Effect of Sulfoaluminate Clinker Addition on Boron Removal During Water Softening
Abstract
1. Introduction
- At an industrial scale, effective boron removal has been observed when the magnesium-to-boron molar ratio exceeds 10 mol/mol. This process, however, results in the formation of gelatinous and hygroscopic solids [43]. Similar to silica removal, the mechanism is believed to involve adsorption. Notably, the preformed magnesium hydroxide is less effective than the in situ formed one [44].
- At a laboratory scale, the addition of hydrogen peroxide (H2O2) has led to the formation of calcium perborate (CaB2O4(OH)4), a compound with low solubility that is inferred to facilitate boron removal. Enhanced performance has been reported when barium hydroxide (Ba(OH)2) is used instead of calcium hydroxide [45].
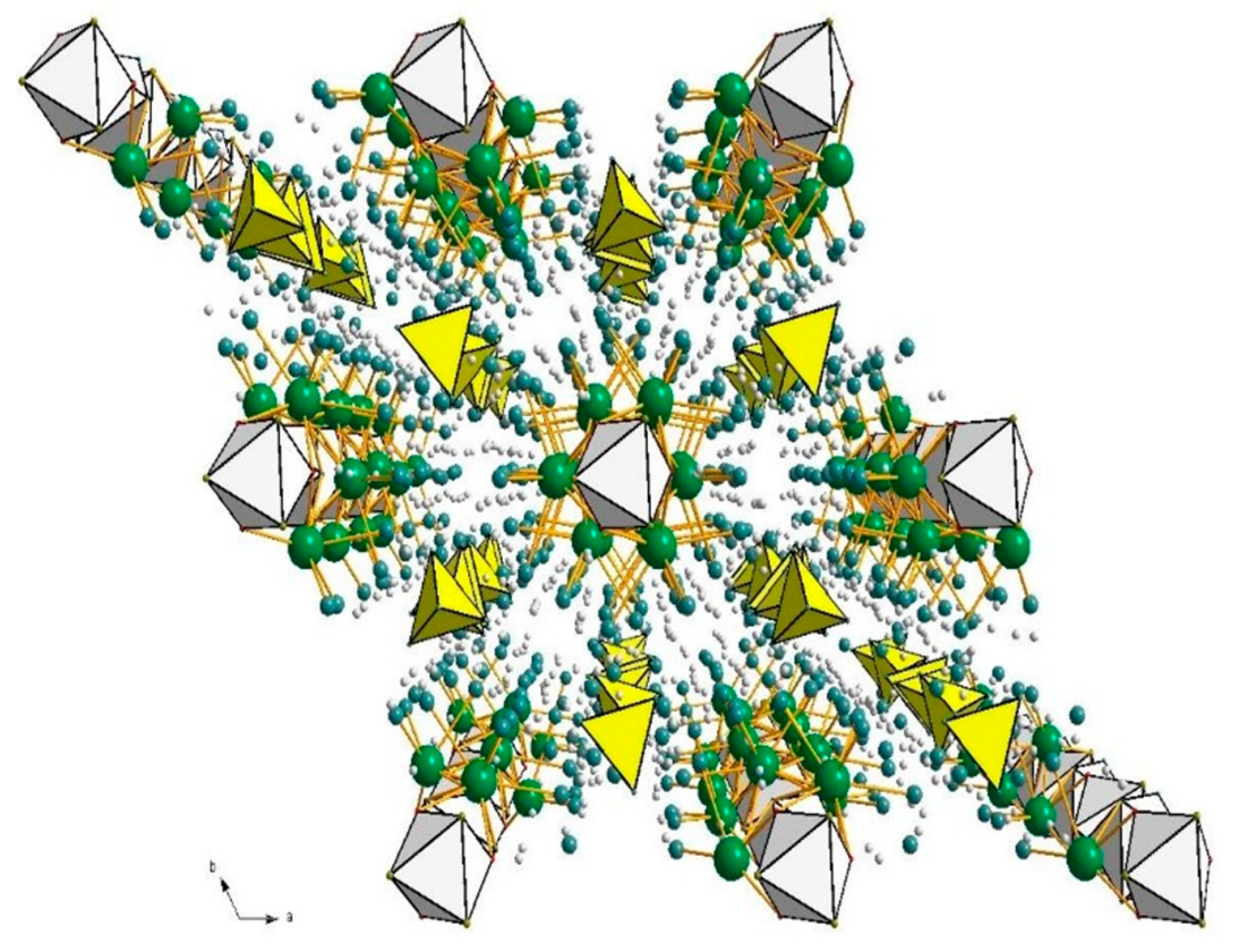
- At an industrial scale, the use of ettringite (Ca6Al2(SO4)3(OH)12·26H2O) has also proven effective [46]. Ettringite, a clay and a key hydration product of Portland cement, can be synthesized from hydrated aluminum sulfate (Al2(SO4)3·18H2O) and calcium hydroxide [47]. Its structure comprises columns of (Ca6[Al2(OH)12·24H2O]6+) aligned along the c-axis, with sulfate (SO42−) and water molecules occupying the intercolumnar channels (Figure 1). The framework is stabilized by hydrogen bonding [48], and its open architecture allows for water mobility and ion exchange [49]. Specifically, ettringite can exchange one sulfate ion for one borate ion, turning into the clay mineral charlesite (Ca6Al2(SO4)2(B(OH)4)(OH,O)12·26H2O), which belongs to the same group [50]. Amorphous meta-ettringite, obtained from ettringite through thermal dehydration at temperature higher than 65 °C, has been claimed as more effective than ettringite as such [51]. Importance of meta-ettringite has also been confirmed in a study dealing with Portland cement waste as a low-cost boron adsorbent [52]. Charlesite cannot be considered an exploitable boron raw material because its theoretical boron content of 0.7% wt. is far less than the 15.0% wt. found in state-of-the-art minerals. According to that, other valorization routes alternative to landfilling have to be considered [53].
2. Materials and Methods
2.1. Raw Water
2.2. Precipitation Softening
2.3. Leaching Test
2.4. Physical–Chemical Characterization
- Boron with a Hack Lange LCK 307TM kit (Hach Lange Italia, Lainate, Italy) based on the Azomethine-H methodology (certified for boron levels from 0.05 mg/L to 0.25 mg/L);
- Aluminum with a Hack Lange LCK 301TM kit using the Chromazurol-S methodology (certified for aluminum levels from 0.02 mg/L to 0.5 mg/L );
- Sulfate with a Hack Lange LCK 153TM kit based on barium sulfate (BaSO4) methodology (certified for sulfate levels from 50 mg/L and 140 mg/L).
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Water Science School. Available online: https://www.usgs.gov/special-topics/water-science-school (accessed on 10 January 2024).
