Abstract
The large yellow croaker (Larimichthys crocea) is one of China’s most economically important marine fish species, with its cage culture production leading the nation for many years. However, the rapid expansion of aquaculture has brought challenges such as germplasm degradation, reduced disease resistance, inconsistent product quality, and low adoption of improved strains, which have hindered the sustainable development of the industry. The primary objective of this review is to summarize the current practices and challenges in seedling selection for L. crocea. The secondary objectives include discussing the influence of genetic, physiological, and environmental factors on growth performance and proposing future research directions for sustainable breeding programs. This review covers key topics including morphological screening, growth performance evaluation, genetic diversity conservation, disease resistance improvement, and adaptation to environmental stress. It also explores the application of modern technologies such as marker-assisted selection, intelligent monitoring, environmental control, precision feeding, and disease prevention. Moreover, it highlights core issues in current breeding practices, such as over-reliance on single-trait selection and insufficient integration of environmental adaptability and disease resistance. Finally, future trends are discussed, emphasizing the integration of genomic tools with artificial intelligence to promote intelligent, precise, and sustainable breeding approaches. These insights aim to enhance aquaculture productivity while supporting long-term ecological balance and industry sustainability.
1. Introduction
The large yellow croaker (Larimichthys crocea), commonly known as the Chinese large yellow croaker, belongs to the order Perciformes, family Sciaenidae, and genus Larimichthys [1]. This species is named for its golden-yellow luster along the lateral body surface and is one of the most important marine aquaculture species in China. Large yellow croaker is highly favored by consumers for its tender texture, delicious flavor, and richness in protein and trace elements [1]. It is widely used in high-end catering markets both domestically and internationally, with stable and continuously growing market demand. With the continuous advancement of aquaculture technology, the large yellow croaker has emerged as a species with significant economic potential in the field of aquaculture [2]. As a warm-water marine fish, the large yellow croaker primarily inhabits sandy-bottomed shallow coastal waters. Its optimal temperature range is 18–25 °C [3]. The species exhibits schooling behavior and a relatively fast growth rate. Spawning typically occurs from April to June and again from September to October each year [3]. Female croakers produce a large number of eggs and demonstrate strong reproductive capabilities. Although the species is highly adaptable to environmental conditions, it has relatively high requirements for water quality. Proper water quality management and scientifically formulated feeding strategies are essential for healthy growth.
With the decline of natural fishery resources, artificial aquaculture has become the main method for supplying large yellow croaker [4]. Within the aquaculture process, the selective breeding of seedlings (juvenile fish) is a critical step that directly affects survival rate, growth performance, disease resistance, and overall farming efficiency [5]. High-quality seedlings should originate from healthy broodstock with favorable genetic backgrounds, exhibiting strong disease resistance and environmental adaptability. They should be capable of rapid growth and high survival rates, thereby improving feed conversion efficiency, shortening the culture cycle, reducing costs, and increasing economic returns. In contrast, low-quality seedlings tend to be more susceptible to disease, show slower growth, and significantly increase farming risks and economic burdens.
Seedling selection is not only vital for economic outcomes but also plays a critical role in ensuring population health and ecological balance. Scientific breeding practices should enhance productive traits while maintaining genetic diversity to prevent genetic degradation and declining adaptability. Preserving genetic diversity helps strengthen the population’s immunity and adaptability, while also reducing the risk of recessive harmful mutations caused by inbreeding. Under ecological aquaculture models, high-quality seedlings should demonstrate superior environmental adaptability and feed conversion efficiency, which can effectively reduce pollution and eutrophication in aquatic systems. This, in turn, promotes environmentally friendly and sustainable development in aquaculture [6]. Therefore, selective breeding of seedlings is not only key to improving farming profitability but also forms the foundation for ecological aquaculture and the sustainable development of the industry [7].
To address these needs, the primary objective of this review is to summarize the current practices, challenges, and research progress in the selective breeding of Larimichthys crocea seedlings. The secondary objectives include: (1) analyzing the effects of genetic, physiological, and environmental factors on seedling quality and growth performance; (2) exploring the integration of intelligent technologies such as computer vision, artificial intelligence, and genomic tools in seedling selection; and (3) proposing future directions for sustainable and intelligent breeding strategies.
2. Selective Breeding Strategies for Large Yellow Croaker Seedlings
The selective breeding of large yellow croaker (Larimichthys crocea) seedlings can be systematically optimized across multiple dimensions, including morphological trait selection, genetic diversity assessment, growth performance evaluation, environmental adaptability analysis, and disease resistance screening [8]. Through integrated breeding approaches, favorable traits in the germplasm resources of large yellow croaker have been significantly improved, enhancing production performance and stress resistance during aquaculture, and thus providing a solid technical foundation for efficient and sustainable marine aquaculture.
2.1. Morphological Trait Selection
Large yellow croaker populations from different geographic regions exhibit significant morphological diversity, particularly in external traits such as body length, body depth, and caudal peduncle height. These traits offer important phenotypic references for seedling selection [9]. Currently, morphological selection in large yellow croaker mainly combines individual selection and family-based breeding to identify healthy individuals with strong growth potential, thereby optimizing the overall growth performance of the population [9]. Building on traditional selection methods, recent studies have introduced modern technologies such as image analysis and geometric morphometrics. Computer vision measurement systems have been applied to quantitatively analyze body shape traits in large yellow croaker, significantly improving the objectivity and precision of morphological assessments (Figure 1) [10,11]. With the continuous advancement of molecular breeding technologies, researchers have begun exploring the association between molecular markers and morphological traits to identify key genetic loci governing body shape, enabling marker-assisted precision breeding. In practical breeding applications, the “Minyou No. 1” strain of large yellow croaker was developed jointly by Jimei University and the Ningde Aquatic Technology Extension Station. This strain exhibits a body shape similar to wild types, yellowish coloration, and favorable commercial traits [12]. Similarly, “Donghai No. 1” was developed by the College of Marine Sciences, Ningbo University, using wild populations from the Daikuyang waters. This strain has been bred through long-term mass selection and displays superior body shape and golden coloration [13]. Furthermore, YU et al. [14] developed an automated morphological parameter detection system that integrates machine vision with weighing sensors, enabling simultaneous measurement of body length, body weight, and body width with high accuracy, thus providing technical support for efficient morphological evaluation. CHEN et al. [15] using a combination of linkage analysis and association analysis, identified quantitative trait loci (QTL) associated with body weight, body length, body height, and caudal peduncle length in the large yellow croaker. Their findings revealed the genetic basis of morphological traits and provided a theoretical foundation for molecular marker development and functional gene studies.
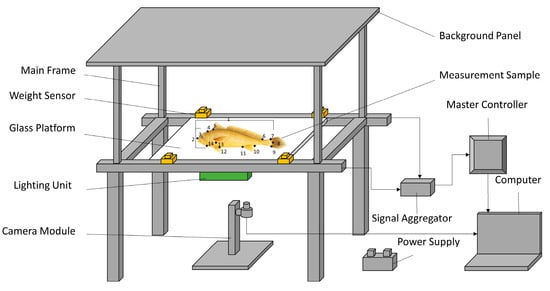
Figure 1.
Computer Vision Measurement System for Morphological Parameters of Croaker [10,11]. Notes:1. body length, 2. body height, 3. muzzle end, 4. upper edge of gill cover, 5. anterior end of dorsal fin base, 6. posterior end of dorsal fin base, 7. upper end of caudal fin base, 8. point of intersection of caudal fin base and lateral line, 9. lower end of caudal fin base, 10. posterior end of anal fin base, 11. anterior end of anal fin base, 12. left ventral fin base, 13. left pectoral fin base, 14. lower edge of the gill cover.
To address these limitations, we propose integrating computer vision-based morphometric analysis with genomic tools as a complementary pre-screening stage. This strategy can improve both efficiency and objectivity in seedling selection, particularly under intensive aquaculture conditions. Relying solely on these traits for germplasm selection may not accurately reflect the true genetic background of individuals, resulting in relatively low efficiency in genetic improvement. Therefore, in modern breeding practices, morphological selection should be integrated with molecular marker-assisted selection and other advanced techniques to enhance the precision and efficiency of breeding.
2.2. Growth Performance Selection
The selective breeding of large yellow croaker (Larimichthys crocea) for growth performance primarily employs a combined strategy of individual selection and family-based selection. By establishing systematic family populations, key growth traits such as body weight, body length, and daily weight gain are quantitatively evaluated to identify and retain superior individuals with outstanding growth potential [16]. Particularly, using wild-caught populations as the base for breeding helps maintain genetic diversity while promoting the continuous accumulation and genetic improvement of favorable growth traits [17]. Several studies have systematically estimated the heritability of growth-related traits in the large yellow croaker, with findings indicating moderate to high heritability for these traits. This provides a solid theoretical and practical foundation for subsequent genetic improvement efforts [18]. In recent years, the rapid development of molecular biotechnology has led to the increasingly widespread application of molecular breeding techniques in the selection for growth performance, serving as a valuable supplement to traditional phenotypic selection. Genome editing (GE) technologies have also shown early promise in the large yellow croaker. Researchers have successfully induced targeted gene mutations by microinjecting Cas9 messenger RNA (mRNA) and its corresponding single-guide RNA (sgRNA) from the CRISPR/Cas9 system, demonstrating potential advantages in accelerating individual growth and enhancing disease resistance [19]. In practical breeding, “Donghai No. 1,” a representative strain derived from wild populations in the Daikuyang sea area, was developed through more than a decade of systematic selection. Experimental results confirm that under identical farming conditions, this strain exhibits significantly better growth performance compared to standard commercial seedlings [20]. In the field of marker-assisted selection (MAS), ZHANG et al. [21] identified significant correlations between mutations in the coding region of the FST gene and growth traits in large yellow croaker using single-nucleotide polymorphism (SNP) screening, providing molecular evidence for developing candidate markers. SUI [22] conducted genetic evaluations of growth traits using microsatellite markers. Although the estimated heritability was relatively low (0.09–0.19), high genetic correlations among traits were observed, and key markers such as LYC0088 and LYC0143, which are closely associated with growth traits, were identified—offering theoretical support for practical breeding efforts.
Growth performance selection is of great significance for improving aquaculture profitability and promoting large-scale production. However, growth traits are highly affected by temperature, salinity, and density. Therefore, precise environmental control is essential during selection [23]. Moreover, due to the long breeding cycles and high financial and technical investments required, improving selection efficiency and achieving multi-technology integration remain key challenges to be addressed in future research and practice.
2.3. Genetic Diversity Selection
Genetic diversity forms the foundation for the genetic improvement and sustainable breeding of the large yellow croaker (Larimichthys crocea), playing a key role in maintaining population vitality, enhancing environmental adaptability, and improving stress resistance. In recent years, with the rapid development of the large yellow croaker aquaculture industry, the population has increasingly relied on artificial breeding systems, leading to a significant decline in genetic diversity, an increase in inbreeding levels, and adverse effects on economically important traits such as growth performance and disease resistance [24]. Multiple studies have shown that wild populations of large yellow croaker generally exhibit high levels of genetic diversity, while farmed populations are prone to allele loss, a decrease in effective population size, and reduced genetic differentiation, indicating that their genetic foundation is becoming increasingly limited [25]. To mitigate these issues, researchers have introduced wild populations into breeding programs through hybridization or backcross strategies, which have effectively restored genetic diversity in farmed populations, thereby enhancing the stability and adaptability of the genetic foundation [26]. With the continuous advancement of molecular marker technologies, molecular-assisted monitoring and evaluation techniques have become important tools for managing genetic diversity. Among these, Amplified Fragment Length Polymorphism (AFLP) and microsatellite markers are widely used in the analysis of genetic diversity in the large yellow croaker. For example, HOU et al. [27] used AFLP technology to evaluate the genetic diversity of the F4 generation of the “Donghai No. 1” population, finding that the proportion of polymorphic loci was 39.67%, the Nei’s gene diversity index was 0.1399, and the Shannon index was 0.2099. These results were similar to those of the original parent population, indicating that scientific breeding strategies can effectively maintain genetic diversity within the population. ZHENG et al. [28] used microsatellite markers to analyze the genetic structure of small yellow croaker populations along China’s coastline, revealing high genetic diversity and low genetic differentiation, providing theoretical support for the conservation and utilization of large yellow croaker germplasm resources.
