Abstract
The early detection of neurological and psychiatric disorders is critical for optimizing patient outcomes and improving the efficacy of healthcare delivery. This study presents a novel multiclass machine learning (ML) framework designed to classify epilepsy, migraine, and schizophrenia simultaneously using electroencephalography (EEG) signals. Unlike conventional approaches that predominantly rely on binary classification (e.g., healthy vs. diseased cohorts), this work addresses a significant gap in the literature by introducing a unified artificial neural network (ANN) architecture capable of discriminating among three distinct neurological and psychiatric conditions. The proposed methodology involves decomposing raw EEG signals into constituent frequency subbands to facilitate robust feature extraction. These discriminative features were subsequently classified using a multilayer ANN, achieving performance metrics of 95% sensitivity, 96% specificity, and a 95% F1-score. To enhance clinical applicability, the model was optimized for potential integration into real-time diagnostic systems, thereby supporting the development of a rapid, reliable, and scalable decision support tool. The results underscore the viability of EEG-based multiclass models as a promising diagnostic aid for neurological and psychiatric disorders. By consolidating the detection of multiple conditions within a single computational framework, this approach offers a scalable and efficient alternative to traditional binary classification paradigms.
1. Introduction
Neuropsychiatric and neurological conditions, particularly schizophrenia, epilepsy, and migraine, constitute a substantial global disease burden, with profound implications for individual wellbeing and healthcare infrastructure worldwide [,]. Current epidemiological studies reveal striking prevalence figures, with schizophrenia affecting about 1% of the global population, migraine disorders impacting nearly 1 billion individuals, and epilepsy exhibiting a lifetime prevalence of 0.5–1% [,]. These statistics highlight the critical necessity for developing precise and accessible diagnostic methodologies.
Modern neurodiagnostic techniques, including structural MRI, fMRI, and PET, are valuable in clinical settings but face challenges such as expense, limited availability in low-resource areas, and suboptimal temporal resolution []. In comparison, electroencephalography (EEG) provides distinct benefits, being non-invasive, cost-effective, mobile, and capable of capturing neural activity with millisecond precision—qualities that enhance its utility for studying cortical activity in neuropsychiatric disorders [,].
The widespread adoption of EEG in research and clinical environments stems from its accessibility and exceptional temporal resolution. These attributes also make EEG a promising input for AI-driven analyses, particularly in diagnosing and categorizing neurological and psychiatric conditions [,]. EEG recordings, obtained via scalp electrodes, measure oscillatory neural activity across key frequency bands—delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–32 Hz), and gamma (32–45 Hz)—each linked to distinct cognitive and physiological processes [].
Progress in computational neuroscience has expanded EEG’s diagnostic potential. Machine learning and deep learning methods are increasingly employed to interpret EEG data, yielding high precision in detecting psychiatric illnesses like major depressive disorder and schizophrenia []. Additionally, hybrid models combining EEG with demographic or clinical information using convolutional neural networks have demonstrated improved diagnostic reliability [].
However, existing research predominantly focuses on binary classification—differentiating healthy subjects from patients—rather than multiclass discrimination. To bridge this gap, our study introduces an integrated EEG-based framework for the concurrent identification of epilepsy, migraine, and schizophrenia. The subsequent section reviews prior computational EEG analyses relevant to these disorders.
The literature on EEG-based epilepsy detection reveals significant methodological diversity. Li et al. (2013) established a robust framework combining empirical mode decomposition (EMD) with support vector machines (SVMs), achieving exceptional performance metrics (98.00% sensitivity, 99.40% specificity) through careful analysis of intrinsic mode functions (IMFs) and their statistical properties []. Subsequent research by Satapathy et al. (2017) demonstrated the effectiveness of transformer architectures in this domain, reporting 99.5% classification accuracy when processing wavelet-transformed EEG data []. Time–frequency analysis approaches have shown particular promise in handling EEG complexity. Zahra et al. (2017) implemented a sophisticated pipeline involving multivariate EMD followed by Hilbert transform analysis, successfully discriminating between five distinct EEG classes with 87.20% accuracy using artificial neural networks []. Wavelet-based methods have also proven effective, as evidenced by Sharmila et al. (2018), who achieved 100% accuracy in classifying seizure and non-seizure EEG signals (Set A vs. Set E) using discrete wavelet transform (DWT) combined with entropy features and an SVM classifier []. Recent advances in deep learning have expanded the methodological toolkit for EEG analysis. Acharya et al. (2018) developed a specialized 13-layer convolutional neural network architecture capable of distinguishing between normal, preseizure, and seizure states with 88.67% overall accuracy []. Hybrid approaches combining multiple signal processing techniques have yielded particularly strong results, with Lu et al. (2018) reporting classification accuracies ranging from 81.96% to 98.75% using an innovative integration of Hilbert–Huang transform (HHT) and Kraskov entropy []. Comparative studies have provided valuable insights into algorithm selection. Chen et al. (2019) conducted a systematic evaluation of six ML classifiers, identifying least squares SVM (LS-SVM) as the optimal choice with 99.5% accuracy for three-state classification []. These collective findings demonstrate substantial progress in automated EEG analysis while highlighting opportunities for further methodological refinement.
Recent advancements in EEG signal processing have demonstrated the efficacy of continuous wavelet transform (CWT)-based depthwise convolutional neural network (CNN) architectures in effectively capturing spatiotemporal patterns for epilepsy detection, with studies reporting high classification accuracy []. Further developments in hybrid modeling approaches, combining wavelet decomposition with one-dimensional CNNs and multihead attention mechanisms, have yielded exceptional performance, achieving classification accuracies exceeding 99.8% in seizure detection tasks []. Moreover, practical implementations of real-time seizure detection systems incorporating short-time Fourier transform (STFT) analysis with transfer learning frameworks, including pretrained architectures such as GoogleNet, have validated their clinical applicability [].
Current research on automated migraine diagnosis through EEG analysis remains limited compared to other neurological disorders, despite migraine’s high global prevalence—estimated at approximately 15% of the global population in 2021, corresponding to about 1.16 billion individuals worldwide [,]. Recent epidemiological data from the Global Burden of Disease Study indicate that the prevalence of migraine has steadily increased from roughly 733 million cases in 1990 to over 1.1 billion in 2021 []. In addition to prevalence estimates, recent EEG-based investigations have identified characteristic neural patterns associated with migraine. For example, a 2024 study reported elevated occipital alpha power (~12 Hz) during migraine attacks compared to interictal periods, suggesting that such spectral characteristics may serve as potential biomarkers for automated migraine detection systems []. This notable research gap highlights the need for more comprehensive studies in this field. Several key studies have contributed to the development of automated migraine diagnosis systems: Yin et al. (2015) developed a decision support system for clinical migraine diagnosis, focusing on differentiating migraine from tension-type headache []. Their approach used expert-selected clinical features with K-nearest neighbors (KNN) classification, achieving 90% accuracy []. In EEG-based studies, Akben et al. (2016) applied photic stimulation at 2 Hz, 4 Hz, and 6 Hz frequencies, analyzing power spectrum characteristics with ANN classification to reach 85% accuracy []. Further advancing this approach, Subasi et al. (2019) employed DWT for feature extraction from EEG signals during photic stimulation, using random forest (RF) classification to achieve 85.95% accuracy []. For migraine subtype differentiation, Frid et al. (2020) implemented a radial basis function SVM classifier that distinguished migraine with aura from that without aura with 84.62% accuracy []. More recently, Aslan (2021) proposed an innovative method combining tunable Q-factor wavelet transform (TQWT) with ensemble learning, attaining 89.6% classification accuracy []. Although migraine detection via EEG has been relatively underexplored, recent CNN-based models using time–frequency representations have achieved F1-scores exceeding 90%, particularly in distinguishing migraine from tension-type headaches [].
