Appraisal of Industrial Pollutants in Sewage and Biogas Production Using Multivariate Analysis and Unsupervised Machine Learning Clustering
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Object
2.1.1. General Information
- Population equivalent (p.e.) of the WWTP: 180,000
- Maximum design hydraulic capacity of the WWTP: 42,200 m3/d
- Length of the combined sewage system: 76 km
- Length of the sanitary sewage system: 477.2 km
- Length of the rainwater system: 72.8 km
- Population equivalent (p.e.) of residents using sewage network: 93,708
- Population equivalent (p.e.) of industry using sewage network: 73,805
- Number of residents using septic tanks: 8821
- Number of residents using domestic treatment plants: 71
2.1.2. Energy Management
2.2. Analysed Parameters
2.3. Research Data Handling and Statistical Analysis
2.4. Data Preparation for Machine Learning
2.5. Unsupervised k-Means Method for Pollution Analysis in Sewage
2.6. The Assessment of Addition of Polyaluminium Chloride and Commercial Products for Struvite Reduction in Processing
3. Results
3.1. Concentrations of Pollution in Sewage at the Subsequent Treatment Stages
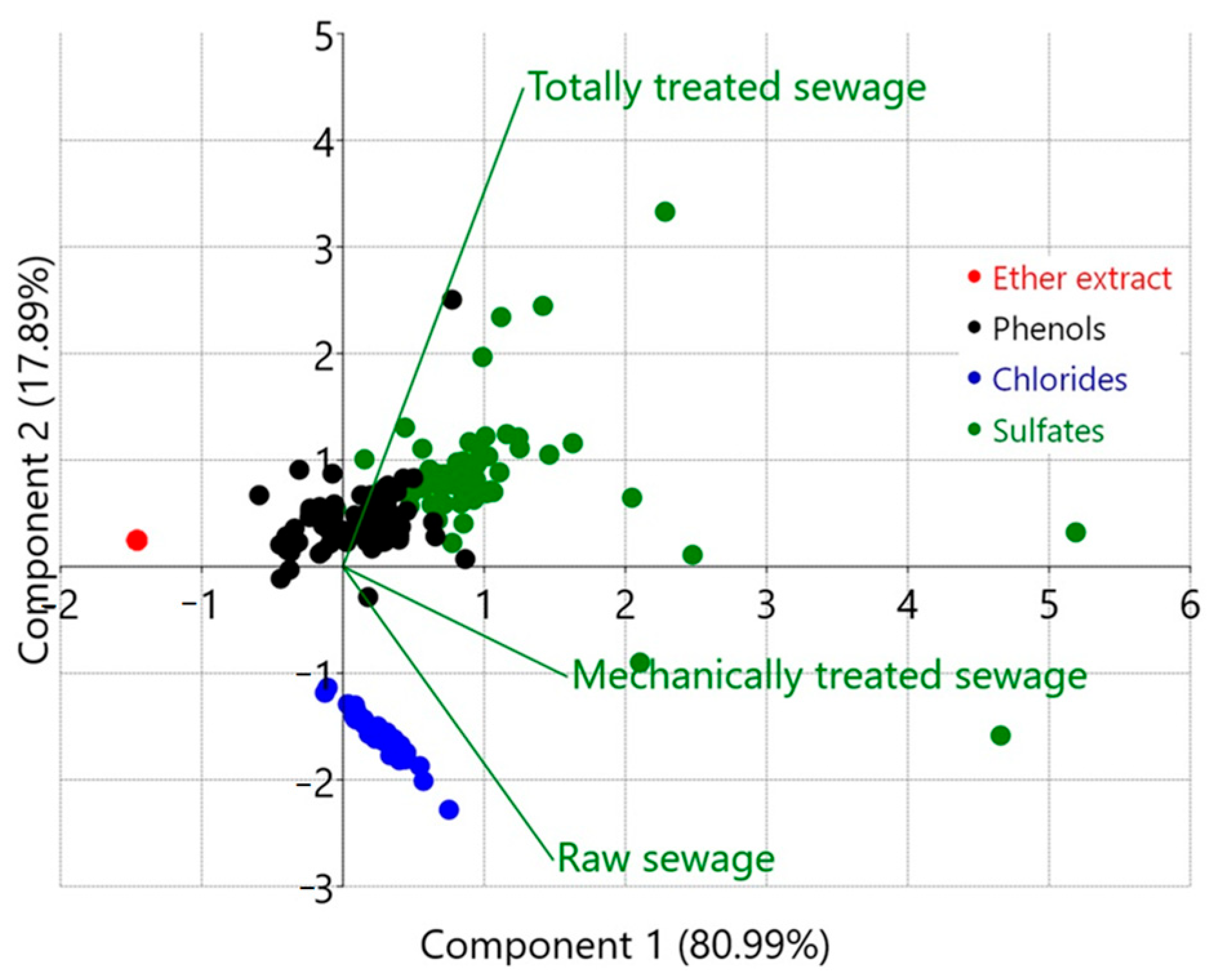
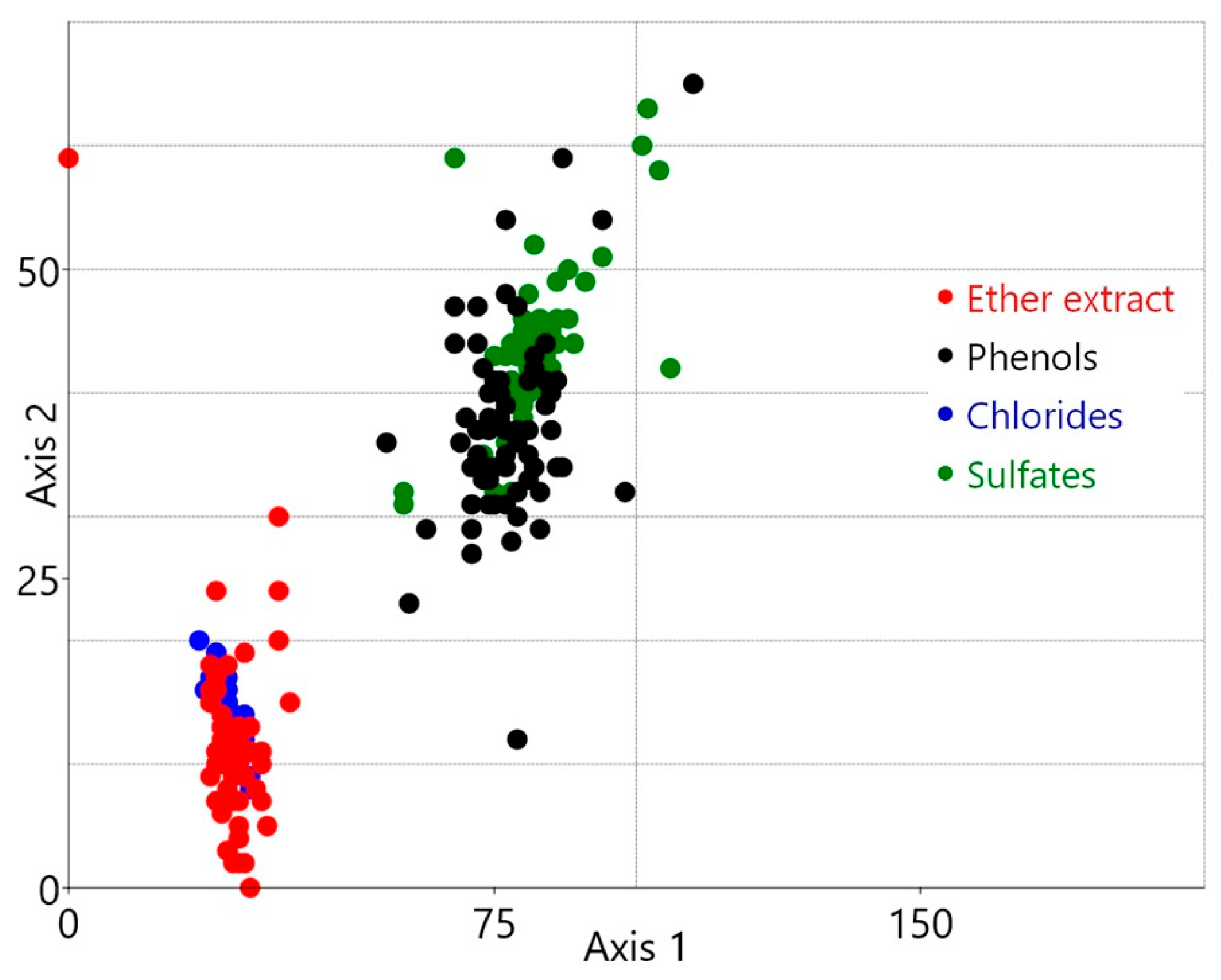
3.2. Selecting Optimal Cluster Number for Sewage Pollution Analysis
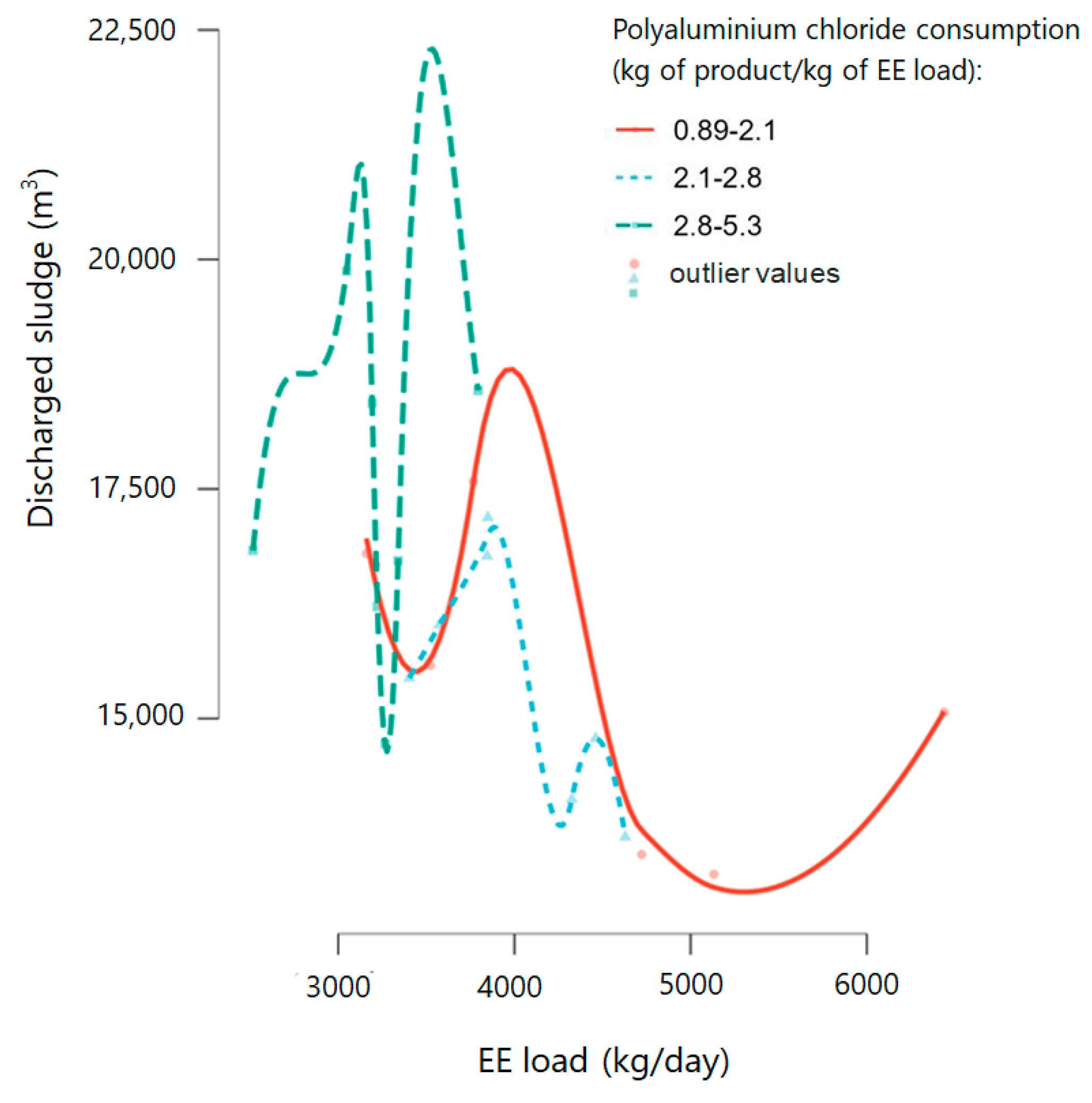
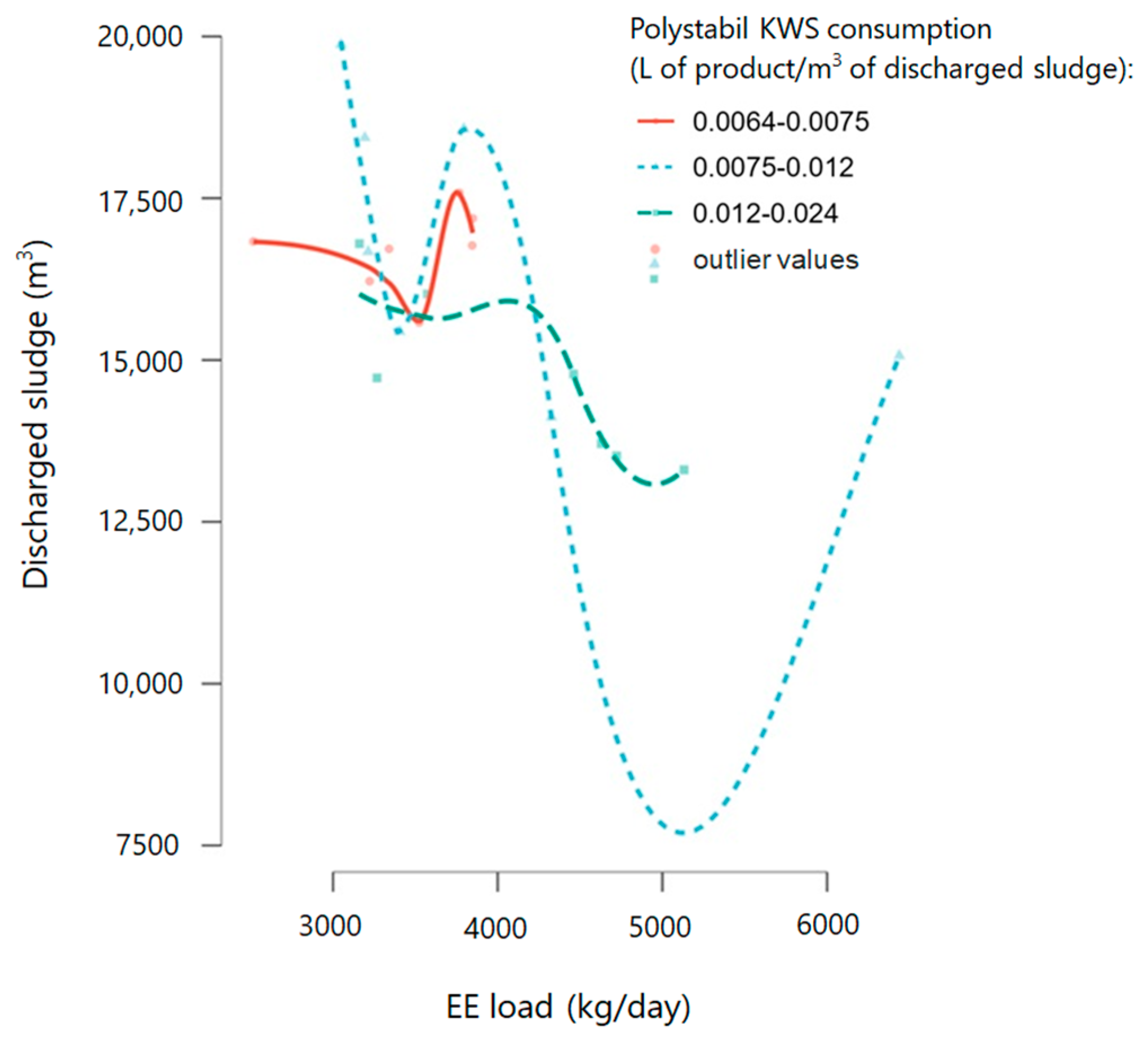
3.3. Technological Process Improvement Through Polyaluminium Chloride Addition and Struvite Reduction
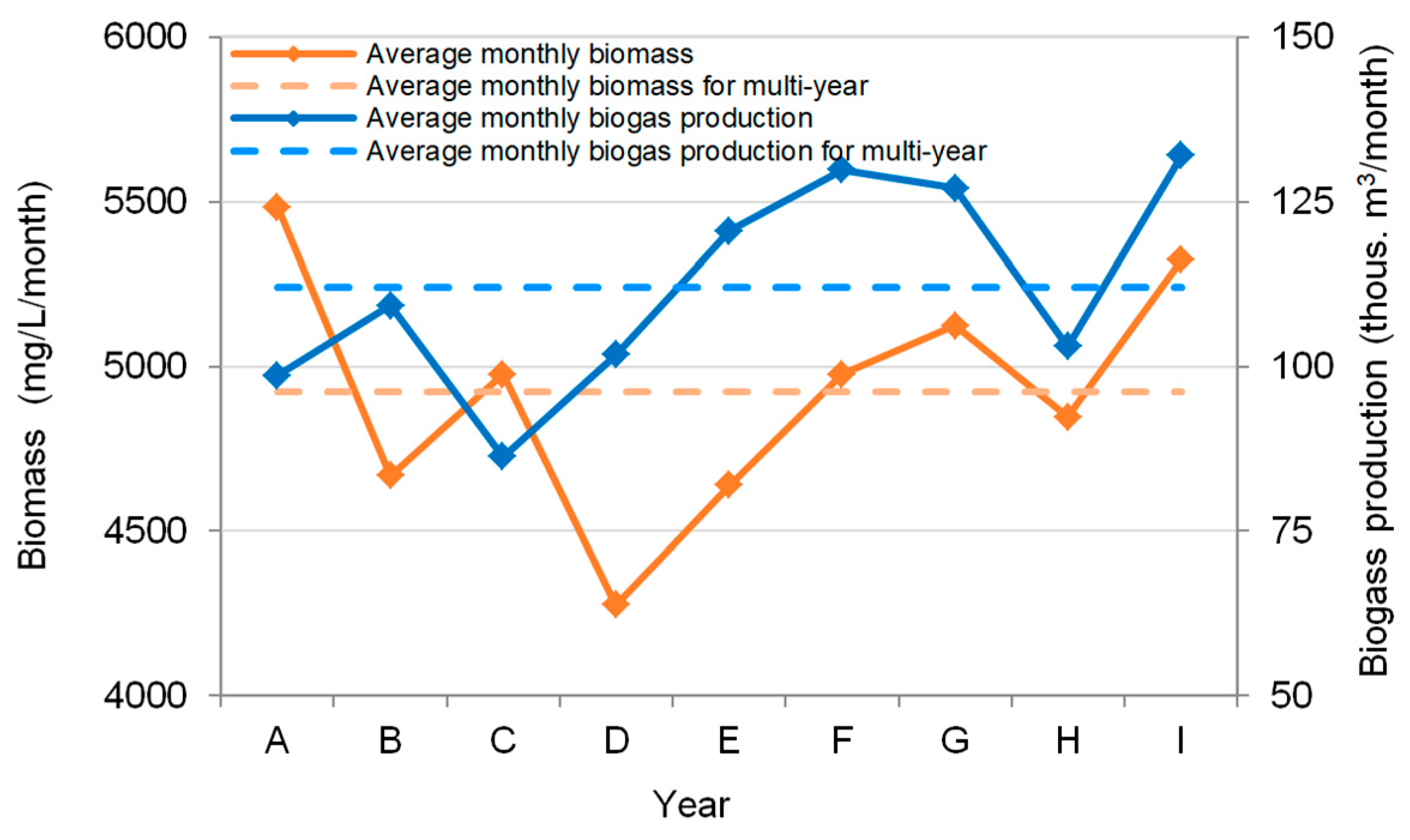
3.4. The Analysis of Biogas Production at the WWTP
4. Discussion
4.1. Removal of the Ether Extract, Phenols, Chlorides and Sulfates from Sewage in Municipal WWTPs
4.2. Biogas Production and Coagulant Assessment to Enhance Treatment Processes
4.3. Segregation of Industrial Pollutants Using a Machine Learning Clustering Algorithm
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Share of Own Energy in Demand | Covering the Demand with Own Energy | Energy Sale | Energy Purchase | Energy Production | Energy Used | Year |
|---|---|---|---|---|---|---|
| (%) | (MWh/Month) | |||||
| 20 | 20 | 12.68 | 215.16 | 34.27 | 249.43 | A |
| 66 | 66 | 40.32 | 119.75 | 115.17 | 234.91 | B |
| 51 | 51 | 4.06 | 120.64 | 119.44 | 240.08 | C |
| 61 | 61 | 1.90 | 86.29 | 135.97 | 221.20 | D |
| 73 | 70 | 7.35 | 64.27 | 160.17 | 217.08 | E |
| 88 | 85 | 6.67 | 32.03 | 198.11 | 223.49 | F |
| 94 | 90 | 9.31 | 22.13 | 209.15 | 222.80 | G |
| 89 | 85 | 8.26 | 35.21 | 209.63 | 236.58 | H |
| 75 | 74 | 2.11 | 71.64 | 201.75 | 271.29 | I |
| 69 | 67 | 10.30 | 85.24 | 153.74 | 235.21 | Avg |
| 0.32 | 0.30 | 1.08 | 0.67 | 0.36 | 0.07 | CV |
| Discharged Sewage Sludge | Composting | Biogas Production | Biomass | Year |