In ongoing breeding practices, it is essential to scientifically avoid the genetic bottleneck effects caused by excessive inbreeding and strong selection pressure. Regular monitoring of population genetic structure and diversity is needed to ensure the genetic stability and potential for genetic improvement within the population. Scientifically and rationally managing genetic diversity not only helps optimize productive traits and reduce the risk of inbreeding depression but also strongly supports the long-term sustainable development of the large yellow croaker industry.
2.4. Disease Resistance Selection
Disease resistance selection has become a key strategy for improving the aquaculture efficiency of large yellow croaker (Larimichthys crocea) and ensuring the sustainable development of the industry, receiving widespread attention in recent years. Due to the constraints of high-density farming practices, disease issues have become increasingly severe, with pathogens spreading rapidly and exhibiting high mortality rates, which severely restrict the stable development of the industry. Therefore, improving the disease resistance of large yellow croaker populations has become one of the primary focuses of current breeding efforts [29]. At present, genetic improvement of disease resistance traits in large yellow croaker is mainly based on traditional phenotypic selection, with the gradual incorporation of modern breeding technologies such as marker-assisted selection (MAS) and genomic selection (GS) to improve the efficiency and precision of disease resistance breeding [30]. With the development of functional genomics and high-throughput sequencing technologies, theoretical support for understanding the genetic basis of complex disease resistance traits has been provided. Researchers have identified multiple key genetic loci associated with disease resistance through quantitative trait loci (QTL) mapping and genome-wide association studies (GWASs), and have combined single-nucleotide polymorphism (SNP) and microsatellite markers to screen for disease-resistant individuals [31]. Additionally, genetic databases constructed from large-scale whole-genome resequencing data have provided important data support for the development of breeding chips, significantly improving trait identification and selection efficiency. Currently, disease resistance assessments for major pathogens such as Vibrio alginolyticus and Iridovirus have been widely applied in breeding practices, and related functional gene research has made significant progress. WEI et al. [32] studied the autophagy-related gene ATG10 in large yellow croaker, discovering that its expression was significantly upregulated under viral infection stress, and its overexpression in vitro effectively inhibited viral replication, indicating its important role in antiviral immune responses. Additionally, FU et al. [33] identified Bacillus amyloliquefaciens NB Lm36, which exhibited broad-spectrum antibacterial activity. This strain showed significant inhibitory effects against multiple aquatic pathogens and was non-toxic to the large yellow croaker, indicating its potential as a biocontrol agent. In the application of disease resistance breeding, the “Ningxin” series breeding chips developed by Xu Peng’s team at Xiamen University integrate molecular markers for multiple traits, and based on this, the “Ningkang No. 1” strain, with significantly enhanced disease resistance, was successfully bred. This provides valuable germplasm resources and efficient technical means for disease resistance breeding of large yellow croaker [34], with genomics-assisted breeding technologies offering multiple approaches to cultivating disease-resistant fry (Figure 2).
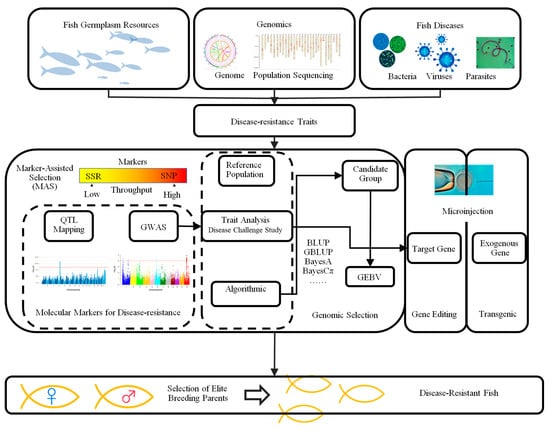
Figure 2.
Workflow of genomics-assisted breeding for disease-resistant fish [35,36]. Notes: Collection of target fish germplasm resources for genomic and population sequencing to identify bacterial/viral/parasitic disease resistance traits. Process and key elements of genomics-assisted breeding technology, including marker-assisted selection, genomic selection, genome editing and transgenesis. Selection of optimal parents based on the results of the analyses and selection of disease-resistant varieties.
Although significant progress has been made in disease resistance breeding for the large yellow croaker, several challenges remain. These include the diversity and variability of pathogen spectra, the complex genetic basis of disease resistance traits, and gene-environment interactions (G × E) [29]. Future research should focus on further optimizing genomic selection models, exploring core functional genes related to pathogen recognition, signal transduction, and immune regulation, and actively investigating the potential application of gene editing technologies such as CRISPR/Cas9 in precision breeding, thereby accelerating the development of a more efficient and precise disease resistance selection system for large yellow croaker.
2.5. Environmental Adaptability Selection
Environmental adaptability is a key indicator for evaluating the overall performance of aquaculture species, directly influencing their survival rate and production efficiency under varying farming conditions. It is of great significance for achieving the stability and sustainable development of large yellow croaker aquaculture. This species is highly sensitive to changes in environmental factors such as water quality, temperature, salinity, and water flow speed. Extreme weather events or high-density farming conditions can lead to stress responses, triggering disease outbreaks, which become major bottlenecks to the industrialization of the species [37]. Currently, research on improving the stress resistance of large yellow croaker mainly focuses on the genetic improvement of key traits such as low-oxygen tolerance, high-temperature tolerance, and disease resistance (Table 1). Some studies have constructed systematic family groups and evaluated survival rates, feeding behavior, and stress-related physiological parameters under different environmental conditions to identify individuals with strong environmental adaptability. For example, the bred variety ‘Donghai No. 1’ exhibits significant advantages in euryhalinity, eurythermy, and cold tolerance, demonstrating markedly enhanced environmental adaptability [13]. ZHANG et al. [38] used a self-designed Annular experimental flume system to screen large yellow croaker for resistance to water flow, finding that individuals with strong flow resistance had more developed muscle fiber structures and better antioxidant capacity, with upregulated expression levels of stress resistance-related genes. Additionally, CHEN et al. [39] employed Specific Length Amplified Fragment Sequencing (SLAF-seq) for genome-wide association studies (GWASs) and identified 38 single-nucleotide polymorphism (SNP) loci significantly related to high-temperature tolerance and 26 key genes involved in transcriptional regulation, metabolic regulation, and immune function, providing a theoretical basis for marker-assisted selection for high-temperature tolerance in large yellow croaker.
As previously discussed, the growth and physiological performance of large yellow croakers are influenced by multiple environmental factors. Here, we focus on how these parameters affect broodstock selection criteria under variable farming conditions [40,41,42,43]. Therefore, systematic environmental adaptability screening helps enhance their physiological stability and production performance under various farming models and environmental stress conditions [44]. Individuals with stronger adaptability are able to maintain basic metabolic functions and reduce growth losses during short-term environmental changes. These findings suggest that gene–environment interactions (G × E) significantly influence the expression of key aquaculture traits. Selection under controlled laboratory conditions may not always predict performance in complex real-world farming environments. Therefore, integrating environmental adaptability data into breeding models is crucial for developing resilient strains.

Table 1.
Molecular markers for tolerance traits in the yellow croaker.
Table 1.
Molecular markers for tolerance traits in the yellow croaker.
| Trait | Species | Marker Type | Marker Number | Marker/Gene | |
|---|---|---|---|---|---|
| temperature tolerance | low temperature tolerance | Larimichthys crocea [45,46,47] | SSR | 1 | LYC002112bp |
| SSR | 1 | LYC0015 | |||
| SSR | 1 | KPC43 | |||
| high temperature tolerance | Larimichthys crocea [40,48] | SSR | 3 | LYC0148, LYC0200, LYC0435 | |
| SNP | 38 | ||||
| Oxygen tolerance | hypoxia-tolerance | Larimichthys crocea [49] | SNP | 4 | chr13:2535902, chr15:11774198, chr18:20360178, chr24:9514192 |
3. Application of Emerging Technologies in Seedling Selection for Large Yellow Croaker
The integrated evaluation system presented in this review connects phenotypic assessment, such as morphology and growth, with genotypic confirmation through MAS and GWAS. This ensures that each selected individual meets both physical and genetic standards, enabling more targeted and sustainable breeding decisions.
Seedling selection is a critical step in enhancing the aquaculture efficiency and production stability of Larimichthys crocea. With the rapid development of aquaculture technology, traditional phenotypic selection methods are no longer sufficient to meet the demands of precise and efficient screening of genetic resources in modern, intensive aquaculture practices. In recent years, a series of modern technological methods, including genomic selection (GS), intelligent monitoring, environmental control, smart feeding, and disease prevention, have been gradually introduced into the breeding system for the large yellow croaker. These technologies have significantly improved the efficiency and accuracy of seedling selection, providing strong support for achieving high-yield, high-efficiency aquaculture models under variable environmental conditions.
3.1. Molecular Breeding Technology
Molecular breeding technology has become an important tool for the genetic improvement of the large yellow croaker. Common methods include Restriction-site Associated DNA Sequencing (RAD-seq), genetic linkage map construction, quantitative trait loci (QTL) mapping, genome-wide association studies (GWASs), whole genome resequencing (WGR), single nucleotide polymorphism (SNP) chips, genomic selection (GS), and genome editing (GE). The combined application of these technologies has significantly enhanced the efficiency and accuracy of breeding large yellow croaker (Table 2).

Table 2.
Characteristics of different genomics-assisted nursery technologies.
With the continuous development of functional genomics and molecular breeding technologies, genome-wide association studies (GWASs) have become one of the core methods for genetic analysis of complex economic traits in aquatic animals. This technology identifies single nucleotide polymorphisms (SNPs) and candidate genes associated with traits such as growth and disease resistance across the entire genome, providing theoretical and molecular foundations for the selection of superior breeding parents and molecular-assisted breeding [58]. XIAO et al. [59] constructed the first physical genome map of the large yellow croaker (total length approximately 727 Mb) based on high-throughput sequencing, laying an important foundation for the discovery of genes related to environmental adaptability and subsequent molecular breeding research. At the same time, the construction of genetic linkage maps and the identification of quantitative trait loci (QTL) have also provided critical support for genetic analysis of important traits. Several studies have constructed genetic linkage maps for the large yellow croaker, identifying multiple QTLs related to growth, disease resistance, and other traits [60]. The map constructed by AO et al. [61] has a total length of 5451.3 cM, with an average marker interval of 0.54 cM, demonstrating high map density and resolution. Simplified genome sequencing technology, RAD-seq (Restriction-site Associated DNA Sequencing), which is cost-effective and suitable for large-scale SNP mining, has been widely applied in the large yellow croaker. Studies show the existence of two major genetic lineages and the identification of multiple SNPs related to growth traits. LIU et al. [62] analyzed 120 large yellow croakers from five cultured and wild populations using RAD-seq, detecting 7161 high-quality SNP markers. whole genome resequencing (WGR) enables comprehensive analysis of genomic variation between individuals, revealing differences in genetic structures of populations from different waters and genetic characteristics caused by domestication. ZHANG et al. [63] compared the effects of 0.5× and 8× sequencing depths in large yellow croaker and found that low-coverage whole genome sequencing (LcWGS) can also be used for genomic selection (GS), showing high practicality. The development and application of SNP chips enable high-throughput genotyping, and the SNP chip for the large yellow croaker has a high proportion of polymorphic sites. ZHOU et al. [64] performed genotyping on 96 large yellow croakers from five populations using the “Ningxin No. 1” SNP chip, finding that 83.38% of the SNPs were polymorphic, with the cross-population effective range ranging from 26.68% to 56.23%. This technology can predict the genomic breeding value (GEBV) of individuals based on whole-genome markers, improving genetic evaluation accuracy while shortening the breeding cycle. QIU et al. [65] estimated the genetic parameters of nine quantitative traits using SNP data, showing that there was a high genetic correlation between some morphological traits. Molecular-assisted breeding has progressed rapidly. A GWAS based on ddRAD sequencing in 220 individuals identified 13 SNPs across 8 linkage groups associated with growth and body-shape traits. Additionally, genomic selection for hypoxia tolerance and marker-assisted selection (MAS) leveraging SNP and SSR markers have been successfully implemented [66]. Although Genome Editing (GE) technology has not yet been applied in large yellow croaker, it has been used for precise trait improvement in various fish species (Figure 3) [57,58,67,68].
