Abstract
Environmental pollution with microplastics (MPs) and nanoplastics (NPs) continues to increase. These pollutants have been found in the environment (water, soil, and air) as well as in human tissues and biological fluids. Oral, inhalation, and dermal pathways play key roles in human exposure to plastic particles. The primary sources of exposure are foods, beverages, air, and dust. Polymers can penetrate the skin primarily via endocytosis, exocytosis, passages through cell-to-cell junctions, and interaction with the extracellular matrix. However, the health effects of dermal exposure remain poorly understood. Microplastics and NPs have been detected in the gastrointestinal, respiratory, circulatory, urinary, reproductive, and nervous systems, causing detrimental effects in each. Such effects include oxidative stress, inflammation, cellular damage, and protein aggregation. Furthermore, their presence has been linked to cardiovascular and neurodegenerative diseases. However, standardized protocols for analyzing NPs and MPs in human organs and tissues have not yet been established or legally regulated. Further research is needed to fully determine exposure thresholds, but legislative and lifestyle changes can already be implemented.
1. Introduction
Microplastics (MPs) are defined as plastic particles 0.1 to 5000 µm in size, whereas nanoplastics (NPs) are typically 1 to 100 nm (0.001–0.1 μm) [1] (Table 1). Although MPs and NPs occur in various shapes, they are commonly classified into fragments, fibers, films, foams, and beads [2,3,4]. Plastic particles are commonly composed of polymers including polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyamide (PA) [5,6,7].
The presence of MPs and NPs has been documented worldwide across environmental matrices, including soil, aquatic systems, sediments, air, drinking water, and food [8,9]. Environmental sources of MPs are typically categorized as primary and secondary sources [10]. Primary MPs are intentionally manufactured at microscopic sizes. They are used as abrasives in certain industrial processes and as microbeads in cosmetics and personal-care products. Secondary MPs form through fragmentation or degradation of larger plastic items that persist as environmental waste [11]. Major sources of secondary MPs include tire wear, synthetic textiles, plastic packaging, and fishing gear [12,13,14,15].
Plastic particles present in the environment can enter the human body through inhalation, ingestion, or dermal absorption [16]. Studies have confirmed their presence in the digestive, respiratory, circulatory, urinary, reproductive, and nervous systems [17,18]. Such accumulation can contribute to inflammation, oxidative stress, gut microbiome dysbiosis, and diseases affecting those organ systems [19,20,21,22,23,24,25].

Table 1.
Comparison of MPs and NPs.
Table 1.
Comparison of MPs and NPs.
| Properties | MPs | NPs | Reference |
|---|---|---|---|
| Size | 0.1 to 5000 µm | 1 to 100 nm (0.001–0.1 μm) | [1] |
| Main sources | Intentionally manufactured, fragmentation/degradation of larger pieces of plastics | Fragmentation of MPs | [11,26] |
| Environmental behavior and transport | Tend to settle, float or be trapped in sediments; mobility depends on shape and size | Tend to create colloidal solution; high mobility through porous media and biological barriers | [27,28] |
| Detection and analytical method | µ-FTIR, μ-Raman, LDIR, PLM, Py-GC/MS, SEM-EDX | AFM, fluorescence microscopy, LIBD, NTA, Py-GC/MS, SERS, TEM | [27,29,30] |
Abbreviations: µ-FTIR—micro-Fourier transform infrared spectroscopy; µ-Raman—micro-Raman spectroscopy; AFM—atomic force microscopy; LDIR—laser direct infrared imaging; LIBD—laser induced breakdown spectroscopy; MPs—microplastics; NPs—nanoplastics; NTA—nanoparticle tracking analysis; Py-GC/MS—pyrolysis-gas chromatography/mass spectrometry; SEM-EDX—scanning electron microscopy-energy dispersive X-ray spectroscopy; SERS—surface enhanced Raman spectroscopy; TEM—transmission electron microscopy.
The high surface-to-volume ratio and strong hydrophobicity of plastic particles allow them to interact with organic contaminants, heavy metals, and pathogens. The coexistence of MPs with other contaminants can affect bioaccumulation and increase toxicity in living organisms [31]. Moreover, MPs can act as carriers of heavy metals, such as arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), and lead (Pb), leading to greater accumulation and synergistic toxic effects [32]. These particles can also transport pathogens within the human body, potentially facilitating infections [33].
Recent review publications have highlighted the potential hazards associated with MPs [34,35,36,37,38], NPs [39,40,41,42,43], and both particle types [44,45,46,47,48,49]. However, these reviews primarily focus on specific aspects, such as environmental pollution, exposure routes, toxicity in animals, or effects on individual human organ systems. Further research is needed to clarify how MPs and NPs may contribute to diseases of the stomach, intestines, lungs, kidneys, and reproductive organs.
In this review, we provide a comprehensive overview of current knowledge and recent advances on the health impacts of plastic particles—including routes of exposure, mechanisms of toxicity, measured concentrations in humans, potential effects on organ systems, and global regulations. We also identify persistent research gaps and propose directions for future studies.
2. Materials and Methods
This narrative review is based on scientific articles retrieved from the ScienceDirect, PubMed, and Google Scholar databases. The search combined the terms “microplastic” and “nanoplastic” with keywords including “human”, “toxicity”, “oxidative stress”, “urine”, “kidney”, “brain”, “feces”, “liver”, “sperm”, “placenta”, “amniotic fluid”, “breast milk”, “lung”, “skin”, “blood”, “intake”, “oral”, “inhalation”, “dermal”, and “exposure”. These terms were applied to searches within the titles, abstracts, and keywords of the publications. The analysis focused on peer-reviewed, English-language articles published from 2020 onward. Particular attention was given to studies published in 2024–2025 that examined the toxicity of MPs and NPs and their impact on the human body.
3. Routes of Exposure to MPs and NPs
Environmental pollution with plastics, driven by their widespread use and insufficient regulation, is a major source of human exposure to MPs and NPs [50]. Figure 1 highlights the most common environmental sources of plastic particles.
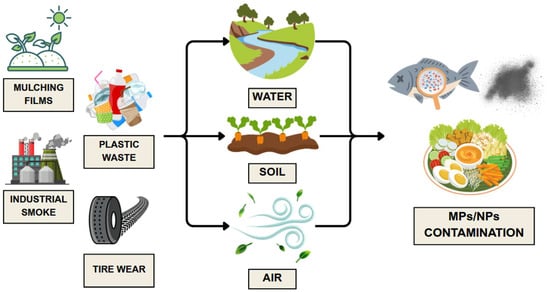
Figure 1.
Environmental sources of MPs and NPs. Developed by the authors based on [51,52,53,54,55]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
The diverse sizes, shapes, and polymer types of MPs and NPs affect their uptake, distribution, and eventual fate within the human body [56,57]. Human exposure also varies substantially depending on the route of entry—ingestion, inhalation, or dermal contact [56,58]. Figure 2 illustrates how polymer particles enter the human body through different exposure pathways.
An increasing number of studies have reported the presence of MPs and NPs in the human body [37,59,60,61,62,63,64,65,66,67,68,69]. Researchers have detected these particles in various tissues and organs using a range of analytical techniques [58,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89]. These analyses often focus on particle size, shape, and polymer type, as summarized in Table 2.
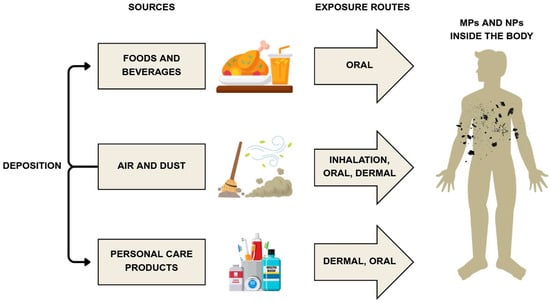
Figure 2.
General sources and human exposure routes to MPs and NPs. Developed by the authors based on [58,90,91]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).

Table 2.
Summary of research findings on MPs and NPs detected in human samples.
Table 2.
Summary of research findings on MPs and NPs detected in human samples.
| Human Body System | Type of Sample | Determination Method | Polymer Composition | Shape | Size | Quantity/Concentration | Region | Reference |
|---|---|---|---|---|---|---|---|---|
| Digestive System | Feces | μ-Raman | PP, HDPE, PS, PET | Unspecified | Unspecified | 10.19 μg/g | Indonesia | [70] |
| µ-FTIR | PP, PET, PS, PE, PVC, PC, PA, PU | Unspecified | 20–800 μm | 1–36 particles/g | China | [71] | ||
| μ-Raman | PS, PP, PE, PET, PVC | Unspecified | 30–1800 µm | 20.4–138.9 particles/g wet weight | China | [72] | ||
| µ-FTIR | PA, PE, PET, PMMA, PCM, PP, PS, PU, PVC | Fragments, fibers | 5–5000 μm | 3.5 particles/g feces | Austria | [73] | ||
| μ-Raman | PET, PA, PP, PE, PC, PVC, POM, PTFE, EVA, PS, PMMA, PBT, AS, PES, TPU | Sheets, fibers, fragments, pellets | 50–300 μm | 41.8 items/g (IBD patients) 28.0 items/g (healthy people) | China | [74] | ||
| LDIR | PET, PA, PE, PP, PC, PS, PVC, POM, PTFE, EVA, PMMA, PES, TPU, PBT, PBAT, PU, AS | Unspecified | 5–500 μm | 62 items/g (CRC patients) 43 items/g (healthy people) | Iran | [75] | ||
| Respiratory System | Bronchoalveolar fluid | μ-Raman | PE, PS, PP, PET | Fibers, fragments | 20–500 μm | 4.1 particles/sample | Iran | [76] |
| µ-FTIR, SEM-EDX | PET, semi-synthetic polymers | Fibers | 2.34 mm (median length) | 9.18 ± 2.45 particles/100 mL | Spain | [77] | ||
| Circulatory System | Serum | Py-GC/MS | PE, PVC, PS, PET, N66, PP | Unspecified | Unspecified | 20.81 μg/g (median) | China | [58] |
| Thrombi | Py-GC/MS, LDIR, SEM | PE, N66, PVC | Irregular fragments | 20–100 μm | 61.75–141.8 μg/g (median) | China | [68] | |
| Blood | Py-GC/MS | PE, PS, PP, PET, PMMA | Unspecified | 0.7–500 μm | 3.53 µg/mL (average) | Netherlands | [78] | |
| Plaques | Py-GC/MS | PE / PVC | Irregular fragments | <1 μm | PE 21.7 ± 24.5 μg/mg of plaque (mean) PVC 5.2 ± 2.4 μg/mg of plaque (mean) | Italy | [79] | |
| Urinary/excretory system | Urine | µ-Raman | PE, PP, PVA, PVC | Irregular fragments, spheres | 4–15 μm | 0–3 particles/sample | Italy | [69] |
| µ-Raman | PE, PS, PP | Fibers | 20–100 μm | 0–2 particles/sample | Iran | [76] | ||
| µ-Raman | PE, PS, Styrene-Isoprene | Fiber, fragments | 3–13 μm | 2.3 particles/sample | Italy | [80] | ||
| µ-Raman | PE, PS, Styrene-Isoprene | Fragments | >0.2 μm | 1.28 ± 0.49/sample (mean) | Italy | [81] | ||
| µ-FTIR, SEM-EDX | PE, PS, PP, PTFE, resins | Fragments, films, fibers | 19–400 μm (length) 10–128 μm (width) 15–>300 μm (length) 9–>300 μm (width) | 0–9600 particles/L (healthy patients) 0–36,000 particles/L (endometriosis patients) | Great Britain | [82] | ||
| Py-GC/MS, LDIR | PE, PVC, N66, PMMA, PU, PP, PET | Diversity of shape | <50 μm | 1.50 mg/kg 15.17 particles/kg | China | [83] | ||
| Kidney | µ-Raman | PE, PS | Fragments | 1–29 μm | 4.3 particles/sample | Italy | [80] | |
| µ-Raman | PE, PS, Styrene-Isoprene | Fragments | >0.2 μm | 1.7 ± 2.11/sample (mean) | Italy | [81] | ||
| Reproductive System | Sperm | µ-Raman | PS, PP, PVC, PTFE, PET, PE, ABS, PC | Unspecified | 1.2–20 μm | Unspecified | China | [84] |
| Placenta | Py-GC/MS | PE, PVC, N66, SBR, ABS, PET, N6, PMMA, PU, PC, PP, PS | Unspecified | 1–20 µm | 6.5–685 µg/g | U.S. | [85] | |
| Amniotic fluid | Raman, Py-GC/MS | PTFE, PS, ABS, PE, PC, PVC | Unspecified | 3.05 ± 1.05 µm | Unspecified | China | [86] | |
| Nervous System | Brain | Py-GC/MS, TEM, SEM-EDS | PE, PP, PVC, styrene-butadiene rubber | Unspecified | 1–5 μm (brain tissues) <1 μm (brain parenchyma) 100–200 nm (brain pellets) | median = 1254 μg/g (East Coast samples) median = 26,076 μg/g (dementia samples) | U.S. | [87] |
| Olfactory bulb | µ-FTIR | PP, PA, NYL, N6, PVA, PE, wool-PP | 83.4% of fragments 16.6% of spheres 25% of fibers | 5.5–26.4 μm (length) 3.0–25.4 μm (width) | 1–4 particles/olfactory bulb | Brazil | [88] | |
| Cerebrospinal fluid | LDIR, Py-GC/MS | PP, PE, PS, PVC | Unspecified | <100 μm | 0–3 μg of polymer/g of cerebrospinal fluid | China | [89] |
Abbreviations: ABS—acrylonitrile butadiene styrene; ASA—acrylonitrile styrene acrylate; EVA—ethylene-vinyl acetate; HDPE—high density polyethylene; N6—nylon 6; N66—nylon 66; NYL—nylon; PA—polyamide; PBAT—poly(butylene adipate terephthalate); PBT—poly(butylene terephthalate); PC—polycarbonate; PCM—phase-change material; PE—polyethylene; PET—poly (ethylene terephthalate); PES—poly(ethylene succinate); PMMA—poly(methyl methacrylate); POM—polyoxymethylene; PP—polypropylene; PS—polystyrene; PTFE—polytetrafluoroethylene; PU—polyurethane; PVA—poly(ethylene vinyl acetate); PVC—poly(vinyl chloride); SBR—styrene-butadiene rubber; TPU—thermoplastic polyurethane; U.S.—United States.
