Abstract
Petroleum pollution can pose a serious threat to soil health and its ecological functions. This study investigated the efficacy of bacterial treatments for bioremediation of diesel-contaminated soil under outdoor conditions for a period of 4 months. Unlike most previous studies conducted under laboratory conditions, this study applied single and multi-bacterial consortia directly into diesel-contaminated soil under outdoor conditions, evaluating both hydrocarbon degradation and soil toxicity changes. Three treatments using a single strain, a 4-strain consortium, and an 8-strain consortium were applied to 2% (v/w) diesel-contaminated soil, and their performance was compared to uncontaminated and untreated controls. Total petroleum hydrocarbon (TPH) degradation was quantified using GC-FID, and the soil toxicity was assessed using Eisenia fetida toxicity test and higher plant germination assays. As the experiment demonstrated, the multi-strain bacterial consortium (BT3) achieved the highest TPH degradation (78.3%) and demonstrated significant reduction in long-chain hydrocarbon fractions (C14-C28). Toxicity measurements showed that all three bioremediation treatments, especially BT3, significantly increased earthworm survival, body weight change and plant germination rate after the bioremediation. Microbial community analysis based on 16S rRNA sequencing revealed significant shifts in the dominant bacterial genera over time, accompanied by a noticeable reduction in alpha diversity. In particular, BT3 showed a significant decrease in Shannon diversity index values from 9.4 at S1 to 6.9 at S3 (p < 0.01), whereas BT1 and BT2 remained relatively stable (p > 0.05). Overall, the results demonstrated that all three bacterial treatments significantly enhanced diesel degradation and reduced soil toxicity under outdoor conditions, highlighting their potential for future large-scale applications in sustainable soil remediation. Importantly, this study combines constructed microbial consortia with multi-level toxicity assessments, providing a comprehensive framework to guide future bioremediation strategies.
1. Introduction
Passive hydrocarbon degradation via natural processes, such as those occurring in soil and water, can alleviate petroleum pollution over time. However, these processes are typically slow and inefficient, particularly in areas with high contamination levels [1,2]. To accelerate bioremediation, the introduction of external microbial agents has been reported to enhance TPH degradation rates by over 30% compared with natural attenuation, while also increasing bacterial diversity and facilitating soil remediation [3,4]. This faster bioremediation method is particularly advantageous in situations that demand urgent environmental interventions to prevent further damage.
Microbial consortia are mixtures of microbes that work together to co-metabolise organic pollutants [5]. However, despite their success in controlled laboratory settings, microbial consortia often face challenges when applied in natural environments. Previous studies suggested that while bacterial consortia degrade hydrocarbons effectively under laboratory conditions, their efficacy tends to be reduced when implemented in field applications [6]. Therefore, it is crucial to conduct field-based trials to validate the practicality and effectiveness of these bioremediation strategies in natural settings.
Furthermore, while the primary goal of bioremediation is hydrocarbon degradation, the process can also alter the chemical and biological properties of the soil, which may increase the toxicity post-remediation [7]. PAH derivatives (e.g., oxygenated, alkylated, and nitro PAH) often coexist with parent compounds in PAH-contaminated soils [8]. However, the concentrations of these intermediates are not usually measured during soil remediation and therefore are not commonly accounted for in risk assessments. Several studies have reported that these intermediate degradation products may pose a greater risk to human and ecological receptors [9]. Such risks may be more severe than previously anticipated [10]. Thus, focusing only on contaminant removal may not fully capture the environmental and health risks posed by the remediated soils. As a result, comprehensive soil toxicity assessments, integrating chemical analysis with various toxicity assays, are essential for accurately evaluating soil safety post-remediation [11,12,13]. Therefore, this study evaluated the effectiveness on single strain and mixed culture consortia to degrade diesel in artificially spiked soil and conducted detailed toxicity evaluations of soil and soil water extracts at the start and end of the trial-plot experiment, to evaluate both bioremediation efficacy and potential residual toxicity of remediated soils.
Bioremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated soils typically include natural attenuation, biostimulation, bioaugmentation, phytoremediation, and composting [14]. Among them, organic amendment and composting methods shown the highest removal rates for polycyclic aromatic hydrocarbons (PAHs) in soil (removal rates typically range from 57% to 89%), which was better than many other bioremediation methods [15]. The degradation efficacy of bacteria consortia typically outperforms single strains, and their degradation performance can be further enhanced through strategies such as consortium design or immobilisation [16,17]. Field applications have confirmed its feasibility, but practical implementation is often limited by reduced microbial activity and slower degradation rates, leading to longer bioremediation times and declines in soil biodiversity [18]. In addition, the performance of introduced consortia can vary due to site heterogeneity, pollutant aging, and limited contaminant bioavailability [19]. Another concern is the formation of oxidized (OPAH) and nitrated (NPAH) derivatives of PAHs, as well as other intermediates generated during degradation [20]. These compounds are often more toxic and persistent than their parent PAHs, and they are rarely included in routine monitoring [21]. These findings motivated us to pair chemical measurements with ecotoxicological analyses, allowing us to assess both removal efficiency and residual risks under outdoor conditions.
Therefore, this study aimed to evaluate the effectiveness of constructed bacterial consortia in the bioremediation of diesel-contaminated soils under natural conditions. To achieve this, outdoor remediation trials were conducted using different bacterial treatments, and both diesel degradation and soil toxicity were assessed before and after treatment. To the best of our knowledge, there is no prior study focus on the outdoor trial while also integrating hydrocarbon degradation analysis with multiple soil toxicity bioassays. This combined analysis provides a more realistic and comprehensive evaluation of bioremediation strategies, providing valuable insights into both bioremediation efficacy and ecological safety.
2. Materials and Methods
Diesel was purchased from a local fuel station and stored at 4 °C in the dark until use. Eisenia foetida (Tiger worms) was purchased from Original Organics (Hitchin, England). Daphtoxkit F kit and Phytotoxkit Solid Samples kit were purchased from MicroBiotest Inc. (Gent, Belgium). Alivibrio fisherii strain ATCC 49387 was purchased from the American Type Culture Collection (Manassas, VA, USA). All the other chemicals and reagents used in this study were purchased from Merck Life Science Limited (Wicklow, Ireland) and used as received.
2.1. Preparation of Petroleum-Degrading Bacterial Consortia
Eight hydrocarbon-degrading bacterial strains, Acinetobacter seifertii (represented by three distinct strains: BHA_1, NA_1, and TRI_1), Acinetobacter vivianii, Bacillus subtilis, Rhodococcus qingshengii, Cellulosimicrobium cellulans, and Microbacterium algeriense were used for the bioremediation process. These strains were previously isolated from petroleum-contaminated sites in Carlow (Ireland) or Nigeria, identified through 16S rRNA gene sequencing and whole genome sequencing analysis, and selected based on their diesel degradation capabilities. Details of the bacterial consortia and corresponding strain IDs are shown in Table 1.

Table 1.
Bacterial composition in three bacterial treatments.
Strains were grown individually in diesel-supplemented Bushnell-Haas broth, adjusted to OD600 = 1.0, and combined to form three bacterial treatments: BT1 (BHA_1 only), BT2 (four strains in equal proportions), and BT3 (all eight strains in equal proportion).
2.2. Preparation of Experimental Soil
Commercial topsoil and horticultural sand (Westland) were mixed at a 3:1 (w/w) ratio, sieved (10–200 mesh), and spiked with diesel (20 mL/kg soil; 16,118 mg/kg), followed by thorough mixing and a two-week aging period in a greenhouse [22]. Soil physicochemical properties were analysed prior to contamination. The soil exhibited neutral pH (7.0 ± 0.1), with 4.7 ± 0.6% organic matter, 0.16 ± 0.02% total nitrogen, 719.4 ± 58.9 mg/kg total phosphorus, and water-holding capacity of 49.3 ± 6.8%. The soil texture was classified as sandy loam, with additional details in Table S1. pH was measured based on the method described by FAO [23]. Soil water-holding capacity (WHC) was determined by the gravimetric method as described by Nelson, Adjuik [24]. Soil texture analysis by the ‘Mason Jar Soil Test’ as described by Deshpande [25]. The soil organic matter content was quantified using the Loss on Ignition (LOI) method following the method reported by Satoh, Ishizuka [26], while total phosphate and total nitrogen concentrations were analysed by an external laboratory (IAS Laboratories, Carlow, Ireland).
2.3. Experimental Design for the Out-Door Soil Remediation Trial
The experiment was carried out in plastic plant pots (volume 10.63 L). Five different treatments were established, and each treatment was replicated five times. Each pot was filled with 3 kg of soil, and the bacterial consortia were inoculated into the diesel-contaminated soil at a ratio of 10% v/w.
To ensure proper nutrient balance for microbial activity, 25 mL of 1 M ammonium chloride solution was added. In addition, 0.23 g of a pre-mixed NPK fertilizer blend was dissolved in 75 mL of deionized water. The fertilizer contained:
- 18% Nitrogen (11.7% ammoniacal nitrogen, 6.3% nitric nitrogen)
- 60% Phosphorus (6% soluble in neutral ammonium citrate and water; 54% water-soluble)
- 12% Potassium (water-soluble)
This nutrient amendment adjusted the carbon, nitrogen, and phosphorus (C:N:P) ratio to 100:10:1, as recommendations by Ouriache, Moumed [27] and Ou, Wu [28].
The soil moisture content was maintained at 50% WHC. The pots were covered with sterilized plastic bags with small openings for aeration and contamination control. The experiment was performed under outdoor environmental conditions from May 2024 to September 2024 (4 months), during which the average rainfall was 47.7 mm, and the average temperature was 14.4 °C (Met Eireann.ie).
The 5 experimental treatments were performed as follows:
- Treatment 1 (CSC): untreated clean soil control;
- Treatment 2 (DCS): 2% (v/w) diesel-contaminated soil;
- Treatment 3 (BT1): 2% (v/w) diesel-contaminated soil + 10% bacterial consortia;
- Treatment 4 (BT2): 2% (v/w) diesel-contaminated soil + 10% bacterial consortia;
- Treatment 5 (BT3): 2% (v/w) diesel-contaminated soil + 10% bacterial consortia.
Experiments were conducted in an open courtyard using a randomized block design (Figure S1). Pots with clean soil were placed around the experimental setup to minimize environmental edge effects. Soil samples were collected in triplicate on days 0 (S1), 60 (S2), and 120 (S3).
