Mesenchymal Stem Cells in Liver Fibrosis: A Dose-Dependent Recovery
Abstract
1. Introduction
2. Materials and Methods
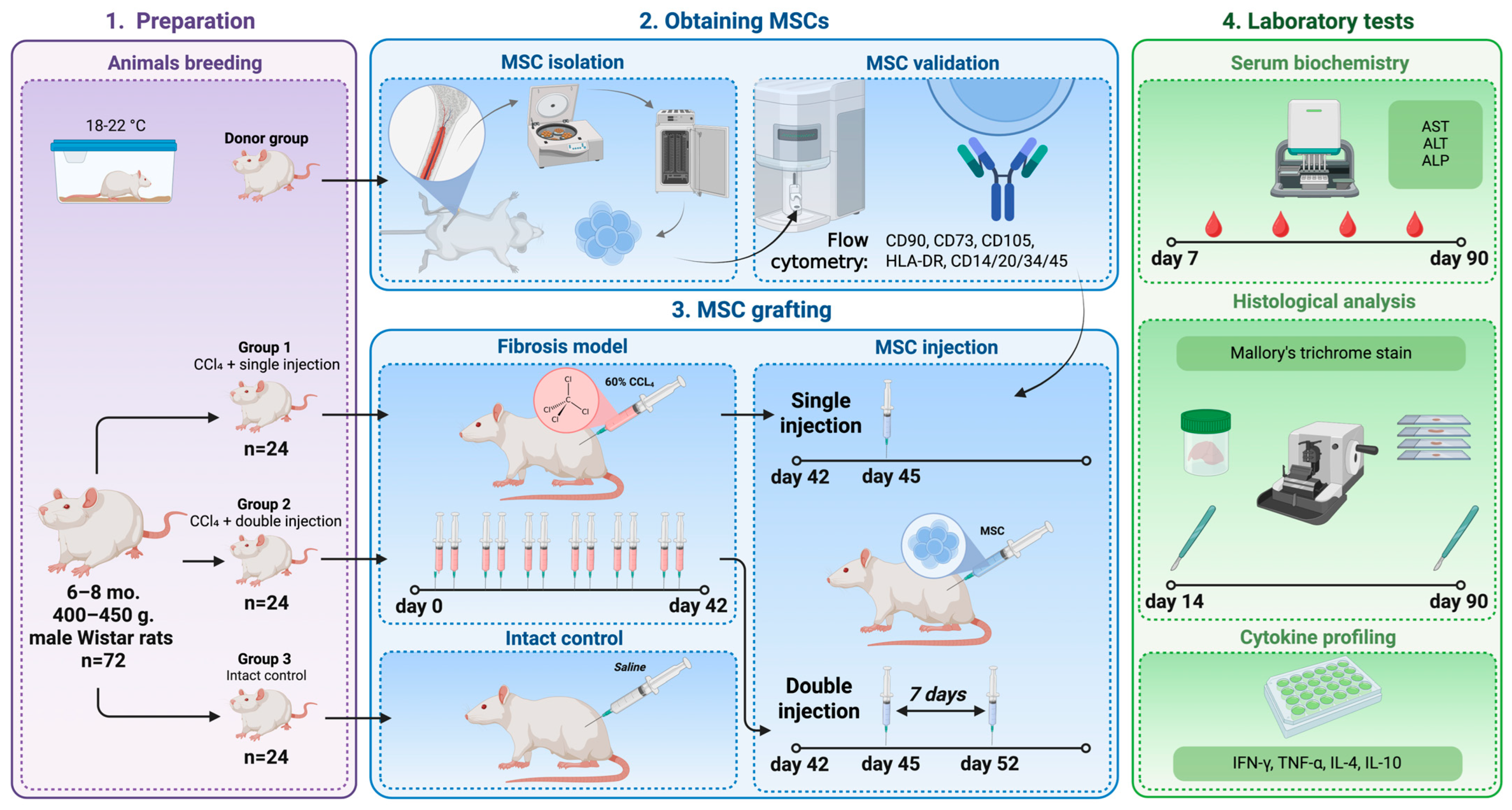
2.1. Animals
2.2. Mesenchymal Stem Cells
2.3. Liver Fibrosis Modeling
2.4. Study Groups
2.5. Blood Tests
2.6. Histological Study
2.7. Statistical Analysis
3. Results
3.1. MSCs Are the Principal Component of Cell Culture in This Study
3.2. Biochemical Markers of Liver Cytolysis Recover Faster After MSC Grafting
3.3. Cytokine Levels Vary Greatly After MSC Grafting
3.4. MSC Grafting Ameliorates Histological Signs of Liver Damage
4. Discussion
Limitations and Perspectives
- The set of diagnostic tools involved is limited. For instance, a recent study with a similar design, although via 9 and not 6 weeks of CCl4 poisoning, revealed antioxidant machinery involved in the MSC effects in liver fibrosis [40]. This research, parallel to ours, implies the need to enlarge the set of methods used, as the current study was obviously limited and may be even biased by the use of fixed tissues not able to reveal the molecular pathways of antioxidation. We propose that an aspiration biopsy via a known method [41] can be appropriate for the widening of research in this field. Hypoxic effects also deserve a detailed study because of their ambiguity in liver fibrosis. For example, a fascinating report by Kuo and colleagues showed MSCs from Wharton’s jelly were able to reverse fibrotic changes in the liver, and this impact has been more evident in hypoxic states [42]. Furthermore, future research should include additional methods of liver fibrosis visualization with higher resolution and/or accuracy (e.g., coherent anti-Stokes Raman scattering microscopy, fluorescence lifetime imaging, or second harmonic generation microscopy) to observe the dynamic recovery of hepatic plates.
