Abstract
In this study, the facile removal of the chromium-complex-based reactive azo dye C. I. Reactive Black 8 (RB8) from model wastewaters by the co-action of alternative sorbents—biochar (BC) and bentonite (BT)—with ionic liquids such as benzalkonium chloride (BAC) or Aliquat 336 (A336) was studied. The experiments using model RB8-containing wastewater proved that the co-action of BAC with BC is the most promising method of RB8 separation from wastewater containing 1 g L−1 of RB8 dye. The application of 2 g L−1 BC in co-action with 1.5 g L−1 BAC or 1 g L−1 BT in co-action with 2 g L−1 BAC enables the removal of more than 98% of contaminant RB8 after 30 min of action. Similar removal efficiency (RE) was achieved using 40 g L−1 of powdered activated carbon (PAC) after 180 min of action. To reach the same RE using real RB8-containing wastewater, a four times higher dose of BC and a four times higher dose of BAC per gram of removed RB8 were required. The proposed mechanism of RB8 removal by the co-action of alternative sorbents with BAC comprises a parallel effect of (i) sorption, (ii) the formation of less polar ion pairs accompanied by their sorption on an alternative sorbent and (iii) the separation of used alternative sorbents covered with ion pairs. The removal efficiency of organic contaminant(s) from both model and real wastewater was evaluated by VIS spectroscopy applying the Lambert–Beer law and by the determination of chemical oxidation demand (COD) and/or adsorbable organically bound halogen (AOX) parameters.
Keywords:
ionic liquids; biochar; bentonite; sorption; ion exchange; reactive dyes; azo dyes; reactive black 8 1. Introduction
Synthetic azo dyes are used in a wide range of industrial applications, such as the textile industry, food colorants, cosmetics, paper printing, etc. [1]. A particular subgroup of azo dyes consists of reactive azo dyes, which includes, among others, chromium-complex-based azo dye Reactive Black 8 (RB8) utilized namely for chemical drawing (see Figure 1) [2,3]. In 2020, about 7 × 107 tons of synthetic dyes were produced worldwide [4]. However, during dye production, usually about 5–10% of produced dyes are discharged in effluent [4]. Therefore, dyes have been found frequently in industrial wastewaters [1].
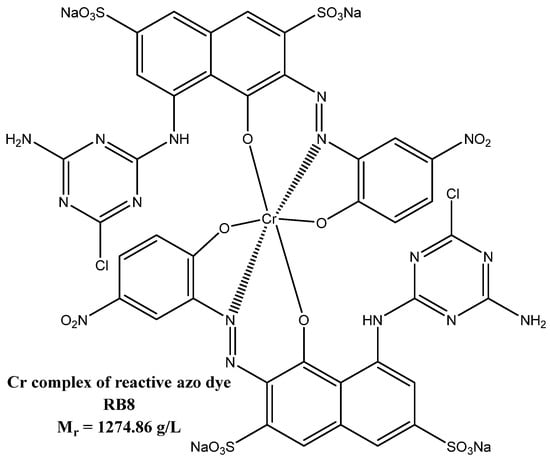
Figure 1.
The chemical structure of RB8 dye (dye–SO3Na).
The wastewaters derived from dye production are highly variable in composition and contain many different compounds such as raw materials, intermediate products and even the dye itself [5]. This type of wastewater is also characterized by a high chemical oxygen demand (COD) as well as a high colorant content [3]. A concentration of dyes in this type of wastewater can reach up to tens of grams per liter [6].
The release of partially treated or untreated wastewater containing such dye into the environment could pose serious health and/or environmental risks. For example, in humans, it has been documented that some dyes can induce various ailments [7]. In addition, with respect to plants, the presence of dyes has become a major challenge, causing, for example, the inhibition of growth and a reduction in the pigment and protein content of microalgae [7]. Thus, even textile dyes can be classified as significant aqueous contaminants [6,7].
Therefore, these negative effects associated with dye-loaded wastewater increase the need for effective removal prior to industrial discharge. As discharge regulations become more stringent, the mechanical-biological treatment processes seem to be unable to comply with COD and color removal requirements [3].
Advanced treatment techniques are therefore urgently required [5]. Several biological treatment methods and various microorganisms such as bacteria, algae, fungi or yeast are available for dye bio-degradation (for detailed information, see [8] and refs. therein). Jha et al. [9] found that the biological degradation of RB8 dye occurred in a low concentration in wastewater using hairy roots of Physalis minima L. The above-mentioned biological methods are generally inexpensive, easy to apply and are currently used to remove organics and colors from wastewater. On the other hand, biological treatments often do not reach the required dye removal efficiency due to the recalcitrant nature of some dyes [5]. Other conventional wastewater treatment methods include coagulation/flocculation [5], advanced oxidation processes such as the Fenton reaction [10] or electrooxidation [11], etc. There are also innovative reductive methods based even on the hydrodehalogenation of halogenated dyes, their by-products and unreacted starting materials using aluminum-based alloys [12,13,14].
