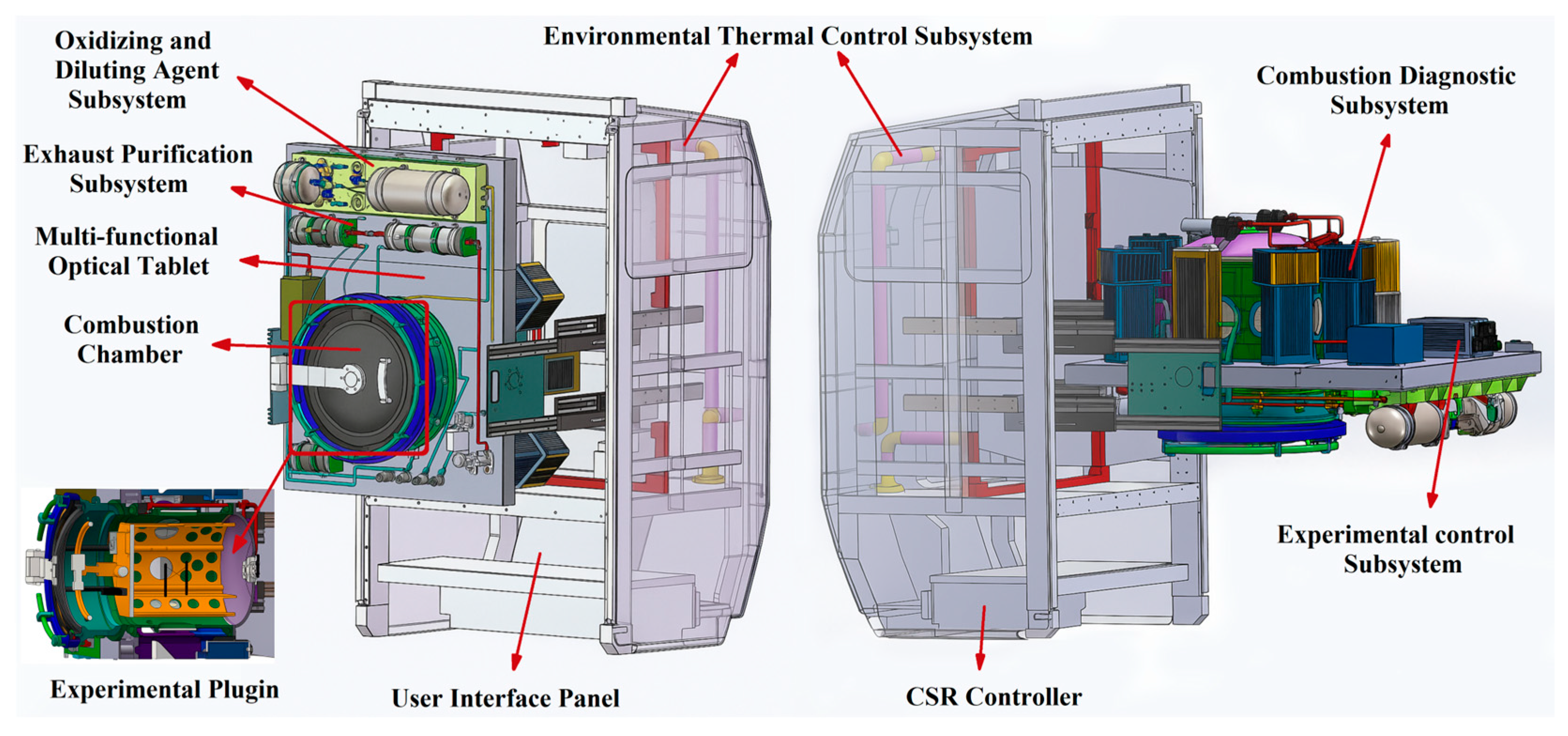
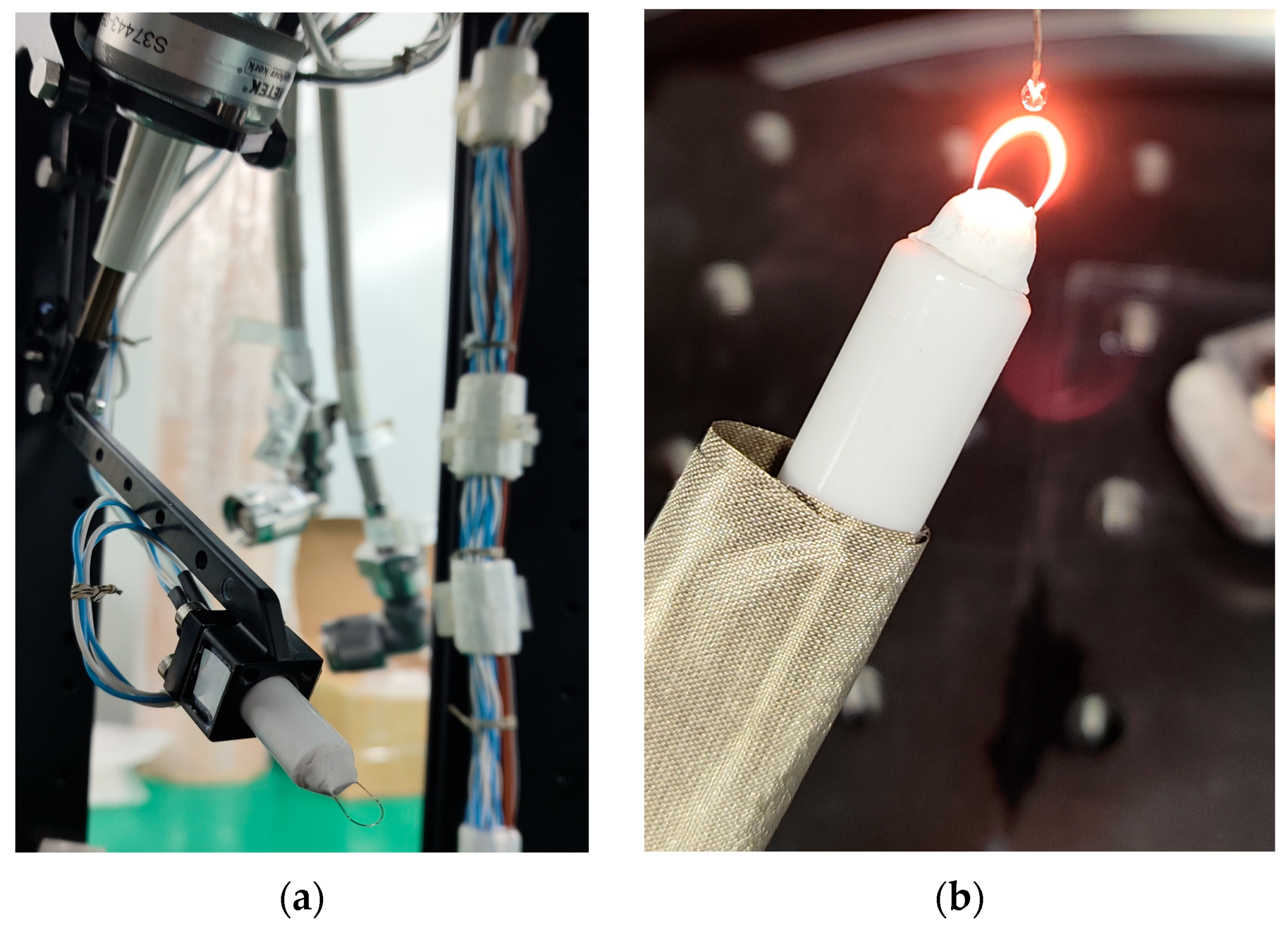
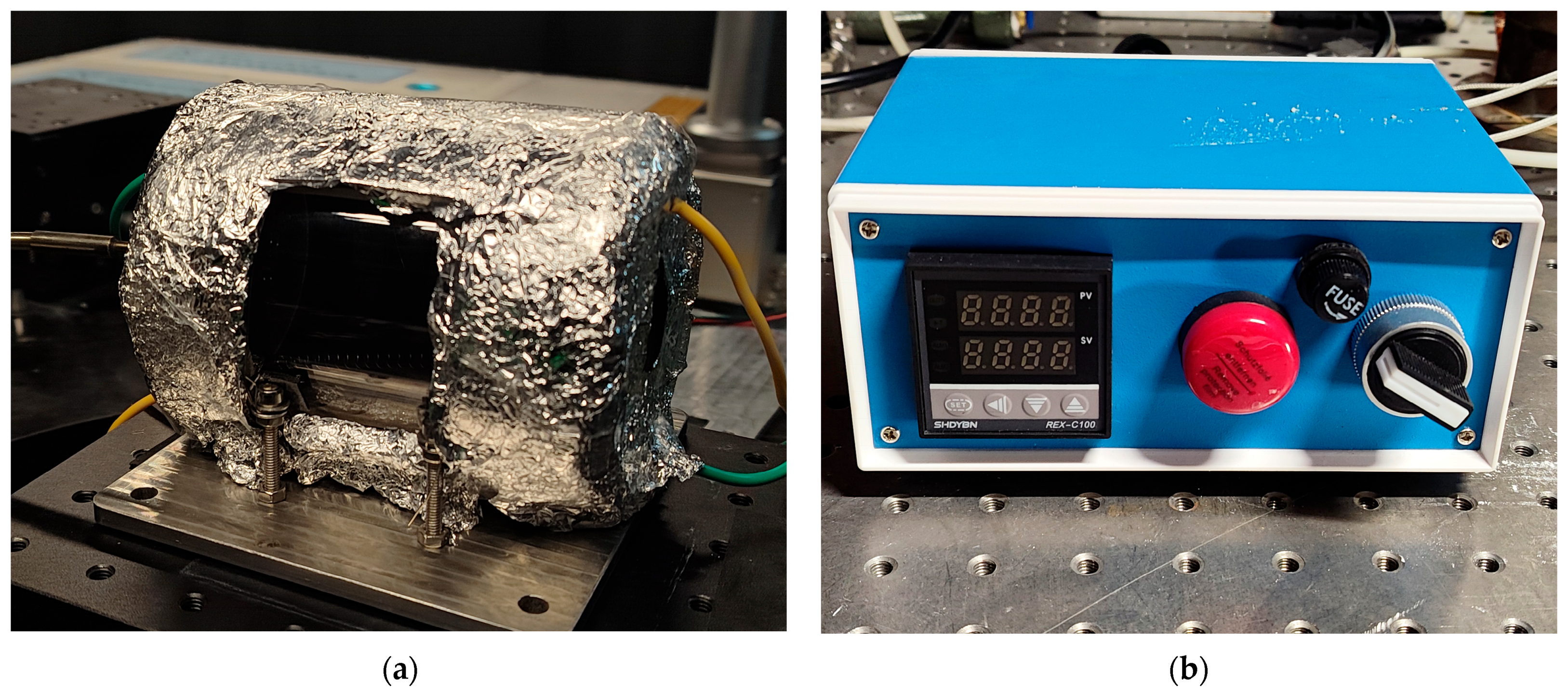
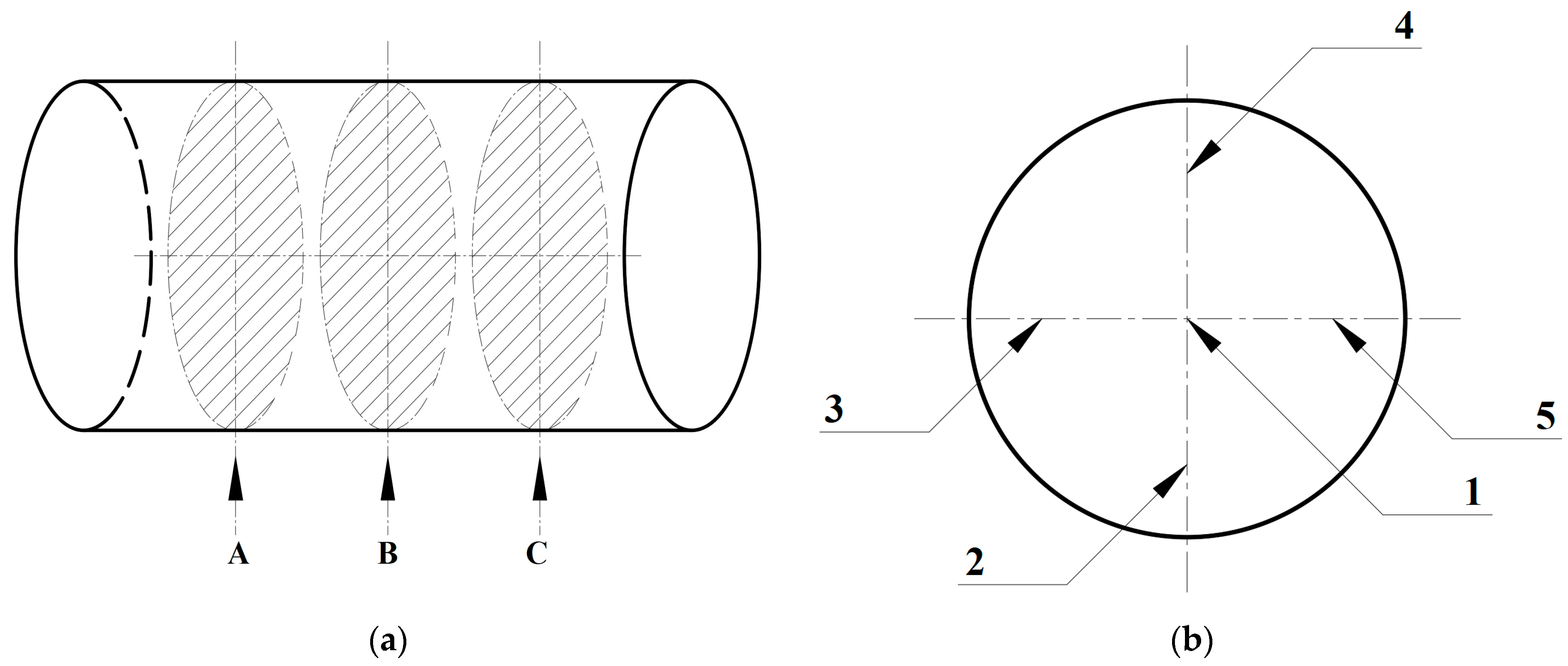
4.1. Droplet Ignition Prediction Model Under Normal Gravity
First, it is assumed that the droplet is stationary relative to the environment, and after vaporization, the fuel molecules only undergo spherically symmetric radial one-dimensional flow caused by the Stefan flow, with the heat radiation and thermal dissociation reactions of the fuel being neglected. In the short time before ignition, the droplet remains in a pure evaporation state, with the fuel vapor diffusing outward from the droplet surface, while the air diffuses inward toward the droplet surface. When the droplet ignites, the reaction is most intense in a thin layer close to the hot boundary, and the temperature gradient at the outer boundary of this layer is zero. Based on the experience of solving ignition problems [
10], the order of magnitude of the parameter changes in the reaction zone thin layer at ignition is approximately
, where
is the gas constant,
is the ambient temperature inside the heating vessel,
is the activation energy of the fuel, parameters with an asterisk ‘
’ denote values at the reactive zone’s inner boundary, subscript ‘
’ indicates values at the droplet surface, and the subscript ‘
’ represents ambient parameters. The boundary conditions for ignition can be approximated as follows.
In the reaction zone, the average concentration of fuel gas is
The average concentration of oxygen is
The boundary temperature of the reaction zone is
Due to the extremely small actual scale of the thin layer, curvature variations can be neglected, and the convective effects caused by temperature changes are negligibly weak, allowing the convective terms in the equations to be ignored. Therefore, we have
In the equation, represents the heat of combustion.
Integrating this equation results in