A comprehensive review of EEG-based schizophrenia detection reveals significant advancements in ML and deep learning approaches. Several studies have demonstrated the potential of various feature extraction and classification techniques for accurate diagnosis. Santos-Mayo et al. (2016) conducted a comparative analysis of classification algorithms, finding that multilayer perceptron (MLP) (93.42%) and SVM (92.23%) achieved the highest diagnostic accuracy []. Johannesen et al. (2016) focused on working memory prediction using EEG-derived features and SVM classification, reporting 87% accuracy []. Thilakvathi et al. (2017) employed entropy and fractal-based features, including spectral entropy, Shannon entropy, and Higuchi fractal dimension, in an SVM model that reached 88.5% accuracy []. Further developments in feature extraction were demonstrated by Aslan and Akın (2019), who applied relative wavelet energy and KNN classification, achieving 90% accuracy []. Deep learning approaches have also shown promise: Oh et al. (2019) developed an 11-layer CNN with classification accuracies ranging from 81% to 98% [], while Shalbaf et al. (2020) converted EEG signals into visual representations, using pretrained CNNs with SVM to achieve 98% accuracy with ResNet-18 []. Recent innovations include Sun et al.’s (2021) hybrid framework, which transformed EEG signals into RGB images and found fuzzy entropy features superior to FFT-based methods []. Supakar et al. (2022) further advanced deep learning applications by implementing a long short-term memory (LSTM)-enhanced recurrent neural network (RNN) model, attaining 93–98% accuracy across 84 subjects [].
Recent studies have demonstrated significant advancements in EEG-based disorder classification through innovative deep learning approaches. Aich and Ahamed (2025) developed a novel hybrid framework employing CWT for EEG signal encoding coupled with EfficientNet-based classification, reporting exceptional accuracy exceeding 99.7% []. Parallel developments include the introduction of a cascaded atrous convolutional network architecture incorporating adaptive weight fusion, which has established new benchmarks for subject-level classification robustness in schizophrenia detection []. Furthermore, the integration of explainable artificial intelligence (XAI) principles with generative data augmentation techniques has yielded remarkable performance in schizophrenia identification, with classification metrics approaching theoretical maximum values [].
Building upon the established literature, this study makes a significant methodological leap by developing a multiclass ML framework capable of concurrently identifying three clinically distinct conditions: epilepsy (a neurological disorder characterized by recurrent seizures), migraine (a complex neurological condition with diverse manifestations), and schizophrenia (a chronic psychiatric disorder). This approach represents a substantial departure from conventional binary classification systems that merely differentiate between healthy and pathological states—a limitation that significantly constrains their clinical utility given the frequent comorbidity and symptom overlap observed in practice.
This study introduces a groundbreaking approach by developing the first unified ML model capable of simultaneously classifying three clinically distinct disorders—epilepsy, migraine, and schizophrenia—through EEG signal analysis. Our comprehensive literature review confirms that no previous research has attempted to integrate these particular conditions within a single multiclass classification framework. This represents a significant advancement beyond existing studies that typically focus on binary classification of individual disorders.
Furthermore, the developed model is not solely aimed at achieving academic classification accuracy but is also designed to be optimized for integration into real-time diagnostic systems. In doing so, the model’s applicability in the healthcare domain is enhanced, with the goal of serving as a fast and reliable diagnostic tool that can be incorporated into clinical decision support systems. The inclusion of real-time analytical functionality increases the model’s effectiveness in practical applications and could lead to more timely and efficient patient management.
2. Materials and Methods
2.1. Dataset
In this study, EEG recordings were obtained using the OpenBCI UltraCortex Mark IV (OpenBCI, Brooklyn, NY, USA) headset equipped with the Cyton+Daisy module, with data collected at a sampling frequency of 125 Hz. The recordings were conducted in compliance with the ethical guidelines approved by the Ethics Committee of Dicle University Faculty of Medicine. As visually demonstrated in Figure 1—an interface screenshot obtained from the OpenBCI GUI v5.2.1 software environment—the electrodes were positioned in accordance with the international 10–20 system, specifically at locations Fp1, Fp2, C3, C4, T5, T6, O1, O2, F7, F8, F3, F4, T3, T4, P3, and P4. Each participant underwent two 4 min EEG recording sessions (totaling 8 min, i.e., 480 s) in a seated, eyes-closed, and relaxed condition. Participants were instructed to remain motionless and silent to minimize movement-related artifacts. The study cohort included 54 individuals: 24 with epilepsy (14 male, 10 female; mean age 36.64 ± 5.2 for males, 38.2 ± 4.8 for females), 11 with migraine (4 male, 7 female; mean age 36.25 ± 5.1 for males, 32.25 ± 4.6 for females), and 19 with schizophrenia (17 male, 2 female; mean age 36.41 ± 4.9 for males, 34.0 ± 3.7 for females).
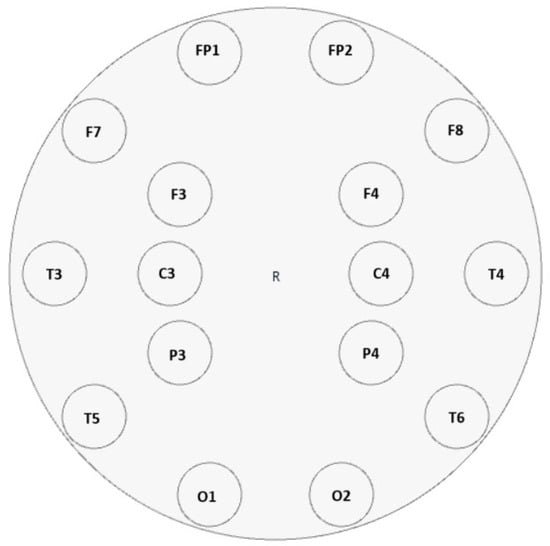
Figure 1.
Electrode placement of the 16-channel OpenBCI UltraCortex Mark IV EEG headset based on the international 10–20 system. Electrodes were positioned at Fp1, Fp2, C3, C4, T5, T6, O1, O2, F7, F8, F3, F4, T3, T4, P3, and P4 to ensure adequate coverage of frontal, temporal, parietal, and occipital regions.
Prior to segmentation, all EEG recordings were visually inspected to identify segments affected by electrode displacement, excessive muscle activity, or other sources of noise. Problematic intervals were then programmatically excluded using Python tools. Following this quality control, the remaining clean recordings were divided into 120 s segments. The resulting number of usable data instances before and after segmentation is presented in Table 1:

Table 1.
Number of Usable EEG Recordings Before and After Segmentation.
In the migraine group, the number of usable segments increased after segmentation due to the successful retention of partially clean portions from recordings initially affected by localized noise. Conversely, no increase was observed in the schizophrenia group, as recordings tended to exhibit more diffuse and persistent artifacts, limiting the recovery of additional clean data. This rigorous preprocessing and segmentation strategy ensured that only high-quality, artifact-free EEG segments were included in the dataset, thereby strengthening the reliability and clinical relevance of the subsequent multiclass classification model.
