Abstract
Glycation in biological systems contributes to the development of chronic diseases, particularly under conditions of hyperglycemia and oxidative stress. This study evaluated the antiglycation and methylglyoxal (MGO)-trapping capacities of aqueous and methanolic extracts of carob flour. The methanolic extract exhibited significantly higher bioactive compounds, containing 1.4-fold more total phenolics and 1.6-fold more flavonoids than the aqueous extract, as well as 1.2- and 1.8-fold-higher antioxidant activity. Antiglycation activity was assessed using bovine serum albumin (BSA)–glucose and BSA–MGO in vitro models, where the methanolic extract consistently outperformed the aqueous extract. At 25 mg/mL, the formation of advanced glycation end-products was inhibited by 81.0% in the BSA–glucose model and nearly 70% in the BSA–MGO model. These findings were supported by lower IC50 values for the methanolic extract (6.6 vs. 10.8 mg/mL and 9.4 vs. 16.6 mg/mL). MGO-trapping capacity was also higher for the methanolic extract, reaching 97% with 25 mg/mL after 168 h. The superior antiglycation and MGO-trapping activities of the methanolic extract are attributed to its higher content of gallic acid and other phenolic compounds with known bioactivities. These results highlight the potential of carob-based formulations as functional ingredients with preventive applications against glycation-associated pathologies.
1. Introduction
Carob flour (Ceratonia siliqua), obtained from the dried and ground pods of the carob tree, is a versatile food ingredient traditionally consumed in Mediterranean countries and increasingly appreciated worldwide for its nutritional, functional, and therapeutic properties [1]. The carob tree, a leguminous evergreen species native to arid and semi-arid climates, produces pods that are rich in sugars, dietary fiber, and a wide array of phytochemicals. These compounds are distributed between the pulp and the seeds, with the pulp being particularly rich in phenolic substances [2]. After dehulling and deseeding, the pulp is dried in ventilated ovens and mechanically milled to obtain commercial carob flour. Carob flour is most commonly derived from the pulp and has been used for decades as a natural sweetener or as a caffeine-free and theobromine-free alternative to cocoa powder, thanks to its mildly sweet flavor and characteristic brown color [3]. However, beyond its traditional uses in confectionery and baking, carob flour has attracted growing scientific attention for its potential health-promoting properties, largely attributed to its content of bioactive compounds, including phenolic acids (such as gallic, caffeic, and ferulic acids) and flavonoids (such as quercetin and catechins) [4].
Polyphenols are well known for their antioxidant activity, which involves several mechanisms such as free radical scavenging, inhibition of lipid peroxidation, and chelation of transition metals. These antioxidant properties play a crucial role in neutralizing oxidative stress, a key factor involved in the pathogenesis of numerous chronic conditions, including cardiovascular diseases, cancer, and diabetes mellitus [5]. In addition to their antioxidant effects, polyphenols are also being studied for their capacity to interfere with the formation of advanced glycation end-products (AGE), a group of harmful compounds that result from the Maillard reaction. The initial stage of AGE formation involves the reaction between reducing sugars and amino groups on proteins, leading to the formation of unstable Schiff bases. These intermediates undergo rearrangement to yield more stable Amadori products. Subsequent oxidative and degradative processes convert these early glycation products into highly reactive dicarbonyl compounds, such as methylglyoxal (MGO) and 3-deoxyglucosone [6]. MGO can rapidly react with nucleophilic groups in proteins, forming stable adducts that compromise protein structure and function. AGE accumulate naturally in the body over time but are markedly elevated in pathological conditions such as hyperglycemia and chronic inflammation, contributing to tissue damage and dysfunction. Their accumulation has been associated with the progression of diabetic complications, atherosclerosis, kidney dysfunction, and neurodegenerative diseases such as Alzheimer’s [7]. Therefore, evaluating the ability of food-derived compounds to trap or neutralize MGO, as well as their effectiveness in inhibiting protein glycation, has become an important research strategy in identifying dietary agents that may counteract glycation-related damage [8].
Therefore, the main objective of this study is to comprehensively evaluate the antioxidant and antiglycation potential of aqueous and methanolic extracts of carob flour. Although carob has been traditionally used in food applications, its comparative effectiveness in mitigating glycation—particularly through methylglyoxal trapping—has not been thoroughly studied. This work provides novel insights by using two in vitro glycation models (bovine serum albumin (BSA)–glucose and BSA–MGO) and assessing the direct MGO-trapping capacity, offering a dual approach to understanding both preventive and direct antiglycation mechanisms.
2. Materials and Methods
The methodology used in this study has been previously described by Mesías et al. [9], with minor modifications as detailed below.
2.1. Reagents and Chemicals
Pyridoxamine dihydrochloride (PM), Folin–Ciocalteu reagent, ferric chloride hexahydrate, sodium acetate, disodium phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, sodium chloride, potassium chloride, sodium carbonate, ethanol, and methanol (99.5%) were obtained from Panreac (Barcelona, Spain). D(+)-glucose, bovine serum albumin, methylglyoxal, sodium azide, aminoguanidine (AG), 5-methylquinoxaline (5-MQ), o-phenylenediamine (OPD), Trolox, phenolic acid standards, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) reagent, potassium persulfate, aluminum chloride hexahydrate, and quercetin hydrate were all purchased from Sigma (St. Louis, MO, USA). All additional chemicals, solvents, and reagents used in this study were of analytical grade.