The parameter ‘
’ in the equation is calculated using the following expression:
In the equation, represents the reaction kinetics parameter, and denotes the environmental density.
Integrating Equation (6) yields
Introducing the analytical expression
for droplet combustion, and assuming that the temperature gradient at the outer boundary of the reaction zone’s thin layer is approximately equal to that under pure evaporation without combustion, we have
In Equation (9),
represents the total mass flux,
is the specific heat capacity at constant pressure,
denotes the latent heat of evaporation,
is the thermal diffusivity, and
is the radius of the reaction zone’s thin layer after being converted to a film. The parameters
and
can be calculated using the following expressions:
Due to the temperature gradient in the atmospheric space near the droplet surface, slight natural convection occurs. The Nusselt number under natural convection conditions can be calculated using an empirical formula, and its expression is
In this expression,
denotes the Rayleigh number, representing the influence of buoyancy induced by the temperature gradient. It can be calculated using the following expressions:
In this equation,
represents the chemical equivalence ratio. Given the relatively low concentration magnitude of fuel vapor within the thin-film reaction zone, and considering that thermal condition
, for ease of application in engineering contexts, certain thermophysical parameters such as
,
, and
are approximated by the corresponding properties of air at the same temperature, as shown in
Table 4. The parameters within temperature ranges are fitted using power functions, and interpolation is performed accordingly.
The ignition conditions for droplets given by Zhou are [
10]
By equating Equations (6) and (9), and substituting Equation (12) into them, the semi-empirical ignition condition for a single fuel droplet in the stationary air environment in normal-gravity conditions can be derived as follows:
Due to the challenges in directly measuring parameters such as fuel vapor and oxygen concentrations within the reaction layer surrounding the droplet under practical conditions, and considering that determining the reaction kinetics parameters and latent heat of vaporization in Equation (15) involves complex calculations and measurement processes, alternative approaches are often employed. These include utilizing simplified models or indirect measurement techniques to estimate the necessary parameters [
10,
35].
To solve engineering problems, the complex components of Equation (15) are simplified in this study by employing a power model based on ambient temperature and summarizing it in the form of a semi-empirical formula.
The empirical constants
A and
n can be determined through experimental data. Regarding the selection of activation energy values for chemical reactions, some existing studies indicate that fuels exhibit negative temperature coefficient (NTC) behavior within certain temperature ranges [
36]. This phenomenon arises from the complex mechanisms of fuel oxidation reactions and may be accompanied by cool-flame combustion [
37]. However, the focus of this study is on the autoignition phenomena of fuel droplets. The flames observed in the experiments are not characteristic of cool flames. Therefore, based on the experimental data obtained in the high-temperature reaction regime [
37,
38,
39], three activation energies for chemical reactions were determined, as shown in
Table 5.
The relative sensitivity coefficient
of
with respect to
is calculated by
At
, the relative sensitivity coefficients for the three fuels are as shown in
Table 6.
The above results indicate that, under the experimental conditions of this study, the minimum ignition diameter of the droplets is minimally influenced by the activation energy.
