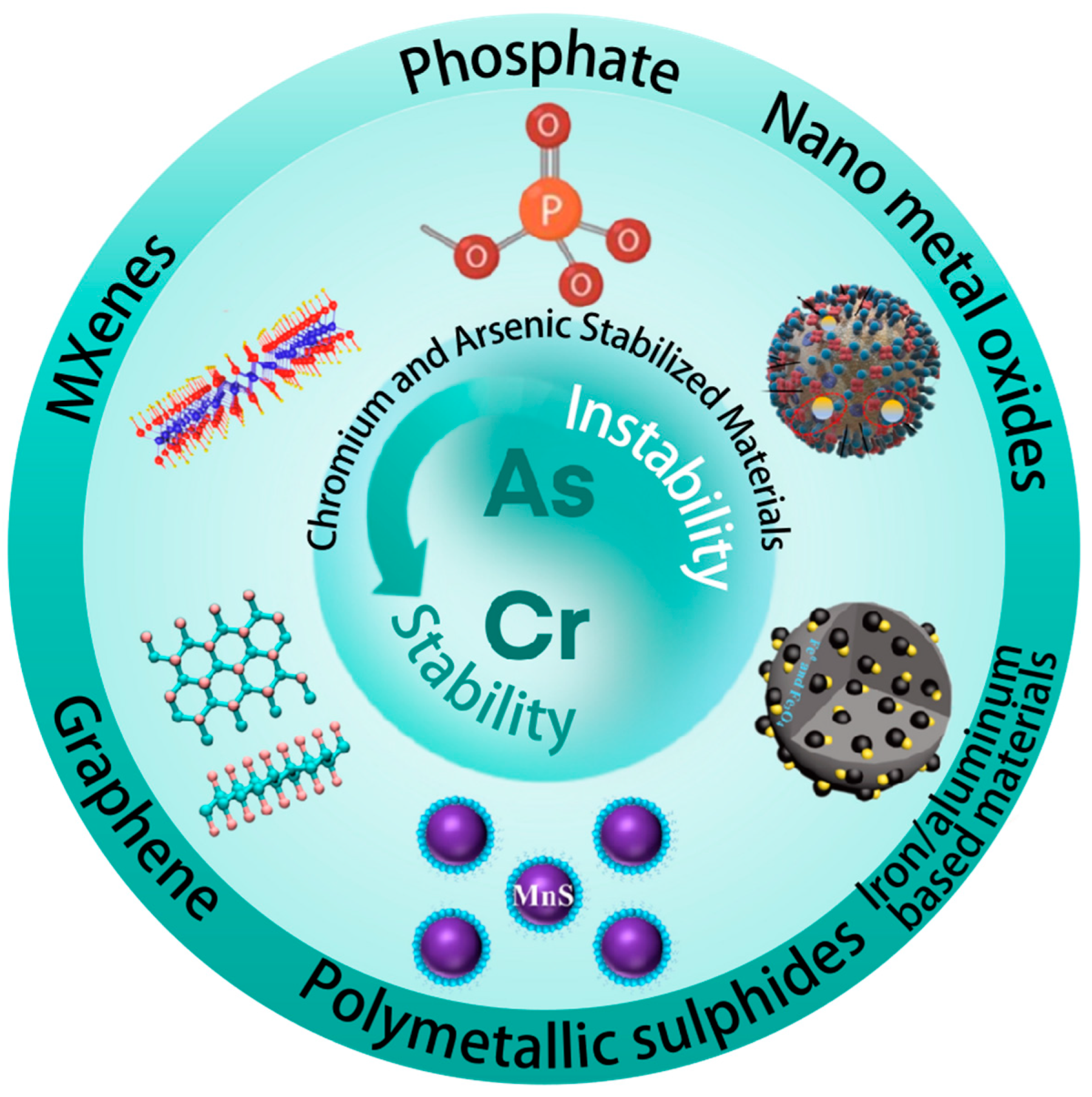
Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil
Abstract
1. Introduction
2. Inorganic Stabilization Materials
2.1. Phosphate Compounds
2.2. Nano-Metal Oxides
2.3. Iron/Aluminum Based Materials
2.4. Polymetallic Sulfide
2.5. Graphene
2.6. MXenes
3. Mechanism of Chromium and Arsenic Stabilization
3.1. Adsorption Mechanism
3.2. Oxidation-Reduction Reaction Mechanism
3.3. Precipitation Mechanism
3.4. Ion Exchange Mechanism
3.5. Ligand Exchange and Surface Complexation Mechanism
4. Factors Affecting the Stabilization Process of Chromium and Arsenic Stabilized Materials
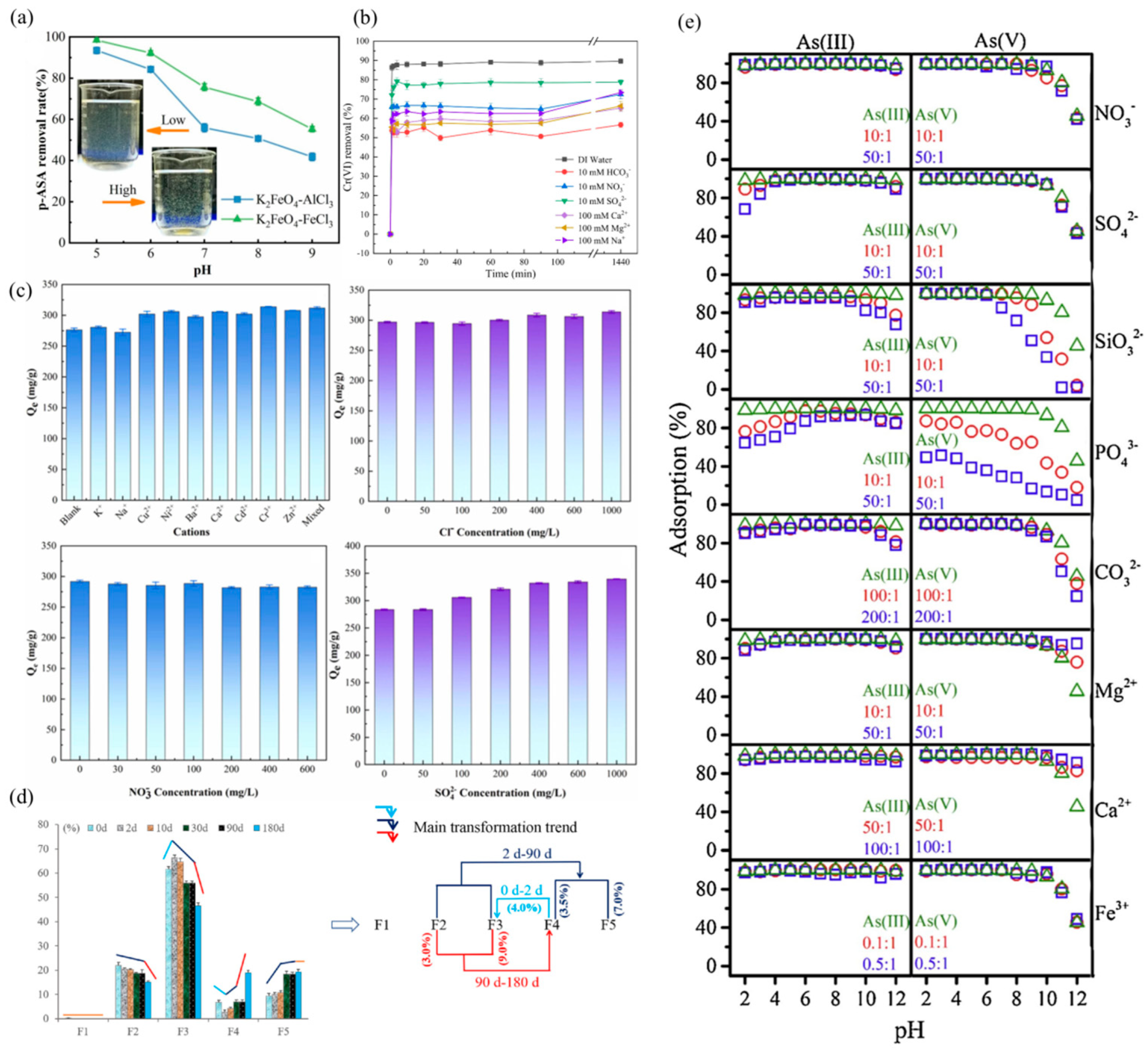
4.1. The Influence of Environmental pH on the Stabilization Process of Chromium and Arsenic
4.2. The Influence of Stabilization Time on the Stabilization Process of Chromium and Arsenic
4.3. The Influence of Coexisting Ions on the Stability Process of Chromium and Arsenic
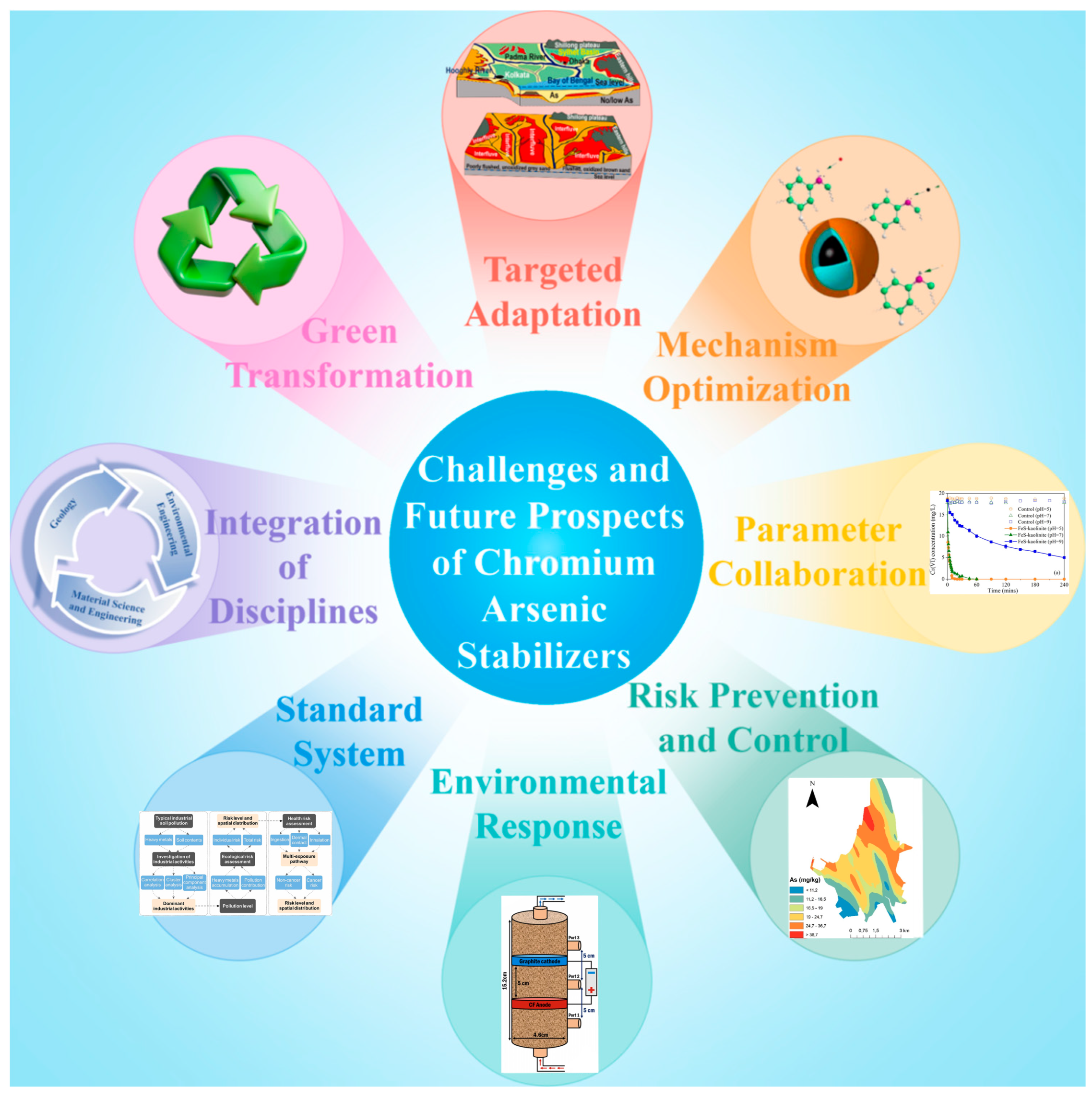
5. Conclusions
- In practical applications, remediation should be carried out based on the form of pollutants and the characteristics of the site. The forms of pollutants, such as the valence and occurrence forms of chromium and arsenic, directly affect toxicity and mobility, determining the stabilization pathway (reduction, adsorption, or physical fixation). The characteristics of the site (pH, organic matter, permeability, interfering ions, and soil type) and climatic conditions (temperature, humidity, and precipitation) determine the suitability and reaction efficiency of the material [117], avoiding stability failure or secondary release caused by unsuitable environmental conditions.
- The selection and targeting of stabilization materials directly determine the stabilization efficiency of chromium and arsenic. Different materials have different mechanisms of action on chromium (such as zero-valent iron reducing Cr(VI)) and iron-based materials adsorbing As(V)), and precise matching of pollutant characteristics is required. In addition, material costs and engineering feasibility also need to be comprehensively balanced.
- When using stabilization materials, the feed material, reaction time, pH range, and cost range should be considered. If these parameters are mismatched, it may result in substandard processing or resource waste. Optimizing parameters can simultaneously improve efficiency and engineering sustainability.
- Risk control and long-term management should be considered before applying chromium and arsenic stabilization materials. It is necessary to prevent and control the risk of secondary release after stabilization and reduce environmental exposure through monitoring frequency and emergency plans (such as supplementing iron-based materials). At the same time, long-term stability depends on the anti-aging ability of materials (such as sulfide failure under oxidation conditions) and environmental fluctuations (such as pH sudden changes).
- Future research should pay more attention to evaluating chromium and arsenic stabilization materials in practical complex environments. Most current research focuses on static experiments, whereas real environments should simulate dynamic processes such as wet–dry alternation, freeze–thaw cycles, and thermal aging. It is recommended that wet–dry cycle experiments be utilized to simulate the stabilization capacity of chromium and arsenic pollutants and structural changes of materials under periodic wetting–drying effects.
- Currently, research on chromium and arsenic stabilization materials requires the establishment of unified performance evaluation standards and engineering application guidelines. In the future, a standardized framework systematically covering material screening, stabilization efficiency verification, and environmental risk control should be developed. Firstly, it is necessary to clarify material application parameters for different contaminated sites (such as acidic mine drainage, alkaline tailings ponds, etc.) and establish standardized stabilization experimental methods. Secondly, an evaluation standard should be developed to define reasonable thresholds for long-term leaching concentration, occurrence forms, and ecological toxicity of chromium and arsenic.
