Abstract
Adequate peri-implant hard and soft tissue volume is critical for ensuring implant stability, long-term functionality, and optimal esthetic results. While autogenous connective tissue grafts (CTGs) remain the gold standard for soft tissue augmentation, volume-stable collagen matrices (VCMXs) have emerged as a promising alternative, offering key advantages such as eliminating donor-site morbidity, reducing postoperative pain, and improving patient acceptance. This review summarizes the evidence on VCMXs from studies published between 1995 and 2024, with a focus on mucosal thickness gain, soft tissue stability, and patient-reported outcomes. To date, the evidence on VCMXs is still conflicting. Randomized controlled trials indicate that VCMXs can effectively increase peri-implant mucosal thickness (ranging from 0.3 to 1.0 mm), although the included clinical studies are very heterogeneous in terms of follow-up time. According to the literature, CTGs demonstrate superior long-term volumetric stability, particularly in highly esthetic zones. On the other hand, VCMXs demonstrated comparable mid-term outcomes while offering reduced postoperative discomfort, as confirmed by multicenter noninferiority trials. While VCMXs may yield slightly inferior clinical outcomes compared to CTGs, their patient-centered benefits make them a viable and often preferable option for soft tissue augmentation in implant dentistry. In conclusion, current evidence supports the use of VCMXs as valuable tools in contemporary implant therapy, particularly for specific indications where their benefits outweigh limitations.
1. Introduction
Dental implants are the standard treatment for tooth replacement, showing 90–95% success after 10 years of functional loading [1]. Success depends on careful case selection and managing risk factors, which can be systemic or local. Systemic risk factors include smoking, which impairs healing through vasoconstriction and reduced neutrophil function, and poorly controlled diabetes, which affects bone metabolism and wound healing [2,3]. The influence of osteoporosis and coagulation disorders on implant outcomes remains uncertain and needs further research [2,3].
Implant failures are clinically significant events that can be classified according to their timing and underlying etiology. Early failures, which occur before or during the osseointegration phase (typically within the first 3–6 months post-placement), are invariably biological in nature [2,4]. These early complications are frequently attributed to surgical trauma during implant placement, which may disrupt the delicate healing process. Inadequate bone volume or density at the recipient site can compromise primary stability, a critical determinant of successful osseointegration [4]. Furthermore, bacterial contamination during surgery or immediate post-operative infection can lead to early implant failure [4]. Late failures, occurring after successful osseointegration and prosthetic loading, may stem from either biological or mechanical causes [2,4]. Peri-implantitis, a biofilm-associated inflammatory disease affecting peri-implant tissues, represents the most common biological cause of late implant failure [4]. Mechanical failures often result from occlusal overload or improper force distribution, particularly in cases of bruxism or inadequate prosthetic design [4].
Moreover, recent advances in implant materials and surface treatments have significantly influenced implant success rates [5,6]. Titanium and its alloys, favored for their biocompatibility and mechanical strength, owe much of their performance to the formation of a stable titanium oxide (TiO2) layer that enhances corrosion resistance and reduces bacterial adhesion. Surface modifications such as plasma electrolytic oxidation, laser treatment, and chemical coatings have been developed to optimize the delicate balance between promoting osseointegration and minimizing biofilm formation, thus addressing both mechanical stability and infection-related complications over the implant’s lifespan [5,6]. These innovations are critical in mitigating the challenges posed by complex microbial biofilms and improving the long-term prognosis of dental implants [7,8].
The long-term success of dental implants relies not only on osseointegration—the direct connection between bone and implant—but also on the health of peri-implant soft tissues. The peri-implant mucosa acts as a barrier against bacterial and mechanical threats. Periodontal plastic surgery, especially autologous connective tissue grafts (CTGs), is the gold standard for soft tissue augmentation [9,10]. CTGs increase keratinized mucosa width, improve soft tissue thickness, and enhance vascularity [9]. Adequate keratinized mucosa (>2 mm) is linked to better plaque control, less inflammation, and improved tissue stability. In aesthetic zones, CTGs prevent mucosal recession and support natural emergence profiles [10]. Despite their efficacy, CTGs have several limitations, including the need for a second surgical site, leading to increased operative time, and donor site morbidity such as pain, bleeding and healing complications [11]. To address these issues, volume-stable collagen matrices (VCMXs) have been developed as alternatives for soft tissue augmentation [11]. These 3D scaffolds mimic connective tissue extracellular matrix, supporting cell migration, proliferation, and angiogenesis [12,13]. Pre-clinical studies showed that VCMXs provide soft tissue augmentation outcomes comparable to CTGs, with the advantage of avoiding donor-site morbidity [12,13]. In addition to volume augmentation, soft tissue management around dental implants often requires increasing the width of keratinized mucosa to reduce biological risks such as inflammation and plaque accumulation. In such cases, xenogeneic collagen matrices (XCMs), typically derived from porcine sources, are preferred. XCMs have demonstrated efficacy in augmenting keratinized mucosal width, offering advantages in terms of reduced surgical time and postoperative morbidity when compared to free gingival grafts (FGGs). Volume-stable collagen matrices (VCMXs) are primarily indicated for enhancing soft tissue thickness and contour; therefore, the choice between VCMX and XCM should be guided by specific clinical objectives—whether focusing on aesthetic contouring through soft tissue thickening or improving peri-implant tissue health via keratinized tissue augmentation [14,15].
