microbial inactivation, or plasma physics aspects, with comparatively limited attention given to the agricultural application. In particular, the role of CP in seed treatment, covering germination enhancement, stress tolerance, and resilience under arid and saline conditions, has not been comprehensively synthesized. Moreover, previous reviews have rarely compared CP directly with conventional priming strategies (chemical or biological) or discussed cultivar-specific responses, and the challenges of scaling up from laboratory to field. The present review therefore aims to bridge these gaps by critically evaluating current evidence, outlining technological limitations, and proposing research directions to facilitate the practical adoption of CP seed treatments in agriculture.
This review aims to: (i) define the basic principles of cold plasma and its interactions with seeds; (ii) critically assess the reported effects of cold plasma seed treatment on germination, plant growth, and overall crop productivity; (iii) examine the key factors influencing treatment efficacy; and (iv) identify current challenges and suggest future research directions necessary for the successful translation of this emerging technology from laboratory settings to field applications. By integrating the available evidence, this review aims to evaluate the overarching question of whether cold plasma constitutes a viable and effective alternative approach for improving crop production via seed treatment.
2. Cold Plasma Generation and Its Active Chemistry
The overall performance of cold plasma treatment is quite closely linked to how the plasma is produced and the complex set of chemically reactive particles that form during its generation. To create plasma, an electric field delivers energy to a neutral gas, referred to as the working gas, which can be a noble species like helium or argon, or simply the gases found in the environment such as air or nitrogen [33]. When researchers aim to treat seeds, the two most common sources of operating atmospheric pressure are the Dielectric Barrier Discharge (DBD) and the Atmospheric Pressure Plasma Jet (APPJ) (Figure 1) [34]. DBD configuration is favored in agricultural work because it uses two electrodes that are arranged with a dielectric layer in between [35]; this setup spreads a thin, steady plasma layer over a wide area, enabling multiple seed trays to be processed simultaneously. The APPJ, by contrast, generates a narrow plasma beam that travels in a directed stream, which permits high-precision treatment but can be a limiting factor in seed throughput when dealing with large volumes. Beyond these, other plasma sources are also employed, including radio-frequency (RF) and microwave discharges that provide stable and diffuse plasmas, gliding arc discharges that generate reactive gas flows suitable for larger-scale treatment, and corona or spark discharges that allow localized surface modifications. Each source type offers a distinct balance of plasma uniformity, reactive species generation, and scalability, with performance strongly dependent on exposure geometry and working gas composition [36].
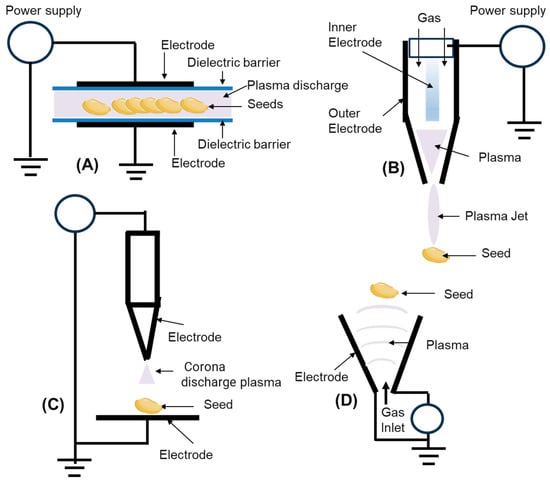
Figure 1.
The most common sources operating at atmospheric pressure; schemes of a DBD (dielectric barrier discharge) plasma (A), diagram of the atmospheric plasma jet source (B), corona discharge plasma (C) and gliding arc discharge (D).
More importantly, the selected generator and the gas surrounding it control the detailed mix of reactive radicals and ions that form during discharge, and it is this chemical profile that underlies the treatment’s biological impact. Although this composition contains a wide range of electrons, ions, UV photons, and electric fields, the highly reactive oxygen and nitrogen species (RONS) are the most important elements for seed alteration [30]. These RONS comprise a range of reactive nitrogen species (RNS) including nitric oxide (NO) and nitrogen dioxide (NO2), as well as reactive oxygen species (ROS) like ozone (O3), atomic oxygen (O), hydroxyl radicals (•OH), and superoxide (O2−) when air is employed as the working gas. These species are distinguished by their brief life spans and intense chemical reactivity. Due to their strong reactivity, they can physically etch the seed coat, making it more permeable, and they can also function as signaling molecules that can enter the seed and start a series of beneficial biochemical reactions that will ultimately affect germination and plant growth [29,30]. In fact, seed germination is a tightly regulated process involving hormonal balance, metabolic reactivation, and signaling by reactive oxygen and nitrogen species (ROS and RNS). Abscisic acid (ABA) enforces dormancy, whereas gibberellic acid (GA) promotes germination; their interplay is strongly influenced by ROS accumulation during seed imbibitions [30]. At optimal levels, ROS facilitate oxidation of regulatory proteins and weakening of the seed coat, thereby accelerating embryo growth. Similarly, nitric oxide (NO) and its derivatives (NO2−, NO3−) contribute by reducing ABA, enhancing GA, and breaking dormancy, often in light-dependent pathways. Cold plasma provides an exogenous source of ROS and RNS (e.g., O•, O2−, H2O2, NO), which can reinforce these endogenous signaling cascades, leading to enhanced germination and vigor. However, excessive plasma exposure may cause ROS overaccumulation, triggering antioxidant defenses and inhibiting germination, highlighting the importance of optimizing treatment duration and intensity.
Plasma-seed interactions can vary considerably depending on the plasma source, exposure geometry, and treatment parameters. Following the terminology proposed by Simek and Homola [36], these interactions are typically classified into two main types: direct and indirect. Direct plasma-seed interaction occurs when seeds are exposed directly to plasma species, resulting in physical surface modifications such as etching, increased wettability, and the generation of reactive species that can enhance germination and seedling vigor. Indirect interaction involves plasma-activated media or effluents, where reactive oxygen and nitrogen species (RONS) produced by the plasma interact with seeds, promoting biochemical responses and improving stress tolerance without direct exposure to the plasma itself. Recognizing these distinctions highlights the critical role of plasma type, power, exposure time, and distance in optimizing seed treatment outcomes.
Argon- and helium-based plasma sources are among the most widely studied in laboratory experiments, mainly because they generate stable, homogeneous, and easily controllable discharges. Their relatively inert nature ensures reproducibility and allows precise attribution of observed biological effects to plasma-generated species rather than gas impurities. However, these noble gases are expensive and not readily available for large-scale agricultural applications, which limits their practicality outside of controlled environments. By contrast, atmospheric air and nitrogen represent more economical and scalable options, although their complex reactive chemistry can introduce variability in seed responses. Thus, while Ar and He plasmas remain valuable for fundamental studies and proof-of-concept experiments, the broader agricultural deployment of CP technology will likely depend on air- or nitrogen-based systems [36].