The electrode montage was designed to ensure extensive cortical sampling while specifically incorporating frontal lobe channels (Fp1, Fp2, F3, F4, F7, F8), based on their documented clinical utility for evaluating neurological and psychiatric disorders, including epilepsy, migraine, and schizophrenia [,,]. This targeted approach was guided by several neurophysiological rationales: first, these prefrontal and frontal sites are critically involved in mediating cognitive control processes, decision-making functions, and emotional regulation mechanisms—core domains that frequently demonstrate impairment across the studied conditions [,]. Second, accumulating electrophysiological evidence has consistently identified these anterior regions as exhibiting distinctive pathological signatures in the examined disorders []. The systematic integration of these clinically relevant electrodes was therefore implemented to maximize the detection of disease-specific neural patterns and enhance the diagnostic accuracy of our computational approach.
This rigorous preprocessing and segmentation strategy ensured that only high-quality, artifact-free EEG segments were included in the dataset, thereby strengthening the reliability and clinical relevance of the subsequent multiclass classification model.
2.2. Data Preprocessing
This research adopted a structured EEG preprocessing methodology comprising four principal phases to generate high-fidelity data appropriate for computational modeling. In the initial stage, extraneous high-frequency noise and biological artifacts—such as electromyographic signals, ocular motion, and electrocardiographic interference—were suppressed through the application of a 20th-order Butterworth low-pass filter (45 Hz cutoff frequency), ensuring preservation of clinically meaningful neural oscillatory patterns. Although the power line interference is nominally centered at 50 Hz, its spectral leakage into adjacent frequency bands due to hardware-induced aliasing and shielding imperfections justifies the selection of a slightly conservative cutoff at 45 Hz, which is a common practice in EEG preprocessing pipelines to retain gamma-band activity while minimizing contamination [,]. This filtering strategy selectively preserves diagnostically relevant low-frequency neural oscillations while attenuating high-frequency noise components, thereby enhancing signal quality for subsequent analysis. The Butterworth filter was specifically chosen for its maximally flat passband characteristics, ensuring minimal distortion of physiological signals within the target frequency range. The subsequent processing stage involved partitioning continuous EEG recordings into standardized 120 s segments to enhance chronological uniformity and support temporal characteristic analysis. Following segmentation, the signals were spectrally decomposed into five canonical frequency bands—delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–45 Hz)—using 20th-order Butterworth bandpass filters to enable frequency-domain analysis of neural activity. Although spectral decomposition was performed across all standard EEG frequency bands, only features derived from the delta and theta bands were retained for analysis due to their stronger clinical relevance to neuropsychiatric disorders and to reduce feature dimensionality in light of the limited dataset size. This segment duration was selected based on its optimal balance between capturing both low- and high-frequency neural oscillations while maintaining diagnostically important temporal relationships, providing superior stability for low-frequency component analysis compared to briefer intervals while avoiding excessive data fragmentation. The final stage encompassed the computation of key statistical descriptors—including harmonics and geometric means along with skewness coefficients—from each spectral subband, achieving effective dimensionality reduction while conserving essential amplitude distribution characteristics. The complete preprocessing framework was implemented using the Python programming language, specifically version 3.12, and capitalized on the optimized numerical computation capabilities of NumPy and signal processing functions within SciPy for efficient execution of filtering, segmentation, and feature extraction operations. Independent component analysis (ICA) was not utilized in this study for artifact removal, as high-frequency noise and biological artifacts were suppressed through the application of a 20th-order Butterworth low-pass filter. The MNE library was not used. This rigorously designed and reproducible processing sequence effectively transformed raw electrophysiological recordings into a curated, machine-learning-compatible dataset while preserving critical neurophysiological information.
2.2.1. Segmentation
EEG signals were divided into standardized 120 s segments to meet ML input requirements while preserving diagnostic information. This segmentation strategy simultaneously enabled data augmentation through temporal variations and ensured dimensional consistency across samples. The fixed window length was optimized to capture clinically meaningful patterns while maintaining computational efficiency, with all segments undergoing quality control to exclude artifacts. The analyzed segments were extracted from continuous, artifact-free portions of the recordings to capture spontaneous brain activity.
2.2.2. Extraction of Statistical Features
The direct classification of high-dimensional EEG time-series data significantly increases computational complexity and impedes effective model training. To address these challenges, we extracted statistical features that provide compact yet meaningful representations of the fundamental signal characteristics. These features facilitate more efficient model learning by reducing dimensionality while preserving discriminative patterns. Specifically, we employed three key statistical measures: harmonic mean (capturing reciprocal relationships in signal amplitudes), geometric mean (representing multiplicative central tendency), and skewness (quantifying distribution asymmetry). Rather than estimating power spectral density (PSD) or relative PSD, we filtered EEG signals into canonical frequency bands using 20th-order Butterworth bandpass filters and directly computed these statistical features from the time-domain amplitude values within each band. This enabled the characterization of neural activity across frequency ranges without requiring spectral power computation. The mathematical formulations for these measures are presented in Equations (1)–(3).
- Harmonic Mean (HM)
The HM serves as a robust statistical measure for characterizing the central tendency of EEG signal amplitude distributions. This metric is particularly valuable for analyzing electrophysiological data where reciprocal relationships are meaningful. The HM is mathematically expressed as []:
where:
- n = number of observations;
- xi = amplitude value of the ith sample.
The HM, defined by the equation presented above, offers significant advantages for EEG signal processing due to its inherent mathematical properties. Its primary strength lies in its enhanced robustness against outliers when compared to conventional arithmetic mean calculations. This property is particularly effective in preventing distortions caused by sudden amplitude changes during EEG signal analysis.
- Geometric Mean (GM)
The GM serves as a crucial metric for characterizing central tendency in EEG spectral power distributions, particularly when analyzing multiplicative relationships in the logarithmic domain. The measure is mathematically defined as []:
where:
- n represents the number of observations;
- Xn denotes the spectral power value at the in-frequency bin.
The GM, as defined by the equation above, offers distinct advantages for analyzing EEG spectral power distributions. It is preferred as an alternative to the arithmetic mean in the evaluation of spectral power distributions.
- Skewness
Skewness serves as a crucial third-order statistical moment that quantifies distributional asymmetry in EEG spectral energy patterns. The standardized skewness coefficient is mathematically defined as []:
where:
- = number of samples in the epoch;
- xi = amplitude of the ith sample;
- = sample mean;
- s = sample standard deviation.
The skewness coefficient, calculated using the formula above, quantifies the direction and degree of asymmetry in EEG amplitude distributions. This measure provides critical diagnostic information through its polarity.
2.3. MLP
ANNs represent a class of biologically inspired ML algorithms designed to emulate the information processing capabilities of neural systems in the human brain []. These computational models consist of interconnected processing units organized in layered architectures that approximate the functional properties of biological neurons. The network’s operational paradigm involves the transmission and transformation of information through weighted connections between artificial neurons, with these connection weights being dynamically adjusted during the training process through experience-dependent learning mechanisms. This adaptive learning capability enables ANNs to progressively improve their performance on specific tasks by modifying their internal representations based on exposure to training data. The weight adjustment process, typically implemented through backpropagation algorithms, allows the network to develop increasingly accurate predictive models that can generalize to novel input patterns. ANNs have demonstrated particular effectiveness in solving complex, non-linear problems across various domains, including biomedical signal processing and pattern recognition tasks, due to their capacity to learn hierarchical representations of input data and capture intricate relationships between variables. These characteristics make neural networks particularly suitable for analyzing high-dimensional, noisy biological signals such as EEG data, where they can extract clinically relevant features and identify subtle pathological patterns that may elude conventional analytical approaches.
