Abstract
The development of hot dry rock needs working fluids, and mineral reactions happen due to contact between hot dry rock minerals and fluids. Mineral reactions influence hot dry rock reservoir characteristics, whereas their impacts on hot dry rock mechanical properties lack understanding. In this study, a simulation study on the influence of reactions between granitic hot dry rock minerals and water on rock non-closed crack compressive shear initiation was conducted. High temperature (180 °C) and high pressure (24 MPa) mineral reaction experiments were performed to obtain reaction kinetics parameters. A model of non-closed crack compressive shear initiation induced by mineral reactions was established. Based on the model, the influences of mineral reactions on the compressive shear initiation of non-closed cracks were analyzed. Results show that the mineral reactions primarily contain feldspar dissolution and quartz precipitation, and their overall effect is crack enlargement. The crack enlargement reduces crack initiation potential for a crack inclination angle of α = 0°, while it increases crack initiation potential for α = 45° and 90°. The difference in crack initiation potential under various α values is attributed to the relative position of the crack to the maximum principal stress direction. This work reveals the influences of mineral reactions on hot dry rock reservoir crack initiation, contributing to achieving sustainable hot dry rock exploitation.
1. Introduction
Water–rock interactions are a common phenomenon due to contacts between fluids and rocks, with one of the major manifestations being mineral reactions [1]. Mineral reactions lead to changes in the types and abundance of minerals in rocks, influencing rock porosity, and subsequently, reservoir permeability and productivity [2,3]. Hot dry rock (HDR) is one typical type of geothermal reservoir, which has a stable energy output, high energy utilization coefficient, and large reserves [4]. The exploitation of HDR has been under investigation since 1974. An HDR reservoir is composed of a volume of rock that contains an interconnected crack network between the injection and production wells. Various fluids can be injected into the crack network to extract heat from the HDR reservoir. The contacts between the HDR and fluids subsequently induce mineral reactions [5,6]. The successful exploitation of an HDR geothermal reservoir relies on the understanding of water–rock interactions between the HDR, which is typically granitic, and the fluids in contact with the HDR. This provides a method to obtain the physical characteristics of the reservoirs, contributing to a long-term and stable exploitation of HDR [7]. Therefore, investigating the influences of mineral reactions on HDR is of crucial importance.
The alterations in rock mechanical characteristics due to mineral reactions have been extensively studied. Mineral reactions change the contents and structures of rocks, thereby leading to significant variations in rock stress state and mechanical properties [8]. The rock surfaces can be altered by mineral reactions, such as mineral dissolution and precipitation [9,10,11]. This morphological alteration further affects the fracture mechanical properties of fractured rocks. The dissolution of minerals at a crack tip leads to crack growth, which could increase stress intensity factors (SIFs) at the crack tip and then induce crack initiation. This is not conducive to the stability of rocks [12]. However, compared to HDR reservoir exploitation, most current studies on the changes of rock fracture mechanical properties induced by mineral reactions have focused on addressing the relatively shallow geological engineering problems, such as rock slop stability and underground oil-gas storage reservoirs with lower temperatures and pressures [13,14]. HDR reservoirs have a high temperature and high pressure, which enhances mineral reactions between rocks and fluids [15,16,17]. In addition, the exploitation of HDR is a long-term project that lasts for decades [1], further increasing the enhancing effect. This enhancement can intensify the influences of mineral reactions on the fracture mechanical properties of rocks, posing a bigger challenge on the sustainable exploitation of HDR reservoirs.
The objective of this paper is to study the influence of reactions between granitic hot dry rock (HDR) minerals and water on rock non-closed crack compressive shear initiation through simulations. First, determination of the HDR mineral characteristics, as well as high-temperature and high-pressure (HTHP) mineral reaction experiments between the HDR and deionized water were carried out to obtain the mineral reaction kinetics parameters. Then, based on rock mechanics, the compression-shear initiation criterion for rock cracks under water pressure [18,19,20], and the model of surface thickness change due to mineral reactions [21], a model of rock non-closed crack compressive shear initiation induced by mineral reactions was established. Finally, the influences of mineral reactions on rock non-closed crack compressive shear initiation were accessed through simulations.
2. Materials and Methods
2.1. Materials
The granitic cuttings were recovered from a geothermal well at a depth of 2350 m in Gonghe Basin, Qinghai Province, where a typical HDR project in China is located [22]. Cuttings with a size of 5 mesh were picked out, followed by cleaning with deionized water and drying under 105 °C for 24 h [23]. Furthermore, deionized water with a pH value of 7 and a density of 1 kg/m3 was utilized as the reactive solution to react with the cuttings, as water is a commonly utilized working fluid for HDR exploitation.