This comprehensive review aims to critically evaluate the biological properties and long-term clinical performance of volume-stable collagen matrices (VCMXs) for peri-implant soft tissue augmentation. Given the increasing demand for minimally invasive alternatives to autogenous connective tissue grafts (CTGs), this review specifically investigates whether the use of VCMXs in patients receiving dental implants can provide comparable outcomes to CTGs in terms of soft tissue volume gain, long-term stability, and patient-reported satisfaction. By integrating current clinical and pre-clinical findings, this review seeks to offer evidence-based guidance for clinicians in selecting optimal soft tissue augmentation strategies tailored to both functional and aesthetic needs.
2. Materials and Methods
This comprehensive review was conducted to explore and synthesize the available literature on the use of volume-stable collagen matrices (VCMXs) for peri-implant soft tissue augmentation. A broad literature search was performed using electronic databases, including PubMed and Google Scholar, covering publications from 1995 to 2024. The PICO (Population, Intervention, Comparator, Outcome) question was the following: “Are VCMXs effective in increasing per-implant soft tissue thickness?”.
The search included various combinations of the following keywords: “volume-stable collagen matrix”, “VCMX”, “connective tissue graft”, “soft tissue augmentation”, “dental implant”, and “peri-implant mucosa”.
Studies were included if they met the following criteria:
- -
- Pre-clinical or clinical studies
- -
- Investigated the clinical or preclinical application of VCMXs in dental implantology
- -
- Reported quantitative or qualitative outcomes related to soft tissue volume, esthetic contour, or patient-reported outcomes
- -
- Were published in English
- -
- Were accessible in full-text format
Studies were excluded if they were:
- -
- In vitro studies
- -
- Irrelevant to VCMXs or soft tissue management
- -
- Published before 1995
The literature screening and study selection process was conducted manually by the authors, following a structured yet non-systematic approach. Studies were selected based on their scientific relevance, methodological soundness, and direct applicability to the clinical use of volume-stable collagen matrices in implant dentistry. Great efforts were made to prioritize high-quality peer-reviewed articles, randomized controlled trials, and long-term clinical studies to ensure the reliability and relevance of the included data.
3. Biology of Soft Tissue Around Dental Implants
Tissues surrounding dental implants are referred to as peri-implant tissues. They are categorized into two types: hard tissue and soft tissue, each playing a crucial role in the long-term function and stability of the implant. The hard tissue forms a direct interface with the implant’s surface, whilst peri-implant mucosa forms as part of the natural healing process after the placement of the implant or the abutment [16,17]. This process results in a stable mucosal seal around the implant’s trans-mucosal area, typically consisting of about 2 mm of epithelial tissue and 1–1.5 mm of connective tissue [17,18,19].
Biological complications associated with osseointegrated implants are a topic of major interest in modern dental research and clinical practice [20]. During everyday clinical function, osseointegrated dental implants and their surrounding tissues are continuously exposed to microbial and mechanical stresses [21], possibly leading to the development of peri-implant diseases. To minimize this risk, the formation of a stable and functional soft tissue seal around the implant is essential. This seal acts as a protective barrier, preventing bacterial invasion from the oral environment into the implant–tissue interface. The critical role of peri-implant soft tissue in ensuring long-term implant stability has led to the introduction of volume-stabilized collagen matrices (VCMXs) as a viable biomaterial for soft tissue augmentation and an alternative to autogenous connective tissue grafts, aiming to reduce patient morbidity while promoting effective soft tissue augmentation around dental implants. Unlike traditional grafts, VCMXs provide a three-dimensional scaffold with interconnected porous architecture that supports cellular infiltration, neovascularization, and tissue integration [10,22]. Clinically, VCMXs have shown promising results in maintaining soft tissue volume and keratinized mucosa width, contributing to the long-term aesthetic and functional success of implant-supported restorations [10].
In summary, VCMXs interact dynamically with peri-implant tissues by supporting cellular infiltration, angiogenesis, and extracellular matrix formation, making them a valuable adjunct in implant soft tissue management when autogenous grafts are contraindicated or undesirable [23].