- The breakthrough of chromium and arsenic stabilization technologies requires the deep integration of environmental engineering, materials science, and geology. Future research requires the construction of a multidisciplinary cross-disciplinary framework: the discipline of environmental engineering should focus on analyzing the migration and transformation laws of pollutants, providing environmental data for material design; materials science needs to be combined with geological mineralization principles to develop mineralized materials and enhance their ability to stabilize chromium and arsenic; geology needs to intervene in the study of hydrogeochemical characteristics of the site and predict the geological compatibility of stabilization materials through models.
- The green transformation of chromium and arsenic stabilization materials has become an important trend. Future research will focus on developing environmentally friendly and low-energy inorganic stabilizers, with priority given to using industrial solid waste such as red mud and fly ash or natural minerals (such as attapulgite and sepiolite) as matrix materials, and improving their ability to stabilize heavy metals of chromium and arsenic through modification, to achieve the goal of “treating waste with waste”.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shanmugam, T.; Selvaraj, J.; Mani, U. An Improved Ion Chromatographic Method for Fast and Sensitive Determination of Hexavalent Chromium and Total Chromium Using Conductivity Detection. J. Chromatogr. Sci. 2019, 57, 939–943. [Google Scholar] [CrossRef]
- Smith, A.H.; Steinmaus, C.M. Health Effects of Arsenic and Chromium in Drinking Water: Recent Human Findings. Annu. Rev. Public Health 2009, 30, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Su, Q.; He, Y.; Pan, H.; Liu, H.; Mehmood, K.; Tang, Z.; Hu, L. Toxicity of inorganic arsenic to animals and its treatment strategies. Comp. Biochem. Physiol. Part C Toxicol. Endocrinol. 2023, 271, 109654. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Quadri, S.S.A.; Ali, R.; Mesidor, A.; Shah, I.A.; Raqeeb, N.; Afzal, M.O.; Rizwan, M.; Sr, T.R.; Nosheen, S. Arsenic in the Food Chain in Pakistan: Assessing Risks to Human Health and Ensuring Food Security Through Comprehensive Contamination Mitigation Strategies. Cureus 2024, 16, e54069. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S.; et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater. 2021, 403, 124027. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-G.; Hu, Q.; Zhao, F.-J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Sheng, Y.; Baars, O.; Guo, D.; Whitham, J.; Srivastava, S.; Dong, H. Mineral-bound trace metals as cofactors for anaerobic biological nitrogen fixation. Environ. Sci. Technol. 2023, 57, 7206–7216. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality Incorporating the First and Second Addenda, 4th ed.; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Karunanidhi, D.; Aravinthasamy, P.; Subramani, T.; Kumar, D.; Venkatesan, G. Chromium contamination in groundwater and Sobol sensitivity model based human health risk evaluation from leather tanning industrial region of South India. Environ. Res. 2021, 199, 111238. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Chiu, K.; Lin, C.; Leu, H. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Sheng, Y.; Shi, J.; Dong, H. Disentangling Microbial Syntrophic Mechanisms for Hexavalent Chromium Reduction in Autotrophic Biosystems. Environ. Sci. Technol. 2021, 55, 6340–6351. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, H.; Zhang, L.; Wu, Y.; Lan, C.; Tang, J.; Wang, J.; Tang, L. Insights into the Mechanism of Selective Removal of Heavy Metal Ions by the Pulsed/Direct Current Electrochemical Method. Environ. Sci. Technol. 2024, 58, 5589–5597. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Z.; Li, H.; Yuan, S.; Zhang, Y.; Dong, Y. Direct current electrochemical method for removal and recovery of heavy metals from water using straw biochar electrode. J. Clean. Prod. 2022, 339, 130746. [Google Scholar] [CrossRef]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.K.; Dragoi, E.N. A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2023, 312, 137099. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, P.; Ni, W.; Zhang, S.; Li, S.; Deng, W.; Hu, W.; Li, J.; Pei, F.; Du, L.; et al. A review of solid wastes-based stabilizers for remediating heavy metals co-contaminated soil: Applications and challenges. Sci. Total Environ. 2024, 920, 170667. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, Q.; Wang, J.; Chen, X.; Mao, X.; He, Z. Inhibition of the bioavailability of heavy metals in sewage sludge biochar by adding two stabilizers. PLoS ONE 2017, 12, e0183617. [Google Scholar] [CrossRef]
- Datta, D.K.; Biswas, T.; Castonguay, E.; Ni, P. Sustainable Stabilizer Derived from Calcium- and Phosphorus-Rich Biowaste for Remediation of Heavy Metal-Contaminated Soil: A Critical Review. Sustainability 2024, 16, 8841. [Google Scholar] [CrossRef]
- Zhou, S.; Du, Y.; Feng, Y.; Sun, H.; Xia, W.; Yuan, H. Stabilization of arsenic and antimony Co-contaminated soil with an iron-based stabilizer: Assessment of strength, leaching and hydraulic properties and immobilization mechanisms. Chemosphere 2022, 301, 134644. [Google Scholar] [CrossRef]
- Cao, S.; Li, J.; Nie, J.; Shi, Y.; Dong, J.; Zhang, L.; Xue, Q. Red mud-based Fe/C nanostructured materials for multi-interface remediation of Cr(vi)-contaminated soil and stabilization. Environ. Sci. Nano 2025, 12, 1116–1125. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, R.; Wang, S.; Wang, Y.; Bi, R.; Jia, Y. Fate of arsenic during up to 4.5 years of aging of FeIII-AsV coprecipitates at acidic pH: Effect of reaction media (Nitrate vs. Sulfate), Fe/As molar ratio, and pH. Chem. Eng. J. 2020, 388, 124239. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; Zhan, L.; Yang, H.; Gong, Y. Removal of Cr(VI) from aqueous solution using ball mill modified biochar: Multivariate modeling, optimization and experimental study. Sci. Rep. 2024, 14, 4853. [Google Scholar] [CrossRef] [PubMed]
- Lindh, P.; Lemenkova, P. Leaching of Heavy Metals from Contaminated Soil Stabilised by Portland Cement and Slag Bremen. Ecol. Chem. Eng. S 2022, 29, 537–552. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Munir, H.M.S.; Sagir, M.; Arif, M.; Hassan, A.; Rachmadona, N.; Rajendran, S.; et al. Remediation techniques for elimination of heavy metal pollutants from soil: A review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, Z.; Di, J.; Wang, D.; Yang, Z.; Wang, Y.; Xie, Z. Study on the Effectiveness of Sulfate Reducing Bacteria to Remove Heavy Metals (Fe, Mn, Cu, Cr) in Acid Mine Drainage. Sustainability 2023, 15, 5486. [Google Scholar] [CrossRef]
- Sekula, P.; Hiller, E.; Šottník, P.; Jurkovič, Ľ.; Klimko, T.; Vozár, J. Removal of antimony and arsenic from circum_neutral mine drainage in Poproč, Slovakia: A field treatment system using low-cost iron-based material. Environ. Earth Sci. 2018, 77, 518. [Google Scholar] [CrossRef]
- Gupta, A.; Aniyery, R.B.; Sharma, A.; Singh, N.; Sharma, B. Iron Functionalized Zinc Peroxide nanomaterial for removal of Arsenic and Chromium from contaminated water. J. Chem. Pharm. Sci. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef]
- Janjhi, F.A.; Ihsanullah, I.; Bilal, M.; Castro-Muñoz, R.; Boczkaj, G.; Gallucci, F. MXene-based materials for removal of antibiotics and heavy metals from wastewater– a review. Water Resour. Ind. 2023, 29, 100202. [Google Scholar] [CrossRef]
- Ou, Y.; Gu, Z.; Luo, Y. Efficient heavy metal ion removal by fluorographene nanochannel templated molecular sieve: A molecular dynamics simulation study. Sci. Rep. 2024, 14, 6298. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, X.; Guo, Y.; Lin, Z.; Su, X.; Liu, B. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur. J. Hazard. Mater. 2021, 404, 124057. [Google Scholar] [CrossRef]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef] [PubMed]