3.1. Oral Exposure
Ingestion is the primary pathway through which MPs and NPs enter the human body, owing to the frequent contamination of food and beverages with polymer particles [9,92]. A pilot study of 26 students in China by Song et al. [58] found that diet was the predominant source, with an estimated intake of 346.65 μg MPs/kg body weight/year compared to 41.17 μg MPs/kg body weight/year from drinking water. Commonly contaminated food products include fruits and vegetables [93], packaged meat [94], and animal-derived products such as honey [95] and eggs [96].
The types of MPs and NPs detected in foods can vary greatly across products and sources. The most commonly identified polymers include PE [96,97,98,99], PP [97,98,99], PET [97,98,99], PS [94], and PA [99].
Multiple mechanisms contribute to the entry of MPs and NPs into the food chain. Currently, no studies directly link food–packaging contact to the release of MPs and NPs. Nevertheless, particles of the same polymer type as the packaging are often the most abundant contaminants detected [100]. Other common mechanisms include deposition on meals from air and dust [90], contamination of water [101] and soil [102], and release from cookware [103]. Additionally, MPs and NPs can move up the food chain, as animals consume contaminated water, plants, and other animals, ultimately resulting in human consumption [102]. Finally, production, storage, and transport affect the type and extent of MPs and NPs contamination in food and beverages [92].
Recent studies have confirmed that humans can also be exposed to MPs and NPs through dental procedures, orthodontic aligners, and denture use [104,105], as well as through various oral–care products [91]. For example, Protyusha et al. [91] estimated annual exposure to MPs in India to range from 111 particles per person (mouth freshener spray) to 48,910 particles (toothbrush).
3.2. Inhalation Exposure
Both indoor and outdoor air, along with dust, are important sources of MPs and NPs [91,106,107]. These particles can also be released from carpets [108] and during dental [104] and laboratory procedures [109]. The extent of contamination and levels of MPs and NPs found in air and dust are closely linked to the deterioration of building materials and products used [108,110]. Additionally, MPs and NPs can become aerosolized via sea spray [111]. Although exposure from this source is much lower than in urban and indoor areas overall [111], these particles can still pose a threat to human health, especially when combined with environmentally persistent free radicals, which may influence the generation of reactive oxygen species (ROS) by MPs [112]. Face masks can also release these particles, although the protection they offer against external sources of MPs generally outweighs the exposure they cause [113,114]. The predominant particle shape is fibers, and the most commonly identified polymer is poly(ethylene terephthalate) (PET) [77,106,108].
Islam et al. [106] quantified MPs intake through inhalation of indoor dust during winter in Dhaka (Bangladesh) at 2986 ± 1035 MPs/kg body weight/day. Song et al. [58] estimated exposure to MPs from general inhalation at 59.57 μg/kg body weight/day. Notably, MPs suspended in air can settle on food, further increasing the potential burden on humans [90]. At the time of writing, no inhalation-related data on NPs are available.
3.3. Dermal Exposure
Skin contact is a significant pathway of exposure to MPs and NPs, with particle size strongly influencing penetration patterns [115]. In a 3D cellular skin model, Eom et al. [115] found that 0.1–0.5 µm PS spheres readily penetrated human dermal fibroblast spheroids, a process attributed to endocytosis, exocytosis, passage through cell-to-cell junctions, and interaction with the extracellular matrix. Conversely, 1–3 µm particles tended to remain on the cell surface [115].
In a similar 3D human skin model, Song et al. [116] observed that PS fragments penetrated the dermal layer within one hour of exposure. For particles smaller than 2 µm, at a dose of 100 μg, the average maximum penetration reached 4.7 μg after six hours of contact. Subsequently, the amount of detected MPs decreased after eight hours, reaching levels comparable to the control group after 24 h. Similar effects were observed in cytotoxicity and inflammatory responses, indicating potential risks from short-term contact. At a lower dose (25 μg), penetration was two orders of magnitude lower and no time-dependent effects were observed, underscoring the importance of the administered dose [116]. Additionally, polymer particles have been found to accelerate skin cell aging [117].
4. Mechanisms of MPs and NPs Toxicity
4.1. Shape and Size
The toxicity of MPs and NPs is influenced by multiple factors, notably particle size, shape, and surface properties [118]. For instance, the small size of NPs enables cellular uptake, potentially disrupting processes such as protein folding and mitochondrial function [119]. Moreover, 25-nm PS particles have been shown to reduce cell viability, induce S-phase cell-cycle arrest, trigger transcription of inflammatory genes, and alter the expression of proteins involved in cell-cycle regulation and apoptotic pathways [120]. Luo et al. [121] exposed RAW 264.7 cells and primary peritoneal macrophages to PS particles and found that smaller particles (0.5 µm) induced greater mitochondrial depolarization, ROS generation, and apoptosis than larger particles (5 µm).
Another important factor is particle size, which influences transmembrane transport and tissue distribution. Phagocytosis is considered the primary uptake mechanism for MPs [122]. In contrast, NPs can enter cells via multiple pathways—including phagocytosis, pinocytosis, and endocytosis—and even via energy-independent membrane penetration to a depth of approximately 50 nm in vitro [122]. Endocytosed NPs are of particular concern because they may disrupt endosomal membrane integrity [123]. Consequently, NPs released into the cytosol may interact with mitochondria or the nucleus, thereby disrupting cellular processes [123]. For instance, Ding et al. [124] demonstrated the presence of PS NPs in the stomach, intestine, and liver of mice, as well as in GES-1 cells exposed to these particles. The study showed that PS NPs accumulated in the cytoplasm, autophagosomes, and lysosomes, and induced cytotoxicity.
Shape is another key determinant. Plastic particles occur as spheres, irregular fragments, fibers, films, or foams [125]. Acute toxicity tests suggest that particle shape strongly influences toxicity [126], with fibers often considered more hazardous than other forms [127]. Kim et al. [128] showed that irregular and fibrillar PET MPs impaired the overall health of Artemia franciscana, reducing swimming ability, decreasing positive phototaxis, and damaging intestinal structure. An et al. [129] observed that PE MPs fragments exhibited higher chronic toxicity to Daphnia magna than PE MPs beads, resulting in reduced survival, growth, and reproduction. Schwarzer et al. [130] reported sublethal effects on life-history traits and morphology of Daphnia magna exposed to small PS beads and fragments. Bobori et al. [131] reported that dietary addition of spherical PP MPs impaired gill and liver cell function in Danio rerio and Perca fluviatilis.
4.2. Chemical Composition
Plastic particles are not composed solely of polymers. They also contain additives such as plasticizers, flame retardants, stabilizers, antioxidants, and dyes [132]. Many of these additives act as endocrine disruptors [133]. For example, phthalates (plasticizers) can alter estrogen and testosterone levels and functions, and they may also inhibit thyroid function. Bisphenol A, in contrast, can bind to estrogen, progesterone, and androgen receptors, thereby disrupting reproduction, metabolism, and neurodevelopment [134]. These additives are not covalently bound to the polymer matrix. As a result, they can migrate from MPs and NPs into the environment under varying conditions, including changes in pH, temperature, ultraviolet radiation, and salinity [132]. Moreover, MPs and NPs can serve as vectors for hazardous substances, including heavy metals, persistent organic pollutants (POPs), and pathogens. These co-transported contaminants can enter the human body and increase health risks [132]. For instance, exposure to polychlorinated biphenyls has been linked to neurotoxicity, endocrine dysfunction, and reproductive disorders [135]. Particular attention should be given to the adsorption of heavy metals by MPs and NPs. Exposure to toxic metals such as Cd, Pb, Cr, As, mercury (Hg), and nickel (Ni) can cause gastrointestinal, respiratory, cardiovascular, reproductive, renal, hematopoietic, and neurological disorders [136]. Such metals can adhere to the surface of plastic particles, enter organisms through ingestion, and gradually accumulate in tissues. Furthermore, increased toxicity has been observed in fish populations due to the presence of heavy metals in MPs and NPs, with detrimental effects on survival rates [137]. Consequently, MPs and NPs are often described as “Trojan horses” [119,132]. This suggests that although MPs and NPs may appear innocuous at first glance, they can exert latent effects due to associated chemical compounds [138].
The release of potentially hazardous substances from MPs may be influenced by factors such as polymer type, plastic origin, and the extraction solvent used. Järvelä et al. [139] reported that low-density polyethylene (LDPE) MPs accumulated and released more substances than high-density polyethylene (HDPE) and PP MPs. Recycled LDPE and HDPE MPs reduced cell viability to a greater extent than non-recycled MPs [139]. In contrast, no significant differences were observed between virgin and recycled PP MPs. Furthermore, the use of an organic solvent such as chloroform resulted in more efficient extraction of substances from MPs than water or methanol.
Another important issue concerns the impact of chemical modifications of NPs on intracellular transport processes. For instance, PS NPs modified with amino groups showed a stronger propensity to interact with biological membranes and accelerated cellular uptake compared with PS NPs modified with carboxyl groups. However, the latter were not detected in lysosomes. Conversely, PS NPs modified with amino groups localized in lysosomes and exhibited toxicity [140].
4.3. Toxicological Effects
Inflammation, oxidative stress, endoplasmic reticulum (ER) stress, apoptosis, and alterations in autophagy are the primary toxicological effects of MPs and NPs [46,141].
Inflammation constitutes the body’s primary defense mechanism against foreign agents. Exposure to MPs has been shown to activate immune responses, specifically inducing the expression of proinflammatory cytokines and histamine in human mast cell and microglial cell lines [142]. In vitro studies have demonstrated that NPs induce alterations in the cell membrane surface, thereby triggering the inflammatory process [143]. Other inflammatory reactions caused by MPs and NPs include cell and tissue damage [142]. For instance, Chen et al. [144] observed that PS NPs activated the inflammatory NF-κB/NLRP3 pathway in mice and induced the expression of IL-1β and IL-18 cytokines in the intestine, recruiting macrophages and neutrophils to this tissue. They also found that impaired liver function positively correlated with intestinal inflammation and barrier damage.
Conversely, ER stress is a temporary process that restores ER homeostasis. This process occurs when misfolded and unfolded proteins accumulate and is associated with metabolic disturbances, apoptosis, inflammation, and insulin resistance [145]. Zhang et al. [145] observed that disruption of lipid metabolism by PS NPs in largemouth bass was associated with the induction of the ER stress response. Furthermore, the extent of this disruption increased with exposure time.
Autophagy is a cellular cleansing system. It protects glial cells by modulating neuroinflammation and regulates key functions in the male and female reproductive systems [146]. Fanghella et al. [146] noted that most studies on autophagy disruption involve PS NPs, limiting understanding of how plastic particles affect autophagy. Liang et al. [147] demonstrated that disruption of the autophagy-lysosomal pathway induced by PS NPs increased pyroptosis in dopaminergic neurons, accelerating the onset and progression of Parkinson’s disease. In addition, the researchers concluded that PS NPs have the potential to serve as autophagy inhibitors in the future. Nevertheless, the effect of other polymers on autophagy remains to be elucidated.
Apoptosis is a programmed cell death process essential for the development of multicellular organisms [148]. Płuciennik et al. [149] hypothesized that MPs and NPs have the capacity to activate both intrinsic (mitochondrial) and extrinsic apoptotic pathways. It has been demonstrated that smaller particles exhibit a higher capacity to trigger both apoptotic pathways. This phenomenon is likely attributable to the induction of ROS production and the activation of the p53 protein [149]. Wu et al. [150] demonstrated that exposure to PS NPs resulted in oxidative stress, subsequently leading to inflammation and apoptosis in the carp heart. These findings indicate that plastic particles elicit multifaceted and interconnected toxic effects.
Furthermore, MPs and NPs can interact with biomolecules in biological fluids, forming protein-coated corona complexes [151,152]. The binding affinity for NPs is contingent upon the component, with proteins exhibiting a lower binding affinity compared to humic acids. Consequently, components with high binding affinity strongly adhere to the NPs surface, resulting in the formation of a hard layer. Above the hard layer, a soft layer may form [153]. Liu et al. [152] observed that the formation of a protein corona (PrC) in zebrafish liver cells reduced the toxicity of PS NPs. They also noted that the type of PrC may significantly influence toxicity. For example, fetal bovine serum mitigated plastic particle-induced metabolic disturbances and stress responses more effectively than bovine serum albumin. Conversely, Fu et al. [154] found that the presence of PrC on MPs increased the maximum antibiotic adsorption capacity by 51.9–64.7%. This effect is further influenced by the stable structure of the PrC, which mediates hydrophobic interactions, van der Waals forces, electrostatic interactions, and hydrogen bonding. During interactions, the protein coating may undergo conformational changes, altering its function and binding affinity [153].
4.4. Oxidative Stress
Oxidative stress is among the most frequently reported adverse effects resulting from exposure to MPs. This condition is characterized by an imbalance between oxidants and antioxidants, with a predominance of oxidants [155]. Harmful stimuli lead to an overproduction of ROS, potentially exceeding the capacity of cellular antioxidant defense mechanisms. Consequently, oxidized biomolecules, DNA mutations, protein denaturation, and increased lipid peroxidation occur, resulting in oxidative cell damage, apoptosis, and necrosis [156]. MPs can generate ROS through both extracellular and intracellular mechanisms [155]. Ma et al. [157] observed that the expression of genes related to oxidative stress, apoptosis, and cellular senescence changed significantly before and after rainbow trout were exposed to MPs-induced stress. The study demonstrated that MPs reduced antioxidant capacity by inhibiting glutathione synthase expression. Mannering et al. [158] reported that Pomacentrus amboinensis exposed to PS MPs containing di(2-ethylhexyl) phthalate (DEHP) experienced greater oxidative stress and damage than those exposed to PS MPs without this plasticizer. Chen et al. [159] showed that MPs, both alone and in combination with Pb, induced oxidative stress, histopathological damage, and immune dysfunction in Mytilus coruscus. Although Pb alone did not cause significant toxic effects, co-exposure with MPs exacerbated oxidative damage and immune dysfunction. Regarding NPs, Babaei et al. [160] demonstrated that exposure of male Wistar rats to PS NPs increased catalase activity and decreased acetylcholinesterase (AChE) activity, indicating neurotoxicity. Feng et al. [161] further observed that NPs-induced oxidative stress responses may contribute to developmental toxicity in zebrafish embryos.