|---|---|---|---|---|
| (Thous. Dry Matter/Month) | (mg/Month) | (Thous. m3/Month) | (mg/L/Month) | |
| 279.35 | 834.5 | 98.62 | 5483 | A |
| 274.30 | 674.9 | 109.22 | 4668 | B |
| 234.85 | 652.5 | 86.25 | 4977 | C |
| 203.33 | 508.4 | 101.71 | 4275 | D |
| 244.97 | 555.1 | 120.60 | 4638 | E |
| 244.07 | 561.9 | 129.79 | 4977 | F |
| 256.35 | 581.9 | 127.02 | 5124 | G |
| 260.00 | 617.4 | 103.05 | 4846 | H |
| 267.32 | 721.6 | 131.97 | 5325 | I |
| 251.62 | 634.2 | 112.03 | 4924 | Avg |
| 0.09 | 0.15 | 0.13 | 0.07 | CV |
Appendix B
| Polyaluminium Chloride Consumption in Relation to EE Load | EE Load in Raw Sewage | Monthly Polyaluminium Chloride Consumption | Year |
|---|---|---|---|
| (kg of Product/kg of EE Load) | (kg/Day) | (kg of Product/Month) | |
| 2.185 | 4096 | 8950 | H |
| 2.745 | 3475 | 9540 | I |
| Polystabil KWS Consumption in Relation to the Volume of Discharged Sewage Sludge | Discharged Sewage Sludge | Monthly Polystabil KWS Consumption | Year |
|---|---|---|---|
| (L of Product/m3 of Discharged Sludge) | (m3/Month) | (L of Product/Month) | |
| 0.0125 | 15,050 | 188 | H |
| 0.0088 | 17,482 | 154 | I |
References
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in wastewater treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Rossi, M.L.; Kremer, P.; Cravotta, C.A.; Scheirer, K.E.; Goldsmith, S.T. Long-term impacts of impervious surface cover change and roadway deicing agent application on chloride concentrations in exurban and suburban watersheds. Sci. Total Environ. 2022, 851, 157933. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Gardeshi, M.; Arab, H.; Benguit, A.; Drogui, P. Runoff water loaded with road de-icing salts: Occurrence, environmental impact and treatment processes. Water Environ. J. 2024, 38, 20–31. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Mousavi, S.Z.; Potapova, A.; McIntyre, J.; Shi, X. Toxicological impacts of roadway deicers on aquatic resources and human health: A review. Water Environ. Res. 2021, 93, 1855–1881. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Al-Mur, B.A.; Jeyakumar, R.B. Cosmetic industrial wastewater treatment and bioelectricity production in upflow microbial fuel cell (UMFC) using extremophilic bacterial consortium. J. Taiwan Inst. Chem. Eng. 2025, 166, 105438. [Google Scholar] [CrossRef]
- Ke, X.; Qin, Z.; Chen, A.; Tian, Y.; Yang, Y.; Zhang, H.; Qiu, G.; Wu, H.; Wei, C. Triple strategies for process salt reduction in industrial wastewater treatment: The case of coking wastewater. Sep. Purif. Technol. 2025, 355, 129614. [Google Scholar] [CrossRef]