2.2. Extractions
Carob flour (Ceratonia siliqua), certified for organic cultivation, was purchased from a local market in Madrid, Spain (Lot HAL051030). The nutritional composition declared on the package label per 100 g of product was as follows: energy, 293 kcal; fat, 0.50 g; saturated fat, 0.0 g; total carbohydrates, 51.0 g; sugars, 37.7 g; total dietary fiber, 35.8 g; protein, 4.46 g; and salt, 0.03 g. Extracts were prepared by homogenizing 250 mg of the flour in either 25 mL of distilled water or a 50% (v/v) methanol solution to obtain aqueous and methanolic extracts, respectively. The mixtures were vortexed for 15 min at 50 °C. After extraction, the solid residue was separated by centrifugation at 3950× g for 10 min at 4 °C. The resulting supernatants were collected and filtered through Whatman No. 1 filter paper. This extraction process was performed twice, and the combined supernatants were used for further analysis. For methanolic extracts, the organic solvent was removed by evaporation using a TurboVap-LV evaporator (Biotage, Uppsala, Sweden) at 40 °C. All final extracts were frozen at −80 °C and subsequently lyophilized. The dried extracts were weighed and stored at 4 °C until analysis. Solubility was calculated and expressed as the g of solids in the soluble fraction per 100 g of sample (%, w/w).
2.3. pH Measurement
A 250 mg portion of the lyophilized extract was mixed with 10 mL of either water (for aqueous extract) or 50% methanol (for methanolic extract). The mixture was vortexed for 3 min, allowed to stand at room temperature for 1 h, and then centrifuged at 3910× g for 10 min at 4 °C. The pH of the resulting supernatants was measured using a CG-837 pH meter (Schott, Mainz, Germany). Analyses were performed in duplicate.
2.4. Determination of Total Phenolic Acids
Phenolic acids—including caffeic acid (CA), chlorogenic acid (CGA), ferulic acid (FA), gallic acid (GA), gentisic acid (GE), protocatechuic acid (PCA), p-coumaric acid (pCU), p-hydroxybenzoic acid (pHB), sinapinic acid (SIN), syringic acid (SYN), and vanillic acid (VA)—were quantified using a chromatographic method based on the procedure described by Mesías et al. [10], with slight modifications. In brief, 150 mg of each lyophilized extract was mixed with 2.5 mL of 2 mol/L sodium hydroxide, 2.5 mL of a 20 mL/L ascorbic acid solution containing 13.4 mmol/L EDTA (ethylenediaminetetraacetic acid), and 0.5 mL of 0.1 mg/mL isoferulic acid, which was used as the internal standard. The mixture was flushed with nitrogen gas and allowed to hydrolyze under constant agitation for 16 h at room temperature. After hydrolysis, the sample was centrifuged at 2370× g for 10 min at 4 °C, and the supernatant was acidified by adding 0.75 mL of acetic acid. Phenolic acids were extracted with ethyl acetate in two steps (4 mL followed by 2 mL). The organic layers containing the liberated phenolic acids were collected by carefully pipetting off the upper layer and then combined. The pooled extracts were evaporated to dryness using a speed-vac concentrator (Thermo Fisher Scientific, Courtaboeuf, France) at 45 °C for 1 h. The resulting residue was reconstituted in 1 mL of methanol/water (75:25, v/v) and filtered through a 0.45 μm membrane before HPLC analysis. The analysis was carried out in duplicate using a Shimadzu HPLC system (Kyoto, Japan) equipped with an LC-20 CE pump, an SIL-10ADvp autosampler, a CTO-10ASVP oven, and an SPD-M20A diode array detector. Results were expressed as μg/g of sample. The limit of quantification was established at 4 μg/g.
2.5. Total Phenolic Content Determination
The total phenolic content (TPC) of the lyophilized extracts was measured using the Folin–Ciocalteu colorimetric method, with slight modifications from the protocol described by Mesías et al. [10]. Each extract (75 mg) was dissolved in 3 mL of distilled water (for aqueous extract) or in a 50% (v/v) methanol solution (for methanolic extract). For the assay, 250 μL of the appropriately diluted sample was combined with 250 μL of Folin–Ciocalteu reagent (pre-diluted 1:1, v/v, in methanol) and vortexed. After 3 min, 500 μL of a 75 g/L sodium carbonate solution and 4 mL of water were added. The mixture was vortexed again for 10 min and left to stand in the dark for 1 h. Absorbance was recorded at 750 nm using a SynergyTM HT-multimode microplate spectrophotometer (BioTek Instruments, Winooski, VT, USA). Gallic acid was used as the calibration standard, and the TPC was expressed as mg of gallic acid equivalents (GAE) per g of extract. Each analysis was performed in duplicate, with four independent measurements conducted per replicate. The limit of quantification was 4.0 mg GAE/g of sample.
2.6. Flavonoid Determination
Flavonoid content was determined using the aluminum chloride method described by Abdel-Hameed [11]. To prepare the sample solution, 75 mg of each lyophilized extract was weighed and dissolved in 3 mL of either deionized water or 50% methanol. For the assay, 80 μL of the sample solution was mixed with 80 μL of aluminum chloride in ethanol and 100 μL of sodium acetate. The mixture was incubated in the dark for 150 min and then centrifuged at 14,926× g for 3 min. Flavonoids were quantified based on the formation of a flavonoid–aluminum complex with a maximum absorbance at 440 nm. Absorbance readings were taken using the previously mentioned BioTek microplate spectrophotometer. Quercetin was used as the reference standard, and the results were expressed as μg quercetin equivalents per 100 mg of extract. Each analysis was performed in duplicate, with four independent measurements conducted per replicate. The limit of quantification was set at 0.025 μg quercetin equivalents per 100 mg of sample.