- Andrunik, M.; Wołowiec, M.; Wojnarski, D.; Zelek-Pogudz, S.; Bajda, T. Transformation of Pb, Cd, and Zn Minerals Using Phosphates. Minerals 2020, 10, 342. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, H.; He, F.; Repo, E.; Yang, W.; Liao, Q.; Zhao, F. Iron-doped hydroxyapatite for the simultaneous remediation of lead-, cadmium- and arsenic-co-contaminated soil. Environ. Pollut. 2022, 312, 119953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Nie, K.; Arinzechi, C.; Li, J.; Liao, Q.; Si, M.; Yang, Z.; Li, Q.; Yang, W. Cooperative effect of slow-release ferrous and phosphate for simultaneous stabilization of As, Cd and Pb in soil. J. Hazard. Mater. 2023, 452, 131232. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Fei, X.; Wang, M.; Liu, S.; Zhang, X.; Xue, Q. Stabilization and strengthening of chromium(VI)-contaminated soil via magnesium ascorbyl phosphate (MAP) and phytase addition. J. Hazard. Mater. 2023, 448, 130860. [Google Scholar] [CrossRef]
- Xiulan, Y.; Jinqiu, S.; Qiqian, W.; Junfeng, S. Stabilization of soil arsenic by natural limonite after mechanical activation and the associated mechanisms. Sci. Total Environ. 2020, 708, 135118. [Google Scholar]
- Yu, Z.; Xiao, M.; Liu, H.; Zhu, W.; Dong, Z.; Wang, W.; Yang, T. FeOOH modified carbon doped TiO2 p-n heterojunction wrapped Fe3O4 for highly efficient removal of As(III) through photocatalytic oxidation and adsorption. J. Water Process. Eng. 2024, 61, 105314. [Google Scholar] [CrossRef]
- Ravbar, M.; Maver, K.; Knaflič, T.; Arčon, I.; Tušar, N.N.; Štangar, U.L.; Šuligoj, A. Nickel-decorated ZnO nanoparticles for effective solar reduction of hexavalent chromium and removal of selected pharmaceuticals. Appl. Surf. Sci. 2025, 681, 161463. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.; Gu, S.; Mei, S.; Zhu, G.; Yu, M.; Wu, Y.; Ping, Y.; Hong, K.; Zhang, J.; et al. Degradation of hexavalent chromium and methyl orange by the synergistic system of graphitic carbon nitride and electron beam irradiation. Chemosphere 2022, 287 Pt 2, 132228. [Google Scholar] [CrossRef]
- Wu, J.; Yan, M.; Lv, S.; Yin, W.; Bu, H.; Liu, L.; Li, P.; Deng, H.; Zheng, X. Preparation of highly dispersive and antioxidative nano zero-valent iron for the removal of hexavalent chromium. Chemosphere 2021, 262, 127733. [Google Scholar] [CrossRef] [PubMed]
- Alidokht, L.; Oustan, S.; Khataee, A.; Neyshaburi, M.R.; Reyhanitabar, A. Stabilization of chromium(VI)by hydroxysulfate green rust in chromium(VI)-contaminated soils. Pedosphere 2021, 31, 645–657. [Google Scholar] [CrossRef]
- Hui, C.; Zhang, Y.; Ni, X.; Cheng, Q.; Zhao, Y.; Zhao, Y.; Du, L.; Jiang, H. Interactions of iron-based nanoparticles with soil dissolved organic matter: Adsorption, aging, and effects on hexavalent chromium removal. J. Hazard. Mater. 2021, 406, 124650. [Google Scholar] [CrossRef]
- Jo, J.Y.; Choi, J.H.; Tsang, Y.F.; Baek, K. Pelletized adsorbent of alum sludge and bentonite for removal of arsenic. Environ. Pollut. 2021, 277, 116747. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Liang, T.; Yan, X.; Zhang, Y.; Zhou, Y.; Sarkar, B.; Ok, Y.S. Ball-milled magnetite for efficient arsenic decontamination: Insights into oxidation-adsorption mechanism. J. Hazard. Mater. 2022, 427, 128117. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Y.; Pi, K.; Liu, C.; Li, J.; Duan, M.; Wang, Y. In situ Fe-sulfide coating for arsenic removal under reducing conditions. J. Hydrol. 2016, 534, 42–49. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, G.; Xiang, H.; Wang, C.; Zeng, X.; Lu, P.; Zhang, J.; Chen, J.; Wang, R. Eliminating arsenic impurities from high-purity antimony trioxide through potassium sulfide co-precipitation. Sep. Purif. Technol. 2024, 333, 125920. [Google Scholar] [CrossRef]
- Kong, L.; Yan, R.; Liu, M.; Xu, J.; Hagio, T.; Ichino, R.; Li, L.; Cao, X. Simultaneous reduction and sequestration of hexavalent chromium by magnetic β-Cyclodextrin stabilized Fe3S4. J. Hazard. Mater. 2022, 431, 128592. [Google Scholar] [CrossRef]
- Pei, C.; Zhu, J.-H.; Lv, L. Enhanced visible-light induced photocatalytic activity in core-shell structured graphene/titanium dioxide nanofiber mats. J. Environ. Chem. Eng. 2024, 12, 113112. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S.; Svintsitskiy, D.A.; Gerasimov, E.Y.; Parmon, V.N. A Method for Synthesis of Nitrogen-Doped Graphene with High Specific Surface Area. Dokl. Phys. Chem. 2020, 495, 159–165. [Google Scholar] [CrossRef]