- Ngulube, K.F.; Abdelhaleem, A.; Osman, A.I.; Peng, L.; Nasr, M. Advancing sustainable water treatment strategies: Harnessing magnetite-based photocatalysts and techno-economic analysis for enhanced wastewater management in the context of SDGs. Environ. Sci. Pollut. Res. 2024, 31, 1–37. [Google Scholar] [CrossRef]
- Singh, A.; Gogoi, R.; Sharma, K.; Kumar, R.; Siril, P.F. Continuous flow synthesis of disordered covalent organic framework for ultra-high removal of industrial pollutants in flow. Sep. Purif. Technol. 2023, 307, 122739. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Wang, M.; Li, B.; Song, X.; Wu, M.; Guangzai, N.; Yin, Y. Efficient separation of biofuel, lignin and alkali from black liquor by acid precipitation and ethanol extraction for valorization. Ind. Crops Prod. 2024, 209, 117987. [Google Scholar] [CrossRef]
- Konieczny, P.; Szymański, M. Food industry wastewater—Characteristics, hazards, benefits. eko-net.pl “Firma i Środowisko” 2005, 10, 16–22. (In Polish) [Google Scholar]
- Rucka, K.; Balbierz, P.; Mańczak, M. Assessment of the possibilities of wastewater treatment from a confectionery plant. In Interdyscyplinarne Zagadnienia w Inżynierii i Ochronie Środowiska; Traczewska, T.M., Ed.; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2012; pp. 429–434. (In Polish) [Google Scholar]
- Rucka, K.; Mańczak, M.; Balbierz, P. Technological study of confectionary industry wastewater treatment in the activated sludge process. In Interdyscyplinarne Zagadnienia w Inżynierii i Ochronie Środowiska; Traczewska, T.M., Kaźmierczak, B., Eds.; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2014; pp. 699–706. (In Polish) [Google Scholar]
- Piekutin, J. Rainwater treatment of petroleum substances. Ekon. Środowisko 2013, 4, 60–66. (In Polish) [Google Scholar]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Boguski, A. Efficiency of grease substances removal in a municipal sewage treatment plant. Rocz. Ochr. Sr. 2008, 10, 481–489. (In Polish) [Google Scholar]
- Bhoi, R.; Shrivastava, A.; Sarkar, A. Chapter 10—Study of microbial communities in degrading toxic pollutants in the wastewater and solid waste treatment industries. In Functional Metagenomics. Microbial Diversity, Interaction, and Application in Bioremediation. Developments in Applied Microbiology and Biotechnology, 1st ed.; Shah, M.P., Ed.; Academic Press: New York, NY, USA, 2024; pp. 205–223. [Google Scholar] [CrossRef]
- Olczak, C.; Ligus, G.; Miodoński, J.M. Contemporary methods for treatment of phenolic coke wastewater. Chemik 2013, 67, 979–984. [Google Scholar]