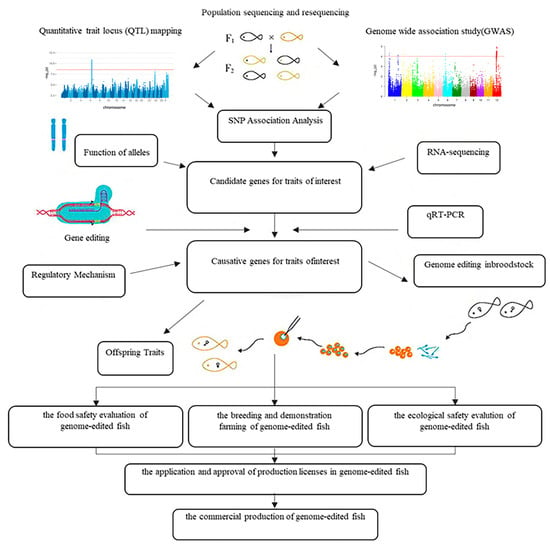
Figure 3.
Methods applied in gene editing in fish [58,67,68]. Notes:The top-left and top-right insets show Manhattan plots from QTL and GWAS analyses. Alternating colors indicate different chromosomes for visual clarity. Each point represents a SNP, with the y-axis showing –log10(p-value) of association with the trait. The red horizontal line denotes the genome-wide significance threshold; points above it are significantly associated loci.
Genomic data can be used for early screening of high-quality seedlings and, combined with environmental factors, can optimize breeding strategies to improve selection accuracy [69]. However, genomic breeding technology still relies on professional support and equipment investment. Due to technical barriers and cost limitations, the application of this technology in aquaculture breeding remains at an early stage, and further technological optimization and resource integration are urgently needed to achieve its widespread application at the industrial level.
3.2. Intelligent Monitoring and Data Analysis Technology
Intelligent monitoring and data analysis technology provides real-time and comprehensive data support for the selection of high-quality Larimichthys crocea seedlings [70]. By relying on Internet of Things (IoT) sensors, video monitoring systems, and data processing platforms, aquaculture managers can fully monitor the growth dynamics, health status, and environmental parameters of the seedlings, providing data-based evidence and decision-making support for scientific selection (Figure 4).
Video monitoring, combined with image recognition algorithms, enables real-time identification and recording of external features, swimming behaviors, and growth status of the fish, aiding in the selection of individuals with superior growth performance [71]. At the same time, IoT technology, by deploying multi-parameter sensors for dissolved oxygen, temperature, pH, ammonia nitrogen, and other factors, ensures continuous monitoring of the aquaculture water environment, providing optimal growth conditions for the seedlings [72]. By integrating big data and artificial intelligence technologies, growth data and environmental adaptability can be modeled and analyzed, assisting in predicting growth trends and health status, thus improving the selection efficiency [73,74]. ZHOU et al. [75] monitored water quality factors, meteorological factors, and biological factors, and established a disease forecasting model for cage-cultured Larimichthys crocea based on multivariate statistical analysis, gray theory, and BP neural networks. The model identified key environmental factors, such as water temperature, transparency, and suspended solids, with a prediction accuracy of up to 81.53%, providing scientific evidence for early warning and prevention of diseases in Larimichthys crocea. WANG et al. [71] proposed an abnormal behavior monitoring method for Larimichthys crocea in recirculating aquaculture systems based on computer vision. Using the improved YOLOX-S algorithm, abnormal behaviors were detected in real time, and the Bytetrack algorithm was used for tracking, achieving a detection accuracy of 98.4% and tracking accuracy over 95%, effectively improving aquaculture monitoring efficiency. ZHOU et al. [70] introduced an automated method based on computer image recognition technology for diagnosing Ichthyophthirius multifiliis disease in industrial marine aquaculture of Larimichthys crocea. The model was trained using transfer learning with the YOLOv3 algorithm, achieving an accuracy of 92% for disease recognition. Notably, the “Conson No. 1” smart aquaculture vessel—equipped with automated waste removal, circulating seawater to simulate natural currents, noise-reduction systems, and IoT-based environment monitoring—has been certified by the Aquaculture Stewardship Council. It sustainably produces approximately 3700 tonnes of large yellow croaker annually [76].
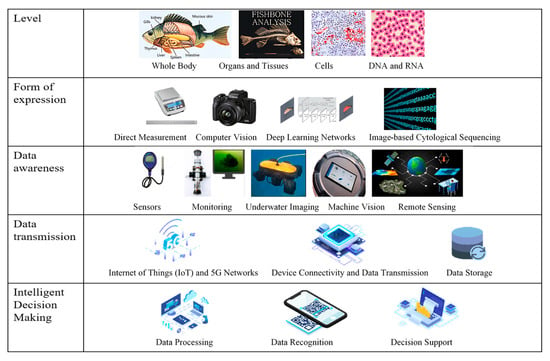
Figure 4.
Overall framework of intelligent monitoring technology and data analysis system [77,78].
Intelligent technology provides real-time, comprehensive monitoring and data analysis, reducing human operational errors, improving the efficiency and accuracy of selection, and enhancing the level of automation in aquaculture management [79]. However, the high capital investment, technical support requirements, and long-term maintenance costs remain significant challenges for its widespread application [80].
3.3. Environmental Control and Optimization Technologies
Environmental factors are crucial for the selection of high-quality seed for large yellow croaker, influencing their growth, health, and breeding efficiency (Table 3). Large yellow croaker seed farming is typically divided into two stages: the early fry stage, which primarily uses factory-based farming methods, and the later juvenile fish stage, which transitions to offshore cage farming. The environmental requirements differ significantly between these stages. In the early fry stage, factory-based farming, environmental factors such as water quality, stocking density, and light intensity significantly impact the survival rate and growth rate of the seed. Key water quality indicators, such as temperature, dissolved oxygen, pH, ammonia nitrogen concentration, and salinity, directly affect the fish’s immune function and metabolic activities [81,82,83]. A stable water environment helps alleviate stress responses and enhance disease resistance [23,84]. Controlling stocking density is also crucial; appropriate density reduces competition and stress, while overcrowding can lead to water quality deterioration and pathogen spread [85]. Additionally, light intensity and photoperiod influence feeding behavior and endocrine regulation. Suitable lighting can enhance the seed’s immunity and promote growth [86]. In recent years, intelligent environmental control technologies have been gradually applied to the early stages of large yellow croaker farming. KOU et al. [87] developed a smart aquaculture monitoring system based on NB-IoT technology, enabling real-time collection of water quality parameters and remote management, providing technical support for precise environmental control. FAN et al. [88] utilized computer vision technology to achieve real-time monitoring of the growth process of the large yellow croaker, which can guide critical operations such as scientific feeding, density regulation, and growth assessment. SONG et al. [89] researched the LED lighting design for aquaculture workshops based on the visual physiological structure of aquatic animals and their light sensitivity, proposing lighting design principles and cost-reduction recommendations. In the later juvenile fish stage, the farming model mainly shifts to offshore cage farming, with farming sites typically located in nearshore areas with relatively calm waters. Daily management requires regular cage operations based on the fish’s growth status, as well as continuous monitoring of key environmental factors such as water temperature, salinity, water flow, and transparency. The feeding, schooling behavior, and disease occurrence of the fish are also observed in real time to ensure timely intervention. In 2016, Zhejiang Ocean University established a 100,000 m3 coastal farming facility based on the “suspended large cage engineering technology” on the southern side of Taohua Island in Zhoushan, significantly improving the management of overwintering, automatic feeding, and efficient harvesting, and promoting the large-scale and standardized development of farming models [90]. Regarding site selection research, ZHANG et al. [91] constructed a site selection evaluation model for deep-water cage farming based on the Analytic Hierarchy Process (AHP). They found that water quality was the most critical natural factor, infrastructure was the most important social factor, and marine functional zoning was the main limiting factor, providing a theoretical basis for scientific site selection.

Table 3.
Influence of environmental factors on the selection of large yellow croaker (Larimichthys crocea) broodstock.
In summary, the intelligent control of environmental factors provides strong support for improving the efficiency and health of seed farming. Current environmental monitoring and control systems cover various aspects, including water quality regulation, temperature control management, and growth status monitoring. However, these technologies still face challenges in their promotion, such as high equipment costs, insufficient system stability, and high technical barriers for operators. It is urgently needed to integrate sensor technologies, optimize control algorithms, and establish training mechanisms for operators to further enhance the system’s practicality and environmental adaptability.
3.4. Intelligent Feeding Technology
Feed, as a critical input in aquaculture, directly affects the growth rate and disease resistance of large yellow croaker fry, with its type, nutritional composition, and particularly the content of animal protein being key factors [97,98]. To improve feed utilization efficiency and reduce farming costs, intelligent feeding technologies are gradually becoming an important development direction in modern aquaculture management. Intelligent feeding systems can dynamically adjust the feed amount and frequency based on real-time monitoring data, including fish feeding behavior and water quality parameters, thereby optimizing feed supply, reducing waste, and enhancing feed conversion efficiency [99,100]. Furthermore, culturists can adjust feed formulations based on real-time monitoring data from intelligent systems, tailoring nutritional profiles according to the growth stage, health status, and environmental conditions of seedlings to ensure adequate and balanced nutritional support [101]. In recent years, the application of artificial intelligence (AI) technology in intelligent feeding has made significant progress. ZHANG et al. [102] achieved accurate identification and tracking of large yellow croaker behavior based on an optimized YOLOv5 object detection algorithm, providing an effective basis for scientific feeding. TIAN et al. [103] tudied the spatial distribution characteristics of large yellow croaker in cage farming areas using a small fish-finding unmanned boat and found that they were mainly concentrated in the middle and lower water layers, where the water flow was slower, rocks were abundant, and light was weaker, providing data support for spatially precise feeding. REN et al. [104] studied the vocalization characteristics of large yellow croaker in different behavioral states and found that the fish emitted sounds with a frequency of approximately 800 Hz and a simple single pulse when foraging, while their spawning behavior was characterized by double or triple pulses with longer intervals between pulses. This finding provides a possible basis for acoustic recognition-based feeding timing judgment.
Despite the significant advantages of intelligent feeding systems in improving management efficiency and feed conversion rates, there are still several challenges, such as high initial investment costs for equipment, high technical skill requirements for operators, and potential biases in data collection and processing. If the system control is inadequate, it could result in either insufficient or excessive feeding, thereby affecting the health and growth of the fry [105]. Therefore, future efforts should focus on improving sensor accuracy and algorithm response efficiency, promoting the integration of multi-source information such as visual recognition, acoustic monitoring, and environmental sensing, enhancing system stability and environmental adaptability, to achieve more precise and efficient intelligent feeding management [106].