2.4. Extraction and Quantification of Petroleum Hydrocarbons in Soil
Soil petroleum hydrocarbons were extracted using the Soxhlet extraction method documented in EPA Method 3540C [29]. Briefly, 10 g of soil samples were extracted for 18 h at 70 °C using 300 mL of hexane: acetone (1:1, v/v) in Soxhlet apparatuses. The extracted samples were then diluted to 5 mL with n-Hexane. Afterward, 200 µL of the sample was diluted with 800 µL of n-Hexane again (five times dilution) and filtered through 0.22 µm PTFE acetate syringe filter. The filtered samples were injected into a GC-FID equipped with a DB-TPH GC column. The injector temperature was set at 300 °C, and samples were injected in splitless mode. Samples (1 µL) were injected at 300 °C in splitless mode with nitrogen and hydrogen as carrier gases (1.5 and 30 mL/min). The oven temperature was set as follows: initial temperature was 50 °C and held for 3 min, then increased to 180 °C and held for 5 min and finally increased to 320 °C and held for 10 min. The final concentration of the extracted samples was calculated using Equation (1) as shown below:
where
CGC (ppm, mg/L): concentration of extracts obtained from the diesel standard curve.
D: dilution factor (which is 5 here).
Vfinal: The final volume of the extracts (which is 5 mL = 0.005 L here).
Msoil: The weight of the soil used (which is 10 g = 0.01 kg here).
For example, if the GC-FID measured extract concentration (CGC) was 200 mg/L, with a dilution factor of 5, a final extract volume of 0.005 L, and a soil sample mass of 0.01 kg (10 g), the TPH concentration was 500 mg/kg.
The diesel hydrocarbon degradation rate (%) was calculated using Equation (2):
where
CSample (mg/kg): diesel hydrocarbon concentration in test soil at the time of sampling.
CS1 (mg/kg): diesel hydrocarbon concentration in test soil at the soil sampling point S1.
2.5. Total Viable Bacterial Cell Counts in Soil
The total viable bacterial cell count in the soil was estimated using the drop plate method as previously described by Lally et al., [30]. Briefly, 1.0 g of soil was suspended in 9 mL of sterile 0.85% saline solution to make a 10−1 dilution, followed by serial dilutions up to 10−9. For each dilution, 10 µL solution was dropped five times onto designated segments of nutrient agar plates. The plates were air-dried in a laminar flow hood, then inverted and incubated at 30 °C for 24 h. Colony-forming units (CFUs) were counted, and the average CFU count per drop was used to calculate the CFU per gram of soil (Equation (3)), expressed as CFU/g soil [31].
where
∑C: total colony counts from multiple drops.
n: number of drops (which is 5 here).
100: conversion factor (to adjust from 10 uL plated to 1 mL).
10−d: dilution factor.
2.6. Estimation of TPHs Degraders Counts in Soil Using the Most Probable Number (MPN) Method
The TPH degraders in soil were measured by the microtiter-based most probable number (MPN) method according to Johnsen, Boe [32] with slight modifications. Specifically, 1.0 g of soil was serially diluted (10−1 to 10−8) in minimal salts broth and inoculated into microtiter wells containing 100 µL of 1% (v/v) diesel solution in n-hexane. Controls included wells with and without diesel or bacterial inoculation, and the microtiter plates were incubated at 30 °C for 7 days. After incubation, 50 µL of an electron donor solution and 10 µL of WST-1 reagent were added to each well. The plates were incubated at room temperature for 5 h with shaking at 300 rpm, and the dehydrogenase activity was assessed by the colorimetric reduction of WST-1 to formazan. Most Probable Number (MPN) values were calculated based on positive wells and dilution series using Equation (4).
2.7. Soil Sampling, DNA Extraction, and Sequencing Analysis
Soil sampling was conducted at three time points: at the beginning of the experiment (S1), at two months (S2), and at 4 months of the experiment (S3). At each sampling point, the soil in each pot was properly mixed with a hand spade. Soil DNA extraction was performed using the FastDNA® Spin Kit for Soil (MPBio, CA, USA), following the kit’s specifications with some modifications. Briefly, 2 g of soil was suspended in 0.85% NaCl, vortexed, and centrifuged to remove debris. The supernatant was further centrifuged, and the pellet resuspended in PBS. Samples were pretreated with lysozyme (60 µL/mL, 1 mg/mL) at 37 °C for 1 h before proceeding with the kit protocol [18].
The bacterial microbiome sequence data generated in this study have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject No. PRJNA1247371. The bacterial 16S rRNA gene sequencing was conducted by Novogene Co., Ltd. (Cambridge, UK) using the Illumina MiSeq platform with a paired-end 250 bp (PE250) sequencing strategy. The V3–V4 hypervariable region of the 16S rRNA gene was amplified using standard primers. The resulting datasets have been archived under BioSample Numbers SAMN47810523–SAMN47810537 and SAMN47812186–SAMN47812215 (release date: 1 November 2026). Sequence data analysis was performed using a standard pipeline, including quality filtering, denoising, and amplicon sequence variant (ASV) inference using DADA2 in QIIME2 [33].
2.8. Earthworm Toxicity Test
Soil toxicity tests were conducted before and after bioremediation soils following OECD 207 [34]. Eisenia fetida were pre-conditioned in clean soil and fed a leaf-oatmeal mix. Ten washed and weighed earthworms were placed into 500 g of test soil in plastic pots (triplicates per treatment). All pots were kept in the dark at room temperature and fed weekly. Mortality and body weight were recorded on days 7 and 14 to calculate mortality and weight loss percentages (Equations (5) and (6)).
2.9. Higher Plant Toxicity Test
The Phytotoxkit was used to assess the phytotoxicity of soil samples before and after bioremediation [35]. Following the manufacturer’s instructions, soil samples (90 cm3) were tested with seeds of Sinapis alba, Lepidium sativum, and Sorghum saccharatum for 3 days. Germination inhibition was measured to determine the toxicity level of the soil samples [36]. The inhibition percentage of seed germination (IG) of the plant was calculated using Equation (7).
where
Mgf: the mean number of germination seeds in reference soil.
Mgt: the mean number of germination seeds in test soil.
2.10. Acute Toxicity Test of Daphnia magna
Water extraction from diesel-contaminated soil was carried out according to the ‘Solid waste-Extraction procedure for leaching toxicity-Horizontal vibration method’ (HJ 557-2010) [37].
The toxicity of the water extracts was assessed using the Daphtoxkit F (Microbiotest, Belgium) following the manufacturer’s instructions [38]. A dilution series (100% to 6.25%) was prepared. Each test well received 10 mL of solution and five D. magna, with four replicates per treatment. After 24–48 h of incubation at 20 °C in the dark, mortality was recorded to assess toxicity [39].
2.11. Acute Toxicity Test of Luminescent Bacteria
The toxicity of soil water extracts was assessed using Aliivibrio fischeri (ATCC 7744) in a short-term luminescence inhibition assay [40]. Cultures were grown overnight in nutrient broth with 3% NaCl at 25 °C and harvested at OD600 > 1.0. For each test, 100 µL of bacterial culture was mixed with 100 µL of soil water extract in a black 96-well plate, with samples tested in triplicate. All samples were adjusted to 3% NaCl. After 15 min of incubation at 25 °C and 120 rpm, luminescence was measured using a microplate reader. Inhibition was calculated using Equation (8).
where
Ls: the luminescence intensity of test soil samples’ water extracts.
Ln: the luminescence intensity of negative control (3% NaCl).
2.12. Statistical Analysis
All data were organized and stored using Excel 2401. The data were checked for normality using the Shapiro–Wilk test (p > 0.05). Data that were normally distributed were analysed for statistical differences using ANOVA with the Tukey post hoc test (p < 0.05), while data that failed the normality test were analysed using the non-parametric Kruskal–Wallis test, followed by Dunn’s multiple comparisons test to determine significant differences (p < 0.05), using GraphPad Prism, version 9.0 for macOS.
3. Results and Discussion
3.1. Diesel Hydrocarbon Biodegradation and Fractional Analysis Across Treatments Diesel Degradation Study with GC-FID Analysis
Soil extracts from five treatments were analysed by GC-FID. The concentration of residual diesel hydrocarbons was calculated using a diesel standard curve (Table S2, Figure S2), and degradation rates are shown in Figure 1. CSC (untreated clean soil control) results were excluded due to negligible diesel content and undetectable GC-FID peaks. Additionally, Octafluoronaphthalene (OFN, 5 ppm) was added as a recovery standard to assess Soxhlet extraction efficiency. Recovery rates ranged from 92.31% to 92.80% across sampling points (Table S3), confirming consistent and reliable of the extraction method.
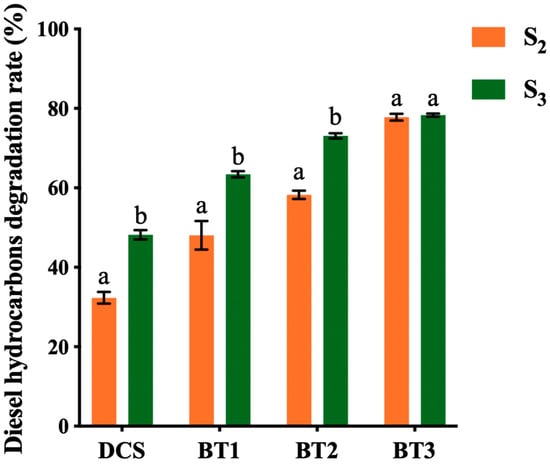
Figure 1.
The diesel hydrocarbon degradation rate (%) of four treatments across two sampling points (S2, and S3) (n = 3). Data are presented as mean ± SD (standard deviation). Soil treatments include DSC (diesel-contaminated soil), BT1 (bacterial treatment 1), BT2 (bacterial treatment 2), and BT3 (bacterial treatment 3). Different letters above the bar indicate a statistical difference between different sampling points within each sample.
The diesel hydrocarbon degradation rates for the DCS, BT1, BT2, and BT3 treatments at sampling points S2 and S3 were calculated using S1 as a reference. As shown in Figure 1, the diesel hydrocarbon degradation rate across all treatments showed significant and continuous increases over the bioremediation process (p < 0.05), indicating that diesel hydrocarbons gradually degraded during the bioremediation period. At S1, all treatments presented an initial degradation rate of approximately 30% (based on the ratio of their measured TPH concentrations in S1 to the theoretical initial concentration of 16,118 mg/kg). This phenomenon may be attributed to the loss during the soil mixing and aging process before the treatments, and the loss of lighter volatile and semi-volatile hydrocarbon fractions by volatilization, as well as the losses that occurred during Soxhlet extraction and sample preparation for GC-FID analysis [41]. Although separate control volatility experiments were not conducted in this study to directly quantify abiotic losses, Octafluoronaphthalene (OFN, 5 ppm) was used as a recovery standard to monitor extraction consistency. The recovery rates remained stable (92–93%; Table S3), confirming that extraction losses were minimal and controlled. Future studies should include abiotic controls to more accurately distinguish physical/chemical hydrocarbon losses from microbial degradation.