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.A.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef]
- Cirrincione, L.; Geropanta, V.; Peri, G.; Scaccianoce, G. Enhance urban energy management and decarbonization through an EC-based approach. In Proceedings of the 2023 IEEE International Conference on Environment and Electrical Engineering, Madrid, Spain, 6–9 June 2023. [Google Scholar]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Scaccianoce, G.; Camarda, M.C. Towards the energy optimization and decarbonization of urban settings: Proposal of a strategy at neighbourhood level to foster nearly zero and positive energy districts. In Proceedings of the 2023 IEEE International Conference on Environment and Electrical Engineering, Madrid, Spain, 6–9 June 2023. [Google Scholar]
- Ferrucci, A.; Vocciante, M. Improved management of water resources in process industry by accounting for fluctuations of water content in feed streams and products. J. Water Process Eng. 2021, 39, 101870. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2023, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, H.M.; Al Sobhi, S.A.; El Naas, M.H. Mapping the desalination journal: A systematic bibliometric study over 54 years. Desalination 2022, 526, 115535. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Lee, D.E.; Khan, A.; Husain, A.; Anwar, K.; Lee, H.C.; Shin, S.H.; Lee, J.-Y.; Danish, M.; Jo, W.K. Synergistic performance of Z-scheme based g-CN/Zn0.5Cd0.5S/GO nanocomposite towards sustainable model organic pollutants detoxification: Mechanistic insights and practical implications. J. Alloys Comp. 2025, 1022, 179820. [Google Scholar] [CrossRef]
- Lee, D.E.; Husain, A.; Khan, A.; Danish, M.; Jo, W.K. Versatile platform of 3D/2D/1D: ZnFe2O4/NiAl-LDH/MWCNTs nanocomposite for photocatalytic purification of dinoseb and electrocatalytic O2 evolution reaction. Environ. Res. 2025, 264, 120367. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Danish, M.; Jo, W.K. Two birds with one stone: Facile fabrication of a ternary ZnIn2S4/BiVO4/MWCNTs nanocomposite for photocatalytic detoxification of priority organic pollutants and hydrogen production with a proposed mechanism. J. Alloys Comp. 2024, 980, 173615. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Rizzo, G.; Scaccianoce, G. The landfilling of municipal solid waste and the sustainability of the related transportation activities. Sustainability 2022, 14, 5272. [Google Scholar] [CrossRef]
- Vocciante, M.; Meshalkin, V. An accurate inverse model for the detection of leaks in sealed landfills. Sustainability 2020, 12, 5598. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Soil and Pollution: An Introduction to the Main Issues. In Soil Pollution, 1st ed.; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: New York, NY, USA, 2018. [Google Scholar]