Several authors presented methods for the removal of the RB8 dye [8,15,16,17,18,19]. These methods comprise biological treatment [8], Fenton oxidation [15], sonification [17], electro-oxidation [17] or photocatalytic oxidation [16]. Zhang et al. [18] and Khoshhesab et al. [19] also published the possibilities of RB8 dye adsorption on nano ZnO or Fe powder, respectively.
The previously mentioned adsorption of dyes (including RB8 dye) using different types of adsorbents is a widely used method for the treatment of contaminated aqueous streams [20]. The typical example of the most widely applied carbonaceous adsorbent is activated carbon (AC) [20]. Despite the high adsorption efficiency of the dyes on this sorbent, activated carbon is quite expensive [21], and the disposal of saturated active carbon can be problematic and costly, too [21].
In the last decade, the emphasis has been placed on the application of cheap alternative carbonaceous sorbents, such as biochar (BC) produced from waste biomass [22]. BC has recently gained an interest for its potential application in agriculture due to great carbon sequestration [23,24]. The physical and chemical properties of BC are dictated by the feedstock and production processes [23,24,25]. As Yargicoglu et al. [25] reported, a wide range of fixed carbon (0–47.8%), volatile matter (28–74.1%) and ash contents (1.5–65.7%) were observed among tested samples of BC. A high variability in the surface area (0.1–155.1 g m2) and PAH and heavy metal contents of the solid phase was also detected (0.7–83 mg kg−1) [25]. Detailed characterizations and descriptions of BC are presented in several research works [23,24,25]; for a graphical representation of the BC structure, see ref. [26].
Due to relatively high cation exchange capacities and low cost, the utilization of a clay sorbent, such as bentonite (BT) or montmorillonite (MTM), is also one of the alternative sorption techniques applicable for the removal of organic pollutants from wastewater [27,28,29]. BT/MTM consists chiefly of crystalline clay minerals belonging to the smectite group, which are hydrated aluminum silicates containing iron and magnesium. Two types of BT—sodium or calcium—are recognized, and the uses of each depend on specific physical properties [28,29]. The main constituents of BT clay, SiO2, Al2O3, K2O, Fe2O3, MgO, Na2O and TiO2, reach approx. 50%, 15%, 0.3%, 12%, 3%, 3% and 0.269%, respectively, with the rest of the impurities [30,31]. The further descriptions, physical and chemical characteristics and structures of BT clay are detailed in refs. [28,30,31]. These clay materials find many applications, e.g., in the construction industry or as cat litter [28].
Despite several different applications of BC and BT (in agriculture, construction industry, etc.), many authors have previously published options for the adsorption of different types of dyes on BC [32,33,34,35] as well as on BT [27,29,36,37,38]. However, BC and BT provided lower adsorption capacities and removal efficiencies (in comparison with AC action) [32,33,34,35,36,37].
The applicable improvement of removal efficiencies serves the co-action of BC or BT together with appropriate ionic liquids (ILs) [32,38]. ILs are usually defined as compounds completely composed of ions with melting points below 100 °C [39]. Some authors reported the application of ILs in the separation of acid dyes from aqueous solutions. Vijayaraghavan et al. [39], for the first time in 2006, reported on the effective extraction of selected acid azo dyes from wastewater using ILs. Since 2006, other authors [40,41,42,43] introduced efficient methods applicable for acid dye separation using ILs.
As we previously published, anionic drugs [44] or anionic azo dyes [45] can be effectively separated from aqueous solutions using ILs based on quaternary ammonium salts (R4NX). This method consists in the addition of commercially available and inexpensive ILs, which is accompanied by the ion exchange of inorganic anions X− of R4NX with anions of acid dyes (according to Equation (1)), producing low-polar ion pairs (dye–SO3NR4) insoluble in aqueous solution and separable by sedimentation and/or filtration.
dye–SO3− + Na+ + R4N+ + X− → dye–SO3NR4 ↓ + Na+ + X−
In this paper, the removal of the chromium complex of reactive azo dye RB8 using alternative sorbents such as BC or BT alone or in the co-action of IL benzalkonium chloride (BAC) or Aliquat 336 (A336) (for chemical drawings, see Figure 2) and the action of sole BAC or A336 was studied in model and real wastewater produced in the dye manufacturing process.
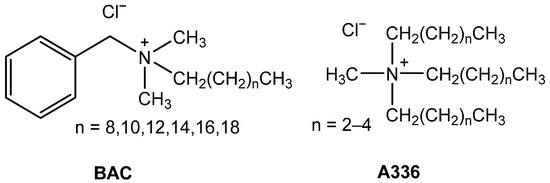
Figure 2.
Chemical drawings of tested ionic liquids (R4NX)—BAC and A336.
2. Materials and Methods
2.1. Chemicals
RB8 dye was purchased from a local supplier (Synthesia Co., Pardubice, Czech Republic) with defined HPLC purity higher than 98%. The content of inorganic species in RB8 is documented by EDXRF Figure S1 in the Supplementary Materials. Model wastewater was prepared with an initial concentration of RB8 c0 = 1 g L−1. The real wastewater produced by manufacturing RB8 (aqueous filtrate produced within separation of dye; the specifications: concentration of RB8 c0 = 7 g L−1, COD = 10.6 g L−1, AOX = 215 mg L−1; for the absorption spectrum, see Figure S2 in the Supplementary Materials) was also obtained from the local dye and pigment producer. Ionic liquids—Aliquat 336 (A336) in purity 98%+ and 50% aq. benzalkonium chloride (BAC)—were purchased from Merck (Prague, Czech Republic). Other chemicals and solvents in p.a. quality were obtained from Lach-Ner Co., Neratovice, Czech Republic.