At S2, BT3 showed the highest improvement in diesel hydrocarbon degradation rate with 77.76% degradation, followed by BT2 and BT1 at 58.20% and 48.03%, respectively. Notably, the DCS (control) treatment exhibited the lowest degradation at 32.27%, representing the lowest remediation efficiency observed. These findings demonstrated that while varying in bioremediation efficacy, all inoculated bacterial treatments, including the single strains and consortia, significantly enhanced hydrocarbon degradation compared to the unamended control (DCS) (Table S4). Particularly, the bacterial consortium BT3 achieved a superior degradation rate during the first 2 months, establishing itself as the most effective biological treatment. At S3, bacteria treatments BT1 and BT2 continuously showed an increase in degradation rate, with 63.42% and 73.08%, respectively. In contrast, the BT3 treatment reached a stable level with no significant difference observed (p > 0.05). This plateau may have been caused by nutrient depletion, reduced bioavailability of the degradable hydrocarbons, oxygen limitation, or the accumulation of recalcitrant intermediates during the later stage of bioremediation [19,42]. As more complex and higher microbial density consortia, BT3 are likely to impose higher metabolic demands on available resources. Additionally, competitive interactions among the eight strains may have further constrained long-term activity, leading to stabilization of degradation levels [6]. Notably, the DCS treatment also presented a 48.19% degradation rate, demonstrating that indigenous bacteria were capable of degrading a significant fraction of petroleum hydrocarbons in soil at low concentrations [43]. These results demonstrated the diesel hydrocarbon degradation capacity of indigenous microorganisms, while also highlighting the efficient degradation ability and sustained degradation performance of the inoculated bacteria over a four-month bioremediation process.
These results aligned with previous studies, which reported that bioaugmentation can effectively enhance diesel hydrocarbon degradation [44,45]. Notably, the improvement in the degradation rate observed in the first two months (S1 to S2) was greater than in the last two months (S2 to S3). Similar results were reported by Miles, Gestler [46] and Kuppusamy, Maddela [47], showing that petroleum hydrocarbons in soil commonly undergo biphasic biodegradation, with rapid degradation in the initial phase followed by a much slower phase. Moreover, the bacterial treatments (BT1, BT2, and BT3) were found to have lower oil residue weights after Soxhlet extraction (Figure S3) and greater diesel hydrocarbon degradation rates compared to non-inoculated DCS control, suggesting that the inoculate bacteria or bacterial consortia can effectively contribute to the petroleum hydrocarbon degradation. This conclusion was supported by findings by Abena, Li [48] and Curiel-Alegre, de la Fuente-Vivas [49], who reported that bioaugmentation treatment exhibited better efficiencies than natural attenuation. Furthermore, during the bioremediation period, both BT1 and BT2 showed consistent and significant enhancement in diesel degradation rate and reduction in Soxhlet extraction weight, highlighting their valuable and sustained biodegradation performance. In contrast, BT3 treatment showed the most significant increase in the first two months (S1 to S2), but no significant differences were observed in the following two months (S2 to S3). This observation was possibly due to the limited contaminant bioavailability, change in soil conditions, decrease in soil nutrient levels, and survival of the inoculated hydrocarbon-degrading bacteria [50]. On the other hand, the petroleum-degrading microorganisms in the soil may lack the capability to degrade certain petroleum compounds, particularly the high-molecular-weight polycyclic aromatic hydrocarbons (PAHs), which are often more resistant to microbial degradation [51,52].
Overall, the results indicate that DCS, BT1, BT2, and BT3 treatments showed a significant decrease in soil diesel hydrocarbon content across all sampling points in the bioremediation process. Over the four-month bioremediation, BT3 exhibited the highest diesel hydrocarbon degradation rate of 78.34%, followed by BT2 at 73.08%, BT1 at 63.42%, and DCS at 48.19%.
To investigate the changes in diesel components during the degradation process, a diesel composition analysis was performed. Table 2 shows the average percentage degradation of hydrocarbons based on carbon chain length within the C8–C28 range, after 2 months (S2) and 4 months (S3) of bioremediation. Hydrocarbons above C28 (C28-C40) were excluded due to the inability to establish a reliable quantitative standard curve. The selected analytical window (C8–C28) aligns with EPA Method 1663, which typically reports diesel hydrocarbons within the C9-C30 range [53]. In addition, previous studies have also reported that C9-C26 is the dominant fraction in diesel [54]. Therefore, to ensure analytical accuracy and relevance, the analytical range was limited to C8-C28.

Table 2.
Percentage degradation of the hydrocarbon fractions (C10-C28) for each treatment at sampling points S2 and S3 (mean ± SD, n = 3). * Indicates statistical significance compared to the DCS treatment (negative control) at p < 0.05.
At S2, all treatments achieved complete degradation (100%) of the C8-C10 fraction, while the C10-C12 fraction also demonstrated a high degradation rate across treatments, ranging from 73.14% to 79.85%. These results indicated that low molecular weight (LMW) hydrocarbons (C8-C12) showed high degradation within the first two months. Similar results were reported by Manaswini, Sivagami [55], suggesting that LMW PAHs were more susceptible to microbial degradation due to their simpler structure and higher bioavailability. For long-chain hydrocarbons (C12-C28), BT1 and BT2 exhibited higher degradation rates than DCS treatment in most fractions. However, in most cases, these differences were not marked as statistically significant. Notably, both BT1 and BT2 showed significantly higher degradation rates for the C22-C24 fractions than DCS, suggesting that the bacteria inoculated in these treatments have a strong ability to target this range of hydrocarbons. In addition, BT3 showed significant improvement over DCS in C14-C16, C18-C20, and C22-C24 fractions, indicating the strong ability of the BT3 consortium to effectively degrade long-chain hydrocarbons. It was worth noting that all bacterial treatments (BT1, BT2, and BT3) achieved over 80% degradation for C20-C22 fraction within two months, while the DCS treatment also showed 70.43% degradation. These results indicated that C20-C22 fraction were more biodegradable under the tested soil conditions and can be efficiently degraded by both indigenous and inoculated bacteria.
At S3, all treatments exhibited a higher degradation rate compared to S2, indicating that hydrocarbon degradation progressed over time. For LMW hydrocarbons, similar trends were observed, with all treatments achieving 82.44% to 87.68% degradation of the C10-C12 fraction. These results confirmed that LMW hydrocarbons can be effectively removed through both bacteria treatments (BT1, BT2 and BT3) and untreated soil (DCS) under the tested soil conditions. For long-chain hydrocarbons (C12-C28), all bacteria treatments (BT1, BT2 and BT3) showed significantly higher degradation rates with C14-C16, C18-C20, C22-C24 and C26-C28 fractions compared to the DCS treatment. This indicates that, after 4 months, the bacterial treatments enhanced the degradation of a broader range of hydrocarbons compared to the control, demonstrating improved degradation efficiency. Moreover, BT2 and BT3 treatments also showed a significantly higher degradation with C16-C18 and C20-C22 fractions. This may be attributed to the inoculation of bacterial consortia in BT2 and BT3, enabling more efficient degradation across a broader range of hydrocarbons [56].
Overall, these results show that the bioremediation of diesel hydrocarbons increased over time, especially in the degradation of medium-long chain hydrocarbons (C12-C28). While the DCS treatment presented high degradation rates for LMW hydrocarbons (C8-C12), it was significantly less effective in long-chain hydrocarbons (C12-C28). Among the treatments, BT3 demonstrated the highest degradation rate at both sampling points, with significant improvements in multiple hydrocarbon fractions compared to DCS treatment. Moreover, all bacterial treatments (BT1, BT2, and BT3) showed improved degradation performance at both sampling points, indicating that inoculated bacteria/bacteria consortia can substantially enhance the degradation of long-chain hydrocarbons (C12-C28).
3.2. Soil Microbial Population Dynamics During Bioremediation
Total viable bacterial counts (log10 CFU/g) were monitored across five treatments over four months. Bacterial treatments (BT1–BT3) showed significantly higher initial counts due to bacterial addition, while control soils (CSC and DCS) had lower baseline levels. A decline in bacterial counts was observed in bacterial treatments from the first to the second sampling point, likely due to environmental stress, competition, and nutrient depletion. From the second to third sampling point, bacterial populations remained stable across all treatments, indicating microbial adaptation to soil conditions during the bioremediation process (Figure S4). As CFU counts quantify total viable bacteria, which do not distinguish inoculated strains from indigenous populations. Future study will use strain-specific qPCR targeting unique markers or genes to quantify the inoculum strains relative to native microbes.
TPH-degrading bacterial populations were also tracked throughout the bioremediation period. At the start, diesel-contaminated soil control (DCS) and bacterial treatments led to higher TPH-degrader counts compared to the untreated clean soil control (CSC), although some TPH degraders were unexpectedly present even in CSC, possibly due to background contamination. Across all treatments, populations of TPH-degrading bacteria showed no significant changes after the initial time point, suggesting a stable degrader population throughout the experiment (Figure S5).
3.3. Soil Bacterial Community Dynamics Analysis During Bioremediation
To analyse the change in soil bacterial community during the bioremediation process, soil samples were collected at each sampling point, and soil DNA was extracted and sent to Novogene Ltd. (Cambridge, UK) for microbiome analysis based on 16S rRNA sequencing.
As demonstrated in Figure 2, significant differences in the relative abundance of bacterial genera across treatments and sampling points were observed. For CSC treatment, the bacterial community was relatively stable over the bioremediation period. The dominant bacteria (relative abundance above 2%) identified were Sphingomonas, Gemmatimonadaceae Gemmatimonas, SWB02, Luteimonas and Acidibacter, which have been reported as commonly found in soil environments [57,58,59]. Notably, many bacterial strains of the genus Sphingomonas and Luteimonas have been reported with polycyclic aromatic hydrocarbons (PAH) degradation ability [60,61]. Moreover, species from the bacterial genus Gemmatimonas and Acidibacter were also found to be the keystone genus in the highly petroleum hydrocarbon-contaminated soils [6,62].
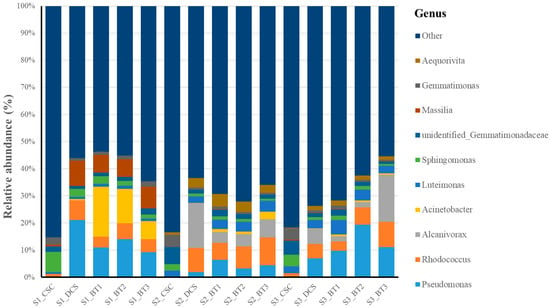
Figure 2.
Relative abundance of the top ten most abundant bacterial genera across five treatments (CSC = clean soil control, DCS = diesel-contaminated soil, BT1-BT3 = bacterial treatments 1–3) at three sampling points (S1 = sampling point 1, S2 = sampling point 2, S3 = sampling point 3). Bars represent mean values of triplicate samples (n = 3).
In the CSC treatment, the top 10 genera accounted for only 23 to 27% of the total abundance, indicating the clean bacterial community was more diverse compared to other treatments. Similar findings were previously reported by Abena, Chen [63] that petroleum pollution significantly reduced bacterial abundance and diversity, making unpolluted soils more diverse in their bacterial communities.
