Abstract
Black chokeberry is a valuable raw material for the food and pharmaceutical industries due to its bioactive compounds and unique health properties. Aronia berries are distinguished by a volatile compound profile that varies depending on the species, cultivation location, and processing method. The aim of this study was to compare changes in selected physical and chemical parameters and the volatile compound profile of Aronia melanocarpa (Michx.) Elliot was subjected to convective drying, freeze-drying, and freezing. To assess changes in chokeberry quality, measurements of the average fruit diameter, average fruit weight, color and pH were used. Total polyphenol content, total antioxidant activity and the profile of volatile compounds were examined. The color parameters of freeze-dried chokeberries retained a color profile most similar to that of fresh fruit. The total phenolic content of freeze-dried fruit (79.99 ± 0.32 mg GAE/g dw) was comparable to that of fresh fruit (79.67 ± 0.54 mg GAE/g dw), while both dried and frozen fruit showed a decrease. A significant increase in antioxidant activity was noted in freeze-dried fruit. The volatile compound profile of fresh fruit was dominated by alcohols (mainly ethanol, hexanol, and (Z)-3-Hexen-1-ol), while aldehydes (mainly benzaldehyde) dominated in processed fruit. It was found that the processing method has a significant impact on both the amount and composition of VOCs in chokeberries.
1. Introduction
The contemporary food industry seeks raw materials that combine health benefits with attractive sensory qualities. Such ingredients not only are appealing to consumers but also provide opportunities for product innovation. They are valued for their overall properties and features, such as exceptional aroma, intense natural pigments, and high antioxidant content [1,2,3]. Chokeberry (Aronia melanocarpa) is gaining recognition worldwide and is considered a promising functional raw material. Its market was valued at USD 1832.34 million in 2024 and is projected to reach USD 2312.56 million by 2033 [4]. Among chokeberry species, black chokeberry (Aronia melanocarpa (Michx.)) is the most widespread. This perennial shrub from the Rosaceae family originates in eastern North America and was introduced to Europe in the early 20th century [5]. Numerous cultivars exist, including Galicjanka, Hugin, Viking, Nero, Rubin, and Aron. Chokeberry shrubs are long-lived and require minimal care [6,7]. Unlike climacteric fruits, berries are harvested at full maturity since they do not continue to ripen. Fully ripe berries have a short shelf life due to high respiration rates, water loss, and susceptibility to decay. Aronia can be harvested at different stages, usually at maximum mass, which leads to variations such as higher anthocyanin content or reduced browning [8,9,10].
Although fresh fruits are rarely consumed because of their astringent taste, chokeberry is widely processed into juices, syrups, concentrates, purees, wines, and dietary supplements rich in anthocyanins [11]. Aronia contains antioxidants, notably polyphenols such as anthocyanins, procyanidins, phenolic acids, flavonols, and phenols. Moreover, it provides vitamin C and essential minerals, including magnesium, copper, iron, manganese, selenium, and zinc, which add to its antioxidant and anti-inflammatory potential [12,13,14].
The seasonal nature of Aronia berries necessitates preservation. Drying, freeze-drying, and freezing are commonly used techniques that help retain the fruit’s beneficial properties. Preservation affects the profile of volatile compounds (VOCs) crucial for the fruit’s aroma. VOCs stimulate human olfactory receptors and influence the sensory perception of fruits and their products. Processors must choose the appropriate method to maintain the desired aroma. Elevated temperatures during preservation may degrade VOCs, leading to unfavorable changes [15,16,17]. Dozens of volatile compounds were identified in fresh black chokeberries. The main compounds include alcohols, ketones, acids, aldehydes, terpenes, esters, and hydrocarbons. The most abundant compounds are 3-penten-2-one, 1-hexanol, and 2-hexen-1-ol [18]. The chokeberry VOC profile is further affected by cultivar, cultivation conditions, post-harvest handling, and processing methods, which complicates comparisons across studies [19]. This highlights the importance of targeted research on a single variety using standardized preservation techniques.
The novelty of this study lies in systematically comparing the effects of convective drying, freeze-drying, and freezing on both physicochemical parameters and the VOC profile of fresh chokeberry (Aronia melanocarpa Elliot). By focusing on fruits of the same variety and controlled processing conditions, we provide new insights into how preservation techniques shape both quality attributes and aroma-related compounds.
2. Materials and Methods
2.1. Sample Preparation
Fruits of Aronia melanocarpa (Michx.) Elliot were obtained from a local plantation (Halinów, Mazowieckie, Poland). Ripe chokeberries were harvested in early September. The fruits were firm, dark navy blue, practically black, and shiny. Some of the leaves on the chokeberries were discolored yellow-orange (Figure 1), and the chokeberries easily detached from the stems. Immediately after harvesting, the berries were transported (in containers preventing mechanical damage) to the laboratory and processed. The fresh fruits were subjected to freezing, convection drying, and freeze-drying. Fruits for freezing were vacuum-packed, placed in a quick-freezer for one hour, and then transferred to a freezer (−20 °C) for 10 days.

Figure 1.
Aronia bush with discolored leaves.
Convection drying of chokeberry fruits was done in a laboratory dryer (BINDER, Tuttlingen, Germany) for 24 h at 65 °C. Fruits for freeze-drying were spread on a tray and placed in the freezer (−25 °C) for 24 h. After this time, the tray with the frozen fruits was inserted into the freeze-dryer chamber (Christ Alpha 2–4 LSCplus, Osterode am Harz, Germany). Freeze-drying was carried out for 48 h under the following conditions: shelf temperature 2.5 °C, vacuum 0.1 mbar, condensate −83.7 °C. Before research, the frozen berries were thawed at room temperature for 30 min.
2.2. Physical Characteristics
Measurements of the average diameter and average weight of chokeberry fruits were carried out on 20 random fruits. The weight of aronia berries was measured by a laboratory weighing balance (RADWAG PS 6000/C/1, Radom, Poland). Fruit size was measured with a vernier caliper with a screw (Gimex MAUa DIN 862, Kaiserslautern, Germany). Dry matter of samples was determined using the oven method (BINDER FP 115 Classic Line, Tuttlingen, Germany) at 105 °C for 20 h.
Color measurements were made in 7 replicates for each sample and carried out using the reflection method with a Konica Minolta Chroma Meter CR-400 (Tokyo, Japan) colorimeter, working in CIELab color space (L*a*b*). A D65 light source, an 8 mm measuring head, and a standard 2° observer were used. Before measurements, the colorimeter was calibrated on a white standard with parameters L* = 98.45, a* = −0.10, and b* = −0.13.
2.3. pH Measurement
Measurements were made with a Testo 205 handheld pH meter (Testo Ltd., Alton, UK), equipped with a penetrating electrode and temperature probe, with temperature compensation. The pH meter was calibrated with two buffers of pH 4.01 and 7.00. 2.5 g of fruit was crushed and mixed with purified water (Hydrolab, HPL 10sp, Straszyn, Poland) before measurement. For fresh and frozen fruit, the amount of water was 0.5 mL, while 1 mL of water was added to freeze-dried and dried fruit. The measurement was made for each sample in triplicate.
2.4. Total Polyphenol Content (TPC)
The TPC of chokeberry fruit was determined using the Folin–Ciocalteu method [20]. First, extraction was performed using 1 g of ground fresh and frozen fruit, 0.5 g of ground dried fruit, and 0.3 g of ground freeze-dried fruit, respectively. 10 mL of 80% methanol in water (v/v) was added to weighed chokeberry fruit. Samples prepared for extraction were placed in a container with ice and water and sonicated for 40 min in a dark place. After 40 min, the samples were centrifuged (3755× g) in a centrifuge (MPW-251, MED. INSTRUMENTS, Warszawa, Poland). The extracts were transferred to screw-capped tubes, and the remaining sediment was re-extracted. Both extracts were combined for further analysis. Next, 2.25 mL of purified water and 0.5 mL of Folin–Ciocalteu’s reagent (Sigma Aldrich Inc., Burlington, MA, USA) were added to 0.25 mL of the extract. The resulting solution was left in the dark for 4 min. Then, 1.5 mL of saturated sodium carbonate solution (at 20 °C: 21.5 g sodium carbonate decahydrate/100 mL water) (Sigma Aldrich Inc., USA) was added. The samples were placed in a water bath (40 °C) for 30 min. Absorbance was measured using a UV-160A spectrophotometer (Shimadzu Cor, Kyoto, Japan) at a wavelength of 765 nm against 80% aqueous methanol. The extracts were analyzed in triplicate. Gallic acid solutions (Sigma Aldrich Inc., USA) were prepared at seven concentrations to generate a calibration curve. The calibration curve was performed three times.