- Li, Z.; Yang, C.; Cui, J.; Ma, Y.; Kan, Q.; Guan, J. Recent Advancements in Graphene-Based Supports of Metal Complexes/Oxides for Epoxidation of Alkenes. Chem.-Asian J. 2018, 13, 3790–3799. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Sakthivel, T.S.; Jeyaranjan, A.; Seal, S.; Bezbaruah, A.N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere 2020, 253, 126702. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Yin, Y.; Liang, Q.; Zhu, Z.; Luo, H. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Gu, G.; Chen, X.; Wei, G.; Xu, M. MXene-reinforced bioactive polymer hydrogels for biomedical applications. APL Mater. 2024, 12, 080602. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Gogotsi, Y.; Taheri, M.L. Direct Correlation of MXene Surface Chemistry and Electronic Properties. Microsc. Microanal. 2018, 24, 1606–1607. [Google Scholar] [CrossRef]
- Ghotia, S.; Kumar, A.; Sudarsan, V.; Dwivedi, N.; Singh, S.; Kumar, P. Multilayered Ti3C2Tx MXenes: A prominent materials for hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 100–107. [Google Scholar] [CrossRef]
- Sun, M.; Chu, S.; Sun, Z.; Jiao, X.; Wang, L.; Li, Z.; Jiang, L. A review of etching methods and applications of two-dimensional MXenes. Nanotechnology 2024, 35, 382003. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Zaman, W.Q.; Anwer, H.; Azad, F.; Long, X.; Miran, W.; Khan, M. Two-dimensional MXene and molybdenum disulphide for the removal of hexavalent chromium from water: A comparative study. Desalin. Water Treat. 2024, 320, 100693. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Hu, B. Electrostatic self-assembly of nanoscale FeS onto MXenes with enhanced reductive immobilization capability for U(VI) and Cr(VI). Chem. Eng. J. 2023, 456, 141100. [Google Scholar] [CrossRef]
- Li, S.; Luo, S.; Wan, S.; Wang, P.; Zhou, G.; Wang, W.; Chen, R. Enhanced Performance of nZVI/MXene@CNTs for Rapid As(III) Removal from Aqueous Solutions. Appl. Sci. 2022, 12, 8206. [Google Scholar] [CrossRef]
- Zang, S.; Zhang, Q.; Hu, B.; Zhang, Y.; Pu, J.H.; Lv, M. Effective Technique and Mechanism for Simultaneous Adsorption of As(III/V) from Wastewater by Fe-ZIF-8@MXene. Toxics 2024, 12, 419. [Google Scholar] [CrossRef]
- Jamaluddin, N.S.; Alias, N.H.; Samitsu, S.; Othman, N.H.; Jaafar, J.; Marpani, F.; Lau, W.J.; Tan, Y.Z. Efficient Chromium (VI) Removal from Wastewater by Adsorption-Assisted Photocatalysis using MXene. J. Environ. Chem. Eng. 2022, 10, 108665. [Google Scholar] [CrossRef]
- Bourestan, N.R.; Nematollahzadeh, A.; Jadid, A.P.; Basharnavaz, H. Chromium removal from water using granular ferric hydroxide adsorbents: An in-depth adsorption investigation and the optimization. Chem. Phys. Lett. 2020, 748, 137395. [Google Scholar] [CrossRef]
- Diephuis, W.R.; Molloy, A.L.; Boltz, L.L.; Porter, T.B.; Aragon Orozco, A.; Duron, R.; Crespo, D.; George, L.J.; Reiffer, A.D.; Escalera, G.; et al. The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles. Nanomaterials 2022, 12, 1598. [Google Scholar] [CrossRef]
- Huang, D.; Han, X.; Zhang, F.; Liu, F.; Shi, Z.; Wang, G. Reduction mechanism of hexavalent chromium in aqueous solution by sulfidated granular activated carbon. J. Clean. Prod. 2021, 316, 128273. [Google Scholar] [CrossRef]
- Hu, B.; Yang, T.Z.; Liu, W.F.; Zhang, D.C.; Chen, L. Removal of arsenic from acid wastewater via sulfide precipitation and its hydrothermal mineralization stabilization. Trans. Nonferrous Met. Soc. China 2019, 29, 2411–2421. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Z.; Li, Z.; Yue, R.; Guo, F.; Xu, Z. Selective separation of chromium from sulphuric acid leaching solutions of mixed electroplating sludge using phosphate precipitation. Hydrometallurgy 2019, 186, 42–49. [Google Scholar] [CrossRef]
- Lofù, A.; Mastrorilli, P.; Dell’Anna, M.M.; Mali, M.; Sisto, R.; Vignola, R. Iron(II) modified natural zeolites for hexavalent chromium removal from contaminated water. Arch. Environ. Prot. 2016, 42, 35–40. [Google Scholar] [CrossRef]
- Nassar, H.F.; Mohamed, M.A. Removal of arsenic(V) using pure zeolite (PZ) and activated dithizone zeolite (ADZ) from aqueous liquids: Application to green analytical chemistry. Anal. Sci. 2024, 40, 755–763. [Google Scholar] [CrossRef]
