Abstract
Horticultural substrates based on coconut fibre are among the most commonly used growing media, but with limited durability. This study presents methods for modifying coconut fibre through surface coating with biopolymers, where polymerisation was initiated in the applied solution. Additionally, the suitability of adding pelletised biochar was analysed. A biomonomer derived from wood processing was used both for fibre surface modification and for binding biochar into pellets. Surface modification through biopolymer coating resulted in changes to selected physicochemical properties. It was found that the coating significantly altered the porosity of the substrate. Depending on the type of coconut fibre, the differences in porosity compared to the unmodified substrate ranged from 12 to 24%. This directly influenced bulk density, which is a crucial parameter in the preparation of substrates for plant growth. The surface properties of the modified substrates also affected sorption and retention capacities. From the perspective of plant production, the supply of bioavailable forms of potassium and phosphorus is essential. Coating and the addition of pelletised biochar, regardless of the type of fibre used, significantly increased the release of PO43− and K+ compared to unmodified substrates. The physiological parameters in plants confirmed the suitability of the modified substrates for plant production.
1. Introduction
Modern agriculture is increasingly developing soilless cultivation methods that allow for plant production under conditions of limited or entirely eliminated contact with soil. In such systems, soil is replaced by mineral substrates, primarily mineral wool and rock wool [1]. These methods, including hydroponics, aeroponics, and cultivation in organic and mineral substrates, aim to improve the efficiency of water, nutrient, and growing space utilisation [2]. However, after the substrates’ period of use, disposal becomes necessary, which generates environmental concerns and affects the profitability of crop production [3].
Among the various materials used as soilless substrates, natural materials hold a particularly important place, chief among them coconut fibre (Cocos nucifera), a by-product of the coconut industry. Coconut fibre is characterised by high porosity, good water retention capacity, appropriate spatial structure, and relatively stable chemical composition. It possesses natural antifungal properties, and its low pH and electrical conductivity (EC) make it suitable for a wide range of vegetable and horticultural crops [4]. Nevertheless, certain limitations such as structural instability over longer periods of use and variability in surface properties have prompted the search for ways to improve these characteristics.
One approach to enhancing substrate durability and extending its lifespan is the use of additives or coatings on coconut fibres. Although synthetic materials can be effective in this regard, their use in coating processes may lead to additional issues due to the non-biodegradable nature of the modified materials. An alternative solution may lie in the use of various biomaterials. Biopolymers, natural polymers derived from plant, animal, or microbial sources, are highly environmentally compatible and biodegradable. Examples include chitosan, sodium alginate, carboxymethyl cellulose, modified starch, and gelatine. Their use in materials engineering, agriculture, and environmental protection is rapidly expanding due to the growing importance of sustainable development [5].
A promising solution may involve the use of natural-origin monomer systems in which the polymerisation process can be initiated in a controlled manner. This is particularly relevant because biopolymers often exhibit properties that hinder coating processes, whereas using monomer solutions allows the coating to be effectively achieved once polymerisation has been initiated [6,7].
An additional component of coconut-fibre-based horticultural substrates may be biochar, obtained through biomass processing. This additive modifies the surface characteristics of the substrates in which it is used, significantly affecting their properties, particularly sorption and retention capabilities [8,9]. To date, research on substrates has primarily focused on the use of unmodified coconut fibre as a cultivation medium [10,11]. The application of biopolymer coatings represents a novel research direction that could significantly enhance the functionality of this material within soilless cultivation systems.
The aim of this study was to demonstrate the applicability of coating coconut fibre—used as a plant-growing substrate—with a polymer coating obtained through the polymerisation of an isolate produced during fibreboard manufacturing, rich in biomonomers, depending on selected physical characteristics of different types of coconut fibre. For this purpose, modified horticultural substrates with varying physicochemical properties were prepared, influencing the mobility of selected nutrients.
2. Materials and Methods
2.1. Characteristics of Coconut Fibres
Two types of coconut fibres were used to produce the substrates: coconut fibre variant A (Ceres, Pyzdry, Poland) and variant B (Legro, Legro Group, Helmond, The Netherlands), differing in their physical parameters.
2.2. Biopolymer Coating Production and the Coating Process
The selected types of coconut fibres used as horticultural substrates were coated with a layer in which the polymerisation of biocomponents was initiated, following the methodology described by Kuboń et al. [12]. The coating was produced through polymerisation using a wood-derived isolate (Fibro Lep, Fibris, Przemyśl, Poland), as described by Kuboń et al. [12].
2.3. Production of Pelletised Biochar
The pelleted biochar was produced from powdered biochar with the addition of a polymerising wood-derived isolate. The powdered biochar was obtained via batch pyrolysis of sunflower husk biomass at a temperature of 450–550 °C. The conversion of biomass into powdered biochar contributed to CO2 sequestration, as the carbon contained in the plant material was stabilised in a solid form (biochar), rather than being released into the atmosphere as carbon dioxide.
The process of producing pelletised biochar, used as an additive to coconut fibre substrates, was carried out in accordance with the methodology described by Kuboń et al. [12].
2.4. Variants of Horticultural Substrates
The following variants of horticultural substrates were produced and analysed:
- ▪
- AW_0: unmodified coconut fibre—variant A
- ▪
- AW_1: coconut fibre A modified with a 10% addition of pelletised biochar
- ▪
- AW_2: coconut fibre A modified by coating with biopolymer layers
- ▪
- AW_3: coconut fibre A modified by coating with biopolymer layers and a 10% addition of pelletised biochar
- ▪
- BW_0: unmodified coconut fibre—variant B
- ▪
- BW_1: coconut fibre B modified with a 10% addition of pelletised biochar
- ▪
- BW_2: coconut fibre B modified by coating with biopolymer layers
- ▪
- BW_3: coconut fibre B modified by coating with biopolymer layers and a 10% addition of pelletised biochar.