2.2. Sorbents
Powdered activated carbon (PAC, granulation < 45 µm) Silcarbon CW20 was purchased from Brenntag Co. (Prague, Czech Republic). Montmorillonite (powder “K10”) was obtained from Sigma-Aldrich (Prague, Czech Republic). The powdered bentonite (BT) material was purchased from the local supplier (Keramost Co., Most, Czech Republic, the waste product from the processing of bentonite clays). Biochar (BC) was a material from the Institute of Chemical Process Fundamentals of the Czech Academy of Sciences [46], produced in a twin-fire gasifier at 700 °C using a four-stage pyrolysis of waste wood biomass. For a detailed description of the BC sample, see our previous work in ref. [32]. The brief specification of sorption materials is given in Table 1. The detailed characteristics and specifications of tested BC sample are available in refs. [23,24].

Table 1.
The specifications of the BC, BT and commercial adsorbents according to refs. [47,48,49,50].
2.3. Separation of Reactive Dye from Model and Real Wastewater
Comparative experiments were carried out in 250 mL round-bottom flasks at 25 °C equipped with magnetic stirring bars on Starfish equipment (Radleys Discovery Technologies, Saffron Walden, UK) installed on a Heidolph Heistandard magnetic stirrer for parallel reactions. A tube filled with granulated charcoal was fitted to the neck of the flasks. The appropriate quantity of sorbents and/or ILs was added to 100 mL of model (1 g L−1) or real (7 g L−1) wastewater containing RB8 dye. After a sufficient time of vigorous stirring (at 400 rpm), the suspensions were immediately filtered. Then, the concentration of RB8 in the aqueous filtrate was analyzed spectrophotometrically. The experimental scope of RB8 separation cycle layout using alternative sorbents with presence/absence of benzalkonium chloride is illustrated and presented in Scheme S1 and Figure S3 in the Supplementary Materials.
2.4. Determination of Partition Coefficient Octan-1-ol/Water
An aqueous solution containing 0.1 mmol of RB8 or 0.1 mmol of ion pair (R4N)4-RB8 was introduced into the round-bottom flask, the total volume of the aqueous phase was adjusted to 100 mL with demineralized water, and the mixture was filled with 100 mL of octan-1-ol. The prepared two-phase mixture was agitated at 400 rpm overnight, the immiscible phases were separated using a separatory funnel, and the concentration of dissolved dye was analyzed using VIS spectroscopy. The partition coefficient (Pow) was calculated according to Equation (2). Each experiment was performed three times; the presented values of log Pow are calculated as mean value. The error bars in the figures dealing with the octan-1-ol/water partition coefficients presented the standard deviation of the log Pow value.
2.5. Chemical Analysis
A Hach DR2800 VIS spectrophotometer was employed within the absorbance measurements using 1 cm glass cuvettes. The concentrations of dye in aqueous solutions were determined by measuring at a wavelength of 587 nm; see Figure S2 in the Supplementary Materials. The AOXs (adsorbable organically bound halogens) were analyzed using Multi X 2500 analyzer (Analytic Jena Co., Jena, Germany). COD analyses were carried out according to the ISO EN 9562 standard, and in aqueous solutions, it was determined using the Hach Lange cuvette test using the Hach DR2800 (Vienna, Austria) VIS spectrometer. Due to the high chloride concentrations, the COD Hach Lange test LCK1014 was used due to the ability to mask the chloride content up to 4 g Cl− L−1 [51]. The contents of BAC or A336 in the reaction mixtures were determined using cationic surfactants, the Hach Lange cuvette test and the Hach DR2800 (Austria) VIS spectrometer.
ED XRF measurements of RB8 and saturated biochar were performed using the ED XRF spectrometer ElvaX (Elvatech, Kyiv, Ukraine). X-ray tube with Pd anode was operated at a current of 50 μA and voltage of 10 kV for the light element spectral region (Na–Ti) and 45 kV for the heavy element spectral region (V–U). Helium micro-flushing of the sample chamber was used for measurements in light element spectral region to suppress the intensity of the Ar signal and increase the sensitivity for measured elements. The acquisition time of 90 s was used for both spectral regions. Samples of BC were simply poured into a sample cup covered with Mylar foil without any kind of other sample preparation step.
The decolorization efficiencies were calculated according to Equation (3):
where DE is the decolorization efficiency of the aqueous dye solution (%), A is the absorbance measured after the removal process, and A0 is the initial absorbance of the dye solution. All the error bars in the figures are calculated as the relative standard deviation (RSD) of the decolorization efficiency. In all cases, the RSDs were less than 6%.