- Li, K.; Yao, J.; Masakorala, K.; Li, X.; Li, S.; Li, X. Innovation and prospects of heavy metal solidification/stabilization techniques: A comprehensive review on materials, mechanisms, and evaluation systems. Environ. Technol. Innov. 2025, 37, 104040. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Y.; Fan, J.; Xu, X.; Cao, X. Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: Immobilization mechanism and long-term stability. J. Hazard. Mater. 2021, 405, 124226. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Liu, H.; Miao, Y.; Zheng, H.; Zhang, G. Investigating the efficacy and mechanistic pathways of mildly alkaline activators in the immobilization and stabilization of high-arsenic metallurgical slag using slag-based cementitious matrices. Process Saf. Environ. Prot. 2025, 198, 107138. [Google Scholar] [CrossRef]
- Huang, X.; Tu, J.; Wang, H.; Xue, F.; Zhao, X.; Xiang, Y.; Lan, J. Activated nickel tailings for remediation of arsenic-contaminated soils: Tailings activation, leaching toxicity and passivation mechanisms. J. Environ. Chem. Eng. 2025, 13, 115912. [Google Scholar] [CrossRef]
- Ugwu, E.; Tursunov, O.; Kodirov, D.; Shaker, L.; Al-Amiery, A.; Yangibaeva, I.; Shavkarov, F. Adsorption mechanisms for heavy metal removal using low cost adsorbents: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 614, 012166. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, G.; Li, X.; Zhao, Q.; Bi, X.; Wu, K.; Chen, H. Effects of hydrogen-peroxide supply rate on schwertmannite microstructure and chromium(VI) adsorption performance. J. Hazard. Mater. 2019, 367, 520–528. [Google Scholar] [CrossRef]
- Khandelwal, N.; Singh, N.; Tiwari, E.; Darbha, G.K. Achieving strong Pb-Cr complexation in Mg/Al LDHs for ultrafast chromate ions separation and chrome recovery from complex water matrices. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100754. [Google Scholar] [CrossRef]
- Zhang, X.; Navarathna, C.M.; Leng, W.; Karunaratne, T.; Thirumalai, R.V.K.G.; Kim, Y.; Pittman, C.U.; Mlsna, T.; Cai, Z.; Zhang, J. Lignin-based few-layered graphene-encapsulated iron nanoparticles for water remediation. Chem. Eng. J. 2021, 417, 129199. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, K.; Li, G.; Zhao, Q.; Wang, W.; Jiang, J.; Wang, Y.; Yuan, L. Stabilization of arsenic, antimony, and lead in contaminated soil with montmorillonite modified by ferrihydrite: Efficiency and mechanism. Chem. Eng. J. 2023, 457, 141182. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, J.F.; Fernandez-Villagomez, G.; Jasso, A.U.L.; Fernandez, J.M.; Navarro-Blasco, I.; Alvarez, J.I. Increasing the Sustainability of the Stabilization/Solidification of Potentially Toxic Elements Contained in Tailings from an Active Mine Using a Modified Lime Mortar. Sustainability 2024, 16, 2320. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, J.; Hu, X.; Xing, L.; Yan, C. Immobilization of Cr(VI) contaminated soil using green-tea impregnated attapulgite. J. Clean. Prod. 2021, 278, 123967. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y. Dual role of pyrogenic carbon in mediating electron transfer from clay minerals to chromium in aqueous and solid media. J. Hazard. Mater. 2024, 470, 134290. [Google Scholar] [CrossRef]
- Su, T.; Zhang, X.; Wang, Z.; Guo, Y.; Wei, X.; Xu, B.; Xia, H.; Yang, W.; Xu, H. Cellulose nanocrystal-based polymer hydrogel embedded with iron oxide nanorods for efficient arsenic removal. Carbohydr. Polym. 2024, 331, 121855. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.; Tsang, D.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.; Ok, Y.; Poon, C. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, Z.; Sun, G.; Zhang, Q.; Cao, M.; Tu, S.; Xiong, S. Simultaneous reduction in cadmium and arsenic accumulation in rice (Oryza sativa L.) by iron/iron-manganese modified sepiolite. Sci. Total Environ. 2022, 810, 152189. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, F.; Tang, B. Ethylenediamine-functionalized MnFe2O4 @ferrihydrite as a magnetic adsorbent for removal of Cr(VI): Adsorption and mechanism studies. J. Environ. Chem. Eng. 2023, 11, 110230. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Lu, Y.; Hu, B. Fe3S4 intercalated alkalized MXenes to enhancement of Au(III) and Cr(VI) removal by reduction and immobilization synergistic effect. Sep. Purif. Technol. 2024, 334, 126012. [Google Scholar] [CrossRef]