4.5. Challenges Related to Human Toxicity Assessment
Estimating human health risks from both MPs and NPs remains challenging, due to the limited data on their occurrence, exposure pathways, and biodistribution [46]. Existing studies [162,163,164,165,166] primarily focus on determining potential toxic effects in plants, mussels, fish, mice, and rats. Furthermore, Brouwer et al. [167] caution against overinterpreting findings that assess MPs toxicity based on a single polymer type. Most recent experimental studies [157,168,169,170,171] on MPs and NPs toxicity (published between 2024 and 2025) focus mainly on PS, which complicates the assessment of actual human health risks. Additionally, the toxicity of true-to-life MPs is believed to differ significantly from that of synthetic PS microbeads [167]. As noted by Xu et al. [172], the complex and diverse physicochemical properties of MPs and NPs make interpreting individual studies on plastic particle toxicity difficult. Figure 3 provides a summary of the toxicity mechanisms of MPs and NPs.
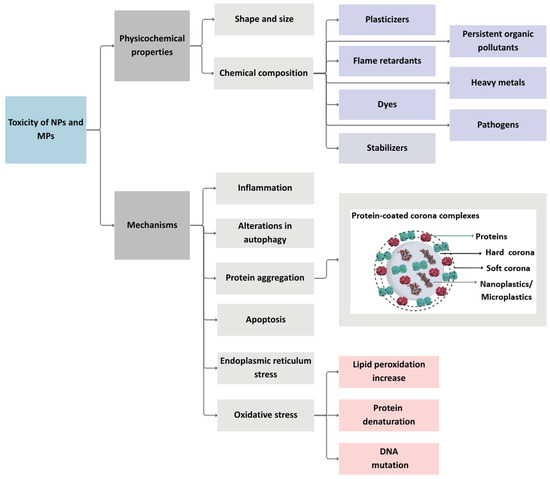
Figure 3.
The fundamental mechanisms of toxicity of MPs and NPs. Developed by the authors based on [119,132,141,151,153,156]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
5. Impact of MPs and NPs on Different Human Body Systems
5.1. Digestive System
The digestive system can be exposed to MPs and NPs through the consumption of food, beverages, and water [173]. These particles can subsequently migrate to other organ systems or accumulate within the digestive tract [21]. This may cause an imbalance in the intestinal microbiome, leading to dysbiosis, defined as changes in its composition and/or function [21,174,175]. Dysbiosis can have wide-ranging effects on digestion, immune responses, and overall health [21,37] (Figure 4).
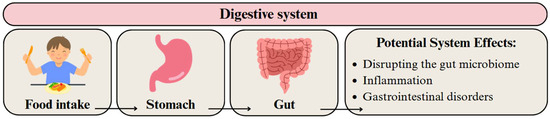
Figure 4.
Migration pathway of MPs and NPs in the digestive system and their potential health effects. Developed by the authors based on [37,59]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
The presence of MPs can influence systemic metabolism and alter the composition of the gut microbiome. A study conducted by Fan et al. [176] found that the nanotoxicity of polylactic acid (PLA) MPs is time-dependent. Short-term exposure to PLA MPs induced inflammation, decreased the diversity of gut microbiota, and increased the dysbiosis index, suggesting an imbalance in the microbiome. Subsequently, metabolic disorders were observed in the liver and jejunum. In contrast, long-term exposure led to adaptive changes in the microbiome and metabolism, establishing a new homeostatic state under prolonged external stress.
Detection of MPs and NPs in the digestive system can be performed by analyzing fecal samples from volunteers. Wibowo et al. [70] reported that MPs were present in approximately 64% of human fecal samples collected from a rural community in Indonesia. Among the detected MPs, PP was the most common type, with an average concentration of 10.19 µg/g of feces. Products commonly used by the study participants were also examined. The highest concentrations of MPs were detected in tempeh (11.08 µg/g) and table salt (2.6 μg/g), suggesting that these products are likely major sources of gastrointestinal contamination. The potential for oral hygiene products to introduce MPs was also examined. Notably, toothpaste contained MPs at concentrations up to 15.42 µg/g, indicating it may contribute to gastrointestinal exposure.
Zhang et al. [71] studied a group of young men from China and detected MPs in 23 samples (95.8%), with particle counts between 1 and 36 per gram and sizes from 20 to 800 μm. Between one and eight types of particles were identified in the samples, with PP being the most abundant polymer. An analysis of the association of dietary habits with the number of MPs detected revealed a moderate correlation related to beverage choices. Specifically, consumption of bottled water and soft drinks was associated with higher excretion of MPs.
A study by Ho et al. [72] identified an average of 50 particles per gram (20.4–138.9 particles per gram wet weight) in feces collected from a healthy Hong Kong population. PS was the most common polymer type, followed by PP and PE. The particles were mainly fragments, whereas about two-thirds of the PET particles were fibers. Over 88% of the particles measured less than 300 µm.
The choice of products used in the daily diet can affect the content of MPs and NPs. Hartman et al. [73] conducted a pilot study dividing the daily menu into three periods: normal (with typical exposure), low (minimizing the use of plastic packaging and utensils), and high (increasing the use of plastic packaging and items). Plastic particles were detected in all samples, with median concentrations up to 3.5 particles per gram of feces, with PE being the most frequently identified polymer. Although the differences between the “low” and “high” periods did not show a consistent dose–response relationship, statistical analysis linked a higher number of particles with greater plastic use.
It remains unclear whether MPs in the body influence the development of digestive diseases. A study by Yan et al. [74] compared the content of MPs in feces among two groups in China: healthy volunteers and patients with inflammatory bowel disease (IBD). Comparative analysis revealed that the concentration of MPs in IBD patients was significantly higher than in healthy volunteers. In healthy individuals, it was 28 items/g, and in patients, it was 41.8 items/g. Fifteen types of MPs were detected in the feces, with PET (22.3–34.0%) and PA (8.9–12.4%) being the most common. The predominant shapes were sheets and fibers. The study suggests that exposure to MPs may be related to the disease process or that IBD increases the retention of MPs.
Research is ongoing, and growing evidence indicates that MPs may contribute to cancer development. A study conducted by Xu et al. [75] examined the relationship between MPs concentration in feces and the risk of developing colorectal cancer (CRC) among Chinese people. The study involved 258 patients with CRC and 493 healthy individuals. Fecal samples collected from both groups contained MPs. The average concentration of MPs in healthy volunteers was 43 particles/g dry weight, and in CRC patients it was 62 particles/g dry weight. Seventeen polymer types were detected across all analyzed stool samples and PET was predominant in both healthy volunteers and patients (30% and 37%, respectively). These results indicate that current environmental exposure to MPs may elevate CRC risk.
5.2. Respiratory System
The general deposition of particulate matter in the airways is largely influenced by its size. Particles between 5 and 10 μm and 1–5 μm in size can reach the upper and lower airways, respectively [63,64]. Particles approximately 1 μm in size behave like gases, reaching the alveoli and translocating into cellular tissue and the circulatory system [64], whereas smaller particles are mostly expelled in exhaled air [177]. According to Riaz et al. [62], breathing patterns can influence the deposition of MPs, with greater deposition in 4–10 μm and 1–3 μm size ranges at lower and higher flow rates, respectively (Figure 5). Additionally, the deposition hotspots of inhaled MPs are the main bronchi and initial airways [62].
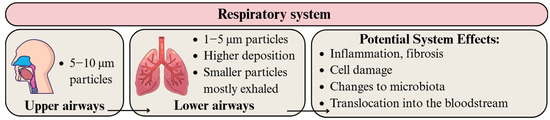
Figure 5.
Migration pathway of MPs and NPs in the respiratory system and their potential health effects. Developed by the authors based on [62,63,64,177,178]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
Polymer particles, mostly in the form of fibers, have been detected in the bronchoalveolar fluid of European and Iranian individuals from diverse occupational backgrounds. Identified polymers included PE, PS, PP, and PET [77]. Baeza-Martínez et al. [77] found a statistically significant elevation in the number of MPs in high-risk occupations (e.g., construction and gas station workers) and smokers compared to low-risk occupations (e.g., civil servants and salesmen) and non-smokers.
Particles originating from PET material were found to cause genotoxicity in human airway epithelial cells (A549) [179]. They also reprogrammed the lung microbiota and exacerbated inflammation and fibrosis after myocardial infarction [180]. In a study by Baeza-Martínez et al. [77], higher levels of microfibers in bronchoalveolar lavage fluid were correlated with limited airflow, which could be associated with reduced lung capacity and obstructive lung diseases. Moreover, Zhu et al. [181] found that lung tissue contained the highest amount of MPs compared to intestines and tonsillar tissues.
Current research provides strong evidence of the potential toxic effects of MPs and NPs on human lungs [77,179,180]. However, larger studies encompassing diverse polymer types and particle shapes are needed for a comprehensive risk assessment.
5.3. Circulatory System
Numerous studies confirm the occurrence of MPs and NPs in human blood, with levels depending on the population tested [58,78]. Leslie et al. [78] detected polymer particles in the blood of 77% of volunteers from the Netherlands, with an average concentration of 3.53 µg/mL. In contrast, Song et al. [58] found MPs in 100% of serum samples from Chinese students, with a median concentration of 20.81 μg/g.
Both MPs and NPs can enter the bloodstream via common pathways, particularly through gastrointestinal absorption and inhalation [58,65]. Uptake may be enhanced when natural biological barriers are compromised due to damage or inflammation in pathological conditions [65] (Figure 6). Additionally, exposure is possible through the use of medical devices and intravenous fluids [182,183]. Particles smaller than 150 μm can cross the gut epithelium, whereas those under 20 μm may penetrate deeper into organs [184]. However, the fate of MPs and NPs in the bloodstream, including their potential breakdown into smaller particles, remains poorly understood.
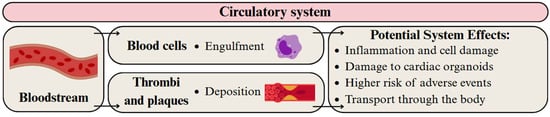
Figure 6.
Migration pathway of MPs and NPs in the circulatory system and their potential health effects. Developed by the authors based on [65,66,67,68,79]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
Polymer particles have been detected in thrombi and plaques from patients, and their presence is linked to increased health risks, including hypertension, stroke, myocardial infarction, and death [65,68,79]. Moreover, MPs induced regional pathological changes in cardiac organoids [66] and oxidative stress in vascular smooth muscle cells [185].
Toxicity of MPs and NPs to immune cells is well documented, with a particular focus on PS [67,186,187]. Park et al. [186] showed that neutrophils readily bind and engulf 1 µm PS spheres, resulting in enhanced proinflammatory effects and cell death. Likewise, Koner et al. [187] found that PS spheres sized 100–450 nm induced intracellular ROS production and DNA damage in THP-1 macrophages. Wolff et al. [67] further confirmed that exposure PS and poly(methyl methacrylate) (PMMA) particles sized 50–1100 nm was cytotoxic to macrophages, which exhibited greater sensitivity than dendritic cells. The documented presence of various polymer particles in the human circulatory system and their associated health risks warrants further investigation into the specific effects of each polymer type, shape, and dose. As PS is one of the most studied plastics [67,186,187], future research should expand to include other commonly detected polymers such as PE, poly(vinyl chloride) (PVC), PET, and PP [58,78].
5.4. Urinary/Excretory System
Upon entering the bloodstream, plastic particles can translocate to various organs, including the kidneys, whose primary function is to filter and excrete metabolic waste [188]. The human body does not have a specific mechanism for the excretion of MPs in the kidneys. However, it can be assumed that the mechanism is similar to that of protein molecules that are selectively filtered through the glomerular filtration membrane based on their molecular weight. On the other hand, renal tubular epithelial cells cannot reabsorb these particles, allowing them to pass into the urine [60] (Figure 7). A challenge arises, however, because the glomerular filtration barrier can only allow the passage of particles smaller than approximately 10 nm. The probable mechanism of MPs excretion involves their transport through the bloodstream, uptake by epithelial cells of the convoluted tubules, passage through the tubular system, and eventual excretion in the urine [69].
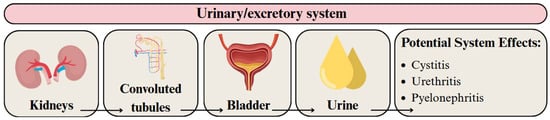
Figure 7.
Migration pathway of MPs and NPs in the urinary/excretory system and their potential health effects. Developed by the authors based on [23,60,69]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
Pironti et al. [69] detected 7 irregularly shaped MPs (4–15 µm) in 6 human urine samples, including PP, PE, PVC, and poly(ethylene vinyl acetate) (PVA). Exacoustos et al. [81] identified 17 MPs in 7 out of 10 samples of healthy human kidney tissue obtained during nephrectomy for renal cancer. They also detected 9 plastic fragments in 7 of 10 human urine samples. Polymers such as PS, styrene-isoprene, and PE were detected. In turn, Massardo et al. [80] analyzed healthy kidney tissue collected during nephrectomy, as well as urine samples from healthy donors. They identified 23 MPs in 10 urine samples and 43 particles in 10 kidney samples. Particle sizes ranged from 3 to 13 μm in urine and from 1 to 29 μm in kidneys. The most frequently detected polymers were PE and PS, while the most common pigments were hematite and Cu-phthalocyanine. Rotchell et al. [82] detected larger, irregularly shaped MPs in urine samples from patients with endometriosis compared to samples from healthy individuals. The hypothesis that larger plastic particles may lead to inflammatory reactions was proposed; however, this finding was not confirmed by toxicological studies. Song et al. [83] observed significantly higher levels of MPs (<50 μm) in urine samples from children and their adult guardians who frequently come into contact with plastic toys and playground equipment. Furthermore, individuals who regularly use skin care products showed a significant increase in MPs in their urine. This finding suggests a potential correlation between the presence of plastic particles in urine and the living environment.