2.2. Tests and Experiments
2.2.1. Determination of the HDR Mineral Characteristics
Thin sections of the HDR were observed using a BX51 polarizing microscope from Olympus Co. Ltd., Tokyo, Japan, to identify the rock minerals. Further, X-ray diffraction (XRD) test was performed on the cuttings to determine the mineral contents using the D/Max-RC type diffractometer from Rigaku Co. Ltd., Tokyo, Japan. In terms of XRD, four samples were utilized for parallel mineral content determination. The cuttings were ground into powders with a size smaller than 300 mesh to guarantee the test accuracy.
2.2.2. HTHP Mineral Reaction Experiments
HTHP mineral reaction experiments were conducted using an HTHP flow reactor, and the details of the reactor are provided in reference [23]. Cuttings with a mass of 33.3439 g were utilized for HTHP mineral reactions. The reaction temperature was set as 180 °C [15,22]; meanwhile, the pressure Δp was determined as follows:
where pw is the static fluid column pressure (MPa), pf is the formation pressure (MPa), which is the pressure of formation water in a reservoir, ρw is the density of deionized water (kg/m3), g is the gravitational acceleration (10 N/kg), and h is the static column depth of drilling fluid (m), which is equivalent to the sampling depth (2350 m) of the HDR. As a limited amount of fluid exists in HDR reservoirs [22], pf is assumed as 0, and Δp is calculated to be 24 MPa. The volume flow rate Q (mL·min−1) was determined as follows:
where Vsol is the volume of the solution, and Δt is the elapsed reaction time, which is 7d, ρsol is the density of the solution, which is 1000 kg·m−3, and F is the solution mass flow rate. The solution volume is 500 mL (Water/Rock 15:1), which is limited by the solution container and guarantees a sufficient amount of water for water–rock interactions [24]. Therefore, Q is 0.05 mL·min−1, and F is 0.072 kg·d−1. The cuttings were placed in the HTHP vessel of the HTHP flow reactor to react with deionized water. During reactions, water samples were obtained at days 1, 3, 5, and 7 to determine their solution element concentrations using the ICAP 7200 Duo inductively coupled plasma optical emission spectrometer (ICP-OES) from Thermo Fisher Scientific Co. Ltd., Waltham, MA, USA, and their pH values using the PHS-3C pH meter from Shanghai INESA Scientific Instrument Co. Ltd., Shanghai, China. Considering the mineral element contents, sodium (Na), potassium (K), silica (Si), and alumina (Al) content in the solutions were obtained; meanwhile, their detection limits were 0.20 mg/L, 0.35 mg/L, 0.20 mg/L, and 0.05 mg/L, respectively. Determination of the solution element content concentrations and pH was performed under room temperature and atmospheric pressure.
2.3. Model of Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
2.3.1. Determination of Mineral Reactions
Determination of mineral reactions was conducted through reaction equilibrium simulations using Phreeqc Interactive 3.6.2-15100, which is a well-developed hydrogeochemical simulation program. Reaction temperature and deionized water were defined in the SOLUTION module. The minerals were defined in the EQUILIBRIUM_PHASES module. The output results contain the changed moles of minerals, indicating the mineral reactions before reaching reaction equilibrium. The simulation program is provided in the Supplementary Materials.
2.3.2. Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
Figure 1 presents a schematic diagram of a non-closed crack with an inclination angle of α under a two-dimensional plane stress state of σ and kσ (where k is the lateral pressure coefficient) and water pressure pw.
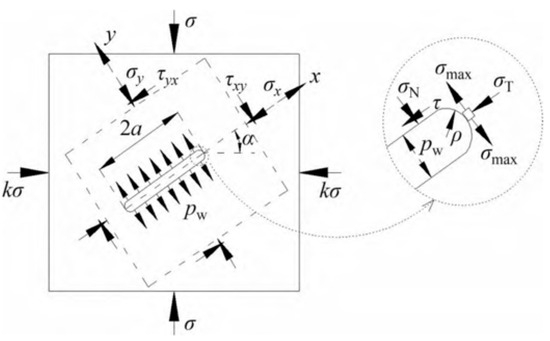
Figure 1.
Non-closed crack with internal water pressure under initial ground stress [19].
The stress on the crack plane of the crack model is given as [19]:
where σN is the normal stress on the crack plane, σT is the transverse compressive stress parallel to the crack plane, and τ is the shear stress parallel to the crack plane. Further, the SIFs at the tip of a non-closed crack are depicted as follows:
where ρ is the curvature radius at the crack tip, which is equal to a half of the crack aperture (b) herein, and a is half of the crack length. Figure 2 shows the alterations in crack size parameters of an equivalent crack induced by mineral reactions.
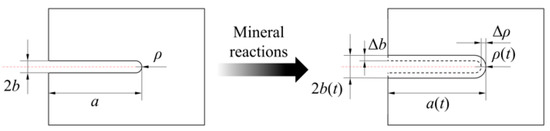
Figure 2.