- Salehi, S.; Abdollahi, K.; Panahi, R.; Rahmanian, N.; Shakeri, M.; Mokhtarani, B. Applications of biocatalysts for sustainable oxidation of phenolic pollutants: A review. Sustainability 2021, 13, 8620. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: A review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Rajan, P.S.; Arun, J.; Brindhadevi, K.; Le, Q.H.; Pugazhendhi, A. A review on recent advancements in extraction, removal and recovery of phenols from phenolic wastewater: Challenges and future outlook. Environ. Res. 2023, 237, 117005. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R. Treatment of wastewater streams containing phenolic compounds using hybrid techniques based on cavitation: A review of the current status and the way forward. Ultrason. Sonochem. 2008, 15, 1–15. [Google Scholar] [CrossRef]
- Szklarek, S.; Górecka, A.; Wojtal-Frankiewicz, A. The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution: A review. Sci. Total Environ. 2022, 805, 150289. [Google Scholar] [CrossRef]
- Duan, L.; Yun, Q.; Jiang, G.; Teng, D.; Zhou, G.; Cao, Y. A review of chloride ions removal from high chloride industrial wastewater: Sources, hazards, and mechanisms. J. Environ. Manag. 2024, 353, 120184. [Google Scholar] [CrossRef]
- Xu, W.; Liu, C. Application of induced crystallization for the mitigation of calcium sulfate in high-sulfate wastewater: A review. J. Water Process Eng. 2025, 69, 106824. [Google Scholar] [CrossRef]
- Khatry, I.S.; Devi, M.G.; Qartoubi, F.A.; Jadidi, A.A.; Al Rashdi, S.A. Investigation on adsorption performance of activated carbon prepared from municipal solid waste for the removal of pollutants from refinery wastewater. Indian J. Chem. Technol. 2024, 31, 288–297. [Google Scholar] [CrossRef]
- Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and Conditions to be Met During Sewage Discharge into the Water or into the Ground and During Rainwater or Snowmelt Discharge into the Water or into the Water Devices (Dz.U. 2019 poz. 1311). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20190001311/O/D20191311.pdf (accessed on 9 February 2025). (In Polish)
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Tahri, M.; Larif, M.; Bachiri, B.; Kitanou, S.; Rajib, B.; Benazouz, K.; Khimani, M.; Taky, M.; Elamrani, M.; Elmidaoui, A. Characterization of heavy metals and toxic elements in raw sewage and their impact on the secondary treatment of the Marrakech wastewater treatment plant. J. Mater. Environ. Sci. 2017, 8, 2311–2321. [Google Scholar]
- Stefanowicz, T.; Lisiecki, J. Trials on phenol decomposition in sewage by unadapted activated sludge. Arch. Environ. Prot. 2000, 26, 159–167. (In Polish) [Google Scholar]
- Zhong, W.; Wang, D.; Xu, X. Phenol removal efficiencies of sewage treatment processes and ecological risks associated with phenols in effluents. J. Hazard. Mater. 2012, 217–218, 286–292. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol removal from industrial wastewaters: A short review. Desalin. Water Treat. 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- Hong, C.C.; Chan, S.-K.; Shim, H. Effect of chloride on biological nutrient removal from wastewater. J. Appl. Sci. Environ. Sanit. 2007, 2, 85–92. [Google Scholar]