- Our study does not consider the variety of subpopulations for MSCs [43]. Perhaps, a more refined selection of MSC subtypes may lead to a more remarkable beneficial impact. For example, Nishina and colleagues have recently demonstrated that Muse cells, a subpopulation of MSCs, exhibit the largest proliferative potential in liver fibrosis after twelve weeks of CCl4 poisoning [44]. Furthermore, not only are MSC subpopulations not regarded in the current work but also immune- and apoptosis-related markers, as the study design was focused on revealing whether there exists a dose-dependent effect. However, further research should elucidate the issue of certain apoptotic and immune-mediated pathways involved in the liver’s recovery from fibrosis in this model.
- The choice of intervention mode for fibrosis modeling is challenging. We preferred CCl4, as it is the most common agent for fibrosis modeling in animals [45], not only with rats but mice [46] and monkeys [47], too. Furthermore, we had tested it successfully in our previous studies [37,38]. However, other chemical models than CCl4 have also been successfully tested in rats, for example, poisoning with thioacetamide [48], diethylnitrosamine, and acetaminophen [45]. Furthermore, surgical, organelle-targeting, immune-mediated, alcohol- or diet-provoked models exist, as well as liver fibrosis modeling via breeding transgenic animals with related genes involved [45]. All these modes require additional testing of our regimen for liver fibrosis recovery in our further research.
- Animals of the same sex were included to obtain consistent results; for ethical reasons, we avoided doubling animal number with both sexes observed. Thus, we preferred male rats, as comparable experiments with male rats have been conducted by other teams [49,50,51]. However, we assume female rats to be also appropriate for such studies, and further research should also account for testing MSCs in female animals.
- There exists a necessity to entirely and comprehensively describe if the MSC-induced changes permit survival of hepatocytes with their compensatory hypertrophy only or if certain hyperplasia can also be seen. In further studies, a detailed evaluation of cell growth is therefore required.