- Wang, Y.-n.; Tsang, Y.F.; Wang, H.; Sun, Y.; Song, Y.; Pan, X.; Luo, S. Effective stabilization of arsenic in contaminated soils with biogenic manganese oxide (BMO) materials. Environ. Pollut. 2020, 258, 113481. [Google Scholar] [CrossRef]
- Álvarez, A.M.; Guerrón, D.B.; Calderón, C.M. Natural zeolite as a chromium VI removal agent in tannery effluents. Heliyon 2021, 7, e07974. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, L.; Yang, L.; Zhou, X.; Zhang, Y. Structural Properties of Graphene Oxide Prepared from Graphite by Three Different Methods and the Effect on Removal of Cr(VI) from Aqueous Solution. Nanomaterials 2023, 13, 279. [Google Scholar] [CrossRef]
- Luo, T.; Wang, R.; Chai, F.; Jiang, L.; Rao, P.; Yan, L.; Hu, X.; Zhang, W.; Wei, L.; Khataee, A.; et al. Arsenite (III) removal via manganese-decoration on cellulose nanocrystal -grafted polyethyleneimine nanocomposite. Chemosphere 2022, 303, 134925. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, Q.; Guo, M.; Zhang, M.; Zhao, N.; Xu, Q.; Zhang, W.; Qiu, R. The effect of the synergistic thermal treatment and stabilization on the transformation and transportation of arsenic, chromium, and cadmium in soil. Sci. Total Environ. 2024, 907, 167948. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, L.; Peng, W.; Fan, Q.; Li, Q.; Dong, Y.; Liu, Y.; Boczkaj, G.; Wang, Z. Enabling simultaneous redox transformation of toxic chromium(VI) and arsenic(III) in aqueous media—A review. J. Hazard. Mater. 2021, 417, 126041. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Huang, A.; Lu, F.; Nie, Y.; Ma, J. Enhanced removal of organic arsenic by using potassium ferrate coupled with metal coagulants: Role of iron species and effect of AlCl3 and FeCl3. Chem. Eng. J. 2023, 475, 146109. [Google Scholar] [CrossRef]
- Kim, C.; An, S.; Lee, J.; Ghosh, A.; Zhong, M.; Fortner, J.D. Photoactive Polyethylenimine-Coated Graphene Oxide Composites for Enhanced Cr(VI) Reduction and Recovery. ACS Appl. Mater. Interfaces 2021, 13, 28027–28035. [Google Scholar] [CrossRef]
- Yang, N.; Qi, X.; Li, Y.; Li, G.; Duan, X. Highly effective remediation of high arsenic-bearing wastewater using aluminum-containing waste residue. J. Environ. Manag. 2023, 325, 116417. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Bi, D.; Xiao, L.; Theng, B.; Wang, H.; Ok, Y. Carbon-coated montmorillonite nanocomposite for the removal of chromium(VI) from aqueous solutions. J. Hazard. Mater. 2019, 368, 541–549. [Google Scholar] [CrossRef]
- Hou, Q.; Han, D.; Zhang, Y.; Han, M.; Huang, G.; Xiao, L. The bioaccessibility and fractionation of arsenic in anoxic soils as a function of stabilization using low-cost Fe/Al-based materials: A long-term experiment. Ecotoxicol. Environ. Saf. 2020, 191, 110210. [Google Scholar] [CrossRef]
- Shan, D.; Shi, Y.; Zhou, B.; Liu, Z.; Yang, L.; Zhu, X.; Luo, Q.; Li, G. Simultaneous and continuous stabilization of As and Cd in contaminated soil by a half wrapping-structured amendment. J. Environ. Chem. Eng. 2021, 9, 105416. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Chen, Y.; Zhang, T.; Zhang, Z.; Li, H.; Shan, F.; Ma, T. Anti-stacking, recyclable and electron-rich Fe3O4 modified L-glutamic acid functionalized graphene oxide towards Cr(VI) removal by adsorption-reduction behavior. Sep. Purif. Technol. 2025, 363, 132090. [Google Scholar] [CrossRef]
- Azzam, A.M.; Shenashen, M.A.; Selim, M.M.; Yamaguchi, H.; El-Sewify, I.M.; Kawada, S.; Alhamid, A.A.; El-Safty, S.A. Nanospherical inorganic α-Fe core-organic shell necklaces for the removal of arsenic(V) and chromium(VI) from aqueous solution. J. Phys. Chem. Solids 2017, 109, 78–88. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Z.; Ding, Z.; Zuo, M.; Cheng, J.; Jing, C. Simultaneous arsenic and fluoride removal using {201}TiO2–ZrO2: Fabrication, characterization, and mechanism. J. Hazard. Mater. 2019, 377, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xie, T.; Liu, Y.; Wu, Y.; Liu, H.; Su, Z.; Huang, Y.; Yuan, X.; Zhang, C.; Li, X. Carboxymethyl cellulose stabilized ferrous sulfide@extracellular polymeric substance for Cr(VI) removal: Characterization, performance, and mechanism. J. Hazard. Mater. 2022, 425, 127837. [Google Scholar] [CrossRef]