Schematic diagram of the alterations in crack size of an equivalent crack induced by mineral reactions.
The rate that the surface changes in thickness due to mineral reactions of mineral i (dXi/dt) can be determined as follows [21]:
where Vi is the molar volume of mineral i, ki is the reaction rate constant of mineral i, and Si is the saturation index of mineral i. As the elapsed time for mineral reactions is short-term (only 7d), which is far from equilibrium, Equation (5) can be simplified as follows:
In a flow reactor, the release rate of J species rJ by reactions of mineral i, which are primarily dissolutions of mineral i, is given as follows [25]:
As the solution is deionized water at the inlet, that is, mJ,in = 0, Equation (7) is simplified as follows:
where mJ,out and mJ,in are the molar concentrations of species J at the outlet and inlet, and A is the total surface area of the cuttings, which is given as follows:
where SBET and mr are the BET specific surface area and mass of the cuttings. Assuming the release of J species rJ primarily results from the dissolution of mineral i, we have the following:
where r+,i is the dissolution rate of mineral i. For dissolution at far-from-equilibrium conditions, there is an additional equivalence [23]:
Combining Equations (8), (10) and (11) gives us the following:
As dissolution enlarges cracks and precipitation narrows cracks, we combine Equations (6), (9) and (12), and give the following:
where p and q are the type numbers of mineral i that result in the mineral dissolving and precipitating, respectively. The boundary condition of Equation (13) is t = 0, X = X(t) = 0. Assuming the mineral reactions cause an even thickness change in a crack, we have the following:
Further:
Combining Equations (16) and (17) with Equation (4) derives the influences of mineral reactions on SIFs at the tip of a non-closed crack:
The rock shear crack toughness under compression-shear conditions(ⅡC) is as follows [20]:
where λ12 is the compression-shear coefficient. And subsequently, Equation (20):
Equation (20) shows that the initiation of non-closed cracks under water pressure is attributed to the evolution of SIFs that results from crack size parameter alterations due to mineral reactions. A schematic diagram of the research process is illustrated in Figure 3.
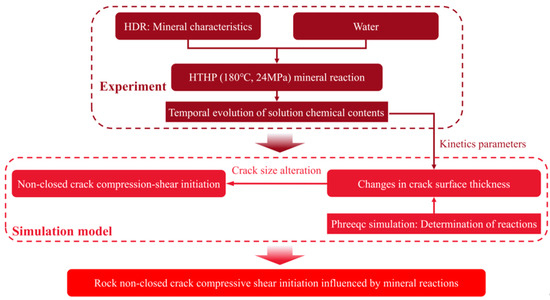
Figure 3.
Schematic diagram of the research process.
3. Results
3.1. Mineral Characteristics of HDR
Figure 4 shows the mineral characteristics of HDR. Through thin section observations, feldspar (Fsp), quartz (Qtz), and biotite (Bt) were identified, and the mineral grains are in close contact with each other, indicating a tight rock matrix of the HDR. XRD results show that the average mineral contents in the HDR include 23.75% Qtz, 43% albite (Ab), 28.5% microcline (Mic), and 4.75% Bt. Ab and Mic belong to Fsp. Based on the mineral contents, the rock is classified as biotite monzonitic granite [23].
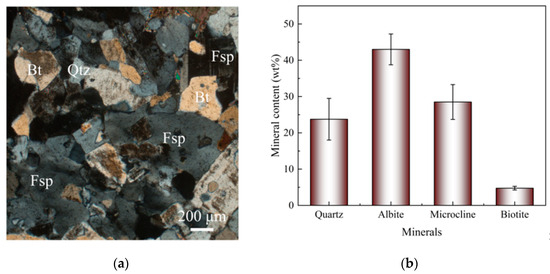
Figure 4.
Mineral characteristics of HDR: (a) A picture of the thin section of the HDR; (b) Mineral contents of the HDR.