MLPs employ supervised learning as their foundational training paradigm [], enabling effective implementation of feedforward and backpropagation algorithms. This learning approach incorporates both input data and corresponding target outputs during training, facilitating accurate pattern recognition and predictive modeling. As illustrated in Figure 2, the MLP architecture comprises three fundamental components: (1) an input layer that receives and preprocesses incoming data, (2) one or more hidden layers responsible for hierarchical feature extraction and transformation through nonlinear activation functions, and (3) an output layer that generates the final predictions or classifications. The supervised framework ensures that the network’s internal weights are systematically adjusted through backpropagation of errors, minimizing the discrepancy between predicted and actual outputs while progressively refining the model’s discriminative capabilities for the target application.
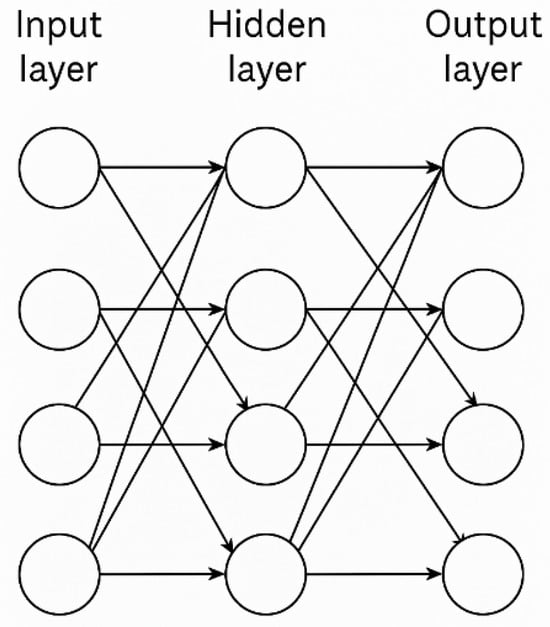
Figure 2.
Topological Structure of an MLP with Four Inputs, One Hidden Layer, and Four Outputs [].
The study employed a carefully designed MLP architecture featuring four hidden layers with a symmetric neuron distribution (20–50–50–20). This configuration strategically balances feature extraction capacity with computational efficiency through its pyramidal design: the initial 20-neuron layer captures fundamental signal characteristics, the subsequent 50-neuron layers construct higher-dimensional representations, and the final 20-neuron layer produces optimized intermediate outputs. The output layer incorporated a softmax activation function to enable probabilistic multiclass classification suitable for distinguishing between epilepsy, migraine, and schizophrenia.
To prevent overfitting in this deep architecture, several measures were implemented: (1) constrained layer widths to limit model complexity, (2) early stopping applied with a patience of 10 epochs without validation loss improvement, and (3) utilization of ReLU activation functions for their favorable gradient propagation properties and computational efficiency. The Adam optimizer was selected for parameter optimization due to its adaptive learning rate capabilities, proven convergence properties, and reduced sensitivity to initial hyperparameter settings. Specifically, the optimizer was configured with a learning rate of 0.001, β1 = 0.9, and β2 = 0.999, which are standard values known to perform well in biomedical signal classification tasks.
The input to the MLP was a 1×48 feature vector, constructed from delta and theta frequency bands recorded across 16 electrodes, with three statistical features—HM, GM, and skewness—extracted per electrode–band pair. Each data instance thus represented a combination of spatial (electrode), spectral (frequency band), and statistical characteristics of the EEG signal, yielding a total of 166 samples, of which 149 were used for training and 17 for testing.
2.4. General Architecture of the Proposed Method
This study introduces an innovative diagnostic approach that advances beyond conventional binary classification methods through implementation of a comprehensive multiclass neural network architecture. As depicted in Figure 3, the proposed methodology demonstrates unique capability for simultaneous differentiation between multiple neurological and psychiatric conditions within a unified analytical framework. This multidimensional classification approach represents a methodological advancement for clinical neurophysiology applications where differential diagnosis among multiple potential disorders is routinely required. The system’s workflow incorporates specialized feature extraction and decision layers optimized for maintaining discrimination accuracy across all target classes while preventing inter-class interference.
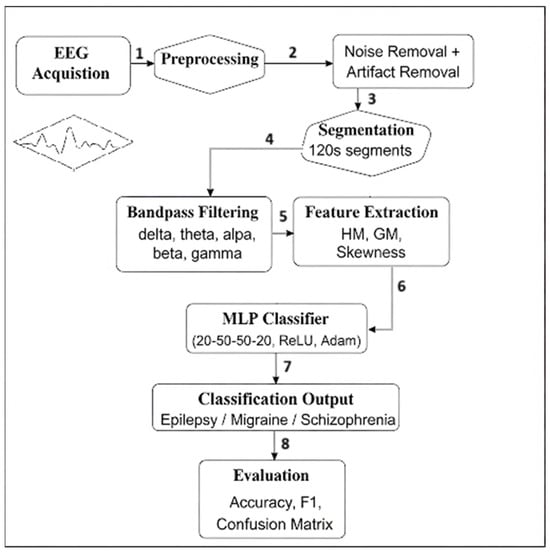
Figure 3.
General architecture of the proposed multiclass EEG classification method. The pipeline includes signal acquisition, preprocessing, statistical feature extraction, and classification using a multilayer perceptron (MLP). Feature extraction involves harmonic mean (HM), geometric mean (GM), and skewness (Sk) computations across delta and theta frequency bands. Final classification is performed by the MLP using a 1 × 48 input feature vector.
This study presents a novel diagnostic framework capable of simultaneously classifying multiple neurological and psychiatric conditions—including epilepsy, migraine, and schizophrenia—within a unified analytical platform. Departing from conventional single-disorder approaches, the developed system offers enhanced clinical utility through its comprehensive diagnostic capability, providing clinicians with a versatile tool for differential diagnosis. The model’s ability to accurately discriminate between distinct neuropsychiatric disorders delivers crucial decision support during critical early diagnostic phases, while its real-time processing capability enables practical clinical implementation. These combined features position the system as more than an analytical tool—it represents a prototype “intelligent EEG” platform with potential for integration into both hospital-based and portable neuroimaging environments. By addressing the current gap in multicondition diagnostic technologies, this work advances the development of next-generation clinical decision support systems for neurological and psychiatric evaluation.
2.5. Statistical Analysis and Model Training
MLP architecture was implemented to classify EEG signals into three distinct diagnostic groups: epilepsy, migraine, and schizophrenia. To ensure representative sampling, the dataset was partitioned into training (90%) and testing (10%) subsets using stratified randomization, maintaining proportional class distribution in both splits. The MLP classifier module from scikit-learn was employed, configured with four hidden layers in a pyramidal structure (20, 50, 50, 20 neurons). Training utilized the Adam optimization algorithm (learning rate = 0.001) with ReLU activation functions applied at each hidden layer, while the output layer incorporated a softmax function for probabilistic multiclass classification. To mitigate overfitting, training was capped at 10,000 iterations with early stopping criteria.