4. Biomaterials for Soft Tissue Augmentation
Following tooth extraction, a series of biological events are triggered, often resulting in alveolar bone resorption and development of localized ridge defects, which may negatively affect future implant-prosthetic rehabilitations [24,25]. Successful implant placement requires adequate bone volume and healthy peri-implant soft tissue to ensure long-term stability and aesthetic integration. Key clinical parameters for evaluating peri-implant tissue health include the presence of interdental papillae, the position of the mucosal margin, and both two-dimensional and three-dimensional alteration in peri-implant soft tissues [26]. Deficiencies in buccal bone or soft tissue volume may jeopardize the implant prognosis, especially in aesthetically sensitive zones. To manage or prevent these complications, various surgical approaches have been developed to correct and reconstruct alveolar ridge defects. Depending on the size and location of the defect and prosthetic plan (Implant vs. bridge, etc.), bone augmentation and soft tissue augmentation techniques or a combination of both procedures may be considered to restore both function and aesthetics [13,27]. One of the methods employed to augment soft tissue around dental implants is autogenous subepithelial connective tissue grafts (CTGs). CTGs taken from the palate or maxillary tuberosity [28] are considered the gold standard according to the literature, because they offer the most predictable results [29]. However, several complications have been reported in association with connective tissue graft harvesting procedures, including technical complexity, pain associated with a second surgical site, limitations in graft size, covering only a few implants or teeth at one time, as well as risks of extended bleeding, and infection [30,31]. To overcome the limitation associated with autogenous connective tissue grafts, various biomaterials have been introduced, including xenogeneic, allogeneic and synthetic substitutes. These materials aim to reduce surgical time, minimize donor site morbidity, and improve patient acceptance [32,33,34]. However, to be effective alternatives, these biomaterials must fulfil two essential criteria, namely (i) a favorable biological behavior that allows for proper tissue modeling and remodeling, and (ii) long-term structure and volume stability [13].
Collagen matrices (CM) have been introduced as effective substitutes for autogenous connective tissue grafts in procedures such as soft tissue augmentation and root coverage. These matrices eliminated the need for donor site surgery, reduce operative time, minimize patient morbidity, and improve overall patient acceptance. Their porous structure supports cell migration, proliferation, and extracellular matrix formation, contributing to favorable regenerative outcomes [35]. A volume-stable collagen matrix (VCMX) is a porcine-derived, cross-linked biomaterial designed to support soft tissue regeneration in oral surgery. Structurally, it consists of a porous, sponge-like network that maintains its dimensional stability over time. Unlike conventional collagen membranes, VCMX does not function as a barrier, but rather serves as a scaffold that promotes the ingrowth of surrounding soft tissues. This unique design combines the mechanical strength typically associated with cross-linked membranes and the excellent tissue integration seen in non-cross-linked materials, making it particularly suitable for soft tissue augmentation around teeth and dental implants [36,37].
5. Structural Characteristics of VCMXs and Clinical Implications
Volume-stable collagen matrices (VCMXs) represent a significant advancement in periodontal and peri-implant soft tissue regeneration, with their clinical performance being fundamentally determined by three interconnected structural characteristics: porosity architecture, biodegradation profile, and mechanical stability [38,39]. The porosity network of VCMXs has been extensively characterized through micro-computed tomography (μCT) analyses, revealing that an optimal pore size range of 100–300 μm promotes superior cellular infiltration and neovascularization [23,40]. Scaffolds with higher porosity have been shown to exhibit decreased compressive (elastic) modulus—for example, a study reported a 25% reduction in compressive modulus for higher-pore scaffolds compared to low-pore variants [41]. Additionally, other work demonstrates a notable mechanical weakening as pore sizes approach 600 μm [42,43].
In vivo degradation studies using fluorescent labeling techniques show a biphasic resorption pattern: initial surface erosion followed by sustained core stability [44]. At 6 months post-implantation, histological and immunohistochemical analyses demonstrated complete host tissue integration with preservation of scaffold structure and favorable biocompatibility [45]. Mechanical stability is essential for clinical success in load-bearing areas. Dynamic mechanical thermal analysis (DMTA) has demonstrated that cross-linked VCMXs maintain effective ridge width stability over extended periods, while also facilitating soft-tissue volume preservation and integration into host tissues, as confirmed by histologic analyses [46].
An example of a VCMX is shown in Figure 1.
Figure 1.
(A) Frontal and (B) lateral view of a VCMX.
Clinical studies from re-entry surgeries confirm that the enhanced stability of cross-linked matrices translates to superior clinical outcomes, with guided bone regeneration (GBR) procedures utilizing non-resorbable membranes demonstrating significantly lower complication rates (6.9%)—a common cause of volume loss—compared to those using resorbable membranes (22.7%) [47].
An example of a clinical application of VCMXs to increase soft tissue thickness is shown in Figure 2.