2.5. DPPH Analysis
The analysis of chokeberry fruit was determined using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical solution (Sigma Aldrich Inc., USA) [20]. Methanolic extracts of chokeberry fruit were prepared as in Section 2.4. The reaction solution was prepared by mixing 0.1 mL of chokeberry fruit extract with 2.9 mL of methanolic (Honeywell International Inc., Morristown, NJ, USA) DPPH solution (0.25 mM). The solution was placed in the dark room for 30 min at room temperature. Absorbance was measured against 0.1 mL of fruit extract supplemented with methanol to a volume of 3 mL, at 517 nm. Extracts from fresh, frozen, dried and freeze-dried fruits were analyzed in 3 replicates.
The antioxidant activity of fruits was calculated as the percentage of DPPH radicals quenched according to Formula (1):
% inhibition = [(ADPPH − Ae)/ADPPH)] × 100
- ADPPH—absorbance of the prepared solution of the DPPH radical
- Ae—Absorbance of the extract after reaction with DPPH
2.6. Volatile Compound Profile
The volatile compound profile was analyzed using the HERACLES II electronic nose (Alpha M.O.S., Toulouse, France) with headspace [21]. Fresh, frozen, dried, and freeze-dried fruits were analyzed, each in 5 replicates. Crushed samples of 2 ± 0.05 g were weighed into 20 mL vials and mixed with 0.9% (w/w) sodium chloride solution (Sigma Aldrich Inc., St. Louis, USA). The vials were closed with aluminum caps and silicone septa. Samples were incubated at 60 °C for 15 min, and then 3500 μL of the headspace was removed for analysis. The injecttion rate was 125 mL/s. Analytes were concentrated on a Tenax-filled trap and then, after being divided in half, simultaneously analyzed on two capillary columns of different polarity (a non-polar MXT 5 and a medium-polar MXT 1701): with a diameter of 0.18 mm, a film thickness of 0.4 µm, and a length of 10 m. Results were recorded using a computer with AlphaSoft V14.0 software (Alpha M.O.S., Toulouse, France). The injection temperature was 200 °C. The carrier gas was hydrogen. Analysis was performed using a temperature program: initially at 40 °C (hold for 5 s), then a temperature increase of 4 °C/s until reaching 270 °C (hold for 30 s). The flame ionization detector (FID) temperature was 270 °C. Calibration was performed using the headspace method using a standard mixture of alkanes (C6 to C16 (Restek Inc., Edmond, OK, USA)). Volatile compound peaks, whose presence was confirmed on both columns simultaneously, were identified based on the Kovats retention indices (RI) for both columns. Additionally, for the recognized analytes with the highest peak areas, single standards were used to confirm retention times (ethanol, 2,3-pentanedione, hexanal, hexanol and benzaldehyde (Sigma Aldrich Inc., Burlington, NJ, USA)). The analysis utilized the original AroChemBase database from ALPHA M.O.S. (44,000 compounds and sensory descriptors).
2.7. Statistical Analysis
Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA) was used to assess the significance of differences in study results using one-way analysis of variance (ANOVA), and Fisher’s LSD test was applied at the α = 0.05 level of significance.
PCA principal component analysis (at α = 0.05) of the volatile chromatographic profiles of all chokeberry groups was used to assess the variation in volatile compound profiles. PCA was performed using the original AlfaSoft software. Visualization of the results is presented in the form of graphs for the first two components (PC1 versus PC2).
3. Results and Discussion
3.1. Physical Properties
Processing significantly affected the physical characteristics of chokeberry fruits, particularly their size and weight. The water content of plant-based raw materials is influenced by factors such as variety, ripeness, geographical origin, and cultivation conditions and extrinsic factors: handling, including harvesting time, storage conditions, and processing techniques. In order to ensure microbiological safety and adequate shelf life, particularly for products not stored under deep-freezing conditions, it is generally recommended that the final moisture content after drying remains below 10% [16,22]. The dry matter content of chokeberries ranges from 15.6% to 17.5% [18]. In our case, the dry matter content of dried and freeze-dried chokeberries was 19.7% and 17.3%, respectively. Water loss leads to changes in fruit appearance because of turgor loss and cell wall damage. The average diameter of fresh chokeberries was 7.8 ± 0.7 mm, which is consistent with literature values reporting a typical size range between 6 and 13 mm [11]. As shown in Table 1, statistically significant differences (p < 0.05) were observed between dried and freeze-dried fruits and between fresh and dried and freeze-dried fruits, as well as between frozen and dried fruits.

Table 1.
Average size and weight of chokeberry fruits.
The smallest diameter was recorded for dried fruits (5.5 ± 0.7 mm), indicating considerable shrinkage during convection drying. Freeze-dried fruits had an average size of 6.7 ± 0.8 mm, which, although smaller than fresh samples, was significantly larger than their hot-air-dried counterparts. These differences reflect the structural effects of the drying method applied. According to Bogusz et al. [23], fruit shrinkage during freeze-drying is typically minimal (5–15%) due to water removal via sublimation, which helps maintain the integrity of the fruit’s cellular matrix. Water that evaporates under vacuum conditions creates additional pores in the raw material, allowing moisture to escape more easily and with less structural damage—more pores result in fewer large disruptions to the cell walls [24]. Convection drying involves liquid water evaporation under elevated temperatures, leading to collapse of cell walls and shrinkage of up to 80% [25]. Frozen fruits (7.4 ± 0.7 mm) were the most similar in size to fresh ones, with no statistically significant difference. This confirms that properly controlled freezing parameters limit the formation of large ice crystals, thereby minimizing cellular damage and maintaining the original fruit structure. The freeze-drying process preserves the fruit’s structure by removing water through sublimation [26]. Moreover, the water loss during frozen storage is relatively low, which allows the fruit to retain many of its physical properties after thawing.
The highest average weight was recorded for fresh fruits (1.04 g), followed by frozen samples (0.81 g). Both drying and freeze-drying led to a substantial reduction in fruit mass (0.25 g), which reflects the extensive water loss during these processes. The markedly greater decrease compared to freezing is a direct result of dehydration, where most of the cellular water is removed. Despite yielding similar final weights, the two dehydration methods differed in their structural effects, with freeze-dried fruits retaining a more intact appearance compared to hot-air-dried samples. A visual comparison of fruits subjected to different treatments is presented in Figure 2.

Figure 2.
Chokeberry fruits—fresh and obtained through different processing methods ((A)—fresh, (B)—frozen, (C)—freeze-dried, (D)—dried).
3.2. Color Analysis
Color is one of the key quality indicators in fruit and plays an essential role in consumer perception. In chokeberry, as in other dark-skinned berries, the external appearance is shaped by anthocyanins accumulated in the skin. These include glycosides of delphinidin, petunidin, malvidin, and their methoxylated derivatives, which contribute to the dark, intense coloration typical of the species [27,28]. A waxy layer on the fruit surface also influences color perception. Cuticular waxes, composed of long-chain aliphatic compounds and triterpenoids, form a whitish coating that modifies how light reflects off the skin. This wax layer gives the fruit a slightly lighter appearance and improves its attractiveness. Studies on blueberries have shown that this effect is related to β-diketones, which are responsible for forming this surface layer [29]. Although the wax layer effectively protects against water loss and pathogens, it is sensitive to mechanical damage and deteriorates under temperature and humidity. Wu et al. [30] presented data showing that storage conditions cause structural changes in skin waxes, leading to reduced integrity and increased permeability, which in turn accelerates water loss and deterioration in quality.
Changes in brightness were reflected in the L* parameter, which showed the highest variation among the analyzed color components (Table 2). The highest L* values were observed in fresh fruits (24.64) and freeze-dried fruits (24.44), indicating good surface structure retention and light reflection. Dried fruits had the lowest L* value (18.56), which reflects darkening of the fruit skin. This effect may be related to pigment concentration in the peel and partial collapse of tissue structure during drying. A decrease in brightness after freezing was also visible and may be caused by water loss from vacuoles due to ice crystal formation, which compresses cell walls and compacts pigments [30,31].