The removal efficiency (%) of AOXs or COD from the wastewater was evaluated with respect to Equation (4):
where RE is the removal efficiency of AOXs or COD (%), c is the concentration of AOXs or COD in solution after the removal process (mg L−1), and c0 is the initial concentration of AOXs or COD in the dye solution before removal process (mg L−1).
3. Results and Discussion
3.1. Removal of RB8 from Model Wastewater by Sorption
In preliminary experiments, we optimized the sorption of RB8 dye on BC and BT alternative sorbents. The aqueous solution of RB8 with an initial concentration of c0 = 1 g L−1 simulated the real industrial wastewater from dye manufacturing [3,5,6]. As Figure 3A illustrates, BC doses higher than 40 g L−1 provided decolorization efficiency DE > 70% and COD removal above 60%. Lower doses of BC led to unsatisfactory removal of RB8 from aqueous solutions (“blank” means DE caused by filtration using filter paper). The fact that lower doses of BC than 40 g L−1 are not able to satisfactorily remove tested dye RB8 from model aqueous solutions can be caused, for example, by the specific surface area of applied BC (see Table 1) or lower affinity of RB8 dye to the surface of BC. On the other hand, BC is a better sorbent for the removal of RB8 than BT. As can be seen in Figure 3B, 25 g L−1 BT shows no effect on COD value and causes only 10% DE. Increasing the amount of used BT (40 g L−1) enabled only 19% decolorization and an 11% decrease in COD from the aqueous solutions of RB8. It could be explained by the lower specific surface area of BT [27,29,36] compared with BC and no effect of ion exchange (BT serves as cation exchanger).
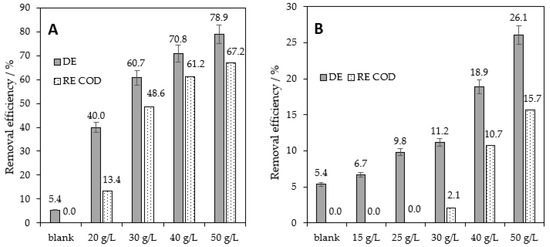
Figure 3.
Removal of RB8 (1 g L−1 model aq. solution) after 5 h of (A) BC and (B) BT action.
Afterward, the virgin and commercially available adsorbents—PAC and MTM—were compared with the above tested alternatives BC and BT, respectively. For a relevant comparison, the uniform dosages of all tested sorbents were set at 40 g L−1. Figure 4 shows the rates of RB8 dye sorption on PAC, BC, MTM and BT. The decolorization efficiencies of RB8 on tested sorbents increased in the following order: BT (19%) < MTM (48%) < BC (71%) < PAC (98%). A comparison of the decolorization and COD removal efficiencies of each sorbent is presented in Figure S4 in the Supplementary Materials. The sufficient removal of COD from the RB8 dye solutions provided only PAC (87%) and BC (61%).
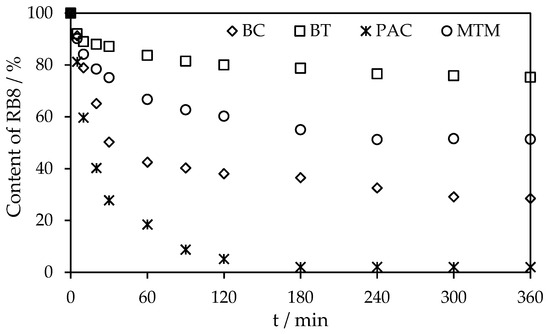
Figure 4.
Comparison of the sorption rate of RB8 (1 g L−1 model aq. solution) on PAC (40 g L−1) and MTM (40 g L−1) and alternative sorbents BC and BT (40 g L−1).
As can be seen, BC enables even better removal efficiency of RB8 than commercial MTM. Again, it can be caused by the much higher specific surface area of BC compared with clay-based sorbents BT or MTM [27,29,32,36,46]. Despite the efficient adsorption of RB8 on PAC, an alternative and cheaper sorbent BC is a good compromise for expensive PAC [21].
For the results of EDXRF analysis of an alternative BC sorbent saturated with RB8 dye, see Figure S5 in the Supplementary Materials Section. It is obvious that the elevated concentration of chromium determined in saturated BC corresponds to the adsorbed chromium complex RB8.
3.2. Separation of RB8 Based on Ion Exchange Using ILs
Our previous works [32,44,45] reported that the addition of appropriate amounts of quaternary ammonium compounds (R4NX, ILs) to the aqueous solutions of acidic halogenated compounds (substituted with -COO− or -SO3− groups), including dyes or drugs, results in ion exchange and the formation of respective insoluble ion pairs (see Equation (1) above and Scheme 1 below) that can be separated from aqueous solutions by simple filtration. The formation of the mentioned ion pairs has been earlier proven by NMR analysis; see refs. [32,44,45].
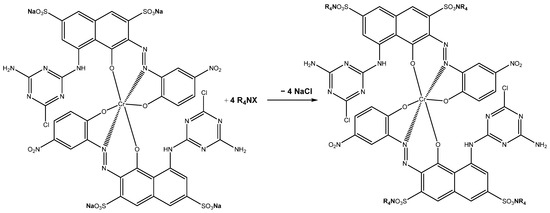
Scheme 1.