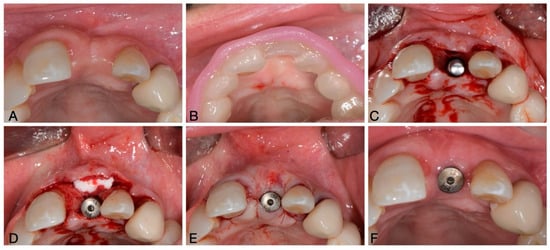
Figure 2.
Example of clinical application of a Volume Stable Collagen Matrix. (A) Edentulous space requiring implant-prosthetic rehabilitation, (B) surgical template for implant positioning, (C) implant placed in the correct prosthetic position, (D) VCMX placement on the buccal aspect, (E) suture placement and (F) one-month post-surgical follow-up.
6. Synthesis of the Current Evidence
Current literature provides increasing pre-clinical and clinical evidence that volume-stable collagen matrices (VCMXs) as a viable biomaterial for soft tissue augmentation around dental implants can offer a balance between clinical effectiveness and patient-centered outcomes and can provide soft tissue volume gain and stability comparable to autogenous connective tissue grafts (CTGs) in the short- and mid-term. However, its long-term predictability remains under investigation [48].
Autogenous connective tissue grafts remain the gold standard for peri-implant soft tissue augmentation due to their superior and consistent volume gain. However, VCMXs have emerged as a less invasive alternative, offering comparable clinical outcomes in the short and mid-term. While tissue gain with VCMXs may be slightly lower, they significantly reduce patient morbidity and eliminate the need for a donor site. Therefore, VCMXs represent a patient-friendly option when minimizing surgical complexity and morbidity is a clinical priority.
6.1. Pre-Clinical Evidence
Sasagawa et al. [49] conducted a preclinical study on six male beagle dog mandibles to evaluate the effects of a volume-stable porcine collagen matrix on peri-implant soft tissue augmentation. After extracting specific teeth and allowing a 6-month healing period, implants were placed, and the collagen matrix was transplanted into the buccal sites. Histological analysis after 3 months showed a significant horizontal increase in soft tissue thickness at the buccal sites. Microscopic examination revealed integration of endogenous tissues within the collagen matrix, indicating effective tissue fusion and biocompatibility. These results suggest that the volume stable collagen matrix can successfully promote soft tissue augmentation around implants in the buccal region. Although promising, these animal model findings may not be directly generalizable to clinical practice.
Thoma et al. [50] initially conducted a preclinical study on dogs to investigate the effectiveness of a volume-stable collagen matrix (VCMX) compared to subepithelial connective tissue grafts (CTG) for soft tissue augmentation around dental implants in dogs. Both treatment modalities resulted in similar short time gains in ridge width, particularly within the first two months. However, by six months, most of the augmented tissue had remodeled, leaving only minimal soft tissue thickness. While VCMX sites showed mild early inflammation, both materials demonstrated favorable biocompatibility and integration.
6.2. Clinical Evidence
In a different study, Thoma et al. [13] performed a randomized clinical trial on 20 patients to evaluate volume-stable collagen matrices versus connective tissue grafts for soft tissue augmentation at single-tooth implant sites. The study reported that VCMX achieved buccal soft tissue thickness gains comparable to CTG at 90 days post-operation, with a median gain of 1.0 mm for VCMX versus 01.5 mm for CTG (p = 0.56). This result demonstrates that VCMX provides a similar increase in soft tissue thickness than CTG at 90 days post-operation. The study design focused on establishing non-inferiority of VCMX, concluding that VCMX is at least as effective as CTG. Additionally, patients treated with VCMX experienced less postoperative pain. Histological analysis confirmed favorable tissue integration for both materials. These studies suggest that VCMX is an effective alternative to autogenous connective tissue grafts for soft tissue augmentation, offering comparable short-term clinical outcomes and enhanced patient comfort, although long-term tissue stability requires further investigation.
Zeltner et al. [30] performed a randomized controlled clinical study comparing the effectiveness of a VCMX versus CTG for soft tissue augmentation around dental implants. The study evaluated linear volumetric soft tissue changes at implant sites up to 3 months after augmentation. Both treatment groups showed significant soft tissue volume gain, with no statistically significant difference between VCMX and CTG. The VCMX was associated with improved patient comfort and reduced surgical morbidity, with comparable short-time augmentation results to the connective tissue grafts. These findings support the use of volume stable collagen matrices as a reliable alternative to autogenous grafts for peri-implant soft tissue augmentation in the early healing phase.
Hämmerle et al. [51] conducted a multicenter, randomized controlled trial comparing a volume-stable collagen matrix to a subepithelial connective tissue graft for soft tissue augmentation after implant placement. Patients were randomly assigned to one of the two treatments following implant surgery. The result showed that both treatments led to similar increases in soft tissue thickness, with no statistically significant differences between them. However, patient-reported outcomes regarding pain and comfort favored the collagen matrix group. At one-year follow-up, aesthetic outcomes and soft tissue volume stability were comparable in both groups. Therefore, according to their results, VCMXs can be considered a suitable alternative to connective tissue grafts, especially when minimizing surgical invasiveness and improving patient comfort are priorities.