Table 2.
Color parameters (L*, a*, b*) of chokeberry fruits subjected to various treatments.
The degradation of the wax layer during processing contributed to the observed changes in brightness. A temperature near 4 °C can alter the wax structure from platelets to granules, while high humidity leads to degradation of surface terpenes and alkaloids, increasing skin permeability and accelerating water loss. In chokeberry, the natural waxy coating is visible on fresh fruit and contributes to how the skin refracts light. Its presence has also been discussed by Oszmiański and Lachowicz [32], who highlighted its role in maintaining color stability.
The a* and b* parameters remained relatively stable across treatments. All fruits showed positive a* values, indicating the presence of red tones, although the lowest value was found in dried fruits. These results suggest a low contribution of red pigment components. The b* values were also low, pointing to the dominance of blue hues. Freeze-dried fruit differed significantly from the other samples and showed L* and a* values closest to fresh fruit, while the b* values were lower and significantly different. The higher a* values observed in frozen and freeze-dried fruits compared to fresh samples may be explained by cell structure disruption and water loss during these processes, which can concentrate anthocyanins and enhance red color expression, while the low-temperature conditions help to limit pigment degradation.
Chokeberry color was resistant to change. The fruit maintained its appearance despite processing, which was confirmed by Samoticha et al. [33], who found that freeze-dried berries retained their color profile better than hot-air dried samples. Similar observations were made by Ceylan et al. [34], who reported that freeze-dried fruits retained natural color better, while drying led to significant degradation. Oszmiański and Lachowicz [32] confirmed that freeze-dried chokeberry had higher and more stable L*, a*, and b* values than dried fruit and contained more anthocyanins. Their study characterized fresh fruits with low L* and b* values and moderate a* values, typical for dark-colored berries.
The observed differences in color parameters may also be related to the natural variability of raw material, including maturity, cultivar, and growing conditions. A lower a* value and a higher b* value suggest a lower anthocyanin concentration and a relatively higher share of other pigments, such as carotenoids. Freeze-drying is the most effective method of preserving chokeberries’ color and pigment integrity [35,36]. Also it is worth noticing that whole berries maintain their color, while the juice (or other products like pulp) may be more vulnerable to changes [37].
3.3. pH Determination
When comparing the pH values of fresh (3.61 ± 0.04), frozen (3.56 ± 0.01), dried (3.57 ± 0.01), and freeze-dried (3.60 ± 0.01) chokeberry fruits, no significant effect of processing on acidity was observed. These results suggest that the applied treatments did not substantially alter the acid-base balance of the fruit. King et al. [9], in their study on the impact of freezing on chokeberry quality, reported a pH of 3.1 for fresh fruit. Their findings also confirmed that freezing had only a limited effect on pH. The acidity of chokeberries typically falls within the range of 3.1 to 3.7, although this can vary depending on fruit ripeness and cultivar differences [38]. The naturally low pH of chokeberry contributes to its microbiological stability, making it less prone to spoilage. This inherent acidity also supports the use of mild preservation techniques such as pasteurization. Moreover, the retention of low pH after processing makes chokeberry a promising candidate for the development of natural fruit-based preservatives. Such formulations could extend shelf life and enhance the microbial safety of other food products [39].
3.4. Determination of TPC
Polyphenols and flavonoids are compounds sensitive to environmental conditions. When plant material is dried at elevated temperatures, partial degradation of these valuable constituents may occur. Choosing a preservation method that minimizes thermal damage is essential for maintaining the quality and functional properties of the plant material [40,41].
Figure 3 presents the results of TPC in fresh and processed chokeberry fruits. Per 1 g of dry weight, fresh (79.67 mg) and freeze-dried (79.99 mg) fruits had the highest polyphenol content, so freeze-dried method did not change the total polyphenol content.
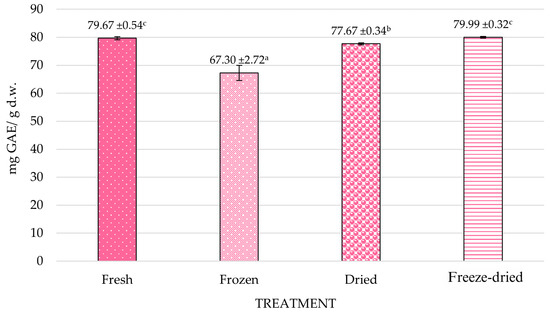
Figure 3.
Effect of processing methods on the total phenolic content (TPC) of chokeberry fruits. a–c—homogeneous groups within columns that are statistically significantly different (p < 0.05); error bars represent standard deviation.
The highest TPC was recorded in freeze-dried fruits (79.99 ± 0.32 mg GAE/g d.w.), followed by fresh (79.67 ± 0.54) and dried samples (77.67 ± 0.34) (Figure 3). The TPC values of freeze-dried and fresh fruits did not differ significantly, indicating that non-thermal drying methods can effectively retain phenolic compounds. This observation is consistent with findings from Nawawi et al. [35], who demonstrated that freeze-drying minimizes degradation due to low processing temperature and oxygen exclusion [36]. Similarly, Uribe et al. [42] reported that peppermint dried under less intense thermal conditions retained a higher flavonoid content. They also showed that drying at 50 °C preserved more flavonoids compared to drying at 90 °C, confirming the importance of gentle drying in maintaining the bioactive potential of plant-based materials. This agrees with our results, where drying at increased temperatures caused loss of polyphenols; however, it did not occur at such a high rate as freezing.
Frozen fruits exhibited a significantly lower TPC (67.30 ± 2.72). Although freezing is generally considered a protective method, this reduction may be attributed to oxidative degradation during thawing and cell membrane rupture caused by ice crystal formation, which exposes polyphenols to oxidative enzymes [43,44]. Furthermore, the variability in frozen samples (as reflected in the higher standard deviation) suggests inconsistencies in structural damage and enzyme interaction at the tissue level.
This suggests that heat dehydration may concentrate phenolics by removing water, while lyophilization—conducted under low temperatures and vacuum—efficiently preserves thermolabile phenolic compounds [45].
In other research blueberry fruit subjected to convection drying and freeze-drying had significantly lower total polyphenol content than fresh and frozen fruit. On a dry weight basis, the polyphenol content of freeze-dried fruit was lower by an average of 2.5% compared to fresh chokeberries. For dried fruit, the loss of polyphenols was 15.9% [46,47]. Polyphenols found in berries are mainly anthocyanins, which are very perishable compounds. During the freeze-drying process, the low temperature and gradual evaporation of water from the frozen material allow for a well-preserved structure and relatively low losses of polyphenolic compounds. During convection drying, the action of the heated air stream causes significant shrinkage of the dried material and high losses of thermolabile components, which include polyphenols [46,47]. Using a shelf-life extension method delays the loss of antioxidant compounds compared to a control sample, as demonstrated by Luo et al. [48] in a study on stored strawberries using edible coatings.
3.5. Antioxidant Activity
Phenolic compounds, including flavonoids, are major contributors to the antioxidant potential of plant raw material. Together with other secondary metabolites, such as terpenoids and phenolic acids, they form a complex system responsible for neutralizing reactive oxygen species and protecting cells from oxidative stress. This synergy between diverse compound groups reflects the plant’s adaptive mechanisms to environmental stress and plays a central role in its biological activity [16,49].
Chokeberry exhibits high antioxidant activity, which is correlated mainly with its high content of phenolic compounds. Figure 4 shows the high antioxidant ability of components present in chokeberry fruit to neutralize the free radical DPPH. In our study, fresh fruits’ DPPH radical scavenging capacity reached 90.64%, which exceeded previous reports of aronia or aronia products’ antioxidant activity. Earlier studies indicated a range of ~55–85% scavenging activity, depending on extraction method and product type [6,50,51,52]. Freeze-dried chokeberries exhibited an even higher antioxidant capacity (approximately 4.6%) than the fresh material. Thi and Hwang [53] also observed a similar trend, with freeze-dried berries showing higher DPPH and FRAP (Ferric Reducing Antioxidant Power) results compared to fresh or hot-air-dried samples.