The effectiveness and suitability of the modified substrates were evaluated in raspberry production [12]. A single-factor experiment was conducted using greenhouse cultivation of the dessert raspberry cultivar Malling™ Bella. Long-cane plants were planted in 11-litre production pots filled with the tested substrates. The pot experiment was carried out in a completely randomised design, in three independent series. In each series, every experimental variant was replicated twelve times. The plants were fertilised using a fertigation system based on a nutrient substrate with an appropriate electrical conductivity (EC), applying the same water dosage across all variants.
2.5. Measurement of Selected Physical Properties of the Produced Substrates
Coconut fibres variant A and B (used as the substrate base), as well as the modified substrates produced, were subjected to an evaluation of selected physical properties to assess the effect of biopolymer coating on the degree of surface modification of horticultural substrates. The following properties were determined: porosity, according to the methodology described by Kuboń et al. [12]; bulk density, following the procedure outlined by Campbell [13]; moisture content, measured gravimetrically at 105 °C using a Ragwag MA50.R.N moisture analyser (Ragwag, Radom, Poland); and susceptibility to compaction, based on the method described by Saffih-Hdadi et al. [14].
2.6. Assessment of Electrical Conductivity (EC) and pH of the Analysed Substrates
The pH of the produced substrates was measured in aqueous suspensions using the potentiometric method, with a Hanna Instruments HI 5222 pH meter (Nusfalău, Romania). The aqueous suspension of the substrate samples was prepared by mixing homogenised substrate with deionised water in a volume-to-volume ratio of 1:10. After preparation, the samples were left to stand for 24 h, after which the pH measurements were taken. The same solutions were used to determine the electrical conductivity (EC), which was measured by the conductometric method using an HI 2316 EC-meter from Hanna Instruments (Nusfalău, Romania).
2.7. Measurement of the Release Capacity of Selected Inorganic Ions and Soluble Forms of Micronutrients and Heavy Metals from the Analysed Substrates
The content of selected inorganic ions—cations and anions (Ca2+, Mg2+, K+, and PO43−)—was determined using a leaching test. For this purpose, individual substrate variants were leached with deionised water over a period of seven weeks. Each day, 1 dm3 of substrate was flushed with 1 dm3 of deionised water, corresponding to the volume of water used under real fertigation conditions, carried out five times per day. In laboratory conditions, deionised water was applied to the substrates five times a day, in 200 mL portions. The resulting leachate was collected cumulatively and then analysed for the content of individual inorganic ions. The results were presented as weekly means over the duration of the experiment. The length of the leaching test was dependent on the water retention properties of the respective substrates, as described in Section 2.7. The content of inorganic ions in the leachates was determined using ion chromatography, with a DIONEX 5000+ system (Thermo Fisher Scientific, Waltham, MA, USA).
The content of soluble forms of micronutrients and potentially toxic trace elements in the produced substrates was determined after extraction in 1M HCl (Merck, Darmstadt, Germany), using a substrate-to-solution ratio of 1:10 (v/v). The extraction was conducted over a period of two hours, with continuous shaking on a rotary shaker. After filtration, the extracts were analysed for soluble forms of Fe, Mn, Zn, Cu, Ni, Pb, and Cd using atomic absorption spectrometry (AAS), with a HITACHI Z-2000 instrument (Tokyo, Japan).
2.8. Water Retention Properties of the Substrates
The produced substrates were analysed for their water retention capacity, which is of considerable importance in horticultural production. For this purpose, the maximum water holding capacity (MWHC) of the analysed substrate variants was determined. To assess MWHC, samples were placed in metal cylinders with a volume of 1 dm3, loosely filled with the prepared substrate variants (with 10 replicates for each variant). The prepared samples were then placed in containers filled with water, gradually submerging the cylinders. The samples were left for 24 to 48 h to reach full water saturation. After this period, the samples were removed from the water and allowed to drain freely for approximately 15 min. The fully saturated substrates were weighed and then placed in a forced-air electric oven, where they were dried at 105 °C to constant weight. MWHC was calculated based on the difference between the mass of the water-saturated sample and its dry mass.
In addition to MWHC, the water retention ability of the substrate variants was assessed from a completely dry state to full saturation. For this purpose, 1 dm3 of water was poured daily through 1 dm3 of each produced substrate. During this test, the water retention capacity of the substrates was monitored. The experiment continued until a decrease in the amount of retained water was observed in all analysed substrates, which, under our conditions, was achieved after a period of seven weeks.
2.9. Statistical Analysis
The obtained results were analysed using the STATISTICA 13.1 software (StatSoft, Palo Alto, CA, USA). One-way analysis of variance (ANOVA) and Tukey’s post hoc test (α = 0.05) were used to show the differences between the effects of the applied variable factors on the analysed properties.
2.10. Determination of the Suitability of Applied Substrate Modifications for Plant Production
The produced substrates were tested in raspberry pot cultivation conducted under protected conditions, following the methodology described by Kuboń et al. [12]. The suitability of the applied substrate modifications for dessert raspberry production was evaluated by measuring chlorophyll content using the SPAD method with a SPAD-502 Plus chlorophyll meter (Konica Minolta Co., Chiyoda, Japan). This parameter serves as an indicator of plant growth, development, and health status. Additionally, the effect of the modified substrates on the fruit yield per raspberry plant was determined. The measurement of relative chlorophyll content was carried out in the first and second years of raspberry cultivation, with 12 repetitions for each experimental variant.
3. Results and Discussion
3.1. Physical Parameters of Modified Horticultural Substrates
Coconut fibre is a natural material with proven suitability as a horticultural substrate for plant cultivation. However, the main drawback of such a substrate is its limited durability. During the cultivation process, coconut fibre loses volume relative to its original state, which is a result of its degradation [15]. This phenomenon restricts the transport of nutrients delivered to plants via the retention system, primarily due to a loss of retention capacity. As a consequence, these substrates have a limited lifespan, typically lasting up to two growing seasons in container cultivation. The defined lifespan of the substrate directly affects the profitability of production.