- Li, Y.; Yang, Z.; Yang, K.; Wei, J.; Li, Z.; Ma, C.; Yang, X.; Wang, T.; Zeng, G.; Yu, G.; et al. Removal of chloride from water and wastewater: Removal mechanisms and recent trends. Sci. Total Environ. 2022, 821, 153174. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, B.; Doucette, E.M.; Kyser, S.J. Centralized softening as a solution to chloride pollution: An empirical analysis based on Minnesota cities. PLoS ONE 2021, 16, e0246688. [Google Scholar] [CrossRef]
- Kyser, S.; Doucette, E. Alternatives for Addressing Chloride in Wastewater Effluent; Minnesota Pollution Control Agency: St. Paul, MN, USA, 2018. Available online: https://www.pca.state.mn.us/sites/default/files/wq-wwprm2-18.pdf (accessed on 9 February 2025).
- El Houari, A.; Ranchou-Peyruse, M.; Ranchou-Peyruse, A.; Bennisse, R.; Bouterfas, R.; Goni Urriza, M.S.; Qatibi, A.-I.; Guyoneaud, R. Microbial communities and sulfate-reducing microorganisms abundance and diversity in municipal anaerobic sewage sludge digesters from a wastewater treatment plant (Marrakech, Morocco). Processes 2020, 8, 1284. [Google Scholar] [CrossRef]
- van den Brand, T.P.H.; Roest, K.; Chen, G.H.; Brdjanovic, D.; van Loosdrecht, M.C.M. Potential for beneficial application of sulfate reducing bacteria in sulfate containing domestic wastewater treatment. World J. Microbiol. Biotechnol. 2015, 31, 1675–1681. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Lee, E.; Kim, K.; Han, J.-I. Sulfate reducing bacteria-based wastewater treatment system integrated with sulfide fuel cell for simultaneous wastewater treatment and electricity generation. Chemosphere 2019, 233, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rincón, F.; Lopez-Vazquez, C.; Welles, L.; van den Brand, T.; Abbas, B.; van Loosdrecht, M.; Brdjanovic, D. Effects of electron acceptors on sulphate reduction activity in activated sludge processes. Appl. Microbiol. Biotechnol. 2017, 101, 6229–6240. [Google Scholar] [CrossRef]
- García de Lomas, J.; Corzo, A.; Gonzalez, J.M.; Andrades, J.A.; Iglesias, E.; Montero, M.J. Nitrate promotes biological oxidation of sulfide in wastewaters: Experiment at plant-scale. Biotechnol. Bioeng. 2006, 93, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Kyser, S.; Wolohan, K.; Hanson, A.; Parrella, A.; Jones, D. Sulfate Treatment; Minnesota Pollution Control Agency: St. Paul, MN, USA, 2018. Available online: https://www.eqb.state.mn.us/sites/eqb/files/documents/FINAL_181107%20Sulfate%20Treatment%20Presentation.pdf (accessed on 9 February 2025).