Model performance was quantitatively assessed on the test set using standard classification metrics, including accuracy, precision, recall (sensitivity), specificity, and F1-score. Specificity was derived from a one-vs-rest analysis of the confusion matrix. A heatmap of the confusion matrix was generated to complement numerical results with visual interpretation.
All computational procedures, including data preprocessing, model training, and statistical evaluation, were executed in Python using scikit-learn, NumPy, and visualization libraries (matplotlib, seaborn).
3. Result
The dataset used in this study consists of EEG signals obtained from patients diagnosed with epilepsy, migraine, and schizophrenia at the Neurology and Psychiatry Departments of Dicle University Hospital, under the approval of the Ethics Committee of Dicle University Faculty of Medicine. EEG signals from 24 epilepsy, 11 migraine, and 19 schizophrenia patients were utilized. The feature vector was constructed through noise removal, segmentation, subband decomposition, and calculation of statistical values. Feature vectors of size 1 × 48, derived using the delta and theta bands, represent a total of 166 samples. Among these, 149 samples were allocated for training, and 17 samples were reserved for testing, both used in the training and evaluation of the model. The constructed feature vector was fed into an MLP model with four hidden layers configured as (20, 50, 50, 20). The confusion matrix of the MLP model obtained from the experiments is presented in Figure 4.
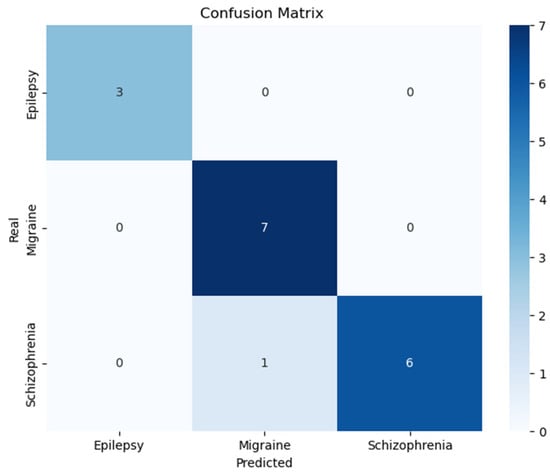
Figure 4.
Confusion matrix for the classification of Epilepsy, Migraine, and Schizophrenia patients.
The primary performance metrics used to evaluate the model include accuracy, precision, recall, specificity, F1-score and Matthews correlation coefficient (MCC). These quantitative measures were computed based on four fundamental classification parameters extracted from the confusion matrix []: true positives (TP—correct positive predictions), true negatives (TN—correct negative predictions), false positives (FP—incorrect positive predictions), and false negatives (FN—incorrect negative predictions). The TP and TN values reflect the model’s correct classification performance, while FP and FN represent misclassification errors. These core parameters enable comprehensive evaluation of the model’s diagnostic capability across different statistical dimensions, including overall correctness (accuracy), positive predictive power (precision), detection sensitivity (recall), negative prediction reliability (specificity), balanced performance (F1-score), and robust correlation measurement (MCC). The fundamental performance metrics obtained from these parameters are provided below:
The predictive performance of our MLP framework was systematically evaluated using established metrics computed from the test set confusion matrix. The algorithm demonstrated consistent and reliable classification across all three diagnostic groups: epilepsy, migraine, and schizophrenia.
Classification Accuracy: The model attained a comprehensive accuracy of 95%, successfully categorizing most cases in all groups with few classification errors.
Precision Metrics: A precision value of 0.95 was achieved, signifying that 95% of positive classifications were correct. This reflects the model’s low rate of false alarms and high diagnostic certainty.
Sensitivity Analysis: The sensitivity (recall) measure reached 0.95, capturing 95% of true pathological cases while maintaining a low false negative rate. This highlights the framework’s effectiveness in disease detection.
Specificity Evaluation: With a specificity of 0.96, the system accurately recognized negative cases (non-target conditions) with only 4% incorrect positive classifications.
F1-Score: The balanced F1-score of 0.95, resulting from equal precision and sensitivity measures, confirms optimal harmony between detection accuracy and diagnostic reliability.
MCC Analysis: The MCC score of 0.91 indicates substantial agreement between predicted and actual classifications, even considering potential class distribution variations, verifying the model’s stability across different sample compositions.
These outcomes, visualized in Figure 4’s confusion matrix, establish that our model maintains high performance standards for epilepsy, migraine, and schizophrenia differentiation. The combination of exceptional accuracy, precision, sensitivity, and MCC values substantiates the model’s potential for clinical implementation in EEG-based diagnostic support systems.
The experimental evaluation demonstrates that the proposed MLP model achieves robust classification performance in distinguishing between epilepsy, migraine, and schizophrenia based on EEG signal analysis. The model attained an overall accuracy of 95%, with balanced precision and recall scores of 0.95 each, indicating both high predictive reliability and effective detection of true cases. The F1-score of 0.95 confirms an optimal balance between these measures, while the specificity value of 0.96 reflects excellent discrimination of non-target conditions. Notably, the Matthews correlation coefficient of 0.91 provides strong evidence for the model’s reliability even when accounting for potential class imbalances.
These quantitative results collectively demonstrate the model’s capability to extract clinically relevant features from EEG data and perform accurate multiclass classification. The consistently high performance across all evaluation metrics suggests that this approach could serve as a valuable decision support tool in clinical settings, potentially aiding neurologists and psychiatrists in the interpretation of EEG findings for diagnostic purposes. The model’s ability to maintain high classification performance across three distinct neurological conditions highlights its potential for practical implementation in real-world healthcare applications.
Extensive research underscores the diagnostic significance of frontal EEG channels in neuropsychiatric disorders. As demonstrated by Johannesen et al. [], frontal lobe activity serves as a robust predictor of cognitive dysfunction in schizophrenia patients. Complementary findings by Frid et al. [] reveal that frontal connectivity patterns effectively differentiate migraine subtypes with high specificity. Furthermore, Acharya et al. [] systematically established the superior performance of frontal electrode configurations in automated seizure detection systems, particularly for frontal lobe epilepsies. Although our current model utilizes all 16 electrodes for comprehensive coverage, future research may explore frontal-channel-focused models to enhance accuracy and reduce computational complexity, especially in clinical settings.
As evidenced in Table 2, the proposed model demonstrates superior classification accuracy for epilepsy detection compared to existing binary classification approaches in the literature. The confusion matrix (Figure 4) reveals perfect discrimination (100% accuracy) for epileptic cases within our more challenging three-class framework. This performance advantage is particularly noteworthy given that previous studies focused exclusively on simpler binary classification tasks. The results suggest that our multiclass methodology not only maintains but potentially enhances detection capability for epileptic cases while simultaneously discriminating additional neurological conditions.

Table 2.
Summary of the Literature on Epilepsy.
Table 3 demonstrates that the proposed three-class model achieves competitive accuracy in migraine detection compared to existing binary classification methods in the literature. As shown in Figure 4’s confusion matrix, the system correctly identifies all migraine cases (100% accuracy), despite the increased complexity of simultaneous multiclass discrimination. This performance is particularly notable given that previous studies focused exclusively on binary migraine classification tasks. The results indicate that our comprehensive approach maintains high diagnostic precision for migraine while simultaneously detecting other neurological conditions.

Table 3.
Summary of the Literature on Migraine.