One approach to extending the durability of coconut substrates is surface modification of the fibres. However, this process involves the introduction of additional components, which should be of natural origin and exhibit a high degree of biodegradability. Such a method was described by Kuboń et al. [12], who demonstrated that such coatings can be successfully applied in horticultural production. To date, studies have confirmed no negative impact of surface modification on plant growth and development, as assessed by physiological parameters, as well as on the quality of raspberry fruits (vitamin C content, total polyphenol content, and total antioxidant capacity). However, the studies were conducted using only one type of coconut fibre and were limited to selected substrate parameters.
The modified substrates produced in this study were assessed in terms of selected physical properties, changes in which reflect the degree of modification of the coconut fibre surface. The addition of pelletised biochar reduced the porosity of the substrate; however, the impact varied depending on the type of coconut fibre used [16]. Coconut fibre of variant A with the addition of biochar showed a 21% reduction in porosity (AW_1), whereas variant B showed only an 11% reduction (BW_1). This decrease was attributed to the type of coconut fibre used, as the biochar addition was identical in both substrate variants, amounting to 10% by weight.
Surface coating of the coconut fibres with biopolymer, as expected, reduced the specific surface area of the fibres by 12.7% (AW_2) and 24% (BW_2), respectively. As reported by Velayutham et al. [17], coating porous fibres with polymer layers generally reduces their specific surface area due to the surface smoothing process. This trend was observed in the present study for the tested coconut fibre types following biopolymer coating. The smaller reduction observed for fibres with higher initial porosity indicates a lower degree of surface modification, which may result in increased susceptibility to substrate degradation during use [18].
The combination of the proposed modifiers for coconut-fibre-based horticultural substrates—namely the biopolymer coating and the addition of pelletised biochar—resulted in increased porosity of the tested substrates. The change in porosity directly influenced the bulk density of the substrates. The trend in bulk density was inversely proportional to that of porosity (Table 1). Materials with a low specific surface area, and consequently low porosity, are characterised by higher bulk density, which is related to a tendency toward fibre agglomeration [19].

Table 1.
Physical parameters of the investigated horticultural substrates.
However, the compaction susceptibility of the substrate depends solely on the type of coconut fibre used. The biopolymer coating process and the addition of pelletised biochar had virtually no effect on this parameter (Table 1).
3.2. Ability to Release Selected Mineral Components into the Aqueous Phase
3.2.1. Electrical Conductivity (EC) and pH of the Substrates
The two types of coconut fibre used to produce the analysed substrate variants differed significantly in terms of pH. In the control treatments based on coconut fibres A and B, the average pH values were 5.85 and 6.40, respectively, indicating an acidic and slightly acidic reaction (Figure 1). Modification of coconut fibre A with the analysed additives significantly increased the pH, with the highest value recorded in variant AW_3. It should be noted that, in the case of fibre A, the applied coating additives led to a notable shift in the substrate’s pH classification—from acidic in AW_0 to slightly acidic in AW_1 and AW_2, and to neutral in AW_3. These changes can be considered beneficial, as such pH levels are optimal for the growth and development of most plants, primarily due to improved nutrient availability, which directly supports plant growth and development [20]. In the case of coconut fibre B, the changes were somewhat less pronounced. Nevertheless, all the additives used to modify the base substrate also resulted in an increase in pH, similar to the effects observed in fibre A (Figure 1).
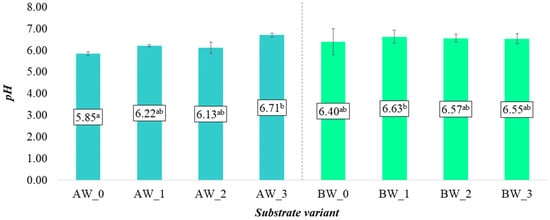
Figure 1.
pH of aqueous solutions from the produced substrates (mean ± SD). NOTE: Mean values ± standard error. (a–b) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
The coating process and additives used to modify the substrates also had a significant effect on changes in the electrical conductivity (EC) of the analysed aqueous solutions (Figure 2). According to the presented data, the average EC values for both types of coconut fibre were lowest in the control treatments and increased following the application of both pelletised biochar and the combination of biochar and isolate. Comparing the two types of coconut fibre, substantially higher EC values were recorded in coconut fibre B. For this variant, average EC values ranged from 0.14 to 0.48 mS cm−1, with the highest value observed in substrate BW_3. In contrast, substrates produced from coconut fibre A exhibited much lower average EC values, ranging from 0.08 mS cm−1 (AW_0) to 0.17 mS cm−1 (AW_1). In all cases, however, the EC values for the substrates in their initial state were relatively low. The observed changes are attributable to both the applied additives and the type of coconut fibre used. The issue of EC in horticultural cultivation is related to the excessive retention of nutrients supplied with irrigation water, which can lead to a high concentration of salts in the upper layers of the substrate. As a result, this may reduce crop yields due to its negative impact on plant development [21].
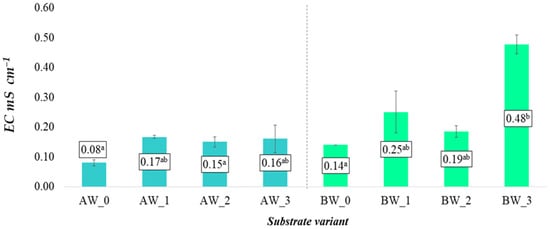
Figure 2.
EC of aqueous solutions from the produced substrates (mean ± SD). Mean values ± standard error. (a–b) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
3.2.2. Retention Properties
The water retention capacity (MWHC) of the substrates was significantly influenced by both the type of coconut fibre used and the nature of the additives incorporated to modify their physical and chemical properties (Figure 3). Comparing the two types of coconut fibres analysed, fibre B exhibited approximately twice the MWHC values compared to fibre A. This suggests that fibre B has superior water retention properties, possibly due to its higher porosity or distinct structural characteristics.
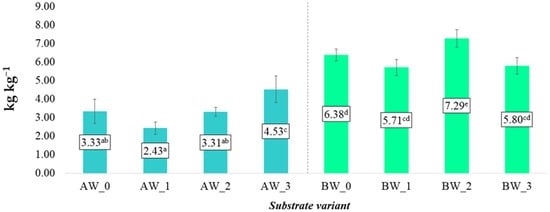
Figure 3.