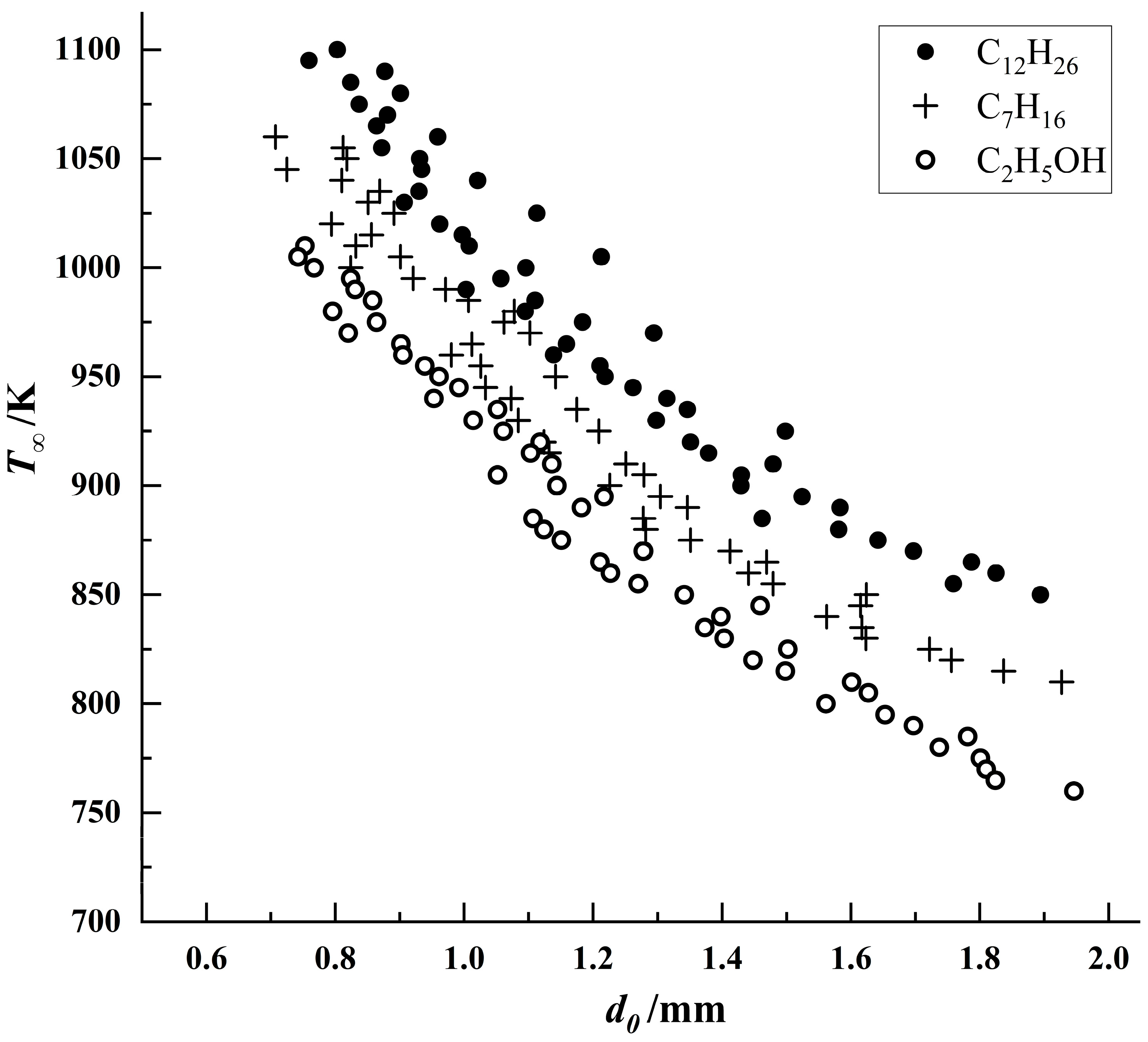
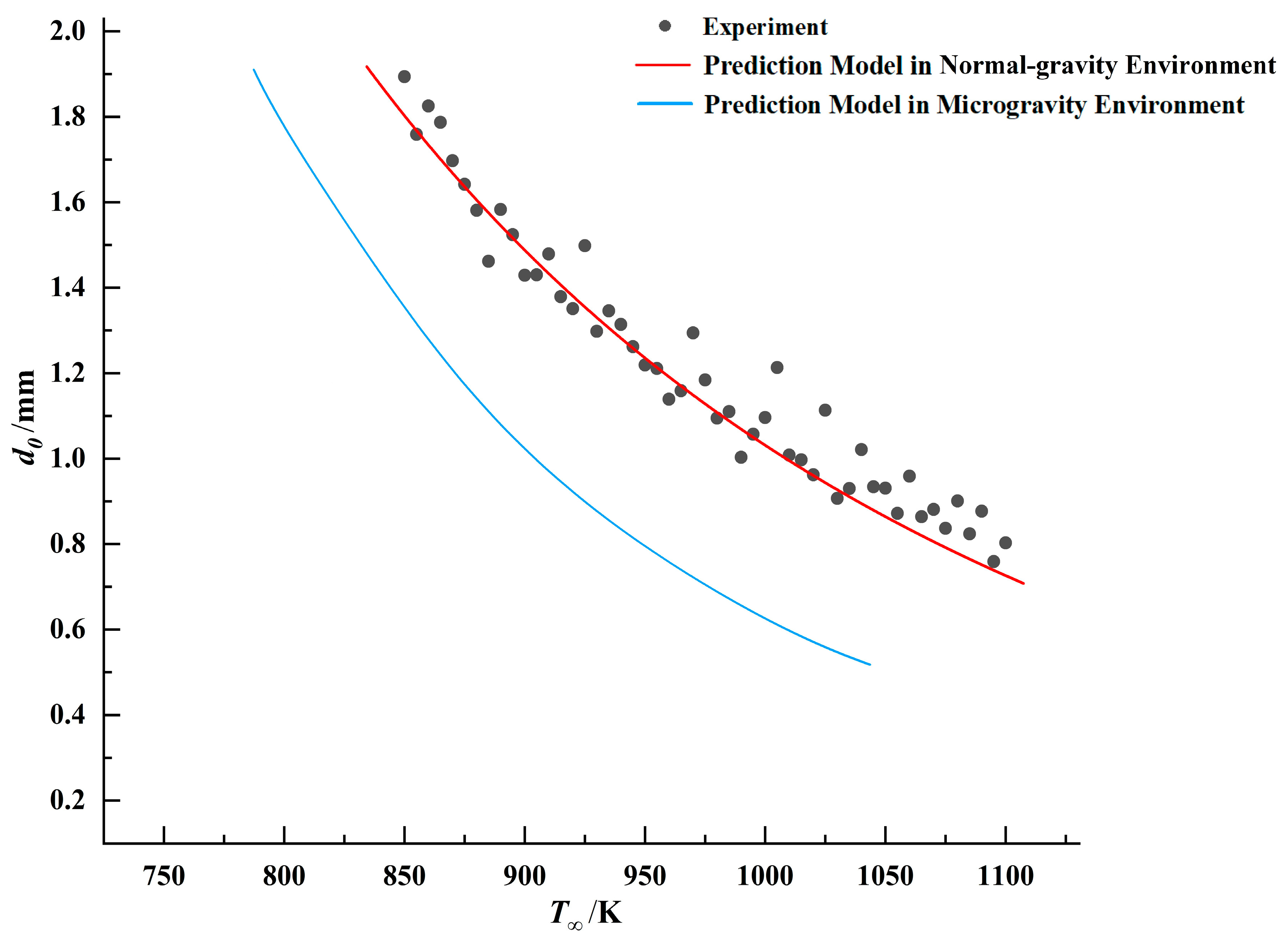
The aforementioned droplet ignition model was fitted against the experimental data for the three fuels, with the comparative results presented in
Figure 11,
Figure 12 and
Figure 13.