Wang et al. [189] used the human kidney proximal tubule epithelial cell line HK-2 for their study. Stable ATG5 knockdown (ATG5KD) HK-2 cells were then generated following puromycin selection. They found that adding PS MPs to stable ATG5KD kidney cells reduced cell viability compared to water-treated controls. Inhibition of autophagy increased inflammatory protein expression and cell death, suggesting that autophagy protects against PS MPs-induced kidney damage. Goodman et al. [188] investigated the potential toxic effects of 1 μm PS MPs using human embryonic kidney cells (HEK 293). They observed that these particles can significantly affect the metabolic activity of kidney cells after long-term exposure. After 72 h, metabolic activity decreased by 61% compared to unexposed cells. Additionally, treating cells with 5 or 50 μg/mL PS MPs induced high ROS levels within 2 h.
MPs and NPs in the urinary tract are believed to increase the risk of cystitis, urethritis, and pyelonephritis [23]. This may be due to enhanced intracellular transport of pathogenic microorganisms [23]. Additionally, PS MPs increased serum blood urea nitrogen, uric acid, and creatinine in various animal models [190]. Treatment of rats with 1 µm PS MPs caused kidney histological changes, including glomerular disruption, inflammatory infiltration, loss of brush border, and detachment of renal tubular epithelial cells [190]. Nevertheless, extrapolating these animal study results to lifelong human exposure remains challenging [191].
5.5. Reproductive System
The reproductive system can be adversely affected by MPs and NPs in both males and females. In males, they can disrupt the blood-testis barrier, which can lead to impaired spermatogenesis and potentially affect fertility [192]. In females, MPs threaten reproductive health by impairing ovarian function, altering hormone levels, and reducing fertility [193,194]. Plastic particles can also affect lipid metabolism and the reproductive health of offspring [195] (Figure 8).
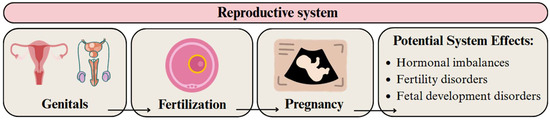
Figure 8.
Migration pathway of MPs and NPs in the reproductive system and their potential health effects. Developed by the authors based on [192,193,194]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
Zhang et al. [84] conducted a study involving volunteers from China, collecting sperm and urine samples. Sperm quality parameters were assessed, including total sperm count, concentration, motility, and morphology. Plastic particles were found in all samples, with the number of types detected ranging from 3 to 5 [84]. The highest detection rates were found for PS, PP, and PE. Samples containing polytetrafluoroethylene (PTFE) were also associated with reduced sperm quality, including decreases in total sperm count, concentration, and progressive motility.
A study conducted by Garcia et al. [85] showed the presence of MPs in all placentas examined. However, concentrations varied widely, ranging from 6.5 to 685 µg of MPs and NPs per gram of placental tissue. The most frequently detected polymer was PE, which was present in almost all samples. PVC and nylon were identified as the next most common polymers.
Wang et al. [196] studied the harmful effects of PS MPs of different sizes (0.1 and 5 μm) on the placenta—a key organ in fetal development—after oral exposure during pregnancy. The results showed that both sizes of PS MPs could spread within mouse placental tissue. However, 0.1 μm particles showed a greater ability to penetrate the placenta and accumulate in fetal liver and brain tissues than 5 μm particles. Additionally, they reduced the surface area of connections, decreased labyrinthine vascularization, and increased cell apoptosis in the placenta, leading to fetal developmental disorders.
In a study by Tian et al. [86] involving 48 pregnant women, MPs were detected in the amniotic fluid of 39 samples. Six types of polymers were identified, with PTFE (31%), PS (21%), and acrylonitrile butadiene styrene (15%) being the most prevalent. However, no correlation has yet been found between the presence of MPs in amniotic fluid and fetal development or postnatal size and weight.
Saraluck et al. [197] investigated the influence of hygiene on the presence of MPs in breast milk and their impact on the microbiome. During the study, MPs were present in only 38.98% of breast milk samples (23 out of 59), and the most common types of plastic were PP, PE, and PVC. It was noted that maternal hygiene affects MPs content. Mothers practicing good hygiene—frequent handwashing and using specialized products for laundering underwear—were significantly less likely to have MPs in their breast milk. Changes in bacterial composition were also noted. Specifically, Gram-positive bacteria, particularly Staphylococcus and Streptococcus, were more abundant in samples containing MPs. This suggests that Gram-positive bacteria may form biofilms and adhere to the surface of MPs. In contrast, samples without detected MPs showed higher abundances of Gram-negative bacteria, particularly Enterobacteriaceae, Moraxellaceae, and Pseudomonadaceae. These findings suggest that the presence of MPs may have an impact on the selective growth of specific bacterial taxa.
5.6. Nervous System
Determining the consequences of MPs and NPs in the brain remains challenging because these particles can undergo biotransformation, including biological corona formation, polymer swelling, and surface functionalization [198]. Plastic particles, particularly NPs, are thought to breach the blood–brain barrier (BBB) and trigger four primary pathogenic mechanisms: oxidative stress, neuroinflammation, acting as chemical carriers, and Aβ oligomer formation [61] (Figure 9). The small size of NPs allows for intracellular uptake, potentially disrupting cellular processes such as protein folding and mitochondrial function, thereby affecting neuronal health [119]. Exposure to MPs and NPs is believed to increase the risk of Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis [198].
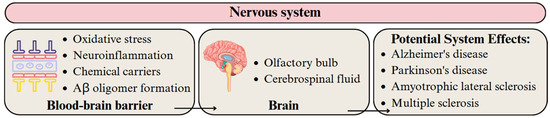
Figure 9.
Migration pathway of MPs and NPs in the nervous system and their potential health effects. Developed by the authors based on [61,198]. Source of images: https://www.canva.com/ (accessed on 21 August 2025).
Nihart et al. [87] observed that postmortem human frontal cortex samples contained significantly higher concentrations of MPs and NPs than liver or kidney samples. Depending on the specific brain sample analyzed (tissue, parenchyma, or pellet), PE particles ranging in size from 100 nm to 5 μm were detected. Notably, brain samples from individuals with vascular dementia contained higher concentrations of plastic particles than those from healthy controls. This likely reflects brain tissue atrophy caused by vascular dementia, which compromises BBB integrity. Amato-Lourenço et al. [88] identified 16 synthetic polymer particles and fibers in the olfactory bulb of 8 out of 15 deceased individuals. The MPs ranged from 5.5 to 26.4 μm, with an average fiber length of 21.4 μm. The most abundant polymer, however, was PP. These findings suggest that the olfactory pathway serves as a key route for the entry of exogenous particles into the brain. Xie et al. [89] analyzed cerebrospinal fluid (CSF) from 28 patients, with and without CNS infections, and found that only PS, PE, PP, and PVC particles could selectively enter the human CNS. Moreover, CSF concentrations of PP and PE were significantly higher in patients with CNS infections, suggesting that the distribution of MPs and NPs within the CNS may be influenced by health status or comorbidities.
Ban et al. [199] exposed human neuroblastoma cells (SH-SY5Y) to PS NPs (50 nm). Neuroblastoma cells exposed to high concentrations of PS NPs exhibited neurite shrinkage, morphological changes, nuclear swelling, and leakage of intracellular components. Kwon et al. [200] found that one week of oral treatment with PS MPs (<2 μm) in mice led to brain accumulation via microglial phagocytosis, potentially triggering immune activation and apoptosis. Transcriptome analysis of human microglial HMC-3 cells treated with PS MPs revealed altered expression of immune response gene clusters, immunoglobulins, and several related microRNAs. Additionally, Zhang et al. [201] found that PE MPs induced oxidative stress, leading to human intervertebral disc degeneration. The redox imbalance could enhance the aging of nucleus pulposus cells and consequently reduce the ability to withstand mechanical loads. This leads to spinal instability and neurogenic pain.
Plastic particles in the brain can also cause AChE inhibition and changes in neurotransmitter levels, potentially contributing to behavioral changes. So et al. [202] observed that exposure to PS MPs impaired social novelty preferences in mouse offspring. Chen et al. [203] also demonstrated that PS NPs and PS MPs induced anxiety-like behaviors in mice. Zhang et al. [204] suggested that PLA and poly(butylene adipate-co-terephthalate) biodegradable plastics may cause behavioral abnormalities in zebrafish by inducing immune dysregulation in the brain. Luo et al. [205] suggest that children may be particularly vulnerable, as MPs and NPs can alter synaptic development, neurotransmission, and hormonal balance. This, in turn, leads to cognitive, behavioral, and developmental disorders. Nevertheless, no studies to date have confirmed this relationship.
5.7. Exposure Risk Assessment
Determining human exposure to MPs and NPs remains scientifically challenging, primarily due to the limited data available for such assessments. For example, Kadac-Czapska et al. [99] evaluated infant exposure to MPs via food consumption. They highlighted that the type, quantity, and frequency of food intake are important considerations. Other important factors include the food packaging material, the purity of water used in preparation, and the types of containers from which the child consumes the food. This indicates that numerous factors influence the assessment of actual exposure in this group, even when considering only dietary sources.
As shown in Table 2, standardized protocols for detecting plastic particles in human organs or tissues remain lacking. To date, only the European Union (EU) [206] and the California State Water Resources Control Board [207] have implemented regulations for measuring MPs in drinking water—none exist yet for human tissue matrices. In practice, researchers detect MPs and NPs in human samples using various spectroscopic and chromatographic techniques, but each comes with significant limitations (Table 3).

Table 3.
Overview of challenges and limitations associated with the analysis of MPs and NPs in human matrices.
Table 3.
Overview of challenges and limitations associated with the analysis of MPs and NPs in human matrices.
| Analytical Methods | Advantages | Limitations | Reference |
|---|---|---|---|
| LDIR | Characterization of MPs by size, shape, and polymer type | Not suitable for determining NPs | [68] |
| μ-Raman | Identification and quantification of MPs | Not suitable for determining NPs | [76] |
| SEM-EDX | Observation of surface morphology and elemental composition of MPs and NPs | - | [77] |
| µ-FTIR | Identification and quantification of MPs | Not suitable for determining NPs | [82] |
| FTIR with attenuated total reflectance | Assessment of the functional chemistry and polymer type of MPs | Limited to particles with a size >1–20 µm | [85] |
| Py-GC/MS | Speciation and quantification of MPs and NPs in native human tissues | Lipids in the human matrix may interfere with analysis Oxidative degradation during pyrolysis may underestimate MPs/NPs | [85,87] |
| TEM | Observation of shapes and sizes of NPs and MPs in different tissue types | Polymer composition of MPs/NPs cannot be confirmed | [87] |
| General limitations and challenges with MPs and NPs analysis in human matrices | Lack of standardization of methodological protocols Lack of consistent concentration units within the matrix Method validation rarely performed Limited number of studies on human matrices | ||
Other key challenges include developing methods to isolate plastic particles from human tissues without loss or contamination and the scarcity of studies applying consistent protocols to the same sample type. Additionally, the units used to report MPs and NPs concentrations vary across studies (e.g., μg/g, μg/mL, or particles per sample), complicating comparisons. Over the past five years, most research has focused on urine [69,76,80] and stool [70,72,73] samples; however, the wide variety of analytical techniques applied to these matrices, often without thorough validation, complicates reliable comparisons. In contrast, few studies have investigated other human matrices—blood, semen, placenta, brain, and olfactory bulb—highlighting them as promising targets for future research [78,84,85,87,88].
Notably, no legal regulations define safe human exposure levels for plastic particles, and comprehensive environmental or epidemiological assessments of MPs and NPs exposure are lacking. Reducing the release of plastics, and consequently MPs, into the environment remains a global priority. Many governments have begun taking action. For example, China [208] and Australia [209] have banned lightweight plastic shopping bags, while the EU has implemented similar restrictions [210]. These regions have also banned the sale of certain single-use plastic items (e.g., straws, cutlery, plates) and restricted the use of expanded PS foodware [209,211]. In the United States (U.S.), similar restrictions currently exist only at the state level [212]. To mitigate MPs contamination, countries such as Australia, China, the United Kingdom, and the U.S. have banned rinse-off cosmetics containing plastic microbeads [208,209,213,214]. The EU plans to ban plastic microbeads in all cosmetic products by 2027 and is the only jurisdiction to announce plans to phase out microbeads in lip, nail, and makeup products by 2035 [215]. However, it is important to note that these regulations generally target larger MPs and still do not cover smaller—and potentially more hazardous—NPs.
6. Conclusions
A growing body of research is currently focused on identifying plastic particles within the human body. The studies referenced in this review have demonstrated that plastic particles composed of various polymers can be found in human organs. Additionally, studies indicate that MPs and NPs may affect multiple human organ systems. Furthermore, these plastic particles may influence the gut microbiome and reproductive processes. Experimental studies further suggest that MPs and NPs in the human brain may be linked to neurological diseases, including dementia, which raises significant concerns. Nevertheless, establishing a definitive correlation between their presence and the potential for disease development remains challenging.
Humans are exposed to MPs and NPs through all major pathways, although certain polymers are more prevalent in specific routes (for example, PET is frequently encountered via inhalation). Overall, ingestion (oral) and inhalation represent the most significant and best-studied exposure routes. By contrast, the significance of dermal exposure is not yet fully understood, particularly with regard to polymer types other than PS. Recent studies, however, have begun to clarify how MPs and NPs penetrate the skin—mainly through endocytosis, exocytosis, passage through cell junctions, and interactions with the extracellular matrix.