3.2. Temporal Evolution of the Solution Element Contents and pH
Figure 5 shows the temporal evolution of the solution element contents and pH under a temperature of 180 °C and a pressure of 24 MPa. As seen in Figure 5a,b,e, Na, K, and pH initially experience an increase and then a decrease at around day 3. The temporal evolution is similar to the results reported by reference [24]. From Figure 5c, Si generally increases within the experiment duration. The equilibrium concentration of Si due to quartz dissolution at 180 °C is about 3.31 mmol/L [21], which indicates that the equilibrium solubility of quartz at 180 °C is about 3.31 mmol/L, and the Si concentration can increase up to a maximum of 3.31 mmol/L. Therefore, Si keeps a gradual increase as the Si concentration is less than the maximum Si concentration. Compared to the quartz dissolution, the Si is primarily released by feldspar dissolution, which contributes to the precipitation of quartz [23]. From Figure 5d, Al experiences a sudden rise at around day 1 and subsequently declines at around day 3. The rise of Na, K, Si, Al, and pH is primarily attributed to element leaching and dissolution of Fsp. The time for the decline in Na, K, and pH corresponds to Al, which could result from the generation of secondary Fsp precipitations that consume Na, K, Al, and OH− [26]. For dilute solutions, molar concentrations (mol/L) are approximately equal to molal concentrations (mol/kg). Moreover, Figure 5 reveals that the mineral reactions are far from equilibrium, as the calculated reaction quotients of quartz and feldspar are significantly smaller than their reaction equilibrium constants [21,27].
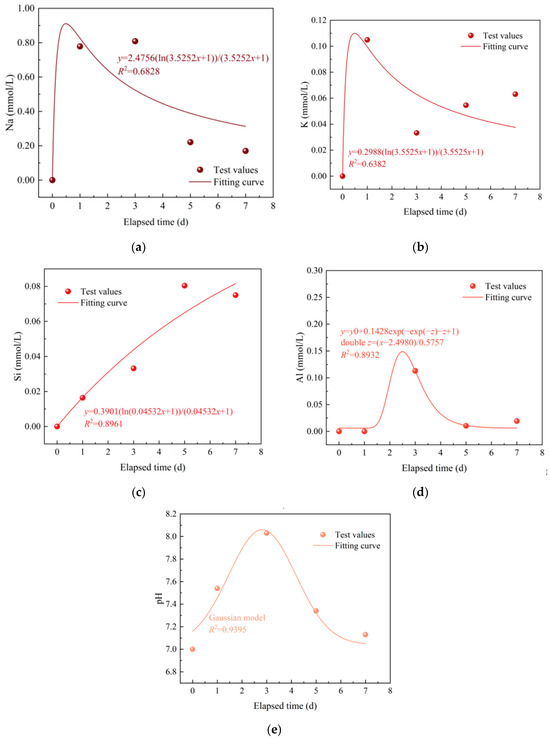
Figure 5.
Temporal evolution of the solution element contents under a temperature of 180 °C and a pressure of 24 MPa: (a) Na; (b) K; (c) Si; (d) Al; (e) pH.
3.3. Simulation Results of Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
3.3.1. Simulation Results of Reaction Equilibrium
Based on the database of Phreeqc, Mic is defined as K-feldspar (Kfs), and Bt is defined as K-mica during simulations. The simulation conditions are consistent with the experiment conditions, including a temperature of 180 °C, as well as water with a pH of 7 and a mass of 0.5 kg. Simulation parameters of the rock minerals are given in Table 1.

Table 1.
Simulation parameters of rock minerals.
Figure 6 demonstrates the simulation results of mineral reaction equilibrium. Upon reaching equilibrium, the changes in mineral moles of Ab and Kfs are negative, indicating the dissolution of Ab and Kfs; meanwhile, those of K-mica and Qtz are positive, indicating the precipitation of K-mica and Qtz. The dissolution of Ab and Kfs, as well as the precipitation of K-mica and Qtz, are depicted as follows [27,28,29]:
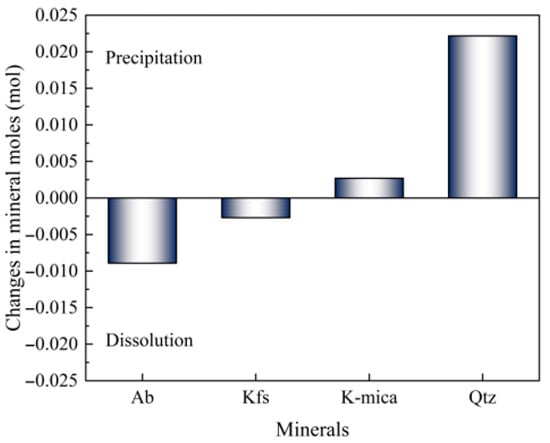
Figure 6.
Simulation results of the changes in mineral moles in the mineral assemblage.
Equations (21) and (22) demonstrate that the dissolution rates of Ab and Mic can be represented by the release rate of Na+ and K+, respectively. As the formation of Bt requires a much more reactive environment [30] compared to the experiment conditions, the dissolution of Fsp and the precipitation of Qtz are considered as the primary mineral reactions for crack size parameter alterations herein.
3.3.2. Simulation Results of the Non-Closed Crack Compressive Shear Initiation
Figure 7 shows the non-closed crack model for simulation calculations. A signal non-closed crack with a length of 1 m, an aperture of 0.55 mm, and an inclination angle α was considered. Besides, α is set to be 0°, 45°, and 90°. The direction of the maximum principal stress is vertical. Therefore, the crack is perpendicular to σ for α = 0° and is parallel to σ for α = 90°. The water pressure within the crack is designed as the experiment pressure, which is 24 MPa. Detailed simulation parameters are provided in Table 2.