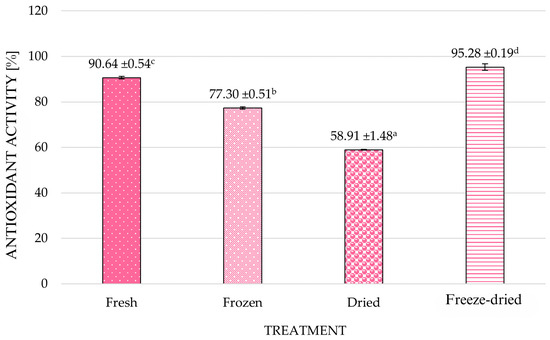
Figure 4.
Antioxidant activity of chokeberry fruits as influenced by different treatments. a–d—homogeneous groups within columns that are statistically significantly different (p < 0.05); error bars represent standard deviation.
Ochmian et al. [54] also found significantly higher antioxidant activity of freeze-dried blueberry fruit than fresh fruit. Raw chokeberries are naturally rich in polyphenols and ascorbic acid, which determine their antioxidant potential. The higher values observed after freeze-drying may be attributed not only to the protection of thermolabile compounds such as vitamin C and anthocyanins, but also to structural disruption and water removal during drying, which enhance the extractability and concentration of these bioactives in analytical assays. Freeze-drying is conducted at low temperatures and under vacuum, minimizing degradation of bioactive molecules [45] while simultaneously improving their accessibility. Recent studies have shown that pre-treatment with ultrasound or protective agents before drying may further improve the retention of phenolic compounds and antioxidant potential in berry products [55].
Frozen chokeberries had significantly lower antioxidant activity (p < 0.05) than fresh and freeze-dried fruits; their ability to neutralize the free DPPH radical was 13.34 percentage points lower than in fresh fruits. Frozen fruits also had lower polyphenol content than other fruits. Dried chokeberries, on the other hand, had significantly lower antioxidant activity than fresh fruit, despite similar polyphenol content. Their ability to neutralize the DPPH free radical was 31.73 percentage points lower. This can be explained by thermal degradation of the most active phenolic compounds and ascorbic acid, along with polymerization and Maillard reaction products, which still react with the Folin–Ciocalteu reagent but exhibit lower radical scavenging efficiency. The study by Li et al. [56] found that the antioxidant activity of sea buckthorn depends on its balanced composition (polyphenols, flavonoids). Changes in the content of individual compounds, which can be caused by drying temperature, result in significant changes in antioxidant activity. Hassimotto et al. [57] also found no relationship between total polyphenol content and antioxidant activity. They suggest that antioxidant activity results from combining different compounds that exhibit synergistic and antagonistic effects. As a result of freezing and thawing of fruits, new compounds with different antioxidant properties may be formed. In particular, increased enzymatic activity during thawing can degrade polyphenols. Also, Nthabiseng et al. [58] found a reduction in the antioxidant activity of marula fruit during frozen storage.
3.6. VOC Profile
Figure 5 shows the contribution of the main groups of compounds to the VOC profiles of chokeberry fruit. Alcohols contributed the most to the VOC profile of fresh chokeberry fruit (67.56%). On the other hand, aldehydes had the largest share in shaping the VOC profile of processed chokeberry fruit, i.e., frozen (60.33%), dried (80.90%), and freeze-dried (88.22%). Ketones and other compounds contributed minimally to the profile of volatile compounds in all chokeberry fruits studied. Depending on the processing method used, the number of identified VOC ranged from 24 (in frozen and freeze-dried samples) to 27 (in fresh and over-dried samples). The most significant changes occurred during convection drying and freeze-drying in the group of aldehydes and alcohols. Recent research confirms that freeze-drying, while optimal for preserving bioactive compounds and colour [59], may lead to selective loss of highly volatile aroma compounds.
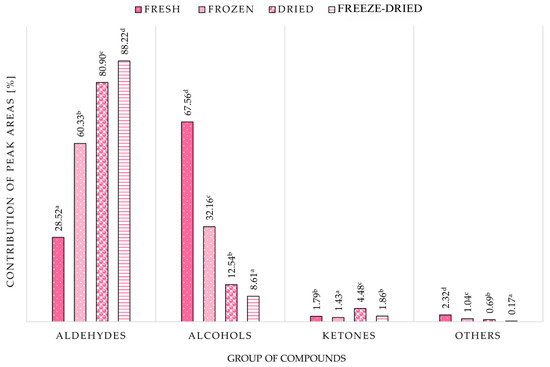
Figure 5.
Volatile compound group contributions in chokeberries under various treatments. a–d—homogeneous groups within columns that are statistically significantly different (p < 0.05).
A summary of the identified compounds in chokeberry fruit is presented in Table 3. The VOC profile of fresh fruit was dominated by ethanol, which accounted for almost 40% of all compounds and was significantly higher than that of processed fruit. Similarly, Qian et al. [60] found that ethanol had the highest concentration among all alcohols in the blackberry VOC profiles analysed.

Table 3.
VOC profiles of chokeberry fruits subjected to different processing methods.
Frozen and freeze-dried fruits and frozen and dried chokeberries did not differ significantly in ethanol content. A significant difference was found between dried and freeze-dried fruits, which had the lowest ethanol content (7.14%). In fresh fruit, sugars are converted to ethanol by fermentation Via alcohol dehydrogenase. Also, the natural microflora of fruit, including fermenting bacteria and yeast, can contribute to converting sugars into ethanol [61]. According to de Silva Nunes et al. [62], the ethanol content of the fruit flesh can be used as a marker of the fermentation processes taking place in the fruit to assess its degree of ripeness. Alcohol dehydrogenase, essential for ethanol synthesis, is sensitive to freezing, inhibiting its activity [63]. The lower proportion of ethanol in the volatile compound profile of frozen and freeze-dried chokeberries may therefore be correlated to a reduction in the activity of the enzymes responsible for the fermentation of sugars to ethanol.
Conventional drying methods using high temperatures allow ethanol to be removed from products. However, the increased temperature used in the process also causes degradation and evaporation of beneficial components. According to Sujka et al. [64], freeze-drying can be a way of getting rid of ethanol from the product while retaining other aromatic components. The process uses a temperature sufficient for ethanol evaporation, while at the same time being lower than conventional drying, without adversely affecting other VOCs. 1-hexanol was the second compound that shapes the volatile compound profile of fresh chokeberries. Their results show that hexanol content was over 15%, which could affect the fruity aroma of chokeberries. D’Agostino et al. [65] analysed the VOC profiles of blackberries and found that 1-hexanol was one of the main compounds, contributing up to 16.39%. Other chokeberry fruits were characterised by a significantly lower proportion of this compound. No differences were found between dried and freeze-dried fruit. Leon-Sanchez et al. [66] found that a decrease in the activity of the enzyme alcohol dehydrogenase (ADH) induced by temperature changes was responsible for changes in hexanol content in tomato fruit. Alcohol dehydrogenase is crucial in the metabolism of alcohols in fruit. This enzyme influences the profile of volatile compounds through the reciprocal conversion of the resulting aldehydic and alcoholic volatiles. Abouelenein et al. [67] recognised (Z)-3-Hexen-1-ol in strawberry fruit, however, at a much lower level of 0.16%. In studies of fresh chokeberries, the proportion of this alcohol was above 11%, then increased significantly in frozen chokeberries to almost 17% and decreased significantly in dried and freeze-dried chokeberries, as did hexanol. Both alcohols have similar boiling points (above 150 °C). Research [68] showed a lower contribution to the volatile profile of (Z)-3-Hexen-1-ol compounds than hexanol. It is important to note that VOC profiles vary depending on environmental conditions and cultivation location, and that postharvest treatments strongly influence composition. This relationship is demonstrated by Butorová et al. [19] in the study of different chokeberries as well as Tabaszewska et al. [69].
The most abundant group of compounds was aldehydes, as reported by previous research [70]. Aldehydes can enhance fruit flavour by imparting fruity, vegetal notes, but can also contribute biting and pungent characteristics [71]. Ten aldehydes were recognised, i.e., acetaldehyde, propanal, 2-methylpropanal, butanal, 2-butenal, 3-methylbutanal, 2-pentenal, hexanal, (E)-2-hexenal, and benzaldehyde.