2.7. Antioxidant Activity Determinations
For the ABTS assay, the procedure followed the method described by Gökmen et al. [12] with minor modifications. To generate ABTS radical cations (ABTS•+), a 7 mmol/L ABTS stock solution was mixed with 2.45 mmol/L potassium persulfate and allowed to react in the dark at room temperature for 12–16 h. The resulting ABTS•+ solution was then diluted with 50% ethanol until it reached an absorbance of 0.70 ± 0.02 at 734 nm. For analysis, 5 mg of each lyophilized extract was dissolved in 10 mL of either distilled water or 50% (v/v) methanol. A volume of 200 μL of each sample or Trolox standard was added to 3.8 mL of 50% ethanol and 1 mL of the diluted ABTS•+ solution. After incubation for 45 min at room temperature, absorbance was measured at 734 nm using the previously mentioned BioTek microplate spectrophotometer. Trolox was used as the calibration standard at concentrations ranging from 0.08 to 0.25 mmol/L. The total antioxidant capacity was expressed as µmol Trolox equivalents antioxidant capacity (TEAC) per g of extract. Each analysis was performed in duplicate, with four independent measurements conducted per replicate. The limit of quantification for this method was 11 μmol TEAC/g sample.
The FRAP (ferric-reducing antioxidant power) assay was performed according to the method described by Morales et al. [13], with slight modifications. Lyophilized extracts (50 mg) were dissolved in 10 mL of either deionized water or 50% methanol (diluted as necessary). The FRAP reagent was prepared by mixing a 40 mM TPTZ solution, a 20 mM ferric chloride hexahydrate solution, and a 0.3 M sodium acetate buffer (pH 3.6). For the assay, 40 μL of the sample solution was combined with 200 μL of acetate buffer and 60 μL of the FRAP reagent. The mixture was incubated at 37 °C for 30 min, after which the absorbance was measured at 595 nm using the previously mentioned BioTek microplate spectrophotometer. Quantification was performed using a Trolox calibration curve, and the results were expressed as μmol TEAC per g of extract. Each analysis was performed in duplicate, with four independent measurements conducted per replicate. The limit of quantification was established at 1.8 μmol TEAC/g sample.
2.8. Assessment of Direct MGO-Trapping Capacity
The direct MGO-trapping capacity was evaluated using a method adapted from Mesías et al. [10]. In this procedure, 100 μL of MGO solution (0.4 mg/mL) was combined with 750 μL of phosphate buffer (0.1 mol/L, pH 7.4), 50 μL of 5-MQ (internal standard, 1 mg/mL), and 100 μL of either buffer (as the blank), carob flour extract solutions (ranging from 1 to 25 mg/mL), or a PM solution (as the positive control with 1 mg/mL). To prepare the sample solutions, each lyophilized extract was dissolved in either water or a 50% methanol solution to obtain final concentrations of 1, 5, 10, and 25 mg/mL. The mixtures were then incubated at 37 °C for 168 h, with samples collected on intermediate days to monitor trapping capacity over time. After each incubation, a total of 200 μL of OPD (derivatization reagent, 10.8 mg/mL) was added to each sample and control. The mixtures were vortexed for 5 s and allowed to react for 30 min. The remaining MGO concentration was determined by measuring the formation of the derivatized product, 2-methylquinoxaline (2-MQ), in each sample. Quantification was carried out using the previously mentioned Shimadzu HPLC system. Chromatographic separation was performed on a Mediterranea-Sea-ODS2 column (150 mm × 3 mm, 5 μm; Tecknokroma, Barcelona, Spain). The amount of unreacted MGO was calculated based on the peak area ratio of 2-MQ and 5-MQ. The percentage reduction in MGO was calculated with the following formula:
MGO trapping (%) = [(MGO in control − MGO in sample with extract or PM solution)/MGO in control] × 100%.
Analyses were performed in duplicate. The IC50 values were calculated from the dose–response curves of each experiment using Microsoft Excel.
2.9. In Vitro Glycation Assay with BSA–Glucose (BSA–Glu) and BSA–Methylglyoxal (BSA–MGO)
The BSA–Glu and BSA–MGO assays, adapted from the method of Mesías et al. [10], were performed to evaluate the inhibitory effects of extracts on protein glycation induced by glucose and methylglyoxal, respectively. To prepare the sample solutions, each lyophilized extract was dissolved in either water or a 50% methanol solution to obtain final concentrations of 1, 5, 10, and 25 mg/mL. For the BSA–Glu assay, 100 μL of the extract solutions was combined with 200 μL of BSA solution (35 mg/mL) and 400 μL of Glu solution (175 mg/mL). Blanks containing BSA–Glu without the test sample were stored at −80 °C until measurement. The positive control was prepared by mixing 100 μL of AG solution (4 mg/mL) with 200 μL of BSA solution and 400 μL of Glu solution. The samples were incubated for 21 days at 37 °C, and fluorescence was measured at 360 nm excitation and 420 nm emission using the previously mentioned BioTek microplate spectrophotometer. For the BSA–MGO assay, 100 μL of the extract solutions was combined with 200 μL of BSA solution (35 mg/mL) and 400 μL of MGO solution (0.4 mg/mL). Blanks containing BSA–MGO without the test sample were stored at −80 °C until measurement. The positive control was prepared by mixing 100 μL of AG solution (4 mg/mL) with 200 μL of BSA solution and 400 μL of MGO solution. The samples were incubated for 14 days at 37 °C, and fluorescence was measured at 340 nm excitation and 420 nm emission using a microplate reader. Each analysis was performed in duplicate, with four independent measurements conducted per replicate.
2.10. Statistical Analysis
Statistical analyses were conducted using SPSS version 26 (SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation (SD). A two-tailed Student’s t-test was used to compare the results of the aqueous and methanolic extracts. Statistical significance was set at p < 0.05.