3. Mechanisms of Cold Plasma Interaction with Seeds
The beneficial effects of CP on seeds are not the result of a single phenomenon, but rather a complex interaction of physical, biochemical, and antimicrobial actions. These mechanisms can occur simultaneously, with the initial physical modifications of seed coat facilitating subsequent and deeper biochemical responses within the embryo.
3.1. Physical Effects: Seed Coat Etching and Enhanced Hydrophilicity
The testa, or seed coat, acts as a protective layer that is frequently hydrophobic and waxy, which may limit the water imbibition, which is a vital and physiological condition for the seed germination. The interaction between cold plasma and a seed occurs primary at its surface induced by the high-energy electrons and, more significantly, the potent reactive oxygen species (ROS) like ozone (O3) and hydroxyl radicals (•OH) generated in the plasma. This action physically erodes the waxy outer layers and can create microscopic cracks and pores. A few studies showing the physical impacts of non-thermal plasma treatment on seed coat characteristics and germination are compiled in Table 1 [29,37,38,39,40,41,42]. In fact, CP treatment directly affects the limitation caused by the testa through a process known as surface etching or ablation. By studying wheat [41] and Maize [41], researchers demonstrated the etching of seeds surface via CP treatment. On pumpkin, the CP induced strong corrugation of seed coat [41]. Seed morphology can be observed by Scanning Electron Microscopy (SEM) [42,43]. Additionally, the surface is functionalized through plasma chemistry by removing hydrophobic hydrocarbon groups and introducing polar, oxygen-containing functional groups such as hydroxyl (-OH) and carboxyl (-COOH) [44,45], which can be analyzed using Fourier-transform infrared (FTIR) spectroscopy. The cumulative result of this etching and functionalization is a significant increase in the surface energy and, consequently, the hydrophilicity (wettability) of the seed coat. Because of this increased affinity for water, the embryo can absorb water much more quickly and uniformly, ensuring that it is well hydrated. This accelerates the breaking of dormancy and the start of the germination process.
3.2. Biochemical and Physiological Effects: RONS as Signaling Molecules
Beyond the physical changes to the seed coat, CP induces a cascade of deep biochemical and physiological responses within the seed itself. The biochemical and physiological reactions triggered by the plasma treatment in seeds are summarized in Figure 2. This is primarily mediated by the diffusion of smaller, relatively stable reactive oxygen and nitrogen species (RONS) through the newly permeable seed coat [45,46,47,48]. Once entering, these species act as effective signaling molecules, like their role in native biological systems. The nitric oxide (NO), a key RNS component of CP, is a well-known signaling molecule in plants that can break seed dormancy by interacting with phytohormone pathways [49] and enhances seed germination. It is widely reported that plasma treatment alters the crucial balance between the growth-promoting hormone gibberellic acid (GA) and the dormancy-inducing hormone abscisic acid (ABA), creating a higher GA/ABA ratio that highly promotes germination [50]. Also, the influx of RONS introduces a slight oxidative stress. This prompts the seed to upregulate its endogenous antioxidant defense systems, leading to an increase in the activity of enzymes like superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) [51]. This response not only mitigates potential damage from the plasma itself but may also boost the seed, making the obtained seedlings more resilient to environmental stresses. Finally, the enhanced water uptake activates metabolic enzymes, such as α-amylase and proteases, which are responsible for breaking down stored starches and proteins into usable energy for the growing embryo [15].
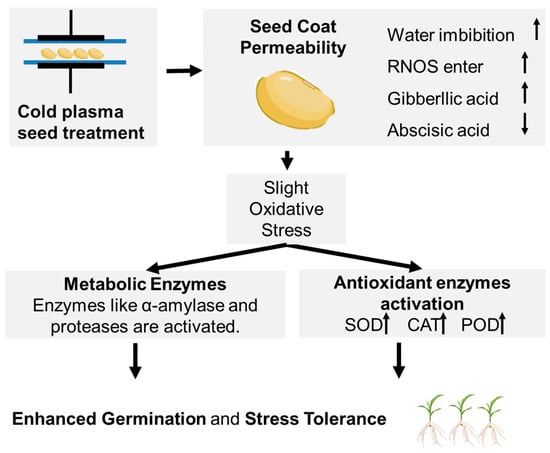
Figure 2.
Diagram summarizing the mechanism of cold plasma-induced seed activation.
3.3. Antimicrobial Effects: A Chemical-Free Approach to Seed Decontamination
The seed treatment with CP has appeared as a promising approach to seed decontamination, offering an effective alternative to traditional disinfectants. In that context, studies have demonstrated its efficacy against a wide range of seed-borne pathogens, including fungi, bacteria, and viruses, without affecting seed viability [52,53]. The antimicrobial effects of CP are mainly explained by the generation of reactive oxygen and nitrogen species (RONS), ultraviolet (UV) radiation, and charged particles, which together disrupt microbial cell membranes, proteins, and DNA, leading to the neutralization of pathogen [54]. In cereal, as an example, CP treatment has been shown to significantly reduce fungal contamination in the seed surface [55]. From the microbes studied, Escherichia coli and Salmonella spp., which are often associated with seed contamination, have shown significant reduction after CP treatment [54]. Also, fungal pathogens such as Aspergillus flavus and Fusarium spp., known for producing mycotoxins, are efficiently inactivated by the CP [55,56]. As well viral particles, including those of Tobacco mosaic virus (TMV), have also been reported to be neutralized by CP, probably due to protein denaturation and nucleic acid damage [57].
4. Agronomic Benefits and Efficacy
4.1. Enhanced Germination and Vigor
Germination is the process by which a seed develops into a new plant and is the physiological and biochemical process that begins when a viable seed absorbs water (imbibition) and ends with the emergence of the embryonic root (radicle) through the seed coat. This process is influenced by various factors, including seed health, environmental conditions, and genetic background. On the other hand, vigor refers to the seed’s ability to germinate rapidly and uniformly under a wide range of environmental conditions, as well as its ability to produce healthy seedlings able to resist biotic and abiotic stress. Improved germination and vigor are critical indicators of seed quality and play an essential role in determining the success of crop establishment and subsequent yield. Many studies have demonstrated that CP treatment of seeds can improve seed water absorption, activate metabolic processes, and activate enzymatic activity, leading to rapid and more uniform germination [15,47].
To demonstrate the impact of cold plasma seed treatment on germination, Figure 3 summarizes findings from fifteen previously published studies covering various crop species. The optimum exposure time reported for each species was used to redraw the comparative histogram between untreated and CP-treated seeds. Almost in all cases, CP treatment obviously improved germination compared with untreated controls. For example, wheat, rice, cotton, soybean, mung bean, cucumber, coriander, and radish all exhibited substantial increases, with CP-treated seeds reaching above 90% germination. In some crops such as barley, alfalfa, tomato, and watermelon, CP treatment more than doubled the germination rate compared with the untreated seeds. Buckwheat represents the only exception, where CP treatment (radio frequency plasma for 90 s) resulted in a slight decrease compared to the untreated seeds. This underscores the species-specific responses to plasma type and treatment conditions. The data demonstrate that CP seed treatment generally improves germination performance across a broad range of crops, confirming its promise as an eco-friendly technology to enhance seed germination. Also, the effectiveness of CP treatment is highly dependent on the plasma type and exposure duration, which vary considerably among species. Detailed data on plasma type and exposure duration for each species are provided in the Supplementary Material (Table S1). Short treatments (e.g., wheat, soybean, cucumber, pepper) were sufficient to enhance germination, indicating that minimal exposure can trigger beneficial physiological changes. Conversely, prolonged exposures (e.g., cotton 27 min, tomato 600 s) were required for optimal effects in some species.