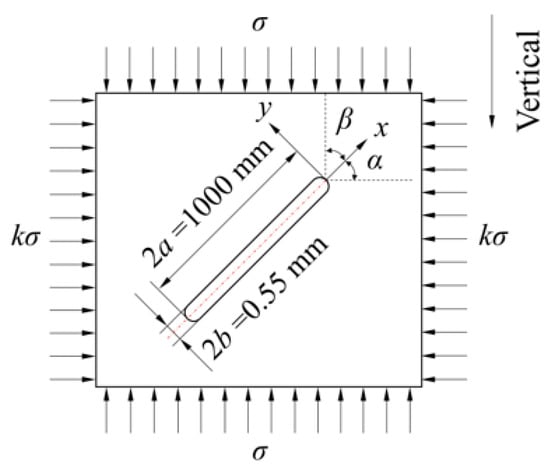
Figure 7.
Schematic diagram of the non-closed crack model for simulation calculation.

Table 2.
Simulation parameters of the non-closed crack compressive shear initiation model.
Figure 8 shows the temporal evolution of thickness changes on crack surfaces due to various mineral reactions. The dissolution of Fsp enlarges cracks; meanwhile, Qtz precipitation narrows cracks. The enlargement due to Fsp dissolution is significantly larger than Qtz precipitation, and hence, the overall result is the enlargement of the crack (e.g., [12]). The influence of mineral reactions on the temporal evolution of thickness changes on a crack surface, in descending order, is Ab dissolution, Mic dissolution, and Qtz precipitation.
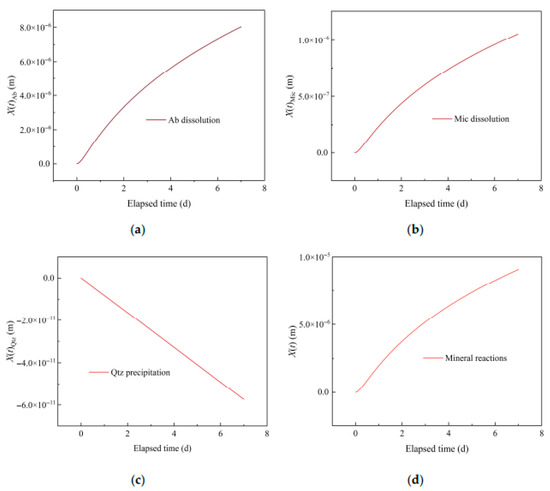
Figure 8.
Temporal evolution of thickness changes on crack surface due to various mineral reactions: (a) Ab dissolution; (b) Mic dissolution; (c) Qtz precipitation; (d) Combined mineral reactions.
Figure 9 shows the temporal evolution of SIFs of a crack with various inclination angles due to mineral reactions. Generally, the changed values of SIFs are small, as the mineral reactions are short-term. When α = 0°, type I SIF decreases over time. When α = 45°, type I SIF increases, and type Ⅱ SIF decreases with time. When α = 90°, type I SIF increases over time. Further, the crack initiation potential is represented by λ12KⅠ+|KⅡ|, as illustrated in Figure 10. As α increases from 0° to 90°, the initial value of λ12KⅠ+|KⅡ| experiences a decline and then increases (e.g., [33,34]). For α = 0°, the crack enlargement induced by mineral reactions reduces λ12KⅠ+|KⅡ|, inhibiting crack initiation potential. In contrast, for α = 45° and 90°, the crack enlargement increases λ12KⅠ+|KⅡ|, intensifying crack initiation potential.
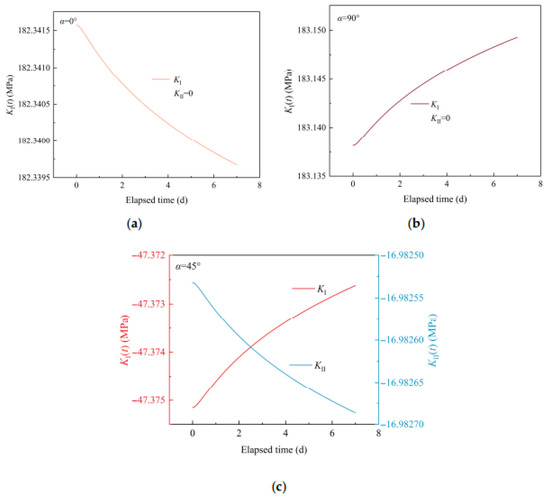
Figure 9.