- Peri, G.; Cirrincione, L.; Mazzeo, D.; Matera, N.; Scaccianoce, G. Building resilience to a warming world: A contribution toward a definition of “Integrated Climate Resilience” specific for buildings—Literature review and proposals. Energy Build. 2024, 315, 114319. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Bioresour. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef]
- Alberti, S.; Basciu, I.; Vocciante, M.; Ferretti, M. Experimental and physico-chemical comparison of ZnO nanoparticles’ activity for photocatalytic applications in wastewater treatment. Catalysts 2021, 11, 678. [Google Scholar] [CrossRef]
- de Folly d’Auris, A.; Rubertelli, F.; Taini, A.; Vocciante, M. A novel polyurethane-based sorbent material for oil spills management. J. Environ. Chem. Eng. 2023, 11, 111386. [Google Scholar] [CrossRef]
- Wang, R.; Tang, Z.; Zhang, C.; Sun, W.; Chen, Z.; Wei, X.; Liu, M. Research progress on heavy metal wastewater treatment in the integrated circuit industry: From pollution control to resource utilization. Sep. Purif. Technol. 2025, 376, 134033. [Google Scholar] [CrossRef]
- The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 8 April 2024).
- Charlot, G. Analisi Chimica Quantitativa: Equilibri in Soluzione, 3rd ed.; Piccin Editore: Padova, Italy, 1977; pp. 1–466. [Google Scholar]
- Guidelines for Drinking Water Quality: Fourth Edition Incorporating the First and Second Addenda. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 26 May 2025).
- Acque Consumo Umano; Ministero dell’Ambiente e della Sicurezza Energetica (MASE) del Governo Italiano: Rome, Italy. Available online: https://www.mase.gov.it/portale/web/guest/acque-consumo-umano (accessed on 7 August 2025).
- Tagliabue, M.; Reverberi, A.P.; Bagatin, R. Boron removal from water: Needs, challenges and perspectives. J. Clean. Prod. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Rahmavati, K.; Ghaffour, N.; Aubry, C.; Amy, G.L. Boron removal efficiency from Red Sea water using different SWRO/BWRO membranes. J. Membr. Sci. 2012, 423–424, 522–529. [Google Scholar] [CrossRef]
- Imbernon Mulero, A.; Gallego Elvira, B.; Martinez Alvarez, V.; Martin Gorritz, B.; Molina del Toro, R.; Jodar Conesa, F.J.; Maestre Valero, J.F. Ion exchange resins to reduce boron in desalinated seawater for irrigation in Southeastern Spain. Agronomy 2022, 12, 1389. [Google Scholar] [CrossRef]
- Khatib, Z.; Verbeek, P. Water to value. Produced water management for sustainable field development of mature green fields. In Proceedings of the International Conference on Health, Safety and Environment in Oil and Gas Exploration and Production, Kuala Lumpur, Malaysia, 20–22 March 2002; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2022. [Google Scholar]
- Ahmadun, F.R.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Water Handbook, Veolia, 2023. Available online: https://www.watertechnologies.com (accessed on 23 June 2023).