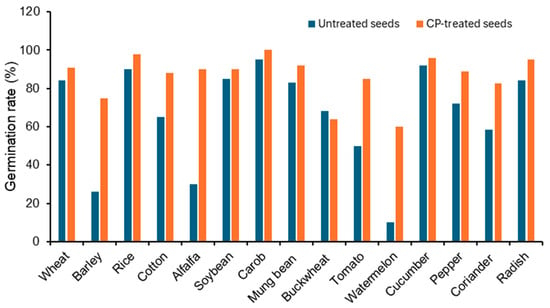
Figure 3.
Effects of cold plasma (CP) seed treatment on germination rate (%) across 15 plant species based on previously published studies. For each species, the optimum exposure time reported in the respective study was selected to redraw the figure. Values reflect data compiled from the literature, and in all studies, the observed differences between untreated and CP-treated seeds were statistically significant (p < 0.05). Buckwheat represents the only exception, where CP treatment (radio frequency plasma for 40 s) resulted in a slight decrease compared with the untreated seeds. The species are wheat [24], barley [15], rice [58], alfalfa [59], soybean [26], carob [60], mung bean [61], buckwheat [21], tomato [28], watermelon [62], cucumber [63], pepper [63], coriander [64], radish [65], andcotton [66].
The enhancement of germination and vigor following cold plasma exposure can be attributed to multiple, interrelated mechanisms driven by plasma-generated reactive oxygen and nitrogen species (RONS). Physically, short-lived radicals such as •OH and O3 etch the seed coat surface, creating micropores and increasing hydrophilicity, which accelerates imbibition and oxygen diffusion into the embryo. Biochemically, NO and NO2 act as signaling molecules that alter the hormonal balance, typically promoting gibberellic acid (GA) synthesis while reducing abscisic acid (ABA), thus favoring dormancy release and radicle protrusion. In parallel, enzymatic activity is stimulated, with α-amylase and proteases breaking down stored macromolecules to provide energy for early growth, while antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) mitigate oxidative stress induced by RONS. Collectively, these mechanisms explain why CP-treated seeds often show faster and more uniform germination, improved seedling vigor, and tolerance to abiotic stress, as consistently reported across diverse plant species [15,29,45,46].
These positive effects are mainly caused by the modification of seed coat permeability, facilitating water absorption and oxygen diffusion, which are critical for legume germination [42]. Furthermore, treated seeds show increased nitrogen fixation ability, leading to healthier seedlings and improved crop yields [55]. Vegetable seeds, such as tomato (Solanum lycopersicum) and lettuce (Lactuca sativa), exhibit marked improvements in germination and early growth following CP treatment [58]. Accordingly, CP seed treatment offers a sustainable approach to improving seed germination and vigor across diverse plant species. Besides biological effects, cost is a key factor for plasma seed treatment. Noble gas systems (Ar, He) are stable but expensive, whereas air and N2 plasmas offer more practical and scalable options. Treatment time and throughput also affect cost efficiency, making economic viability as important as biological performance.
4.2. Stress Tolerance
The application of CP on seeds has been shown to improve resistance to biotic stresses such as diseases, and abiotic stresses, such as drought and salinity, making it a promising pre-sowing strategy. CP technologies, such as dielectric barrier discharge (DBD), radio frequency (RF) discharge, and plasma jets, have been shown in recent research to have the potential to improve plant resistance to biotic and abiotic stressors (Table 2). The mechanisms behind this improved resilience are multifaceted, involving a cascade of interrelated biochemical and molecular responses within the seed and later seedling [28,67,68,69]. In the genetic level, CP treatment has been shown to upregulate stress-responsive genes and proteins, thereby improving the plant’s ability to resist adverse conditions [41]. Studies have shown that seeds treated with cold plasma exhibit significantly increased activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) [70,71]. This enzymatic upregulation helps to alleviate the detrimental effects of reactive oxygen species (ROS) that accumulate during stress conditions, thus protecting cellular components from oxidative damage. As well, studies have shown that CP can alter the balance of important hormones like gibberellins (GAs), which stimulate germination, and abscisic acid (ABA), which is typically linked to stress reactions and dormancy [68]. The RONS generated by the plasma, including NO, are thought to act as signaling molecules that promote these enzymatic and hormonal responses [68,72]. In general, CP seed treatment represents a new and sustainable approach to improving plant stress tolerance, with significant potential for application in agriculture under different environmental conditions.

Table 1.
The effects of non-thermal plasma treatment on seed coat properties, hydrophilicity and germination.
Table 1.
The effects of non-thermal plasma treatment on seed coat properties, hydrophilicity and germination.
| Species | Plasma Type | Effects on Seed Coats | Hydrophilicity | Germination | Ref |
|---|---|---|---|---|---|
| Wheat | DBD/helium | Surface etching, removal of waxy layer. Confirmed with SEM imaging. | Significantly increased. Water contact angle decreased from ~120° to <40°. | Increased germination rate and speed. Enhanced seedling growth and vigor. | [29,70] |
| Soybean | DBD plasma/O2 and N2 | Beneficial effect on seed coating with rhizobia. | Significantly increased. Water absorption rate was markedly higher post-treatment. | Accelerated germination and enhanced early seedling growth. | [35] |
| Peper | DBD/argon | Interaction between seed coat and plasma affected seedling abnormalities. | Increased seed coat hydrophilicity. | Enhanced germination and seedling vigor | [38] |
| Pumpkin | CAPJ/helium or argon | Strong corrugation of seed coat | Improve seed water uptake during imbibitions | CAPJ accelerates the germination and radicle development | [39] |
| Camelina | DBD/air | Partial external structures disappeared and became disorganized | - | CP pretreatment led to an increase in the efficiency of the oil extraction process | [40] |
| Maize | GAD/air | Modification of the etched surface of the starch caryopsis at 900 s exposure time | Improve seed water uptake | Germination parameters increased by 4.6% and 17.4% | [41] |
| Lentils, beans, and wheat | CRF/air | Effectively modification of seed coat surface properties | Significantly increased wettability, allowing faster hydration of the embryo. | Increase in the germination speed. | [42] |
Dielectric Barrier Discharge (DBD); Cold Atmospheric Plasma Jet (CAPJ); Gliding Arc Discharge (GAD); Cold radiofrequency air plasma (CRF).

Table 2.
Effects of different cold plasma types on plant stress responses.
Table 2.