Other authors [52] conducted a randomized controlled clinical trial on 20 patients to assess mid-term outcomes following peri-implant soft tissue augmentation using VCMX and CTG. After abutment connection and prosthetic procedures, buccal mucosal thickness and ridge contour were evaluated at 6 months, 1 year, and 3 years. The result showed a modest increase in buccal mucosal thickness for both groups at 3 years (median +0.5 mm for VCMX and +0.8 mm for CTG), without statistically significant differences between them. Importantly, peri-implant soft tissues and bone levels remained stable, and patient-reported outcome measures (PROMs) showed no significant differences during follow-up. These results indicate that both VCMX and CTG provide lasting soft tissue volume and contour stability around implants over a 3-year period.
Finally, the same group [53] presented the five-year follow-up of implant sites treated using either VCMX or CTG. At five years post-restoration, both groups exhibited similar increases in buccal mucosal thickness and minimal ridge contour loss, with no statistically significant difference between treatments. Peri-implant tissues remained healthy, esthetic outcomes were favorable, and patient-reported measures showed comparable comfort and satisfaction. Despite limitations like sample size and dropouts, the study supports VCMX as an effective alternative to CTG, offering long-term tissue stability and aesthetic outcomes with reduced surgical invasiveness.
The main results of the five clinical studies are summarized in Table 1.

Table 1.
Main findings from the clinical studies comparing VCMXs and CTGs.
According to the results of this review, several well-designed randomized controlled trials have consistently shown that volume-stable collagen matrices (VCMXs) can be a reliable alternative to autogenous connective tissue grafts (CTGs) for soft tissue augmentation around implants. These findings suggest that VCMXs may offer similar clinical benefits—such as stable soft tissue volume and improved peri-implant health—while being less invasive for patients.
On the other hand, many of these studies come from the same research groups and were conducted on the same pool of patients at different timepoints, which might limit how broadly the results can be applied to everyday clinical settings. Also, while the available data up to five years are encouraging, we still need more long-term evidence. Histological findings (e.g., Thoma et al., 2017 [50]) offer valuable insight into tissue behavior, but it’s not entirely clear how these results translate to long-term clinical success. Differences in surgical technique, patient populations, and follow-up timeframes between studies also make it challenging to draw firm, across-the-board conclusions. Overall, VCMXs appear to be a promising option with clear advantages, but further multicenter studies with more diverse patient groups and standardized methods are needed to confirm their effectiveness in broader practice.
It is important to note, however, that the current evidence is limited by a lack of high-quality, long-term studies to fully establish the predictability and stability of VCMXs over time. Moreover, despite growing clinical evidence supports their use, there is still a significant gap in the literature regarding the cost-effectiveness analyses comparing VCMXs with autogenous connective tissue grafts. Such economic evaluations are crucial for informed clinical decision-making and should be a focus of future research to fully assess the value of VCMXs in routine practice.
7. Discussion
A comprehensive evaluation of preclinical and clinical evidence supports the judicious use of VCMXs in modern implant dentistry, while clearly delineating their advantages and limitations compared to autogenous grafts [14,54]. Indeed, clinical outcomes from randomized controlled trials and longitudinal cohort studies provide robust evidence for VCMX applications in specific scenarios [51,55].
Moreover, systematic reviews demonstrate VCMXs achieve predictable soft tissue thickness augmentation, representing approximately 85–90% of the volume gain attained with connective tissue grafts (Zeltner et al., 2017 [30]). For soft tissue augmentation, VCMXs produce comparable results to autografts, with increases of 2.0–2.5 mm versus 2.3–2.8 mm at twelve months, while eliminating donor-site morbidity [15].
In clinical practice, these matrices are particularly indicated for moderate soft tissue deficiencies requiring 1–2 mm augmentation, in posterior regions with lower aesthetic demands, in medically compromised patients such as diabetics or elderly individuals, for full-arch rehabilitations needing extensive augmentation, and cases where avoiding donor-site morbidity is prioritized [56].
To maximize clinical effectiveness, optimal surgical protocols should include meticulous recipient site preparation with thorough debridement, creation of well-vascularized wound bed, tension-free flap closure, adequate hydration of the VCMX, and secure fixation with non-resorbable sutures [57]. The use of adjunctive fibrin glue may enhance initial stabilization [15].
Postoperative management should include antibiotic coverage for 5–7 days, delayed mechanical cleaning for 14–21 days, an extended healing period of 8–10 weeks before prosthetic loading, and long-term monitoring for volume stability [30]. Nonetheless, differences in patient populations and defect characteristics across studies may influence these results, and variability in surgical techniques limits the universal applicability of current recommendations.