- Mundim, B.; Volschan Junior, I.; Hoffmann, B.S. Evaluation of the environmental and economic performance of wastewater treatment technologies of warm climate regions. J. Sustain. Dev. Energy Water Environ. Syst. 2023, 11, 1110472. [Google Scholar] [CrossRef]
- Puttock, A.; Auster, R.E.; Gatis, N.; Brazier, R.E. Human, or Nature-led? A Spectrum of Nature-based Solutions. Nat.-Based Solut. 2025, 8, 100249. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Franchi, E.; Samà, C.; Gila, L.; Zanardi, S.; Palmery, S.; Proto, A.; et al. New light on phytoremediation: The use of luminescent solar concentrators. Appl. Sci. 2021, 11, 1923. [Google Scholar] [CrossRef]
- Farzi, A.; Borghei, S.M.; Vossoughi, M. The use of halophytic plants for salt phytoremediation in constructed wetlands. Int. J. Phytoremediation 2017, 19, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Capitano, C.; Cirrincione, L.; Peri, G.; Rizzo, G.; Scaccianoce, G. A simplified method for the indirect evaluation of the ‘embodied pollution’ of natural stones (marble) working chain to be applied for achieving the Ecolabel brand of the product. J. Clean. Prod. 2022, 362, 132576. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Sabathianakis, G.; Kritsotakis, I.; Kabourakis, E.M.; Manios, T. Halophytes as vertical flow constructed wetland vegetation for domestic wastewater treatment. Sci. Total Environ. 2017, 583, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Franchi, E.; Fusini, D.; Pietrini, I.; Bretzel, F.; Scartazza, A.; Barbafieri, M.; Vocciante, M. Low-impact management of produced water: Assessing phytodepuration with Halocnemum strobilaceum and Suaeda fruticosa. J. Sustain. Dev. Energy Water Environ. Syst. 2024, 12, 1120494. [Google Scholar] [CrossRef]
- Marsawa, A.; Meyerstein, D.; Daltrophe, N.; Kedem, O. Compact accelerated precipitation softening (CAPS) as pretreatment for membrane desalination (II). Lime softening with concomitant removal of silica and heavy metals. Desalination 1997, 113, 73–84. [Google Scholar] [CrossRef]
- Tagliabue, M.; Bagatin, R.; Conte, A.; Congiu, A.; Perucchini, S.; Zanardi, S.; Bellettato, M.; Carati, A. Metal-rich sludge from mine water treatment: From waste to effective arsenate adsorbent. Desal. Water Treat. 2017, 69, 294–301. [Google Scholar] [CrossRef]
- Rioyo, J.; Aravinthan, V.; Bundschuh, J.; Linch, M. High pH softening pretreatment for boron removal in inland desalination systems. Sep. Purif. Technol. 2018, 205, 308–316. [Google Scholar] [CrossRef]
- Doran, G.F.; Williams, K.L.; Drago, J.A.; Huang, S.S.; Leong, L.Y.C. Pilot study results to convert oil field produced water to drinking water or reuse quality. In Proceedings of the Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27–30 September 1998; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 1998. [Google Scholar]
- Parks, J.L.; Edwards, M. Boron removal via formation of magnesium silicate solids during precipitative softening. J. Environ. Eng. 2007, 133, 149–156. [Google Scholar] [CrossRef]
- Shih, Y.J.; Liu, C.H.; Lan, W.C.; Huan, Y.H. A novel chemical oxo-precipitation (COP) process for efficient remediation of boron wastewater at room temperature. Chemosphere 2014, 111, 232–237. [Google Scholar] [CrossRef]
- Assett, D.J.; Thompson, J.S. Enhanced Ettringite Formation for the Treatment of Hazardous Liquid Waste. U.S. Patent No. 5,547,588, 20 August 1996. [Google Scholar]