The highest proportion of aldehydes was recorded in freeze-dried fruit (88.22%), and the lowest in fresh fruit (28.52%). This result was significantly influenced by benzaldehyde. Benzaldehyde in chokeberry fruit is mainly formed by the breakdown of amygdalin, i.e., a cyanogenic glycoside present in the seeds and pulp of the fruit. The enzymatic breakdown of amygdalin releases benzaldehyde-cyanohydrin, followed by benzaldehyde and hydrocyanic acid [19,38,68]. During freezing of chokeberries, cell breakdown and the release of enzymes (e.g., β-glucosidase) occur, which promotes the breakdown of amygdalin to benzaldehyde. In contrast, lighter water-soluble compounds are evaporated during thawing, which relatively increases the proportion of benzaldehyde in the VOC profile. During drying, the enzymes can continue to function with reduced water content, leading to further degradation of amygdalin. Furthermore, drying can concentrate volatile compounds, and some, such as aromatic aldehydes, may be more stable than ester or phenolic ones. The highest proportion of benzaldehyde for freeze-dried fruit may result from different mechanisms. Residual enzymatic activity can persist during freezing, enabling further amigdaline hydrolysis. In addition, benzaldehyde is relatively stable and less volatile in a vacuum and at low temperature, while other volatile substances (e.g., esters and alcohols) are more susceptible to loss or degradation. However, removal of almost all water causes a relative enrichment effect. More volatile or thermolabile compounds are lost to a greater extent, increasing the percentage of benzaldehyde in the chromatographic profile. In addition, the structural breakdown of plant tissues during sublimation further increases the release of bound precursors. Similar arrangements were reported for strawberries, in which frozen and freeze-dried fruit contained a higher relative amount of aldehyde compared to fresh or dried heat fruit [67].
The aldehyde that also contributed significantly to the VOC profile of fresh and frozen chokeberries was hexanal. In the case of frozen chokeberry fruit, it was the main compound shaping the volatile compound profile (approximately 28%). A significantly lower proportion of hexanal was characterised in dried and freeze-dried fruit. In fresh fruit, the proportion of hexanal was about 9%. Fatty acids, linoleic and linolenic, are converted to volatile aldehydes such as hexanal by oxidative degradation catalysed by lipoxygenase (LOX) or hydrogen peroxide lyase (HPL). Hexanal is then converted to alcohol by dehydrogenase [72]. Both of the above-mentioned fatty acids are present in chokeberry fruit, and linoleic acid is the main fatty acid found in chokeberries [38]. Low temperature affects the inhibition of the ADH enzyme, which prevents the reduction of hexanal. Fatty acids may also have been oxidised before freezing, and the very low temperature inhibited further transformation. This is why the proportion of hexanal can be so high in frozen chokeberry fruit. Significant losses of hexanal can be seen with processes using high temperatures; the higher the temperature, the lower the proportion of this compound in the chromatographic profile. Drying and freeze-drying may have inhibited the oxidation of said fatty acids by reducing the water content of the fruit, resulting in a lower aldehyde content. Hexanal is one of the main compounds responsible for strawberries’ green, unripe aroma [73]. It also contributes significantly to the aroma of cranberries [74].
Fresh chokeberry fruit was also distinguished by a significantly higher proportion of acetaldehyde, 4.7%. A low proportion of this compound characterised fruits subjected to drying, freeze-drying, and freezing. Acetaldehyde is a natural aromatic component of most fruits and is formed due to fruit metabolism during ripening. It is the last important compound of the alcoholic fermentation chain reaction, as it is reduced to ethanol. However, a certain amount of acetaldehyde is not converted and remains in the raw material [71]. It is a very volatile compound with a low boiling point, which makes it easy to evaporate. In addition, it is a compound that easily reacts with other fruit components. Acetaldehyde in fruit is obtained from pyruvate with the involvement of pyruvate decarboxylase. By oxidation, it is converted to acetic acid, while by reduction, acetaldehyde is converted to ethanol [75]. Given that the proportion of these compounds did not increase during processing, it can be concluded that the reduced proportion of acetaldehyde is not the result of the chemical reactions that occurred. Ubeda et al. [76] found that adding pectolytic enzymes to the fruit promotes the release of acetaldehyde and increases its content. Therefore, the low acetaldehyde values obtained for processed fruit could be correlated with the inhibition of enzymatic reactions in the fruit or the loss of acetaldehyde due to the application of low and high temperatures.
The proportion of propanal in the VOC profile differed significantly between the selected processing methods. Freeze-dried fruit had the lowest proportion of propanal (0.37%). There were no statistically significant differences for frozen fruit compared to fresh fruit, which had the highest proportion of this compound, i.e., around 1.5%. Fruit that had been exposed to high temperatures was characterised by a reduced propanal content. This aldehyde has a low boiling point (48.8 °C), so it may have evaporated together with water. Wang et al. [77] state that propanal is one of the aldehydes that gives an unpleasant odour to black chokeberry fruit. A low proportion of butanal was recorded in fresh fruit (0.56%), while its presence decreased significantly in other groups. Butanal, also known as butyraldehyde, gives chokeberry fruit an aroma described as chocolatey and buttery. It may be a by-product of carbohydrate and lipid metabolism in plants or produced by microbial metabolism [78]. The temperature used in the processing operations likely reduced the proportion of butanal. 2-Pentenal was one of the main compounds shaping the chromatographic profile of dried chokeberry fruit (30.54%) and was significantly higher than in the profiles of other chokeberry fruit. 2-Pentenal imparts an apple-like aroma to the fruit. Given that the proportion of 2-pentenal is many times higher only in dried fruit, it can be concluded that this is related to the process temperature. The dried chokeberries were subjected to a much higher temperature than the freeze-dried chokeberries, and changes only occurred at 65 °C. One reason could be the oxidation of polyunsaturated fatty acids present in the chokeberry, leading to the formation of aldehydes, which include 2-pentenal. Antoszkiewicz et al. [79] state that drying contributes to oxidation, which synthesizes aldehydes. The remaining aldehydes, i.e., 2-methylpropanal, 2-butenal, 3-methylbutanal, (E)-2 hexenal, summed up to less than 1% in the chromatographic profile for fresh chokeberry 0.76%, frozen 0.53%, dried 0.87% and freeze-dried 0.48%.
The group of ketones and other compounds, such as esters, sulphur compounds, or terpenes, accounted for a minor share of the chokeberry volatile compound profile, with shares of 1.79% and 2.32% for ketones and other compounds, respectively, in fresh chokeberry. Though a minor group, ketones increased in dried fruits (4.48%), and pentane-2-one and 2,3-pentanedione were identified as dominant representatives. These are commonly formed Via Maillard reactions, Strecker degradation of amino acids, or thermal oxidation of lipids. The presence of these compounds has been associated with buttery and creamy notes, and their increase in dried samples corresponds to the elevated temperatures used in air drying [80].
The group labelled “others” (Figure 5), which included esters, terpenes, and sulfur compounds, contributed minimally across all treatments. The highest proportion was recorded in fresh samples (2.32%), with a steep decline in freeze-dried fruits (0.17%). Esters such as ethyl acetate and methyl isobutyrate are highly volatile and thermolabile; they are known to degrade during thermal processing or be lost under vacuum conditions [81]. Terpenes like linalool and p-cymenene decreased after drying, consistent with their instability in high-temperature or low-pressure environments [82].