Effects of different cold plasma types on plant stress responses.
| Plasma Type | Effects of Plasma Under Stress Conditions | Ref |
|---|---|---|
| Drought stress | ||
| Radio Frequency (RF) Discharge | Enhanced seed germination under PEG-induced drought conditions. | [24] |
| DBD | Improved nodulation, symbiotic nitrogen fixation, root and shoot growth. | [71] |
| RF | CP had a significant effect on the adaptability of alfalfa seeds in different drought environment | [73] |
| Plasma Jet | Induced drought stress tolerance with improved growth and biochemical alterations in tomato seedlings. | [28] |
| Salinity | ||
| DBD | Plasma treatment of barley seeds induced high germination and more chlorophylls a and b in leaves | [74] |
| Cold Plasma (10 kV) | CP treatment either alone or combined with SA improved plant uptake of nutrients in rice (Oryza sativa) under salinity conditions. | [75] |
| DBD/8 min | CP increases the production of Prosopis koelziana pigments, further strengthening their ability to resist salt stress | [76] |
| RF | One-minute CP treatment of wheat seeds significantly enhanced grain yield and quality under moderate to high salinity stress | [77] |
| Cadmium | ||
| DBD/1.5 min | Plasma technology mitigates Cd toxicity in wheat plants | [78] |
| Temperature | ||
| Cold Plasma (20 kV for 20 min) | Highest germination, seedling dry weight, vigor index, and field emergence under cold stress (18 °C). | [79] |
| CP | CP can significantly improve the germination of rice seeds affected by heat stress. | [80] |
| Biotic Stress | ||
| Radio Frequency (RF) Discharge | Increased resistance to Ralstonia solanacearum (bacterial wilt). | [25] |
| Dielectric Barrier Discharge (DBD) | Effectively removed seed-borne fungal pathogens, enhancing seed health and germination. | [81] |
| CP | Boosts plant protection against pathogens. | [82] |
4.3. Crop Production
Many studies have demonstrated that CP seed treatment significantly improves crop growth, yield, and quality. Numerous crops have been shown to benefit from CP treatment in terms of yield and growth. Notably, it increased important growth parameters in soybeans by 13.8% to 27.5%, including shoot length, root length, and seedling dry weight. In some cases, it even promoted root growth more than shoot development [26]. This positive effect on plant biomass is linked to increased antioxidant enzyme activity, which likely improves stress resilience and metabolic activity in early growth stages. Likewise, CP treatment improved growth in field pea by enhancing root length and nodulation, which supports nutrient acquisition and can increase yield under optimal moisture conditions [72]. It enhances plant vigor and biomass accumulation by promoting growth biochemical activities such as increasing soluble sugar and protein contents in seedling and activating seed reserve mobilization [15]. For example, CP treatment increased seed reserve mobilization and seedling growth traits, allowing better seedling production and potential for higher yield in barley and soybean [15,42,66]. In wheat, CP has been reported to considerably improve plant height, root length, seedling fresh weight, chlorophyll, nitrogen, and water content, leading to enhanced growth and yield [65]. In tomato, the seed treatment with CP boosts plant height, stem diameter, leaf thickness, and dry weight by approximately 9 to 17%, along with augmented nutrient uptake and enhanced resistance to bacterial wilt, thereby contributing to better growth and yield [67]. Many crops reported to benefit from cold plasma treatment in terms of growth or yield including rice, barley, oats, spinach, oilseed rape, green onion, chickpeas, and cucumber, showing variable but all growth stimulation effects [15,73].
5. Assessment of CP-Seed Treatment
5.1. Comparison with Chemical Treatment and Biological Priming
Both cold plasma and traditional priming techniques share common objectives. They aim to increase germination rates, enhance plant growth, and improve yield potential. To provide a clearer understanding of emerging seed treatment technologies, a comparative analysis was conducted across the three major approaches: cold plasma treatment, chemical seed treatment, and biological priming. Each method presents distinct advantages and limitations in terms of environmental impact, effectiveness, scalability, and practical application. As shown in Figure 4, cold plasma offers a promising, non-chemical alternative with rapid treatment potential and environmental benefits, though it still faces challenges in large-scale adoption [74,83]. Unlike chemical and biological priming, cold plasma uniquely generates reactive oxygen and nitrogen species, modulates seed coat permeability, and provides antimicrobial effects simultaneously, offering a rapid, residue-free, and environmentally friendly alternative for enhancing germination and early seedling vigor. Chemical and biological priming involves the pre-treatment of seeds with various substances to induce early physiological responses. The chemical treatments of seeds remain widely used due to their established protocols and immediate effectiveness but raise concerns over residues and long-term sustainability [77]. Biological priming often employs microbial inoculants or natural plant extracts to enhance seed performance. The microbial inoculation represents a balanced, eco-friendly option, supporting soil health and plant-microbe interactions, yet may face variability due to microbial viability and field conditions [78]. A key issue to consider is the potential environmental impacts of chemical and biological techniques, which may contribute to soil and water pollution due to the substances released during application.
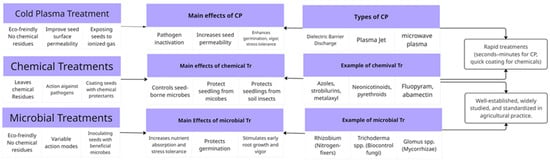
Figure 4.
Diagram of comparison between cold plasma, chemical, and microbial seed treatments.
5.2. Limitations
Despite its advantages, CP technology faces limitations that have hindered widespread adoption. Unlike conventional chemical and biological treatments, this technique does not yet have established protocols or sufficient field-scale validation. Numerous studies delineate the limitations related to cold plasma seed treatments, including:
- (a)
- Excessive or prolonged plasma exposure can damage seed surfaces and even internal structures, reducing seed viability and growth. A recent study demonstrated that plasma treatments beyond an optimal duration caused cracking and rupturing of seed coats, negatively affecting water uptake and the development of seedling. For soybean, cultivar-specific responses further illustrate the risks of excessive plasma exposure. According to the study by [84], significant variation was observed among cultivars: some showed enhanced germination and vigor at shorter exposure times (≤3 min), while others demonstrated declines in germination percentage, seedling length, and vigor index when treated for longer durations (≥5 min) or under higher discharge power. In addition, prolonged exposure was associated with altered antioxidant enzyme activities, indicating stress responses [84]. These findings emphasize that optimization of CP treatment is strongly cultivar-dependent, and that exceeding threshold exposure levels can shift effects from beneficial to detrimental.
- (b)
- Although reactive oxygen and nitrogen species (RONS) play a central role, the precise biochemical and molecular mechanisms remain unclear [85,86,87,88,89,90]. As shown in a study on Arabidopsis thaliana [84], short exposures (0.5–1 min) at 60 W stimulated root elongation and fresh weight, suggesting beneficial signaling from plasma-generated RONS. In contrast, longer exposures (≥5 min) significantly reduced root length and biomass, with severe inhibition observed at 10 min attributed to oxidative stress caused by RONS generated during the plasma treatment [86].