- An additional limitation raises the possibility of extrapolation between our studies and human disease. For sure, conventional models of liver fibrosis have already been regarded as partially available for translation to human pathology [52]. To bring these models closer to human liver structure, liver fibrosis models have been developed in primates in recent years [47,53]. However, not only do the dosage regimens between rodent/primate studies and clinical studies of MSCs in liver pathology differ, but also it is challenging in clinical studies to find a proper dosage (various clinical studies used doses differing in billions of ways) [54,55,56]; thus, the importance of our study is of high level.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Man, S.; Deng, Y.; Ma, Y.; Fu, J.; Bao, H.; Yu, C.; Lv, J.; Liu, H.; Wang, B.; Li, L. Prevalence of liver steatosis and fibrosis in the general population and various high-risk populations: A nationwide study with 5.7 million adults in China. Gastroenterology 2023, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Terai, S.; Tsuchiya, A. Status of and candidates for cell therapy in liver cirrhosis: Overcoming the “point of no return” in advanced liver cirrhosis. J. Gastroenterol. 2017, 52, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.E.; Luger, D.; Lipinski, M.J. Large animal model efficacy testing is needed prior to launch of a stem cell clinical trial: An evidence-lacking conclusion based on conjecture. Circ. Res. 2017, 121, 496–498. [Google Scholar] [CrossRef]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-stem-cell-derived hepatic cells: Hepatocytes and organoids for liver therapy and regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Liu, P.; Mao, Y.; Xie, Y.; Wei, J.; Yao, J. Stem cells for treatment of liver fibrosis/cirrhosis: Clinical progress and therapeutic potential. Stem Cell Res. Ther. 2022, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Shi, X.; Cao, H.; Li, L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res. Ther. 2018, 9, 72. [Google Scholar] [CrossRef]
- Van der Helm, D.; Barnhoorn, M.C.; de Jonge-Muller, E.S.M.; Molendijk, I.; Hawinkels, L.J.A.C.; Coenraad, M.J.; van Hoek, B.; Verspaget, H.W. Local but not systemic administration of mesenchymal stromal cells ameliorates fibrogenesis in regenerating livers. J. Cell Mol. Med. 2019, 23, 6238–6250. [Google Scholar] [CrossRef]
- Zhou, G.P.; Jiang, Y.Z.; Sun, L.Y.; Zhu, Z.J. Therapeutic effect and safety of stem cell therapy for chronic liver disease: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2020, 11, 419. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhang, L.; Li, J.; Zhu, Z. Mesenchymal stem cells: Potential application for the treatment of hepatic cirrhosis. Stem Cell Res. Ther. 2018, 9, 59. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y.Y.; Xu, R.N.; Meng, F.P.; Yu, S.J.; Fu, J.L.; Hu, J.H.; Li, J.X.; Wang, L.F.; Jin, L.; et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol. Int. 2021, 15, 1431–1441. [Google Scholar] [CrossRef]
- Yang, S.; Liu, J.; Khe, J.S.; Lu, A.J.H.; Lim, V. Emerging insights into mesenchymal stem cells and exosome-based therapies for liver injury. Biomol. Biomed. 2025, 25, 1691–1708. [Google Scholar] [CrossRef]
- Hisada, M.; Zhang, X.; Ota, Y.; Cameron, A.M.; Burdick, J.; Gao, B.; Williams, G.M.; Sun, Z. Fibrosis in small syngeneic rat liver grafts because of damaged bone marrow stem cells from chronic alcohol consumption. Liver Transpl. 2017, 23, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.D.; Chen, E.Q. Mesenchymal stem cell-derived exosomes in the treatment of end-stage liver disease. Curr. Stem Cell Res. Ther. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Moayedfard, Z.; Bagheri, L.K.; Alizadeh, A.A.; Nekooeian, A.A.; Dara, M.; Koohpeyma, F.; Parsa, S.; Nikeghbalian, S.; Hosseinpouri, A.; Azarpira, N. The ameliorative effect of adipose-derived mesenchymal stem cells and their exosomes in non-alcoholic steatohepatitis by simultaneously enhancing autophagic flux and suppressing endoplasmic reticulum stress. Iran. J. Med. Sci. 2025, 50, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.A.; Kaiser Junior, R.L.R.; Piron-Ruiz, G.; de Quadros, L.G. Are mesenchymal stem/stromal cells a novel avenue for the treatment of non-alcoholic fatty liver disease? World J. Stem Cells. 2025, 17, 99638. [Google Scholar] [CrossRef]
- Pînzariu, A.C.; Moscalu, R.; Soroceanu, R.P.; Maranduca, M.A.; Drochioi, I.C.; Vlasceanu, V.I.; Timofeiov, S.; Timofte, D.V.; Huzum, B.; Moscalu, M.; et al. The therapeutic use and potential of MSCs: Advances in regenerative medicine. Int. J. Mol. Sci. 2025, 26, 3084. [Google Scholar] [CrossRef]
- GOST 33216-2014; Guidelines for Accommodation and Care of Animals. Species-Specific Provisions for Laboratory Rodents and Rabbits. Standartinform: Moscow, Russia, 2016.
- GOST 33215-2014; Guidelines for Accommodation and Care of Animals. Environment, Housing and Management. Standartinform: Moscow, Russia, 2019.