- Liu, Y.; Takaya, T.; Ohuchi, A. Boron removal from wastewater via coagulation sedimentation with ettringite: An experimental and mechanism study. Desal. Water Treat. 2017, 58, 435–441. [Google Scholar] [CrossRef]
- Clark, S.M.; Colas, B.; Kunz, M.; Speziale, S.; Monteiro, P.J.M. Effect of pressure on the crystal structure of ettringite. Cem. Concr. Res. 2008, 38, 19–26. [Google Scholar] [CrossRef]
- Zhang, M.; Reardon, E. Removal of boron, chromium, molybdenum, and selenium from wastewater by incorporation into hydrocalumite and ettringite. Environ. Sci. Technol. 2003, 37, 2947–2952. [Google Scholar] [CrossRef]
- Dunn, P.J.; Peacor, D.R.; Leavens, P.B.; Baum, J.L. Charlesite, a new mineral of the ettringite group, from Franklin, New Jersey. Am. Mineral. 1983, 68, 1033–1037. [Google Scholar]
- Iizuka, A.; Takahashi, M.; Nakamura, T.; Yamasaki, A. Preparation of metaettringite from ettringite and its performance for boron removal from boric acid solution. Mater. Trans. 2017, 58, 1761–1767. [Google Scholar] [CrossRef]
- Iizuka, A.; Takahashi, M.; Nakamura, T.; Yamasaki, A. Boron removal performance of a solid sorbent derived from waste concrete. Ind. Eng. Chem. Res. 2014, 53, 4046–4051. [Google Scholar] [CrossRef]
- Parks, J.L.; Edwards, M. Boron in the environment. Crit. Rev. Environ. Sci. Technol. 2012, 35, 81–114. [Google Scholar] [CrossRef]
- Gougar, M.L.D.; Scheetz, B.E.; Roy, D.M. Ettringite and C-S-H Portland cement phase for waste ion immobilization: A review. Waste Manage. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Madzivire, G.; Petrik, L.F.; Gitari, W.M.; Ojumu, T.V.; Balfour, G. Application of coal fly ash to circumneutral mine waters for the removal of sulphates as gypsum and ettringite. Miner. Eng. 2010, 23, 252–257. [Google Scholar] [CrossRef]
- Alvarez Ajuso, E.; Nugteren, H.W. Synthesis of ettringite: A way to deal with the acid wastewaters of aluminium anodizing industry. Water Res. 2005, 39, 65–72. [Google Scholar] [CrossRef]
- Corazza, F.; Cepolina, S.; di Dio, M.; Capannelli, G.; Comite, A.; Capannelli, G. An environmentally sustainable cement composition, its use for inert dredging sediments/sludges, related method for inerting. EP 3,259,239 B1, 1 July 2020. [Google Scholar]
- Tagliabue, M.; Baric, M.; Zubin, N.; Montanari, E.; Marra, G.L.; Perucchini, S.; Mazzara, C.; Lagrotta, E. Boron removal through precipitation softening in presence of ettringite. Desal. Water Treat. 2023, 309, 165–170. [Google Scholar] [CrossRef]
- Ali Pre Green™ Technical Data Sheet; Heidelberg Materials Italia Cementi: Peschiera Borromeo, Italy. Available online: https://www.heidelbergmaterials.it/sites/default/files/2023-10/HM_Technical_data_sheet_-_ALI_PRE_GREEN_-_EN.pdf (accessed on 21 May 2025).
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- UNI EN 12457-2:2004; Characterization of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges: Part 2. One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or with Size Reduction). Ente Italiano di Normazione (EN): Milano, Italy, 2004. Available online: https://store.uni.com/en/uni-en-12457-2-2004 (accessed on 21 May 2025).
- Canizares, P.; Martinez, P.; Jimenez, C.; Lobato, J.; Rodrigo, M.A. Comparison of the aluminum speciation in chemical and electrochemical dosing processes. Ind. Eng. Chem. Res. 2006, 45, 8749–8756. [Google Scholar] [CrossRef]
- Attuazione Direttiva 91/271/CEE; Ministero dell’Ambiente e della Sicurezza Energetica (MASE) del Governo Italiano: Rome, Italy. Available online: https://www.mase.gov.it/portale/attuazione-direttiva-91-271-cee (accessed on 7 August 2025).