- (c)
- Practical challenges limit field adoption, as farmers are familiar with chemical or microbial seed treatments; CP requires new infrastructure and training. The challenges of practical field application include the need of specialized, often costly equipment and technical expertise to operate plasma treatment systems effectively [86]. Limited field-scale data and standardized protocols further complicate widespread acceptance in agricultural practice [71,83].
- (d)
- While CP treatment enhances early seedling vigor, these advantages may diminish over time. Some studies show faster germination and enhanced early seedling growth, treated and untreated plants reach comparable final growth and biomass at later stages [91]. This suggests that the positive effects of CP are often limited to early developmental stages, highlighting the need for long-term monitoring to determine whether initial benefits translate into lasting agronomic improvements.
Among these limitations, the most critical is the lack of field validation, which is discussed in detail in Section 5.3.
5.3. Lack of Field Validation
Despite the research findings highlighting the potential benefits of CP technology in agriculture, its field validation in crop production remains in the early stages and is not yet extensively explored [92,93,94]. Most studies to date have been conducted under controlled laboratory or greenhouse conditions, with only a limited number of large-scale or multi-location field trials [93]. Field environments are inherently heterogeneous, with fluctuating soil properties, climatic conditions, and biotic interactions that may strongly influence the efficacy of plasma treatments. Moreover, long-term assessments of ecological impacts, including effects on soil health, microbial diversity, and seed–microbe interactions, are still lacking. While promising outcomes have been reported for root development, nodulation, and yield following CP seed treatments, these results require further confirmation under real agricultural conditions, encompassing diverse crops, soil types, climates, and management practices. Therefore, coordinated multi-location field trials across diverse agroecological zones are urgently needed to validate reproducibility and scalability. Such validation is essential not only for scientific credibility but also as a prerequisite for farmer adoption and policy-level consideration of plasma-based seed technologies.
The limited number of insights into field studies points to several critical challenges that must be addressed before CP can be reliably integrated into conventional farming systems. Translating cold plasma-based seed treatments to field conditions is the strong dependence on fluctuating environmental parameters. Variations in soil moisture regimes, ambient temperature, and the presence of concurrent biotic pressures (e.g., seed-borne and soil-borne pathogens) can markedly influence treatment efficacy and reproducibility [75,94,95,96,97,98,99]. Furthermore, the long-term impacts of CP on crop performance, soil health, and overall agroecosystem stability beyond a single growing season remain largely unknown, underscoring the need for multi-season monitoring. Another major challenge is the technological transition from laboratory-scale experiments to field-scale applications, which require robust, scalable, and cost-effective plasma equipment, along with standardized treatment protocols adapted to different crops and stress conditions [71]. In addition, economic feasibility and user acceptance must be thoroughly evaluated. Large-scale participatory field trials involving farmers and stakeholders are essential not only to confirm the agronomic benefits of CP treatments but also to assess practical feasibility, adoption potential, and sustainability in commercial agriculture.
6. Perspectives and Conclusions
Despite its numerous advantages, several challenges must be addressed before cold plasma seed treatment can be widely adopted in agricultural systems. One of the primary barriers is the high initial investment required for equipment and infrastructure, which varies depending on treatment capacity and automation level, and may limit accessibility for smallholder farmers [98]. Therefore, designing cost-effective, scalable plasma reactors capable of treating large volumes of seeds efficiently and reliably remains a significant engineering challenge [99]. Although existing studies report promising results, the long-term effects of CP treatment on seed physiology, plant development, and overall crop performance are still not well understood. Comprehensive, large-scale field trials across diverse agroecological conditions are essential to validate laboratory findings and ensure the reliability of this technology in real-world farming environments. At the agricultural research level, continued interdisciplinary efforts are needed to optimize treatment parameters and effectively integrate CP technology into existing agricultural practices. Treatment duration is a key parameter; when optimized according to species, it can significantly enhance germination rates, seedling vigor, nutrient uptake, and crop productivity. However, with increasing treatment time, there was a progressive decrease in seed germination [99]. Also, excessive or prolonged exposure to cold plasma can induce oxidative damage and impair seed viability, emphasizing the importance of precise calibration of treatment parameters to avoid destructive effects [100]. In addition, to improve reproducibility and facilitate technology transfer, the development of standardized protocols and reference parameters across different CP systems is critical. This can be achieved through interdisciplinary research projects involving collaboration among experts in plasma physics, agronomy, engineering, plant physiology, and agricultural economics. Agrophysics is an interdisciplinary branch of science that applies the principles and methods of physics to study agricultural systems, integrating physics, biology, and agronomy to better understand soil–plant–atmosphere interactions [101]. It could be a highly required perspective for investigating cold plasma seed treatments.
Despite these challenges, cold plasma holds high potential to transform agriculture by offering sustainable, non-chemical solutions to enhance seed quality, increase crop yields, and reduce environmental impacts. Finally, we concluded that the cold plasma seed treatment is a real promise technology that improves seed germination, vigor, stress tolerance, and overall crop productivity. Its chemical-free nature makes it a strong alternative to conventional methods. However, widespread adoption is limited by the lack of standardized protocols, scalable equipment, and field-scale validation. Future research should prioritize the development of standardized plasma treatment protocols that account for device type, gas composition, and treatment parameters (time and power), enabling reproducibility across laboratories. Interdisciplinary studies combining plant physiology, molecular biology, and plasma physics are essential to elucidate underlying mechanisms at the cellular and molecular levels. Furthermore, large-scale field trials across diverse agroecological zones are urgently needed to validate laboratory findings under real-world farming conditions. Long-term assessments of soil health, microbial communities, and ecological safety will be critical to ensure sustainable adoption. Finally, techno-economic analyses and farmer-participatory studies should be undertaken to evaluate the cost-effectiveness, scalability, and acceptance of plasma-based seed technologies in agricultural practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app152010996/s1, Table S1: Effects of cold plasma on germination rate based on previously published studies.
Author Contributions
M.A.B.: Writing—original draft, M.A.B., I.B. and M.R.; review and validation; M.A.B. and M.R.; review and editing, M.R.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CP | Cold Plasm |
| RONS | Reactive oxygen and nitrogen species |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| DBD | Dielectric Barrier Discharge |
| APPJ | Atmospheric Pressure Plasma Jet |
| NO | Nitric oxide |
| ABA | Abscisic acid |
| GA | Gibberellic acid |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
References
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T. An ecologically sustainable approach to agricultural production intensification: Global perspectives and developments. Field Actions Science Reports. J. Field Actions 2012, 6, 1–6. Available online: http://journals.openedition.org/factsreports/1382 (accessed on 5 October 2025).