Temporal evolution of SIFs of a crack with various inclination angles due to mineral reactions: (a) 0°; (b) 45°; (c) 90°.
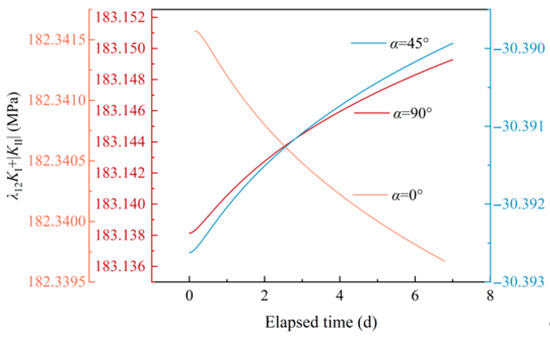
Figure 10.
Simulation results of the influence of mineral reactions on the compressive shear initiation of a non-closed crack under various inclination angles.
4. Discussions
Mineral reactions pose challenges to the development of HDR, such as scaling due to the temperature dependent dissolution-precipitation of silica [35]. In this paper, alterations in crack size parameters due to mineral reactions were determined. Subsequently, simulations of non-closed crack compressive shear initiation under water pressure were carried out. From the simulation results, the variations in non-closed crack size parameters exert different influences on the crack compressive shear initiation between a crack that is perpendicular to the maximum principal stress σ and a crack that is parallel to σ. For a crack that is perpendicular to σ, that is, α = 0°, the stress concentration at the crack tip is reduced, inhibiting the crack initiation, and the influence of crack length on fractured rock strength is weakened [36]. Therefore, the decrease in non-closed crack initiation potential is attributed to the blunting of the crack tip, which results from the increased crack tip curvature radius due to mineral reactions. When α = 45° and 90°, the influence of crack length on the fractured rock strength is significant, and the fractured rock maximum strength reduces as crack length increases [33,36], which indicates an enhanced crack initiation potential. When t = 0, the λ12KⅠ+|KⅡ| for a crack with α = 45° is less than that for a crack with α = 90°, demonstrating that the crack with α = 45° is relatively hard to initiate compared to the crack with α = 90° at the very beginning. However, the variations in crack initiation potential within the α range from 0° to 90° cannot be revealed in this paper and need further investigation.
5. Conclusions
The main conclusions are as follows:
- The primary mineral reactions between granitic HDR minerals and water contain Ab and Mic (Fsp) dissolution, which enlarges cracks, as well as Qtz precipitation, which narrows cracks. The crack enlargement due to Fsp dissolution is significantly larger than the crack narrowing due to Qtz precipitation.
- The mineral reactions between granitic HDR minerals and water influence the rock non-closed crack compressive shear initiation by enlarging the length and aperture of a crack.
- For cracks with an inclination angle α of 0°, the crack tip blunting due to mineral reactions reduces the crack initiation potential, as the enlarged crack length is oriented vertically to the maximum principal stress direction, which inhibits stress concentration at the crack tip. For cracks with α of 45° and 90°, the crack length increase promotes the crack initiation potential.
- In this paper, feldspar dissolution and quartz precipitation were considered, whereas the formation of clay minerals and zeolite due to short-term HTHP mineral reactions is also possible [22]. As the mechanical properties of clay minerals and zeolite are distinct from the minerals in the granitic HDR, studying the variations in mineral compositions after HTHP mineral reactions is of crucial importance to further comprehend the influence of mineral reactions on rock non-closed crack compressive shear initiation.
- This paper elucidates the influences of mineral reactions on rock non-closed crack compressive shear initiation with consideration for HDR exploitation, which provides insights into HDR reservoir damages induced by working fluids. In future works, compressive shear cracking experiments on rock specimens with non-closed cracks before and after HTHP mineral reactions will be conducted to further investigate the influencing mechanisms of mineral reactions on non-closed crack compressive shear initiation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15179695/s1, Phreeqc input file.