- Abali, M.; Ait Ichou, A.; Zaghloul, A.; Chiban, M.; Sinan, F.; Zerbet, M. Evaluation and improvement of the WWTP performance of an agricultural cooperative by adsorption on inert biomaterial: Case of orthophosphate, nitrate and sulfate ions. Appl. Water Sci. 2022, 12, 125. [Google Scholar] [CrossRef]
- Hou, J.; Guo, W.; Wen, Y. Effect of sulfate load on sulfur removal in model constructed wetlands. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012078. [Google Scholar] [CrossRef]
- Ruszel, M.; Masłoń, A.; Ogarek, P. Analysis of biogas from sewage sludge digestion in terms of diversification in the natural gas production structure in Poland. Desalin. Water Treat. 2021, 232, 298–307. [Google Scholar] [CrossRef]
- Ciuła, J.; Wiewiórska, I.; Banaś, M.; Pająk, T.; Szewczyk, P. Balance and energy use of biogas in Poland: Prospects and directions of development for the circular economy. Energies 2023, 16, 3910. [Google Scholar] [CrossRef]
- Guilera, J.; Andreu, T.; Basset, N.; Boeltken, T.; Timm, F.; Mallol, I.; Morante, J.R. Synthetic natural gas production from biogas in a waste water treatment plant. Renew. Energy 2020, 146, 1301–1308. [Google Scholar] [CrossRef]
- Michalski, K.; Kośka-Wolny, M.; Chmielowski, K.; Bedla, D.; Petryk, A.; Guzdek, P.; Dąbek, K.A.; Gąsiorek, M.; Grübel, K.; Halecki, W. Examining the potential of biogas: A pathway from post-fermented waste into energy in a wastewater treatment plant. Energies 2024, 17, 5618. [Google Scholar] [CrossRef]
- Hernandez, J.E.; Edyvean, R.G.J. Inhibition of biogas production and biodegradability by substituted phenolic compounds in anaerobic sludge. J. Hazard. Mater. 2008, 160, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, Z.; Liu, D.; Wu, Y.; Wang, J.; Wang, Q. Coagulation behavior of polyaluminum chloride: Effects of pH and coagulant dosage. Chin. J. Chem. Eng. 2015, 23, 1041–1046. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Luo, H.; Yan, S.; Dai, J.; Bai, Z. Efficient recovery of phenol from coal tar processing wastewater with tributylphosphane/diethyl carbonate/cyclohexane: Extraction cycle and mechanism study. Chem. Eng. Res. Des. 2020, 157, 104–113. [Google Scholar] [CrossRef]
- Libecki, B.; Wardzyńska, R.; Kalinowski, S.; Kuźbik, K. Purification of a reactive dyes mixture solution by means of pre-hydrolyzed coagulants in the standard tests and coagulating flow system. Chem. Eng. Sci. 2025, 301, 120784. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Morrissey, K.G.; Anari, Z.; Daneshpour, R.; Greenlee, L.F.; Thoma, G. Sustainable electroless nutrient recovery from natural agro-industrial and livestock farm wastewater effluents with a flow cell reactor. Resour. Conserv. Recycl. 2025, 212, 107972. [Google Scholar] [CrossRef]
- Sultana, N.; Roddick, F.; Pramanik, B.K. Innovative grease interceptor to enhance fat, oil, and grease removal from the wastewater generated from food service establishments. Chemosphere 2025, 370, 143987. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.A.; Pasquali, C.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero, Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef]