3. Results and Discussion
The functional characterization of the aqueous and methanolic extracts obtained from carob flour is presented in Table 1. The solubility of the raw material in the resulting extracts was nearly identical for the aqueous (48.9%) and methanolic (49.1%) extracts. A similar trend was observed in the pH values, which were 5.1 and 5.6 for the aqueous and methanolic extracts, respectively. Hydrophilic carob flour extracts contain mostly dietary fiber and a phenolic fraction that includes both water-soluble and insoluble tannins, flavanol glycosides, and high contents of different forms of gallic acid [14]. Despite the comparable solubility, the extracts differed in total phenolic content, flavonoid content, and antioxidant activity, all of which were significantly higher in the methanolic extract. Specifically, the extract prepared with methanol showed a 1.4-fold-higher total phenolic content and a 1.6-fold-higher flavonoid content compared to the aqueous extract. Antioxidant activity was evaluated using the ABTS assay, which measures free radical-scavenging capacity, and the FRAP assay, which assesses electron-donating ability. Once again, significant differences were observed, with the methanolic extract exhibiting significantly greater antioxidant activity in both assays, consistent with previous studies focused on cereals, pseudocereals, and seeds [9]. More precisely, the methanolic extract displayed 1.2-fold-higher activity in the ABTS assay and 1.8-fold-greater activity in the FRAP assay than the aqueous extract. These results contradict previous reports suggesting that methanol and ethyl acetate are no more effective than water in extracting polyphenolic compounds from carob [15].

Table 1.
Characterization of aqueous and methanolic extracts of carob flour.
Carob flour is primarily composed of carbohydrates—mainly sucrose, glucose, and fructose—alongside dietary fiber, small amounts of protein, and a variety of polyphenolic compounds, particularly gallic acid, flavonoids, and tannins [3,16,17]. Carob has been widely recognized as a rich source of phenolic compounds and antioxidants [16,18]. Incorporating carob products or their extracts into various foods—such as durum wheat pasta [19], bread [20], and cookies [21]—has been shown to enhance total phenolic content and antioxidant activity. In the present study, the TPC values obtained for carob flour extracts were higher than those previously reported by Ioannou et al. [14] for carob powder (18.19–23.18 mg GAE/g) and by Goulas and Georgiou [15] for various carob extracts.
To evaluate the antiglycation capacity of carob flour extracts, two in vitro models were employed: the BSA–Glu and BSA–MGO systems. The BSA–Glu model simulates the early stages of non-enzymatic glycation, where glucose reacts slowly with proteins to form AGE over time. This system allows for the assessment of the ability of the extracts to inhibit glycation under hyperglycemic-like conditions. In contrast, the BSA–MGO system represents a more advanced stage of glycation using methylglyoxal, a highly reactive dicarbonyl compound that rapidly reacts with amino groups in proteins to form AGE [22]. The use of both models provides a comprehensive evaluation of antiglycation activity, enabling differentiation between the extracts’ ability to prevent glycation through sugar-derived pathways and their capacity to trap reactive carbonyl intermediates. Concentrations ranging from 1 to 25 mg/mL were tested and compared with aminoguanidine (4 mg/mL), a compound commonly used as a positive control in antiglycation studies due to its well-documented ability to inhibit AGE formation [22].
AGE inhibition increased with higher extract concentrations, ranging from 8.3% at 1 mg/mL to 81.0% at 25 mg/mL in the BSA–Glu assay (Figure 1A).
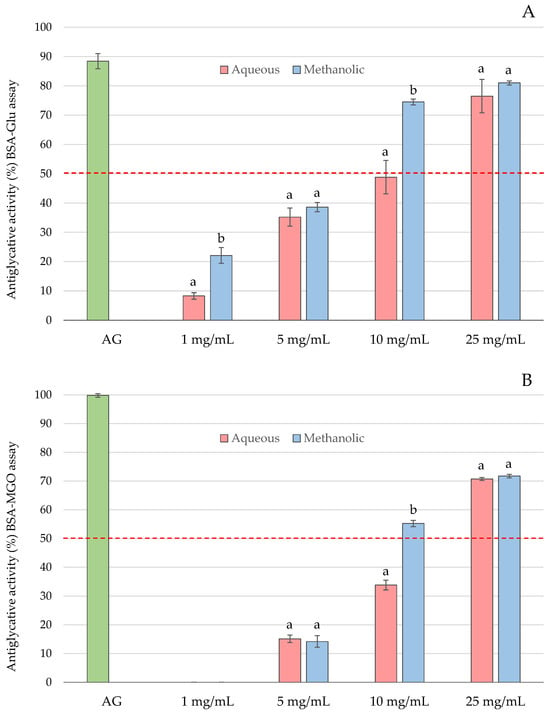
Figure 1.
Effect of carob flour extracts on AGE formation in BSA–Glu (A) and BSA–MGO (B) models. Results are expressed as mean ± SD. Different letters indicate statistically significant differences between the aqueous and methanolic extracts (p < 0.05). The dashed line represents 50% inhibition.
In the BSA–MGO assay, 1 mg/mL showed no significant inhibitory effect, while 25 mg/mL achieved inhibition levels close to 70% (Figure 1B). In both models, methanolic extracts exhibited significantly greater antiglycation activity than aqueous extracts, with statistically significant differences observed at 1 and 10 mg/mL in the BSA–Glu assay and at 10 mg/mL in the BSA–MGO assay. None of the extracts matched the inhibitory potency of aminoguanidine, which showed approximately 90% inhibition in the BSA–Glu assay and nearly 100% inhibition in the BSA–MGO assay. Based on these data, IC50 values were calculated as 10.8 mg/mL and 6.6 mg/mL for aqueous and methanolic extracts, respectively, in the BSA–Glu assay, and 16.6 mg/mL and 9.4 mg/mL, respectively, in the BSA–MGO assay.