It is worth emphasizing that MPs and NPs encompass a broad spectrum of chemical compounds, complicating the assessment of potential toxicity. The mechanisms by which plastic particles affect human cells, and consequently organs, are also of significant concern. However, studies on the toxicity of MPs and NPs often focus on a single polymer type. This approach does not provide a comprehensive view of the risks posed by these particles. Humans are exposed to a wide variety of MPs and NPs throughout their lives. Currently, no established threshold values exist for MPs in human biological samples, limiting the scope of risk assessment. Additionally, standardized methods for detecting MPs and NPs in tissues and body fluids are lacking. Further studies are needed to clarify the relationship between human exposure to MPs and NPs, their presence in the body, and resulting health effects. Significant knowledge gaps remain regarding the effects and behavior of different polymer types and shapes. Future research should include commonly identified polymers, such as PE, PET, PP and PVC, in fragment and fiber forms to ensure a more thorough assessment of human exposure risks. Given the potential threat of MPs and NPs, it is imperative to take action. This can be achieved, among other measures, by limiting the release of plastics into the environment and food chain, thereby reducing the transfer of plastic particles to flora and fauna. An example of such action is emerging global legislation that restricts or bans small single-use plastic packaging, significantly reducing environmental pollution. The potential toxicity of MPs and NPs should remain a major focus for current and future generations.
Author Contributions
Conceptualization, K.K.-C., J.O., N.N., K.J., P.K. and M.G.; writing—original draft preparation, N.N., K.J. and P.K.; writing—review and editing, K.K.-C., J.O. and M.G.; visualization, N.N., K.J. and P.K.; supervision, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Marrone, A.; La Russa, M.F.; Randazzo, L.; La Russa, D.; Cellini, E.; Pellegrino, D. Microplastics in the Center of Mediterranean: Comparison of the Two Calabrian Coasts and Distribution from Coastal Areas to the Open Sea. Int. J. Environ. Res. Public Health 2021, 18, 10712. [Google Scholar] [CrossRef] [PubMed]
- Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. [Google Scholar] [CrossRef] [PubMed]
- D’Hont, A.; Gittenberger, A.; Leuven, R.S.E.W.; Hendriks, A.J. Dropping the Microbead: Source and Sink Related Microplastic Distribution in the Black Sea and Caspian Sea Basins. Mar. Pollut. Bull. 2021, 173, 112982. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nag, R.; Cummins, E. Ranking of Potential Hazards from Microplastics Polymers in the Marine Environment. J. Hazard. Mater. 2022, 429, 128399. [Google Scholar] [CrossRef]
- Yu, R.S.; Singh, S. Microplastic Pollution: Threats and Impacts on Global Marine Ecosystems. Sustainability 2023, 15, 13252. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef]
- Gan, Q.; Cui, J.; Jin, B. Environmental Microplastics: Classification, Sources, Fates, and Effects on Plants. Chemosphere 2023, 313, 137559. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2024, 64, 3502–3521. [Google Scholar] [CrossRef]
- Song, J.; Wang, C.; Li, G. Defining Primary and Secondary Microplastics: A Connotation Analysis. ACS ES&T Water 2024, 4, 2330–2332. [Google Scholar] [CrossRef]
- Ziani, K.; Ioniță-Mîndrican, C.B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Lindfors, S.; Österlund, H.; Lorenz, C.; Vianello, A.; Nordqvist, K.; Gopinath, K.; Lykkemark, J.; Lundy, L.; Vollertsen, J.; Viklander, M. Microplastics and Tyre Wear Particles in Urban Runoff from Different Urban Surfaces. Sci. Total Environ. 2025, 980, 179527. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Hu, Y.; Abidi, N. Microfibers from Synthetic Textiles as a Major Source of Microplastics in the Environment: A Review. Text. Res. J. 2021, 91, 2136–2156. [Google Scholar] [CrossRef]
- Huber, M.; Archodoulaki, V.M.; Pomakhina, E.; Pukánszky, B.; Zinöcker, E.; Gahleitner, M. Environmental Degradation and Formation of Secondary Microplastics from Packaging Material: A Polypropylene Film Case Study. Polym. Degrad. Stab. 2022, 195, 109794. [Google Scholar] [CrossRef]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential Microplastic Release from Beached Fishing Gear in Great Britain’s Region of Highest Fishing Litter Density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the Human Body: A Comprehensive Review of Exposure, Distribution, Migration Mechanisms, and Toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Anuar, S.T.; Yusof, K.M.K.K.; Lai, L.A.; Brentnall, T. Detection of Microplastics in Human Tissues and Organs: A Scoping Review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Sofield, C.E.; Anderton, R.S.; Gorecki, A.M. Mind over Microplastics: Exploring Microplastic-Induced Gut Disruption and Gut-Brain-Axis Consequences. Curr. Issues Mol. Biol. 2024, 46, 4186–4202. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, C.; Duan, X.; Liang, B.; Genbo Xu, E.; Huang, Z. Micro- and Nanoplastics: A New Cardiovascular Risk Factor? Environ. Int. 2023, 171, 107662. [Google Scholar] [CrossRef]
- Saha, S.C.; Saha, G. Effect of Microplastics Deposition on Human Lung Airways: A Review with Computational Benefits and Challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu; Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and Human Health: Unveiling the Gut Microbiome Disruption and Chronic Disease Risks. Front. Cell Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Prabhu, K.; Ghosh, S.; Sethulekshmi, S.; Shriwastav, A. In Vitro Digestion of Microplastics in Human Digestive System: Insights into Particle Morphological Changes and Chemical Leaching. Sci. Total Environ. 2024, 934, 173173. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, L.; Olsen, M.; Tajouri, L.; Beaver, D.; Hudson, C.; Alghafri, R.; McKirdy, S.; Goldsworthy, A. Plastic Induced Urinary Tract Disease and Dysfunction: A Scoping Review. J. Expo. Sci. Environ. Epidemiol. 2024, 35, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Xiong, Y.; Zhang, Y.; Zhang, M. The Hidden Threat: Unraveling the Impact of Microplastics on Reproductive Health. Sci. Total Environ. 2024, 935, 173177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, S.; Liu, J.; Liu, Z. The Effects of Micro- and Nanoplastics on the Central Nervous System: A New Threat to Humanity? Toxicology 2024, 504, 153799. [Google Scholar] [CrossRef]
- Yee, M.S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Strojny, W.; Gruca-Rokosz, R.; Cieśla, M. Microplastics in Water Resources: Threats and Challenges. Appl. Sci. 2025, 15, 4118. [Google Scholar] [CrossRef]
- Wang, S.; AL-Hasni, N.S.; Liu, Z.; Liu, A. Multifaceted Aquatic Environmental Differences between Nanoplastics and Microplastics: Behavior and Fate. Environ. Health 2024, 2, 688–701. [Google Scholar] [CrossRef]
- Pandey, B.; Pathak, J.; Singh, P.; Kumar, R.; Kumar, A.; Kaushik, S.; Thakur, T.K. Microplastics in the Ecosystem: An Overview on Detection, Removal, Toxicity Assessment, and Control Release. Water 2023, 15, 51. [Google Scholar] [CrossRef]
- Ośko, J.; Kadac-Czapska, K.; Jażdżewska, K.; Nowak, N.; Kowalczyk, P.; Grembecka, M. Nanoplastics: From Separations to Analysis—Challenges and Limitations. Separations 2025, 12, 185. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between Microplastics and Organic Pollutants: Effects on Toxicity, Bioaccumulation, Degradation, and Transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Jeyanthi Kumari, V. Hazardous Microplastic Characteristics and Its Role as a Vector of Heavy Metal in Groundwater and Surface Water of Coastal South India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous Hitchhikers? Evidence for Potentially Pathogenic Vibrio Spp. on Microplastic Particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Y.; Shao, Y.; Ray, S.S.; Wang, B.; Zhao, Z.; Yu, B.; Zhang, X.; Li, W.; Ding, J.; et al. A Review on the Occurrence, Detection Methods, and Ecotoxicity of Biodegradable Microplastics in the Aquatic Environment: New Cause for Concern. Trends Anal. Chem. 2024, 178, 117832. [Google Scholar] [CrossRef]
- Muñiz, R.; Rahman, M.S. Microplastics in Coastal and Marine Environments: A Critical Issue of Plastic Pollution on Marine Organisms, Seafood Contaminations, and Human Health Implications. J. Hazard. Mater. Adv. 2025, 18, 100663. [Google Scholar] [CrossRef]
- Kumar, M.; Chaudhary, V.; Kumar, R.; Chaudhary, V.; Srivastav, A.L. Microplastics, Their Effects on Ecosystems, and General Strategies for Mitigation of Microplastics: A Review of Recent Developments, Challenges, and Future Prospects. Environ. Pollut. Manag. 2025, 2, 87–105. [Google Scholar] [CrossRef]
- Sinha, P.; Saini, V.; Varshney, N.; Pandey, R.K.; Jha, H.C. The Infiltration of Microplastics in Human Systems: Gastrointestinal Accumulation and Pathogenic Impacts. Heliyon 2025, 11, e42606. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, L. Microplastic Migration and Transformation Pathways and Exposure Health Risks. Environ. Pollut. 2025, 368, 125700. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J. From Gonads to Generations: Mechanistic Insights into Reproductive Disruption by Polystyrene Nanoplastics and Co-Contaminants in Fish. Environ. Chem. Ecotoxicol. 2025, 7, 1871–1883. [Google Scholar] [CrossRef]
- Thakur, R.; Joshi, V.; Sahoo, G.C.; Jindal, N.; Tiwari, R.R.; Rana, S. Review of Mechanisms and Impacts of Nanoplastic Toxicity in Aquatic Organisms and Potential Impacts on Human Health. Toxicol. Rep. 2025, 14, 102013. [Google Scholar] [CrossRef]
- Permana, R.; Chakraborty, S.; Valsami-Jones, E. Nanoplastics in Aquatic Environments: The Hidden Impact of Aging on Fate and Toxicity. Environ. Chem. Ecotoxicol. 2025, 7, 429–444. [Google Scholar] [CrossRef]
- Barría, C.; Balasch, J.C.; Brandts, I.; Oliva, D.; Iriarte, J.L.; Teles, M. Immunological Responses, Oxidative Stress, and Histopathological Effects of Nanoplastics on Commercially Relevant Mussel Species: A Review. J. Hazard. Mater. Adv. 2025, 17, 100540. [Google Scholar] [CrossRef]
- Gupta, C.; Kaushik, S.; Himanshu; Jain, S.; Dhanwani, I.; Mansi; Garg, S.; Paul, A.; Pant, P.; Gupta, N. Bioaccumulation and Toxicity of Polystyrene Nanoplastics on Marine and Terrestrial Organisms with Possible Remediation Strategies: A Review. Environ. Adv. 2022, 8, 100227. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Lin, Y.-C.; Liu, W.-C.; Lee, Y.-H.; Chiu, H.-W. Air Pollution and Its Impacts on Health: Focus on Microplastics and Nanoplastics. Ecotoxicol. Environ. Saf. 2025, 299, 118402. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, N.; Gao, Q.; Peijnenburg, W.J.G.M.; Yin, K.; Li, L.; Wang, Y. Microplastics and Nanoplastics in the Ocular Environment: Pathways, Toxic Effects, and Future Challenges. Curr. Res. Toxicol. 2025, 9, 100251. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.D.; Gika, H.; Theodoridis, G.; Maragou, N.; Thomaidis, N.; Corredig, M. Microplastics and Nanoplastics: Exposure and Toxicological Effects Require Important Analysis Considerations. Heliyon 2024, 10, e32261. [Google Scholar] [CrossRef]
- Jahedi, F.; Jaafarzadeh Haghighi Fard, N. Micro- and Nanoplastic Toxicity in Humans: Exposure Pathways, Cellular Effects, and Mitigation Strategies. Toxicol. Rep. 2025, 14, 102043. [Google Scholar] [CrossRef]
- Sadique, S.A.; Konarova, M.; Niu, X.; Szilagyi, I.; Nirmal, N.; Li, L. Impact of Microplastics and Nanoplastics on Human Health: Emerging Evidence and Future Directions. Emerg. Contam. 2025, 11, 100545. [Google Scholar] [CrossRef]
- Kochanek, A.; Grąz, K.; Potok, H.; Gronba-Chyła, A.; Kwaśny, J.; Wiewiórska, I.; Ciuła, J.; Basta, E.; Łapiński, J. Micro- and Nanoplastics in the Environment: Current State of Research, Sources of Origin, Health Risks, and Regulations—A Comprehensive Review. Toxics 2025, 13, 564. [Google Scholar] [CrossRef]
- Choudhury, M.; Roy, P. Challenges with Microplastic Pollution in the Regime of UN Sustainable Development Goals. World Dev. Sustain. 2025, 6, 100216. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef]
- Ghosh, S.; Sane, A.; Gohil, S.; Vashishtha, V.; Kumar, S.K.; Kumaraswamy, G. Mechanism of Microplastic and Nanoplastic Emission from Tire Wear. Soft Matter 2025, 21, 2782–2786. [Google Scholar] [CrossRef]