- Sarfraz, S.; Ali, F.; Hameed, A.; Ahmad, Z.; Riaz, K. Sustainable agriculture through technological innovations. In Sustainable Agriculture in the Era of the OMICs Revolution; Springer International Publishing: Cham, Switzerland, 2023; pp. 223–239. [Google Scholar] [CrossRef]
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Watrin, C.; Scheller, M.; Zeun, R.; Olaya, G. Benefits of chemical seed treatments on crop yield and quality. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Springer: Dordrecht, The Netherlands, 2014; pp. 89–103. [Google Scholar] [CrossRef]
- Chatterjee, R. Fundamental concepts and discussion of plasma physics. Techno Rev. J. Technol. Manag. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Yasoob, A.N.; Abdalameer, N.K.; Mohammed, A.Q. Plasma production and applications: A review. Int. J. Nanosci. 2022, 21, 2230003. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Iqbal, M.M.; Ashfaq, M.; Raza, N.; Wang, J.; Hafeez, A.; Kayani, S.B.; Ali, Q. Impact of Antibacterial Agents in Horticulture: Risks to Non-Target Organisms and Sustainable Alternatives. Horticulturae 2025, 11, 753. [Google Scholar] [CrossRef]
- Pal, P.; Sehgal, H.; Joshi, M.; Arora, G.; Simek, M.; Lamba, R.P.; Maurya, S.; Pal, U.N. Advances in using non-thermal plasmas for healthier crop production: Toward pesticide and chemical fertilizer-free agriculture. Planta 2025, 261, 109. [Google Scholar] [CrossRef] [PubMed]
- Akishev, Y.; Machala, Z.; Koval, N. Special issue on recent developments in plasma sources and new plasma regimes. J. Phys. D Appl. Phys 2019, 52, 130301. [Google Scholar] [CrossRef]
- Bruggeman, P.; Brandenburg, R. Atmospheric pressure discharge filaments and microplasmas: Physics, chemistry and diagnostics. J. Phys. D Appl. Phys. 2013, 46, 464001. [Google Scholar] [CrossRef]
- Desai, M.; Chandel, A.; Chauhan, O.P.; Semwal, A.D. Uses and future prospects of cold plasma in agriculture. Food Humanit. 2024, 2, 100262. [Google Scholar] [CrossRef]
- Harry, J.E. Introduction to Plasma Technology: Science, Engineering, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; Available online: https://www.wiley-vch.de/en/areas-interest/natural-sciences/introduction-to-plasma-technology-978-3-527-32763-8?utm_source=chatgpt.com (accessed on 5 October 2025).
- Waghmare, R. Cold plasma technology for fruit based beverages: A review. Trends Food Sci. Technol. 2021, 114, 60–69. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Bettaieb, I.; Hannachi, H.; Rejili, M.; Dufour, T. Cold plasma treatment boosts barley germination and seedling vigor: Insights into soluble sugar, starch, and protein modifications. J. Cereal Sci. 2024, 116, 103852. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability increase in plasma-treated agricultural seeds and its relation to germination improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.Y.; Kim, Y.S.; Balaraju, K.; Mok, Y.S.; Yoo, S.J.; Jeon, Y. Enhancement of seed germination and microbial disinfection on ginseng by cold plasma treatment. J. Ginseng Res. 2021, 45, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Guragain, R.P.; Kierzkowska-Pawlak, H.; Fronczak, M.; Kędzierska-Sar, A.; Subedi, D.P.; Tyczkowski, J. Germination improvement of fenugreek seeds with cold plasma: Exploring long-lasting effects of surface modification. Sci. Hortic. 2024, 324, 112619. [Google Scholar] [CrossRef]
- Orlowski, J.; Motyka-Pomagruk, A.; Dzimitrowicz, A.; Pohl, P.; Terefinko, D.; Lojkowska, E.; Jamroz, P.; Sledz, W. Application of Cold Atmospheric Pressure Plasma Jet Results in Achievement of Universal Antibacterial Properties on Various Plant Seeds. Appl. Sci. 2025, 15, 1255. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Boehm, D.; Bourke, P. Effects of cold plasma on wheat grain microbiome and antimicrobial efficacy against challenge pathogens and their resistance. Int. J. Food Microbiol. 2020, 335, 108889. [Google Scholar] [CrossRef] [PubMed]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold plasma affects germination and fungal community structure of buckwheat seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef]
- Pauzaite, G.; Malakauskiene, A.; Nauciene, Z.; Zukiene, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1700068. [Google Scholar] [CrossRef]
- Cherif, M.M.; Assadi, I.; Khezami, L.; Ben Hamadi, N.; Assadi, A.A.; Elfalleh, W. Review on recent applications of cold plasma for safe and sustainable food production: Principles, implementation, and application limits. Appl. Sci. 2023, 13, 2381. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Min, S.; Yanhui, L.; Hanliang, S. Cold plasma treatment enhances oilseed rape seed germination and seedling growth. J. Zhejiang Univ. Sci. B 2015, 16, 51–58. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol. 2014, 16, 54. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.F.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Katsenios, N.; Efthimiadou, A.; Stergiou, P.; Xanthou, Z.-M.; Giannoglou, M.; Dimitrakellis, P.; Gogolides, E.; Katsaros, G. Pre-sowing treatment of maize seeds by cold atmospheric plasma and pulsed electromagnetic fields: Effect on plant and kernels characteristics. Aust. J. Crop Sci. 2021, 15, 251–259. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free. Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Lalueza, A.; López-Santos, C.; Ghobeira, R.; Cools, P.; Morent, R.; De Geyter, N.; González-Elipe, A.R. Physicochemical surface analysis and germination at different irrigation conditions of DBD plasma-treated wheat seeds. Plasma Process. Polym. 2021, 18, 2000086. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Shome, M.; Kvitky, J.; Fridman, A.; Staack, D. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Process. Polym. 2021, 18, e2000162. [Google Scholar] [CrossRef]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019, 9, 8649. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Film. 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Arnold, A.; von Podewils, S.; Haase, H.; Emmert, S.; von Woedtke, T.; Weltmann, K.D.; Jünger, M. In vitro susceptibility of important skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD). Plasma Process. Polym. 2012, 9, 380–389. [Google Scholar] [CrossRef]
- Stryczewska, H.D.; Boiko, O. Applications of plasma produced with electrical discharges in gases for agriculture and biomedicine. Appl. Sci. 2022, 12, 4405. [Google Scholar] [CrossRef]
- Šimek, M.; Homola, T. Plasma-assisted agriculture: History, presence, and prospects—A review. Eur. Phys. J. D 2021, 75, 210. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed]