Numerous studies have highlighted the critical role of AGE in mediating the harmful effects of hyperglycemia, particularly in the development and progression of diabetes-related complications. Among various glycation precursors, MGO has been identified as a major contributor to intracellular AGE accumulation, underscoring its importance as a pathogenic factor in diabetic conditions [23]. One of the primary strategies to inhibit AGE formation involves reducing reactive carbonyl species and scavenging oxidative free radicals. In this context, in vitro studies have demonstrated that trapping MGO can significantly reduce AGE formation, positioning this mechanism as a promising approach to glycation mitigation. In light of this, the MGO-trapping capacity of carob flour extracts was evaluated by testing a range of concentrations (1 to 25 mg/mL) over incubation periods of 24 to 168 h for both aqueous (A) and methanolic (B) extracts (Figure 2). Pyridoxamine was employed as a positive control due to its high reactivity and strong ability to bind and neutralize methylglyoxal effectively [24]. All extracts exhibited MGO-trapping capacity, with higher concentrations resulting in greater MGO-trapping efficacy. In aqueous extracts, 1 mg/mL trapped less than 20% of MGO after 24 h, whereas 25 mg/mL trapped over 60% within the same period, reaching 94% after 168 h. Similarly, the methanolic extract at 1 mg/mL achieved less than 30% trapping, while 25 mg/mL concentration captured 80% of MGO at 48 h and peaked at 97% after one week of incubation.
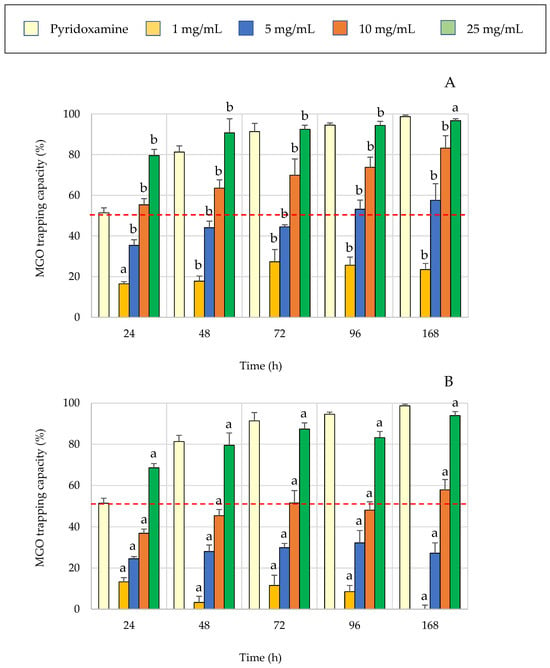
Figure 2.
MGO-trapping capacity of aqueous (A) and methanolic (B) extracts from carob flour at various concentrations (1–25 mg/mL) during incubation periods ranging from 24 to 168 h. Results are expressed as mean ± SD. Different letters indicate statistically significant differences between the aqueous (A) and methanolic (B) extracts (p < 0.05).
The IC50 values for MGO-trapping activity are presented in Table 2, expressed both in terms of concentration (mg/mL) and incubation time (h). The methanolic extract exhibited lower IC50 values than the aqueous extract, indicating higher efficiency in MGO scavenging. Over time, the concentration required to trap 50% of MGO decreased significantly from 16.4 to 8.6 mg/mL for the aqueous extract and from 8.8 to 4.0 mg/mL for the methanolic extract. At fixed concentrations, the methanolic extract reached an IC50 at 87 h with 5 mg/mL, which decreased significantly to 21 and 15 h at 10 and 25 mg/mL, respectively. Although less effective at lower concentrations (e.g., 10 mg/mL), the aqueous extract achieved a comparable IC50 to that of the methanolic extract at 25 mg/mL, suggesting a similar trapping capacity at higher doses.

Table 2.
MGO-trapping capacity of aqueous and methanolic extracts of carob flour evaluated at different time points and concentrations.
The results obtained for both antiglycation capacity and MGO-trapping are consistent with previous findings reported for polyphenol-rich plant extracts. Numerous studies have emphasized the role of antioxidant compounds in effectively inhibiting AGE formation by neutralizing reactive oxygen species and chelating metal ions that catalyze oxidative reactions [25]. In particular, polyphenols have been shown to exert anti-AGE effects by interacting with key substrates in the glycation process, such as binding to functional groups involved in glycation initiation or inhibiting the oxidation of reducing sugars and intermediate compounds like Amadori products [26]. Among these polyphenols, phenolic acids and flavonoids have consistently demonstrated inhibitory effects on AGE formation and the ability to mitigate their harmful effects [27,28,29]. In this context, the superior antiglycation and MGO-trapping capacities observed in the methanolic extract are consistent with its higher TPC, flavonoid levels, and antioxidant activity as measured by the ABTS and FRAP assays (Table 1).
Several recent studies support the strong correlation between antioxidant activity, phenolic/flavonoid content, and the ability to trap MGO or inhibit AGE. For example, Spondias purpurea hexane extract showed significant antiglycation activity [30], while extracts from Ilex paraguariensis and wild fruits demonstrated similar effects linked to high polyphenol content [31,32]. In Ephedra fragilis, the ethyl acetate fraction—rich in phenols and flavonoids—exhibited the most potent antioxidant and antiglycation activities [33]. Likewise, Fecka et al. [26] reported that peppermint leaf extract inhibited glycation by up to 77.2% and formed adducts with MGO. Brassica vegetable extracts, especially cauliflower, showed strong MGO-scavenging ability correlating with high TPC and antioxidant capacity [34]. Gao et al. [35] found that polyphenol-rich extracts of Zijuan tea efficiently trapped MGO, primarily due to their catechin content. Similarly, the aqueous extract of Phyllanthus emblica reduced AGE formation by 87.3%, attributed to its richness in polyphenols and flavonoids [36].
It is important to note that the MGO-scavenging capacity of phenolic compounds varies depending on their chemical structure, as specific structural features are necessary for effective interaction with MGO. For instance, among phenolic acids, compounds such as ferulic—which possess only a single hydroxyl group on the benzene ring—have been shown to lack reactivity with MGO [37]. Similarly, o-phenylphenols like caffeic acid do not directly form adducts with MGO but can undergo dehydration to generate reactive intermediates capable of reacting with MGO [28]. In the case of flavonoids, structural elements also play a crucial role in their ability to trap MGO [38].