As for the DCS treatment, the bacterial community composition experienced significant changes compared to CSC. The dominant bacteria (relative abundance above 2%) were composed of Pseudomonas, Alcanivorax, Rhodococcus, Massilia, Aquabacterium, Sphingobium and Luteimonas. A few bacteria genera (Pseudomonas, Alcanivorax, and Rhodococcus) showed a notable increase in relative abundance compared to the CSC treatment. Although these genera have been previously reported with hydrocarbon degradation ability [64], their functional roles in this study remained unknown. Nevertheless, their enrichment suggested that selective variations in the bacterial community occurred as a result of the introduction of diesel.
In the bacterial inoculated treatments (BT1, BT2, and BT3), the composition of the bacterial community varied significantly during the bioremediation process. Specifically, at S1, Acinetobacter, Pseudomonas, Massilia and Rhodococcus were the most dominant genera, accounting for 28 to 40% of the total bacteria. When reaching the second sampling point (S2), the bacterial community structure was notably changed. In all three bacterial treatments, the relative abundance of genera such as Rhodococcus, Aequorivita, Alcanivorax, and Luteimonas increased substantially within 2 months of bioremediation [65]. The increased relative abundance of these genera may reflect their potential participation in the degradation of petroleum hydrocarbons. In contrast, Acinetobacter and Massilia were the dominant genera in S1 while showing a significant decline by S2, demonstrating that the inoculated Acinetobacter may have struggled to adapt to the soil environment or was outcompeted by other bacteria [66]. As 16S rRNA sequencing cannot distinguish inoculated strains from indigenous members of the same genus, this decline reflects changes at the genus level. Although primer bias cannot be fully excluded, the consistent pattern across different treatments suggests that ecological factors, such as nutritional competition and succession by other bacteria, or the inoculated Acinetobacter struggling to adapt to the soil environment, may have led to the reduced relative abundance of Acinetobacter. In a similar study reported by Li, Liang [67], Massilia may have played an important role in the early stages of degradation but was gradually replaced by other bacteria. In S3, the bacterial community composition continued to shift, with Pseudomonas, Rhodococcus, Alcanivorax, and Luteimonas, becoming the most abundant bacteria, accounting for approximately 20 to 41% of the total bacteria. This variation indicated that these bacteria may better adapt to the soil environment and may play crucial roles in the later stage of degradation.
Temporal changes in bacterial communities were observed at different sampling points across all treatments, suggesting a microbial succession process [68]. These changes may be due to the degradation of diesel hydrocarbons and the variations in environmental conditions and nutrient supplements [69]. To demonstrate these dynamics, the bacterial Shannon diversity index and principal coordinate analysis (PCoA) results are shown in Figure 3 and Figure 4 respectively.
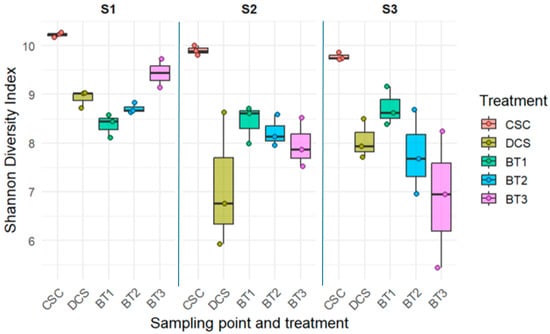
Figure 3.
Boxplot represents the bacterial Shannon diversity index of bacteria communities from each sampling point and treatment. Boxplots contain the Shannon index values of the three replicates of each sample (n = 3). Colours of boxplots according to the various treatments. CSC represents clean soil control, DCS represents diesel-contaminated soil treatment, BT1 represents bacterial treatment 1 sample, BT2 represents bacterial treatment 2 sample, and BT3 represents bacterial treatment 3 sample. S1 represents sampling point 1, S2 is sampling point 2, and S3 is equal to sampling point 3.
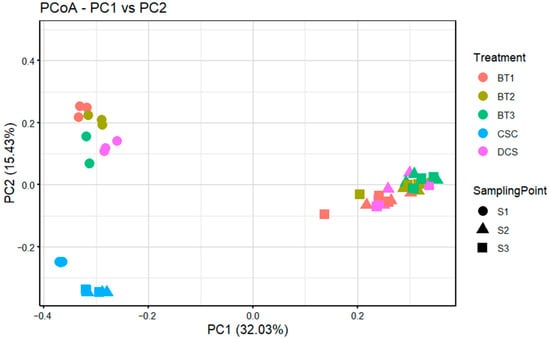
Figure 4.
The principal-coordinate analysis (PCoA) plots were based on the Bray–Curtis dissimilarity of bacterial communities of five treatments (CSC represents clean soil control, DCS represents diesel-contaminated soil treatment, BT1 represents bacterial treatment 1 sample, BT2 represents bacterial treatment 2 sample, and BT3 represents bacterial treatment 3 sample) across three sampling points (S1, S2 and S3). Colours and shapes according to each sample.
The Shannon index was used to characterize the diversity and evenness of bacterial species in the community, which is shown in Figure 3. In this study, the Shannon diversity index of CSC was found to have the highest value across all sampling points, with values ranging from 9.77 to 10.22. The consistently high diversity in CSC treatment indicated that under the natural environment, uncontaminated soil maintained a high level of species diversity and evenness over the 4-month period. Conversely, the DCS treatment showed a significant reduction in bacterial diversity from S1 to S2, with the Shannon index value dropping from 8.92 to 7.10. Afterward, the diversity remained stable from S2 to S3. Similarly, Li, Li [70] reported that petroleum pollution can significantly reduce the abundance and diversity of bacteria in the soil, which further demonstrated the negative impact of diesel contamination on soil health and the soil bacteria community. Additionally, diverse effects on soil bacterial diversity were noticed from BT1, BT2, and BT3. Among them, both BT1 and BT2 remained stable (no significant difference, p> 0.05) with the Shannon index value across all sampling points (ranging from 7.9 to 9.1). This consistency demonstrated that BT1 and BT2 possessed the potential to maintain the bacterial community diversity and evenness in soil. On the other hand, BT3 exhibited a significant decrease (p< 0.05) in the Shannon diversity index values from S1 to S3, implying a decline in soil bacteria diversity and evenness over the four months of bioremediation. This reduction was likely driven by the dominance of a few hydrocarbon-degrading genera, particularly Rhodococcus, Alcanivorax, and Luteimonas (Figure 2). These genera notably increased during bioremediation and accounted for a large proportion of the BT3 community at later stages. Their dominance reduced overall evenness and may have displaced less competitive native taxa, leading to the observed loss of diversity. These results suggest that more complex bacterial consortia may create stronger ecological interactions and competitive pressures on native microbial communities, resulting in reduced overall soil microbial diversity [71,72]. The Shannon diversity index in BT3 was lower than or comparable to DCS at both S2 and S3, suggesting that the BT3 consortium did not significantly enhance overall microbial diversity during this period.
Additionally, these results indicate a negative correlation between the number of strains included in the bacterial consortia and the soil microbial diversity. Bacterial consortia BT3 (containing eight strains) showed a significant decrease in Shannon diversity from S1 to S2 (p = 0.0406) and from S1 to S3 (p = 0.0003), with no significant difference observed between S2 and S3 (p = 0.1004). In contrast, bacterial consortia BT1 and BT2 (containing 1 and 4 strains, respectively) showed no significant difference (p > 0.05) during the whole bioremediation process. These results indicate that more complex bacterial consortia may create stronger ecological interactions and competitive pressure on native microbial communities, which potentially leads to a reduction in overall soil microbial diversity [73,74]. However, it is also possible that the observed reduction in diversity was driven by one or more specific strains uniquely present in BT3. Therefore, further investigation into the individual contributions of each strain would be required to understand these effects.
Principal Coordinate Analysis (PCoA) based on Bray–Curtis dissimilarity revealed distinct patterns in bacterial community dynamics across the three sampling points (Figure 4). The first two principal coordinates, PCo1 (32.03%) and PCo2 (15.43%), collectively accounted for 47.46% of the total variance in community composition. Notably, samples from the CSC treatment formed a tightly clustered group, segregated from all other treatments, indicating both temporal stability in its bacterial community structure and significant variance from other treatments. In contrast, treatments DCS, BT1, BT2, and BT3 exhibited spatial clustering correlated with sampling points: S1 samples grouped in the upper-left quadrant, while S2 and S3 samples coalesced in the mid-right region. This spatial segregation demonstrated higher intra-point community similarity, with S1 communities being significantly different from those in S2 and S3.
Overall, significant differences in bacterial community composition and diversity were observed across different treatments and sampling points. The CSC treatment showed consistently high diversity over the bioremediation process, demonstrating its ability to sustain a stable and diverse microbial community in the natural environment during the experiment. In contrast, the DCS treatment demonstrated significant changes in bacterial community structure and reduction in diversity, indicating the negative impact of diesel contamination on soil health and the soil bacteria community [75]. As for bacterial treatments (BT1, BT2, and BT3), their bacterial community composition changed over time, with a few known hydrocarbon-degrading genera (Rhodococcus, Alcanivorax, Aequorivita, and Luteimonas) becoming dominant as bioremediation progressed. However, the microbial communities in BT1, BT2, and BT3 showed high similarity to each other in terms of overall composition (β-diversity), indicating limited changes in community structure during the bioremediation process. Nevertheless, differences in α-diversity were observed, as Shannon diversity was significantly reduced in BT3 compared to BT1 and BT2. This distinction indicates that while the inoculated consortia enhanced diesel degradation without significantly altering the taxonomic structure of the communities, the more complex BT3 community influenced the richness and evenness by promoting the dominance of a few hydrocarbon-degrading taxa.
Additionally, the strong selective pressure from diesel contamination may have initially reduced bacterial diversity, as observed in the DCS and BT1 treatments. However, diversity in these treatments increased in later stage, suggesting partial recovery. In contrast, no such recovery was observed in BT2 and BT3, indicating that the microbial consortia introduced in these treatments may have had a more lasting impact on community diversity. Surprisingly, microbial diversity did not increase even as TPH levels declined, which may be due to the competitive dynamics within the microbial community, where the dominance of a few fast-growing or well-adapted species limited the re-establishment of a more diverse microbial community [76]. Moreover, the rapid decline in the relative abundance of inoculated bacteria over time suggests they may have struggled to survive in the diesel-contaminated soil or may have mainly participated in the early stages of bioremediation.