- Uslu, T.; Arol, A.I. Use of boron waste as an additive in red bricks. Waste Manage. 2004, 24, 217–220. [Google Scholar] [CrossRef]
- Perez Bravo, R.; Morales Cantero, A.; Bruscolini, M.; Aranda, M.A.G.; Santacruz, I.; de La Torre, A.G. Effect of boron and water-to-cement ratio on the performances of laboratory prepared belite-ye’elimite-ferrite cements. Materials 2021, 14, 4862. [Google Scholar] [CrossRef] [PubMed]
- Runcevski, T.; Dinnebier, R.E.; Magdysyuk, O.V.; Poellmann, H. Crystal structures of calcium hemicarboaluminate and carbonated calcium hemicarboaluminate from synchrotron powder diffraction data. Acta Cryst. B 2012, 68, 493–500. [Google Scholar] [CrossRef]
- Lothenbach, B.; Geiger, C.A.; Dachs, E.; Winnefeld, F.; Pisch, A. Thermodynamic properties and hydration behavior of ye’elimite. Cem. Concr. Res. 2022, 162, 106995. [Google Scholar] [CrossRef]
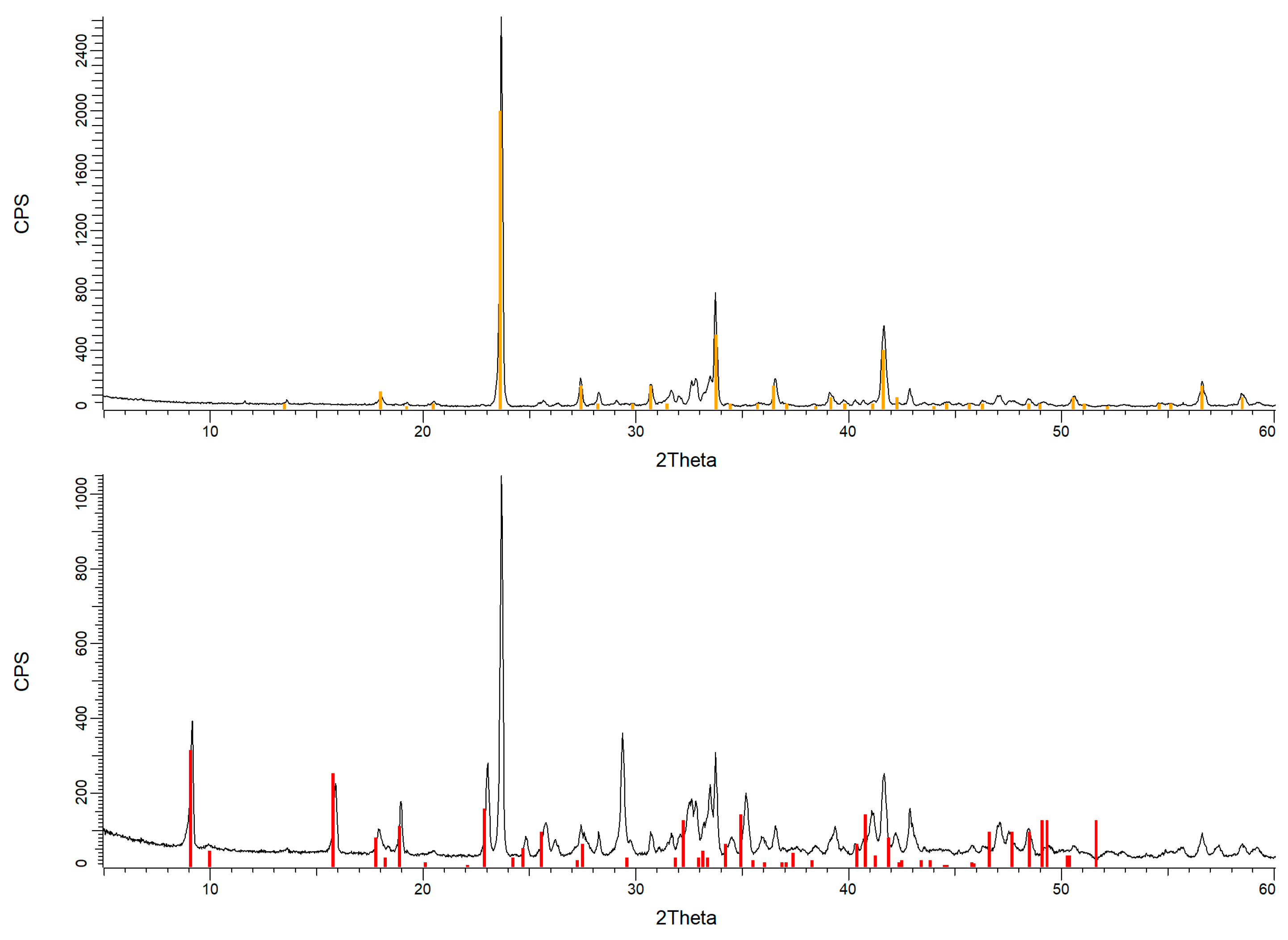

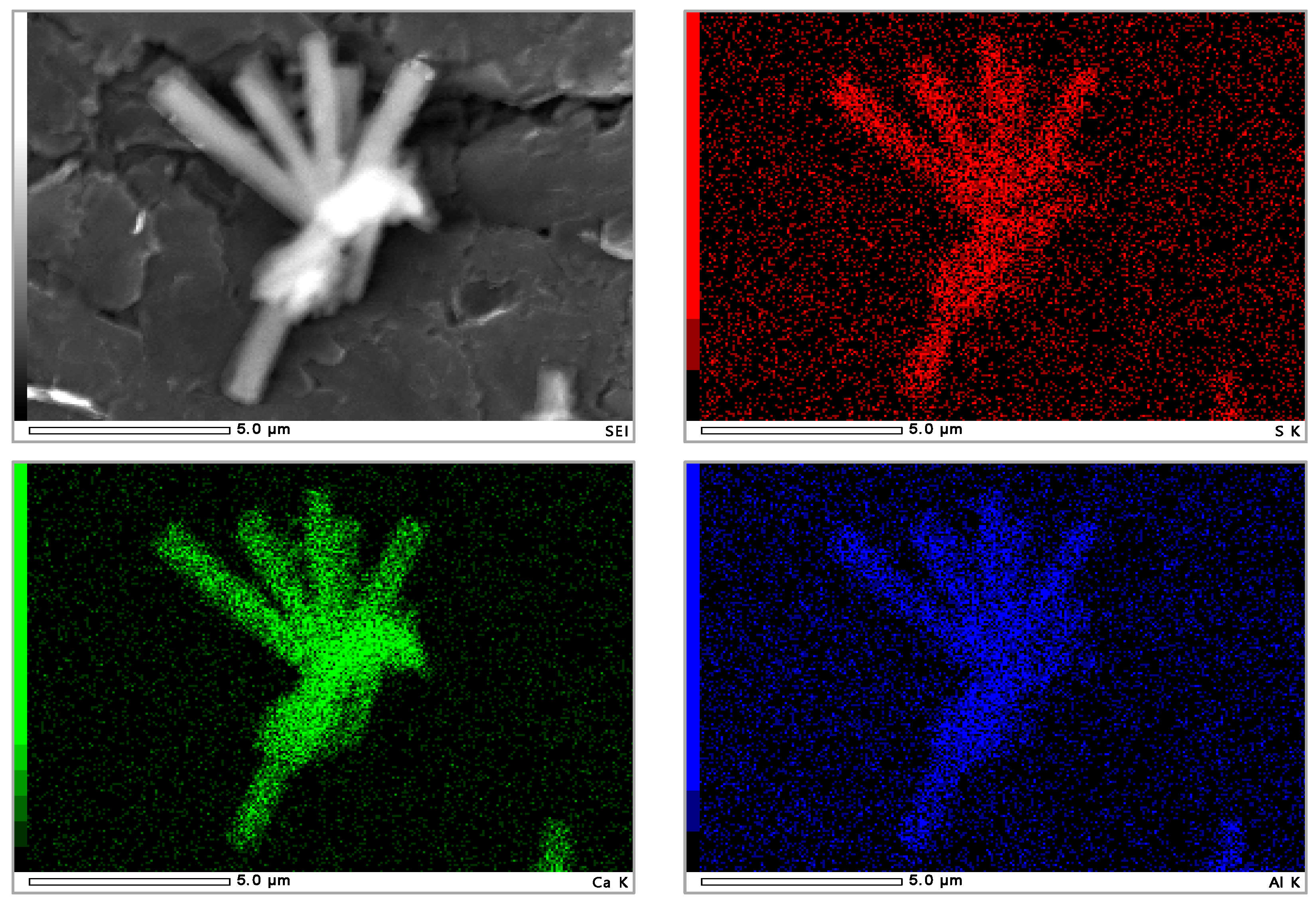
| Element | Ca | Al | S | Si | Mg | Fe | K |
|---|---|---|---|---|---|---|---|
| [-] | [% wt.] | [% wt.] | [% wt.] | [% wt.] | [% wt.] | [% wt.] | [% wt.] |
| Actual | 27.9 | 16.2 | 4.6 | 4.0 | 2.8 | 1.0 | 0.6 |
| Declared | 25.7–29.3 | 14.2–17.5 | 4.0–5.6 | 0.0–4.3 | 0.0–3.0 | 0.0–1.4 | --- |
| Test | EC | Dosing | t | ΔB | BF | AlF | SO4F | Solids |
|---|---|---|---|---|---|---|---|---|
| [-] | [mS/cm] | [-] | [min] | [%] | [mg/L] | [mg/L] | [mg/L] | [g/L] |
| A | 400 | 2.0X | 90 | −12.3 | 13.2 | 20.7 | --- | 1.7 |