The distribution of individual phenolic acids within the total phenolic acid content in the carob flour extracts is presented in Table 3. Except for its CGA content, the aqueous extract showed significantly lower levels of phenolic compounds compared to the methanolic extract, consistent with the TPC values and antioxidant capacity reported in Table 1. In agreement with findings reported for carob syrups [14], gallic acid was the predominant compound in both extracts, with concentrations up to 100 to 1000 times higher than those of the other phenolic acids. The levels of p-hydroxybenzoic, syringic, p-coumaric, and ferulic acids ranged between 40 and 80 µg/g, while the remaining phenolic acids were present at lower concentrations, ranging from 4 to 12 µg/g. These results align with the previous literature [39], which also identifies coumaric and gallic acids as the main phenolic acids in carob. Benkovic et al. [17] highlighted that the gallic acid content and antioxidant capacity were significantly higher in carob flour samples without seeds. The gallic acid content and antioxidant capacity of samples were clearly dependent on the presence of carob seeds: carob flour samples without seeds showed a significantly higher gallic acid content and greater antioxidant capacity (p < 0.05).

Table 3.
Phenolic acid content (μg/g) in aqueous and methanolic extracts of carob flour.
The phenolic acid profile of the extracts may help explain their antiglycation activity and MGO-trapping capacity, as several of these compounds are known for such bioactivities. Among the phenolic compounds identified, gallic acid likely plays a central role in the MGO-trapping capacity of the methanolic extract. Its chemical structure, characterized by three hydroxyl groups attached to a benzoic acid core, facilitates nucleophilic attack on electrophilic dicarbonyl compounds such as MGO. These hydroxyl groups act as electron-donating substituents, enhancing the reactivity of the aromatic ring toward electrophilic aromatic substitution. This mechanism enables gallic acid to form stable adducts with MGO, thereby limiting its ability to participate in AGE formation. MGO-trapping rates for gallic acid under physiological conditions have been reported to range from 28.5% to 87.7% [40,41]. In contrast, chlorogenic and caffeic acids exhibit significantly lower MGO-trapping capacities—approximately fourfold lower than that of gallic acid [41]. Sinapic and p-hydroxybenzoic acids, commonly found in Brassica vegetable extracts, have also been shown to inhibit fluorescent AGE formation by up to 67% [34]. Although ferulic acid lacks MGO-trapping ability [37], other phenolic acids such as syringic acid have demonstrated inhibitory effects on AGE formation [42]. Additionally, in silico studies have indicated that caffeic acid can inhibit AGE-related fluorescence [43]. These findings suggest that the antioxidant and antiglycation properties of carob flour are largely attributable to its phenolic compounds, with additional mechanisms—such as the trapping of reactive carbonyl species like MGO—also likely contributing to its bioactivity.
The lower activity observed in the aqueous extract can be attributed to its reduced content of phenolic compounds, particularly flavonoids and phenolic acids, which are more efficiently extracted with methanol due to their intermediate polarity. These compounds are known to exert their antiglycation and MGO-trapping effects through mechanisms such as carbonyl trapping, metal chelation, and radical scavenging, which are closely linked to their antioxidant capacity. Since water preferentially extracts highly polar components such as sugars, amino acids, and certain organic acids, it may result in a lower concentration of bioactive polyphenols, thereby explaining the reduced activity of the aqueous extract. This suggests that the antiglycation effect is at least partially mediated by the antioxidant mechanisms of polyphenolic compounds, which are less abundant in the aqueous extract.
This investigation has certain limitations as it focuses on the in vitro evaluation of the biological activity of carob flour extracts without considering the economic feasibility of their use as a functional food ingredient. Additionally, further in vivo studies, including animal experiments and clinical trials, are necessary to fully confirm their health benefits. In addition, extraction conditions were intentionally standardized to allow a direct solvent comparison. However, this approach did not account for the impact of variables such as temperature or extraction time. Future studies should address these factors to optimize the extraction process, assess large-scale feasibility, and validate the functionality of the extract under industrial conditions.
4. Conclusions
This study demonstrated that carob flour extracts exhibit notable antiglycation and MGO-trapping capacities, particularly when methanol is used as the extraction solvent. Although both extracts exhibited similar solubility (~49%), the methanolic extract exhibited significantly higher levels of total phenolics (1.4-fold) and flavonoids (1.6-fold), as well as greater antioxidant activity—1.2-fold in the ABTS assay and 1.8-fold in the FRAP assay.
These compositional differences translated into stronger antiglycation effects, with the methanolic extract (25 mg/mL) inhibiting AGE formation by up to 81.0% in the BSA–Glu model and around 70% in the BSA–MGO model. IC50 values further confirmed the superior potency of the methanolic extract in both assays (6.6 vs. 10.8 mg/mL and 9.4 vs. 16.6 mg/mL, respectively). Overall, these findings highlight the enhanced antiglycation potential of methanolic carob extract, supporting its application in the development of functional foods aimed at mitigating glycation-related pathologies. Furthermore, MGO-trapping experiments revealed a maximum scavenging efficiency of 97% after 168 h for the methanolic extract with 25 mg/mL, with a minimum IC50 of 4.0 mg/mL. These properties suggest that carob flour phenolics, particularly gallic acid, contribute both directly—via dicarbonyl trapping—and indirectly—via antioxidant mechanisms—to the inhibition of AGE formation. This dual functionality supports their potential application in functional foods or nutraceuticals targeting glycation-associated oxidative damage.