Formation of insoluble ion pairs (R4N)4RB8.
Therefore, the subsequent experiments were focused on the separation of RB8 dye using two ILs—BAC [52] and A336 [53] (for chemical drawings, see Figure 2 above). We established that 2 g L−1 of BAC (50% aq. solution) enables the decolorization of the RB8 solutions up to 99% and the elimination of COD up to 88%; see Figure 5A. Furthermore, we also observed rapid precipitation of the tested dye using BAC within 30 min of action; see Figure 5A. On the other hand, A336 provided a comparable efficiency (DE = 99%) of the RB8 dye after 30 min using 6 g L−1. Furthermore, the COD removal efficiency reached only 39% using the mentioned dosage of A336; see Figure 5B. The hydrophobic A336 is extremely viscous, and thus its precise application is troublesome [53]. The low solubility in water, along with high viscosity, can cause problems with the application of A336 in practice.
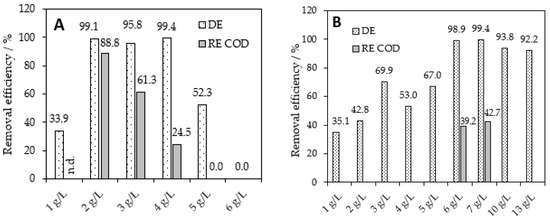
Figure 5.
Separation of RB8 (1 g L−1 model aq. solution) using (A) 50% BAC and (B) A336 within 30 min of action.
The better separation of RB8 using BAC can be caused by the better solubility of BAC in water and thus a better contact of BAC and RB8 dissolved in water. Based on these findings, BAC combines both sufficient aqueous solubility and the removal efficiency of acid halogenated pollutants such as RB8 dye; for more information, see our previous studies [32,44,45].
The better solubility of BAC in water than A336 can be supported with the octan-1-ol/water partition coefficient Pow determined for the ion pair of RB8 with BAC with A336; see Figure S6 in the Supplementary Materials. The octan-1-ol/water partition coefficient for (BAC)4RB8 is lower than the octan-1-ol/water partition coefficient for (A336)4RB8; see Figure S6 in the Supplementary Materials.
The formation of ion pairs can also be supported by the content of BAC or A336 after RB8 dye separation using these ILs. According to the cationic surfactant tests, the content of BAC or A336 after the formation of respective ion pairs within the separation of the RB8 decreased almost by 80% or by 69%, respectively. It is in good agreement with the separation efficiency of the RB8 dye using BAC and A336 (see Figure 5) and the octan-1-ol/water partition coefficient for the relevant ion pairs (see Figure S6 in the Supplementary Materials).
In addition, the mentioned cationic surfactant tests proved that the content of R4NX after the separation of RB8 dye using BAC (in optimized dosages) does not reach the limits of surfactant contents in sewage waters nor the levels of no-observed-adverse-effect concentration (NOAEC) for aquatic organisms [54], and it is under concentrations that can cause acute or chronic toxicity problems for aquatic organisms [55]. The removal of the cationic surfactant also corresponds with the great removal of COD (see Figure 5) after the mentioned treatment (in the second case, the remaining BAC in treated water would increase the content of COD). Therefore, most of the BAC applied for the separation of dyes does not remain in the aqueous solution, and these treated aqueous streams can be discharged into the biological WWTP.
3.3. Co-Action of ILs and Alternative Sorbents for RB8 Separation
Subsequently, our next research was focused on the application of BAC or A336 to enhance BC or BT sorption capacity. Several authors tested the impregnation of BC [33] or BT [27,29,36] using quaternary ammonium compounds such as cetyltrimethylammonium bromide/chloride. These conventional impregnation methods are usually based on the intensive stirring of the adsorbent with impregnation agents such as quaternary ammonium compounds. After the mentioned impregnation, the modified sorbents are applied for the separation of dyes [27,29,33,36]. The authors [27,33,36] reported an improvement in the sorption capacity of these sorbents via impregnation prior to adsorption.
However, we have earlier [32,44] discovered the simple and technologically undemanding method of the co-action of carbonaceous sorbents with ILs for the effective separation of halogenated organic acids from aqueous solutions. The RB8 separation cycle layout using the co-action of alternative sorbents with benzalkonium chloride is illustrated and presented in Scheme S1 and Figure S3 in the Supplementary Materials. The comparison and description of impregnation methods and the co-action of sorbents with ILs are also detailed in our previous works; see refs. [32,44].
Based on these previous observations, we optimized the co-action of BC as well as BT using A336 or BAC. Figure 6 compares the sorption of RB8 on BT or BC, the separation of this dye using A336 alone and the co-action of A336 with BT or BC. The addition of A336 (3 g L−1) to 15 g L−1 of BC or BT does not lead to an increase in the decolorization efficiency compared to the action of A336 alone (3 g L−1). Moreover, not even two times higher doses of A336 (6 g L−1) and the respective sorbent (30 g L−1) per the same amount of RB8 in an aqueous solution did not result in a significant increase in the dye removal efficiency; see Figure 6.