- Sriruksa, C.; Sawangrat, C.; Sansongsiri, S.; Boonyawan, D.; Thanapornpoonpong, S.N. Influence of Seed Coat Integrity on the Response of Pepper Seeds to Dielectric Barrier Discharge Plasma Treatment. Plants 2025, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Hairston, J.S.; Patel, D.; Gott, R.P.; Xu, K.G. Cold plasma poration and corrugation of pumpkin seed coats. Bioelectrochemistry 2019, 128, 175–185. [Google Scholar] [CrossRef]
- Rezaei, S.; Ghobadian, B.; Ebadi, M.T.; Ghomi, H. Qualitative and quantitative assessment of extracted oil from Camelina sativa seed treated by dielectric-barrier discharge cold plasma. Contrib. Plasma Phys. 2020, 60, e202000032. [Google Scholar] [CrossRef]
- Kamseu-Mogo, J.P.; Kamgang-Youbi, G.; Djepang, S.A.; Tamo, B.S.; Laminsi, S. Treatment of maize seeds (Zea mays) by nonthermal plasma generated by gliding electric discharge for application in agriculture. IEEE Trans. Plasma Sci. 2021, 49, 2318–2328. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Kanwal, K.; Zafar, M.; Khan, A.M.; Mahmood, T.; Abbas, Q.; Ozdemir, F.A.; Ahmad, M.; Sultana, S.; Rozina; Fatima, A.; et al. Implication of scanning electron microscopy and light microscopy for oil content determination and seed morphology of Verbenaceae. Microsc. Res. Tech. 2022, 85, 789–798. [Google Scholar] [CrossRef]
- Ghodsimaab, S.P.; Makarian, H.; Ghasimi Hagh, Z.; Gholipoor, M. Scanning electron microscopy, biochemical and enzymatic studies to evaluate hydro-priming and cold plasma treatment effects on the germination of Salvia leriifolia Benth. seeds. Front. Plant Sci. 2023, 13, 1035296. [Google Scholar] [CrossRef]
- Grainge, G.; Nakabayashi, K.; Steinbrecher, T.; Kennedy, S.; Ren, J.; Iza, F.; Leubner-Metzger, G. Molecular mechanisms of seed dormancy release by gas plasma-activated water technology. J. Exp. Bot. 2022, 73, 4065–4078. [Google Scholar] [CrossRef]
- Dufour, T.; Gutierrez, Q.; Bailly, C. Sustainable improvement of seeds vigor using dry atmospheric plasma priming: Evidence through coating wettability, water uptake, and plasma reactive chemistry. J. Appl. Phys. 2021, 129, 084902. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, V.; Subramaniyan, V.; Nagarajan, K.; Sekar, M.; Chinni, S.V.; Ramachawolran, G. Plasma modification techniques for natural polymer-based drug delivery systems. Pharmaceutics 2023, 15, 2066. [Google Scholar] [CrossRef]
- Zhao, J.; Nie, L. Five gaseous reactive oxygen and nitrogen species (RONS) density generated by microwave plasma jet. Phys. Plasmas 2019, 26, 073503. [Google Scholar] [CrossRef]
- Bethke, P.C.; Libourel, I.G.; Jones, R.L. Nitric oxide reduces seed dormancy in Arabidopsis. J. Exp. Bot. 2006, 57, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. Dielectric barrier discharge plasma treatment-induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and physiological plant processes affected by seed treatment with non-thermal plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Fojlaley, M.; Aghajani, A.; Ranji, A.; Bahlooli, E. Low-temperature cold plasma and decontamination of cereals and fruits: A review. World J. Environ. Biosci. 2020, 9, 133–142. Available online: https://environmentaljournals.org/article/low-temperature-cold-plasma-and-decontamination-of-cereals-and-fruits-a-review (accessed on 5 October 2025).
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef]
- Sultan, S.M.; Yousef, A.F.; Ali, W.M.; Mohamed, A.A.; Ahmed, A.R.M.; Shalaby, M.E.; Teiba, I.I.; Hassan, A.M.; Younes, N.A.; Kotb, E.F. Cold atmospheric plasma enhances morphological and biochemical attributes of tomato seedlings. BMC Plant Biol. 2024, 24, 420. [Google Scholar] [CrossRef]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. Available online: https://pubs.acs.org/doi/abs/10.1021/acsami.6b04555 (accessed on 5 October 2025). [CrossRef]
- Benabderrahim, M.A.; Hannachi, H.; Elfalleh, W.; Dufour, T. Nutritional and growth enhancement of alfalfa sprouts through cold plasma and UV seed treatments. Ital. J. Food Sci. 2025, 37, 160–173. [Google Scholar] [CrossRef]
- Othman, K.B.; Cherif, M.M.; Assadi, I.; Elfalleh, W.; Khezami, L.; Ghorbal, A.; Assadi, A.A. Exploring Cold plasma technology: Enhancements in Carob seed germination, phytochemical Composition, and antioxidant activity. Heliyon 2024, 10, e28966. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Mishra, A.; Mishra, R.; Pandey, S.; Prakash, R. Transformative impact of atmospheric cold plasma on mung bean seeds: Unveiling surface characteristics, physicochemical alterations, and enhanced germination potential. AIP Adv. 2024, 14, 075215. [Google Scholar] [CrossRef]
- Lotfy, K. Effects of cold atmospheric plasma jet treatment on the seed germination and enhancement growth of watermelon. Open J. Appl. Sci. 2017, 7, 705. [Google Scholar] [CrossRef][Green Version]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Guragain, D.P.; Subedi, D.P. Exploring the effects of non-thermal plasma pre-treatment on coriander (Coriander sativum L.) seed germination efficiency. Heliyon 2024, 10, e28763. [Google Scholar] [CrossRef] [PubMed]
- Guragain, R.P.; Baniya, H.B.; Dhungana, S.; Chhetri, G.K.; Sedhai, B.; Basnet, N.; Shakya, A.; Pandey, B.P.; Pradhan, S.P.; Joshi, U.M.; et al. Effect of plasma treatment on the seed germination and seedling growth of radish (Raphanus sativus). Plasma Sci. Technol. 2021, 24, 015502. [Google Scholar] [CrossRef]
- de Groot, G.J.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef] [PubMed]
- Le, T.Q.X.; Nguyen, L.N.; Nguyen, T.T.; Choi, E.H.; Nguyen, Q.L.; Kaushik, N.K.; Dao, N.T. Effects of cold plasma treatment on physical modification and endogenous hormone regulation in enhancing seed germination and radicle growth of mung bean. Appl. Sci. 2022, 12, 10308. [Google Scholar] [CrossRef]
- Waskow, A.; Guihur, A.; Howling, A.; Furno, I. RNA sequencing of Arabidopsis thaliana seedlings after non-thermal plasma-seed treatment reveals upregulation in plant stress and defense pathways. Int. J. Mol. Sci. 2022, 23, 3070. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Vasudevan, S.N. Plasma treatment and seed quality advancement: A review. Agric. Rev. 2021, 42, 197–202. [Google Scholar] [CrossRef]
- Holubová, Ľ.; Švubová, R.; Slováková, Ľ.; Bokor, B.; Chobotová Kročková, V.; Renčko, J.; Uhrin, F.; Medvecká, V.; Zahoranová, A.; Gálová, E. Cold atmospheric pressure plasma treatment of maize grains—Induction of growth, enzyme activities and heat shock proteins. Int. J. Mol. Sci. 2021, 22, 8509. [Google Scholar] [CrossRef] [PubMed]