Maximum water holding capacity (MWHC) of the substrates (kg kg−1) (mean ± SD). NOTE: Mean values ± standard deviation. (a–e) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
For substrates based on fibre A, the addition of biochar (AW_1) negatively affected the substrate’s water retention capacity, reducing MWHC compared to the control (AW_0). A similar trend was observed in the fibre-B-based substrates, where the inclusion of biochar (BW_1) led to a decrease in average MWHC values. This phenomenon can be attributed to the high porosity of biochar, which, while increasing the specific surface area of the substrate, may simultaneously limit capillary water retention within its structure.
The application of a biopolymer coating (AW_2 and BW_2) had a varied impact on MWHC depending on the type of fibre. In the case of fibre A (AW_2), MWHC values were slightly lower than for the control, possibly due to pore sealing and reduced wettability. Conversely, in variant BW_2, the use of the biopolymer significantly increased MWHC, achieving the highest level compared to all other variants. This indicates a synergistic interaction between the structure of fibre B and the biopolymer coating, enhancing its ability to bind water.
The most significant improvement in water retention for fibre A was observed when biochar was combined with the biopolymer (AW_3), where MWHC was substantially higher than in other variants. The synergistic effect of both additives likely led to favourable changes in pore structure and the fibre’s capacity to retain water molecules. In contrast, for fibre B in variant BW_3, despite the presence of both additives, MWHC decreased compared to the control, possibly indicating a compensatory effect or an unfavourable interaction between the additives within the specific structure of fibre B.
Changes in the water retention capacity of the analysed substrates are presented in Figure 4. In the control sample made from coconut fibre A (AW_0), an average and relatively unstable ability to retain water was observed, with a distinct decline noted in the 1st and 7th weeks of the experiment. The addition of biochar in variants AW_1 and AW_3 led to an improvement in initial water retention. However, no unequivocal positive effect of biochar on the maintenance of the substrate’s retention capacity over time was observed, as evidenced by a significant decrease in the amount of absorbed water, especially in the 7th week (60.91% for AW_1 and 67.58% for AW_3). The highest retention capacity among the AW variants was achieved in substrate AW_2 (with biopolymer) in the 4th week (91.21%) and 3rd week (88.79%), indicating that the biopolymer may significantly enhance the water properties of the substrate, particularly during the initial phase of plant growth.
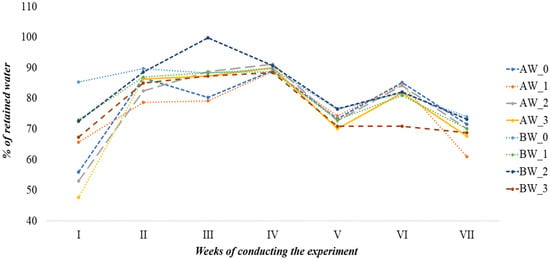
Figure 4.
Water retention dynamics of the produced substrates (%).
In contrast, for substrates based on coconut fibre B, a varied dynamic of water retention changes was also noted. In the control (BW_0), water retention was highest in the first weeks of the experiment, with the peak value in the 2nd week (89.67%). The addition of biochar in variants BW_1 and BW_3 did not unequivocally improve retention parameters. Variant BW_2, coated with biopolymer, achieved the greatest water retention capacity in the 3rd week of the experiment (99.77%).
The obtained results indicate that the applied biopolymer coatings have a significantly positive effect on the retention capacities of the substrates, especially during the most intensive period of plant growth (weeks 3–5). Conversely, despite biochar being a material with good sorption properties, it did not demonstrate a clearly positive impact on the retention parameters of the substrate in the conducted studies. Changes in the water holding capacity of the substrate after the addition of biochar were described in the study by Mejía et al. [20].
3.2.3. Content of Inorganic Ions (Ca2+, Mg2+, K+, PO43−) in the Produced Substrates (Leaching Tests)
The produced substrates were also analysed for the content of major nutrients in ionic forms available to plants. The analyses concerned the content of Ca, Mg, K, and P in forms accessible to plants.
The highest average content of Ca2+ extracted with water was found in the control variants for both types of coconut fibre, A and B (Figure 5). After the application of biochar and biopolymer, the average Ca2+ content decreased, which was observed for both analysed coconut fibre variants. This relationship may be due to the fact that the applied additives promote greater sorption of this element, meaning it is not easily leached from the substrate, which is characterised by high permeability, thereby resulting in increased retention and availability for the developing plant roots. A pertinent study by Chen et al. [22] investigated the effects of biochar amendments on the sorption and leaching of nitrate, ammonium, and phosphate in sandy soils. The researchers found that certain biochars significantly reduced the leaching of these nutrients, indicating enhanced sorption and retention within the soil matrix. This effect was particularly notable in sandy soils, which are characterised by high permeability and typically low nutrient retention [23].
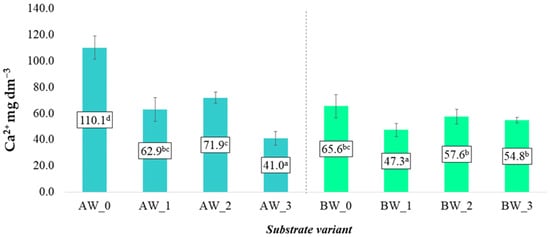
Figure 5.
Calcium cation content in aqueous solutions from the produced substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
In the case of Mg2+ ions, a similar trend was observed; however, this applied exclusively to substrates produced from coconut fibre A. Conversely, the opposite relationship was noted for substrates made from coconut fibre B. In this variant, the average content of Mg2+ ions was lowest in the BW_0 control variant and increased noticeably following the application of the tested additives, reaching the highest values in variant BW_3 (Figure 6).
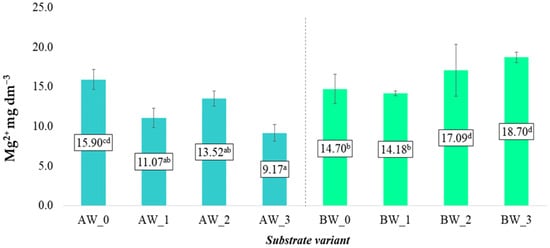
Figure 6.