- Kuçi, Z.; Piede, N.; Vogelsang, K.; Pfeffermann, L.M.; Wehner, S.; Salzmann-Manrique, E.; Stais, M.; Kreyenberg, H.; Bonig, H.; Bader, P.; et al. Expression of HLA-DR by mesenchymal stromal cells in the platelet lysate era: An obsolete release criterion for MSCs? J. Transl. Med. 2024, 22, 39. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y. Immunomodulatory and regenerative effects of MSC-derived extracellular vesicles to treat acute GVHD. Stem Cells 2022, 40, 977–990. [Google Scholar] [CrossRef]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef]
- Kouroupis, D.; Bowles, A.C.; Willman, M.A.; Perucca Orfei, C.; Colombini, A.; Best, T.M.; Kaplan, L.D.; Correa, D. Infrapatellar fat pad-derived MSC response to inflammation and fibrosis induces an immunomodulatory phenotype involving CD10-mediated Substance P degradation. Sci. Rep. 2019, 9, 10864. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.Q.; Meng, X.H.; Fang, X.; Liu, H.Y.; Mwindadi, H.H. MiR-126 regulates the effect of mesenchymal stem cell vascular repair on carotid atherosclerosis through MAPK/ERK signaling pathway. World J. Stem Cells. 2025, 17, 106520. [Google Scholar] [CrossRef]
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266. [Google Scholar] [CrossRef]
- Yao, L.; Hu, X.; Dai, K.; Yuan, M.; Liu, P.; Zhang, Q.; Jiang, Y. Mesenchymal stromal cells: Promising treatment for liver cirrhosis. Stem Cell Res. Ther. 2022, 13, 308. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Hu, Y.; Rao, S.S.; Wang, Z.X. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Wei, P.; Zhong, C.; Yang, X.; Shu, F.; Xiao, S.; Gong, T.; Luo, P.; Li, L.; Chen, Z.; Zheng, Y.; et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma 2020, 8, tkaa020. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chakraborty, A.; Broughton, B.R.S.; Ferens, D.; Widdop, R.E.; Ricardo, S.D.; Samuel, C.S. Comparing the renoprotective effects of BM-MSCs versus BM-MSC-exosomes, when combined with an anti-fibrotic drug, in hypertensive mice. Biomed. Pharmacother. 2021, 144, 112256. [Google Scholar] [CrossRef]
- Sabry, D.; Mohamed, A.; Monir, M.; Ibrahim, H.A. The Effect of Mesenchymal Stem Cells Derived Microvesicles on the Treatment of Experimental CCL4 Induced Liver Fibrosis in Rats. Int. J. Stem Cells. 2019, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Seok, J.; Bae, S.H.; Hwang, S.G.; Kim, G.J. Increased PRL-1 in BM-derived MSCs triggers anaerobic metabolism via mitochondria in a cholestatic rat model. Mol. Ther. Nucleic Acid. 2023, 31, 512–524. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Lu, J.; Feng, Z.; Liu, Z.; Song, H.; Wang, H.; Zhou, Y.; Xu, J. Erythropoietin-Modified Mesenchymal Stem Cells Enhance Anti-Fibrosis Efficacy in Mouse Liver Fibrosis Model. Tissue Eng. Regen. Med. 2020, 17, 683–693. [Google Scholar] [CrossRef]
- Fukushima, K.; Itaba, N.; Kono, Y.; Fukushima, K.; Itaba, N.; Kono, Y.; Okazaki, S.; Enokida, S.; Kuranobu, N.; Murakami, J.; et al. Secreted matrix metalloproteinase-14 is a predictor for antifibrotic effect of IC-2-engineered mesenchymal stem cell sheets on liver fibrosis in mice. Regen. Ther. 2021, 26, 292–301. [Google Scholar] [CrossRef]
- Duman, D.G.; Zibandeh, N.; Ugurlu, M.U.; Celikel, C.; Akkoc, T.; Banzragch, M.; Genc, D.; Ozdogan, O.; Akkoc, T. Mesenchymal stem cells suppress hepatic fibrosis accompanied by expanded intrahepatic natural killer cells in rat fibrosis model. Mol. Biol. Rep. 2019, 46, 2997–3008. [Google Scholar] [CrossRef]