The chromatographic profiles of all the chokeberries tested were subjected to principal component analysis (PCA) (Figure 6) at a significance level of α = 0.05 [23]. The results of the PCA are shown in the graph of the first two components, PC1 and PC2. Principal component analysis showed significant differences in the VOC profiles of the tested fruits. The first component accounted for 72.2% of the variance, while the second accounted for 15.6%. The distribution in the graph of the different chokeberry groups tested reflects the differences in volatile compound profiles. The first principal component (PC1) explains a significant part of the variance (72.2%) and is mainly influenced by benzaldehyde and ethanol. Benzaldehyde shows a significant increase in relative percentage in freeze-dried samples (84.02%). Conversely, the remaining more volatile compounds decrease in their contribution, primarily ethanol, which is reflected in the furthest position of the freeze-dried fruit group in the negative PC1 range. The second principal component (PC2) accounts for 15.6% of the variance. Compounds with a lower profile contribution, such as (E)-2-hexenal, 2-methylfuran, and 1-propanethiol, influence the differentiation of samples from PC2. The contribution of (E)-2-hexenal to the profile of fresh samples is 0.48% and decreases significantly in freeze-dried samples (0.08%). Frozen fruit had the most similar chromatographic profile to fresh fruit. Both groups were distributed on the side of positive PC1 values. The profiles of freeze-dried and fresh chokeberries, and freeze-dried and frozen chokeberries, showed the most significant variation. Freeze-drying caused a significant increase in the percentage of benzaldehyde and a decrease in ethanol. Freeze-dried samples are located far from the other groups, which is due to the lower levels of other compounds in the freeze-dried samples. The variation in profiles can be expressed numerically by distances (Table 4), i.e., the distance between the centers of gravity of the tested groups in the PC1/PC2 plot.
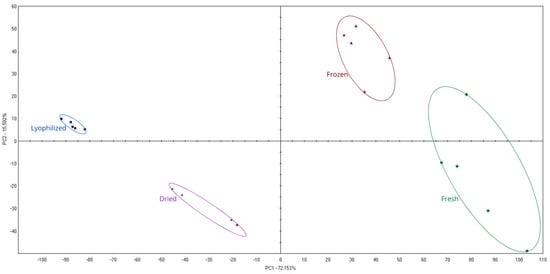
Figure 6.
PCA of chromatographic profiles of different treatment chokeberry fruit.

Table 4.
Distances for PCA of chromatographic profiles of fresh, frozen, convection-dried, and freeze-dried chokeberries.
Halagarda et al. [83], when assessing the effect of fixation method on apple fruit quality using principal component analysis, found that freeze-dried and dried fruit were the most different and varied compared to fresh fruit. In contrast, frozen fruit showed the least differences from fresh and freeze-dried fruit. This study similarly indicates that freeze-dried fruits show the greatest differences in the profile of volatile compounds compared to other fruit groups, mainly due to the presence of benzaldehyde, which dominates in freeze-dried chokeberries.
4. Conclusions
Black chokeberry is a valuable raw material for the food industry due to its bioactive compounds. Processing methods such as freezing, drying, and freeze-drying affect fruit quality parameters, the content of bioactive compounds, and the composition of volatile organic compounds in Aronia melanocarpa (Michx.) Elliot fruit. Color parameters of freeze-dried chokeberry fruits retained a color profile most similar to fresh fruit. Total phenolic content in freeze-dried fruit (79.99 ± 0.32 mg GAE/g d.m.) was comparable to that in fresh fruit (79.67 ± 0.54 mg GAE/g d.m.), while both dried and frozen fruit showed a decrease. Freeze-dried fruit showed a significant increase in antioxidant activity. The volatile compound profile of fresh fruit was dominated by alcohols (mainly ethanol, hexanol, and (Z)-3-hexen-1-ol), while aldehydes (mainly benzaldehyde) dominated in processed fruit. From the perspective of chokeberry applications, the choice of processing method should be consistent with the desired aromatic profile and the type of target product. Freeze-drying is optimal when color, phenolic content, and fresh appearance are important, while drying or freezing is preferred to preserve fresh aromas. Fresh and frozen fruit retained higher levels of short-chain alcohols and esters, which are responsible for fruity and fresh aromatic notes, making them suitable for use in smoothies, juices, and fresh fruit desserts. Dried and freeze-dried chokeberries were dominated by aldehydes, which, depending on their structure and quantity, can impart sweet, nutty notes and may be beneficial in liqueurs, jams or baked goods where a richer aroma is desired.
Author Contributions
Conceptualization, E.G.-H. and E.J.; methodology, E.G.-H., E.J., K.Ż., A.M.-R. and M.Z.; formal analysis, E.G.-H. and E.J.; investigation, E.G.-H. and E.J.; resources, E.G.-H., E.J., K.Ż., A.M.-R. and M.Z.; data curation, E.J. and K.Ż.; writing—original draft preparation, E.G.-H., E.J., K.Ż., A.M.-R. and M.Z.; writing—review and editing, E.G.-H., E.J., K.Ż., A.M.-R. and M.Z.; visualization, E.J. and K.Ż.; supervision, E.G.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the Polish Ministry of Science and Higher Education within the funds of the Institute of Human Nutrition Sciences, Department of Technique and Food Product Development, Warsaw University of Life Sciences (WULS-SGGW), for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roy, S.; Nisha, P.; Chakraborty, R. (Eds.) Traditional and Unconventional Food Crops with the Potential to Boost Health and Nutrition with Special Reference to Asian and African Countries. In Traditional Foods: The Reinvented Superfoods; Springer Nature: Cham, Switzerland, 2024; pp. 45–67. ISBN 978-3-031-72756-6. [Google Scholar]
- Sharma, P.; Bratley, K.; Ford, T.; Ristvey, A.G.; Volkis, V.V. Aronia Mitschurinii—New Generation of Super Fruits with Multiple Health Benefits. J. Berry Res. 2025, 15, 12–25. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.-S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.-M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera Caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef]
- Aronia Berries Market Size, Share, Growth, And Industry Analysis, By Type (Aronia Prunifolia (Purple Chokeberry), Aronia melanocarpa (Black Chokeberry), Aronia arbutifolia (Red Chokeberry)), By Application (Supermarket, Convenience Stores), And Regional Forecast To 2033; Global Market Statistics; India, 2024. p. 113. Available online: https://www.globalmarketstatistics.com/market-reports/aronia-berries-market-13180 (accessed on 24 September 2025).
- Szopa, A.; Kubica, P.; Ekiert, H. Ekologia, Skład Chemiczny, Działanie Prozdrowotne Oraz Badania Biotechnologiczne Aronii Czarnoowocowej (Aronia melanocarpa (Michx.) Elliott), Aronii Czerwonej (Aronia arbutifolia (L.) Pers.) i Aronii Śliwolistnej (Aronia × prunifolia (Marsh.) Rehd.). Postępy Fitoter. 2017, 18, 145–157. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Šnebergrová, J.; Čížková, H.; Neradová, E.; Kapci, B.; Rajchl, A.; Voldřich, M. Variability of Characteristic Components of Aronia. Czech J. Food Sci. 2014, 32, 25–30. [Google Scholar] [CrossRef]
- Jeppsson, N.; Johansson, R. Changes in Fruit Quality in Black Chokeberry (Aronia melanocarpa) during Maturation. J. Hortic. Sci. Biotechnol. 2000, 75, 340–345. [Google Scholar] [CrossRef]
- King, E.S.; Noll, A.; Glenn, S.; Bolling, B.W. Refrigerated and Frozen Storage Impact Aronia Berry Quality. Food Prod. Process Nutr. 2022, 4, 3. [Google Scholar] [CrossRef]
- Engin, S.P.; Mert, C. The Effects of Harvesting Time on the Physicochemical Components of Aronia Berry. Turk. J. Agric. 2020, 44, 361–370. [Google Scholar] [CrossRef]