Magnesium cation content in aqueous solutions from the produced substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
Unlike the observed trends for calcium (Ca2+) and magnesium (Mg2+) cations, the behaviour of potassium (K+) ions in the tested substrates showed different tendencies. Potassium is an element with high mobility and very good solubility in water, which makes it highly susceptible to leaching from the rhizosphere, particularly under conditions of intensive irrigation. For this reason, its availability to plants can vary significantly depending on the substrate composition and the presence of additives with sorption properties.
In this study, the lowest content of K+ ions in aqueous solutions was recorded in the control samples without modifying additives (AW_0 and BW_0), confirming the limited ability of coconut fibre, as a base material, to effectively retain and release potassium. The use of additives such as biochar and biopolymer significantly influenced the dynamics of K+ ion release into the soil solution. In all variants containing biochar (AW_1, AW_3, BW_1, BW_3), a significantly higher content of K+ in the solutions was observed, which can be explained by the fact that biochar itself constitutes a source of potassium. Moreover, its porous structure and cation sorption capacity may promote retention and gradual release of these ions [24].
Similarly, in the variants where only biopolymer coating was applied (AW_2, BW_2), elevated concentrations of K+ ions were found compared to the control samples, although these values were noticeably lower than those in the biochar variants. This may suggest that the biopolymer does not act as a direct source of potassium, and its influence on the K+ content in the solution may result from modifications to the physicochemical properties of the substrate (improving its structure or water retention), which in turn affects the solubility and mobility of mineral nutrients (Figure 7).
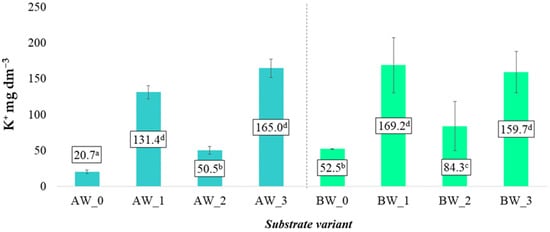
Figure 7.
Potassium cation content in aqueous solutions from the produced substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
The average content of phosphorus in the form of phosphate anions (PO43−) in aqueous solutions obtained from substrates based on coconut fibre types A and B ranged from 2.16 to 9.01 mg dm−3 and 3.36 to 7.61 mg dm−3, respectively (Figure 8). These values indicate a varied availability of phosphorus depending on the applied substrate modification variant.
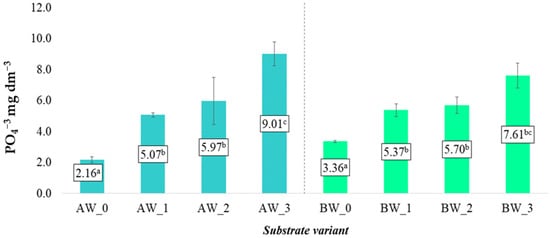
Figure 8.
Phosphate anion content in aqueous solutions from the produced substrates (mean ± SD). Mean values ± standard deviation. (a–c) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
Similarly to the observations regarding potassium ions (K+), the use of additives—including biochar and/or biopolymer—significantly influenced the increase in average concentrations of phosphate anions in the soil solution. The highest PO43− content was recorded in variants AW_3 and BW_3, i.e., in substrates where both biochar and a biopolymer coating were applied simultaneously (Figure 8). The elevated levels of PO43− in these variants may result from the synergistic effect of both additives. Although biochar itself is not a significant source of phosphorus, it can indirectly enhance its availability by altering the sorption and buffering properties of the substrate, as well as by limiting phosphorus fixation. Meanwhile, the biopolymer coating may reduce the leaching rate of nutrients and influence the stability of the microenvironment around the root zone, which could contribute to better phosphorus retention [25].
Compared to the control samples (AW_0, BW_0), all variants with additives showed an increase in PO43− content, confirming the beneficial effect of substrate modifications on improving the availability of this nutrient. The results indicate the potential effectiveness of biochar and biopolymers in optimising phosphorus release and retention in horticultural substrates.
3.2.4. Content of Soluble Forms of Microelements and Trace Elements
The produced substrates were also evaluated for the content of soluble (available) forms of microelements and potentially toxic elements. In particular, the concentrations of soluble forms of iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu), as well as nickel (Ni), lead (Pb), and cadmium (Cd), were analysed.
In the case of iron (Fe), which plays an important role in metabolic processes and photosynthesis, significant differences were observed between the two types of coconut fibre [26]. Coconut fibre A was characterised by an average soluble Fe content approximately twice as high as that of fibre B. These differences may result both from the different origins of the raw material and from the methods of its processing. Additionally, the applied modifiers in the form of biochar and/or coatings by biopolymer influenced the iron content in the aqueous solutions extracted from the substrates. In most cases, an increase in soluble Fe concentration was recorded compared to the control samples, suggesting that these additives may improve the availability of microelements in the plant root zone (Figure 9). This effect may be related to biochar’s ability to adsorb and slowly release nutrients, as well as the influence of the biopolymer on the physicochemical properties of the substrate, including its sorption capacity.
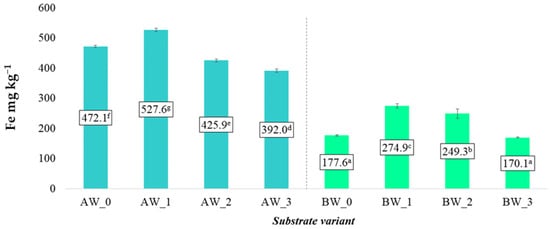
Figure 9.
Content of soluble iron forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–g) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
Similar trends were also observed regarding the content of soluble manganese (Mn) forms. It was demonstrated that the content of available Mn in the substrates varied significantly depending on the additive used and the type of coconut fibre (Figure 10). The greatest increase in soluble manganese content was recorded in variants with the addition of biopolymer isolate, where the average Mn content in aqueous extracts was up to five times higher compared to the control samples (AW_0 and BW_0) (Figure 10). This effect was consistent for both fibre A and B, suggesting that the additive used, rather than the base raw material, had the greatest influence on the mobility and availability of this microelement.