- Abeysingha, D.N.; Dhaliwal, H.K.; Du, L.; De Silva, C.; Szczyglowski, K.; Roopesh, M.S.; Thilakarathna, M.S. The potential of cold plasma-based seed treatments in legume–rhizobia symbiotic nitrogen fixation: A review. Crops 2024, 4, 95–114. [Google Scholar] [CrossRef]
- Feng, J.; Wang, D.; Shaao, C.; Tang, X. Effects of cold plasma treatment on alfalfa seed growth under simulated drought stress. Plasma Sci. Technol. 2018, 20, 035505. [Google Scholar] [CrossRef]
- Perea-Brenes, A.; Garcia, J.L.; Cantos, M.; Cotrino, J.; Gonzalez-Elipe, A.R.; Gomez-Ramirez, A.; Lopez-Santos, C. Germination and first stages of growth in drought, salinity, and cold stress conditions of plasma-treated barley seeds. ACS Agric. Sci. Technol. 2023, 3, 760–770. Available online: https://pubs.acs.org/doi/10.1021/acsagscitech.3c00121?goto=supporting-info (accessed on 5 October 2025). [CrossRef]
- Sheteiwy, M.S.; An, J.; Yin, M.; Jia, X.; Guan, Y.; He, F.; Hu, J. Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 2019, 256, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, Z.M.; Nasibi, F.; Noori, H. Cold plasma technology as a pre-treatment for seed priming enhances germination and reduces salinity stress in Prosopis Koelziana. Sci. Rep. 2025, 15, 26250. [Google Scholar] [CrossRef]
- Saudy, H.S.; Fawzy, M.; Abd El-Momen, W.R.; Mubarak, M.; Ibrahim, M.F.; Elgendy, A.T. Potentiality of non-thermal plasma as an innovative technique for ameliorating wheat productivity under saline soil conditions. Egypt. J. Agron. 2025, 47, 471–480. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.; Das, U.; Sarkar, U.; Roy, N.C.; Reza, A.; Talukder, M.R.; Uddin, A. Reduction of cadmium toxicity in wheat through plasma technology. PLoS ONE 2019, 14, e0214509. [Google Scholar] [CrossRef]
- Shilpa, B.; Priya, P.B.; Pallavi, M.; Rao, P.J.M. Effect of Cold Plasma Treatment on Seed Quality Parameters under Cold Stress in Rice (Oryza sativa L.). J. Exp. Agric. Int. 2024, 46, 943–953. [Google Scholar] [CrossRef]
- Starič, P.; Mravlje, J.; Mozetič, M.; Zaplotnik, R.; Šetina Batič, B.; Junkar, I.; Vogel Mikuš, K. The influence of glow and afterglow cold plasma treatment on biochemistry, morphology, and physiology of wheat seeds. Int. J. Mol. Sci. 2022, 23, 7369. [Google Scholar] [CrossRef] [PubMed]
- Suriyasak, C.; Hatanaka, K.; Tanaka, H.; Okumura, T.; Yamashita, D.; Attri, P.; Koga, K.; Shiratani, M.; Hamaoka, N.; Ishibashi, Y. Alterations of DNA methylation caused by cold plasma treatment restore delayed germination of heat-stressed rice (Oryza sativa L.) seeds. ACS Agric. Sci. Technol. 2021, 1, 5–10. Available online: https://pubs.acs.org/doi/10.1021/acsagscitech.0c00070?goto=supporting-info (accessed on 5 October 2025). [CrossRef]
- Kocira, S.; Pérez-Pizá, M.C.; Bohata, A.; Bartos, P.; Szparaga, A. Cold Plasma as a Potential Activator of Plant Biostimulants. Sustainability 2022, 14, 495. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.d.D.; et al. Can cold plasma be used for boosting plant growth and plant protection in sustainable plant production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The effects of plasma on plant growth, development, and sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Sayahi, K.; Sari, A.H.; Hamidi, A.; Nowruzi, B.; Hassani, F. Evaluating the impact of Cold plasma on Seedling Growth properties, seed germination, and soybean antioxidant enzyme activity. BMC Biotechnol. 2024, 24, 93. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yin, Y.; Wang, J.; Wang, Z.; Ding, H.; Ma, R.; Jiao, Z. Research on the physio-biochemical mechanism of non-thermal plasma-regulated seed germination and early seedling development in Arabidopsis. Front. Plant Sci. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yin, Y.; Sun, H.; Wang, X.; Zhuang, J.; Wang, L.; Ma, R.; Jiao, Z. Regulation of cellular redox homeostasis in Arabidopsis thaliana seedling by atmospheric pressure cold plasma-generated reactive oxygen/nitrogen species. Ecotoxicol. Environ. Saf. 2022, 240, 113703. [Google Scholar] [CrossRef]
- Ucar, Y.; Ceylan, Z.; Durmus, M.; Tomar, O.; Cetinkaya, T. Application of cold plasma technology in the food industry and its combination with other emerging technologies. Trends Food Sci. Technol. 2021, 114, 355–371. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (bacterial wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Suwannarat, S.; Homkanchan, S.; Puttha, J.; Srisonphan, S. Nonthermal plasma engineering for seed disinfection and germination using streamer corona and dielectric barrier discharges. Results Eng. 2025, 26, 104884. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Ahmed, N.; Siow, K.S.; Wee, M.M.R.; Patra, A. A study to examine the ageing behaviour of cold plasma-treated agricultural seeds. Sci. Rep. 2023, 13, 1675. [Google Scholar] [CrossRef]
- Harish, M.S.; Yadav, S.; Jain, B.T. Effect of Cold Plasma Seed Treatment on Physiological and Biochemical Changes in Aged Mustard Seed Variety-RH0749. Epigenetics 2024, 7, 8. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Recek, N.; Zaplotnik, R.; Vesel, A.; Primc, G.; Gselman, P.; Mozetič, M.; Holc, M. Germination and growth of plasma-treated maize seeds planted in fields and exposed to realistic environmental conditions. Int. J. Mol. Sci. 2023, 24, 6868. [Google Scholar] [CrossRef] [PubMed]
- Barjasteh, A.; Lamichhane, P.; Dehghani, Z.; Kaushik, N.; Gupta, R.; Choi, E.H.; Kaushik, N.K. Recent progress of non-thermal atmospheric pressure plasma for seed germination and plant development: Current scenario and future landscape. J. Plant Growth Regul. 2023, 42, 5417–5432. [Google Scholar] [CrossRef]
- Ďurčányová, S.; Slováková, Ľ.; Klas, M.; Tomeková, J.; Ďurina, P.; Stupavská, M.; Kováčik, D.; Zahoranová, A.; Kováčik, D. Efficacy comparison of three atmospheric pressure plasma sources for soybean seed treatment: Plasma characteristics, seed properties, germination. Plasma Chem. Plasma Process. 2023, 43, 1863–1885. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Misra, N.N.; Schlüter, O.; Cullen, P.J. (Eds.) Cold Plasma in Food and Agriculture: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2016; Available online: https://cir.nii.ac.jp/crid/1971712334776425020 (accessed on 5 October 2025).
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination rate of plant seeds. Adv. Contact Angle Wettability Adhes. 2013, 1, 243–258. [Google Scholar] [CrossRef]
- Dobrzański, B.; Grundas, S.; Stępniewski, A. Introduction to Scientific Discipline Agrophysics—History and Research Objects. In Advances in Agrophysical Research; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).