- Bolinas, D.K.M.; Barcena, A.J.R.; Mishra, A.; Bernardino, M.R.; Lin, V.; Heralde, F.M., 3rd; Chintalapani, G.; Fowlkes, N.W.; Huang, S.Y.; Melancon, M.P. Mesenchymal stem cells loaded in injectable alginate hydrogels promote liver growth and attenuate liver fibrosis in cirrhotic rats. Gels 2025, 11, 250. [Google Scholar] [CrossRef]
- Shagidulin, M.; Onishchenko, N.; Grechina, A.; Nikolskaya, A.; Krasheninnikov, M.; Lyundup, A.; Volkova, E.; Mogeiko, N.; Venediktov, A.; Piavchenko, G.; et al. Recombinant spidroin microgel as the base of cell-engineered constructs mediates liver regeneration in rats. Polymers 2022, 14, 3179. [Google Scholar] [CrossRef]
- Shagidulin, M.; Onishchenko, N.; Sevastianov, V.; Krasheninnikov, M.; Lyundup, A.; Nikolskaya, A.; Kryzhanovskaya, A.; Voznesenskaia, S.; Gorelova, M.; Perova, N.; et al. Experimental correction and treatment of chronic liver failure using implantable cell-engineering constructs of the auxiliary liver based on a bioactive heterogeneous biopolymer hydrogel. Gels 2023, 9, 456. [Google Scholar] [CrossRef]
- Onishchenko, N.; Shagidulin, M.; Gonikova, Z.; Nikolskaya, A.; Kirsanova, L.; Venediktov, A.; Pokidova, K.; Kuzmin, E.; Kuznetsova, N.; Kozlov, I.; et al. Hematopoietic stem cells of bone marrow and their total RNA in tat liver regeneration. Appl. Sci. 2025, 15, 3782. [Google Scholar] [CrossRef]
- Fotouh, A.; Elbarbary, N.K.; Momenah, M.A.; Khormi, M.A.; Mohamed, W.H.; Sherkawy, H.S.; Ahmed, A.E.; Diab, M.; Elshafae, S. Hepatoprotective effects of mesenchymal stem cells in carbon tetrachloride-induced liver toxicity in rats: Restoration of liver parameters and histopathological evaluation. Am. J. Vet. Res. 2025, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.; Potapova, E.; Zherebtsov, E.; Kozlov, I.; Seryogina, E.; Kandurova, K.; Alekseyev, A.; Piavchenko, G.; Kuznetsov, S.; Mamoshin, A.; et al. Optical fine-needle aspiration biopsy in a rat model. In Proceedings of the Dynamics and Fluctuations in Biomedical Photonics XVI, San Francisco, CA, USA, 2–3 February 2019; Volume 10877. [Google Scholar] [CrossRef]
- Kuo, W.T.; Hsiao, C.Y.; Chiu, S.H.; Chen, T.H.; Shyu, J.F.; Lin, C.H.; Tsai, P.J. Hypoxia-enhanced Wharton’s jelly mesenchymal stem cell therapy for liver fibrosis: A comparative study in a rat model. Kaohsiung J. Med. Sci. 2025, 41, e70053. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Wang, S.; Zhou, Y.; Li, H.; Wu, Y. Mesenchymal stem cell subpopulations: Phenotype, property and therapeutic potential. Cell Mol. Life Sci. 2016, 73, 3311–3321. [Google Scholar] [CrossRef]
- Nishina, T.; Haga, H.; Wakao, S.; Maki, K.; Mizuno, K.; Katsumi, T.; Hoshikawa, K.T.; Saito, T.; Iseki, M.; Unno, M.; et al. Safety and effectiveness of muse cell transplantation in a large-animal model of hepatic fibrosis. Stem Cells Int. 2025, 2025, 6699571. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.L.; Wang, L.; Pan, H.T.; Zhang, T.R.; Chen, Y.H.; Xu, S.J.; Mao, X.L.; Li, S.W. Animal and organoid models of liver fibrosis. Front. Physiol. 2021, 12, 666138. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Yin, L.; Huang, C.; Fan, S. Mangiferin relieves CCl4-induced liver fibrosis in mice. Sci. Rep. 2023, 13, 4172. [Google Scholar] [CrossRef]