- Tanjil, R.H.; Islam, M.S.; Islam, Z.; Roy, S.; Nahian, S.; Salam, A. Atmospheric Microplastic Pollution in Textile Industrial Areas: Source, Composition, and Health Risk Assessment. Bull. Environ. Contam. Toxicol. 2025, 114, 51. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, Q.; Kumar, A.; Zhang, W.; Yu, Z.; Hui, D.; Zhang, C.; Shan, S. Effect of Degradable Microplastics, Biochar and Their Coexistence on Soil Organic Matter Decomposition: A Critical Review. Trends Anal. Chem. 2025, 183, 118082. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, S.; Gao, W.; Zhou, J.; Cai, H.; Wu, L.; Liu, J.; Wang, Z.; Liu, T. Microplastics Occurrence and Distribution Characteristics in Mulched Agricultural Soils of Guizhou Province. Sci. Rep. 2024, 14, 21505. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, S.Y.; Liao, C.M. Regional and Population-Scale Trends in Human Inhalation Exposure to Airborne Microplastics: Implications for Health Risk Assessment. Environ. Pollut. 2025, 371, 125950. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kamineni, V.N.; Lin, Z. A Physiologically Based Toxicokinetic Model for Microplastics and Nanoplastics in Mice after Oral Exposure and Its Implications for Human Dietary Exposure Assessment. J. Hazard. Mater. 2024, 480, 135922. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Yang, L.; Huang, Y.; Zhang, N.; Ma, G. Internal and External Microplastic Exposure in Young Adults: A Pilot Study Involving 26 College Students in Changsha, China. Environ. Res. 2024, 263, 120250. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Koutsari, C.; Samsonraj, R.M. Microplastics and Nanoplastics and the Digestive System. Gastro Hep Adv. 2025, 4, 100694. [Google Scholar] [CrossRef]
- Tan, R.Y.; She, Q.Y.; Ma, Y.C.; Liu, M.H.; Li, L.J.; Huang, L.L.; Zhong, Y.W.; Bi, H.X. The Threat of Microplastics to Human Kidney Health: Mechanisms of Nephrotoxicity and Future Research Directions. Environ. Res. 2025, 283, 122124. [Google Scholar] [CrossRef]
- Gecegelen, E.; Ucdal, M.; Dogu, B.B. A Novel Risk Factor for Dementia: Chronic Microplastic Exposure. Front. Neurol. 2025, 16, 1581109. [Google Scholar] [CrossRef] [PubMed]
- Riaz, H.H.; Lodhi, A.H.; Munir, A.; Zhao, M.; Ali, M.H.; Sauret, E.; Gu, Y.T.; Islam, M.S. Breath of Pollutants: How Breathing Patterns Influence Microplastic Accumulation in the Human Lung. Int. J. Multiph. Flow. 2025, 185, 105156. [Google Scholar] [CrossRef]
- Löndahl, J.; Pagels, J.; Swietlicki, E.; Zhou, J.; Ketzel, M.; Massling, A.; Bohgard, M. A Set-up for Field Studies of Respiratory Tract Deposition of Fine and Ultrafine Particles in Humans. J. Aerosol Sci. 2006, 37, 1152–1163. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A Review on the Human Health Impact of Airborne Particulate Matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Geppner, L.; Hellner, J.; Henjakovic, M. Effects of Micro- and Nanoplastics on Blood Cells In Vitro and Cardiovascular Parameters In Vivo, Considering Their Presence in the Human Bloodstream and Potential Impact on Blood Pressure. Environ. Res. 2025, 273, 121254. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, Y.; Feng, Y.; Wang, Y.; Cheng, W. Low-Dose of Polystyrene Microplastics Induce Cardiotoxicity in Mice and Human-Originated Cardiac Organoids. Environ. Int. 2023, 179, 108171. [Google Scholar] [CrossRef]
- Wolff, C.M.; Singer, D.; Schmidt, A.; Bekeschus, S. Immune and Inflammatory Responses of Human Macrophages, Dendritic Cells, and T-Cells in Presence of Micro- and Nanoplastic of Different Types and Sizes. J. Hazard. Mater. 2023, 459, 132194. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal Detection and Analysis of Microplastics in Human Thrombi from Multiple Anatomically Distinct Sites. eBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First Evidence of Microplastics in Human Urine, a Preliminary Study of Intake in the Human Body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Wibowo, A.T.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Sugiyo, P.W.W.; Putro, Y.K.; Fauzia, F.N.; Santoso, H.; et al. Microplastic Contamination in the Human Gastrointestinal Tract and Daily Consumables Associated with an Indonesian Farming Community. Sustainability 2021, 13, 12840. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.B.; He, H.R.; Zhang, J.F.; Ma, G.S. You Are What You Eat: Microplastics in the Feces of Young Men Living in Beijing. Sci. Total Environ. 2021, 767, 144345. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.W.; Lim, J.Y.; Yeoh, Y.K.; Chiou, J.C.; Zhu, Y.; Lai, K.P.; Li, L.; Chan, P.K.S.; Fang, J.K.H. Preliminary Findings of the High Quantity of Microplastics in Faeces of Hong Kong Residents. Toxics 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Lomako, I.; Schachner, C.; El Said, E.; Abert, J.; Satrapa, V.; Kaiser, A.M.; Walch, H.; Köppel, S. Assessment of Microplastics in Human Stool: A Pilot Study Investigating the Potential Impact of Diet-Associated Scenarios on Oral Microplastics Exposure. Sci. Total Environ. 2024, 951, 175825. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Zhang, T.; Zhang, F.; Ren, H.; Zhang, Y. Analysis of Microplastics in Human Feces Reveals a Correlation between Fecal Microplastics and Inflammatory Bowel Disease Status. Environ. Sci. Technol. 2022, 56, 414–421. [Google Scholar] [CrossRef]
- Xu, J.; Qu, J.; Jin, H.; Mao, W. Associations between Microplastics in Human Feces and Colorectal Cancer Risk. J. Hazard. Mater. 2025, 495, 139099. [Google Scholar] [CrossRef]
- Jahedi, F.; Haghighi Fard, N.J.; Ahmadi, M.; Takdastan, A.; Shoushtari, M.H.; Dehbandi, R.; Turner, A. Microplastics in Urine, Sputum and Lung Lavage Fluid from Patients with Respiratory Illnesses. Environ. Res. 2025, 274, 121278. [Google Scholar] [CrossRef]
- Baeza-Martínez, C.; Olmos, S.; González-Pleiter, M.; López-Castellanos, J.; García-Pachón, E.; Masiá-Canuto, M.; Hernández-Blasco, L.; Bayo, J. First Evidence of Microplastics Isolated in European Citizens’ Lower Airway. J. Hazard. Mater. 2022, 438, 129439. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Eng. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Massardo, S.; Verzola, D.; Alberti, S.; Caboni, C.; Santostefano, M.; Eugenio Verrina, E.; Angeletti, A.; Lugani, F.; Ghiggeri, G.M.; Bruschi, M.; et al. MicroRaman Spectroscopy Detects the Presence of Microplastics in Human Urine and Kidney Tissue. Environ. Int. 2024, 184, 108444. [Google Scholar] [CrossRef]
- Exacoustos, O.; Artini, C.; Massardo, S.; Caboni, C.; Pastorino, A.; Chiarenza, S.; Zaza, G.; Stallone, G.; Ghiggeri, G.M.; Angeletti, A.; et al. #6111 First Identification And Characterization Of Microplastics In Human Kidney And Urine. Nephrol. Dial. Transplant. 2023, 38, gfad063a_6111. [Google Scholar] [CrossRef]
- Rotchell, J.M.; Austin, C.; Chapman, E.; Atherall, C.A.; Liddle, C.R.; Dunstan, T.S.; Blackburn, B.; Mead, A.; Filart, K.; Beeby, E.; et al. Microplastics in Human Urine: Characterisation Using ΜFTIR and Sampling Challenges Using Healthy Donors and Endometriosis Participants. Ecotoxicol. Environ. Saf. 2024, 274, 116208. [Google Scholar] [CrossRef]
- Song, X.; Chen, T.; Chen, Z.; Du, L.; Qiu, X.; Zhang, Y.; Li, Y.; Zhu, Y.; Tan, Z.; Mo, Y.; et al. Micro(Nano)Plastics in Human Urine: A Surprising Contrast between Chongqing’s Urban and Rural Regions. Sci. Total Environ. 2024, 917, 170455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, G.; Sun, K.; Ren, J.; Zhou, J.; Liu, X.; Lin, F.; Yang, H.; Cao, J.; Nie, L.; et al. Association of Mixed Exposure to Microplastics with Sperm Dysfunction: A Multi-Site Study in China. eBioMedicine 2024, 108, 105369. [Google Scholar] [CrossRef]
- Garcia, M.A.; Liu, R.; Nihart, A.; El Hayek, E.; Castillo, E.; Barrozo, E.R.; Suter, M.A.; Bleske, B.; Scott, J.; Forsythe, K.; et al. Quantitation and Identification of Microplastics Accumulation in Human Placental Specimens Using Pyrolysis Gas Chromatography Mass Spectrometry. Toxicol. Sci. 2024, 199, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liang, L.; Li, Q.; Li, N.; Zhu, X.; Zhang, L. Association between Microplastics in Human Amniotic Fluid and Pregnancy Outcomes: Detection and Characterization Using Raman Spectroscopy and Pyrolysis GC/MS. J. Hazard. Mater. 2025, 482, 136637. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Dantas, K.C.; Júnior, G.R.; Paes, V.R.; Ando, R.A.; De Oliveira Freitas, R.; Da Costa, O.M.M.M.; Rabelo, R.S.; Soares Bispo, K.C.; Carvalho-Oliveira, R.; et al. Microplastics in the Olfactory Bulb of the Human Brain. JAMA Netw. Open 2024, 7, e2440018. [Google Scholar] [CrossRef]
- Xie, J.; Ji, J.; Sun, Y.; Ma, Y.; Wu, D.; Zhang, Z. Blood-Brain Barrier Damage Accelerates the Accumulation of Micro- and Nanoplastics in the Human Central Nervous System. J. Hazard. Mater. 2024, 480, 136028. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Y.; Liu, G.; Huang, X.; Chai, G. Probabilistic Estimation of Airborne Micro- and Nanoplastic Intake in Humans. Environ. Sci. Technol. 2024, 58, 9071–9081. [Google Scholar] [CrossRef]
- Protyusha, G.B.; Kavitha, B.; Robin, R.S.; Nithin, A.; Ineyathendral, T.R.; Shivani, S.S.; Anandavelu, I.; Sivasamy, S.; Samuel, V.D.; Purvaja, R. Microplastics in Oral Healthcare Products (OHPs) and Their Environmental Health Risks and Mitigation Measures. Environ. Pollut. 2024, 343, 123118. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Urooj, Z.; Noreen, S.; Baig, M.M.F.A.; Zhang, X.; Li, B. Micro- and Nanoplastics: Contamination Routes of Food Products and Critical Interpretation of Detection Strategies. Sci. Total Environ. 2023, 891, 164596. [Google Scholar] [CrossRef] [PubMed]
- Oliveri Conti, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as Active Samplers for Microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics Contamination in Eggs: Detection, Occurrence and Status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Vega-Herrera, A.; Garcia-Torné, M.; Borrell-Diaz, X.; Abad, E.; Llorca, M.; Villanueva, C.M.; Farré, M. Exposure to Micro(Nano)Plastics Polymers in Water Stored in Single-Use Plastic Bottles. Chemosphere 2023, 343, 140106. [Google Scholar] [CrossRef]
- Pham, D.T.; Kim, J.; Lee, S.H.; Kim, J.; Kim, D.; Hong, S.; Jung, J.; Kwon, J.H. Analysis of Microplastics in Various Foods and Assessment of Aggregate Human Exposure via Food Consumption in Korea. Environ. Pollut. 2023, 322, 121153. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Jutrzenka Trzebiatowska, P.; Mazurkiewicz, M.; Kowalczyk, P.; Knez, E.; Behrendt, M.; Mahlik, S.; Zaleska-Medynska, A.; Grembecka, M. Isolation and Identification of Microplastics in Infant Formulas—A Potential Health Risk for Children. Food Chem. 2024, 440, 138246. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, X.; Zhao, N.; Jiang, S.; Jin, H. Microplastics in Polystyrene-Made Food Containers from China: Abundance, Shape, Size, and Human Intake. Environ. Sci. Pollut. Res. 2023, 30, 40084–40093. [Google Scholar] [CrossRef]
- Vega-Herrera, A.; Llorca, M.; Borrell-Diaz, X.; Redondo-Hasselerharm, P.E.; Abad, E.; Villanueva, C.M.; Farré, M. Polymers of Micro(Nano) Plastic in Household Tap Water of the Barcelona Metropolitan Area. Water Res. 2022, 220, 118645. [Google Scholar] [CrossRef]
- Huerta Lwanga, E.; Mendoza Vega, J.; Ku Quej, V.; Chi, J.D.L.A.; Sanchez del Cid, L.; Chi, C.; Escalona Segura, G.; Gertsen, H.; Salánki, T.; van der Ploeg, M.; et al. Field Evidence for Transfer of Plastic Debris along a Terrestrial Food Chain. Sci. Rep. 2017, 7, 14071. [Google Scholar] [CrossRef]
- Cole, M.; Gomiero, A.; Jaén-Gil, A.; Haave, M.; Lusher, A. Microplastic and PTFE Contamination of Food from Cookware. Sci. Total Environ. 2024, 929, 172577. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J.; Yao, M.; Liu, Y.; Qian, J.; Ma, Q. Release of Microplastics during Dental Procedures and Denture Wear: Impact on Dental Personnel and Patients. J. Hazard. Mater. 2025, 494, 138463. [Google Scholar] [CrossRef] [PubMed]
- Panayi, N.; Papageorgiou, S.N.; Eliades, G.; Eliades, T. Microplastics and Orthodontic Aligners: The Concerns Arising from the Modernization of Practice Through Polymers and Plastics. J. World Fed. Orthod. 2024, 13, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Zaman, S.U.; Sami, N.I.; Roy, S.; Jeba, F.; Islam, M.S.; Salam, A. Human Inhalation Exposure Assessment of the Airborne Microplastics from Indoor Deposited Dusts during Winter in Dhaka, Bangladesh. Heliyon 2024, 10, e36449. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne Microplastics in Indoor and Outdoor Environments of a Coastal City in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Ageel, H.K.; Harrad, S.; Abdallah, M.A.E. Microplastics in Indoor Air from Birmingham, UK: Implications for Inhalation Exposure. Environ. Pollut. 2024, 362, 124960. [Google Scholar] [CrossRef]