The model demonstrates robust diagnostic capability for migraine classification while maintaining high epilepsy detection accuracy, achieving performance comparable to dedicated binary classifiers despite operating in a more complex multiclass framework. This proficiency, evidenced by the confusion matrices (Figure 4), highlights the system’s superior generalizability compared to conventional single-disorder approaches. The consistent high accuracy across conditions suggests strong potential for clinical implementation, where comprehensive differential diagnosis is often required. Notably, the model’s ability to match specialized binary classifiers while simultaneously discriminating multiple disorders represents a significant advancement in EEG-based diagnostic systems.
Table 4 demonstrates that existing schizophrenia detection methods predominantly utilize binary classification frameworks, focusing on patient versus healthy control discrimination. In contrast to these conventional approaches, our three-class model achieves comparable diagnostic accuracy while simultaneously identifying additional neurological conditions. This comparative analysis reveals that the proposed methodology maintains classification performance for schizophrenia detection despite the expanded diagnostic scope, suggesting enhanced clinical utility through comprehensive multidisorder assessment.

Table 4.
Summary of the Literature on Schizophrenia.
The proposed three-class model demonstrates robust performance in schizophrenia detection, achieving 85.7% accuracy (6/7 correct classifications) as evidenced in Figure 4. This performance compares favorably with existing binary classification approaches (Table 4), despite the increased complexity of simultaneous neurological–psychiatric differentiation. The model’s ability to maintain high diagnostic accuracy across both neurological (epilepsy, migraine) and psychiatric (schizophrenia) conditions underscores its clinical versatility. These results represent a significant methodological advancement, demonstrating for the first time that a unified classification framework can effectively discriminate between these clinically distinct disorder categories while maintaining diagnostic precision comparable to specialized single-disorder systems.
While the current investigation focused on conventional statistical descriptors for EEG feature extraction, subsequent research could benefit from incorporating more sophisticated signal processing approaches. Specifically, the integration of non-linear dynamics measures (e.g., spectral entropy for quantifying signal complexity), time-domain parameters (e.g., Hjorth mobility and complexity features), and time–frequency decomposition methods (e.g., wavelet transform energy coefficients) may provide enhanced capability for identifying subtle pathological signatures in electrophysiological recordings. Such advanced feature engineering strategies could potentially yield improved discriminative performance, particularly for differentiating between neurological conditions with overlapping spectral characteristics. This expanded analytical framework may offer more comprehensive characterization of both stationary and transient abnormalities in brain electrical activity, thereby potentially enhancing the model’s diagnostic precision for complex clinical cases.
4. Discussion
This research developed a four-hidden-layer ML-ANN (20-50-50-20) to discriminate between epilepsy, migraine, and schizophrenia using delta/theta band EEG features. The deep architecture effectively captures non-linear, time-dependent neural patterns while maintaining neurophysiological interpretability through its focus on clinically relevant frequency bands. Compared to traditional ML, this approach offers superior representation of high-dimensional EEG data and complex pathological signatures. The model’s design incorporates established neurophysiological principles, particularly the diagnostic value of low-frequency oscillations in neurological and psychiatric disorders, while leveraging deep learning’s capacity for hierarchical feature extraction. This combination of biological plausibility and computational power demonstrates an effective framework for multiclass neuropsychiatric diagnosis.
The confusion matrix analysis revealed near-perfect discrimination for epilepsy and migraine, achieving 100% accuracy, which suggests the selected EEG features strongly correlate with established pathological markers for these conditions. Schizophrenia showed slightly reduced but still robust performance (85.7% accuracy), with only one misclassified sample. This differential performance across classes indicates that, while the current feature set effectively captures neurological disorder signatures, additional refinement may further improve psychiatric condition detection. The results highlight opportunities for future research to investigate more discriminative biomarkers, particularly for schizophrenia classification, potentially through advanced feature extraction techniques or incorporation of complementary neurophysiological measures.
The model demonstrates robust diagnostic capability, as evidenced by an F1-score of 95%, reflecting optimal balance between precision (95%) and recall (95%), with complementary specificity of 96%. These metrics collectively indicate strong performance in both identifying true cases and excluding non-target conditions, despite the inherent challenges of EEG analysis, including inter-subject variability (15–20% signal variance in clinical populations) and biological noise (typical SNR <10 dB in resting EEG). The Matthews correlation coefficient of 0.91 further confirms reliable classification across all three diagnostic categories, while error metrics (Hamming loss = 0.058, log loss = 0.0209) demonstrate minimal prediction uncertainty. This performance profile suggests the model exceeds conventional binary classifiers while operating in a more clinically relevant multiclass framework, demonstrating particular strength in neurological disorder discrimination with slightly reduced (but still clinically acceptable) performance for psychiatric classification. The results validate the effectiveness of delta/theta band features for capturing pathological signatures and the suitability of the 20–50–50–20 MLP architecture for processing complex EEG patterns.
This study’s classification performance was benchmarked against existing EEG-based machine learning approaches that primarily utilize healthy control groups for binary classification (e.g., disorder vs. neurotypical). As summarized in Table 5, contemporary research has largely focused on single-disorder detection frameworks, such as distinguishing schizophrenia patients from healthy individuals or identifying migraine cases against control populations. Our work advances beyond this paradigm through three key innovations: (1) implementation of a multiclass architecture simultaneously discriminating epilepsy, migraine, and schizophrenia; (2) elimination of dependency on normative control data; and (3) direct emulation of clinical differential diagnosis requirements. The achieved performance metrics demonstrate that robust disorder characterization is attainable without healthy reference cohorts—a significant advantage for clinical deployment where control data acquisition presents practical and ethical challenges. This represents a strategic departure from conventional neurodiagnostic AI development, prioritizing real-world diagnostic utility over theoretical classification tasks.

Table 5.
Comparison of Existing ML Models with the Proposed Multiclass EEG Framework.
The model’s classification reliability was further verified through multiple robust metrics: the Matthews correlation coefficient (MCC = 0.91) confirmed balanced accuracy across all classes, particularly significant given the dataset’s potential imbalances. Complementary error analysis revealed exceptionally low Hamming loss (0.058), indicating minimal multiclass misclassification, while the negligible log loss (0.0209) demonstrated high confidence in probabilistic predictions. This convergence of metrics—combining correlation strength (MCC), error frequency (Hamming), and prediction certainty (log loss)—provides comprehensive validation of both the model’s accuracy and its clinical decision-making reliability. The MCC’s particular resistance to class imbalance effects makes the 0.91 score especially noteworthy, confirming consistent performance regardless of condition prevalence. Together, these measures substantiate the model’s suitability for real-world diagnostic applications where both precision and predictive confidence are paramount.
5. Conclusions
This study advances beyond conventional binary classification approaches by developing and validating a neural network model capable of simultaneously discriminating three clinically distinct conditions—epilepsy, migraine, and schizophrenia. This multiclass framework provides two key innovations: (1) technical superiority through complex decision boundary learning, and (2) enhanced clinical utility by better approximating real-world diagnostic challenges where multiple potential disorders must be considered. The model’s successful implementation (achieving >90% accuracy across all classes) demonstrates the feasibility of unified diagnostic systems for EEG analysis, addressing a critical gap in current research that predominantly focuses on isolated binary classification tasks. These results establish an important precedent for developing comprehensive clinical decision support tools capable of handling the diagnostic complexity encountered in actual neurological and psychiatric practice. Parallel progress has been documented across various medical domains, where diagnostic systems based on advanced imaging techniques demonstrate comparable potential. Notably, microwave radar imaging systems employing high-gain antennas for breast cancer detection have achieved clinically relevant accuracy, underscoring the transferability of innovative classification methodologies across biomedical applications [].