- Corominas, L.; Garrido-Baserba, M.; Villez, K.; Olsson, G.; Cortés, U.; Poch, M. Transforming data into knowledge for improved wastewater treatment operation: A critical review of techniques. Environ. Model. Softw. 2018, 106, 89–103. [Google Scholar] [CrossRef]
- Beckers, L.M.; Brack, W.; Dann, J.P.; Krauss, M.; Müller, E.; Schulze, T. Unraveling longitudinal pollution patterns of organic micropollutants in a river by non-target screening and cluster analysis. Sci. Total Environ. 2020, 727, 138388. [Google Scholar] [CrossRef]
- Choi, J.M.; Manthapuri, V.; Keenum, I.; Brown, C.L.; Xia, K.; Chen, C.; Vikesland, P.J.; Blair, M.F.; Bott, C.; Pruden, A.; et al. A machine learning framework to predict PPCP removal through various wastewater and water reuse treatment trains. Environ. Sci. Water Res. Technol. 2025, 11, 481–493. [Google Scholar] [CrossRef]
- Peng, Q.; Yu, J.; Pang, Y.; Deng, L.; Tang, J.; Wang, J.; Wu, N.; Tang, L. Prospects of advanced oxidation processes for high-salinity coking wastewater treatment: A strategy to support sustainable management. Resour. Conserv. Recycl. 2025, 212, 107880. [Google Scholar] [CrossRef]
- Hanjra, M.A.; Blackwell, J.; Carr, G.; Zhang, F.; Jackson, T.M. Wastewater irrigation and environmental health: Implications for water governance and public policy. Int. J. Hyg. Environ. Health 2012, 215, 255–269. [Google Scholar] [CrossRef] [PubMed]

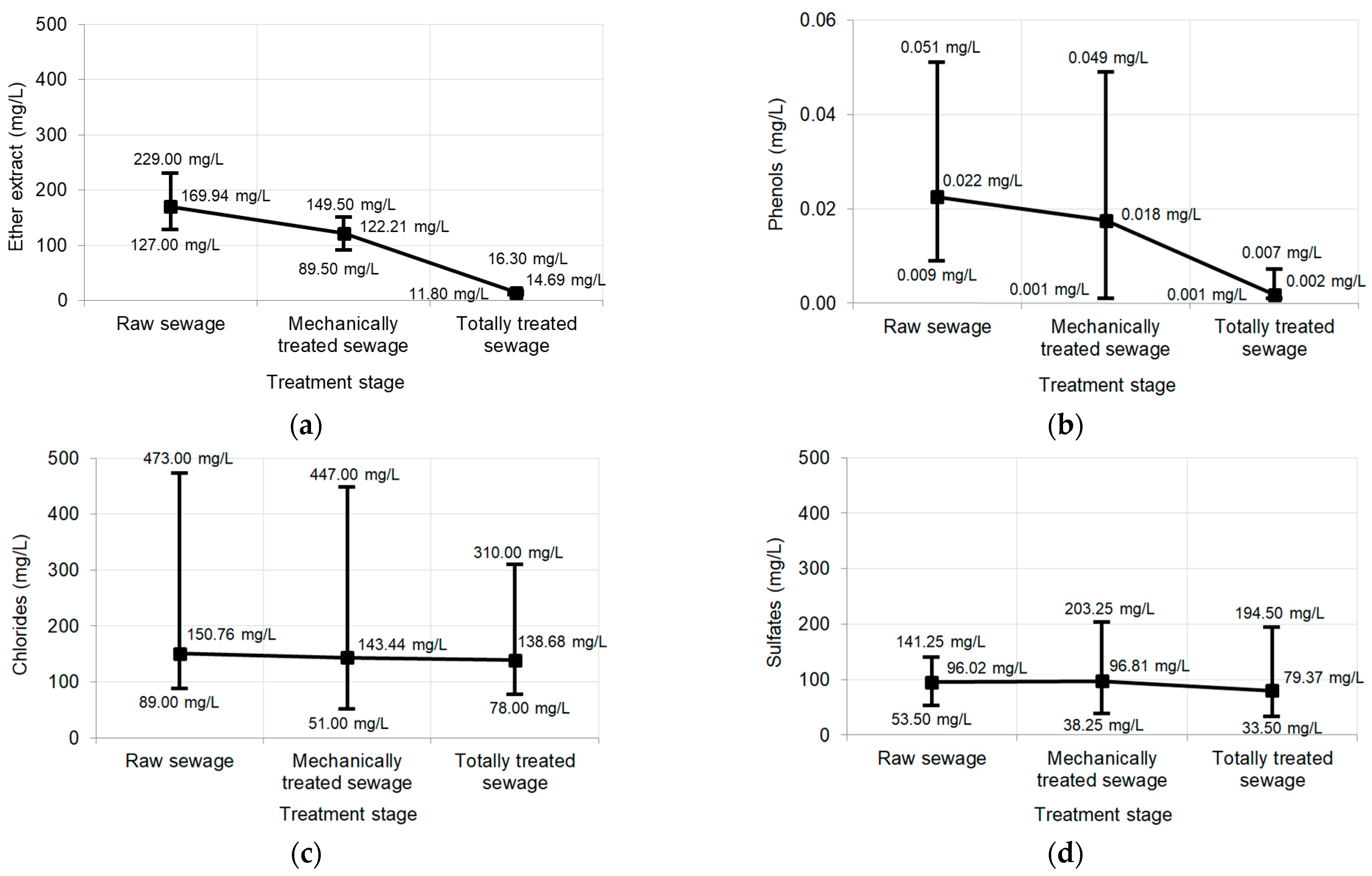
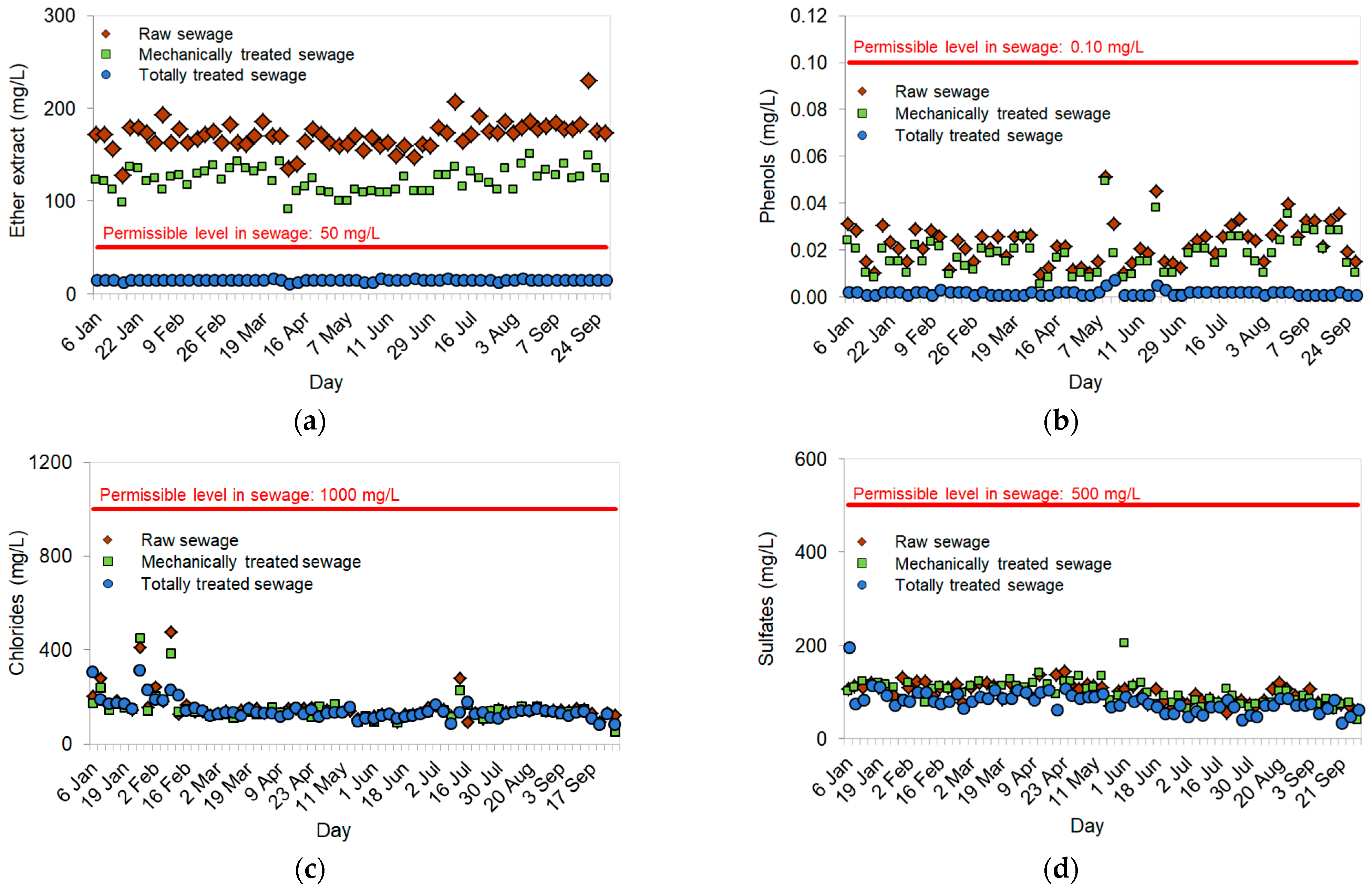
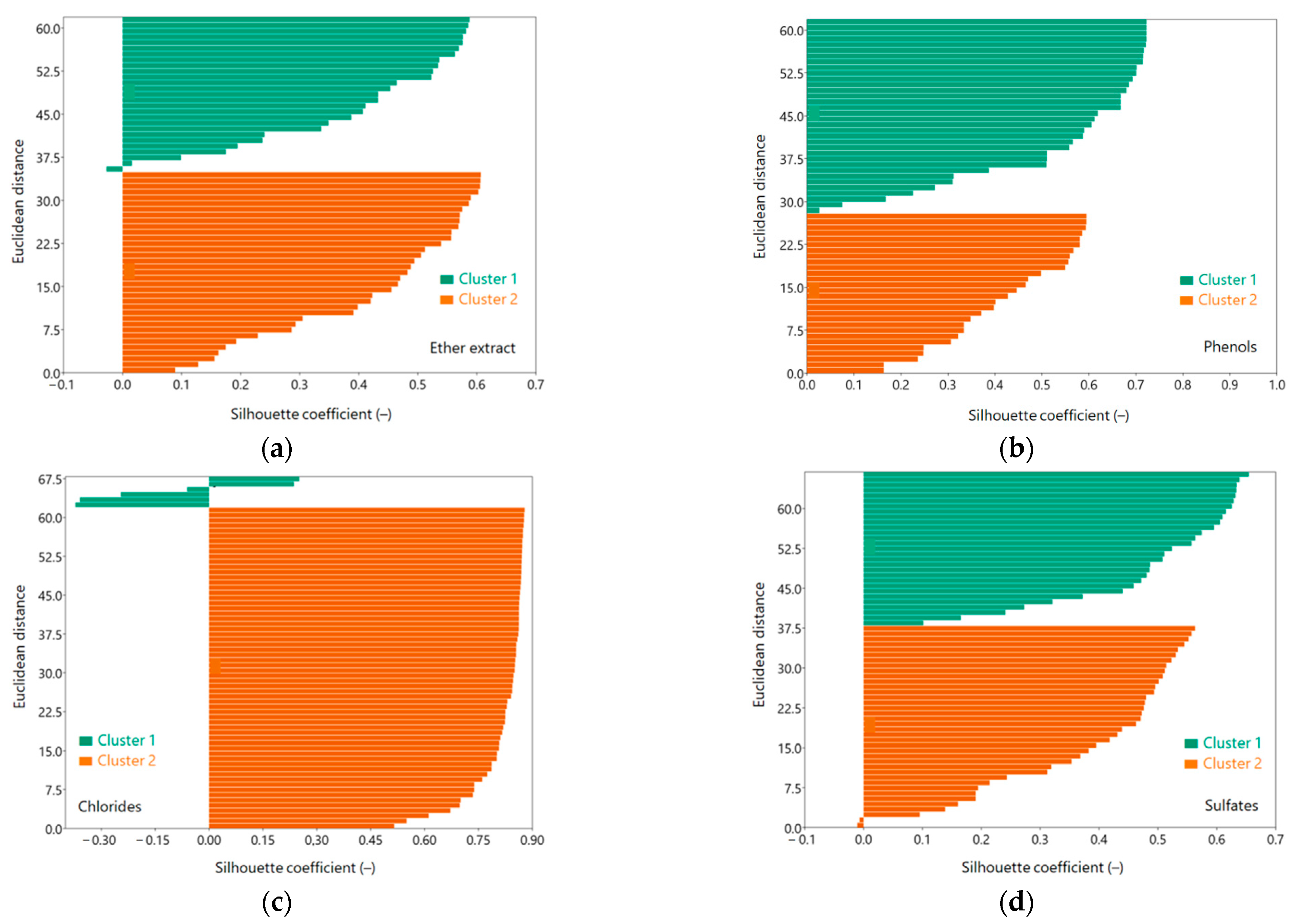
Pollutants tested in raw sewage, mechanically treated sewage and totally treated sewage:
|
Coagulant tested for suspended particles removal:
|
Coagulant tested for struvite reduction:
|
Parameters tested for biogas production:
|
| Sk | Kurt | CV | STD | Max | Avg | Min | Polluting Substance |
|---|---|---|---|---|---|---|---|
| (−) | (mg/L) | ||||||
| Raw sewage | |||||||
| 0.45 | 3.68 | 0.09 | 15.33 | 229.00 | 169.94 | 127.00 | Ether extract |
| 0.76 | 1.06 | 0.38 | 0.009 | 0.051 | 0.022 | 0.009 | Phenols |
| 3.68 | 15.63 | 0.40 | 60.50 | 473.00 | 150.76 | 89.00 | Chlorides |
| −0.04 | −0.76 | 0.21 | 20.23 | 141.25 | 96.02 | 53.50 | Sulfates |
| Mechanically treated sewage | |||||||
| −0.17 | −0.40 | 0.10 | 12.78 | 149.50 | 122.21 | 89.50 | Ether extract |
| 1.09 | 2.60 | 0.47 | 0.008 | 0.049 | 0.018 | 0.001 | Phenols |
| 3.68 | 17.23 | 0.39 | 55.25 | 447.00 | 143.44 | 51.00 | Chlorides |
| 1.05 | 4.41 | 0.25 | 24.10 | 203.25 | 96.81 | 38.25 | Sulfates |
| Totally treated sewage | |||||||
| −1.15 | 1.72 | 0.06 | 0.87 | 16.30 | 14.69 | 11.80 | Ether extract |
| 2.85 | 10.69 | 0.60 | 0.001 | 0.007 | 0.002 | 0.001 | Phenols |
| 2.25 | 6.91 | 0.29 | 40.90 | 310.00 | 138.68 | 78.00 | Chlorides |
| 1.76 | 8.77 | 0.29 | 22.63 | 194.50 | 79.37 | 33.50 | Sulfates |