While other vegetables or their polyphenols are well documented for their antiglycation potential, carob flour offers several distinctive advantages. It is a naturally sweet, caffeine-free, and gluten-free ingredient widely used in food products, making it highly suitable for functional food applications. Additionally, carob flour contains in specific phenolic compounds that exhibit strong antiglycation and MGO-trapping activities, as demonstrated in this study. Moreover, carob is an underutilized crop that is gaining interest in sustainable agriculture, particularly in arid and semi-arid regions, positioning it as an attractive and environmentally friendly source of bioactive compounds. Consequently, carob flour may offer a milder sensory profile and easier incorporation into various food matrices without negatively affecting taste or color. These in vitro findings support the use of carob flour as a functional ingredient for glycation-targeted nutritional strategies. Future studies should not only focus on optimizing extraction parameters but also evaluate the economic feasibility of large-scale production and ensure the validation of its functionality under industrial processing conditions.
Author Contributions
Conceptualization, M.M. and F.J.M.; methodology, F.H.; software, M.M.; validation, M.M.; formal analysis, F.H.; investigation, M.M. and F.J.M.; resources, M.M. and F.J.M.; writing—original draft, M.M.; writing—review and editing, F.J.M.; supervision, M.M.; project administration, F.J.M.; funding acquisition, M.M. and F.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is part of the project PID2022-137697NB-I00, funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AGE | Advanced glycation end-products |
| AG | Aminoguanidine |
| BSA | Bovine serum albumin |
| CA | Caffeic acid |
| CGA | Chlorogenic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| FA | Ferulic acid |
| FRAP | Ferric-reducing antioxidant power |
| GA | Gallic acid |
| GE | Gentisic acid |
| Glu | Glucose |
| HPLC | High-performance liquid chromatography |
| MGO | Methylglyoxal |
| MQ | Methylquinoxaline |
| OPD | o-phenylenediamine |
| PCA | Protocatechuic acid |
| pCU | p-coumaric acid |
| pHB | p-hydroxybenzoic acid, |
| PM | Pyridoxamine |
| SIN | Sinapinic acid |
| SYN | Syringic acid |
| TEAC | Trolox equivalent antioxidant capacity |
| TCP | Total phenolic content |
| TPTZ | 2,4,6-tris(2-pyridyl)-s-triazine |
| VA | Vanillic acid |
References
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability opportunities for Mediterranean food products through new formulations based on carob flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Ghanemi, F.Z.; Belarbi, M. Phytochemical compounds and biological properties of carob pods (Ceratonia siliqua L.) extracts at different ripening stages. Waste Biomass Valorization 2023, 14, 2271–2283. [Google Scholar]
- Ramos-Ruiz, J.A.; Martínez-Romero, J.M.; Rodríguez-Rojo, S. Carob pulp: A nutritional and functional by-product worldwide spread in the formulation of different food products and beverages. Foods 2021, 9, 1146. [Google Scholar]
- Benito-Vázquez, I.; Garrido-Romero, M.; Hontoria-Caballo, G.; García-García, C.; Díez-Municio, M.; Moreno, F.J. Carob (Ceratonia siliqua) flour as source of bioactive compounds: Production, characterization and nutraceutical value. Foods 2024, 13, 3024. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Zhang, Y. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef]
- Bansal, S.; Burman, A.; Tripath, A.K. Advanced glycation end products: Key mediator and therapeutic target of cardiovascular complications in diabetes. World J. Diabetes 2023, 14, 1146–1162. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L.; et al. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Mesías, M.; Holgado, F.; Olombrada, E.; Morales, F.J. In Vitro Bioactivities of Cereals, Pseudocereals and Seeds: Assessment of Antiglycative and Carbonyl-Trapping Properties. Appl. Sci. 2024, 14, 5684. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S. Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 2009, 114, 1271–1277. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Morales, F.J.; Martin, S.; Açar, O.C.; Arribas-Lorenzo, G.; Gökmen, V. Antioxidant activity of cookies and its relationship with heat-processing contaminants: A risk/benefit approach. Eur. Food Res. Technol. 2009, 228, 345–354. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Goulas, V.; Georgiou, E. These results contradict previous reports suggesting that methanol and ethyl acetate are no more effective than water in extracting polyphenolic compounds from carob. Foods 2020, 9, 20. [Google Scholar] [CrossRef]
- Fidan, H.; Stankov, S.; Petkova, N.; Petkova, Z.; Iliev, A.; Stoyanova, M.; Ivanova, T.; Zhelyazkov, N.; Ibrahim, S.; Stoyanova, A. Evaluation of chemical composition, antioxidant potential and functional properties of carob (Ceratonia siliqua L.) seeds. J. Food Sci. Technol. 2020, 57, 2404–2413. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob (Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- El Chami, M.A.; Palacios-Rodríguez, G.; Ordóñez-Díaz, J.L.; Rodríguez-Solana, R.; Navarro-Cerrillo, R.M.; Moreno-Rojas, J.M. Proximate Analysis, Total Phenolic Content, and Antioxidant Activity of Wild Carob Pulp from Three Mediterranean Countries. Appl. Sci. 2025, 15, 1340. [Google Scholar]
- Lupu, M.I.; Canja, C.M.; Padureanu, V.; Boieriu, A.; Maier, A.; Badarau, C.; Padureanu, C.; Croitoru, C.; Alexa, E.; Poiana, M.A. Insights on the Potential of Carob Powder (Ceratonia siliqua L.) to Improve the Physico-Chemical, Biochemical and Nutritional Properties of Wheat Durum Pasta. Appl. Sci. 2023, 13, 3788. [Google Scholar] [CrossRef]