3.4. Toxicity Evaluation of Soil and Soil Water Extracts During Bioremediation
3.4.1. Effect of Diesel-Contaminated Soil (Before and After Remediation) on Earthworm (Eisenia foetida) Survival and Body Mass
Earthworms are widely regarded as one of the most suitable organisms for assessing soil toxicity [77,78]. This experiment employed earthworms Eisenia foetida to detect changes in the toxicity of diesel-contaminated soil before and after bioremediation. This test followed the 14-day acute exposure framework of OECD Test Guideline 207 (Earthworm, Acute Toxicity Tests) [34]. OECD 207 requires a control mortality rate ≤ 10% for validity, which was met in our controls (Figure 5). Additionally, as the soil test exhibited a single contamination level, it was not possible to derive an LC50.
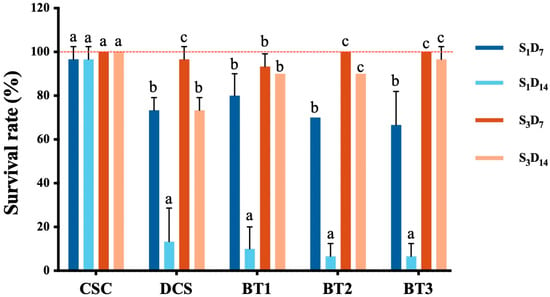
Figure 5.
The survival rate of earthworms (E. foetida after 7 and 14 days) of exposure in 5 treatments soil at the start (S1) and after 4 months (S3) of the experiment, values represent mean ± SD (n = 3, each replicate contains 10 earthworms). CSC represents clean soil control, DCS is diesel-contaminated soil treatment, BT1 is bacterial treatment 1 sample, BT2 is bacterial treatment 2 sample, and BT3 is bacterial treatment 3 sample. Letters a, b, c indication statistical significance.
In the CSC treatment, earthworm survival rates remained consistently high (nearly 100%), demonstrating the non-toxic nature of the uncontaminated control soil (Figure 5). Conversely, the DCS treatment at S1 exhibited a dramatic reduction in survival rates, declining from approximately 73% on day 7 to 13% by day 14. Similarly, bacteria treatments (BT1, BT2 and BT3) also showed a reduction in survival rates between day 7 and day 14. These observations were in agreement with findings by Almutairi [77], who reported a 60% survival rate for earthworms in 2% oil-contaminated soil after 7 days of exposure. Comparable results were stated by Geissen, Gomez-Rivera [79], with 70–90% mortality observed in earthworms exposed to 2% oil-contaminated soil. These collective results confirmed the toxic effects of diesel-contaminated soil on earthworm survival [80]. Furthermore, the declining survival rates under prolonged exposure may result from both the bioaccumulation of petroleum hydrocarbons and the inherent toxicity of diesel compounds, which adversely affect earthworm physiology and ultimately lead to mortality [81,82].
Following the 4-month bioremediation period (S3), earthworm survival rates in bacterial treatment soils (BT1, BT2, and BT3) demonstrated significant improvement, exceeding 90% viability at both 7- and 14-day exposure intervals. These survival rates in remediated soils were statistically comparable to those observed in the CSC control treatment. This demonstrated that the 4-month bioremediation process effectively reduced soil toxicity to levels that did not affect earthworm survival. While the DCS treatment exhibited increased earthworm survival relative to earlier sampling points, its final survival rate (approximately 73% at S3D14) remained substantially lower than other treatments. This differential performance confirmed that while natural attenuation can reduce soil toxicity, its efficacy remained limited compared to active bioremediation approaches [83].
Figure 6 shows the change in body mass of the earthworm at the difference sampling times. In CSC treatment, earthworms consistently gained weight, indicating a suitable environment with adequate nutrients for their growth. Specifically, after a 7-day exposure in non-contaminated soil, earthworms showed a 21.3% increase in body mass, in line with findings by Pochron, Mezic [84], who reported a 10.1% body mass increase in earthworms after their exposure for 7 days in clean soil. For diesel-contaminated treatments (DCS, BT1, BT2 and BT3), earthworms exhibited a continuous decline in body mass, with a significant reduction after 14 days due to high mortality rates. Li, Wang [78] and Zhou, Liang [85] also found that earthworms’ weight decreased significantly after exposure to petroleum-contaminated soil. This weight reduction suggested that the accumulation of toxic petroleum hydrocarbons in earthworms contributed to their continuous body mass loss and the increase in mortality rates.
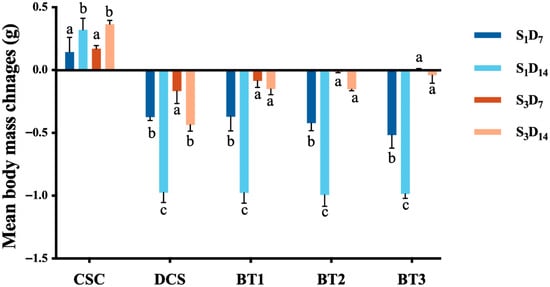
Figure 6.
Change in body mass of earthworms (after 7 days and 14 days) of exposure to five different soil treatments, which were collected at the start (S1) and after 4 months (S3) of the experiment; values represent mean ± SD (n = 3, each replicate contains 10 earthworms). Bars indicate the change in earthworm body mass from the start of each exposure period. Soil treatments include CSC (clean soil control), DCS (diesel-contaminated soil), BT1 (bacterial treatment 1), BT2 (bacterial treatment 2), and BT3 (bacterial treatment 3). Different letters above the bar indicate a statistical difference between different sampling points within each sample (p < 0.05).
In the experiment assessing the impact of soil toxicity on earthworm body mass change, notable differences were observed across treatments and time points. In both bacterial treatments (BT1, BT2, and BT3) and DCS treatment, the soil from S3 resulted in significantly less weight loss in earthworms than S1 soil (Table S4). These findings indicated a reduction in soil toxicity over 4 months of bioremediation. In addition, at S3, after 14-day exposure (S3D14), all bacterial treatments presented a significantly higher earthworm body mass than the DCS treatment (Table S5), suggesting that bacteria treatments can contribute to the decrease in toxicity. This finding aligned with the study by Kooliv and, Saeedi [86], who reported that as the TPH concentration decreased, the earthworm weight loss correspondingly declined. In our study, correlation analysis showed a significant positive relationship between TPH reduction and earthworm biomass changes at S3, both at Day 7 (r = 0.95, p = 0.047) and Day 14 (r = 0.99, p = 0.012), further confirming that enhanced hydrocarbon degradation directly reduced soil toxicity.
Overall, the results indicated a reduction in soil toxicity across the five treatments before and after the 4-month remediation period, as evidenced by earthworm survival rates and body weight changes after 7 and 14 days of exposure. It was worth noticing that bacterial-inoculated treatments (BT1, BT2, and BT3) exhibited substantially greater hydrocarbon degradation efficacy compared to the un-inoculated contaminated control (DCS).
3.4.2. Phytotoxicity of Diesel-Contaminated Soil (Before and After Remediation)
In this experiment, soil toxicity was determined using the Phytotoxkit (Microbiotest, Belgium), with a three-day bioassay to assess seed germination inhibition in three higher plant species exposed to contaminated soil. The inhibited percentage of seed germination was summarized in Table 3, and the result of the seed germination rate in five treatments soil were presented in Table S5.

Table 3.
Percentage inhibition of seed germination (IG), values represent mean ± SD (n = 3, each replicate contains 10 seeds). Five treatment soil samples were collected at the start (S1) and after 4 months (S3) of the experiment. Soil treatments include CSC (clean soil control), DSC (diesel-contaminated soil), BT1 (bacterial treatment 1), BT2 (bacterial treatment 2), and BT3 (bacterial treatment 3).
In this study, three higher plant species, Lepidium sativum, Sinapis alba, and Sorghum saccharatu (seeds provided in the Phytotoxkit) were utilized to conduct the soil toxicity test. Previous studies have demonstrated the sensitivity of these three plant species to petroleum-contaminated soils, thereby making them effective indicators for monitoring the changes in soil TPH content [87,88].
Among the three tested species, Lepidium sativum was the most sensitive to diesel contamination, showing the highest seed germination inhibition (36.7%) in untreated diesel-contaminated soil (DCS) at the start of the experiment. In contrast, Sorghum saccharatum and Sinapis alba showed greater resistance, with inhibition rates of 18.5% and 22.2%, respectively, indicating better tolerance to petroleum hydrocarbons in the early stage of exposure.
In the CSC treatment, seed germination rates across all plant species remained high at both sampling points, with inhibition values consistently low (0–7.4%), confirming its low or non-toxic characteristics. In contrast, all treatments with diesel-contaminated soil (DCS, BT1, BT2, and BT3) demonstrated toxic effects at S1, with relatively low seed germination rates compared to the CSC treatment across all three plant species. Among them, the DCS treatment showed the highest toxicity, with maximum inhibition values of 36.7% (Lepidium sativum), 22.2% (Sinapis alba), and 18.5% (Sorghum saccharatum). These findings align with previous reports by da Silva Correa, Blum [89] and Dib and Ahmed [90], who found that the presence of diesel initially reduced seed germination, and consequently limited their development. The DCS treatment showed the highest toxic effect with the maximum inhibition of seed germination across all plant species at both sampling points. In addition, bacterial treatments BT1, BT2 and BT3 presented comparable levels of toxicity with similar percentages of inhibition of seed germination in all sampling points. Moreover, for treatments with diesel-contaminated soil (DCS, BT1, BT2, and BT3), a considerable improvement was observed in seed germination rate at S3 compared to S1. Especially for bacterial treatments (BT1, BT2 and BT3), the results showed a substantial reduction in phytotoxicity over the 4 months of bioremediation and obtained near-complete recovery of seed germination in Sinapis alba and Lepidium sativum, and strong reductions in phytotoxicity in Sorghum saccharatum. Additionally, the correlation analysis confirmed that TPH reduction was strongly and significantly associated with reduced inhibition in Lepidium sativum (r = −0.98, p = 0.015). Although Sinapis alba (r = −0.88, p = 0.116) and Sorghum saccharatum (r = −0.66, p = 0.344) also showed negative trends, these were not statistically significant, likely due to the small sample size (n = 4). This observation suggests that the bioremediation process reduced the soil toxicity and consequently resulted in soil from S3 being less toxic to all three plant species compared to soil from S1. These results further indicate that different species have varied sensitivity and responses to diesel contamination [91].
Overall, the results indicated notable changes in soil toxicity across the five treatments following the 4-month bioremediation period, as collectively reflected in seed germination rate. These observations were consistent with the studies reported by Roy, Rutter [92] and Haider, Ejaz [93], who found that petroleum hydrocarbons significantly inhibited seed germination. After 4 months of bioremediation, only minor toxic effects were observed in bacterial treatments (BT1, BT2, and BT3) on plant species, demonstrating the effectiveness of these bacterial treatments in reducing the toxicity of diesel-contaminated soil.