- Jones, N.R.; de Jersey, A.M.; Lavers, J.L.; Rodemann, T.; Rivers-Auty, J. Identifying Laboratory Sources of Microplastic and Nanoplastic Contamination from the Air, Water, and Consumables. J. Hazard. Mater. 2024, 465, 133276. [Google Scholar] [CrossRef]
- Yuk, H.; Jo, H.H.; Nam, J.; Kim, Y.U.; Kim, S. Microplastic: A Particulate Matter(PM) Generated by Deterioration of Building Materials. J. Hazard. Mater. 2022, 437, 129290. [Google Scholar] [CrossRef]
- Lambert, S.; Vercauteren, M.; Catarino, A.I.; Li, Y.; Van Landuyt, J.; Boon, N.; Everaert, G.; De Rijcke, M.; Janssen, C.R.; Asselman, J. Aerosolization of Micro- and Nanoplastics via Sea Spray: Investigating the Role of Polymer Type, Size, and Concentration, and Potential Implications for Human Exposure. Environ. Pollut. 2024, 351, 124105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, F.; Fu, H.; Guo, H. Characterisation of Environmentally Persistent Free Radicals and Their Contributions to Oxidative Potential and Reactive Oxygen Species in Sea Spray and Size-Resolved Ambient Particles. NPJ Clim. Atmos. Sci. 2025, 8, 27. [Google Scholar] [CrossRef]
- Shahsavani, A.; Pasalari, H.; Kermani, M.; Tangestani, M.; Ahmadi, F. Health Outcomes Attributed to Inhalation of Microplastic Released from Mask During COVID-19 Pandemic: A Systematic Review. J. Hazard. Mater. Adv. 2025, 18, 100696. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Li, Z.; Song, K. COVID-19: Performance Study of Microplastic Inhalation Risk Posed by Wearing Masks. J. Hazard. Mater. 2021, 411, 124955. [Google Scholar] [CrossRef]
- Eom, S.; Shim, W.; Choi, I. Microplastic-Induced Inhibition of Cell Adhesion and Toxicity Evaluation Using Human Dermal Fibroblast-Derived Spheroids. J. Hazard. Mater. 2024, 465, 133359. [Google Scholar] [CrossRef]
- Song, G.B.; Nam, J.; Ji, S.; Woo, G.; Park, S.; Kim, B.; Hong, J.; Choi, M.G.; Kim, S.; Lee, C.; et al. Deciphering the Links: Fragmented Polystyrene as a Driver of Skin Inflammation. J. Hazard. Mater. 2024, 480, 135815. [Google Scholar] [CrossRef]
- Han, W.; Cui, J.; Sun, G.; Miao, X.; Pufang, Z.; Nannan, L. Nano-Sized Microplastics Exposure Induces Skin Cell Senescence via Triggering the Mitochondrial Localization of GSDMD. Environ. Pollut. 2024, 349, 123874. [Google Scholar] [CrossRef]
- Menichetti, A.; Mordini, D.; Montalti, M. Penetration of Microplastics and Nanoparticles Through Skin: Effects of Size, Shape, and Surface Chemistry. J. Xenobiot. 2025, 15, 6. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Norouzzadeh, R. Microplastic Neurotoxicity: Pathways, Mechanisms, and Implications for Neurodegenerative Diseases. Pers. Med. J. 2025, 10, 31–38. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity Assessment of Microplastic (MPs); a Threat to the Ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.Q.; Cao, M.T.; Sun, C.X.; Wang, J.J.; Gao, M.X.; He, X.R.; Dang, L.N.; Geng, Y.Y.; Li, B.Y.; Li, J.; et al. Size-Dependent Internalization of Polystyrene Microplastics as a Key Factor in Macrophages and Systemic Toxicity. J. Hazard. Mater. 2025, 490, 137701. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, Q.; Li, J.; Li, B.; Liang, W.; Su, L.; Shi, H. Distribution and Translocation of Micro- and Nanoplastics in Fish. Crit. Rev. Toxicol. 2021, 51, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public. Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, R.; Li, B.; Du, Y.; Li, J.; Tong, X.; Wu, Y.; Ji, X.; Zhang, Y. Tissue Distribution of Polystyrene Nanoplastics in Mice and Their Entry, Transport, and Cytotoxicity to GES-1 Cells. Environ. Pollut. 2021, 280, 116974. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Park, J.S.; Yoo, J.W.; Lee, Y.H.; Park, C.; Lee, Y.M. Size- and Shape-Dependent Ingestion and Acute Toxicity of Fragmented and Spherical Microplastics in the Absence and Presence of Prey on Two Marine Zooplankton. Mar. Pollut. Bull. 2024, 206, 116768. [Google Scholar] [CrossRef]
- Matavos-Aramyan, S. Addressing the Microplastic Crisis: A Multifaceted Approach to Removal and Regulation. Environ. Adv. 2024, 17, 100579. [Google Scholar] [CrossRef]
- Kim, L.; Kim, H.; Song, Y.; An, Y.J. Chronic Effects of Irregular and Fibril Microplastics on Artemia Franciscana in a Benthic Environment: Size and Shape-Dependent Toxicity. Mar. Pollut. Bull. 2024, 209, 117270. [Google Scholar] [CrossRef]
- An, D.; Na, J.; Song, J.; Jung, J. Size-Dependent Chronic Toxicity of Fragmented Polyethylene Microplastics to Daphnia Magna. Chemosphere 2021, 271, 129591. [Google Scholar] [CrossRef]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, Size, and Polymer Dependent Effects of Microplastics on Daphnia Magna. J. Hazard. Mater. 2022, 426, 128136. [Google Scholar] [CrossRef]
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878. [Google Scholar] [CrossRef] [PubMed]
- Samaei, S.H.A.; Mojahednia, P.; Chen, J.; Li, Z.; Jaszczyszyn, K.; Kiedrzyńska, E.; Xue, J. What Does the “Trojan Horse” Carry? The Pollutants Associated with Microplastics/Nanoplastics in Water Environments. ACS ES&T Water 2025, 5, 1530–1545. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A Review of the Endocrine Disrupting Effects of Micro and Nano Plastic and Their Associated Chemicals in Mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, I.; Anca, E.D.; Rubin, L.P.; Magen-Molho, H.; Miodovnik, A.; van der Hal, N. Adverse Health Effects of Exposure to Plastic, Microplastics and Their Additives: Environmental, Legal and Policy Implications for Israel. Isr. J. Health Policy Res. 2024, 13, 44. [Google Scholar] [CrossRef]
- Martínez, A. Toxicity of Persistent Organic Pollutants: A Theoretical Study. J. Mol. Model. 2024, 30, 97. [Google Scholar] [CrossRef]
- Mitra, P.; Gupta, S.; Sharma, P. Double Trouble: Unravelling the Health Hazards of Microplastics and Heavy Metals. Ind. J. Clin. Biochem. 2024, 39, 447–449. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, H.; Liu, Y.; Jin, L.; Peng, R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics 2023, 11, 490. [Google Scholar] [CrossRef]
- Cârdan, I.; Nesterovschi, I.; Barbu-Tudoran, L.; Pînzaru, S.C. Blue Micro-/Nanoplastics Abundance in the Environment: A Double Threat as a Trojan Horse for a Plastic-Cu-Phthalocyanine Pigment and an Opportunity for Nanoplastic Detection via Micro-Raman Spectroscopy. Environ. Sci. Nano 2025, 12, 2357–2370. [Google Scholar] [CrossRef]
- Järvelä, E.; Peräniemi, S.; Vepsäläinen, J.; Hrovat, B.; Raninen, K.; Tomppo, L.; Koistinen, A.; Rysä, J. A Study Protocol for Chemical Analysis and Toxicity Testing of Virgin and Recycled Microplastics and Associated Chemicals. Sci. Total Environ. 2025, 975, 179287. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D. Cellular Uptake, Transport, and Organelle Response After Exposure to Microplastics and Nanoplastics: Current Knowledge and Perspectives for Environmental and Health Risks. Rev. Environ. Contam. Toxicol. 2022, 260, 12. [Google Scholar] [CrossRef]
- Bridgeman, L.; Cimbalo, A.; López-Rodríguez, D.; Pamies, D.; Frangiamone, M. Exploring Toxicological Pathways of Microplastics and Nanoplastics: Insights from Animal and Cellular Models. J. Hazard. Mater. 2025, 490, 137795. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, E.; Ferrante, M.; Barbera, N.; Favara, C.; Aquilia, E.; Palella, M.; Cristaldi, A.; Conti, G.O.; Fiore, M. Effects of Nano and Microplastics on the Inflammatory Process: In Vitro and In Vivo Studies Systematic Review. Front. Biosci. Landmark 2022, 27, 287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xuan, Y.; Chen, Y.; Yang, F.; Zhu, M.; Xu, J.; Chen, J. Polystyrene Nanoplastics Induce Intestinal and Hepatic Inflammation through Activation of NF-ΚB/NLRP3 Pathways and Related Gut-Liver Axis in Mice. Sci. Total Environ. 2024, 935, 173458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, J.; Wang, Y.; Chen, M.; Bao, X.; Chen, X.; Xie, S.; Lin, Z.; Yu, Y. Investigating Polystyrene Nano-Plastic Effects on Largemouth Bass (Micropterus salmoides) Focusing on MRNA Expression: Endoplasmic Reticulum Stress and Lipid Metabolism Dynamics. Fishes 2024, 9, 432. [Google Scholar] [CrossRef]
- Fanghella, F.; Pesce, M.; Franceschelli, S.; Panella, V.; Elsallabi, O.; Lupi, T.; Rizza, B.; Di Battista, M.G.; Bruno, A.; Ballerini, P.; et al. Biological Modulation of Autophagy by Nanoplastics: A Current Overview. Int. J. Mol. Sci. 2025, 26, 7035. [Google Scholar] [CrossRef]
- Liang, X.; Zeng, Y.; Zhang, P.; Zhu, B.; Feng, J.; Deng, T.; Fu, Z.; Liu, C.; Chen, C.; Zhang, Y. Polystyrene Nanoplastics Trigger Pyroptosis in Dopaminergic Neurons through TSC2/TFEB-Mediated Disruption of Autophagosome-Lysosome Fusion in Parkinson’s Disease. J. Transl. Med. 2025, 23, 631. [Google Scholar] [CrossRef]
- Ornelas-Cruces, M.; Escalona-Montaño, A.R.; Salaiza-Suazo, N.; Sifontes-Rodríguez, S.; Aguirre-García, M.M. The Potential Role of Sanguinarine as an Inhibitor of Leishmania PP2C in the Induction of Apoptosis. Acta Parasitol. 2025, 70, 35. [Google Scholar] [CrossRef]
- Płuciennik, K.; Sicińska, P.; Misztal, W.; Bukowska, B. Important Factors Affecting Induction of Cell Death, Oxidative Stress and DNA Damage by Nano- and Microplastic Particles In Vitro. Cells 2024, 13, 768. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene Nanoplastics Induced Cardiomyocyte Apoptosis and Myocardial Inflammation in Carp by Promoting ROS Production. Fish Shellfish Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef]
- Luo, H.; Du, Q.; Zhong, Z.; Xu, Y.; Peng, J. Protein-Coated Microplastics Corona Complex: An Underestimated Risk of Microplastics. Sci. Total Environ. 2022, 851, 157948. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Ma, Y.; Duan, F.; Wang, C.; Feng, J.; Yin, H.; Sun, L.; Li, P.; Li, Z.H. Fate of Polystyrene Micro- and Nanoplastics in Zebrafish Liver Cells: Influence of Protein Corona on Transport, Oxidative Stress, and Glycolipid Metabolism. J. Hazard. Mater. 2025, 489, 137596. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Z.; Wei, W.; Chen, J.; Ni, B.J. Toxicity of Micro/Nanoplastics in the Environment: Roles of Plastisphere and Eco-Corona. Soil. Environ. Health 2023, 1, 100002. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Y.; Wang, S. Protein Corona as a Mediator in Antibiotic Adsorption onto Microplastics: Mechanisms and Implications. Int. J. Biol. Macromol. 2025, 311, 143982. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef]
- Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. [Google Scholar] [CrossRef]
- Ma, J.; Ladd, D.M.; Kaval, N.; Wang, H.S. Toxicity of Long Term Exposure to Low Dose Polystyrene Microplastics and Nanoplastics in Human IPSC-Derived Cardiomyocytes. Food Chem. Toxicol. 2025, 202, 115489. [Google Scholar] [CrossRef]
- Mannering, A.M.; Burritt, D.J.; Komyakova, V.; Ferrari, M.C.O.; Vamvounis, G.; Gulizia, A.M.; Staples, A.; McCormick, M.I.; Allan, B.J.M. Microplastic Consumption Elevates Fish Oxidative Stress but Does Not Affect Predator-Driven Mortality. Sci. Total Environ. 2025, 983, 179644. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Jin, Y.; Yao, Y.; Li, S.; Liao, Z.; Zhang, X.; Yan, X. Simultaneous Exposure to Microplastics and Heavy Metal Lead Induces Oxidative Stress, Histopathological Damage, and Immune Dysfunction in Marine Mussel Mytilus Coruscus. Ecotoxicol. Environ. Saf. 2025, 289, 117493. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Nanoplastics-Induced Oxidative Stress, Antioxidant Defense, and Physiological Response in Exposed Wistar Albino Rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 11332–11344. [Google Scholar] [CrossRef]
- Feng, M.; Luo, J.; Wan, Y.; Zhang, J.; Lu, C.; Wang, M.; Dai, L.; Cao, X.; Yang, X.; Wang, Y. Polystyrene Nanoplastic Exposure Induces Developmental Toxicity by Activating the Oxidative Stress Response and Base Excision Repair Pathway in Zebrafish (Danio rerio). ACS Omega 2022, 7, 32153–32163. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Q.; Zhang, J.; Zhang, X.; Yang, N.; Feng, L. Toxic Effects of Microplastics and Nanoplastics on Plants: A Global Meta-Analysis. Environ. Pollut. 2023, 337, 122593. [Google Scholar] [CrossRef]
- Jeong, J.; Thi Quynh Mai, N.; Moon, B.S.; Choi, J.K. Impact of Polystyrene Microplastics (PS-MPs) on the Entire Female Mouse Reproductive Cycle: Assessing Reproductive Toxicity of Microplastics through in Vitro Follicle Culture. Ecotoxicol. Environ. Saf. 2025, 297, 118228. [Google Scholar] [CrossRef]
- Ghosh, T. Microplastics Bioaccumulation in Fish: Its Potential Toxic Effects on Hematology, Immune Response, Neurotoxicity, Oxidative Stress, Growth, and Reproductive Dysfunction. Toxicol. Rep. 2025, 14, 101854. [Google Scholar] [CrossRef] [PubMed]