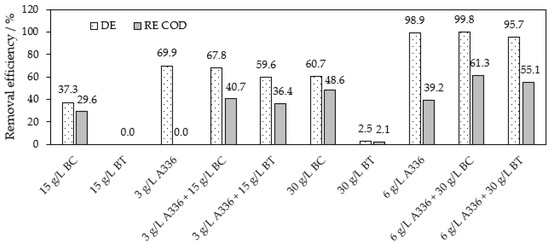
Figure 6.
Optimization of the co-action of A336 and BC or BT within the separation of RB8 (1 g L−1 model aq. solution). Action of sorbents alone within 5 h or within 30 min in co-action with ILs, respectively.
However, the co-action of BAC with the examined alternative sorbents proved very satisfactory results; see Figure 7. For example, the co-action of 1.5 g L−1 of BAC with 2 g L−1 of BC provides 99% decolorization of the RB8 dye solution, an almost 95% COD decrease and 98% removal of AOXs (for AOX results, see Figure S7 in the Supplementary Materials). The action of 1.5 g L−1 of BAC or 2 g L−1 of BC alone does not reach these high removal efficiencies of RB8; see Figure 7. Even 50 g L−1 of sole BC does not allow the described separation capacity compared with the co-action of BC (2 g L−1) and BAC (1.5 g L−1).
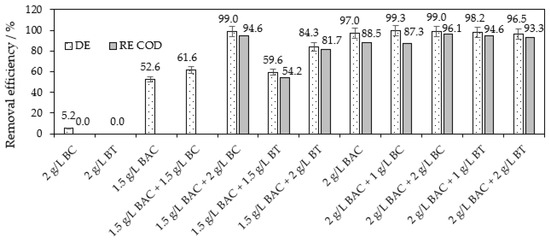
Figure 7.
Optimization of the co-action of BAC (50%) and BC or BT within the separation of RB8 (1 g L−1 model aq. solution). Action of sorbents alone within 5 h or within 30 min in co-action with ILs, respectively.
For similar efficiencies of decolorization and COD decrease, BAC can also be co-acted with BT. The application of BT (1 g L−1) in co-action with BAC (2 g L−1) required only the slightly higher doses of this IL than the application of BC with BAC (1.5 g L−1); for a comparison of the co-action of BAC with BC or BT, see Figure 7.
On the basis of the obtained results, the most promising method of RB8 dye removal is the direct co-action of BAC with the tested alternative sorbent (BC or BT) in verified optimized dosages. However, BT is an easily available material on many markets, and this alternative sorbent is also cheaper than BC [21,56].
Furthermore, Figure 8 presents the comparison of the sorption rates of the RB8 dye sorption on PAC, MTM, BC and BT alone or BC/BT in co-action with BAC. As can be seen, only the co-action of BC or BT with BAC and PAC alone provided an almost 100% removal efficiency of RB8 from the aqueous solution (1 g L−1). The removal efficiencies of RB8 dye increase in the following order: BT < BC < MTM < PAC ≤ BT + BAC ≤ BC + BAC. As we mentioned above (Section 3.1), BT and BC reached the lowest removal efficiencies due to the low specific surface area (see Table 1 above). The satisfactory removal of RB8 from aqueous solutions was achieved using commercial PAC with a high specific surface area. The highest separation capacity was provided by the co-action of alternative sorbents and BAC. It can be caused by the parallel effect of several separation mechanisms detailed in the section below.
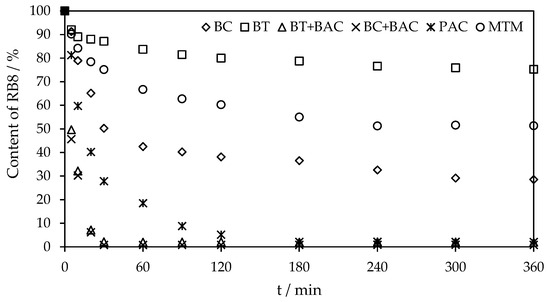
Figure 8.
A rate of sorption of RB8 (1 g L−1 model aq. solution) on different types of sorbents (40 g L−1) and co-action of BC (2 g L−1) with BAC (1.5 g L−1) or BT (2 g L−1) with BAC (2 g L−1).
In addition, the equilibrium of RB8 sorption on BC or BT sorbents in co-action with BAC is established after 30 min. In comparison, the state of equilibrium of RB8 adsorption on other sorbents alone (PAC, MTM, BC and BT) is attained from 3 to 5 h. The co-action of BC or BT with BAC is not only efficient and consumes significantly lower amounts of reagents, but it is also very rapid. Moreover, it provides an improved technological implementation (agitation of reaction mixture, filtration after the separation process, etc.) compared to the action of sole ILs.
The time dependencies of the sorption capacity of RB8 are shown in Figure S8 in the Supplementary Materials. Clearly, the equilibrium sorption capacities (qe in mg g−1; derived from the experimental data) decrease in the following order: BC + BAC ≥ BT + BAC ≥ PAC > BC > MTM > BT. It corresponds well with the above-mentioned removal efficiency sequence.