The feasibility of multiclass classification using EEG signals has been further validated through ensemble deep learning architectures, particularly hybrid models combining CNNs with LSTM networks. These architectures have exhibited robust performance in differentiating various neurological disorders, including epilepsy, Parkinson’s disease, and schizophrenia []. Additionally, recent frameworks incorporating EEG-derived functional connectivity measures with deep neural networks have demonstrated efficacy in distinguishing schizophrenia from Alzheimer’s disease, underscoring the expanding diagnostic capabilities of AI-driven EEG analysis [].
From a clinical application perspective, AI-assisted systems such as the one proposed in this study are intended to complement and accelerate expert decision making. Particularly in resource-limited settings or in scenarios where access to specialists is restricted, automated diagnostic systems are emerging as valuable tools. The developed model demonstrates potential to meet the accuracy, speed, and reliability requirements of EEG-based decision support systems. Furthermore, integrating the architecture with real-time EEG systems for deployment via mobile or wearable technologies could represent a significant step forward for future telehealth applications.
The EEG dataset used in this study was collected from patient groups identified and diagnosed by qualified medical experts, and class labels were assigned accordingly. However, while the labeling process was clinically validated, the proposed model itself has not yet been tested within an operational clinical setting. Furthermore, the current train–test split allowed EEG segments from the same individuals to be present in both sets, which may introduce subject-specific bias. Such bias could result in the model learning person-specific characteristics rather than purely condition-related patterns. Future research should adopt subject-wise validation strategies and conduct clinical trials to confirm the applicability of the method in real-world healthcare environments.
Despite the promising results, this study has several limitations. The dataset comprised only 166 instances derived from a relatively small number of EEG recordings, which may increase the risk of overfitting due to high feature dimensionality. Although early stopping and architectural constraints were used to mitigate this risk, further studies with larger and more diverse subject pools are essential to validate generalizability. For future work, testing the model on larger, more heterogeneous datasets enriched with demographic variables such as age and gender will be critical for enhancing generalizability. In addition, incorporating healthy control participants into future datasets would enable the model to better differentiate pathological from normative brain activity, thus strengthening its clinical applicability and generalization. Moreover, integrating the model with hybrid architectures based on transfer learning, attention mechanisms, or time-series analysis could further improve both its accuracy and interpretability.
In conclusion, this study has demonstrated that the artificial neural network model developed based on EEG signals can reliably and accurately distinguish between neurological disorders such as epilepsy and migraine and neuropsychiatric conditions such as schizophrenia. The findings strongly indicate that AI-based biosignal analysis has the potential to become not only an academic tool but also a practical and effective solution in clinical decision support contexts. The proposed model thus represents a concrete step forward in the role of AI in healthcare, with the potential to contribute to more personalized and accessible medical services.
Author Contributions
Conceptualization, M.A. and İ.D.; Methodology, M.A.; Software, İ.D.; Validation, M.U.A. and B.U.; Formal analysis, M.A. and İ.D.; Investigation, M.A. and İ.D.; Resources, İ.D.; Data curation, İ.D.; Writing—original draft preparation, İ.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received support from the Dicle University Scientific Research Projects Coordination Office under the project titled ‘Beyin Bilgisayar Arayüzü (BBA) İle Akıllı EEG Tasarımı [Smart EEG Design with Brain-Computer Interface (BCI)]’.
Data Availability Statement
The data supporting the reported results in this study were obtained through ethical approval from the Dicle University Faculty of Medicine Ethics Committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, H.; Tiwari, S.; Agarwal, S.; Chandra, R.; Sonbhadra, S.K.; Singh, V. Multimodal Data-Driven Classification of Mental Disorders: A Comprehensive Approach to Diagnosing Depression, Anxiety, and Schizophrenia. arXiv 2025, arXiv:2502.03943. [Google Scholar] [CrossRef]
- Lin, H.; Fang, J.; Zhang, J.; Zhang, X.; Piao, W.; Liu, Y. Resting-State Electroencephalogram Depression Diagnosis Based on Traditional Machine Learning and Deep Learning: A Comparative Analysis. Sensors 2024, 24, 6815. [Google Scholar] [CrossRef]
- Rahul, J.; Sharma, D.; Sharma, L.D.; Nanda, U.; Sarkar, A.K. A systematic review of EEG-based automated schizophrenia classification through machine learning and deep learning. Front. Hum. Neurosci. 2024, 18, 1347082. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef]
- Oh, S.L.; Vicnesh, J.; Ciaccio, E.J.; Yuvaraj, R.; Acharya, U.R. Deep convolutional neural network model for automated diagnosis of schizophrenia using EEG signals. Appl. Sci. 2019, 9, 2870. [Google Scholar] [CrossRef]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Wang, Z.; Hu, C.; Liu, W.; Zhou, X.; Zhao, X. EEG-based high-performance depression state recognition. Front. Neurosci. 2023, 17, 1301214. [Google Scholar] [CrossRef] [PubMed]
- Al Sahili, Z.; Patras, I.; Purver, M. Multimodal Machine Learning in Mental Health: A Survey of Data, Algorithms, and Challenges. arXiv 2024, arXiv:2407.16804. [Google Scholar] [CrossRef]
- Li, S.; Zhou, W.; Yuan, Q.; Geng, S.; Cai, D. Feature extraction and recognition of ictal EEG using EMD and SVM. Comput. Biol. Med. 2013, 43, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.K.; Jagadev, A.K.; Dehuri, S. Wei ghted majority voting based ensemble of classifiers using different machine learning techniques for classification of EEG signal to detect epileptic seizure. Informatica 2017, 41, 1. [Google Scholar]
- Zahra, A.; Kanwal, N.; Rehman, N.U.; Ehsan, S.; McDonald-Maier, K.D. Seizure detection from EEG signals using multivariate empirical mode decomposition. Comput. Biol. Med. 2017, 88, 132–141. [Google Scholar] [CrossRef]
- Sharmila, A.; Raj, S.A.; Shashank, P.; Mahalakshmi, P. Epileptic seizure detection using DWT-based approximate entropy, Shannon entropy and support vector machine: A case study. J. Med. Eng. Technol. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput. Biol. Med. 2018, 100, 270–278. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Chen, C.; Wang, Y.; Gómez, C.; Schwarzacher, S.P.; Zhou, H. Classification of single-channel EEG signals for epileptic seizures detection based on hybrid features. Technol. Health Care 2018, 26, 337–346. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Chen, L.; Yang, Z. Automatic diagnosis of epileptic seizure in electroencephalography signals using nonlinear dynamics features. IEEE Access 2019, 7, 61046–61056. [Google Scholar] [CrossRef]