- Kulling, S.; Rawel, H. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Szaniawska, M.; Taraba, A.; Szymczyk, K. Budowa, Właściwości i Zastosowanie Antocyjanów. Eng. Sci. Technol. 2015, 17, 63–78. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Cyuńczyk, M.; Zujko, M.E. Antioxidant Properties of Chokeberry Products—Assessment of the Composition of Juices and Fibers. Foods 2023, 12, 4029. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Lech, K. The Influence of Different the Drying Methods on Chemical Composition and Antioxidant Activity in Chokeberries. LWT—Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Żbik, K.; Górska-Horczyczak, E.; Onopiuk, A.; Kurek, M.; Zalewska, M. Vacuum and Convection Drying Effects on Volatile Compounds Profile and Physicochemical Properties of Selected Herbs from Lamiaceae Family. Eur. Food Res. Technol. 2023, 249, 2569–2581. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Z.; Li, M.; Xu, H.; Xiao, H.; Xu, B.; Zhao, X. Effect of Different Vinification Processes on Chemical and Sensory Properties of Aronia melanocarpa Wines. Food Res. Int. 2025, 217, 116755. [Google Scholar] [CrossRef]
- Romani, A.; Vignolini, P.; Ieri, F.; Heimler, D. Polyphenols and Volatile Compounds in Commercial Chokeberry (Aronia melanocarpa) Products. Nat. Prod. Commun. 2016, 11, 99–102. [Google Scholar] [CrossRef]
- Butorová, L.; Vítová, E.; Polovka, M. Comparison of Volatiles Identified in Aronia melanocarpa and Amelanchier alnifolia Using Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry. J. Food Nutr. Res. 2016, 55, 57–68. [Google Scholar]
- Zalewska, M.; Górska-Horczyczak, E.; Marcinkowska-Lesiak, M. Effect of Applied Ozone Dose, Time of Ozonization, and Storage Time on Selected Physicochemical Characteristics of Mushrooms (Agaricus bisporus). Agriculture 2021, 11, 748. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Zalewska, M.; Wierzbicka, A. Chromatographic Fingerprint Application Possibilities in Food Authentication. Eur. Food Res. Technol. 2022, 248, 1163–1177. [Google Scholar] [CrossRef]
- Caputo, L.; Amato, G.; De Bartolomeis, P.; De Martino, L.; Manna, F.; Nazzaro, F.; De Feo, V.; Barba, A.A. Impact of Drying Methods on the Yield and Chemistry of Origanum Vulgare L. Essential Oil. Sci. Rep. 2022, 12, 3845. [Google Scholar] [CrossRef]
- Bogusz, R.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D.; Gondek, E. Foam-Mat Freeze Drying of Kiwiberry (Actinidia Arguta) Pulp: Drying Kinetics, Main Properties and Microstructure. Appl. Sci. 2024, 14, 5629. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Khan, M.I.H.; Kumar, C.; Rahman, M.M.; Karim, M.A. Shrinkage of Food Materials During Drying: Current Status and Challenges. Comp. Rev. Food Sci. Food Safe 2018, 17, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Bonat Celli, G.; Ghanem, A.; Su-Ling Brooks, M. Influence of Freezing Process and Frozen Storage on the Quality of Fruits and Fruit Products. Food Rev. Int. 2016, 32, 280–304. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium Spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Yan, Y.; Castellarin, S.D. Blueberry Water Loss Is Related to Both Cuticular Wax Composition and Stem Scar Size. Postharvest Biol. Technol. 2022, 188, 111907. [Google Scholar] [CrossRef]
- Yan, Y.; Dossett, M.; Castellarin, S.D. Cuticular Waxes Affect Fruit Surface Color in Blueberries. Plants People Planet. 2023, 5, 736–751. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, B.; Liu, R.; Han, Y.; Fang, X.; Mu, H.; Farag, M.A.; Simal-Gandara, J.; Prieto, M.A.; Chen, H.; et al. Structures and Functions of Cuticular Wax in Postharvest Fruit and Its Regulation: A Comprehensive Review with Future Perspectives. Engineering 2023, 23, 118–129. [Google Scholar] [CrossRef]
- Dawson, P.; Al-Jeddawi, W.; Rieck, J. The Effect of Different Freezing Rates and Long-Term Storage Temperatures on the Stability of Sliced Peaches. Int. J. Food Sci. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Samoticha, J.; Wojdyło, A.; Golis, T. Phenolic Composition, Physicochemical Properties and Antioxidant Activity of Interspecific Hybrids of Grapes Growing in Poland. Food Chem. 2017, 215, 263–273. [Google Scholar] [CrossRef]
- Ceylan, C.M.; Cakmakoglu, S.K.; Bekiroglu, H.; Yaman, M.; Akcicek, A.; Sagdic, O.; Karasu, S. Effects of Drying Methods on Different Characteristics of Chokeberry. J. Sci. Ind. Res. 2024, 83, 1284–1294. [Google Scholar] [CrossRef]
- Nawawi, N.; Ijod, G.; Abas, F.; Ramli, N.; Mohd Adzahan, N.; Mohamad Azman, E. Influence of Different Drying Methods on Anthocyanins Composition and Antioxidant Activities of Mangosteen (Garcinia mangostana L.) Pericarps and LC-MS Analysis of the Active Extract. Foods 2023, 12, 2351. [Google Scholar] [CrossRef]
- Thi, N.D.; Hwang, E.-S. Effects of Drying Methods on Contents of Bioactive Compounds and Antioxidant Activities of Black Chokeberries (Aronia melanocarpa). Food Sci. Biotechnol. 2016, 25, 55–61. [Google Scholar] [CrossRef]
- Lv, X.; Lan, T.; Wang, S.; Li, X.; Bao, S.; Li, T.; Sun, X.; Ma, T. Comparative Study on the Physicochemical Properties, Functional Components, Color and Anthocyanins Profile of Aronia melanocarpa Juice Using Different Sterilization Methods. Food Innov. Adv. 2024, 3, 64–74. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Yen, P.P.; Kitts, D.D.; Pratap Singh, A. Natural Acidification with Low-pH Fruits and Incorporation of Essential Oil Constituents for Organic Preservation of Unpasteurized Juices. J. Food Sci. 2018, 83, 2039–2046. [Google Scholar] [CrossRef]
- Al Hasani, S.; Al-Attabi, Z.; Waly, M.; Al-Habsi, N.; Al-Subhi, L.; Shafiur Rahman, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Tiho, T.; Yao, J.C.N.; Brou, C.Y.; Adima, A.A. Drying Temperature Effect on Total Phenols and Flavonoids Content, and Antioxidant Activity of Borassus aethiopum Mart Ripe Fruits’ Pulp. JFR 2017, 6, 50. [Google Scholar] [CrossRef]
- Uribe, E.; Marín, D.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, A. Assessment of Vacuum-Dried Peppermint (Mentha piperita L.) as a Source of Natural Antioxidants. Food Chem. 2016, 190, 559–565. [Google Scholar] [CrossRef]
- Simkova, K.; Grohar, M.C.; Pelacci, M.; Veberic, R.; Jakopic, J.; Hudina, M. The Effect of Freezing, Frozen Storage and Thawing on the Strawberry Fruit Composition. Int. J. Fruit. Sci. 2024, 24, 186–199. [Google Scholar] [CrossRef]
- Sielicka, M.; Pawlak, S. Wpływ rozpuszczalnika zastosowanego do ekstrakcji na oznaczoną zawartość związków fenolowych i aktywność prze-ciwutleniającą wytłoków z aronii. In Zagospodarowanie ubocznych produktów przemysłu spożywczego; Górecka, D., Pospiech, E., Eds.; Uniwersytet Przyrodniczy w Poznaniu: Poznań, Poland, 2016; ISBN 978-83-7160-836-0. [Google Scholar]
- Coşkun, N.; Sarıtaş, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Appl. Sci. 2024, 14, 9183. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The Influence of Temperature, Storage Conditions, pH, and Ionic Strength on the Antioxidant Activity and Color Parameters of Rowan Berry Extracts. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Luo, P.; Li, F.; Liu, H.; Yang, X.; Duan, Z. Effect of Fucoidan-based Edible Coating on Antioxidant Degradation Kinetics in Strawberry Fruit during Cold Storage. J. Food Process Preserv. 2020, 44, e14381. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Tolić, M.T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Ivanišová, E.; Brindza, J. Comparative Analysis of Antioxidant Activity and Phenolic Compounds in the Fruits of Aronia Spp. Slovak J. Food Sci. 2020, 14, 393–401. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Shelepova, O.; Vergun, O.; Grygorieva, O.; Kuklina, A.; Brindza, J. Differences Between Aronia medik. Taxa on the Morphological and Biochemical Characters. Environ. Res. Eng. Manag. 2019, 74, 43–52. [Google Scholar] [CrossRef]