| Sulfates | Chlorides | Phenols | Ether Extract | Treatment Stage | ||||
|---|---|---|---|---|---|---|---|---|
| ηSTD | ηAvg | ηSTD | ηAvg | ηSTD | ηAvg | ηSTD | ηAvg | |
| (%) | ||||||||
| N/A | N/A | N/A | N/A | 12.87 | 23.97 | 5.64 | 27.98 | Mechanically treated sewage |
| 11.90 | 22.10 | 11.36 | 13.02 | 3.83 | 91.81 | 0.73 | 91.31 | Totally treated sewage |
| a | ||||||
| p | df2 | df1 | F | Wilks’ Lambda | Independent Variables | |
| <0.001 | 256 | 1 | 2119 | 0.10 | Mechanically treated sewage | |
| <0.001 | 256 | 1 | 117.4 | 0.68 | Totally treated sewage | |
| b | ||||||
| p | R2 | t | Standard Error | Coefficient | Independent Variables | Dependent Variable |
| 0.051 | − | 1.956 | 2.30 | 4.51 | Constant | Raw sewage |
| <0.001 | 0.88 | 46.03 | 0.028 | 1.30 | Mechanically treated sewage | |
| <0.001 | 0.24 | −10.83 | 0.029 | −0.318 | Totally treated sewage | |
| Polystabil KWS Consumption | Ether Extract Load | Discharged Sewage Sludge | Polyaluminium Chloride Consumption | Parameter of Correlation | |
|---|---|---|---|---|---|
| — | — | — | — | Polyaluminium chloride consumption | Spearman’s Rho |
| — | — | — | — | p-value | |
| — | — | — | — | Effect size (Fisher’s z) | |
| — | — | — | — | SE Effect size | |
| — | — | — | 0.488 | Discharged sewage | Spearman’s Rho |
| — | — | — | 0.026 | p-value | |
| — | — | — | 0.534 | Effect size (Fisher’s z) | |
| — | — | — | 0.238 | SE Effect size | |
| — | — | −0.614 | −0.443 | Ether extract load | Spearman’s Rho |
| — | — | 0.004 | 0.046 | p-value | |
| — | — | −0.716 | −0.476 | Effect size (Fisher’s z) | |
| — | — | 0.241 | 0.237 | SE Effect size | |
| — | 0.260 | −0.569 | −0.388 | Polystabil KWS consumption | Spearman’s Rho |
| — | 0.254 | 0.008 | 0.083 | p-value | |
| — | 0.266 | −0.646 | −0.410 | Effect size (Fisher’s z) | |
| — | 0.234 | 0.240 | 0.236 | SE Effect size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halecki, W.; Młyńska, A.; Chmielowski, K. Appraisal of Industrial Pollutants in Sewage and Biogas Production Using Multivariate Analysis and Unsupervised Machine Learning Clustering. Appl. Sci. 2025, 15, 6222. https://doi.org/10.3390/app15116222
Halecki W, Młyńska A, Chmielowski K. Appraisal of Industrial Pollutants in Sewage and Biogas Production Using Multivariate Analysis and Unsupervised Machine Learning Clustering. Applied Sciences. 2025; 15(11):6222. https://doi.org/10.3390/app15116222
Chicago/Turabian StyleHalecki, Wiktor, Anna Młyńska, and Krzysztof Chmielowski. 2025. "Appraisal of Industrial Pollutants in Sewage and Biogas Production Using Multivariate Analysis and Unsupervised Machine Learning Clustering" Applied Sciences 15, no. 11: 6222. https://doi.org/10.3390/app15116222
APA StyleHalecki, W., Młyńska, A., & Chmielowski, K. (2025). Appraisal of Industrial Pollutants in Sewage and Biogas Production Using Multivariate Analysis and Unsupervised Machine Learning Clustering. Applied Sciences, 15(11), 6222. https://doi.org/10.3390/app15116222