Despite their advantages in reducing patient morbidity and simplifying surgical procedures, VCMXs present important limitations including higher material costs compared to autografts, reduced predictability in aesthetic zones, requirements for optimal surgical conditions, and a slower integration timeline [58].
In interpreting this evidence, it is important to recognize that the present review has several limitations due to the restricted availability of data and the absence of a formal assessment of publication bias.
Nevertheless, current evidence supports VCMXs as valuable tools in contemporary implant therapy [59]. Looking ahead, a stratified approach to clinical decision-making that carefully considers both biological and patient-centered factors will be essential to optimize outcomes when incorporating VCMXs into treatment planning [60].
8. Conclusions
At present, evidence from preclinical and clinical studies supports the use of VCMXs as effective tools for enhancing peri-implant soft tissue thickness, particularly in cases where their benefits outweigh limitations. Their application is especially valuable for reducing patient morbidity and avoiding autogenous soft tissue harvesting. While clinical outcomes appear promising, long-term predictability has not yet been firmly established. Well-designed randomized clinical trials on larger cohorts are still needed to assess long-term stability and to better define their role in different implant placement protocols.
Author Contributions
Conceptualization, S.G. and C.C.; methodology, C.C.; validation, N.B., E.C. and S.S.; data curation, N.B.; writing—original draft preparation, S.G., C.C. and R.I.; writing—review and editing, M.P., E.C., N.B. and S.S.; visualization, E.C. and N.B.; supervision, A.B.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Papi, P.; Pompa, G. The Use of a novel porcine derived acellular dermal matrix (mucoderm) in peri-implant soft tissue augmentation: Preliminary results of a prospective pilot cohort study. BioMed Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Papi, P.; Carlo, S.D.; Mencio, F.; Rosella, D.; Angelis, F.D.; Pompa, G. Dental implants placed in patients with mechanical risk factors: A long-term follow-up retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, S48–S51. [Google Scholar] [CrossRef]
- Marchio, V.; Derchi, G.; Cinquini, C.; Miceli, M.; Gabriele, M.; Alfonsi, F.; Barone, A. Tissue level implants in healthy versus medically compromised patients: A cohort comparative study. Minerva Stomatol. 2020, 69, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mencio, F.; De Angelis, F.; Papi, P.; Rosella, D.; Pompa, G.; Di Carlo, S. A randomized clinical trial about presence of pathogenic microflora and risk of peri-implantitis: Comparison of two different types of implant-abutment connections. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1443–1451. [Google Scholar]
- Linkevicius, T.; Apse, P. Influence of abutment material on stability of peri-implant tissues: A systematic review. Int. J. Oral Maxillofac. Implant. 2018, 23, 449–456. [Google Scholar]
- Cinquini, C.; Marchio, V.; Di Donna, E.; Alfonsi, F.; Derchi, G.; Nisi, M.; Barone, A. Histologic Evaluation of Soft Tissues around Dental Implant Abutments: A Narrative Review. Materials 2022, 15, 3811. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants. Mater. Res. Express. 2020, 7, 015417. [Google Scholar]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 32–49. [Google Scholar] [CrossRef]
- Stefanini, M.; Barootchi, S.; Sangiorgi, M.; Pispero, A.; Grusovin, M.G.; Mancini, L.; Zucchelli, G.; Tavelli, L. Do soft tissue augmentation techniques provide stable and favorable peri-implant conditions in the medium and long term? A systematic review. Clin. Oral Implant. Res. 2023, 34 (Suppl. 26), 28–42. [Google Scholar] [CrossRef] [PubMed]
- Zuhr, O.; Bäumer, D.; Hürzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41, S123–S142. [Google Scholar] [CrossRef]
- Mathes, S.H.; Wohlwend, L.; Uebersax, L.; Mentlen, R.V.; Thoma, D.S.; Jung, R.E.; Görlach, C.; Graf-Hausner, U. A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol. Bioeng. 2010, 107, 1029–1039. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Atieh, M.A.; Shah, M.; Hakam, A.; Alshaali, S.; Kasouha, R.; Tawse-Smith, A.; Alsabeeha, N.H.M. Xenogeneic Collagen Matrix Versus Free Gingival Graft for Augmenting Peri-Implant Keratinized Mucosa Around Dental Implants: A Systematic Review and Meta-Analysis. Clin. Exp. Dent. Res. 2024, 10, e932. [Google Scholar] [CrossRef] [PubMed]