- Thi, N.D.; Hwang, E.-S. Bioactive Compound Contents and Antioxidant Activity in Aronia (Aronia melanocarpa) Leaves Collected at Different Growth Stages. Prev. Nutr. Food Sci. 2014, 19, 204–212. [Google Scholar] [CrossRef]
- Ochmian, I.; Figiel-Kroczyńska, M.; Lachowicz, S. The Quality of Freeze-Dried and Rehydrated Blueberries Depending on Their Size and Preparation for Freeze-Drying. Acta Univ. Cibiniensis. Ser. E Food Technol. 2020, 24, 61–78. [Google Scholar] [CrossRef]
- Piecko, J.; Mieszczakowska-Frąc, M.; Celejewska, K.; Szwejda-Grzybowska, J. Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit. Foods 2024, 13, 1231. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Yang, K.; He, Q.; Wang, Y.; Sun, Y.; He, C.; Xiao, P. Impact of Drying Methods on Phenolic Components and Antioxidant Activity of Sea Buckthorn (Hippophae rhamnoides L.) Berries from Different Varieties in China. Molecules 2021, 26, 7189. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant Activity of Dietary Fruits, Vegetables, and Commercial Frozen Fruit Pulps. J. Agric. Food Chem. 2005, 53, 2928–2935. [Google Scholar] [CrossRef]
- Nthabiseng, L.K.; Adeyanju, A.A.; Bamidele, O.P. Effects of Frozen of Marula Fruits (Sclerocarya birrea) on Chemical, Antioxidant Activities, and Sensory Properties of Marula Fruit Juice. Heliyon 2023, 9, e20452. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.; Kratchanova, M.; Petrova, I.; Klisurova, D.; Georgiev, Y.; Ognyanov, M.; Yanakieva, I. Black Chokeberry (Aronia melanocarpa (Michx.) Elliot) Fruits and Functional Drinks Differ Significantly in Their Chemical Composition and Antioxidant Activity. J. Chem. 2018, 2018, 9574587. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal Variation of Volatile Composition and Odor Activity Value of‘Marion’(Rubus Spp. hyb) and‘Thornless Evergreen’(R. Laciniatus L.) Blackberries. J. Food Sci. 2005, 70, C13–C20. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent Advances of Fermented Fruits: A Review on Strains, Fermentation Strategies, and Functional Activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Nunes, W.; De Oliveira, C.S.; Alcantara, G.B. Ethanol Determination in Frozen Fruit Pulps: An Application of Quantitative Nuclear Magnetic Resonance: qNMR for Ethanol Quantification in Frozen Fruit Pulps. Magn. Reson. Chem. 2016, 54, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, K.; Lee, J.H.; Kim, H.J. Protection of Alcohol Dehydrogenase against Freeze–Thaw Stress by Ice-Binding Proteins Is Proportional to Their Ice Recrystallization Inhibition Property. Mar. Drugs 2020, 18, 638. [Google Scholar] [CrossRef]
- Sujka, K.; Koczon, P.; Górska, A.; Wirkowska, M.; Reder, M. Sensoryczne i Spektralne Cechy Wybranych Wyrobów Spirytusowych Poddanych Procesowi Liofilizacji. Żywność Nauka Technol. Jakość 2013, 20, 184–194. [Google Scholar]
- D’Agostino, M.F.; Sanz, J.; Sanz, M.L.; Giuffrè, A.M.; Sicari, V.; Soria, A.C. Optimization of a Solid-Phase Microextraction Method for the Gas Chromatography–Mass Spectrometry Analysis of Blackberry (Rubus ulmifolius Schott) Fruit Volatiles. Food Chem. 2015, 178, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Díaz De León-Sánchez, F.; Pelayo-Zaldívar, C.; Rivera-Cabrera, F.; Ponce-Valadez, M.; Ávila-Alejandre, X.; Fernández, F.J.; Escalona-Buendía, H.B.; Pérez-Flores, L.J. Effect of Refrigerated Storage on Aroma and Alcohol Dehydrogenase Activity in Tomato Fruit. Postharvest Biol. Technol. 2009, 54, 93–100. [Google Scholar] [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Angeloni, S.; Borsetta, G.; Vittori, S.; Maggi, F.; Sagratini, G.; Caprioli, G. Influence of Freezing and Different Drying Methods on Volatile Profiles of Strawberry and Analysis of Volatile Compounds of Strawberry Commercial Jams. Molecules 2021, 26, 4153. [Google Scholar] [CrossRef]
- Hirvi, T.; Honkanen, E. Analysis of the Volatile Constituents of Black Chokeberry (Aronia melanocarpa Ell.). J. Sci. Food Agric. 1985, 36, 808–810. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive Components, Volatile Profile and In Vitro Antioxidative Properties of Taxus baccata L. Red Arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef]
- Kraujalytė, V.; Leitner, E.; Venskutonis, P.R. Characterization of Aronia melanocarpa Volatiles by Headspace-Solid-Phase Microextraction (HS-SPME), Simultaneous Distillation/Extraction (SDE), and Gas Chromatography-Olfactometry (GC-O) Methods. J. Agric. Food Chem. 2013, 61, 4728–4736. [Google Scholar] [CrossRef]
- Bonin, S. Związki Powstające w Czasie Fermentacji Winiarskiej. Ferment. FRUITS Veg. Ind. 2018, 1, 23–25. [Google Scholar] [CrossRef]
- Yan, J.; Ban, Z.; Lu, H.; Li, D.; Poverenov, E.; Luo, Z.; Li, L. The Aroma Volatile Repertoire in Strawberry Fruit: A Review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Lu, H.; Ban, Z.; Wang, K.; Li, D.; Li, D.; Poverenov, E.; Li, L.; Luo, Z. Aroma Volatiles, Sensory and Chemical Attributes of Strawberry (Fragaria × Ananassa Duch.) Achenes and Receptacle. Int. J. Food Sci. Tech. 2017, 52, 2614–2622. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium macrocarpon Ait.) Using Gas Chromatography–Olfactometry (GC-O) and Odor Activity Value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Silva, L.R.; Guedes De Pinho, P.; Gil-Izquierdo, A.; Valentão, P.; Silva, B.M.; Pereira, J.A.; Andrade, P.B. Volatile Profiling of Ficus carica Varieties by HS-SPME and GC–IT-MS. Food Chem. 2010, 123, 548–557. [Google Scholar] [CrossRef]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of Major Volatile Compounds during the Production of Fruit Vinegars by Static Headspace Gas Chromatography–Mass Spectrometry Method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Wang, J.; Wei, B.; Xu, J.; Jiang, H.; Xu, Y.; Wang, C. Influence of Lactic Acid Fermentation on the Phenolic Profile, Antioxidant Activities, and Volatile Compounds of Black Chokeberry (Aronia melanocarpa) Juice. J. Food Sci. 2024, 89, 834–850. [Google Scholar] [CrossRef]
- Dennis, J.A.; Johnson, N.W.; Thorpe, T.W.; Wallace, S. Biocompatible α-Methylenation of Metabolic Butyraldehyde in Living Bacteria. Angew. Chem. Int. Ed. 2023, 62, e202306347. [Google Scholar] [CrossRef]
- Antoszkiewicz, Z.; Lipiński, K.; Purwin, C.; Pysera, B. Wpływ Kiełkowania Oraz Suszenia Ziarna Owsa Na Jakość Tłuszczu i Zawartość α-Tokoferolu. Zesz. Nauk. Uniw. Przyr. We Wrocławiu. Biol. I Hod. Zwierząt 2011, 62, 89–99. [Google Scholar]
- Yang, H.; Pu, R.; Yang, Y.; Dong, T.; Hua, Y.; Guo, L. Impact of Drying Method on Sensory, Physicochemical Properties and Biology Activity Evaluation of Boletus edulis Soup and Their Changes in Volatile Compounds. LWT 2025, 222, 117676. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, K.; Wang, M.; Li, R.; Dai, X.; Liu, Y.; Jiang, X.; Xia, T.; Gao, L. The Functional Characterization of Carboxylesterases Involved in the Degradation of Volatile Esters Produced in Strawberry Fruits. Int. J. Mol. Sci. 2022, 24, 383. [Google Scholar] [CrossRef]
- Das, P.C.; Heydari, M.M.; Baik, O.-D.; Zhang, L.; Tabil, L.G. Enhancing Drying Efficiency and Terpene Retention of Cannabis Using Cold Plasma Pretreatment. Ind. Crops Prod. 2025, 226, 120669. [Google Scholar] [CrossRef]
- Halagarda, M.; Kowa-Halagarda, K.; Popek, S. Ocena Wpływu Metody Utrwalania na Wybrane Determinanty Jakości Jabłek. Żywność Nauka Technol. Jakość 2023, 30, 211–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).