3.4.3. Daphnid (Daphnia magna) Toxicity Test
The acute toxicity of soil water extracts was assessed using Daphnia magna and the Daphtoxkit F (Microbiotest, Belgium). Daphnia magna was exposed to 100% soil water extracts for 24 and 48 h, and mortality was recorded (Figure 7). The theoretically maximum concentration of the diesel in water extract was 1611.8 mg/L However, since many diesel components were not water-soluble, the actual concentration of diesel in the water extracts was expected to be lower than this theoretical value [94].
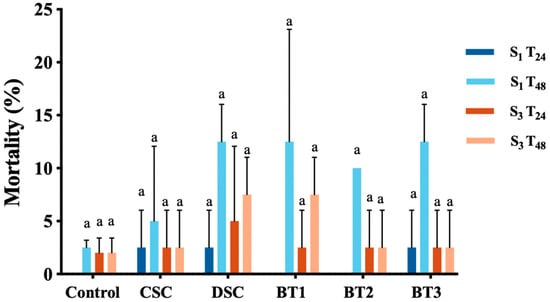
Figure 7.
Mortality (%) of D. magna after 24 and 48 h of exposure to 100% soil water extracts from five treatments, with samples collected at the start (S1) and after 4 months (S3) of the experiment, values represent mean ± SD (n = 4, each replicate contains 4 wells with 5 D. magna in each well). Soil treatments include CSC (clean soil control), DSC (diesel-contaminated soil), BT1 (bacterial treatment 1), BT2 (bacterial treatment 2), and BT3 (bacterial treatment 3). Different letters above the bar indicate a statistical difference between different sampling points within each sample (p < 0.05).
No significant differences in the mortality rates observed within each treatment over a four-month period (p > 0.05). Indicated that the difference in mortality rates was not substantial enough to indicate a measurable effect. In addition, all samples showed low average mortality rates (below 15%) with 100% soil water extracts, revealing that D. magna may not be sufficiently sensitive to the levels of diesel water soluble fractions (WSFs) present in this study. As a 10% mortality rate was acceptable for control validation (refer to the manufacturer’s protocol), any sample that led to mortality rates over 10% was considered toxic to D. magna. In this experiment, CSC showed relatively low mortality rates (<10%) with samples from both sampling times, suggesting that CSC was non-toxic to D. magna. In contrast, DCS, BT1, BT2 and BT3 exerted toxic effects on D. magna at S1 after exposure for 48 h, none of these samples showed toxic effects at S3 after 24 or 48 h. Moreover, Müller, Melegari [95] showed that D. magna mortality increases with diesel concentration. Thus, the reduced toxicity in these treatments are likely to reflect lower diesel levels after four months of bioremediation.
Overall, CSC remained non-toxic throughout the 4 months of remediation, whereas DCS, BT1, BT2 and BT3 showed toxic effects after 48 h of 100% WSFs in S1, and then subsequently decreased to non-toxic in S3.
3.4.4. Luminescent Bacterial (Aliivibrio fischeri) Toxicity Test
Aliivibrio fischeri was used to assess the acute toxicity of soil water extracts, based on luminescence inhibition after 15 min of exposure. The luminescence intensity of all samples was presented in Table S6, and the luminescence inhibition result of A. fischeri to various soil water extracts was presented in Figure 8.
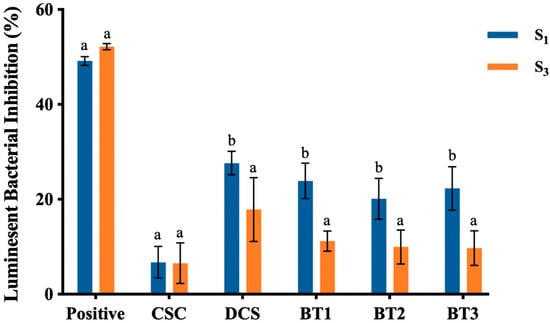
Figure 8.
Luminescence inhibition (%) of A. fischeri after 15 min of exposure to soil water extracts from five different soil treatments collected at the beginning (S1) and after 4 months (S3) of the experiment; values represent mean ± SD (n = 15). The positive control consisted of 100 µL A. fischeri culture mixed with 100 µL of diesel, and the negative control included 100 µL of A. fischeri culture with 100 µL of nutrient broth (containing 3% NaCl). The luminescence inhibition for each sample was calculated based on the luminescence level of the negative control. Soil treatments are CSC (clean soil control), DCS (diesel-contaminated soil), BT1 (bacterial treatment 1), BT2 (bacterial treatment 2), and BT3 (bacterial treatment 3). Different letters above the bar indicate a statistical difference between different sampling points within each sample (p < 0.05).
The CSC treatment showed minor luminescence inhibition, with a value of 6.74% at S1 and 6.54% at S3, probably due to the weakly acidic nature of the soil water extract (pH value 6.21 and 6.37 for S1 and S3). As demonstrated by Silva, Sousa [96] light production of A. fischeri was highly pH dependent, and the optimal pH was 6.8–7.0. Therefore, the weakly acidic environment could influence the luminescence ability of A. fischeri.
When comparing the samples from S1 to S3, a significant decrease in luminescence inhibition rate was exhibited in the treatments with diesel-contaminated soil (DCS, BT1, BT2, and BT3), indicating reduced soil water extract toxicity after four months of bioremediation. While DCS treatment showed a significant reduction in soil toxicity. Which could be due to the degradation of petroleum compounds by indigenous soil microorganisms or the natural weathering and volatilization process [97]. Nevertheless, when compared with bacterial treatments BT1, BT2, and BT3, the soil toxicity for DCS treatment remained at a higher level.
Overall, the bacterial treatments (especially BT3) exhibited higher soil toxicity reduction than the DCS treatment, which may correlate with their hydrocarbon degradation performance. These results confirmed that bacterial treatments (BT1, BT2, and BT3) can effectively degrade diesel hydrocarbons and help to reduce the toxicity of soil and soil water extracts during the bioremediation process.
4. Conclusions
This study demonstrated that inoculated bacterial consortia effectively enhanced diesel hydrocarbon degradation and reduced soil toxicity in diesel-contaminated soils. The GC-FID analyses confirmed significant TPH reductions across treatments, with BT3 showing the highest remediation efficiency. Microbial diversity analyses revealed partial recovery in DCS and BT1 but not in BT2 or BT3, suggesting that while some treatments supported a rebound of native microbial communities, others may have undergone more persistent structural shifts due to the introduced consortia and pot system constraints. Toxicity assessments demonstrated that bacterial treatments, particularly BT3, not only enhanced diesel degradation but also significantly reduced soil and soil water toxicity, confirming their effectiveness in improving environmental safety during bioremediation. Overall, the introduced bacterial consortia proved effective for diesel bioremediation under natural conditions. Future studies should explore longer-term effects, broader hydrocarbon targets, and field-scale applications to assess the practical applicability of this approach.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app151810143/s1: Table S1. Physicochemical characteristics of the diesel-contaminated soil in the study. Table S2. Configuration of diesel standard curve concentration gradient. Table S3. Average peak area, OFN concentration, and extraction efficiency of CSC samples across three sampling points. Table S4. Total petroleum hydrocarbon (TPH) concentrations (mg/kg) for each treatment at three sampling points. Table S5. Seeds germination rate—GR (%). Table S6. Luminescence intensity of all samples at sampling points S1 and S3. Figure S1. Randomized block design of the experiment set-up. Figure S2. Diesel standard curve used for quantifying diesel concentration based on total peak area. Error bars represent the standard deviation of replicate measurements. Figure S3. Gravimetric analysis of the extract’s weight of the 5 different treatments over the remediation. Figure S4. The total viable bacterial cell changes of 5 treatments during 4 months of bioremediation. Figure S5. The TPHs degraders bacterial cell counts of 5 treatments during 4 months of bioremediation.
Author Contributions
Conceptualization, M.W. and K.J.G.; methodology, M.W.; validation, M.W., K.J.G. and D.N.D.; formal analysis, M.W.; resources, K.J.G. and D.N.D.; data curation, K.J.G.; writing—original draft preparation, M.W.; writing, review and editing, K.J.G. and D.N.D.; supervision, K.J.G. and D.N.D.; project administration, K.J.G.; funding acquisition, K.J.G. and D.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project has been funded by SETUs Presidents Award Fellowship, Research Irelands Postgraduate Scholarship programme 2021: project number GOIPG/2021/1238 and the European Union’s Horizon 2020 research and innovation programme “GREENER” under grant agreement number 826312.
Data Availability Statement
The bacterial microbiome sequence data generated in this study have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject No. PRJNA1247371. The resulting datasets have been archived under BioSample Numbers SAMN47810523–SAMN47810537 and SAMN47812186–SAMN47812215. [NCBI] [https://www.ncbi.nlm.nih.gov/bioproject/advanced] [PRJNA1247371].