- Ashurko, I.; Tarasenko, S.; Magdalyanova, M.; Bokareva, S.; Balyasin, M.; Galyas, A.; Khamidova, M.; Zhornik, M.; Unkovskiy, A. Comparative analysis of xenogeneic collagen matrix and autogenous subepithelial connective tissue graft to increase soft tissue volume around dental implants: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 741. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45, S230–S236. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Tessarolo, F.; Caola, I.; Wennström, J.; Nollo, G.; Berglundh, T. Morphogenesis of peri-implant mucosa revisited: An experimental study in humans. Clin. Oral Implant. Res. 2014, 25, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Tessarolo, F.; Caola, I.; Piccoli, F.; Wennström, J.L.; Nollo, G.; Berglundh, T. Early healing of peri-implant mucosa in man. J. Clin. Periodontol. 2016, 43, 816–824. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J.; Lindhe, B.T. Dimension of the periimplant mucosa. Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar]
- Schwarz, F.; Mihatovic, I.; Becker, J.; Bormann, K.H.; Keeve, P.L.; Friedmann, A. Histological evaluation of different abutments in the posterior maxilla and mandible: An experimental study in humans. J. Clin. Periodontol. 2013, 40, 807–815. [Google Scholar] [CrossRef]
- Katagiri, H.; Tawil YEl Lang, N.P.; Imber, J.C.; Sculean, A.; Fujioka-Kobayashi, M.; Saulacic, N. Collagen-Based Matrices for Osteoconduction: A Preclinical In Vivo Study. Biomedicines 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue response to a porous collagen matrix used for soft tissue augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Weijden FVan Der Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: The pink esthetic score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Lissek, M.; Boeker, M.; Happe, A. How thick is the oral mucosa around implants after augmentation with different materials: A systematic review of the effectiveness of substitute matrices in comparison to connective tissue grafts. Int. J. Mol. Sci. 2020, 21, 5043. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Chambrone, D.; Pustiglioni, F.E.; Chambrone, L.A.; Lima, L.A. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J. Dent. 2008, 36, 659–671. [Google Scholar] [CrossRef]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.F.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative Complications Following Gingival Augmentation Procedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brook, I.M.; Noort RVan Scutt, A.M.; Smith, K.G.; Thornhill, M.H. Development, optimization and characterization of a full-thickness tissue engineered human oral mucosal model for biological assessment of dental biomaterials. J. Mater. Sci. Mater. Med. 2008, 19, 1793–1801. [Google Scholar] [CrossRef]
- Moraschini, V.; de Almeida, D.C.F.; Sartoretto, S.; Guimarães, H.B.; Cavalcante, I.C.; Calasans-Maia, M.D. Clinical efficacy of xenogeneic collagen matrix in the treatment of gingival recession: A systematic review and meta-analysis. Acta Odontol. Scand. 2019, 77, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Puzio, M.; Błaszczyszyn, A.; Hadzik, J.; Dominiak, M. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix—1-year randomised follow-up. Ann. Anat. 2018, 217, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Rodriguez-Archilla, A.; Osorio, R. Collagen matrix vs. Autogenous connective tissue graft for soft tissue augmentation: A systematic review and meta-analysis. Polymers 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Sancho-Puchades, M.; Ettlin, D.A.; Hämmerle, C.H.F.; Jung, R.E. Impact of a collagen matrix on early healing, aesthetics and patient morbidity in oral mucosal wounds—A randomized study in humans. J. Clin. Periodontol. 2012, 39, 157–165. [Google Scholar] [CrossRef]
- Alccayhuaman, K.A.A.; Tangl, S.; Blouin, S.; Hartmann, M.A.; Heimel, P.; Kuchler, U.; Lee, J.-S.; Gruber, R. Osteoconductive properties of a volume-stable collagen matrix in rat calvaria defects: A pilot study. Biomedicines 2021, 9, 732. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef]
- Aldhohrah, T.; Qin, G.; Liang, D.; Song, W.; Ge, L.; Mashrah, M.A.; Wang, L.; Al-Moraissi, E. Does simultaneous soft tissue augmentation around immediate or delayed dental implant placement using sub-epithelial connective tissue graft provide better outcomes compared to other treatment options? A systematic review and meta-analysis. PLoS ONE 2022, 17, e0261513. [Google Scholar] [CrossRef]
- Yamahara, S.; Montenegro Raudales, J.L.; Akiyama, Y.; Ito, M.; Chimedtseren, I.; Arai, Y.; Wakita, T.; Hiratsuka, T.; Miyazawa, K.; Goto, S.; et al. Appropriate pore size for bone formation potential of porous collagen type I-based recombinant peptide. Regen. Ther. 2022, 21, 294–306. [Google Scholar] [CrossRef]