- Irnidayanti, Y.; Soegianto, A.; Ramdhany, F.A.; Afifudin, A.F.M.; Payus, C.M.; Hartl, M.G.J. Microplastic Contamination in Green Mussels (Perna viridis Linnaeus, 1858) from Traditional Seafood Markets in Jakarta, Indonesia, and an Evaluation of Potential Hazards. Mar. Pollut. Bull. 2025, 214, 117818. [Google Scholar] [CrossRef] [PubMed]
- Suljević, D.; Karlsson, P.; Fočak, M.; Brulić, M.M.; Sulejmanović, J.; Šehović, E.; Särndahl, E.; Engwall, M.; Alijagic, A. Microplastics and Nanoplastics Co-Exposure Modulates Chromium Bioaccumulation and Physiological Responses in Rats. Environ. Int. 2025, 198, 109421. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, H.; Busch, M.; Yang, S.; Venus, T.; Aalderink, G.; Crespo, J.F.F.; Villacorta, A.; Hernández, A.; Estrela-Lopis, I.; Boeren, S.; et al. Toxicity of True-to-Life Microplastics to Human IPSC-Derived Intestinal Epithelia Correlates to Their Protein Corona Composition. J. Hazard. Mater. 2025, 495, 138908. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, C.; Chen, Z.; Guo, Y.; Jing, Y.; Chen, X.; Liang, H. Polystyrene Microplastics Exacerbate Acetochlor-Induced Reproductive Toxicity and Transgenerational Effects in Zebrafish. Aquat. Toxicol. 2025, 279, 107208. [Google Scholar] [CrossRef]
- Liang, J.; Niu, T.; Zhang, L.; Yang, Y.; Li, Z.; Liang, Z.; Yu, K.; Gong, S. Polystyrene Microplastics Exhibit Toxic Effects on the Widespread Coral Symbiotic Cladocopium Goreaui. Environ. Res. 2025, 268, 120750. [Google Scholar] [CrossRef]
- Zangene, S.; Morovvati, H.; Anbara, H.; Hye Khan, M.A.; Goorani, S. Polystyrene Microplastics Cause Reproductive Toxicity in Male Mice. Food Chem. Toxicol. 2024, 194, 115083. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, H.; Bao, M.; Tang, S.; Gu, X.; Han, M.; Li, P.; Jiang, Q. Polystyrene Microplastics Induce Molecular Toxicity in Simocephalus Vetulus: A Transcriptome and Intestinal Microorganism Analysis. Aquat. Toxicol. 2024, 275, 107046. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Wang, J.J.; Gowen, A.A. A Review of Potential Human Health Impacts of Micro- and Nanoplastics Exposure. Sci. Total Environ. 2022, 851, 158111. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Trzebiatowska, P.J.; Knez, E.; Zaleska-Medynska, A.; Grembecka, M. Microplastics in Food—A Critical Approach to Definition, Sample Preparation, and Characterisation. Food Chem. 2023, 418, 135985. [Google Scholar] [CrossRef]
- Carías Domínguez, A.M.; de Jesús Rosa Salazar, D.; Stefanolo, J.P.; Cruz Serrano, M.C.; Casas, I.C.; Zuluaga Peña, J.R. Intestinal Dysbiosis: Exploring Definition, Associated Symptoms, and Perspectives for a Comprehensive Understanding—A Scoping Review. Probiot. Antimicrob. Proteins 2025, 17, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Hobbs, A.L.V.; Lodise, T.P. Gut Microbiome Health and Dysbiosis: A Clinical Primer. Pharmacotherapy 2022, 42, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qu, Y.; Qu, L.; Shen, D.; Liu, H.; Nie, Z. Oral Exposure to PLA Microplastics Induces Time-Dependent Nanotoxicity via the Gut-Liver Axis. J. Hazard. Mater. 2025, 495, 138931. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Kaundle, B.; Singh, I. Mucoadhesive Drug Delivery Systems in Respiratory Diseases. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 475–491. ISBN 9780128206584. [Google Scholar]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne Particulate Matter and Human Health: Toxicological Assessment and Importance of Size and Composition of Particles for Oxidative Damage and Carcinogenic Mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Alzaben, M.; Burve, R.; Loeschner, K.; Møller, P.; Roursgaard, M. Nanoplastics from Ground Polyethylene Terephthalate Food Containers: Genotoxicity in Human Lung Epithelial A549 Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 892, 503705. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, Z.; Zhao, L.; Lu, L.; Lu, X.; Li, Y.; Gu, T.; Huang, X.; Huang, G.; Liang, Y.; et al. Exposure to Polyethylene Terephthalate Micro(Nano)Plastics Exacerbates Inflammation and Fibrosis after Myocardial Infarction by Reprogramming the Gut and Lung Microbiota and Metabolome. J. Hazard. Mater. 2025, 488, 137410. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue Accumulation of Microplastics and Potential Health Risks in Human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Du, D.; Tang, B.; Yi, W.; He, M.; Liu, R.; Yu, H.; Yu, Y.; Zheng, J. Characteristics and Influencing Factors of Microplastics Entering Human Blood through Intravenous Injection. Environ. Int. 2025, 198, 109377. [Google Scholar] [CrossRef] [PubMed]
- Çağlayan, U.; Gündoğdu, S.; Ramos, T.M.; Syberg, K. Intravenous Hypertonic Fluids as a Source of Human Microplastic Exposure. Environ. Toxicol. Pharmacol. 2024, 107, 104411. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; ISBN 9789251098820. [Google Scholar]
- Lomonaco, T.; Persiani, E.; Biagini, D.; Gisone, I.; Ceccherini, E.; Cecchettini, A.; Corti, A.; Ghimenti, S.; Di Francesco, F.; Castelvetro, V.; et al. Type-Specific Inflammatory Responses of Vascular Cells Activated by Interaction with Virgin and Aged Microplastics. Ecotoxicol. Environ. Saf. 2024, 282, 116695. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, B.; Woo, W.; Kim, L.K.; Hyun, Y.M. Polystyrene Microplastics Induce Activation and Cell Death of Neutrophils through Strong Adherence and Engulfment. J. Hazard. Mater. 2024, 480, 136100. [Google Scholar] [CrossRef]
- Koner, S.; Florance, I.; Mukherjee, A.; Chandrasekaran, N. Cellular Response of THP-1 Macrophages to Polystyrene Microplastics Exposure. Toxicology 2023, 483, 153385. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hua, T.; Sang, Q.X.A. Effects of Polystyrene Microplastics on Human Kidney and Liver Cell Morphology, Cellular Proliferation, and Metabolism. ACS Omega 2022, 7, 34136–34153. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Huang, C.C.Y.; Huang, C.C.; Chia, Z.C.; Lee, C.P.; Lin, Y.F.; Chiu, H.W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells Hk-2 and Male C57bl/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef]
- Aditya, M.R.; Muhammad, A.R.; Adriansyah, V.; Samsu, N.; Sulistomo, H.W. Microplastic Exposure and Its Consequences for Renal and Urinary Health: Systematic Review of In Vivo Studies. All. Life 2025, 18, 2457760. [Google Scholar] [CrossRef]
- de Oliveira, R.B.; Pelepenko, L.E.; Masaro, D.A.; Lustosa, G.M.M.M.; de Oliveira, M.C.; Roza, N.A.V.; Marciano, M.A.; dos Reis, L.M.; Kamel, S.; Louvet, L.; et al. Effects of Microplastics on the Kidneys: A Narrative Review. Kidney Int. 2024, 106, 400–407. [Google Scholar] [CrossRef]
- Marcelino, R.C.; Cardoso, R.M.; Domingues, E.L.B.C.; Gonçalves, R.V.; Lima, G.D.A.; Novaes, R.D. The Emerging Risk of Microplastics and Nanoplastics on the Microstructure and Function of Reproductive Organs in Mammals: A Systematic Review of Preclinical Evidence. Life Sci. 2022, 295, 120404. [Google Scholar] [CrossRef]
- Dusza, H.M.; Katrukha, E.A.; Nijmeijer, S.M.; Akhmanova, A.; Vethaak, A.D.; Walker, D.I.; Legler, J. Uptake, Transport, and Toxicity of Pristine and Weathered Micro-and Nanoplastics in Human Placenta Cells. Environ. Health Perspect. 2022, 130, 097006. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, Y.; Liu, S.; Yuan, H.; You, T.; Xu, H. Microplastics-Perturbed Gut Microbiota Triggered the Testicular Disorder in Male Mice: Via Fecal Microbiota Transplantation. Environ. Pollut. 2022, 309, 119789. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal Exposure to Polystyrene Nanoplastics during Gestation and Lactation Induces Hepatic and Testicular Toxicity in Male Mouse Offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, R.; Wang, R.; Ma, Z.; Jiang, S.; Zhang, F.; Wu, W. Gestational Exposure to Polystyrene Microplastics Incurred Placental Damage in Mice: Insights into Metabolic and Gene Expression Disorders. Ecotoxicol. Environ. Saf. 2025, 294, 118056. [Google Scholar] [CrossRef]
- Saraluck, A.; Techarang, T.; Bunyapipat, P.; Boonchuwong, K.; Pullaput, Y.; Mordmuang, A. Detection of Microplastics in Human Breast Milk and Its Association with Changes in Human Milk Bacterial Microbiota. J. Clin. Med. 2024, 13, 4029. [Google Scholar] [CrossRef]
- Moiniafshari, K.; Zanut, A.; Tapparo, A.; Pastore, P.; Bogialli, S.; Abdolahpur Monikh, F. A Perspective on the Potential Impact of Microplastics and Nanoplastics on the Human Central Nervous System. Environ. Sci. Nano 2025, 12, 1809–1820. [Google Scholar] [CrossRef]
- Ban, M.; Shimoda, R.; Chen, J. Investigation of Nanoplastic Cytotoxicity Using SH-SY5Y Human Neuroblastoma Cells and Polystyrene Nanoparticles. Toxicol. Vitr. 2021, 76, 105225. [Google Scholar] [CrossRef]
- Kwon, W.; Kim, D.; Kim, H.Y.; Jeong, S.W.; Lee, S.G.; Kim, H.C.; Lee, Y.J.; Kwon, M.K.; Hwang, J.S.; Han, J.E.; et al. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis In Vitro and In Vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Wang, Z.; Liu, K.; Huang, S.; Liang, J.; Dai, Z.; Guo, W.; Mao, C.; Chen, S.; et al. Polyethylene Microplastics Promote Nucleus Pulposus Cell Senescence by Inducing Oxidative Stress via TLR4/NOX2 Axis. Ecotoxicol. Environ. Saf. 2025, 292, 117950. [Google Scholar] [CrossRef]
- So, Y.H.; Shin, H.S.; Lee, S.H.; Moon, H.J.; Jang, H.J.; Lee, E.H.; Jung, E.M. Maternal Exposure to Polystyrene Microplastics Impairs Social Behavior in Mouse Offspring with a Potential Neurotoxicity. Neurotoxicology 2023, 99, 206–216. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Chen, Q.; Su, S.; Zhuang, J.; Qiao, D. Polystyrene Micro- and Nanoparticles Exposure Induced Anxiety-like Behaviors, Gut Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2023, 259, 115000. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Lu, Y.; Guo, L. Behavioral Toxicity and Neurotoxic Mechanisms of PLA-PBAT Biodegradable Microplastics in Zebrafish. Sci. Total Environ. 2024, 928, 172354. [Google Scholar] [CrossRef]
- Luo, Q.; Tan, H.; Ye, M.; Jho, E.H.; Wang, P.; Iqbal, B.; Zhao, X.; Shi, H.; Lu, H.; Li, G. Microplastics as an Emerging Threat to Human Health: An Overview of Potential Health Impacts. J. Environ. Manag. 2025, 387, 125915. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance), 435(1). Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj/eng (accessed on 21 August 2025).
- Microplastics Drinking Water|California State Water Resources Control Board. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/microplastics.html (accessed on 21 August 2025).
- Liu, J.; Yang, Y.; An, L.; Liu, Q.; Ding, J. The Value of China’s Legislation on Plastic Pollution Prevention in 2020. Bull. Environ. Contam. Toxicol. 2022, 108, 601–608. [Google Scholar] [CrossRef]
- New South Wales. Plastic Reduction and Circular Economy Act 2021 No 31. 2021. Available online: https://legislation.nsw.gov.au/view/html/inforce/current/act-2021-031 (accessed on 21 August 2025).
- Directive (EU) 2015/720 of the European Parliament and of the Council of 29 April 2015 Amending Directive 94/62/EC as Regards Reducing the Consumption of Lightweight Plastic Carrier Bags (Text with EEA Relevance), 115(11). Available online: https://eur-lex.europa.eu/eli/dir/2015/720/oj/eng (accessed on 21 August 2025).
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment (Text with EEA Relevance), 155(1). Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj/eng (accessed on 21 August 2025).
- Secretary Haaland Issues Order to Phase Out Single-Use Plastics, Protect Public Lands and Waters|U.S. Department of the Interior. Available online: https://www.doi.gov/pressreleases/secretary-haaland-issues-order-phase-out-single-use-plastics-protect-public-lands-0 (accessed on 21 August 2025).[Green Version]
- The Environmental Protection (Microbeads) (England) Regulations 2017. Available online: https://www.legislation.gov.uk/uksi/2017/1312/contents/made (accessed on 22 August 2025).[Green Version]
- Usman, S.; Abdull Razis, A.F.; Shaari, K.; Azmai, M.N.A.; Saad, M.Z.; Mat Isa, N.; Nazarudin, M.F. The Burden of Microplastics Pollution and Contending Policies and Regulations. Int. J. Environ. Res. Public Health 2022, 19, 6773. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/2055 of 25 September 2023 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Synthetic Polymer Microparticles (Text with EEA Relevance), 238(67). Available online: https://eur-lex.europa.eu/eli/reg/2023/2055/oj/eng (accessed on 21 August 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).