3.5. Disease Prevention and Immunization Technologies
In aquaculture, disease prevention is a critical aspect of ensuring farming profitability, especially in high-density farming environments, where the disease resistance of fry directly determines their survival rate and economic returns. Vaccination, as an effective means of enhancing fish immunity, has become an essential component in the selection of fry [107,108]. Currently, the development of vaccines for the large yellow croaker is advancing towards enhancing the duration of immunity and expanding the spectrum of pathogens covered, significantly improving the fry’s resistance to major pathogens [109,110]. At the same time, immunostimulants can activate both the innate and adaptive immune responses in fish, further improving the health of the fry. By combining genetic testing and rapid pathogen diagnostic technologies, early pathogen identification and precise intervention can be achieved, significantly reducing farming risks [111,112]. In recent years, significant progress has been made in large yellow croaker vaccine research. A study demonstrated that dietary Astragalus polysaccharides (APS) significantly enhance the immune efficacy of a formalin-inactivated Pseudomonas plecoglossicida vaccine in large yellow croaker (Larimichthys crocea). After 14 days of APS feeding, vaccinated fish showed higher survival rates post-challenge, elevated serum IgM titers, increased antioxidant enzyme activities (T-AOC, SOD), and lower pro-inflammatory cytokines (IL-1β, IL-6), alongside upregulated anti-inflammatory (IL-10) and T-cell cytokines (IFN-γ, IL-4/13A, IL-4/13B) [113]. ZHNANG et al. [114] developed an oral nano-vaccine for Vibrio alginolyticus based on mesoporous silica nanoparticles (MSN) as a carrier and evaluated its antigen absorption and immune response effects in the intestines. The study showed that the vaccine induced time-dependent expression of immune factors such as interferon-gamma (IFN-γ) and interleukin-1β (IL-1β) in multiple tissues, verifying its feasibility as an oral vaccine. LI et al. [115] developed a subunit vaccine based on recombinant dihydrolipoamide dehydrogenase (rDLD), and the results indicated that rDLD effectively activated both specific and non-specific immune responses in the large yellow croaker, significantly enhancing its resistance to infections caused by Vibrio alginolyticus and Vibrio parahaemolyticus. HE et al. [116] developed an attenuated mutant strain of Pseudomonas plecoglossicida (ΔOmpRΔrpoS), which serves as a candidate live attenuated vaccine (LAV). Experimental results showed that this vaccine strain significantly induced immune responses and improved the resistance of large yellow croaker to pathogen infections without affecting growth performance, demonstrating strong application prospects. These advancements underscore the growing diversity of immunization strategies in aquaculture (Table 4).

Table 4.
Advantages and disadvantages of different vaccination methods are common to the fish farming industry.
Although current vaccines and immunization technologies have significantly improved the immunity and health status of fry, reduced disease incidence, and ensured stable aquaculture profitability, they still face challenges in practical implementation, such as high costs and limited vaccine adaptability. In the future, further integration of precision farming management, environmental control, and multi-omics data should be pursued to optimize vaccine usage strategies, enhancing both the effectiveness of disease prevention and the economic feasibility.
4. Key Issues in the Selection of Large Yellow Croaker Fry
The selection of large yellow croaker fry plays a crucial role in improving aquaculture profitability and ensuring farming success. However, in practice, some farmers have misconceptions during the fry selection process, which leads to poor selection outcomes and can even negatively impact the overall profitability of the aquaculture industry (Table 5).

Table 5.
Misunderstandings in the preferential selection of large yellow croaker broodstock.
4.1. Over-Reliance on Single Trait Selection
In the current breeding practices for large yellow croaker fry, some farmers tend to focus solely on phenotypic traits such as growth rate and body shape, while neglecting the synergistic optimization of key traits such as disease resistance, environmental adaptability, and feed utilization efficiency [121]. Although single-trait selection can enhance economic benefits in the short term, it may lead to a degradation of the fry’s overall traits in the long term, particularly in environments with significant fluctuations in temperature, salinity, or dissolved oxygen. This could, in turn, reduce the overall stability of the aquaculture system. To improve fry suitability, both growth traits and stress resistance should be considered comprehensively, and a multi-trait evaluation system should be established to achieve dual improvements in fry quality and aquaculture profitability.
4.2. Neglecting Genetic Diversity and Inbreeding Risks
The genetic resources of large yellow croaker are relatively singular, and long-term inbreeding and directional selection can lead to a decline in genetic diversity, manifested by reduced adaptability, weakened disease resistance, and degraded reproductive performance [125]. In particular, in situations where there is insufficient parental stock, genetic bottlenecks can occur within the population. To mitigate this issue, molecular tools such as microsatellite markers and mitochondrial D-loop sequences can be used to genetically track and manage parental relationships. Additionally, introducing external superior genetic resources, implementing optimized mating strategies, and building a genetic diversity conservation system can help enhance population vitality and genetic stability, ensuring the long-term sustainability of fry selection efforts.
4.3. Ignoring the Impact of Environmental Factors
Although genetic traits play a decisive role in fry selection, environmental factors (such as water temperature, salinity, dissolved oxygen, ammonia concentration, etc.) also profoundly affect the phenotypic performance and health status of the fry. Some farmers overemphasize genetic potential while neglecting environmental regulation, often resulting in abnormal fry performance under high environmental stress, which can lead to high mortality rates and disease outbreaks. For example, the large yellow croaker is highly sensitive to low dissolved oxygen, and environmental fluctuations can induce stress responses, thus affecting survival rates and growth performance. Therefore, during the breeding process, environmental management should be improved simultaneously by using technologies such as real-time water quality monitoring systems and intelligent temperature control devices to stabilize farming conditions and fully realize the genetic potential of the fry.
4.4. Over-Reliance on Traditional Breeding Methods
Although modern molecular breeding technologies such as marker-assisted selection (MAS) and genomic selection (GS) have matured, traditional breeding methods, such as phenotypic selection and morphological evaluation, remain the mainstream approach in large yellow croaker fry breeding practices [121]. While these methods can improve certain observable traits, they cannot precisely improve recessive or low heritability traits (such as disease resistance), and they involve long breeding cycles and slow progress. Modern breeding technologies, through the integration of multi-omics data, can predict genetic potential early in the individual’s life, thus improving selection efficiency and shortening breeding cycles [126]. Therefore, accelerating the integration and application of molecular tools in breeding systems is a key direction for improving breeding levels.
4.5. Insufficient Attention to Disease Resistance Breeding
Disease resistance, as a core economic trait in aquaculture, has not received adequate attention in large yellow croaker fry selection processes. Some fast-growing fry are prone to pathogen infections in high-density farming environments, exposing issues such as poor disease resistance and low survival rates [127]. Particularly in conditions where water quality is unstable or pathogen load is high, the lack of systematic identification and utilization of disease-resistant genes can lead to significant economic losses. To improve fry disease resistance, genomic-assisted breeding, molecular marker screening, and the application of immunostimulants should be strengthened. Additionally, integrating comprehensive disease prevention measures such as vaccination and the use of immune modulators can effectively enhance the immune capabilities of fry populations and reduce the risk of disease outbreaks [128,129].
5. Problems and Development Trends
5.1. Problems and Challenges
5.1.1. High Technical Costs
Currently, the introduction of advanced technologies such as genomics, marker-assisted selection (MAS), and intelligent monitoring in the selection of large yellow croaker fry has significantly improved breeding efficiency and accuracy. However, these technologies also come with high technical thresholds and financial costs [47,130]. These technologies depend on high-precision instruments and professional talent support, requiring significant initial investment and high maintenance costs, which particularly pose a considerable economic burden for small and medium-sized farms. Moreover, most farming units are still in the early stages of mastering and applying these technologies, and the adoption rate remains low. Therefore, a key challenge in promoting industrial applications is how to reduce technical costs while ensuring the effectiveness of breeding, and to improve the accessibility and practicality of these technologies.
5.1.2. Uncontrollable Environmental Factors
Environmental factors significantly affect the growth and health of the large yellow croaker. Water temperature, salinity, dissolved oxygen, and water quality directly influence the growth performance and health status of the fry [93,131]. Although environmental control technologies, such as automated water quality management systems and intelligent temperature control devices, have made progress, practical production is still limited by factors such as climate change, regional water differences, and sudden pollution events, making it difficult to fully control the environment. Environmental fluctuations can not only affect the phenotypic expression of selected fry but also interfere with the stable release of their genetic potential [72,77]. Therefore, improving the fry’s adaptability to complex environmental conditions and developing breeding strategies suitable for different ecosystems are important directions for optimizing fry selection outcomes.
5.1.3. Risk of Loss of Genetic Diversity
Long-term inbreeding and single-trait selection may gradually narrow the genetic base of large yellow croaker populations, increasing the risks of genetic drift and inbreeding depression [125]. The loss of genetic diversity not only weakens the population’s resistance to diseases and environmental changes but may also lead to degradation in fry performance and reduced reproductive capacity. Particularly under high selection pressure, excessive focus on a few superior individuals can create a genetic bottleneck [46]. Therefore, while improving target traits, it is essential to strengthen the collection and evaluation of genetic resources and build a scientific genetic management system to ensure the long-term maintenance of genetic diversity and population health.
5.1.4. Limited Effectiveness of Disease Resistance Breeding
Large yellow croaker farming faces various pathogen threats, including viruses, bacteria, and parasites, with the high-density farming model further exacerbating the risk of disease transmission [64,132]. Although vaccination and immune enhancement measures have achieved preliminary results, the current disease resistance breeding still primarily relies on traditional genetic selection, and the analysis and utilization of key disease resistance genes are still in the early stages [133]. Molecular breeding techniques, such as marker-assisted selection (MAS) and genomic selection (GS), can improve screening efficiency, but their adoption is limited by technological complexity and implementation costs [134]. A current technical bottleneck in fry selection is how to further improve the accuracy of identifying disease resistance-related genes and develop cost-effective breeding processes.
5.1.5. Lack of Standardized Systems
At present, there is a lack of a systematic and standardized technical framework for large yellow croaker fry selection. Significant differences exist among different farming units and research institutions in terms of breeding processes, trait evaluation methods, and data standards [135,136]. Most breeding technologies are still at the laboratory stage and have not been scaled up for industry-wide application or unified operational standards. The absence of a standardized system not only limits the industrialization efficiency of these technologies but also affects the reproducibility and comparability of selection results. Establishing a comprehensive technical specification system that includes target trait definitions, evaluation index systems, data collection standards, and operational procedures is a key prerequisite for achieving the industrialization of large yellow croaker fry selection.
5.2. Future Prospects and Trends
5.2.1. Widespread Application of Genomics and Genome Editing Technologies
In the future, the selection of large yellow croaker fry will increasingly rely on the deep integration of genomics and genome editing (GE) technologies. With the advancement of genomic research, researchers can use whole-genome data to predict the growth potential, disease resistance, and environmental adaptability of fry at an early stage, enabling precise selection [137]. The combination of genomics and genome editing technologies can not only significantly improve breeding efficiency and accuracy but also effectively reduce the risk of genetic degeneration, providing molecular-level technical support for sustainable aquaculture [138,139].
5.2.2. Popularization of Intelligent Technologies and Precision Aquaculture
The continuous development of artificial intelligence (AI), the Internet of Things (IoT), and big data technologies is driving aquaculture into an era of intelligence and precision. In large yellow croaker fry selection, the integration of smart sensors and algorithmic models enables real-time monitoring and dynamic management of fry health, growth dynamics, and aquaculture environment parameters. Additionally, the widespread application of intelligent feeding systems, water quality control devices, and automatic early warning mechanisms will further enhance the efficiency and decision-making accuracy of the selection process. AI-driven multi-dimensional data analysis also provides key support for scientific breeding, driving the shift from experience-driven to data-driven farming models.