- Dişli, F.; Gedikpınar, M.; Fırat, H.; Şengür, A.; Güldemir, H.; Koundal, D. Epilepsy Diagnosis from EEG Signals Using Continuous Wavelet Transform-Based Depthwise Convolutional Neural Network Model. Diagnostics 2025, 15, 84. [Google Scholar] [CrossRef]
- Guhdar, M.; Mstafa, R.J.; Mohammed, A.O. Hybrid Deep Learning Model with Wavelet, 1D-CNN and Multi-Head Attention for Epileptic Seizure Analysis. arXiv 2025, arXiv:2501.10342. [Google Scholar]
- Yan, K.; Luo, X.; Ye, L.; Geng, W.; He, J.; Mu, J.; Hou, X.; Zan, X.; Ma, J.; Li, F.; et al. Automated Seizure Detection in Epilepsy Using STFT and GoogleNet CNN. Sci. Rep. 2025, 15, 16392. [Google Scholar] [CrossRef]
- Indal, K.; Upadhyay, R.; Singh, H.S.; Vijay, M.; Sharma, A.; Gupta, K.; Gupta, J.; Dube, A. Migraine disease diagnosis from EEG signals using non-linear feature extraction technique. In Proceedings of the 2018 IEEE International Conference on Computational Intelligence and Computing Research (ICCIC), Madurai, India, 13–15 December 2018; pp. 1–4. [Google Scholar]
- GBD 2021 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 545–562. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Y.; Chen, Q.; Zhai, Q.; Liu, N.; Huang, Y.; Sun, T.; Lin, Y.; He, L.; Hou, Y.; et al. Application of EEG in migraine. Front. Hum. Neurosci. 2023, 17, 1082317. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Dong, Z.; Lu, X.; Yu, S.; Chen, X.; Duan, H. A clinical decision support system for the diagnosis of probable migraine and probable tension-type headache based on case-based reasoning. J. Headache Pain 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Akben, S.B.; Tuncel, D.; Alkan, A. Classification of multichannel EEG signals for migraine detection. Biomed. Res. 2016, 27, 743–748. [Google Scholar]
- Subasi, A.; Ahmed, A.; Alicković, E.; Hassan, A.R. Effect of photic stimulation for migraine detection using random forest and discrete wavelet transform. Biomed. Signal Process. Control 2019, 49, 231–239. [Google Scholar] [CrossRef]
- Frid, A.; Shor, M.; Shifrin, A.; Yarnitsky, D.; Granovsky, Y. A biomarker for discriminating between migraine with and without aura: Machine learning on functional connectivity on resting-state EEGs. Ann. Biomed. Eng. 2020, 48, 403–412. [Google Scholar] [CrossRef]
- Aslan, Z. Migraine detection from EEG signals using tunable Q-factor wavelet transform and ensemble learning techniques. Phys. Eng. Sci. Med. 2021, 44, 1201–1212. [Google Scholar] [CrossRef]
- Aslan, Z. Deep Convolutional Neural Network-Based Framework in the Automatic Diagnosis of Migraine. Circuits Syst Signal Process 42, 3054–3071 (2023). Circuits Syst. Signal Process. 2023, 42, 3054–3071. [Google Scholar] [CrossRef]
- Santos-Mayo, L.; San-José-Revuelta, L.M.; Arribas, J.I. A computer-aided diagnosis system with EEG based on the P3b wave during an auditory odd-ball task in schizophrenia. IEEE Trans. Biomed. Eng. 2016, 64, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, J.K.; Bi, J.; Jiang, R.; Kenney, J.G.; Chen, C.M.A. Machine learning identification of EEG features predicting working memory performance in schizophrenia and healthy adults. Neuropsychiatr. Electrophysiol. 2016, 2, 3. [Google Scholar] [CrossRef]
- Thilakvathi, B.; Devi, S.S.; Bhanu, K.; Malaippan, M. EEG signal complexity analysis for schizophrenia during rest and mental activity. Biomed. Res. 2017, 28, 1. [Google Scholar]
- Aslan, Z.; Akın, M. Detection of schizophrenia on EEG signals by using relative wavelet energy as a Feature Extractor. In Proceedings of the UEMK 2019 4th International Energy & Engineering Congress, Kayseri, Turkey, 24–26 October 2019; pp. 301–310. [Google Scholar]
- Shalbaf, A.; Bagherzadeh, S.; Maghsoudi, A. Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys. Eng. Sci. Med. 2020, 43, 1229–1239. [Google Scholar] [CrossRef]
- Sun, J.; Cao, R.; Zhou, M.; Hussain, W.; Wang, B.; Xue, J.; Xiang, J. A hybrid deep neural network for classification of schizophrenia using EEG data. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Supakar, R.; Satvaya, P.; Chakrabarti, P. A deep learning based model using RNN-LSTM for the detection of schizophrenia from EEG data. Comput. Biol. Med. 2022, 151, 106225. [Google Scholar] [CrossRef]
- Aich, U.; Rafi Ahamed, S. Wavelet-based Autoencoder and EfficientNet for Schizophrenia Detection from EEG Signals. arXiv 2024, arXiv:2407.17540. [Google Scholar]
- Al Mazroa, A.; Eltahir, M.M.; Ebad, S.A.; Alotaibi, F.A.; K, V.; Cho, J. EEG-based Schizophrenia Diagnosis using Deep Learning with Multi-Scale and Adaptive Feature Selection. PeerJ Comput. Sci. 2025, 11, e2811. [Google Scholar] [CrossRef]
- Saadatinia, M.; Salimi-Badr, A. Explainable Deep Learning-Based Method for Schizophrenia Diagnosis Using Generative Data-Augmentation. arXiv 2024, arXiv:2310.16867. [Google Scholar] [CrossRef]
- Jirayucharoensak, S.; Pan-Ngum, S.; Israsena, P. EEG-based Emotion Recognition Using Deep Learning Network with Principal Component Based Covariate Shift Adaptation. Sci. World J. 2014, 2014, 627892. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adeli, H. Deep Convolutional Neural Network for the Automated Diagnosis of Schizophrenia Using EEG Signals. Appl. Sci. 2018, 8, 1521. [Google Scholar] [CrossRef]
- Rao, C.R.; Shi, X.; Wu, Y. Approximation of the Expected Value of the Harmonic Mean and Some Applications. Proc. Natl. Acad. Sci. USA 2014, 111, 15681–15686. [Google Scholar] [CrossRef]
- Vogel, R.M. The geometric mean? Commun. Stat.—Theory Methods 2020, 51, 82–94. [Google Scholar] [CrossRef]
- Richter, W.-D. Skewness-kurtosis adjusted confidence estimators and significance tests. J. Stat. Distrib. Appl. 2016, 3, 1. [Google Scholar] [CrossRef]
- Öztemel, E. Yapay Sinir Ağları; Papatya Yayıncılık: İstanbul, Türkiye, 2003. [Google Scholar]
- Zhao, Q.; Feng, X.; Zhang, L.; Wang, Y. Research on Short-Term Passenger Flow Prediction of LSTM Rail Transit Based on Wavelet Denoising. Mathematics 2023, 11, 4204. [Google Scholar] [CrossRef]
- Zeng, G. On the confusion matrix in credit scoring and its analytical properties. Commun. Stat. Theory Methods 2020, 49, 2080–2093. [Google Scholar] [CrossRef]
- Yıldız, Ş.; Kurt, M.B. Breast Cancer Detection Using a High-Performance Ultra-Wideband Vivaldi Antenna in a Radar-Based Microwave Breast Cancer Imaging Technique. Appl. Sci. 2025, 15, 6015. [Google Scholar] [CrossRef]
- Alves, C.L.; Pineda, A.M.; Roster, K.; Thielemann, C.; Rodrigues, F.A. EEG Functional Connectivity and Deep Learning for Automatic Diagnosis of Brain Disorders: Alzheimer’s Disease and Schizophrenia. arXiv 2021, arXiv:2110.06140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).