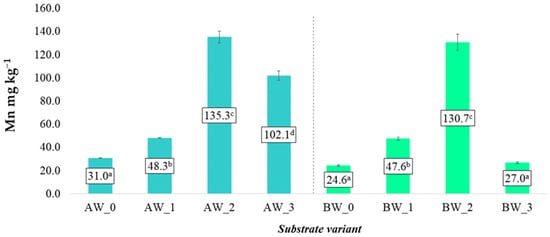
Figure 10.
Content of soluble manganese forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
This phenomenon may result from interactions between the biopolymer and the organic fraction of the substrate, leading to increased solubility of Mn or reduced sorption [27]. Additionally, changes in the physicochemical parameters of the substrate—such as pH or cation exchange capacity—may have promoted the enhanced release of manganese into the soil solution.
In the case of soluble zinc (Zn) content, the observed changes were more varied and dependent on the type of coconut fibre used as well as the type of modifying additives (Figure 11). For fibre A, the average Zn content in aqueous solutions did not undergo significant changes regardless of the variant applied (AW_1–AW_3), indicating a limited impact of additives such as biochar or biopolymer on the mobility of this element.
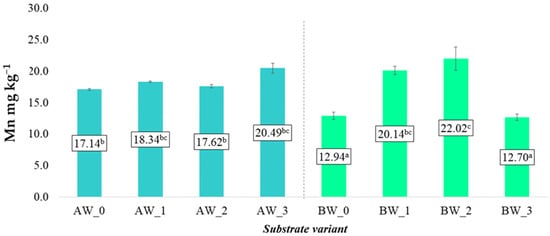
Figure 11.
Content of soluble zinc forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–c) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
In the case of fibre B, a significant increase in Zn content was observed only in variants BW_1 and BW_2, i.e., following the application of biochar and coating biopolymer, respectively. This may suggest that fibre B, due to its different physicochemical structure, exhibits greater susceptibility to changes in Zn bioavailability under the influence of the applied additives.
The increased solubility of Zn in these variants may be linked to improved sorption conditions or changes in the pH of the extracts, which affects the greater mobility of microelements in the root zone. The obtained results indicate that appropriate modification of the substrate composition—also dependent on the type of fibre used—may play a significant role in optimising Zn availability for plants under horticultural cultivation conditions.
In the case of soluble forms of copper (Cu) and lead (Pb), the application of the examined modifying additives did not result in significant changes in their concentrations in aqueous extracts, regardless of the type of coconut fibre used (A or B). The average Cu and Pb levels in the aqueous solutions remained at a similar level across all experimental variants compared to the control samples, indicating a limited impact of the applied additives on the mobility of these elements under laboratory conditions (Figure 12 and Figure 13).
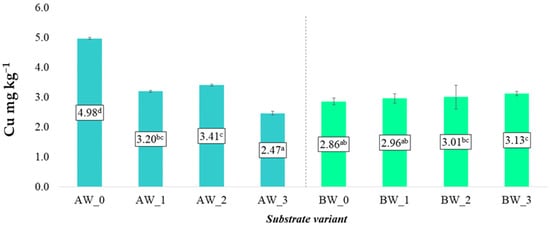
Figure 12.
Content of soluble copper forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
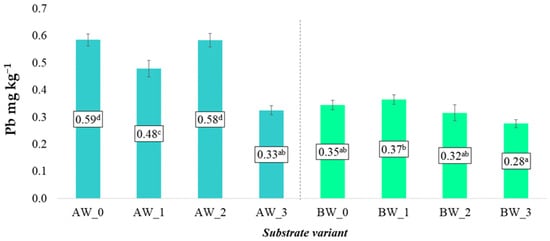
Figure 13.
Content of soluble lead forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
However, it should be noted that in some variants (particularly BW_1 and AW_2), a tendency towards a slight reduction in Cu and Pb content was observed compared to the respective control samples. This may suggest partial binding of these elements by the biochar structures, which are characterised by high porosity and adsorption capacity for binding heavy metals. A similar mechanism may have occurred with the addition of the biopolymer, which could limit metal mobility by forming complexes or by influencing the redox potential of the root environment [28].
From the perspective of food crop safety, the tendency to limit the availability of Cu and Pb may be considered beneficial, especially in the context of the accumulation of these elements in the edible parts of plants. Although Cu and Pb concentrations in the analysed substrates were within safe ranges, further studies should consider long-term changes in the availability of these metals under real cultivation conditions, as well as their potential accumulation in plant tissues.
Different trends were observed in the case of soluble forms of nickel (Ni) and cadmium (Cd) (Figure 14 and Figure 15). Contrary to the previously discussed elements, the application of the modifying additives—both biochar and the biopolymer coating—significantly increased the content of these potentially toxic elements in the obtained aqueous extracts. This relationship was evident for both types of coconut fibre analysed (A and B), suggesting that the increased bioavailability of Ni and Cd was a result of the properties of the additives rather than the base material.
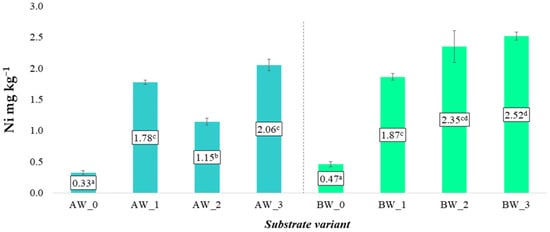
Figure 14.
Content of soluble nickel forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–d) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
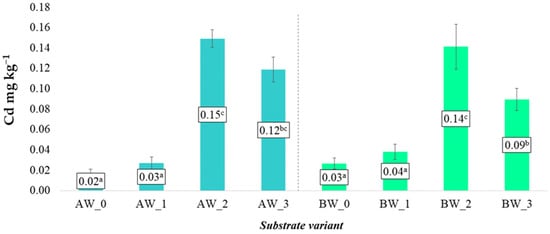
Figure 15.