- Wei, J.; Duan, Y.; Li, M.; Lin, H.; Lv, J.; Chen, Z.; Lin, J.; Song, H.; Zhang, R.; Li, L.; et al. A novel manganese sulfide encapsulating biochar-dispersed zero-valent iron composite for high removal ability of Cr(VI) in water and its mechanism. Colloids Surf. A 2023, 658, 130556. [Google Scholar] [CrossRef]
- Uliana, A.A.; Pezoulas, E.R.; Zakaria, N.I.; Johnson, A.S.; Smith, A.; Lu, Y.; Shaidu, Y.; Velasquez, E.O.; Jackson, M.N.; Blum, M.; et al. Removal of Chromium and Arsenic from Water Using Polyol-Functionalized Porous Aromatic Frameworks. J. Am. Chem. Soc. 2024, 146, 23831–23841. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, G.; Qin, Y.; Cao, M.; Liu, Y.; Li, J.; Qu, J.; Liu, H. Redox Conversion of Chromium(VI) and Arsenic(III) with the Intermediates of Chromium(V) and Arsenic(IV) via AuPd/CNTs Electrocatalysis in Acid Aqueous Solution. Environ. Sci. Technol. 2015, 49, 9289–9297. [Google Scholar] [CrossRef]
- Guan, X.; Dong, H.; Ma, J.; Lo, I.; Dou, X. Performance and mechanism of simultaneous removal of chromium and arsenate by Fe(II) from contaminated groundwater. Sep. Purif. Technol. 2011, 80, 179. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B.; Darbha, G.K. The groundwater arsenic contamination in the Bengal Basin-A review in brief. Chemosphere 2022, 299, 134369. [Google Scholar] [CrossRef]
- Wu, J.; Chen, K.; Tan, X.; Fang, M.; Hu, X.; Tang, Z.; Wang, X. Core-shell CMNP@PDAP nanocomposites for simultaneous removal of chromium and arsenic. Chem. Eng. J. 2018, 349, 481–490. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Liao, Y.; Huang, J.; Dang, Z.; Guo, C. Removal of hexavalent chromium using biogenic mackinawite (FeS)-deposited kaolinite. J. Colloid Interface Sci. 2020, 572, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Tume, P.; Melipichún, T.; Ferraro, F.; Sepúlveda, B.; Roca, N.; Bech, J. Contamination of As, Cd, Cr, Hg and Pb in soils in Arica commune (Chile). Environ. Geochem. Health 2023, 45, 9199–9213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Sarrouf, S.; Ehsan, M.F.; Alshawabkeh, A.N.; Baek, K. In-situ groundwater remediation of contaminant mixture of As(III), Cr(VI), and sulfanilamide via electrochemical degradation/transformation using pyrite. J. Hazard. Mater. 2024, 473, 134648. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z.; Tan, X.; Xu, H.; Zhu, D.; Shen, R.; Ding, K.; Li, H.; Xiang, L.; Yang, Z. Integrated assessment of the pollution and risk of heavy metals in soils near chemical industry parks along the middle Yangtze River. Sci. Total Environ. 2024, 917, 170431. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, C.; Guo, H.; Sheng, Y.; Liu, X. Hydrologic changes induced by groundwater abstraction lead to arsenic mobilization in shallow aquifers. J. Hazard. Mater. 2024, 480, 136133. [Google Scholar] [CrossRef]

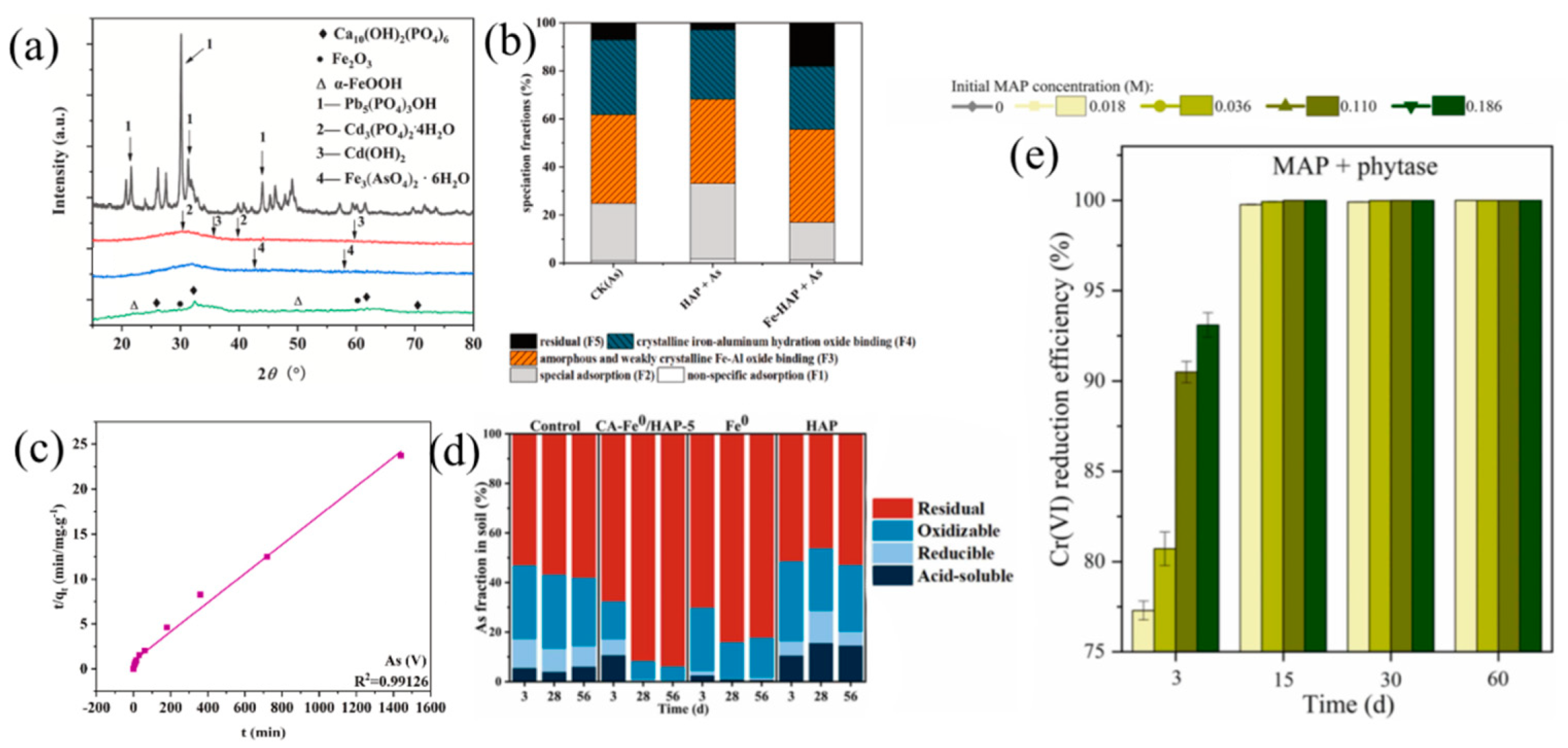
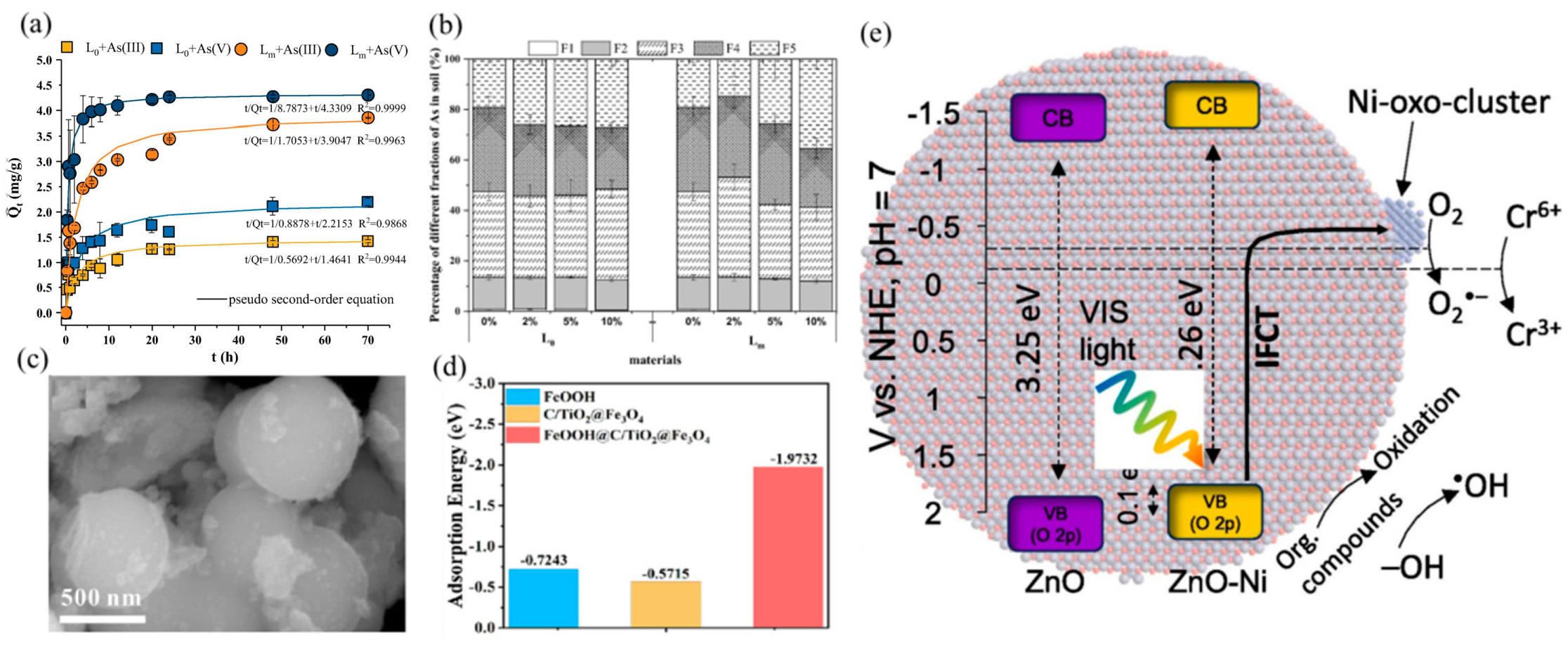
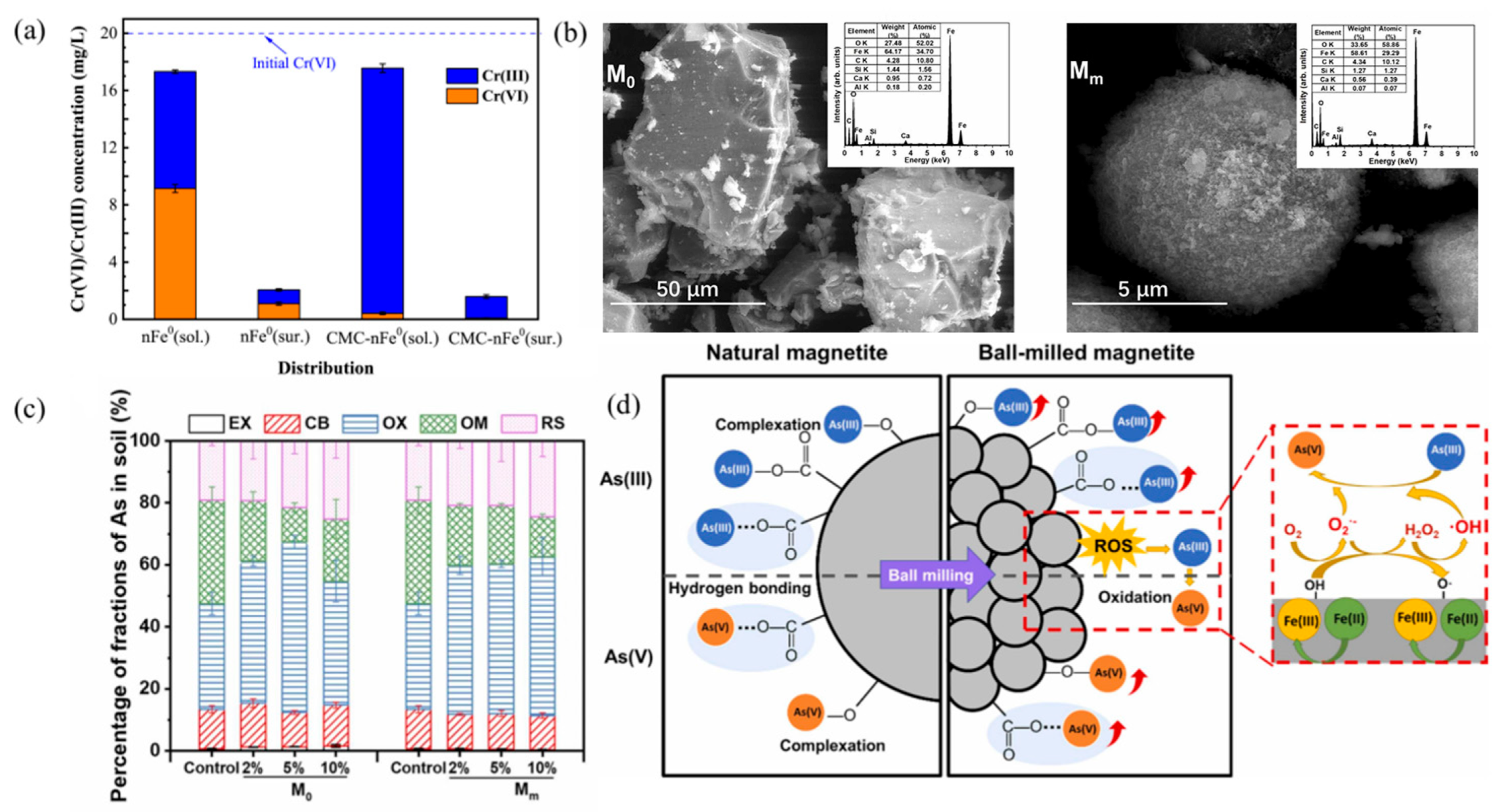
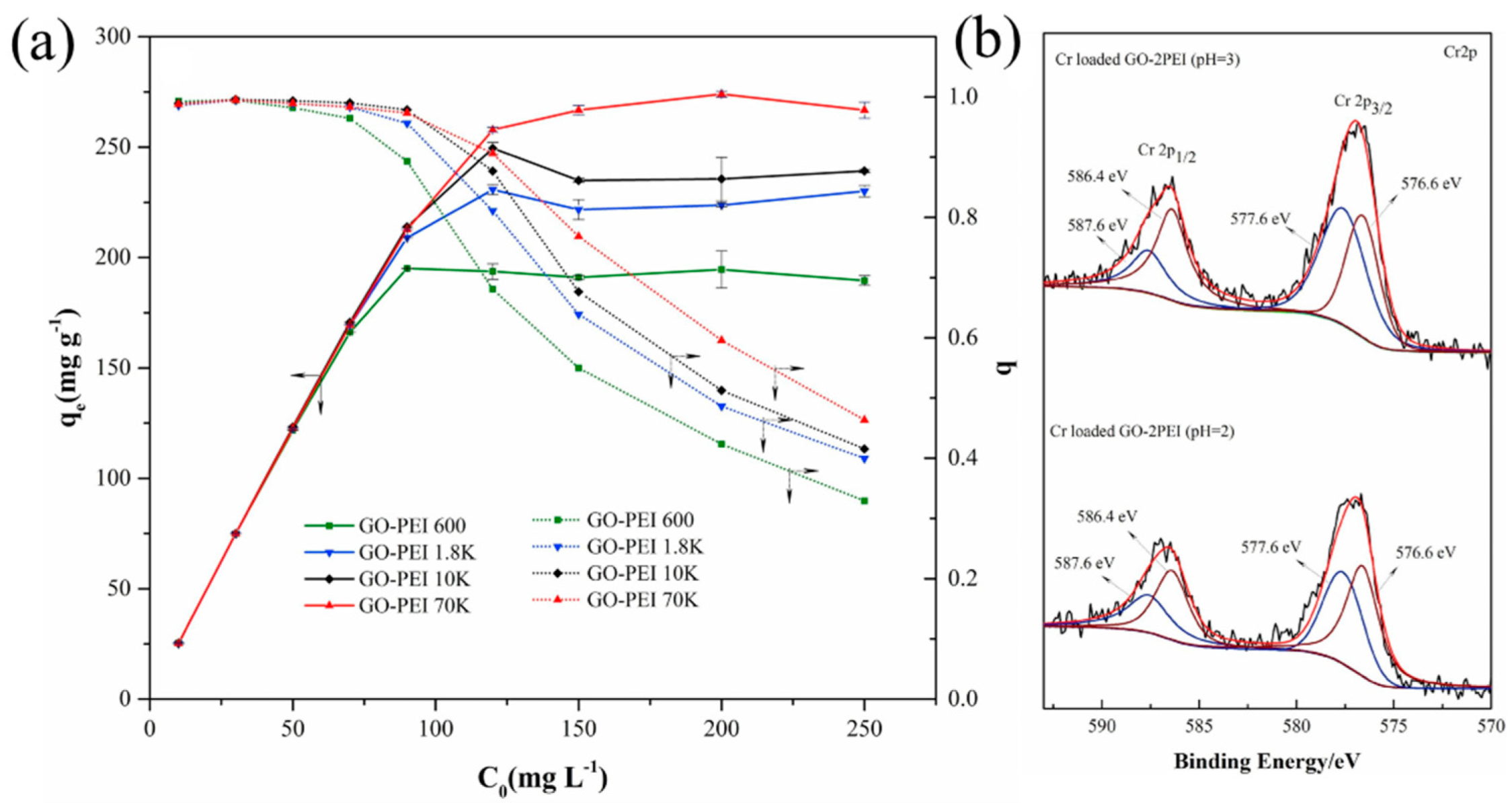
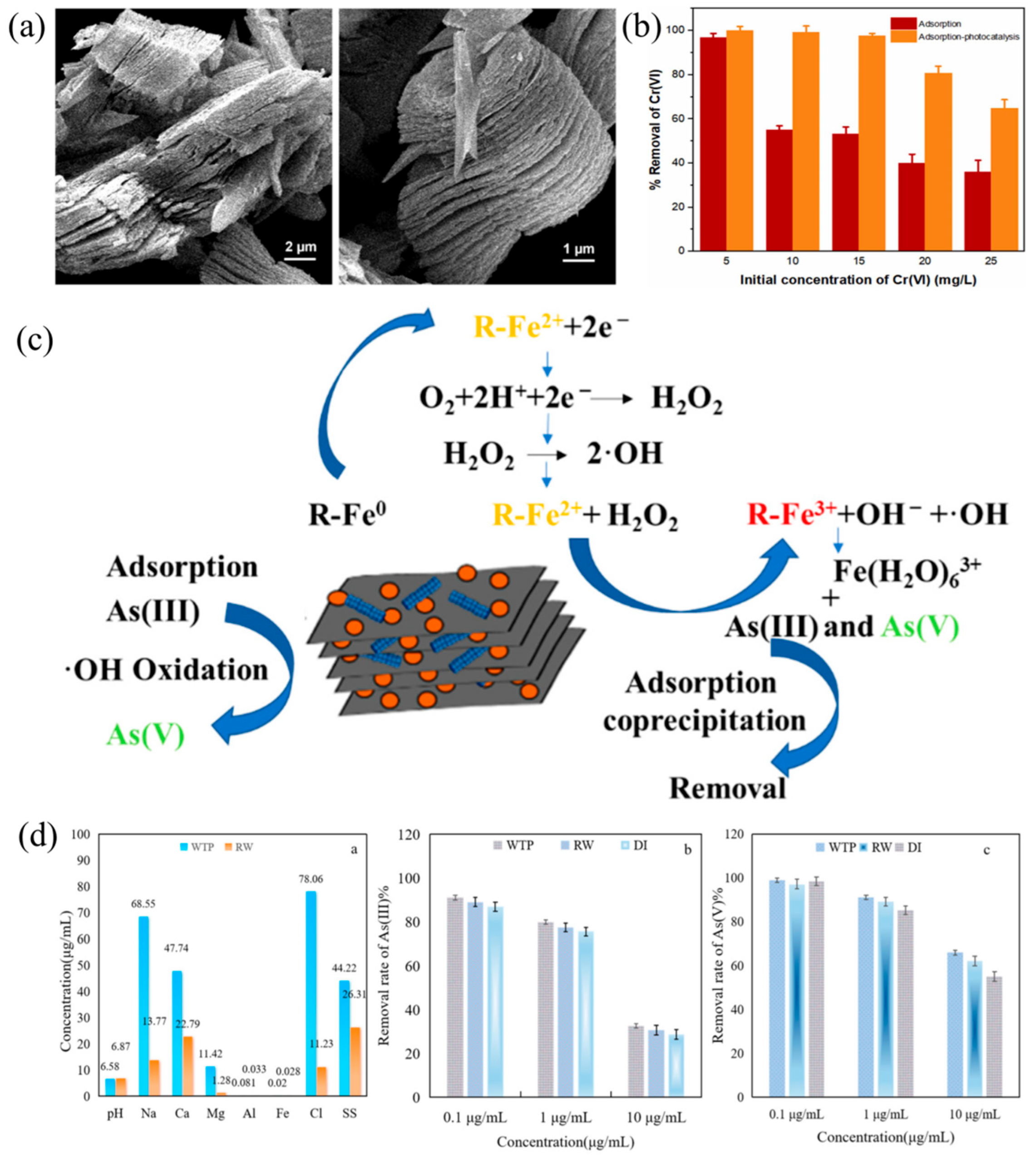
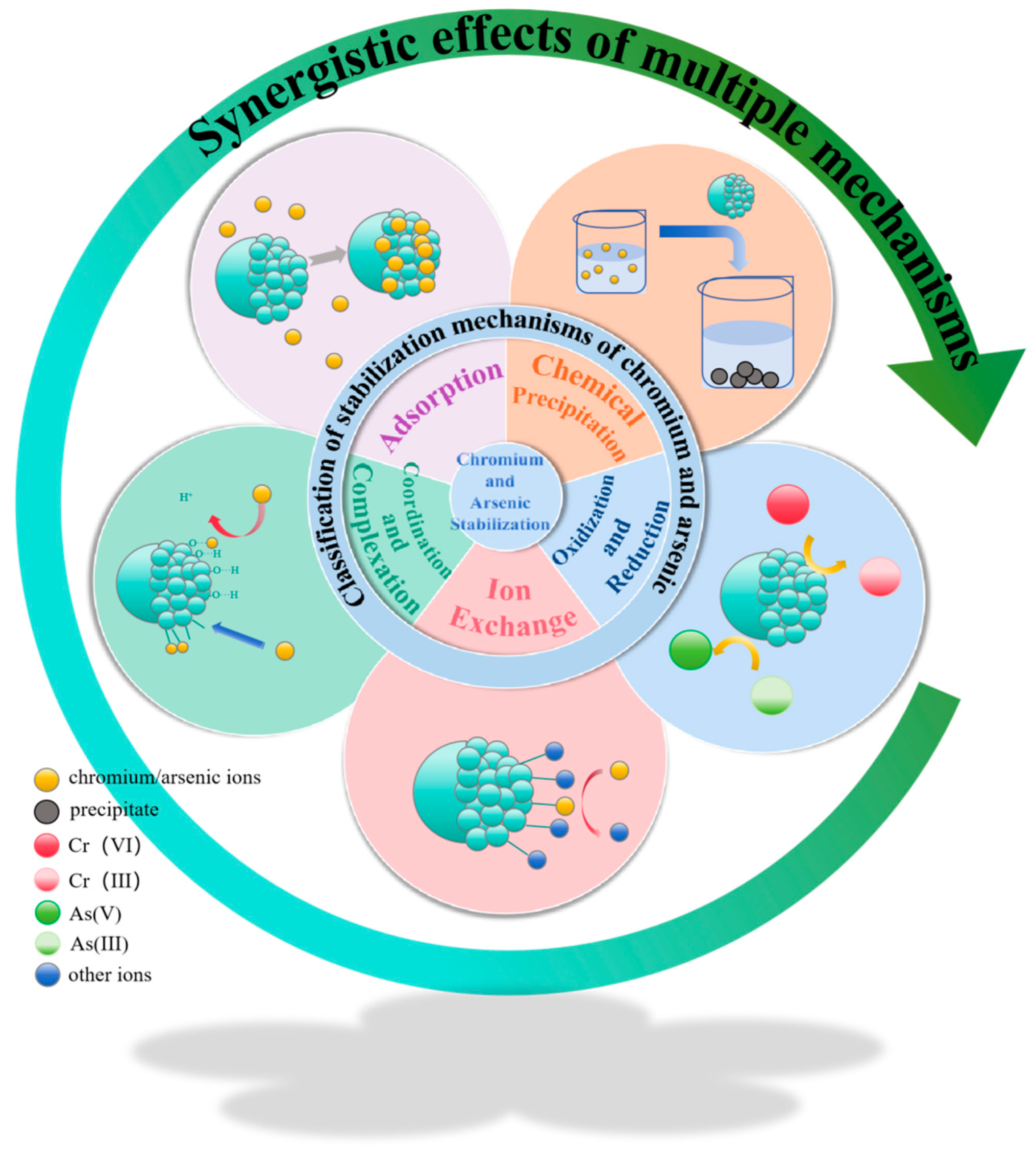
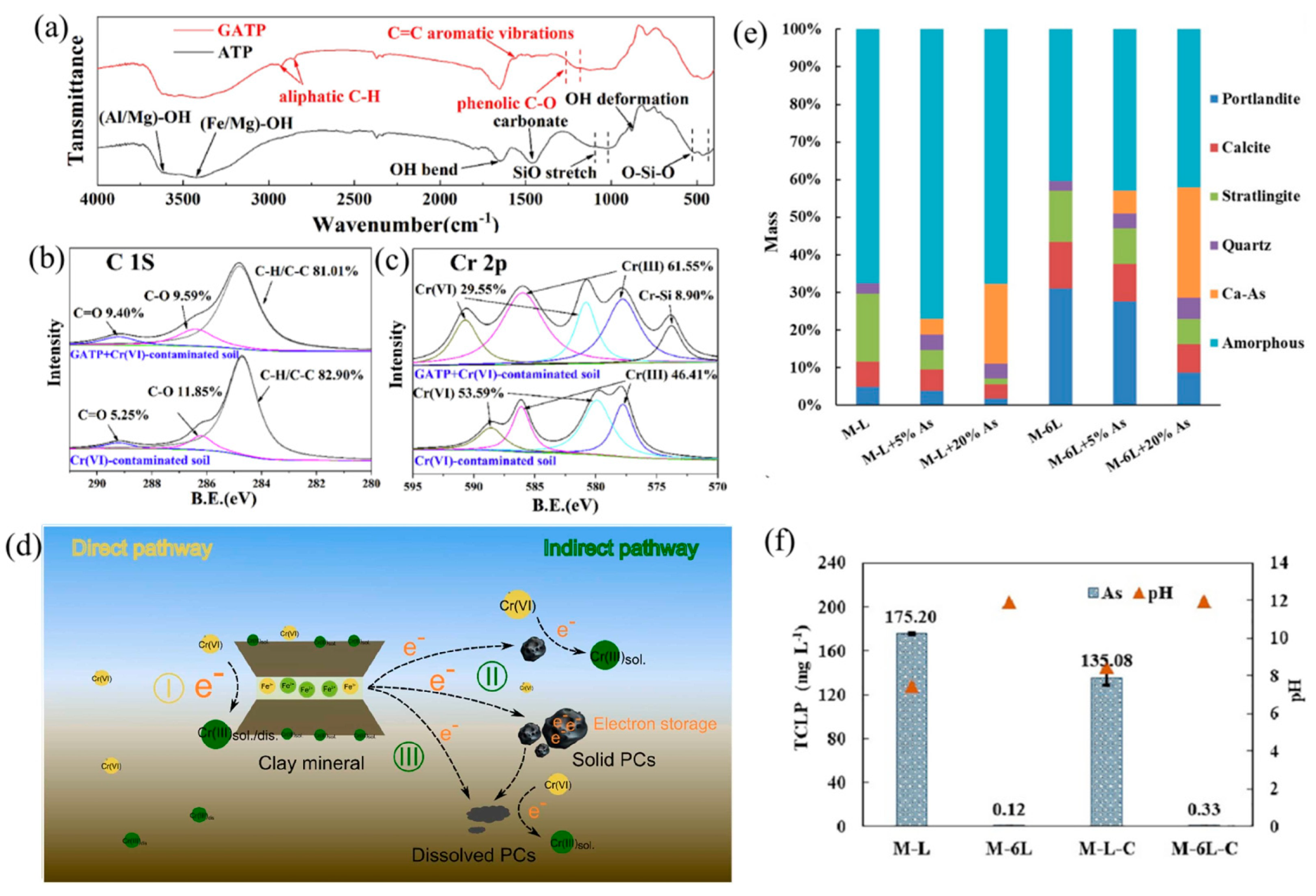

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Dang, Z.; Wang, Y.; Fan, H.; Miao, S. Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil. Appl. Sci. 2025, 15, 7069. https://doi.org/10.3390/app15137069
Wang A, Dang Z, Wang Y, Fan H, Miao S. Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil. Applied Sciences. 2025; 15(13):7069. https://doi.org/10.3390/app15137069
Chicago/Turabian StyleWang, Anqi, Zhiwen Dang, Yibo Wang, Hui Fan, and Shiding Miao. 2025. "Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil" Applied Sciences 15, no. 13: 7069. https://doi.org/10.3390/app15137069
APA StyleWang, A., Dang, Z., Wang, Y., Fan, H., & Miao, S. (2025). Efficient Inorganic Stabilization Materials for Chromium and Arsenic Pollution in Water and Soil. Applied Sciences, 15(13), 7069. https://doi.org/10.3390/app15137069