5.2.3. Application of Green and Sustainable Technologies
With the widespread adoption of environmental sustainability concepts, green aquaculture technologies are becoming an important direction in large yellow croaker breeding. By reducing feed waste, lowering antibiotic usage, and improving resource utilization efficiency, an environmentally friendly aquaculture system can be achieved [140]. The development and application of eco-friendly feed not only help alleviate water pollution but also contribute to improving the health levels and survival rates of fry. Combined with precise feeding systems and water environment control technologies, this can further optimize fry growth conditions and reduce energy consumption. Furthermore, natural immune regulation strategies based on immune stimulants and environmental adaptability screening are gradually replacing traditional chemical drugs, enhancing disease resistance and supporting the aquaculture industry’s transition toward a green, low-carbon, and sustainable direction.
5.2.4. Genetic Diversity Protection and Germplasm Resource Management
Genetic diversity is the foundation for the long-term stability and sustainable development of aquatic species. In large yellow croaker breeding, there will be an increased focus on the systematic management and genetic evaluation of germplasm resources in the future [125]. By establishing gene banks, constructing genetic lineages, and conducting molecular-level genetic diversity analysis, breeders can scientifically select breeding materials with desirable traits and adaptability, ensuring the richness and stability of the genetic background. Protecting genetic diversity not only helps enhance the population’s resistance to stress but also provides important genetic support for the long-term selection of fry.
5.2.5. Cross-Disciplinary Technological Integration Driving Innovative Development
The future of large yellow croaker fry selection will feature a trend of interdisciplinary and cross-technological field integration. The synergistic development of genomics and artificial intelligence, as well as the deep intersection of intelligent aquaculture and environmental science, will accelerate breeding efficiency improvements and breakthroughs in key technologies [68]. Through precision breeding strategies supported by big data, significant progress can be made in various dimensions, such as genetic improvement, environmental adaptability enhancement, and health trait optimization. Moreover, the collaborative application of ecology and bioengineering will provide feasible pathways for the development of low-carbon and efficient aquaculture models. This integration trend signals that future fry selection will not only optimize a single technology but will be a systematic breakthrough driven by multi-disciplinary integrated innovations [141,142].
Future studies should consider developing integrated platforms combining image-based phenotype screening, real-time AI decision-making, and molecular databases to enhance the precision and scalability of selective breeding in aquaculture.
6. Conclusions
With ongoing advancements in Larimichthys crocea fry selection technologies, future research must blend efficiency, precision, and sustainability. Cutting-edge tools—genomics, marker-assisted selection (MAS), intelligent monitoring, and environmental regulation—have established a robust foundation for genetic enhancement of the large yellow croaker. Genomic selection (GS) and genome editing (GE) are poised to accelerate gains in growth traits, disease resistance, and adaptability.
Green aquaculture innovations—including precision feeding, eco-friendly feed, and intelligent disease surveillance—can minimize resource wastes and environmental impacts, steering aquaculture toward a circular, sustainable paradigm. Early MAS successes in species like Japanese flounder and Pacific white shrimp demonstrate the feasibility of molecular breeding in marine systems, yet barriers remain for L. crocea, such as genotyping costs, infrastructure limitations, and lack of standardized protocols.
Protecting genetic diversity and managing germplasm strategically will be indispensable for long-term breeding resilience amidst climate and environmental stresses. The convergence of genomics, artificial intelligence (AI), bioinformatics, smart aquaculture, and environmental sciences is reshaping breeding practices.
In conclusion, optimizing L. crocea fry selection necessitates harmonizing technological innovation, ecological stewardship, and standardized operations. This holistic integration—bridging genetic gain with environmental responsibility—charts a course toward efficient, precise, and sustainable aquaculture. Future directions should prioritize integrating AI-based image analysis, molecular/genomic data, and decision-making platforms to enhance the accuracy, scalability, and real-world applicability of seedling selection systems.
Author Contributions
Conceptualization, X.H., S.Z., Y.W. and Z.W.; literature search and analysis, X.H., H.F., S.P. and S.Y.; writing—original draft preparation, X.H.; writing—review and editing, S.Z., Y.W. and Z.W.; visualization, X.H.; project administration, S.Z.; supervision, S.Z. and Y.W.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central Public-interest Scientific Institution Basal Research Fund, East China Sea Fisheries Research Institute (ECSFR), Chinese Academy of Fishery Sciences (CAFS) (NO. 2024TD04).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Wang, J.; Jing, Y. Larimichthys crocea (large yellow croaker): A bibliometric study. Heliyon 2024, 10, e37393. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ke, Q.-Z.; Su, Y.-Q.; Liu, J.-Q.; Zheng, W.-Q. Protection and utilization status and prospect of large yellow croaker (Larimichthys crocea) germplasm resources. Aquac. Fish. 2022, 46, 674–682. [Google Scholar]
- Gong, D.; Cui, X.; Song, M.; Xing, B.; Xu, P.; Tang, Y.; Yin, L. Behavior of large yellow croaker (Larimichthys crocea) in pen aquaculture as measured by meter-scale telemetry. Front. Mar. Sci. 2023, 10, 1177037. [Google Scholar] [CrossRef]
- Yuan, J.; Lin, H.; Wu, L.; Zhuang, X.; Ma, J.; Kang, B.; Ding, S. Resource status and effect of long-term stock enhancement of large yellow croaker in China. Front. Mar. Sci. 2021, 8, 743836. [Google Scholar] [CrossRef]
- Janssen, K. The Economic Optimization of Breeding Programs in Aquaculture. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Yao, J.-X.; Lin, H.-D.; Wu, L.-S.; Wu, L.-N.; Yuan, J.-G.; Ding, S.-X. Stability of population genetic structure in large yellow croaker (Larimichthys crocea): Insights from temporal, geographical factors, and artificial restocking processes. Ecol. Evol. 2024, 14, e70207. [Google Scholar] [CrossRef]
- Azra, M.N.; Okomoda, V.T.; Ikhwanuddin, M. Breeding technology as a tool for sustainable aquaculture production and ecosystem services. Front. Mar. Sci. 2022, 9, 679529. [Google Scholar] [CrossRef]
- Liu, H.; Fan, S.-J.; Xu, Q.-L.; Wang, X.; Zhang, Y.-L.; Chen, W.; Hu, Y.; Deng, X.-Y.; Liu, H.-Y.; Yang, C.-Z.; et al. Germplasm innovation of large yellow croaker and its research progress. Reprod. Breed. 2025, 5, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Guo, H.-Y.; Liu, B.-S.; Zhang, N.; Zhu, K.-C.; Zhang, D.-C. Analysis of morphological differences in five large yellow croaker (Larimichthys crocea) populations. Isr. J. Aquac.-Bamidgeh 2024, 76, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wang, J.-Y.; Xin, R.; Ke, Q.-Z.; Jiang, P.-X.; Zhou, T.; Xu, P. Application of computer vision in morphological and body weight measurements of large yellow croaker (Larimichthys crocea). J. Fish. China 2023, 47, 207–216. (In Chinese) [Google Scholar]
- Yu, X.-J.; Wu, X.-F.; Shen, W.-L. Pattern recognition method for the identification of Daiqu large yellow croaker based on computer vision. J. Zhejiang Univ. 2018, 44, 490–498. (In Chinese) [Google Scholar]
- Yu, X. “Minyou No. 1” Large Yellow Croaker Passed the Provincial Evaluation for Original and Fine Aquatic Seed. Mod. Fish. Inf. 2010, 25, 28. (In Chinese) [Google Scholar]
- Li, M. Large Yellow Croaker “Donghai No. 1”. Rural. Inf. 2016, 36. (In Chinese) [Google Scholar]
- Yu, X.-J.; Wu, X.-F.; Wang, J.-P.; Chen, L.; Wang, L. Rapid Detecting Method for Pseudosciaena crocea Morphological Parameters Based on the Machine Vision. J. Integr. Technol. 2014, 3, 45–51. (In Chinese) [Google Scholar]
- Chen, J.-W. QTL Analysis of Some Morphological Traits in the Large Yellow Croaker (Larimichthys crocea). Master’s Thesis, Jimei University, Xiamen, China, 2016. (In Chinese). [Google Scholar]
- Jiang, S.; Wang, J.-Y.; Yang, W.-L.; Kong, D.-W.; Yang, Q.-B.; Huang, J.-H.; Yang, Y.-D.; Zhou, F.-L. Evaluation of genetic parameters of growth traits in G2 selected generation of Penaeus monodon. Isr. J. Aquac.-Bamidgeh 2024, 76. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Han, K.-H.; Chen, S.-X.; Zhang, Y.; Zhou, S.-F.; Zhou, R.-F.; Luo, F. Realized Heritability and Growth of Offsprings of Wild Large Yellow Croaker Pseudosciaena crocea. Fish. Sci. 2016, 35, 204–209. (In Chinese) [Google Scholar]
- Dong, L.; Fang, M.; Wang, Z. Prediction of genomic breeding values using new computing strategies for the implementation of MixP. Sci. Rep. 2017, 7, 17200. [Google Scholar] [CrossRef]
- Yan, M.-Z.; Li, B.-J.; Wang, J.-Y.; Bai, Y.-L.; Ke, Q.-Z.; Zhou, T.; Xu, P. Disruption of mstn gene by CRISPR/Cas9 in large yellow croaker (Larimichthys crocea). Mar. Biotechnol. 2022, 24, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Q.-Y.; Zheng, Y.; Sun, W.-B.; Yang, L.; Song, X.-Y. Analysis of the Muscle Structure and Protein Composition of Different Varieties of Cultured Large Yellow Croaker (Larimichthys crocea). Sci. Technol. Food Ind. 2025, 46, 316–323. (In Chinese) [Google Scholar]