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| TPH | Total Petroleum Hydrocarbons |
| ONF | Octofluoronaphthalene |
| PAH | Polycylic Aromatic Hydrocarbons |
| LOI | Loss on Ignition |
| CFU | Colony Forming Unit |
| TVC | Total Viable Count |
| MPN | Most Probable Number |
| IG | Germination Index |
| LMW | Low Molecular Weight |
| ASV | Amplicon Sequence Variant |
References
- Gruiz, K. Natural attenuation in contaminated soil remediation. Eng. Tools Environ. Risk Manag. 2019, 4, 95–201. [Google Scholar]
- Lv, H.; Su, X.; Wang, Y.; Dai, Z.; Liu, M. Effectiveness and mechanism of natural attenuation at a petroleum-hydrocarbon contaminated site. Chemosphere 2018, 206, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Su, Y.; Chen, S.; Cui, W.; Zhao, C.; Liu, Q. Bioremediation of oil-contaminated soil: Exploring the potential of endogenous hydrocarbon degrader Enterobacter sp. SAVR S-1. Appl. Soil Ecol. 2022, 173, 104387. [Google Scholar] [CrossRef]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioagumentation—Assistited Landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial consortia are needed to degrade soil pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors influencing the bacterial bioremediation of hydrocarbon contaminants in the soil: Mechanisms and impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Aziz, Z.S.; Jazza, S.H.; Dageem, H.N.; Banoon, S.R.; Balboul, B.A.; Abdelzaher, M. Bacterial biodegradation of oil-contaminated soil for pollutant abatement contributing to achieve sustainable development goals: A comprehensive review. Results Eng. 2024, 22, 102083. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Dai, Y.; Wu, Y.; Lin, X. Effects of polycyclic aromatic hydrocarbon structure on PAH mineralization and toxicity to soil microorganisms after oxidative bioremediation by laccase. Environ. Pollut. 2021, 287, 117581. [Google Scholar] [CrossRef]
- Sakshi; Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- Bekins, B.A.; Brennan, J.C.; Tillitt, D.E.; Cozzarelli, I.M.; Illig, J.M.; Martinović-Weigelt, D. Biological effects of hydrocarbon degradation intermediates: Is the total petroleum hydrocarbon analytical method adequate for risk assessment? Environ. Sci. Technol. 2020, 54, 11396–11404. [Google Scholar] [CrossRef] [PubMed]
- Cipullo, S.; Prpich, G.; Campo, P.; Coulon, F. Assessing bioavailability of complex chemical mixtures in contaminated soils: Progress made and research needs. Sci. Total. Environ. 2018, 615, 708–723. [Google Scholar] [CrossRef]
- Chai, X.; Wang, M.; Fu, X.; Zhang, W.; Huang, Y.; Germaine, K.J.; Wang, J. Shift of combined ecotoxicity index in petroleum polluted soils during a bacterial remediation. Front. Environ. Sci. 2023, 11, 333. [Google Scholar] [CrossRef]
- de la Parra, S.; González, V.; Vives, P.S.; Curiel-Alegre, S.; Velasco-Arroyo, B.; Rad, C.; Barros, R.; Tamayo-Ramos, J.A.; Rumbo, C. Comparative toxicological assessment of three soils polluted with different levels of hydrocarbons and heavy metals using in vitro and in vivo approaches. Environ. Pollut. 2022, 315, 120472. [Google Scholar] [CrossRef]
- Dehnavi, S.; Ebrahimipour, G. Comparative remediation rate of biostimulation, bioaugmentation, and phytoremediation in hydrocarbon contaminants. Int. J. Environ. Sci. Technol. 2022, 19, 11561–11586. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. Bioremediation of PAH-contaminated soils: Process enhancement through composting/compost. Appl. Sci. 2020, 10, 3684. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Li, W.; Xi, B.; Huang, C. A novel immobilized bacteria consortium enhanced remediation efficiency of PAHs in soil: Insights into key removal mechanism and main driving factor. J. Hazard. Mater. 2025, 486, 137144. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, L.; Yang, H.; Zhang, J. Leveraging indigenous Bacillus consortia for heavy oil biodegradation and soil bioremediation. Environ. Technol. Innov. 2025, 40, 104415. [Google Scholar] [CrossRef]
- Martínez-Cuesta, R.; Conlon, R.; Wang, M.; Blanco-Romero, E.; Durán, D.; Redondo-Nieto, M.; Dowling, D.; Garrido-Sanz, D.; Martin, M.; Germaine, K.; et al. Field scale biodegradation of total petroleum hydrocarbons and soil restoration by Ecopiles: Microbiological analysis of the process. Front. Microbiol. 2023, 14, 1158130. [Google Scholar] [CrossRef] [PubMed]
- Behera, I.D.; Nayak, M.; Biswas, S.; Meikap, B.C.; Sen, R. Enhanced biodegradation of total petroleum hydrocarbons by implementing a novel two-step bioaugmentation strategy using indigenous bacterial consortium. J. Environ. Manag. 2021, 292, 112746. [Google Scholar] [CrossRef]
- Pulleyblank, C. Analysis of Oxygenated Polycyclic Aromatic Hydrocarbons in Contaminated Soil and Water Systems to Inform Remediation Strategy and Risk Assessment. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2021. [Google Scholar]
- Cao, W.; Yuan, J.; Geng, S.; Zou, J.; Dou, J.; Fan, F. Oxygenated and nitrated polycyclic aromatic hydrocarbons: Sources, quantification, incidence, toxicity, and fate in soil—A review study. Processes 2022, 11, 52. [Google Scholar] [CrossRef]
- Barati, M.; Safarzadeh, S.; Mowla, D.; Bakhtiari, F. Improvement of petroleum hydrocarbon remediation using the oat plant in the soil treated by poultry manure. J. Adv. Environ. Health Res. 2018, 6, 253–261. [Google Scholar]
- FAO. Standard Operating Procedure for Soil pH Determination; FAO: Rome, Italy, 2021. [Google Scholar]
- Nelson, J.T.; Adjuik, T.A.; Moore, E.B.; VanLoocke, A.D.; Reyes, A.R.; McDaniel, M.D. A Simple, Affordable, Do-It-Yourself Method for Measuring Soil Maximum Water Holding Capacity. Commun. Soil Sci. Plant Anal. 2024, 55, 1190–1204. [Google Scholar] [CrossRef]
- Deshpande, H. Soil Physical Analysis: Tools and Techniques; Educohack Press: Delhi, India, 2025. [Google Scholar]
- Satoh, Y.; Ishizuka, S.; Hiradate, S.; Atarashi-Andoh, M.; Nagano, H.; Koarashi, J. Sequential loss-on-ignition as a simple method for evaluating the stability of soil organic matter under actual environmental conditions. Environ. Res. 2023, 239, 117224. [Google Scholar] [CrossRef]
- Ouriache, H.; Moumed, I.; Arrar, J.; Namane, A.; Lounici, H. Influence of C/N/P ratio evolution on biodegradation of petroleum hydrocarbons-contaminated soil. Alger. J. Environ. Sci. Technol. 2020, 6, 1604–1611. [Google Scholar]
- Ou, Y.; Wu, M.; Yu, Y.; Liu, Z.; Zhang, T.; Zhang, X. Low dose phosphorus supplementation is conducive to remediation of heavily petroleum-contaminated soil—From the perspective of hydrocarbon removal and ecotoxicity risk control. Sci. Total. Environ. 2024, 929, 172478. [Google Scholar] [CrossRef]
- EPA. Method 3540C: Soxhlet Extraction. In Test Methods for Evaluating Solid Waste, Physical/Chemical Methods (SW-846), 3rd ed.; U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1996. [Google Scholar]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-S.; Jo, H.Y.; Kwon, M.J.; Maghsoudlou, P. Revisiting soil bacterial counting methods: Optimal soil storage and pretreatment methods and comparison of culture-dependent and -independent methods. PLoS ONE 2021, 16, e0246142. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Boe, U.S.; Henriksen, P.; Malmquist, L.M.; Christensen, J.H. Full-scale bioremediation of diesel-polluted soil in an Arctic landfarm. Environ. Pollut. 2021, 280, 116946. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals, Section 207: Earthworm, Acute Toxicity Tests; OECD Publishing: Paris, France, 1984. [Google Scholar]
- Steliga, T.; Kluk, D. Assessment of the suitability of Melilotus officinalis for phytoremediation of soil contaminated with pe-troleum hydrocarbons (TPH and PAH), Zn, Pb and Cd based on toxicological tests. Toxics 2021, 9, 148. [Google Scholar] [PubMed]
- Petroleum-gas University of Ploiesti; Sorana, I.Ţ.; Mihăilescu, S.; Strat, D.; Gheorghe, I.F. Effects of oil pollution on seed germination and seedling emergence toxicity. Rom. Biotechnol. Lett. 2020, 25, 1194–1201. [Google Scholar] [CrossRef]
- HJ 557-2010; Solid Waste—Extraction Procedure for Leaching Toxicity–Horizontal Vibration Method; Ministry of Environmental Protection of the People’s Republic of China, Beijing, China, 2010.
- Suliman, I.; Tudor, I.-M.; Ibram, O. Toxicity Screening of Surface Waters in Zaghen Restoration Area with Toxkit Microbiotests. Transylv. Rev. Syst. Ecol. Res. 2023, 25, 13–22. [Google Scholar] [CrossRef]
- Rybak, J.; Ziembik, Z.; Wróbel, M.; Bihałowicz, J.S.; Rogula-Kozłowska, W.; Mudiyanselage, N.D.; Majewski, G. Seasonal toxicity of urban road dust in runoff process-studies in Poland. Environ. Sci. Pollut. Res. 2024, 31, 38485–38499. [Google Scholar] [CrossRef] [PubMed]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbons in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2021, 207, 111554. [Google Scholar] [CrossRef]
- Bahar, M.; Samarasinghe, S.V.A.C.; Bekele, D.; Naidu, R. Residual hydrocarbons in long-term contaminated soils: Implications to risk-based management. Environ. Sci. Pollut. Res. 2024, 31, 22759–22773. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, W.; Wang, Y.; Guo, J.; Zhang, H.; Hu, X. Nutrient depletion is the main limiting factor in the crude oil bioaugmentation process. J. Environ. Sci. 2021, 100, 317–327. [Google Scholar] [CrossRef]
- Galitskaya, P.; Biktasheva, L.; Blagodatsky, S.; Selivanovskaya, S. Response of bacterial and fungal communities to high petroleum pollution in different soils. Sci. Rep. 2021, 11, 164. [Google Scholar] [CrossRef]
- Ummara, U.; Noreen, S.; Afzal, M.; Ahmad, P. Bacterial bioaugmentation enhances hydrocarbon degradation, plant colonization and gene expression in diesel-contaminated soil. Physiol. Plant. 2021, 173, 58–66. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.-W.; Kim, J. Effect of consortium bioaugmentation and biostimulation on remediation efficiency and bacterial diversity of diesel-contaminated aged soil. World J. Microbiol. Biotechnol. 2021, 37, 46. [Google Scholar] [CrossRef]
- Miles, S.M.; Gestler, R.; Dworatzek, S.M. Bioremediation of Petroleum Hydrocarbons in the Subsurface. In Advances in the Characterisation and Remediation of Sites Contaminated with Petroleum Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2023; pp. 479–502. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Bioavailability of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Cham, Switzerland, 2019; pp. 79–94. [Google Scholar]
- Abena, M.T.B.; Li, T.; Shah, M.N.; Zhong, W. Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere 2019, 234, 864–874. [Google Scholar] [CrossRef]
- Curiel-Alegre, S.; de la Fuente-Vivas, D.; Khan, A.H.A.; García-Tojal, J.; Velasco-Arroyo, B.; Rumbo, C.; Soja, G.; Rad, C.; Barros, R. Unveiling the capacity of bioaugmentation application, in comparison with biochar and rhamnolipid for TPHs degradation in aged hydrocarbons polluted soil. Environ. Res. 2024, 252, 118880. [Google Scholar] [CrossRef]
- Chicca, I.; Becarelli, S.; Di Gregorio, S. Microbial involvement in the bioremediation of total petroleum hydrocarbon polluted soils: Challenges and perspectives. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Mandree, P.; Masika, W.; Naicker, J.; Moonsamy, G.; Ramchuran, S.; Lalloo, R. Bioremediation of polycyclic aromatic hydrocarbons from industry contaminated soil using indigenous Bacillus spp. Processes 2021, 9, 1606. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef]
- EPA. Method 1663: Differentiation of Diesel and Crude Oil by GC/FID; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1997.