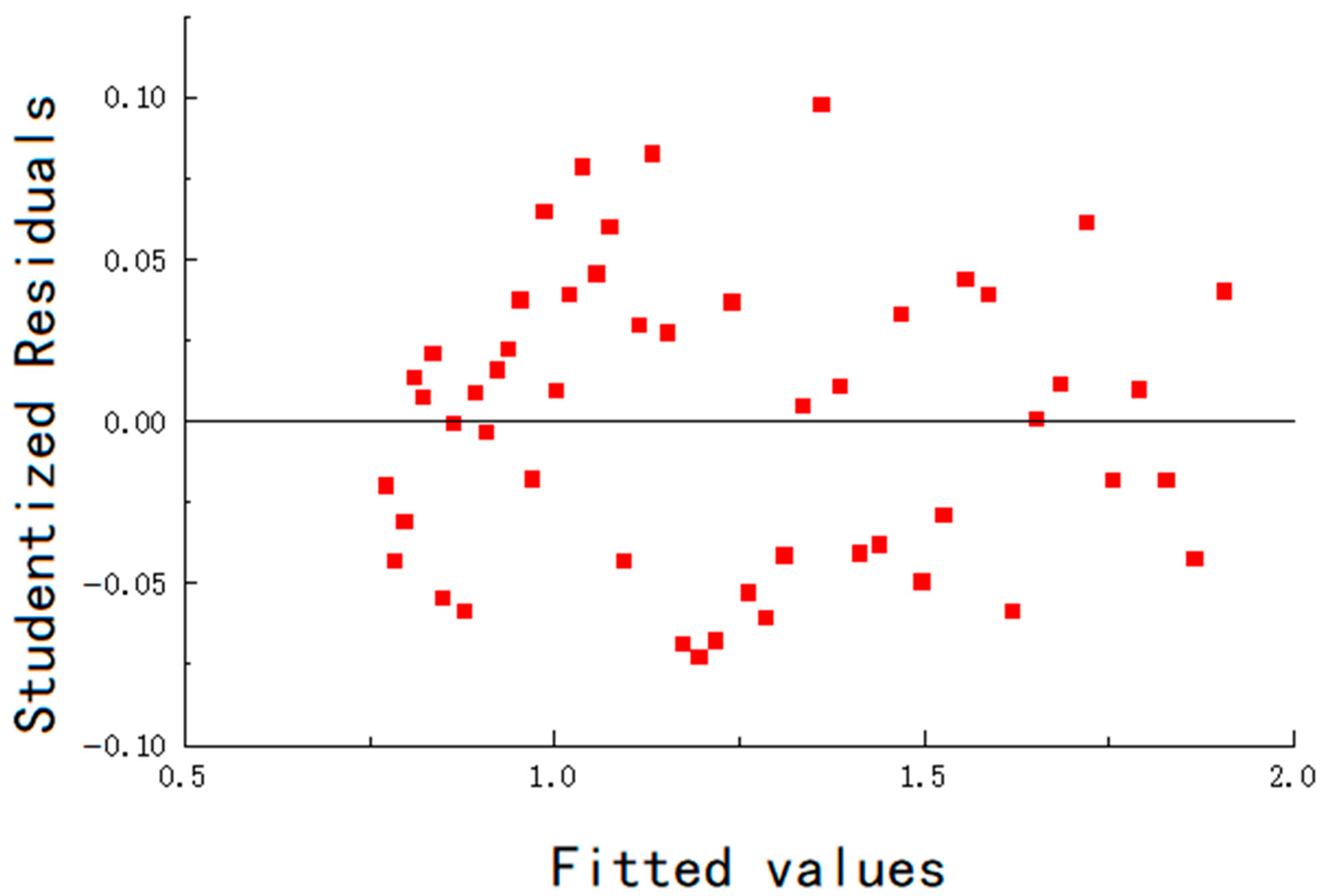
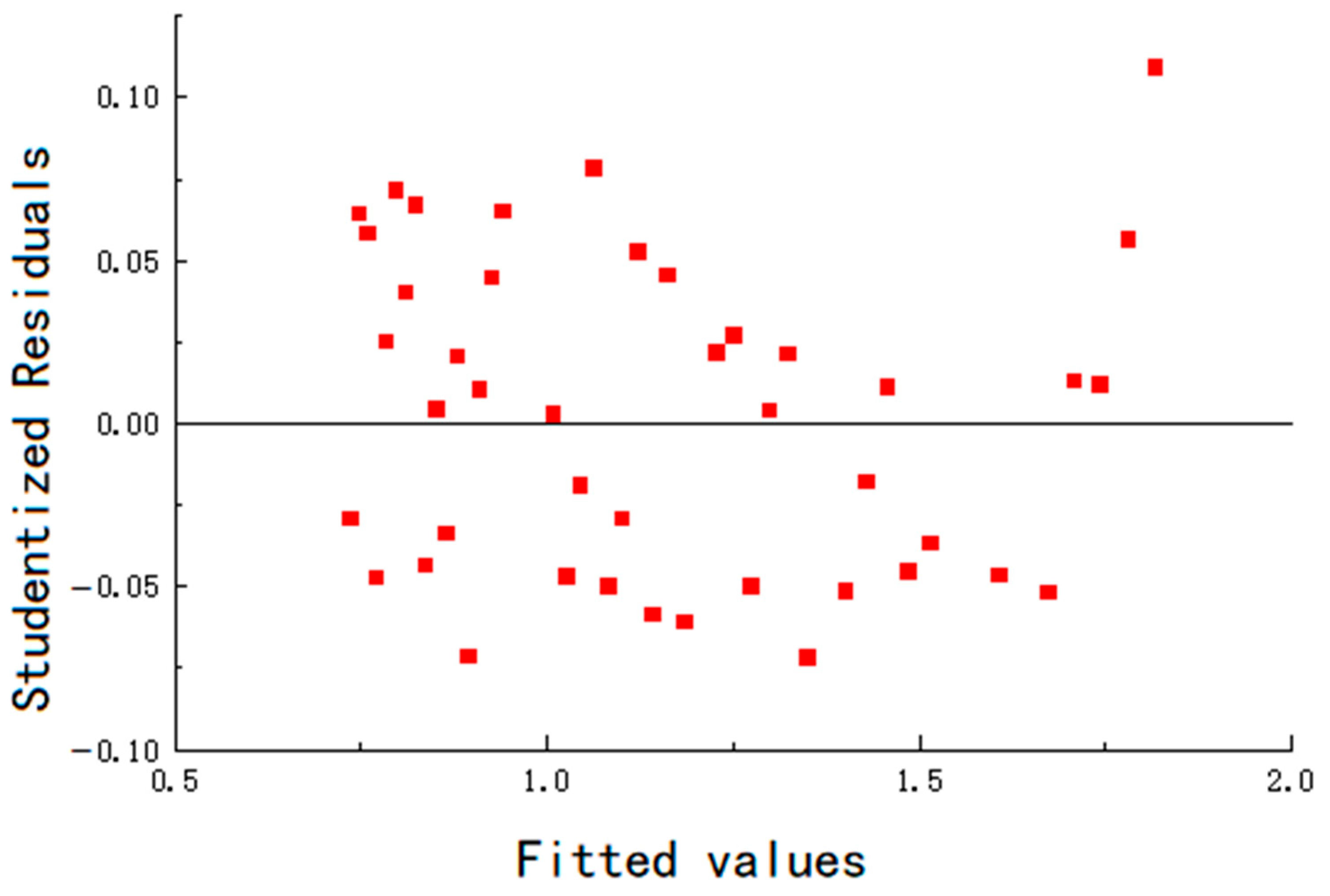
The residuals of the fitting are shown in
Figure 14,
Figure 15 and
Figure 16. The standard errors of the empirical parameters
A and
n are shown in
Table 7. The maximum error remains below 6.5%, and the goodness of fit is greater than 0.96, indicating that the mathematical model fits the experimental data well.