- Zhang, Y.; Han, M.-X.; Cao, M.-Y.; Zhao, Y.-Y.; Li, J.; Xue, L.-Y. SNP Detection of FST Gene and Its Association With Growth Traits in Larimichthys crocea. J. Nucl. Agric. Sci. 2018, 32, 883–891. (In Chinese) [Google Scholar]
- Sui, B.-L. Genetic Parameters Estimation and Correlation Analysis Between Growth-Related Traits and Microsatellite Markers in Large Yellow Croaker Larimichthys crocea. Master’s Thesis, Jimei University, Xiamen, China, 2012. (In Chinese). [Google Scholar]
- Wei, B.; Zheng, S.; Gao, Y.; Zheng, Y.; Yang, X.; Jiang, Z.; Guo, Q. A comparative study on the quality of large yellow croaker (Larimichthys crocea) of different sizes cultured in different cage systems. Aquac. Res. 2023, 2023, 6628371. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liu, L.X.; Wang, W.Z.; Cai, M.-Y.; Yao, C.-L. Genetic structure and genetic diversity analysis of four consecutive breeding generations of large yellow croaker (Pseudosciaena crocea) using microsatellite markers. J. Fish. China 2010, 34, 500–507. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, W.; Ma, L. Genetic diversity and population structure of small yellow croaker (Larimichthys polyactis) in the Yellow and East China seas based on microsatellites. Aquat. Living Resour. 2019, 32, 16. [Google Scholar] [CrossRef]
- Guo, D.-D.; Liu, F.; Niu, B.-L.; Zhan, W.; Xie, Q.-P.; Zhang, Y.; Lou, B. Establishment of diploid hybrid strains derived from female Larimichthys crocea× male Larimichthys polyactis and transmission of parental mtDNA in hybrid progenies. Aquaculture 2022, 561, 738693. [Google Scholar] [CrossRef]
- Hou, H.-H.; Miao, L.; Li, M.-Y.; Mu, F.-S.; Xu, Y.-M. The genetic diversity of F4 generations of “Donghai No. 1” large yellow croaker (Larimichthys crocea) analyzed by AFLP. J. Ningbo Univ. 2018, 31, 31–35. (In Chinese) [Google Scholar]
- Zheng, J.; Yan, Y.-R.; Li, Z.-L.; Song, N. Genetic structure of the small yellow croaker (Larimichthys polyactis) across the Yellow Sea and the East China Sea by microsatellite DNA variation: Implications for the division of management units. PeerJ 2022, 10, e13789. [Google Scholar] [CrossRef]
- Xu, S.-W.; Ge, M.-F.; Feng, J.; Wei, X.-X.; Tan, H.-L.; Liang, Z.; Tong, G.-X. Epidemiological investigation on diseases of Larimichthys crocea in Ningbo culture area. Front. Cell. Infect. Microbiol. 2024, 14, 1420995. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-L.; Chen, X.-T.; Qu, A.; Liu, Y.; Zhao, J.; Ke, Q.-Z.; Pu, F.; Wu, L.-N.; Chi, H.-S.; Gong, H.; et al. Identification and Expression Analysis of LncRNAs Reveal the Immune Mechanism of Visceral White-Nodules Disease Resistance in Large Yellow Croaker. Mar. Biotechnol. 2023, 25, 57–69. [Google Scholar] [CrossRef]
- Li, F.-X.; Yin, X.-L.; Lu, D.-Z.; Lu, D.-Z.; Liu, C.; Zhang, J.-S.; Shen, B. Single nucleotide polymorphisms and its association with disease resistant trait of ISG15 genes in Larimichthys crocea. J. Oceanol. Limnol. 2023, 54, 173–182. (In Chinese) [Google Scholar]
- Wei, Z.-Y.; Lu, L.-X.; Ren, Q.-L.; He, T.-L.; Chen, X.-H. Molecular characteristics and antiviral effects of ATG10 in large yellow croaker (Larimichthys crocea). Acta Hydrobiol. Sin. 2022, 46, 521–528. (In Chinese) [Google Scholar]
- Fu, C.-Y.; Wang, J.-P.; Sun, C.; Chen, L.; Qian, D. Isolation, Identification, Inhibition Spectrum and Safety Test of Antagonistic Bacteria to Major Pathogenic Bacteria in Larimichtys crocea. aBIOTECH 2019, 35, 67–75. (In Chinese) [Google Scholar]
- Liu, L.-D. “Core” technology breaks through the genetic code of large yellow croaker and largemouth bass. Sci. Fish Farming 2024, 8–11. (In Chinese) [Google Scholar]
- Huang, Y.; Li, Z.-Y.; Li, M.-C.; Zhang, X.-H.; Shi, Q.; Xu, Z. Fish Genomics and Its Application in Disease-Resistance Breeding. Rev. Aquac. 2025, 17, e12973. [Google Scholar] [CrossRef]
- Yanez, J.M.; Barria, A.; Lopez, M.E.; Moen, T.; Garcia, B.F.; Yoshida, G.M.; Xu, P. Genome-wide association and genomic selection in aquaculture. Rev. Aquac. 2023, 15, 645–675. [Google Scholar] [CrossRef]
- Tetsuo, K.; Liyi, P.; Ryota, I.; Chunyan, C.; Ping, W.; Ikuyo, T.; Yingying, Y.; Xiaojun, Y.; Baoying, G.; Weiye, L. Whole-genome resequencing of large yellow croaker (Larimichthys crocea) reveals the population structure and signatures of environmental adaptation. Sci. Rep. 2021, 11, 11235. [Google Scholar]
- Zhang, J.-J.; Wang, Y.-B.; Qiao, G.-D.; Wang, Q.; Han, D.-C.; Peng, S.-M. Comparison of antioxidant capacity in tissues, musele ultrastructure and related gene expression of Larimichthys crocea with different anti-flowing abilities. Mar. Fish. 2025, 47, 20–28. (In Chinese) [Google Scholar]
- Chen, X.-M.; Li, J.-K.; Wang, Z.-Y.; Cai, M.-Y.; Han, F.; Liu, X.-D. Genome-wide association study of thermal tolerance in large yellow croaker (Larimichthys crocea) based on SLAF-seq technology. Acta Hydrobiol. Sin. 2017, 41, 735–740. (In Chinese) [Google Scholar]
- Ji, Q.; Xie, Z.-l.; Li, L.-Z.; Han, X.-L.; Song, W. A Characterization of the RNA Modification Response to Starvation under Low Temperatures in Large Yellow Croaker (Larimichthys crocea). Fishes 2024, 9, 41. [Google Scholar] [CrossRef]
- Zhang, H.; Ceng, L.; Xiong, Y.-F.; Song, W. Mechanism of salinity acclimation in Larimichthys crocea improving tolerance to salinity stress. J. Fish. Sci. China 2023, 30, 334–343. (In Chinese) [Google Scholar]
- Ding, J.; Zhang, Y.-B.; Wang, J.-Y.; Liu, C.; Gao, X.-M.; Wu, Y.-J.; Wang, J.-Q.; Wu, X.-F.; Zhu, J.-Q.; Shen, W.-L. Genome-wide association study identified candidate SNPs and genes associated with hypoxia tolerance in large yellow croaker (Larimichthys crocea). Aquaculture 2022, 560, 738472. [Google Scholar] [CrossRef]
- Han, D.-C.; Zhang, J.-J.; Wang, Y.-B.; Qiao, G.-D.; Wang, Q.; Peng, S.-M. Comparative analysis of energy metabolism differences among populations of Larimichthys crocea with different flow resistances. Mar. Fish. 2024, 46, 608–615. (In Chinese) [Google Scholar]
- Guo, H.-H.; Hu, Z.; Zhang, J.-G.; Zou, G.-W.; Liang, H.-W. Advances in environmental tolerance and resistance breeding in fish. J. Fish. China 2023, 47, 70–90. (In Chinese) [Google Scholar]
- Gao, G.-Q.; Chang, Y.-M.; Han, Q.-X.; Chi, B.-J.; Li, M.-Y.; Xue, L.-Y.; Liang, L.-Q. Screening of microsatellite markers associated with cold tolerance oflarge yellow croaker (Pseudosciaena crocea R.). Hereditas 2010, 32, 248–253. (In Chinese) [Google Scholar]
- Wang, H.-R.; Liu, M.-H.; You, J.-J.; Luo, H.-Z.; Xu, Y.-A.; Fu, R.-B. Genetic Diversity Analysis and Screening of Microsatellite Markers Associated with Cold Tolerance of Large Yellow Croaker Pseudosciaena crocea Richardson. J. Zhejiang Ocean Univ. 2014, 33, 6–13. (In Chinese) [Google Scholar]
- Mu, F.-S.; Miao, L.; Ming-Yunli et, a.l. Screening of microsatellite markers associated with cold tolerance of large yellow croaker (Pseudosciaena crocea). J. Biol. 2017, 34, 34–38. (In Chinese) [Google Scholar]
- Li, J.-K. Effects of High Temperature on Physiology Biochemistry and Screening Related Microsatellite Markers in Large Yellow Croaker Larimichthys crocea. Master’s Thesis, Jimei University, Xiamen, China, 2015. (In Chinese). [Google Scholar]
- Wu, Y.-D.; Zhou, Z.-X.; Pan, Y.; Zhao, J.; Bai, H.-Q.; Chen, B.-H.; Zhang, X.-Y.; Pu, F.; Chen, J.; Xu, P. GWAS identified candidate variants and genes associated with acute heat tolerance of large yellow croaker. Aquaculture 2021, 540, 736696. [Google Scholar] [CrossRef]
- Jiang, D.; Li, W.-B.; Wang, Z.-Y.; Fang, M. Genome-wide identification of cis-acting expression QTLs in large yellow croaker. Mar. Biotechnol. 2021, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, Y.; Liu, X.-D.; Wang, X.-Q.; Wang, Z.-Y. Genetic mapping and QTL analysis of growth traits in the large yellow croaker Larimichthys crocea. Mar. Biotechnol. 2014, 16, 729–738. [Google Scholar] [CrossRef]
- Zeng, J.-J.; Long, F.; Wang, J.-Y.; Zhao, J.; Ke, Q.-Z.; Gong, J.; Bai, Y.-L.; Deng, Y.-C.; Jiang, P.-X.; Qu, A.; et al. GWAS reveals heritable individual variations in the inherent swimming performance of juvenile large yellow croaker. Aquaculture 2022, 559, 738419. [Google Scholar] [CrossRef]
- Ke, Q.-Z.; Wang, J.-Y.; Bai, Y.-L.; Zhao, J.; Gong, J.; Deng, Y.-C.; Qu, A.; Suo, N.; Chen, J.; Zhou, T.; et al. GWAS and genomic prediction revealed potential for genetic improvement of large yellow croaker adapting to high plant protein diet. Aquaculture 2022, 553, 738090. [Google Scholar] [CrossRef]
- Yu, M.; Xie, Q.-P.; Wei, F.-L.; Wu, X.-F.; Xu, W.-T.; Zhan, W.; Liu, F.; Guo, D.-D.; Niu, B.-L.; Lou, B. Development and identification of a sex-specific molecular marker in Dai-qu stock large yellow croaker (Larimichthys crocea). Aquaculture 2022, 555, 738172. [Google Scholar] [CrossRef]
- Ke, Q.-Z.; Liu, J.-X.; Zhao, J.; Wang, J.-Y.; Jiang, P.-X.; Deng, Y.-C.; Zhou, X.-Y.; Zeng, J.-J.; Zhou, T.; Xu, P. Genomic Selection of Large Yellow Croaker (Larimichthys crocea) with a High Plant Protein Diet Enhances the Growth Performance of Offspring. Mar. Biotechnol. 2024, 26, 732–740. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Wang, J.-Y.; Zhao, J.; Ke, Q.-Z.; Qu, A.; Deng, Y.-C.; Zeng, J.-J.; Gong, J.; Chen, J.; Pan, Y.; et al. Genomic selection for visceral white-nodules diseases resistance in large yellow croaker. Aquaculture 2022, 559, 738421. [Google Scholar] [CrossRef]
- Li, Q.-H.; Shao, G.-M.; Ding, Y.-Y.; Xu, L.-B.; Shao, J.-C.; Ao, J.-Q.; Chen, X.-H. Effective CRISPR/Cas9-based genome editing in large yellow croaker (Larimichthys crocea). Aquac. Fish. 2023, 8, 26–32. [Google Scholar] [CrossRef]
- Cao, B.-R.; Huang, T.-Q.; Gu, W.; Liu, E.-H.; Wang, G.-C.; Pan, Y.-C.; Wang, B.-Q.; Xu, G.-F. Advances of Gene Editing Technology in Genetic Breeding of Fish. Chin. J. Fish. 2024, 37, 100–108. (In Chinese) [Google Scholar]