Content of soluble cadmium forms in the prepared substrates (mean ± SD). NOTE: Mean values ± standard deviation. (a–c) Identical superscripts denote no significant (p < 0.05) differences between the experimental objects according to the post hoc Tukey HSD test.
The increase in Ni and Cd content in the leachates following the application of biochar may be due to its chemical composition—particularly if the raw material used for biochar production contained trace amounts of these metals. In the case of the biopolymer coating, the increased mobility of Ni and Cd may also be a consequence of the formation of soluble organic complexes or changes in the sorption potential of the substrate matrix.
Despite the observed increases in concentrations, the average levels of nickel and cadmium did not exceed the permissible limits set by applicable soil and earth quality standards. This means that all developed substrate variants are safe for use in agricultural and horticultural cultivation. However, from the perspective of long-term environmental and health safety, it is necessary to monitor the potential accumulation of these elements over time, especially with repeated use of the substrates or in closed systems where leaching and nutrient exchange are limited [29].
3.3. Usefulness of the Applied Substrate Modifications in Plant Production
The relative chlorophyll content in leaves, measured using the SPAD method, is a sensitive and non-invasive indicator of the physiological status of plants and the efficiency of photosynthesis. In crop cultivation on coconut-based substrates, this parameter can serve as a reliable indicator of substrate quality, reflecting its ability to provide adequate water–air and nutrient conditions for plant growth [30].
To determine the effect of the applied substrate modifications, SPAD measurements of the relative chlorophyll content were conducted on raspberry plant leaves. The developed substrate modifications did not significantly affect the chlorophyll content in raspberry leaves (Figure 16), indicating that all tested substrates provided a comparable growth environment to that of the traditional substrate composed solely of coconut fibres. Moreover, the use of the substrate, including the coating process involving the wood-derived isolate, had a positive effect on the quality of the produced fruit, as confirmed by Kuboń et al. [12]. The harvested raspberry fruit exhibited increased levels of phenolic compounds and vitamin C, as well as enhanced antioxidant potential.
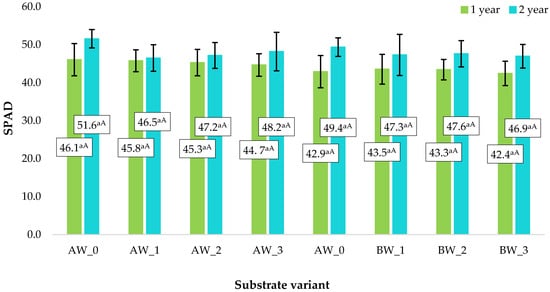
Figure 16.
Relative SPAD chlorophyll content in raspberry leaves depending on the applied substrate. NOTE: Different lowercase letters indicate significant differences between modifications of the same type of fibre, whereas uppercase letters denote significant differences between the same modifications applied to different fibres, with a significance level of α = 0.05.
4. Conclusions
This study presents novel modification methods for coconut-fibre-based horticultural substrates aimed at extending their durability and enhancing their physicochemical properties. The main modifications included coating fibres with biopolymers through in-solution polymerisation and the addition of pelletised biochar derived from wood-processing biomonomers.
- ▪
- The results demonstrated that biopolymer coatings significantly increased substrate porosity by 12–24%, which improves aeration and root respiration. Bulk density was reduced by up to 15%, enhancing substrate lightness and ease of handling. Water retention capacity increased by approximately 18%, contributing to better moisture availability during cultivation. Moreover, electrical conductivity values rose, indicating enhanced ion retention.
- ▪
- Crucially, nutrient dynamics were improved: the modified substrates exhibited a greater capacity to adsorb and gradually release essential macronutrients, notably phosphate ions (PO43−) and potassium ions (K+), with their availability increased by 20–30% compared to unmodified substrates. This improvement translated into better nutrient uptake by plants.
- ▪
- Physiological measurements of raspberries confirmed these benefits. The relative chlorophyll content in leaves increased by 15%, indicating improved plant health and nutrient status. These outcomes suggest that the modified substrates provide a more favourable growing medium for horticultural plants.
- ▪
- Additionally, the enhanced surface properties and structural stability of the modified coconut fibre substrates resulted in an operational lifespan exceeding two growing seasons, reducing the need for frequent substrate replacement and contributing to sustainability and cost-effectiveness in horticultural production.
The integration of biopolymer coatings and pelletised biochar into coconut fibre substrates effectively improved their physical and chemical characteristics, enhanced nutrient availability, and supported healthy plant growth over multiple cycles. These findings highlight the potential for commercial application and warrant further investigation into crop-specific optimisation and environmental impacts.