| B | 400 | 2.0X | 150 | −19.6 | 12.1 | 45.3 | --- | 2.0 |
| C | 400 | 2.5X | 150 | −70.4 | 4.4 | 62.0 | 3.0 | 6.5 |
| D | 400 | 9.0X | 90 | −43.9 | 8.4 | 58.1 | --- | 5.3 |
| E | 400 | 9.0X | 150 | −99.7 | 0.05 | 57.2 | 6.0 | 6.6 |
| F | 400 | 13.0X | 150 | −94.3 | 0.9 | 120.0 | 7.0 | 9.4 |
| G | 15 | 13.0X | 150 | −77.1 | 3.4 | 104.0 | --- | 8.7 |
| P | 400 | 2.5X | 150 | −94.6 | 0.8 | 0.2 | 534.0 | 3.8 |
| Item | Test E | Test P | |||
|---|---|---|---|---|---|
| Type | Specific Cost [EUR/kg] | Amount [kg/m3] | Cost [EUR/m3] | Amount [kg/m3] | Cost [EUR/m3] |
| Aluminum sulfate hydrated | 0.60 | --- | --- | 2.27 | 1.36 |
| Alipre GreenTM | 0.32 | 4.96 | 1.59 | --- | --- |
| Calcium hydroxide | 0.60 | 0.98 | 0.59 | 2.63 | 1.58 |
| Solids disposal | 0.35 | 6.60 | 2.31 | 3.80 | 1.33 |
| Total | --- | --- | 4.49 | --- | 4.27 |
| Phase | Formula | Clinker [% wt.] | A [% wt.] | E [% wt.] | E’ [% wt.] | P [% wt.] |
|---|---|---|---|---|---|---|
| Aragonite | CaCO3 | --- | --- | --- | 13.1 | --- |
| Bredigite | Ca14Mg2(SiO4)8 | 31.0 | 29.5 | 28.7 | 14.6 | --- |
| Calcite | CaCO3 | --- | 24.6 | 13.3 | 23.2 | 23.7 |
| Dellaite | Ca6(Si2O7)(SiO4) (OH)2 | --- | 3.6 | 4.4 | --- | --- |
| Dolomite | CaMg(CO3)2 | --- | 3.2 | --- | --- | --- |
| Ettringite | Ca6Al2(SO4)3(OH)12·26(H2O) | 20.8 | 4.4 | 31.1 | 45.8 | 76.3 |
| Hc | (Ca4Al2(OH)12)(OH(CO3)0.5·4(H2O)) | --- | 7.4 | --- | --- | --- |