- Xiao, S.-J.; Li, J.-T.; Ma, F.-S.; Fang, L.-J.; Xu, S.-B.; Chen, W.; Wang, Z.-Y. Rapid construction of genome map for large yellow croaker (Larimichthys crocea) by the whole-genome mapping in BioNano Genomics Irys system. BMC Genom. 2015, 16, 670. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, J.-J.; Liu, S.-F.; Liu, H.-W.; Zhang, T.-L.; Ye, T.; Lou, B.; Liu, F. QTL Mapping-Based Identification of Visceral White-Nodules Disease Resistance Genes in Larimichthys polyactis. Int. J. Mol. Sci. 2024, 25, 10872. [Google Scholar] [CrossRef]
- Ao, J.-Q.; Li, J.; You, X.-X.; Mu, Y.-M.; Ding, Y.; Mao, K.-Q.; Bian, C.; Mu, P.-F.; Shi, Q.; Chen, X.-H. Construction of the high-density genetic linkage map and chromosome map of large yellow croaker (Larimichthys crocea). Int. J. Mol. Sci. 2015, 16, 26237–26248. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, H.; Chen, J.; Ma, J.; Liu, R.; Ding, S. Genetic variation and population genetic structure of the large yellow croaker (Larimichthys crocea) based on genome-wide single nucleotide polymorphisms in farmed and wild populations. Fish. Res. 2020, 232, 105718. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Li, W.-B.; Liu, G.-J.; Gu, L.-L.; Ye, K.; Zhang, Y.-J.; Li, W.; Jiang, D.; Wang, Z.-Y.; Fang, M. Evaluation for the effect of low-coverage sequencing on genomic selection in large yellow croaker. Aquaculture 2021, 534, 736323. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, B.-H.; Ke, Q.-Z.; Zhao, J.; Pu, F.; Wu, Y.-D.; Chen, L.; Zhou, Z.-X.; Bai, Y.-L.; Pan, Y.; et al. Development and evaluation of a high-throughput single-nucleotide polymorphism array for large yellow croaker (Larimichthys crocea). Front. Genet. 2020, 11, 571751. [Google Scholar] [CrossRef]
- Qiu, C.-L.; Dong, L.-S.; Xiao, S.-J.; Xu, S.-B.; Fang, M.; Wang, Z.-Y. Genetic parameter estimation of nine quantitative traits by a marker-based method in Large Yellow Croaker, Larimichthys crocea (Richardson). Aquac. Res. 2017, 48, 5892–5900. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Han, K.-H.; Wu, Y.-D.; Bai, H.-Q.; Ke, Q.-Z.; Pu, F.; Wang, Y.-L.; Xu, P. Genome-wide association study of growth and body-shape-related traits in large yellow croaker (Larimichthys crocea) using ddRAD sequencing. Mar. Biotechnol. 2019, 21, 655–670. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Tay, Y.X.; Yue, G.H. Genome editing and its applications in genetic improvement in aquaculture. Rev. Aquac. 2022, 14, 178–191. [Google Scholar] [CrossRef]
- Chen, S.-L.; Wang, D.-S.; Kuang, Y.-Y.; Cui, Z.-K.; Li, M.-H. Fish genome editing breeding in china: Status, problems and prospects. J. Fish. China 2023, 47, 13–26. (In Chinese) [Google Scholar]
- Zhao, J.; Feng, M.-S.; Ke, Q.-Z.; Wang, J.-S.; Jiang, T.-S.; Wu, X.-F.; Peng, S.-M.; Bai, Y.-L.; Shen, W.-L.; Zhou, T.; et al. Accurate identification of Larimichthys crocea genetic resources based on “NingXin III” chip and machine learning method. J. Fish. China 2024, 48, 30–41. (In Chinese) [Google Scholar]
- Zhou, L.-Y.; Xie, X.; Jiang, L.-H.; Kurt, B.; Yin, F. Diagnosis of cryptocaryoniasis in large yellow croaker (Larimichthys crocea) by real-time object detection based on YOLOv3. Aquaculture 2024, 581, 740418. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Zhang, X.; Su, Y.-X.; Li, W.-Y.; Yin, X.-L.; Li, Z.-H.; Ying, Y.-F.; Wang, J.-C.; Wu, J.-P.; Miao, F.-J.; et al. Abnormal Behavior Monitoring Method of Larimichthys crocea in Recirculating Aquaculture System Based on Computer Vision. Sensors 2023, 23, 2835. [Google Scholar] [CrossRef]
- Wei, X.-M. Intelligent Monitoring System of Fisher Aquaculture Based on Internet of Things. Master’s Thesis, Tianjin University of Technology, Tianjin, China, 2019. (In Chinese). [Google Scholar]
- Chang, C.-C.; Ubina, N.A.; Cheng, S.-C.; Lan, H.-Y.; Chen, K.-C.; Huang, C.-C. A two-mode underwater smart sensor object for precision aquaculture based on AIoT technology. Sensors 2022, 22, 7603. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Dong, Y.-H.; Zhang, Z.-J. Design and Development of an Intelligent Monitoring System for Marine Aquaculture Environment Based on AIoT. Comput. Knowl. Technol. 2024, 20, 137–139. (In Chinese) [Google Scholar]
- Zhou, R.-J. Studies on the Forecasting of the Main Disease in Cage-Cultured Pseudosciaena crocea. Master’s Thesis, Ningbo University, Ningbo, China, 2011. (In Chinese). [Google Scholar]
- Yang, Z.; Lu, N.; Zhai, L. Study on production strategies for marine aquaculture in China at different scales: A case study of large yellow croaker (Larimichthys crocea). Aquac. Int. 2025, 33, 1–16. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, H.; Zhou, Z.-G.; Chen, N.; Bao, Y.-D. Progress and prospects of the application of digital technology in aquaculture production. China Agric. Inform. 2024, 36, 56–67. (In Chinese) [Google Scholar]
- Fu, G.; Yuna, Y. Phenotyping and phenomics in aquaculture breeding. Aquac. Fish. 2022, 7, 140–146. [Google Scholar] [CrossRef]
- Zou, H. Intelligent Environmental Monitoring and Control Technologies in Digital Aquaculture. Sci. Fish Farming 2023, 78–80. (In Chinese) [Google Scholar]
- Gan, Q.-L. Innovations in Intelligent Technologies and Digital Management in Aquaculture. J. Agric. Catastrophol. 2024, 14, 115–117. (In Chinese) [Google Scholar]
- Liu, C.; Ding, J.; Gao, X.-M.; Du, C.; Hou, C.-C.; Wu, X.-F.; Shen, W.-L.; Zhu, J.-Q. Effects of acute low temperature stress on the hormones and gene expression of glucocorticoid receptor of large yellow croaker Larimichthys crocea. J. Therm. Biol. 2021, 99, 103018. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Wang, Y.-B.; Chen, R.; Yue, Y.-F.; Gao, Q.-X.; Wang, C.-H.; Peng, S.-M. Proteomic and transcriptomic analysis of large yellow croaker (Larimichthys crocea) during early development under hypoxia and acidification stress. Aquaculture 2023, 577, 739982. [Google Scholar] [CrossRef]
- Ruan, S.-J.; Lu, Z.; Huang, W.-Q.; Zhang, Y.; Shan, X.-J.; Song, W.; Ji, C.-L. Renal metabolomic profiling of large yellow croaker Larimichthys crocea acclimated in low salinity waters. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101083. [Google Scholar] [CrossRef]
- Su, X.; Sutarlie, L.; Loh, X.J. Sensors, biosensors, and analytical technologies for aquaculture water quality. Research 2020, 2020, 8272705. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Zhou, R.-L.; Xie, X.; Yin, F. Characteristics and risk assessment of cryptocaryoniasis in large yellow croaker (Larimichthys crocea) at different densities in industrialized aquaculture. Aquaculture 2024, 582, 740501. [Google Scholar] [CrossRef]
- Guo, J.-L.; Yang, D.-F.; Xue, J.-G.; Song, M.-Y.; Xu, P.-X. Bibliometric Study On Fish Color Vision. J. Anhui Agric. Sci. 2024, 52, 10–14. (In Chinese) [Google Scholar]
- Kou, F.; Ma, Z.-J.; Wang, S. Research on Intelligent Aquaculture Monitoring System Based on Internet of Things. Sci. Technol. Inf. 2024, 22, 79–81+85. (In Chinese) [Google Scholar]
- Fan, L.-Z. Research on Real-Time Monitoring Technology for the Growth Process of Large Yellow Croaker Based on Computer Vision; Scientific Research Report; NingboTech University: Ningbo, China, 2015. (In Chinese) [Google Scholar]
- Song, C.-B.; Liu, L.-L.; Lu, P.-Z.; Yang, H.; Li, X.; Gao, X.-L.; Chen, T.; Xiong, W.; Lei, B.-L.; Ma, H.; et al. Design of artificial LED lighting in aquaculture workshop. J. Dalian Ocean Univ. 2018, 33, 145–150. (In Chinese) [Google Scholar]
- Song, W.; Chen-Han, X.; Zheng, L.-X.; Wang, L.; Liu, Y.-L.; Wang, L.-M. Development Status and the Prospect of Deep SeaLarge-Scale Fence Culture in China. Prog. Fish. Sci. 2022, 43, 111–120. (In Chinese) [Google Scholar]
- Zhang, P.-P.; Li, Z.-R.; Song, H.-Y.; Cai, H.-W.; Xia, F.-F. AHP-based evaluation index system on site selection for offshore cage cul-ture. South China Fish. Sci. 2023, 19, 1–9. (In Chinese) [Google Scholar]
- Ren, Z.-Y.; Cui, Y.-Q.; Chen, Y.-F.; Shi, L.-F.; Hao, G.-X.; Yang, S.; Qiu, X.-J.; Liu, S.-J.; Weng, W.-Y. Effect of pH on the structural properties and emulsification of myofibrillar proteins of large yellow croaker (Larimichthys crocea). J. Fish. China 2024, 48, 164–173. [Google Scholar]
- Ceng, L.; Xiong, Y.-F.; Song, W.; Xie, Z.-L.; Wang, Y.-H. Effect of salinity stress on energy metabolism and mitochondrial autophagy in large yellow croaker. Acta Hydrobiol. Sin. 2024, 48, 725–733. (In Chinese) [Google Scholar]
- Björn, B.; Lisa, H.; Alexander, R.; Carolina, W.L.; MarcChristopher, H.; Marieke, V.; Wilhelm, P.H. Effects of stocking density, size, and external stress on growth and welfare of African catfish (Clarias gariepinus Burchell, 1822) in a commercial RAS. Fishes 2023, 8, 74. [Google Scholar]
- Zhang, G.-B.; Ye, Z.-Q.; Jiang, Z.-J.; Wu, C.-Q.; Ge, L.-F.; Wang, J.-X.; Xu, X.-W.; Wang, T.-M.; Yang, J.-W. Circadian patterns and photoperiodic modulation of clock gene expression and neuroendocrine hormone secretion in the marine teleost Larimichthys crocea. Chronobiol. Int. 2024, 41, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Chávez, C.C.; Rodríguez-Ibarra, L.E.; Rodríguez Montes de Oca, G.; Abdo-de la Parra, M.I. Evaluación de diferentes fotoperiodos en el cultivo larvario del botete diana (Sphoeroides annulatus). Ecosistemas Y Recur. Agropecu. 2022, 9, 1–7. [Google Scholar]
- Feng, M.-S.; Jiang, P.-X.; Ke, Q.-Z.; Liu, S.-Y.; Chen, Y.-W.; Du, Y.-Q.; Luo, W.-J.; Liu, Y.-X.; Cai, Q.-X.; Zeng, Z.-H.; et al. A computer vision and RFID fusion-based method for measuring individual feed intake and its application for detecting individual differences in feed efficiency of large yellow croaker (Larimichthys crocea). Water Biol. Secur. 2024, 4, 100332. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Yao, C.-W.; Cui, K.; Hao, T.-T.; Yin, Z.-Y.; Xu, W.-X.; Huang, W.-X.; Mai, K.-S.; Ai, Q.-H. Nutritional programming of large yellow croaker (Larimichthys crocea) larvae by dietary vegetable oil: Effects on growth performance, lipid metabolism and antioxidant capacity. Br. J. Nutr. 2023, 129, 967–980. [Google Scholar] [CrossRef]