For example, virgin PAC provides the equilibrium sorption capacity of around 24.5 mg g−1 for the RB8 dye. On the other hand, BC enables this parameter to be only approximately 17.8 mg g−1. However, the qe for the BC in co-action with BAC reaches a value over 495.8 mg g−1. Analogically, the qe for the BT in co-action with BAC achieved a comparable value (489.8 mg g−1), such as the BC + BAC system. It can be noted that MTM alone provided the equilibrium adsorption capacities of only ca. 12 mg g−1. The great sorption capacities of alternative sorbents co-acted with BAC are caused by the high separation efficiency of RB8 dye using only 2 g L−1 of BC or 1 g L−1 of BT in co-action with BAC (1.5–2 g L−1).
The high sorption capacities of the tested alternative sorbents in the co-action of BAC can be explained by the parallel effect of the proposed mechanisms (a–c):
- (a)
- A sorption-based mechanism: The sorption of dyes on different types of biochar or bentonite is a well-known separation process [22,57]. However, (ad)sorption is also a multistage process, and sorption models are not able to describe these complex mechanisms and distinguish between physical and chemical sorption [58].
- (b)
- The rapid formation of less polar ion pairs: The formation of water-insoluble ion pairs was discussed in Section 3.2. Separation of RB8 using ILs above and was also proved in our previous studies [44,45] using analytical techniques such as NMR.
- (c)
- (i) In the case of BC application, the better affinity of formed ion pairs to the BC surface: As Gray et al. [59] suggested, the biochar-based sorbents show rather the non-polar nature of its surface. Therefore, the less soluble ion pairs can be well adsorbed on the described BC surface. We also suggested this mechanism in our earlier publications [32,44]. Furthermore, BAC alone can be well adsorbed on BC. We observed 81.3% removal of cationic surfactants from an aqueous solution of BAC within this IL sorption on BC. It is also in good agreement with several researchers [60].(ii) In the case of BT application, the entrapment of formed ion pairs using BT: It has been previously reported [38] that BT has a good entrapment capability for cationic surfactants using a cationic exchanging process. Furthermore, we verified that BT provided relatively good separation of BAC (almost 79% removal of cationic surfactants from an aqueous solution of BAC within this IL sorption on BT). These facts explain the comparable removal efficiency of the co-action of BAC with BT and BC. We suppose that the formed ion pairs can be entrapped using BT, e.g., via cationic exchanges or different forces. Optionally, BT improves the separation of formed ion pairs and plays a role as the supporting material in the removal of ion pairs from aqueous solutions.
Based on the observed rapid ion exchange (see Figure 5 above and also refs. [32,44]) and the non-polar character of formed ion pairs (see Figure S6 in Supplementary Materials and also refs. [32,44]), we suppose that the main separation mechanism is the formation of ion pairs and the sorption/entrapment of these ion pairs using alternative sorbents. However, it is possible that the conventional sorption and/or separation of dye using BAC alone participates simultaneously in the separation of RB8 too. The separation mechanisms are depicted in Scheme S2 in the Supplementary Materials.
The adsorption mechanisms can be also potentially established using several analytical methods, i.e., FTIR or other analytical methods. Therefore, BC samples before and after the adsorption of RB8 were characterized using ED XRF. XRF measurements showed a significantly higher content of chromium in BC samples saturated with RB8 dye. For the results of ED XRF analysis of an alternative BC sorbent saturated with RB8 dye or (BAC)4RB8, see Figures S5 and S9 in the Supplementary Materials.
However, an evident relationship between the sorption/separation mechanisms and the effect of sorbents and the BAC action can be hardly postulated from the available data. Despite this, the further explanation and investigation of detailed separation processes may be the subject of our future work.
3.4. Separation of RB8 from Real Wastewater
Finally, we verified the separation of the RB8 dye from real wastewater (aqueous filtrate obtained from industrial RB8 separation). The initial concentration of RB8 in this wastewater is 7 g L−1; for further specifications, see Section 2.1. Chemicals.
Based on the optimization experiments within the separation of RB8 dye from model wastewater, we tested the most promising methods—the co-action of BC or BT with BAC for real wastewater purification. As Figure 9 depicts, the BC alone in an appropriate dosage of 280 g per one liter of real wastewater proved approx. 20% lower decolorization and 15% lower COD removal than experiments with the separation of the tested dye from model wastewater. The lower efficiencies of RB8 removal may be caused by the complex matrix of real wastewater which contains not only RB8 but even different by-products or unreacted reactants. The increasing of BC dosage above 280 g L−1 would not be economical. On the other hand, the same dose of PAC (280 g L−1) is able to reach 92% decolorization and 74% removal of COD; see Figure 9.
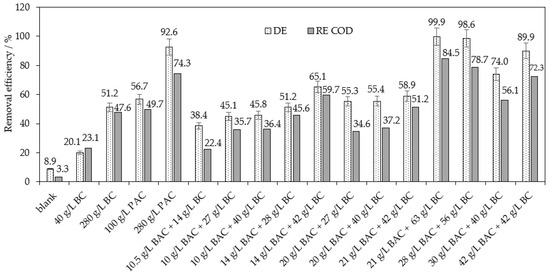
Figure 9.