- Zahorec, J.; Šoronja-Simovic, D.; Petrovic, J.; Šereš, Z.; Pavlic, B.; Sterniša, M.; Možina, S.S.; Ackar, D.; Šubaric, D.; Jozinovic, S. The Effect of Carob Extract on Antioxidant, Antimicrobial and Sensory Properties of Bread. Appl. Sci. 2024, 14, 3603. [Google Scholar] [CrossRef]
- Babiker, E.E.; Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Ahmed, I.A.M.; Almusallam, I.A. Physico-chemical and bioactive properties, fatty acids, phenolic compounds, mineral contents, and sensory properties of cookies enriched with carob flour. J. Food Process. Preserv. 2020, 44, e14745. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 2021, 53, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Advanced glycation end products in diabetes-related macrovascular complications: Focus on methylglyoxal. Trends Endocrinol. Metab. 2022, 33, 898–911. [Google Scholar]
- Bao, X.; Miao, J.; Huang, Y.; Lai, K. Revealing a key inhibitory mechanism of 2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline via trapping of methylglyoxal. J. Food Sci. 2020, 85, 2090–2097. [Google Scholar] [CrossRef]
- Maisto, M.; Tenore, G.C. Polyphenols as a Useful Tool to Ameliorate Advanced Glycation End-product Formation: A Focus on Molecular Mechanisms of Action. Front. Biosci. 2024, 29, 424. [Google Scholar] [CrossRef]
- Fecka, I.; Bednarska, K.; Kowalczyk, A. In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols. Molecules 2023, 28, 2865. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.C.; Derbre, S.; Blanchard, P.; Dadar, M.; Le Ray, A.M.; Richomme, P. Anti-AGE activity of poplar-type propolis: Mechanism of action of main phenolic compounds. Int. J. Food Sci. Technol. 2020, 55, 453–460. [Google Scholar] [CrossRef]
- Lee, S.M.; Zheng, L.W.; Jung, Y.; Hwang, G.S.; Kim, Y.S. Effects of hydroxycinnamic acids on the reduction of furan and α-dicarbonyl compounds. Food Chem. 2020, 312, 126085. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.W.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Muñiz, A.; Garcia, E.; Gonzalez, D.; Zuñiga, L. Antioxidant Activity and In Vitro Antiglycation of the Fruit of Spondias purpurea. Evid. Based Complement. Alternat Med. 2018, 2018, 5613704. [Google Scholar] [CrossRef]
- Martins, G.R.; Bronzel Junior, K.L.; Granero, F.O.; Figueiredo, C.C.M.; Silva, L.P.; Da Silva, R.M.G. Phytoconstituents, antioxidant and antiglycation activity of Chrysophyllum cainito L., Hancornia speciosa Gomes and Plinia glomerata Berg. Fruits. An. Acad. Bras. Ciências 2023, 95, e20201853. [Google Scholar] [CrossRef] [PubMed]
- Cogoi, L.; Marrassini, C.; Saint Martin, E.M.; Alonso, M.R.; Filip, R.; Anesini, C. Inhibition of Glycation End Products Formation and Antioxidant Activities of Ilex paraguariensis: Comparative study of fruit and leaves extracts. J. Pharmacopunct. 2023, 26, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Guenaou, I.; Irahal, I.N.; Errami, A.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Bioactive Compounds from Ephedra fragilis: Extraction Optimization, Chemical Characterization, Antioxidant and AntiGlycation Activities. Molecules 2021, 26, 5998. [Google Scholar] [CrossRef]
- Thilavech, T.; Marnpae, M.; Mäkynen, K.; Adisakwattana, S. Phytochemical Composition, Antiglycation, Antioxidant Activity and Methylglyoxal-Trapping Action of Brassica Vegetables. Plant Foods Hum. Nutr. 2021, 76, 340–346. [Google Scholar] [CrossRef]
- Gao, X.; Ho, C.T.; Li, X.; Lin, X.; Zhang, Y.; Chen, Z.; Li, B. Phytochemicals, Anti-Inflammatory, Antiproliferative, and Methylglyoxal Trapping Properties of Zijuan Tea. J. Food Sci. 2018, 83, 517–524. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chiang, I.C.; Chen, Y.Y.; Hsu, Y.H.; Yen, G.H. Recent advances in the potential of Phyllanthus emblica L. and its related foods for combating metabolic diseases through methylglyoxal trapping. Food Res. Int. 2024, 194, 114907. [Google Scholar] [CrossRef]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Ferulic acid prevents methylglyoxal-induced protein glycation, DNA damage, and apoptosis in pancreatic β-cells. J. Physiol. Biochem. 2017, 73, 121–131. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Aspalathin and Other Rooibos Flavonoids Trapped α-Dicarbonyls and Inhibited Formation of Advanced Glycation End Products In Vitro. Int. J. Mol. Sci. 2022, 23, 14738. [Google Scholar] [CrossRef]
- Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring Carob (Ceratonia siliqua L.): A Comprehensive Assessment of Its Characteristics, Ethnomedicinal Uses, Phytochemical Aspects, and Pharmacological Activities. Plants 2023, 12, 3303. [Google Scholar] [CrossRef]
- Cui, H.; Tao, F.; Hou, Y.; Lu, Y.; Zheng, T.; Sang, S.; Lv, L. Dual effects of propyl gallate and its methylglyoxal adduct on carbonyl stress and oxidative stress. Food Chem. 2018, 265, 227–232. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Kinetic evaluation of the reaction between methylglyoxal and certain scavenging compounds and determination of their in vitro dicarbonyl scavenging activity. Food Res. Int. 2019, 121, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Valle-Sánchez, S.L.; Rodríguez-Ramírez, R.; Ávila-Villa, L.A.; Villa-Lerma, A.G.; Davidov-Pardo, G.; Wall-Medrano, A.; González-Córdova, A.F. Natural inhibitory compounds of advanced glycation end products (AGEs) from the Maillard reaction. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Research Institute of Chemistry, University of Karachi: Karachi, Pakistan, 2023; Volume 79, pp. 341–381. [Google Scholar]
- Khan, M.S.; Alokail, M.S.; Alenad, A.M.H.; Altwaijry, N.; Alafaleq, N.O.; Alamri, A.M.; Zawba, M.A. Binding Studies of Caffeic and p-Coumaric Acid with α-Amylase: Multispectroscopic and Computational Approaches Deciphering the Effect on Advanced Glycation End Products (AGEs). Molecules 2022, 27, 3992. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).