Author Contributions
Conceptualisation, M.B. and N.M.; methodology, M.B., N.M. and M.S.; investigation, M.B., N.M. and M.S.; formal analysis, M.K.; resources, M.K.; data curation, M.B., N.M., M.S. and M.K.; writing—original draft preparation M.B., N.M. and M.S.; writing—review and editing, M.B. and N.M.; visualisation, N.M. and M.S.; supervision, M.B. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to various file formats.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bougoul, S.; Ruy, S.; de Groot, F.; Boulard, T. Hydraulic and physical properties of stonewool substrates in horticulture. Sci. Hortic. 2005, 104, 391–405. [Google Scholar] [CrossRef]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the roots of aeroponic indoor farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques—Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.M.; Kunhamu, T.K. Nature-based solutions in agriculture: A review of the coconut (Cocos nucifera L.)-based farming systems in Kerala, “the Land of Coconut Trees”. Nat. Based Solut. 2022, 2, 100012. [Google Scholar] [CrossRef]
- Valle, C.; Voss, M.; Calcio Gaudino, E.; Forte, C.; Cravotto, G.; Tabasso, S. Harnessing Agri-Food Waste as a Source of Biopolymers for Agriculture. Appl. Sci. 2024, 14, 4089. [Google Scholar] [CrossRef]
- Niekraszewicz, A.; Wiśniewska-Wrona, M.; Kopania, E.; Orlikowski, L.; Pospieszny, H.; Krawczyk, K. Biopolymer compositions for ecological protection and growth stimulation of plants. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 149–162. [Google Scholar]
- Salachna, P.; Zawadzińska, A. Effect of chitosan on plant growth, flowering and corms yield of potted freesia. J. Ecol. Eng. 2014, 15, 97–102. [Google Scholar]
- Altland, J.E.; Locke, J.C. Effect of biochar type on macronutrient retention and release from soilless substrate. HortScience 2013, 48, 1397–1402. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Mejía, P.A.; Salas, M.C.; López, M. Coconut fiber: Evaluation of physicochemical properties and enzymatic activity in soilless culture during three cycles of organic cultivation. In XXX International Horticultural Congress IHC 2018, II International Symposium on Soilless Culture and VIII International; ISHS: Korbeek-Lo, Belgium, 2018; Volume 1273. [Google Scholar]
- Domeno, I.; Irigoyen, I.; Muro, J. New wood fibre substrates: Characterisation and evaluation in hydroponic tomato culture. Eur. J. Hortic. Sci. 2010, 75, 89–94. [Google Scholar] [CrossRef]
- Kuboń, M.; Matłok, N.; Szostek, M.; Wróbel, M.; Mudryk, K.; Sikora, J.; Marczuk, A.; Saletnik, B.; Balawejder, M. Modification of Coconut Fibers through Impregnation with Eco-Friendly Wood Based Isolate as a Method to Increase the Sustainability of Dessert Raspberries Production. Sustainability 2024, 16, 5878. [Google Scholar] [CrossRef]
- Campbell, D.J. Chapter 6—Determination and Use of Soil Bulk Density in Relation to Soil Compaction. In Developments in Agricultural Engineering; Soane, B.D., van Ouwerkerk, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 11, pp. 113–139. [Google Scholar] [CrossRef]
- Saffih-Hdadi, K.; Défossez, P.; Richard, G.; Cui, Y.J.; Tang, A.M.; Chaplain, V. A method for predicting soil susceptibility to the compaction of surface layers as a function of water content and bulk density. Soil Tillage Res. 2009, 105, 96–103. [Google Scholar] [CrossRef]
- Rayburn, L.; Jackson, B.E.; Mays, J.; Hewitt, J.; Fernandez, G. Pine bark as an alternative to coco coir for substrate production of long-cane raspberry in southeastern USA. Acta Hortic. 2024, 1388, 141–144. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Velayutham, T.; Manikandan, G.K.; Aruchamy, K.; Mylsamy, B.; Palaniappan, S.K.; Siengchin, S. 17—Coating of fibers and polymers with synthetic materials. In Elsevier Series on Tribology and Surface Engineering, Surface Modification and Coating of Fibers. Polym. Compos. 2005, 371–398. [Google Scholar] [CrossRef]
- Elfadel, R.G.; Refat, H.M.; El-Wahab, H.A.; Salem, S.S.; Owda, M.E.; Abdel Reheim, M.A.M. Preparation of new surface coating based on modified oil-based polymers blended with ZnO and CuZnO NPs for steel protection. Sci. Rep. 2023, 13, 7268. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Mejía, P.A.; Ruíz-Zubiate, J.L.; Correa-Bustos, A.; López-López, M.J.; Salas-Sanjuán, M.d.C. Effects of Vermicompost Substrates and Coconut Fibers Used against the Background of Various Biofertilizers on the Yields of Cucumis melo L. and Solanum lycopersicum L. Horticulturae 2022, 8, 445. [Google Scholar] [CrossRef]
- Méndez-Cifuentes, A.; Valdez-Aguilar, L.A.; Cadena-Zapata, M.; Alvarado-Camarillo, D.; González-Fuentes, J.A. Nutrient Solution Electrical Conductivity Affects Yield and Growth of Sub-Irrigated Tomatoes. Horticulturae 2023, 9, 826. [Google Scholar] [CrossRef]
- Chen, Z.; Kamchoom, V.; Chen, R.; Prasittisopin, L. Investigating the Impacts of Biochar Amendment and Soil Compaction on Unsaturated Hydraulic Properties of Silty Sand. Agronomy 2023, 13, 1845. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, A.; Moreno, F.J.; Cameán, A.M. Alterations observed in the endothelial HUVEC cell line exposed to pure Cylindrospermopsin. Chemosphere 2012, 89, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.; Ablouh, E.H.; El Bouchtaoui, F.Z.; Hannache, H.; Ghalfi, H.; Sehaqui, H.; El Achaby, M. Cellulose Nanofibers/Engineered Biochar Hybrid Materials as Biodegradable Coating for Slow-Release Phosphate Fertilizers. ACS Sustain. Chem. Eng. 2022, 10, 15250–15262. [Google Scholar] [CrossRef]
- Mogale, J.T.; Maluleke, M.K. The effect of varying coconut coir substrates and loam soil on the physiology, and biochemical constituents of tomato (Solanum lycopersicum var. esculentum) grown under greenhouse environment. Discov. Sustain. 2025, 6, 143. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Tao, S.; Xing, B. Sorption of Aromatic Organic Contaminants by Biopolymers: Effects of pH, Copper (II) Complexation, and Cellulose Coating. Environ. Sci. Technol. 2007, 41, 185–191. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, W.; Yuan, J.; Zhang, Q. Adsorption of Cu(II) and Pb(II) in Aqueous Solution by Biochar Composites. ACS Omega 2025, 10, 13816–13828. [Google Scholar] [CrossRef]
- Rahi, A.A.; Younis, U.; Ahmed, N.; Ali, M.A.; Fahad, S.; Sultan, H.; Zarei, T.; Danish, S.; Taban, S.; El Enshasy, H.A.; et al. Toxicity of Cadmium and nickel in the context of applied activated carbon biochar for improvement in soil fertility. Saudi. J. Biol. Sci. 2022, 29, 743–750. [Google Scholar] [CrossRef]
- Silva, J.D.S.; Mendes, J.D.O.; Costa, R.S.D.; Oliveira, A.R.F.; Braga, M.D.M.; Mesquita, R.O. Development of soybean plants using a substrate based on green coconut fiber. Agron. Colomb. 2021, 39, 47–58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).