- Krieghoff, J.; Picke, A.K.; Salbach-Hirsch, J.; Rother, S.; Heinemann, C.; Bernhardt, R.; Kascholke, C.; Möller, S.; Rauner, M.; Schnabelrauch, M.; et al. Increased pore size of scaffolds improves coating efficiency with sulfated hyaluronan and mineralization capacity of osteoblasts. Biomater Res. 2019, 23, 26. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Liu, L.; Xu, D.; Liu, Y.; Li, S.; Hou, W.; Wang, J.; Chen, X.; Sheng, L.; et al. Improving osteoinduction and osteogenesis of Ti6Al4V alloy porous scaffold by regulating the pore structure. Front Chem. 2023, 11, 1190630. [Google Scholar] [CrossRef]
- Chao, L.; Jiao, C.; Liang, H.; Xie, D.; Shen, L.; Liu, Z. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front Bioeng Biotechnol. 2021, 9, 779854. [Google Scholar] [CrossRef]
- Artzi, N.; Oliva, N.; Puron, C.; Shitreet, S.; Artzi, S.; bon Ramos, A.; Groothuis, A.; Sahagian, G.; Edelman, E.R. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat. Mater. 2011, 10, 890. [Google Scholar] [CrossRef]
- Mohseni, M.; Cometta, S.; Klein, L.; Wille, M.L.; Vaquette, C.; Hutmacher, D.W.; Savi, F.M. In vitro and in vivo degradation studies of a dual medical-grade scaffold design for guided soft tissue regeneration. Biomater. Sci. 2025, 13, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Imber, J.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of vertical ridge augmentation interventions: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef]
- Thoma, D.S.; Buranawat, B.; Hämmerle, C.H.F.; Held, U.; Jung, R.E. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: A systematic review. J Clin Periodontol. 2014, 41, S77–S91. [Google Scholar] [CrossRef]
- Sasagawa, A.; Igarashi, K.; Ueda, K.; Hiroyasu, K.; Watanabe, F. Peri-implant tissue augmentation by volume-stable collagen matrix transplantation: A study of dog mandibles. Odontology 2022, 110, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Naenni, N.; Benic, G.I.; Hämmerle, C.H.F.; Jung, R.E. Soft tissue volume augmentation at dental implant sites using a volume stable three-dimensional collagen matrix—Histological outcomes of a preclinical study. J. Clin. Periodontol. 2017, 44, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jepsen, K.; Sailer, I.; Strasding, M.; Zeltner, M.; Cordaro, L.; di Torresanto, V.M.; Schwarz, F.; Zuhr, O.; Akakpo, D.; et al. Efficacy of a collagen matrix for soft tissue augmentation after implant placement compared to connective tissue grafts: A multicenter, noninferiority, randomized controlled trial. Clin. Oral Implant. Res. 2023, 34, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gasser, T.J.W.; Jung, R.E.; Hämmerle, C.H.F. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.W.; Hämmerle, C.H.F.; Strauss, F.J.; Jung, R.E. Soft tissue augmentation with a volume-stable collagen matrix or an autogenous connective tissue graft at implant sites: Five-year results of a randomized controlled trial post implant loading. J. Periodontol. 2023, 94, 230–243. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Ravidà, A.; Oh, T.J.; Wang, H.L. What Is the Safety Zone for Palatal Soft Tissue Graft Harvesting Based on the Locations of the Greater Palatine Artery and Foramen? A Systematic Review. J. Oral Maxillofac. Surgery. 2019, 77, 271.e1–271.e9. [Google Scholar] [CrossRef]
- Santamaria, M.P.; Miguel, M.M.V.; Rossato, A.; Bonafé, A.C.F.; de Souza, I.V.; Martins, T.M.; Nunes, M.P.; Mathias-Santamaria, I.F. Volume-stable collagen matrix to treat gingival recession associated with non-carious cervical lesions: Randomized clinical trial. J. Periodontol. 2015. Online ahead of print. [Google Scholar] [CrossRef]
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Janakievski, J.; Scheyer, E.T.; Velásquez, D.; Gunsolley, J.C.; Heard, R.H.; Morelli, T. Efficacy of a harvest graft substitute for recession coverage and soft tissue volume augmentation: A randomized controlled trial. J. Periodontol. 2022, 93, 333–342. [Google Scholar] [CrossRef]
- Puisys, A.; Deikuviene, J.; Vindasiute-Narbute, E.; Razukevicus, D.; Zvirblis, T.; Linkevicius, T. Connective tissue graft vs porcine collagen matrix after immediate implant placement in esthetic area: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2022, 24, 141–150. [Google Scholar] [CrossRef]
- Del Amo, F.S.L.; Yu, S.H.; Sammartino, G.; Sculean, A.; Zucchelli, G.; Rasperini, G.; Felice, P.; Pagni, G.; Iorio-Siciliano, V.; Grusovin, M.G.; et al. Peri-implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int. J. Environ. Res. Public Health 2020, 17, 2281. [Google Scholar] [CrossRef]
- Jepsen, K.; Sculean, A.; Jepsen, S. Complications and treatment errors related to regenerative periodontal surgery. Periodontol. 2000 2023, 92, 120–134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).