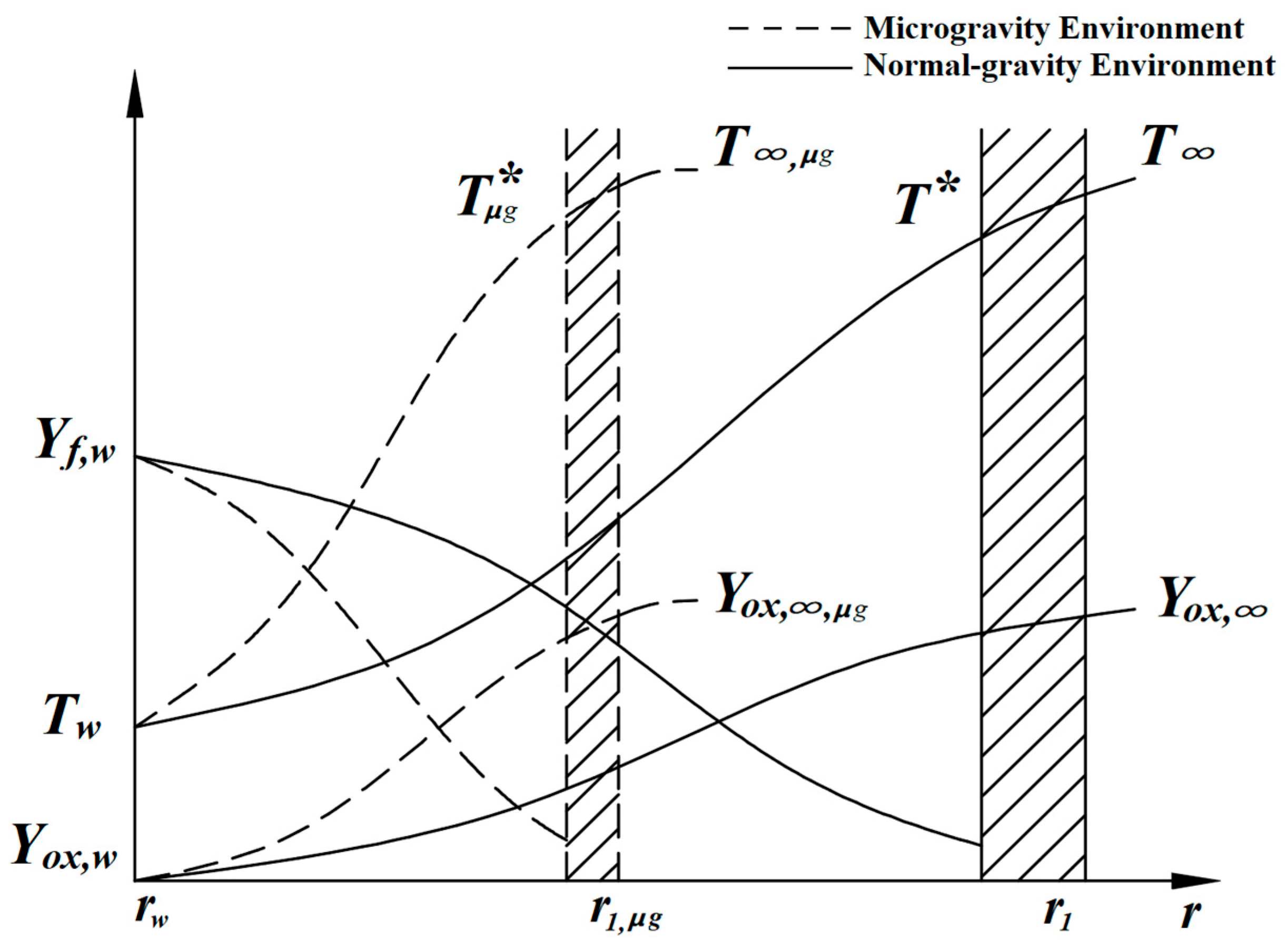
In microgravity environments, natural convection driven by buoyancy is nearly eliminated. The movement of fuel vapor occurs primarily through diffusion driven by molecular thermal motion. Because the flow pattern remains the spherically symmetric radial one-dimensional flow caused by the Stefan flow, the previously assumed boundary conditions are still applicable. Due to the absence of natural convection, the diffusion rate of fuel vapor significantly decreases compared to the normal-gravity environment. Consequently, the scale of the mixed vapor field surrounding the droplet decreases markedly, and the reaction zone becomes closer to the droplet’s surface [
12,
13]. In the Stefan flow, the temperature field can be analyzed using the generalized Reynolds analogy and concentration distribution.
4.2. Droplet Ignition Prediction Model Under Microgravity
In the microgravity environment, thermal motion is the primary cause of the formation of diffusion mass flow. Therefore, the Lewis number is [
10]
The generalized Reynolds analogy between the temperature field and the fuel vapor concentration field is expressed as
Through the Reynolds analogy, combined with the distribution trends of fuel vapor concentration, by referring to
Figure 9, approximate parameter profiles in the gas-phase region surrounding the droplet in the microgravity environment can be plotted, as in
Figure 17. It can be seen that the variation trends of all parameters are basically the same as those in the normal-gravity environment, but their scales are compressed due to reduced diffusion speed. In the microgravity environment, the convective effects are extremely weak.