Author Contributions
Conceptualization, O.J. and X.Z.; methodology, O.J. and X.Z.; validation, W.Z. and H.L.; formal analysis, O.J.; investigation, O.J.; Resources, H.W.; writing—original draft preparation, O.J.; writing—review and editing, X.Z. and W.Z.; visualization, O.J.; supervision, X.Z. and H.W.; project administration, X.Z.; funding acquisition, X.Z. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 42172342), the Science and Technology Innovation Special Project of Xiong’an New Area (Task No. 2022XAGG0500), and the Natural Science Foundation of Henan Province—Youth Science Foundation Project (Grant No. 252300420834).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Acknowledgments
We thank Gong Qingjie and his team members from the School of Earth Sciences and Resources, China University of Geosciences (Beijing), for providing us with the HTHP flow reactor for the HTHP mineral reaction experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HDR | Hot dry rock |
| HTHP | High temperature and high pressure |
| XRD | X-ray diffraction |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| SIF | Stress intensity factor |
| Fsp | Feldspar |
| Qtz | Quartz |
| Bt | Biotite |
| Ab | Albite |
| Mic | Microcline |
| Kfs | K-feldspar |
References
- Feng, B.; Song, D.; Fu, L.; Zhang, S.Q.; Chen, M.T.; Jiang, Z.J. Formation laws of hydrothermal alteration minerals and the genesis of travertine in the Zhacang geothermal field, Guide Basin. Nat. Gas Ind. 2019, 39, 133–142. [Google Scholar]
- Chen, J.Y.; Xu, T.F.; Jiang, Z.J.; Feng, B.; Xu, L. Reducing formation damage by artificially controlling the fluid-rock chemical interaction in a double-well geothermal heat production system. Renew. Energy 2020, 149, 455–467. [Google Scholar] [CrossRef]
- Yasuhara, H.; Kinoshita, N.; Ohfuji, H.; Lee, D.S.; Nakashima, S.; Kishida, K. Temporal alteration of fracture permeability in granite under hydrothermal conditions and its interpretation by coupled chemo-mechanical model. Appl. Geochem. 2011, 26, 2074–2088. [Google Scholar] [CrossRef]
- Hou, Z.M.; Wu, X.N.; Luo, J.S.; Zhang, L.H.; Li, Z.Y.; Cao, C.; Wu, L.; Chen, Q.J. Major challenges of deep geothermal systems and an innovative development mode of REGS integrated with energy storage. Coal Geol. Explor. 2024, 52, 1–13. [Google Scholar]
- Richards, H.G.; Savage, D.; Andrews, J.N. Granite-water reactions in an experimental Hot Dry Rock geothermal reservoir, Rosemanowes test site, Cornwall, U.K. Appl. Geochem. 1992, 7, 193–222. [Google Scholar] [CrossRef]
- He, M.; Gong, W.Z.; Xu, M.B.; Song, J.J. Research status and prospect analysis of hot dry rock development technology. Renew. Energy Resour. 2021, 39, 1447–1454. [Google Scholar]
- Edmunds, W.M.; Abdrews, J.N.; Darbyshire, D.P.F.; Hussain, N.; Savage, D.; Shepherd, T.J. Granite-water interactions in relation to Hot Dry Rock geothermal development. In Proceedings of the Fourth International Seminar on the Results of European Community Geothermal Energy Research and Demonstration, Florence, Italy, 27–30 April 1989. [Google Scholar]
- Tang, L.S.; Wang, S.J. Discussion on the mechanism and quantification method of rock hydrochemical damage. Chin. J. Rock Mech. Eng. 2002, 21, 314–319. [Google Scholar]
- Sanchez-Roa, C.; Saldi, G.D.; Mitchell, T.M.; Iacoviello, F.; Bailey, J.; Shearing, P.R.; Oelkers, E.H.; Meredith, P.G.; Jones, A.P.; Striolo, A. The role of fluid chemistry on permeability evolution in granite: Applications to natural and anthropogenic systems. Earth Planet. Sci. Lett. 2021, 553, 116641. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, W.Q. Finite element analysis of influence of pressure solution of fracture aperture on T-H-M coupling in dual-porosity medium. Rock Soil Mech. 2010, 31, 1269–1275. [Google Scholar]
- Yang, J.B.; Feng, X.T.; Pan, P.Z.; Shen, L.F. Aperture evolution of single fracture in granite under triaxial compressive stress and chemical solution seepage. Chin. J. Rock Mech. Eng. 2012, 31, 1869–1878. [Google Scholar]
- Yang, H.; Cao, P.; Jiang, X.L. Micromechanical model for equivalent crack propagation under chemical corrosion of water-rock interaction. Rock Soil Mech. 2010, 31, 2104–2110. [Google Scholar]
- Wang, Z.C.; Wei, R.L.; Bi, J.C.; Wu, Z.H. Characteristics of water-rock reaction and effect of Reynolds number on dissolution rate for fractures. J. Northeast. Univ. (Sci. Technol.) 2020, 41, 1174–1179. [Google Scholar]
- Miao, S.J.; Cai, M.F.; Ji, D.; Guo, Q.F.; Bai, Y.B. Aging features and mechanism of Granite’s damage under the action of acidic chemical solutions. J. China Coal Soc. 2016, 41, 1137–1144. [Google Scholar]