| Kuzelite | Ca3Al6(OH)18(SO4)1.5·9(H2O) | --- | 6.2 | --- | --- | --- |
| Periclase | MgO | --- | 2.6 | 1.9 | 2.8 | --- |
| Silvite | KCl | --- | --- | --- | 0.5 | --- |
| Ye’elimite | Ca4Al6O12(SO4) | 48.2 | 18.5 | 20.6 | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliabue, M.; de Folly d’Auris, A.; Pacini, A.; Bellettato, M.; Marra, G.; Perucchini, S.; Mazzara, C.; Lagrotta, E.; Vocciante, M. Effect of Sulfoaluminate Clinker Addition on Boron Removal During Water Softening. Appl. Sci. 2025, 15, 8890. https://doi.org/10.3390/app15168890
Tagliabue M, de Folly d’Auris A, Pacini A, Bellettato M, Marra G, Perucchini S, Mazzara C, Lagrotta E, Vocciante M. Effect of Sulfoaluminate Clinker Addition on Boron Removal During Water Softening. Applied Sciences. 2025; 15(16):8890. https://doi.org/10.3390/app15168890
Chicago/Turabian StyleTagliabue, Marco, Alessandra de Folly d’Auris, Andrea Pacini, Michela Bellettato, Gianluigi Marra, Sara Perucchini, Cinzia Mazzara, Emanuele Lagrotta, and Marco Vocciante. 2025. "Effect of Sulfoaluminate Clinker Addition on Boron Removal During Water Softening" Applied Sciences 15, no. 16: 8890. https://doi.org/10.3390/app15168890
APA StyleTagliabue, M., de Folly d’Auris, A., Pacini, A., Bellettato, M., Marra, G., Perucchini, S., Mazzara, C., Lagrotta, E., & Vocciante, M. (2025). Effect of Sulfoaluminate Clinker Addition on Boron Removal During Water Softening. Applied Sciences, 15(16), 8890. https://doi.org/10.3390/app15168890