- Wang, X.-M.; Liu, H.; Zhang, C.-L.; Zhu, C.; Liu, H.-Y. Mechanisms of the Effect of Starvation Duration on the Regulation of Feeding Rhythm and Metabolic Physiology of Cultured Large Yellow Croaker (Larimichthys crocea). J. Mar. Sci. Eng. 2025, 13, 90. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Zhao, J.-Y.; Yao, M.-Y.; Liu, X.; Huo, Y.-K.; Chen, Y.-Y.; Wang, H.-H. Research advances on fish feeding behavior recognition and intensity quantification methods in aquaculture. arXiv 2025, arXiv:250215311. [Google Scholar]
- He, Y.-L.; Tang, Y.-H.; Xu, N.; Yao, C.-W.; Gong, Y.; Yin, Z.-Y.; Li, Q.-F.; Zhang, Y.-Q.; Lai, W.-C.; Liu, Y.-T. Effects of supplemental phytosterol on growth performance, body composition, serum biochemical indexes and lipid metabolism of juvenile large yellow croaker (Larimichthys crocea) fed with high lipid diet. Aquaculture 2022, 551, 737889. [Google Scholar] [CrossRef]
- Zhang, C. Behavior Tracking of Large Yellow Croaker Based on Machine Vision; Zhejiang Ocean University: Zhoushan, China, 2022. (In Chinese) [Google Scholar]
- Tian, Y.-X.; Feng, D.-J.; Zhang, H.; Gui, F.-K.; Qu, X.-Y. Distribution detection of Larimichthys crocea cultured in largenet-enclosure aquaculture by Small Unmanned Surface Vehicle. J. Fish. China 2022, 46, 2084–2096. (In Chinese) [Google Scholar]
- Ren, X.-M.; Gao, D.-Z.; Yao, Y.-L.; Yang, F.; Liu, J.-F.; Xie, F.-J. Occurrence and characteristic of sound in large yellow croaker (Pseudosciaena crocea). J. Dalian Ocean. Univ. 2007, 22, 123–128. (In Chinese) [Google Scholar]
- Dhinakaran, D.; Gopalakrishnan, S.; Manigandan, M.; Anish, T. IoT-Based Environmental Control System for Fish Farms with Sensor Integration and Machine Learning Decision Support. arXiv 2023, arXiv:231104258. [Google Scholar] [CrossRef]
- Yang, Y.-X.; Yu, H.; Zhang, X.; Zhang, P.; Tu, W.; Gu, L.-S. Fish behavior recognition based on an audio-visual multimodal interactive fusion network. Aquac. Eng. 2024, 107, 102471. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Miryala, K.R.; Swain, B. Advances and Challenges in Aeromonas hydrophila Vaccine Development: Immunological Insights and Future Perspectives. Vaccines 2025, 13, 202. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Zhu, C.-H.; Xiao, F.-N.; Liu, X.-D.; Xie, A.-H.; Chen, F.-M.; Dong, P.-P.; Lin, P.-D.; Zheng, C.-Y.; Zhang, H.; et al. PH-controlled release of antigens using mesoporous silica nanoparticles delivery system for developing a fish oral vaccine. Front. Immunol. 2021, 12, 644396. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.-M.; Miao, L.; Chen, J. Current status and development prospects of aquatic vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; Cui, K.; Wu, M.-J.; Xu, D.; Mai, K.-S.; Ai, Q.-H. Polyunsaturated fatty acids influence LPS-induced inflammation of fish macrophages through differential modulation of pathogen recognition and p38 MAPK/NF-κB signaling. Front. Immunol. 2020, 11, 559332. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Chen, H.; An, H.-M.; Wang, Y.-Y.; Shao, J.-C.; Yan, M.-J.; Ao, J.-Q.; Chen, X.-H.; Zhang, W.-N. Dietary Astragalus polysaccharides enhance potency of inactivated Pseudomonas plecoglossicida vaccine in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 157, 107–110. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, C.; Chi, H.; Mweemba, M.H.; Xu, C.; Qin, P.; Chen, X.-H. Early immune response in large yellow croaker (Larimichthys crocea) after immunization with oral vaccine. Mol. Cell. Probes 2021, 56, 101708. [Google Scholar] [CrossRef]
- Li, X.-M.; Tan, Y.-Z.; Zhang, Z.; Huang, Y.-P.; Mu, P.-F.; Cui, Z.-W.; Chen, X.-H. Development of recombinant dihydrolipoamide dehydrogenase subunit vaccine against Vibrio infection in large yellow croaker. Fishes 2022, 7, 17. [Google Scholar] [CrossRef]
- He, L.-Y.; Li, Y.-S.; Kang, J.-L.; Li, J.-X. Development and potential use of Pseudomonas plecoglossicida mutant ΔOmpRΔrpoS as a live attenuated vaccine against visceral white nodules disease in large yellow croaker (Larimichthys crocea). Aquaculture 2023, 574, 739718. [Google Scholar] [CrossRef]
- Bøgwald, J. DALMORA Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef]
- Su, H.; Yakovlev, I.A.; André, V.E.; Jianguo, S.; Liu, C.J. Plant-produced vaccines: Future applications in aquaculture. Front. Plant Sci. 2021, 12, 718775. [Google Scholar] [CrossRef]
- Clarke, J.L.; Waheed, M.T.; Lössl, A.G.; Martinussen, I.; Daniell, H. How can plant genetic engineering contribute to cost-effective fish vaccine development for promoting sustainable aquaculture? Plant Mol. Biol. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Nuryati, S.; Juliadiningtyas, A.D. Potential transmission test of GP25 vaccine in normal flora bacteria of common carp culture media. J. Akuakultur Indones. 2015, 14, 90–97. [Google Scholar] [CrossRef]
- Zhang, K.-F.; Zhou, Y.-D.; Song, W.-H.; Jiang, L.-H.; Yan, X.-J. Genome-wide radseq reveals genetic differentiation of wild and cultured populations of large yellow croaker. Genes 2023, 14, 1508. [Google Scholar] [CrossRef]
- Xu, Z.; Dou, S.-Z.; Ding, S.-X.; Liu, J.-X. Temporal genetic stability despite decades of overexploitation for large yellow croaker in the East China sea. Front. Mar. Sci. 2022, 9, 861840. [Google Scholar] [CrossRef]
- Xinxiu, Y.; Rajesh, J.; Magnus, H.G.; Zhenming, L.; Matthew, K. Construction of genetic linkage maps from a hybrid family of large yellow croaker (Larimichthys crocea). Front. Genet. 2022, 12, 792666. [Google Scholar]
- Liu, R.-Z.; Wang, S.; Huang, D.-L.; Huang, Y.-L.; He, T.-L.; Chen, X.-H. The probiotic roles of Lactiplantibacillus plantarum E2 as a dietary supplement in growth promotion and disease resistance of juvenile large yellow croaker (Larimichthys crocea). Aquaculture 2024, 578, 740082. [Google Scholar] [CrossRef]
- Jia, C.-F.; Liu, H.-L.; Xu, J.; Zhang, Z.-Y.; Zhang, Z.-W.; Chen, S.-Y.; Zhu, F. A review on the germplasm genetic diversity of large yellow croaker (Larimichthys crocea). Mar. Sci. Bull. 2017, 36, 12–18. (In Chinese) [Google Scholar]
- Zhao, J.; Bai, H.-Q.; Ke, Q.-Z.; Li, B.-J.; Zhou, Z.-X.; Wang, H.; Chen, B.-H.; Pu, F.; Zhou, T.; Xu, P. Genomic selection for parasitic ciliate Cryptocaryon irritans resistance in large yellow croaker. Aquaculture 2021, 531, 735786. [Google Scholar] [CrossRef]
- Nguyen, N.H. Genetics and Genomics of Infectious Diseases in Key Aquaculture Species. Biology 2024, 13, 29. [Google Scholar] [CrossRef]
- Shao, J.-C.; Wang, X.-X.; Liu, Q.-Q.; Lv, H.-Y.; Qi, Q.; Li, C.-H.; Zhang, J.-N.; Chen, X.-J.; Chen, X.-H. Eucommia ulmoides leaf extracts combined with Astragalus polysaccharides: Effects on growth, antioxidant capacity, and intestinal inflammation in juvenile large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2025, 161, 110229. [Google Scholar] [CrossRef]
- Zhu, A.-J.; Li, W.-Y.; Zhang, X.-L.; Yan, X.-J. Dietary ursodeoxycholic acid supplementation enhanced nonspecific immunity, antioxidant capacity, and anti-inflammatory-related gene expression in juvenile large yellow croaker (Larimichthys crocea). Aquac. Rep. 2025, 41, 102698. [Google Scholar] [CrossRef]
- Peng, S.-M.; Wang, Y.-B.; Wang, Q.; Gao, Q.-X.; Zheng, H.-F.; Xu, J.; Wang, L.-M. A review: Molecular breeding related techniques of Larimichthys crocea. Mar. Fish. 2022, 44, 375. (In Chinese) [Google Scholar]
- Zhang, Y. Effects of Different Carbon-to Nitrogen Ratios on Biofloc Formation and Water Quality in Larval Rearing Ponds of Large Yellow Croaker. Sci. Fish Farm. 2023, 12, 77–79. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.-X.; Li, Y.; Tang, J.-C.; Li, K.-Q.; Shen, J.-J.; Liu, C.; Jiang, Y.-H.; Zhang, Z.-P.; Wang, Y.-L.; Zou, P.-F. SARM suppresses TRIF, TRAF3, and IRF3/7 mediated antiviral signaling in large yellow croaker Larimichthys crocea. Front. Immunol. 2023, 13, 1021443. [Google Scholar] [CrossRef]
- Muktar, Y.; Tesfaye, S.; Tesfaye, B. Present Status and Future Prospects of Fish Vaccination: A Review. J. Vet. Sci. Technol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Wan, L.; Wang, W.-J.; Liu, G.-J.; Dong, L.-S.; Li, W.-B.; Han, Z.-F.; Ye, K.; Wang, Z.-Y. A genome-wide association study of resistance to Pseudomonas plecoglossicida infection in the large yellow croaker (Larimichthys crocea). Aquac. Int. 2019, 27, 1195–1208. [Google Scholar] [CrossRef]
- Shen, W.-L.; Wu, X.-F.; Shen, T.-J.-K.; Lin, S.-Q. The Effects of Different Diets and Culture Environments on the Morphological Variations in the Large Yellow Croaker (Larimichthys crocea). Prog. Fish. Sci. 2017, 38, 70–77. (In Chinese) [Google Scholar]
- Wang, Y. Paradigm shift of fish nutrition and feed: The necessity revealed by the application of formulated feed in Micropterus salmoides and Larimichthys crocea farming. J. Fish. China 2024, 48, 222–240. (In Chinese) [Google Scholar]
- Zhou, X.; Gao, F.-Y.; Lu, M.-X. Progress on breeding of disease-resistant fishes: A review. J. Dalian Ocean Univ. 2021, 36, 510–523. (In Chinese) [Google Scholar]
- Houston, R.D.; Christina, K.; Diego, R. Animal board invited review: Widespread adoption of genetic technologies is key to sustainable expansion of global aquaculture. Animal 2022, 16, 100642. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-J. Research Progress in Precision Feeding Technologies for Aquaculture. Anim. Breed. Feed 2024, 23, 58–60. (In Chinese) [Google Scholar]
- Rather, M.A.; Ahmad, I.; Shah, A.; Hajam, Y.A.; Amin, A.; Khursheed, S.; Ahmad, I.; Rasool, S. Exploring opportunities of Artificial Intelligence in aquaculture to meet increasing food demand. Food Chem. X 2024, 22, 101309. [Google Scholar] [CrossRef]
- Xu, Y.-B.; Zhang, X.-P.; Li, H.-H.; Zheng, J.-N.; Olsen Michael, S.; Varshney Rajeev, K.; Prasanna Boddupalli, M.; Qian, Q. Smart breeding driven by big data, artificial intelligence and integrated genomic-enviromic prediction. Mol. Plant 2022, 15, 1664–1695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).