- Li, J.F. Simulation research and performance analysis of hot dry rock power generation technology. Shandong Chem. Ind. 2021, 50, 114–119. [Google Scholar]
- Zhao, Y.H.; Feng, B.; Zhang, G.B.; Shangguan, S.T.; Qi, X.F.; Li, X.; Qiao, Y.C.; Xu, J.N. Study of the interaction between the granitic hot-dry rock (HDR) and different injection water. Acta Geol. Sin. 2020, 94, 2115–2123. [Google Scholar]
- Chen, D.G.; Zhi, X.C.; Yang, H.T. Geochemistry, 2nd ed.; University of Science and Technology of China Press: Hefei, China, 2009; pp. 124–126. [Google Scholar]
- Hou, G.Y. Advanced Course in Rock Mechanics; Science Press: Beijing, China, 2018; pp. 374–375. [Google Scholar]
- Liu, H.Y.; Zhou, Y.Z.; Zhang, G.X.; Xue, L.; Zheng, X.H. Compression-shear initiation criterion for rockmass crack under water pressure. J. Cent. South Univ. (Sci. Technol.) 2023, 54, 920–929. [Google Scholar]
- Zhou, Q.L. Compressive shear fracture criterion of rock and its application. J. Geotech. Eng. 1987, 9, 33–37. [Google Scholar]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Et Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar]
- Lin, W.J.; Wang, G.L.; Shao, J.L.; Gan, H.N.; Tan, X.F. Distribution and exploration of hot dry rock resources in China: Progress and inspiration. Acta Geol. Sin. 2021, 95, 1366–1381. [Google Scholar]
- Jiang, O.; Zheng, X.H.; Gong, Q.J.; Wu, H.D.; Chu, B.Z. Research on mass transfer mechanisms due to short-term water-rock interactions between granite cuttings and alkaline NaCl solution and their patterns. Geoenergy Sci. Eng. 2025, 245, 213512. [Google Scholar] [CrossRef]
- Savage, D.; Bateman, K.; Milodowski, A.E.; Hughes, C.R. An experimental evaluation of the reaction of granite with streamwater, seawater and NaCl solutions at 200 °C. J. Volcanol. Geotherm. Res. 1993, 57, 167–191. [Google Scholar] [CrossRef]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Et Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, R.C.; Lu, X.C.; Gao, J.F.; Xu, S.J. Experimental study on the low-temperature dissolution of microperthite in alkaline solution. Acta Mineral. Sin. 2003, 23, 333–340. [Google Scholar]
- Yang, F.J.; Wang, G.L.; Hu, D.W.; Zhou, H.; Tan, X.F. Influence of water-rock interaction on permeability and heat conductivity of granite under high temperature and pressure conditions. Geothermics 2022, 100, 102347. [Google Scholar] [CrossRef]
- Hellmann, R.; Tisserand, D. Dissolution kinetics as a function of the Gibbs free energy of reaction: An experimental study based on albite feldspar. Geochim. Et Cosmochim. Acta 2006, 70, 364–383. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of the feldspars: Part II dissolution at conditions close to equilibrium. Hydrometallurgy 2015, 151, 163–171. [Google Scholar] [CrossRef]
- Zhang, S.Y.; He, W.Y.; Gao, X.; Tian, C.H.; Xiao, Y.W. Alteration zonation and metallogenic mechanism of porphyry copper deposits: A case study of thermodynamic equilibrium simulation of fluid-rock interactions in Yulong deposit. Acta Petrol. Sin. 2024, 40, 1837–1852. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhang, Y.J.; Lei, Z.H.; Zhang, Q.; Zhang, S.Q.; Fu, L. Inversion analysis of 3D geostress field in GR2 well area of Gonghe hot rock geothermal well. Chin. Q. Mech. 2019, 40, 115–123. [Google Scholar] [CrossRef]
- Lei, Z.H.; Zhang, Y.J.; Zhang, S.Q.; Fu, L.; Hu, Z.J.; Yu, Z.W.; Li, L.Z.; Zhou, J. Electricity generation from a three-horizontal-well enhanced geothermal system in the Qiabuqia geothermal field, China: Slickwater fracturing treatments for different reservoir scenarios. Renew. Energy 2020, 145, 65–83. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, X.D.; Wang, Y.B.; Song, C.P. Study on fracture characteristics of fractured rock mass under unidirectional compression. Coal Geol. China 2025, 37, 65–71. [Google Scholar]
- Li, T.Y.; Zhao, Z.Y.; Wu, L. Study on the damage mode and law of single-fissure rock specimens under hydraulic pressure. J. Univ. South China (Sci. Technol.) 2024, 38, 50–57. [Google Scholar]
- Longval, R.; Meirbekova, R.; Fisher, J.; Maignot, A. An overview of silica scaling reduction technologies in the geothermal market. Energies 2024, 17, 4825. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, K.X.; Yao, X.L.; Liang, P.; Liu, X.X. Simulation experiment study on the effect of fracture geometry on rock strength. China Min. Mag. 2019, 28, 141–148. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).