- Ding, K.; Liu, M.R.; Li, J.; Huang, K.; Liang, Y.; Shang, X.; Chen, J.; Mu, J.; Liu, H. Establishment of a liver fibrosis model in cynomolgus monkeys. Exp. Toxicol. Pathol. 2014, 66, 257–261. [Google Scholar] [CrossRef]
- Enciso, N.; Amiel, J.; Fabián-Domínguez, F.; Pando, J.; Rojas, N.; Cisneros-Huamaní, C.; Nava, E.; Enciso, J. Model of liver fibrosis induction by thioacetamide in rats for regenerative therapy studies. Anal. Cell Pathol. 2022, 2022, 2841894. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, C.; Li, Y.; Wu, Q.; Gao, R. Mest attenuates CCl4-induced liver fibrosis in rats by inhibiting the Wnt/β-catenin signaling pathway. Gut Liver 2014, 8, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, F.; Li, S.; Wu, B.; Gu, Y.; Yuan, Y. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des. Devel Ther. 2019, 13, 1889–1900. [Google Scholar] [CrossRef]
- Alejolowo, O.O.; Oluba, O.M.; Adeyemi, O.S. Anogeissus leiocarpus (DC.) Guill. & Perr. extract-protected rats against CCl4-induced liver fibrosis. J. Taibah Univ. Med. Sci. 2025, 20, 474–486. [Google Scholar] [CrossRef]
- Weber, S.N.; Wasmuth, H.E. Liver fibrosis: From animal models to mapping of human risk variants. Best. Pract. Res. Clin. Gastroenterol. 2010, 24, 635–646. [Google Scholar] [CrossRef]
- Lai, C.; Feng, T.; Wei, L.; Zhang, T.; Zhou, G.; Yang, Q.; Lan, T.; Xiang, G.; Yao, Y.; Zhou, L.; et al. Development and validation of a primate model for liver fibrosis. J. Pharmacol. Toxicol. Methods 2019, 100, 106600. [Google Scholar] [CrossRef]
- Eom, Y.W.; Shim, K.Y.; Baik, S.K. Mesenchymal stem cell therapy for liver fibrosis. Korean J. Intern. Med. 2015, 30, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Wang, Z.R.; Yao, W.Q.; Linghu, E.Q.; Wang, F.S.; Shi, L. Stem cell therapies for chronic liver diseases: Progress and challenges. Stem Cells Transl. Med. 2022, 11, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Li, Y.C.; Chen, P.X.; Ma, K.S.; Wang, L.T. Mesenchymal stem cell therapy as a game-changer in liver diseases: Review of current clinical trials. Stem Cell Res. Ther. 2025, 16, 3. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyundup, A.; Shagidulin, M.; Onishchenko, N.; Beregovykh, V.; Krasheninnikov, M.; Venediktov, A.; Pokidova, K.; Nikolskaya, A.; Kuzmin, E.; Kostin, A.; et al. Mesenchymal Stem Cells in Liver Fibrosis: A Dose-Dependent Recovery. Appl. Sci. 2025, 15, 10471. https://doi.org/10.3390/app151910471
Lyundup A, Shagidulin M, Onishchenko N, Beregovykh V, Krasheninnikov M, Venediktov A, Pokidova K, Nikolskaya A, Kuzmin E, Kostin A, et al. Mesenchymal Stem Cells in Liver Fibrosis: A Dose-Dependent Recovery. Applied Sciences. 2025; 15(19):10471. https://doi.org/10.3390/app151910471
Chicago/Turabian StyleLyundup, Aleksey, Murat Shagidulin, Nina Onishchenko, Valery Beregovykh, Mikhail Krasheninnikov, Artem Venediktov, Ksenia Pokidova, Alla Nikolskaya, Egor Kuzmin, Andrey Kostin, and et al. 2025. "Mesenchymal Stem Cells in Liver Fibrosis: A Dose-Dependent Recovery" Applied Sciences 15, no. 19: 10471. https://doi.org/10.3390/app151910471
APA StyleLyundup, A., Shagidulin, M., Onishchenko, N., Beregovykh, V., Krasheninnikov, M., Venediktov, A., Pokidova, K., Nikolskaya, A., Kuzmin, E., Kostin, A., Arzhanova, A., Fadeev, P., Kuznetsova, N., Piavchenko, G., & Gautier, S. (2025). Mesenchymal Stem Cells in Liver Fibrosis: A Dose-Dependent Recovery. Applied Sciences, 15(19), 10471. https://doi.org/10.3390/app151910471