Therefore, the empirical expression for the Nusselt number calculated using Equation (14) is no longer applicable. In the droplet evaporation process dominated by low Reynolds numbers and Stefan flow, the Nusselt number can be modified using the Spalding number correction. The modified empirical formula is [
40]
By substituting the modified
into Equation (17), the ignition condition for stationary air droplets in the microgravity environment can be derived as
In microgravity, the scale of the reaction zone has changed; however, the concentration of fuel vapor within the reaction zone remains largely unaffected. Because the parameters
,
, and
in Equation (16) are determined by the physical properties of the fuel, the parameters
,
, and
are determined by the chemical reaction pathway of the fuel and the enthalpy changes in the reactants and products. The influence of variations in gravity on these parameters is relatively minor and can be considered negligible. Therefore, the empirical coefficients
A and
n remain applicable in the microgravity environment. By combining the empirical coefficients derived from experiments conducted in normal gravity with the modified
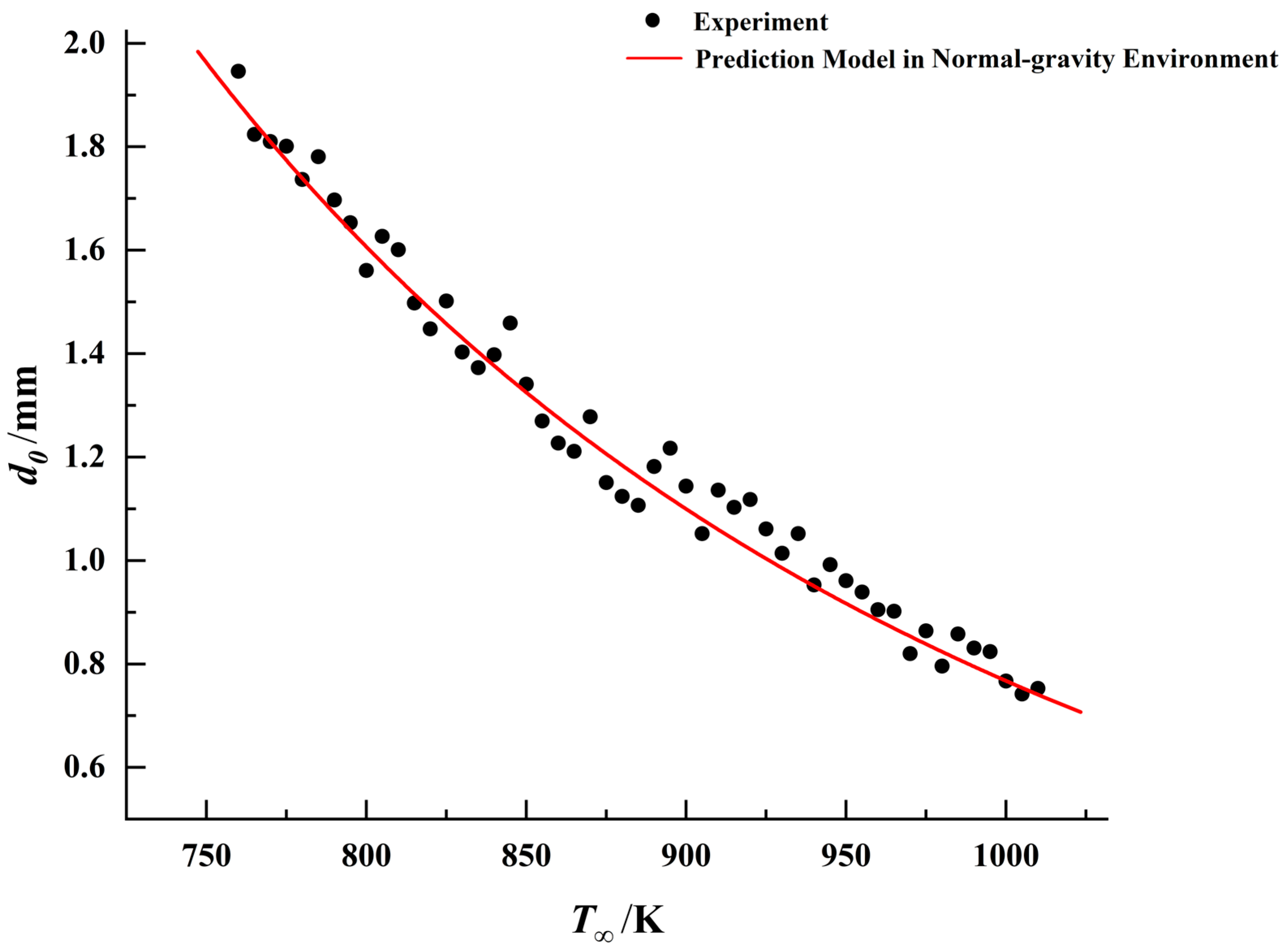
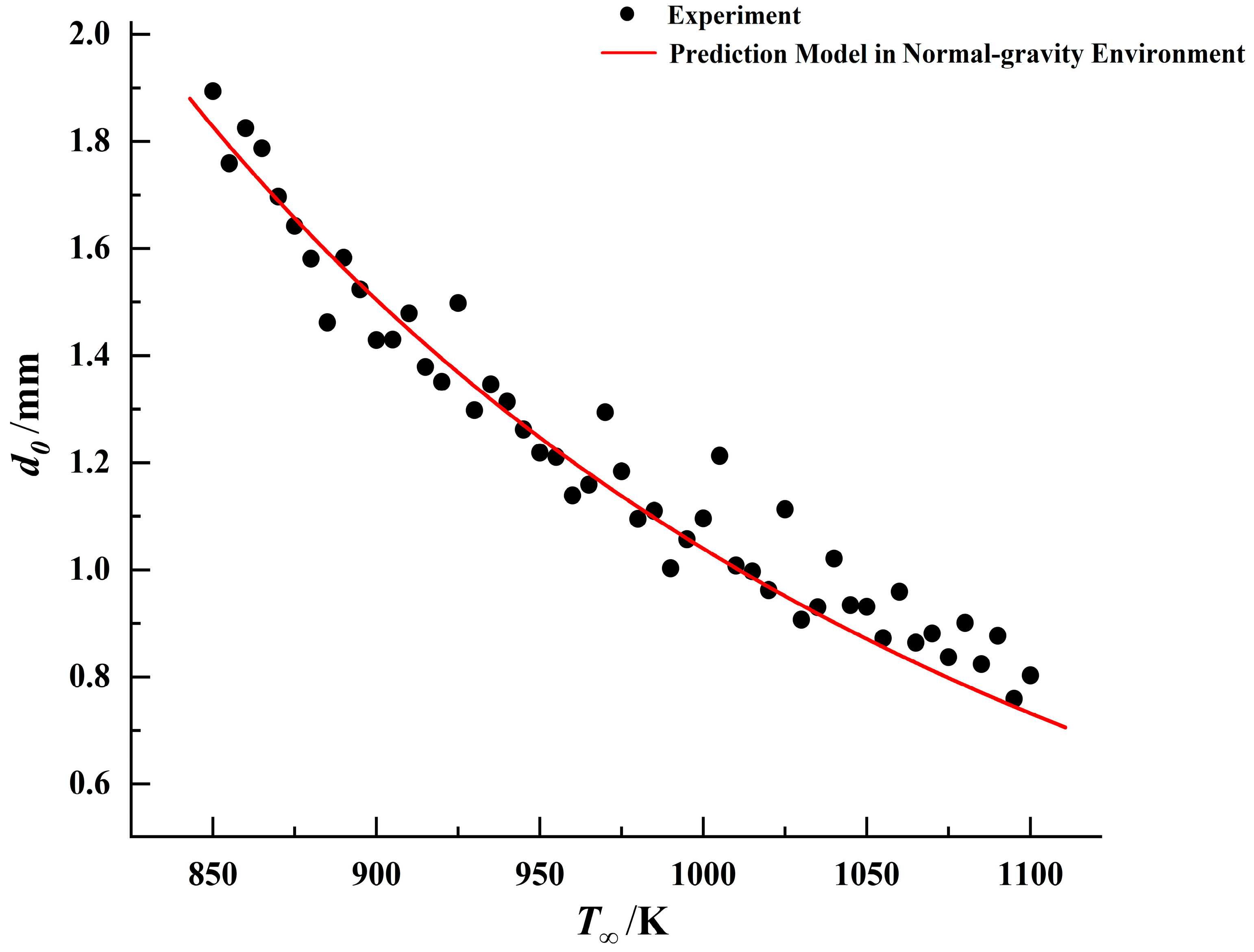
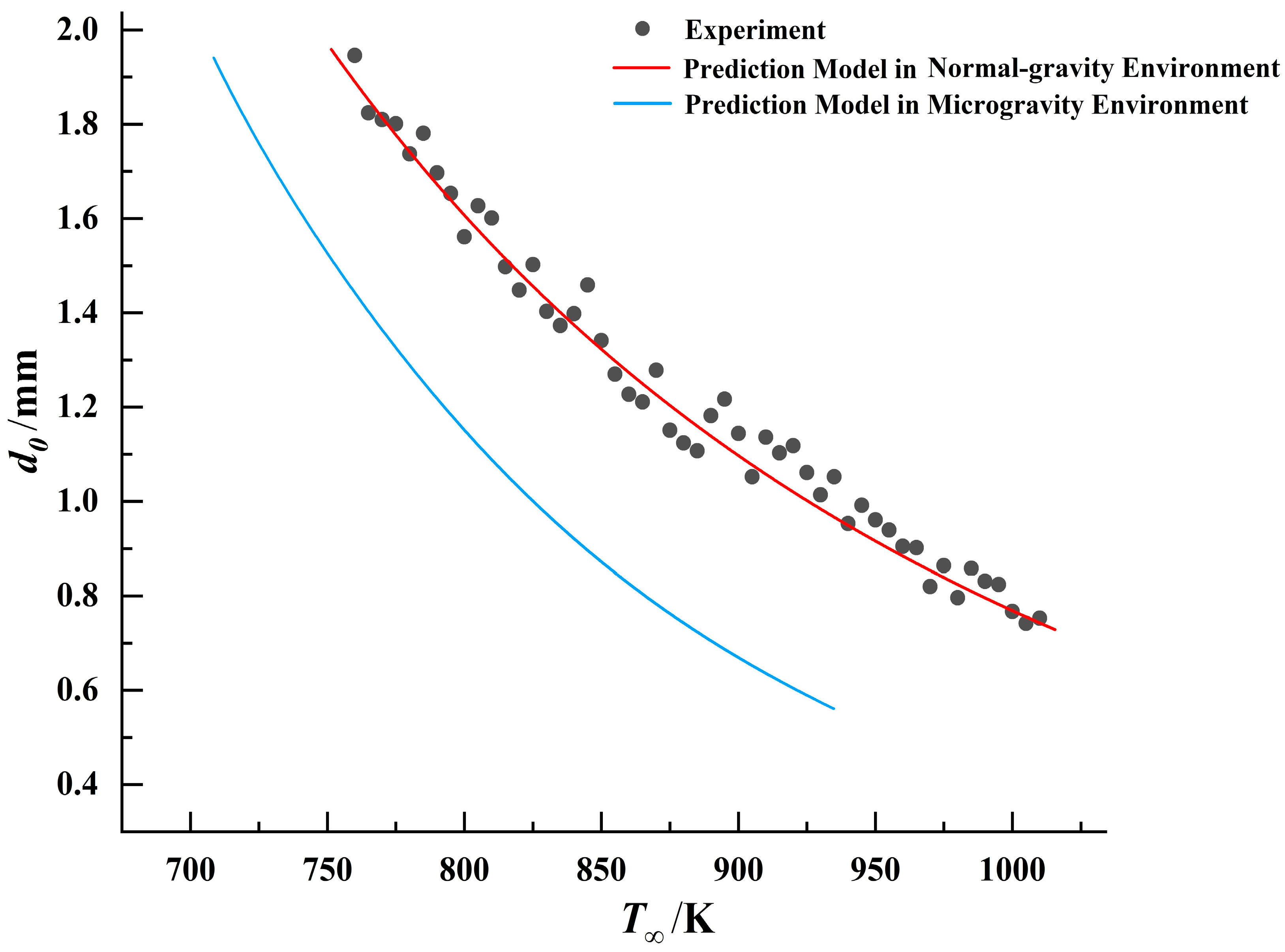
, a predictive model for droplet ignition in microgravity can be established. Comparisons between the ignition models in different gravitational environments and experimental data for the three fuels are presented in
Figure 18,
Figure 19 and
Figure 20.
By comparing the results, it was observed that under identical droplet diameter conditions, all three fuels exhibited lower ignition threshold temperatures in the microgravity environment. This indicates that droplets are more readily ignited in this environment. This conclusion aligns with phenomena observed in numerous prior microgravity combustion experiments [
15,
16,
18,
19,
40,
41]. Therefore, the proposed model effectively predicts the ignition boundaries of droplets in microgravity. However, due to the current lack of specific experimental data available from the microgravity environment, certain parameters in the derivation of empirical coefficients were approximated using the thermophysical properties of the fuels in normal gravity and those of air. Consequently, the semi-empirical and semi-theoretical predictive model presented in this study requires further experiments for refinement. However, the microgravity liquid combustion experiment module intended for use on the China Space Station’s Combustion Science Experimental System is not yet operational. As a result, there is a lack of direct experimental data to refine and optimize the mathematical model.