- Chaida, A.; Bensalah, F.; Trari, B. Potential for Crude Oil and Diesel Biodegradation by the Indigenous Pseudomonas sp. Strain LGMS7 Using GC-MS and GC-FID Analyses. Jordan J. Biol. Sci. 2022, 15, 441–448. [Google Scholar]
- Manaswini, G.; Sivagami, K.; Gopalakrishnan, M.; Harshini, P.; Janjaroen, D.; Ganesan, S. Biodegradation of low molecular weight polycyclic aromatic hydrocarbons in soil: Insights into bacterial activities and bioremediation techniques. Sustain. Chem. Environ. 2024, 7, 100146. [Google Scholar] [CrossRef]
- Ebadi, A.; Ghavidel, A.; Sima, N.A.K.; Heydari, G.; Ghaffari, M.R. New strategy to increase oil biodegradation efficiency by selecting isolates with diverse functionality and no antagonistic interactions for bacterial consortia. J. Environ. Chem. Eng. 2021, 9, 106315. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Q.; Zhang, N.; Wu, Y.; Wei, Q.; Huang, Z.; Dong, C. Sandy loam soil maintains better physicochemical parameters and more abundant beneficial microbiomes than clay soil in Stevia rebaudiana cultivation. PeerJ 2024, 12, e18010. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Shade, A. Soil bacteria and archaea. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 41–74. [Google Scholar]
- Zhou, M.; Liu, Z.; Wang, J.; Zhao, Y.; Hu, B. Sphingomonas relies on chemotaxis to degrade polycyclic aromatic hydrocarbons and maintain dominance in coking sites. Microorganisms 2022, 10, 1109. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, X.; Wu, J.; Chen, K. Effect of compost amendment and bioaugmentation on PAH degradation and microbial community shifting in petroleum-contaminated soil. Chemosphere 2020, 256, 126998. [Google Scholar] [CrossRef]
- Jia, W.; Cheng, L.; Tan, Q.; Liu, Y.; Dou, J.; Yang, K.; Yang, Q.; Wang, S.; Li, J.; Niu, G.; et al. Response of the soil microbial community to petroleum hydrocarbon stress shows a threshold effect: Research on aged realistic contaminated fields. Front. Microbiol. 2023, 14, 1188229. [Google Scholar] [CrossRef]
- Abena, M.T.B.; Chen, G.; Chen, Z.; Zheng, X.; Li, S.; Li, T.; Zhong, W. Microbial diversity changes and enrichment of potential petroleum hydrocarbon degraders in crude oil-, diesel-, and gasoline-contaminated soil. 3 Biotech 2020, 10, 42–48. [Google Scholar] [CrossRef]
- Santisi, S.; Cappello, S.; Catalfamo, M.; Mancini, G.; Hassanshahian, M.; Genovese, L.; Giuliano, L.; Yakimov, M.M. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium. Braz. J. Microbiol. 2015, 46, 377–387. [Google Scholar] [CrossRef]
- Kumar, V.; AlMomin, S.; Al-Aqeel, H.; Al-Salameen, F.; Nair, S.; Shajan, A.; Melcher, U. Metagenomic analysis of rhizosphere microflora of oil-contaminated soil planted with barley and alfalfa. PLoS ONE 2018, 13, e0202127. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Li, P.; Liang, X.; Shi, R.; Wang, Y.; Han, S.; Zhang, Y. Unraveling the functional instability of bacterial consortia in crude oil degradation via integrated co-occurrence networks. Front. Microbiol. 2023, 14, 1270916. [Google Scholar] [CrossRef]
- Fu, X.; Song, Q.; Li, S.; Shen, Y.; Yue, S. Dynamic changes in bacterial community structure are associated with distinct priming effect patterns. Soil Biol. Biochem. 2022, 169, 108671. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Bajagain, R.; Jeong, S.-W.; Kim, J. Insights into the biodegradation of diesel oil and changes in bacterial communities in diesel-contaminated soil as a consequence of various soil amendments. Chemosphere 2021, 285, 131416. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Xin, Y.; Huang, T.; Liu, J. Petroleum pollution affects soil chemistry and reshapes the diversity and networks of microbial communities. Ecotoxicol. Environ. Saf. 2022, 246, 114129. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Dong, Y.; Fan, H.; Bai, Z.; Zhuang, X. Engineering microbial consortia towards bioremediation. Water 2021, 13, 2928. [Google Scholar] [CrossRef]
- Bôto, M.L.; Magalhães, C.; Perdigão, R.; Alexandrino, D.A.M.; Fernandes, J.P.; Bernabeu, A.M.; Ramos, S.; Carvalho, M.F.; Semedo, M.; LaRoche, J.; et al. Harnessing the potential of native microbial communities for bioremediation of oil spills in the Iberian Peninsula NW coast. Front. Microbiol. 2021, 12, 633659. [Google Scholar] [CrossRef]
- Liu, X.; Salles, J.F. Drivers and consequences of microbial community coalescence. ISME J. 2024, 18, wrae179. [Google Scholar] [CrossRef]
- Zegeye, E.K.; Brislawn, C.J.; Farris, Y.; Fansler, S.J.; Hofmockel, K.S.; Jansson, J.K.; Wright, A.T.; Graham, E.B.; Naylor, D.; McClure, R.S.; et al. Selection, succession, and stabilization of soil microbial consortia. Msystems 2019, 4, e00055-19. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; A van der Heijden, M.G.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Valiei, A.; Dickson, A.M.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Bacterial community dynamics as a result of growth-yield trade-off and multispecies metabolic interactions toward understanding the gut biofilm niche. BMC Microbiol. 2024, 24, 441. [Google Scholar] [CrossRef]
- Almutairi, M. Vermiremediation strategy for remediation of Kuwaiti oil contaminated soil. SN Appl. Sci. 2019, 1, 1312. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sun, Z. Ecotoxicological effects of petroleum-contaminated soil on the earthworm Eisenia fetida. J. Hazard. Mater. 2020, 393, 122384. [Google Scholar] [CrossRef]
- Geissen, V.; Gomez-Rivera, P.; Lwanga, E.H.; Mendoza, R.B.; Narcías, A.T.; Marcías, E.B. Using earthworms to test the efficiency of remediation of oil-polluted soil in tropical Mexico. Ecotoxicol. Environ. Saf. 2008, 71, 638–642. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef]
- Srivastava, S.; Gautam, K.; Prakash, V.; Tripathi, V.; Thakur, R.S.; Patel, D.K.; Singh, D.; Anbumani, S.; Manickam, N. Life cycle responses of earthworm, Eisenia fetida to soil contaminated with crude oil sludge containing polycyclic aromatic hydrocarbons (PAHs). Environ. Pollut. 2025, 381, 126645. [Google Scholar] [CrossRef]
- Sun, N.; Liu, Q.; Wang, J.; He, F.; Jing, M.; Chu, S.; Zong, W.; Liu, R.; Gao, C. Probing the biological toxicity of pyrene to the earthworm Eisenia fetida and the toxicity pathways of oxidative damage: A systematic study at the animal and molecular levels. Environ. Pollut. 2021, 289, 117936. [Google Scholar] [CrossRef]
- Yaman, C. Performance and kinetics of bioaugmentation, biostimulation, and natural attenuation processes for bioremediation of crude oil-contaminated soils. Processes 2020, 8, 883. [Google Scholar] [CrossRef]
- Pochron, S.T.; Mezic, M.; Byrne, S.; Sasoun, S.; Casamassima, A.; Kilic, M.; Nuzzo, A.; Beaudet, C.-E. Exposure to Roundup increases movement speed and decreases body mass in earthworms. Front. Environ. Sci. 2022, 10, 991494. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, J.; Pan, H.; Liu, J.; Liu, Y.; Zhao, Y. A model of the physiological and biochemical characteristics of earthworms (Eisenia fetida) in petroleum-contaminated soil. Ecotoxicol. Environ. Saf. 2019, 174, 459–466. [Google Scholar] [CrossRef]
- Koolivand, A.; Saeedi, R.; Coulon, F.; Kumar, V.; Villaseñor, J.; Asghari, F.; Hesampoor, F. Bioremediation of petroleum hydrocarbons by vermicomposting process bioaugmentated with indigenous bacterial consortium isolated from petroleum oily sludge. Ecotoxicol. Environ. Saf. 2020, 198, 110645. [Google Scholar] [CrossRef]
- Wojtowicz, K.; Steliga, T.; Kapusta, P.; Brzeszcz, J. Oil-contaminated soil remediation with biodegradation by autochthonous microorganisms and phytoremediation by maize (Zea mays). Molecules 2023, 28, 6104. [Google Scholar] [CrossRef]
- Zawierucha, I.; Malina, G.; Herman, B.; Rychter, P.; Biczak, R.; Pawlowska, B.; Bandurska, K.; Barczynska, R. Ecotoxicity and bioremediation potential assessment of soil from oil refinery station area. J. Environ. Health Sci. Eng. 2022, 20, 337–346. [Google Scholar] [CrossRef]
- Correa, H.d.S.; Blum, C.T.; Galvão, F.; Maranho, L.T. Effects of oil contamination on plant growth and development: A review. Environ. Sci. Pollut. Res. 2022, 29, 43501–43515. [Google Scholar] [CrossRef]
- Dib, D.; Ahmed, D.S.A. Comparative study of the diesel fuel contamination effects of different types of soils on the growth and germination of four plant species. Braz. Arch. Biol. Technol. 2024, 67, e24230728. [Google Scholar] [CrossRef]
- Kucaj, W.F.; Rygielski, K.; Cybulska, K. Optimizing the use of the phytotoxkit test to assess the toxicity of soil contaminated with creosote. Pol. J. Soil Sci. 2019, 52, 153. [Google Scholar] [CrossRef]
- Roy, P.; Rutter, A.; Gainer, A.; Haack, E.; Zeeb, B.A. Phytotoxicity of weathered petroleum hydrocarbons in soil to boreal plant species. Environ. Res. 2023, 238, 117136. [Google Scholar] [CrossRef]
- Haider, F.U.; Ejaz, M.; Alam Cheema, S.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef]
- Yu, W.; Wan, Y.; Zhou, W.; An, J.; Tian, L.; Ma, J. Molecular composition of water soluble fraction of petroleum products and crude oils: Insights into groundwater contamination potential and environmental forensics. J. Environ. Sci. 2025, 159, 437–444. [Google Scholar] [CrossRef]
- Müller, J.B.; Melegari, S.P.; Perreault, F.; Matias, W.G. Comparative assessment of acute and chronic ecotoxicity of water soluble fractions of diesel and biodiesel on Daphnia magna and Aliivibrio fischeri. Chemosphere 2019, 221, 640–646. [Google Scholar] [CrossRef]
- Silva, A.R.; Sousa, C.; Exner, D.; Schwaiger, R.; Alves, M.M.; Petrovykh, D.Y.; Pereira, L. pH-induced modulation of Vibrio fischeri population life cycle. Chemosensors 2021, 9, 283. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Yue, G.; Liu, S.; Liu, H.; Song, Q.; Wu, B. Comparisons of four methods for measuring total petroleum hydrocarbons and short-term weathering effect in soils contaminated by crude oil and fuel oils. Water Air Soil Pollut. 2021, 232, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).