Optimization of co-action of BAC (50%) and BC within the separation of RB8 from real wastewater (containing 7 g L−1 of RB8 dye among others) after 5 h of action. (Removal efficiencies of sole PAC are added for comparison of removal robustness of BAC/BC system).
Afterward, the method of the co-action of BC with BAC was investigated for the purpose of enhancing the separation ability of RB8 dye from real wastewater. For model wastewaters containing 1 g L−1 of RB8 dye, the optimal dosage was established at 1.5 g L−1 of BAC and 2 g L−1 of BC. Therefore, we tested seven times higher doses of these reagents for real wastewater containing 7 g L−1 of RB8 dye (seven times higher RB8 content than in model wastewater). However, these previously optimized doses of BC and BAC achieved only 40% decolorization and 22% removal of COD from real wastewater; see Figure 9.
Therefore, increasing BAC and BC doses was examined to enhance the removal of the RB8 dye. BAC (28 g L−1) in co-action with 56 g L−1 of BC is able to achieve almost 99% decolorization and 78% COD removal. In other words, for a comparable removal effectiveness of the RB8 dye from real wastewater, the addition of a 2.7 times higher dose of BAC and 4 times higher dose of BC compared with model wastewater is required; see Figure 9.
Analogously, we scrutinized the optimized method of direct combination of BAC with the second alternative sorbent BT for the separation of RB8 from real wastewater; see Figure 10. In contrast to BC (see Figure 9), the previously optimized dosages of 14 g L−1 of BAC and 28 g L−1 of BT applied for the treatment of real wastewater containing 7 g L−1 of RB8 dye achieved satisfactory removal efficiency (90.6% decolorization and 84.5% COD removal). The optimal dose of BT + BAC was verified as 20 g L−1 of BAC and 28 g L−1 of BT which offer 96.1% decolorization and 90.7% removal of COD; see Figure 10. Therefore, it is required to use only a 1.4 times higher dose of BAC and a 4 times higher dose of BT than in the case of model wastewater.
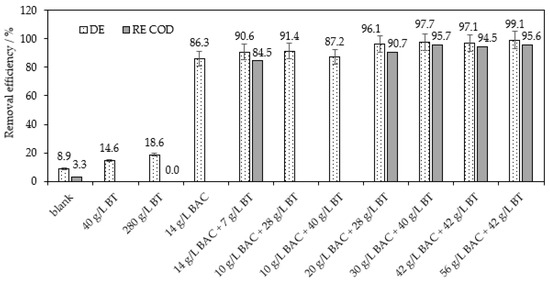
Figure 10.
Optimization of co-action of BAC (50%) and BT within the separation of RB8 from real wastewater (containing 7 g L−1 of RB8 dye among others) after 5 h of action.
Figure 11 illustrates the rates of RB8 sorption on PAC alone and alternative sorbents in co-action with BAC from real wastewater using the above-optimized doses of sorbents. The equilibrium of RB8 sorption on virgin PAC was established after 240 min. On the other hand, upon the action of BC or BT with BAC, the equilibrium state was gained after 90 min or 60 min, respectively.
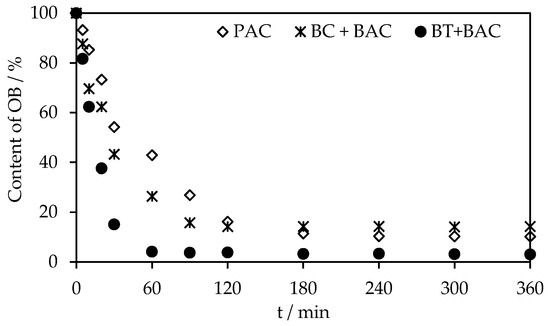
Figure 11.
Comparison of sorption rate of RB8 (7 g L−1) from real wastewater on PAC (280 g L−1) and BC (42 g L−1) in co-action with BAC (42 g L−1) or BT (28 g L−1) in co-action with BAC (20 g L−1).
From the obtained results, it is evident that the most promising method of direct combination of BAC with BT for RB8 dye from real wastewater is rapid and as effective as the more expensive PAC treatment [21].
The economic costs of the respective methods are presented in Table S1 in the Supplementary Materials. Although RB8 separation using BAC alone is the economically acceptable method, the co-action of BAC with BT applied for RB8 removal from model and real wastewater offers the most effective decolorization and COD removal; see Table S1 in the Supplementary Materials. Moreover, the direct combination of BT and BAC enables the cheapest separation of RB8 dye or ion pairs, respectively.
The new approach of the circular economy and the requirements of the price decrease of treatment processes aim at the facile and effective recycling of materials applied, for example, in treating wastewater. Therefore, our previous article [45] proposed new innovative methods of recycling ion pairs formed in dye separation processes. We also designed a process for prolonging the life cycle of spent carbonaceous sorbents using BAC [44]. Based on these findings, it can be assumed that the exhausted sorbents applied in co-action with BAC have great potential for regeneration and recycling too. However, the direct and simultaneous recycling of both materials—BAC and respective alternative sorbents—is a challenge for our future research.
Finally, for a comparison of the above-described results of the separation of RB8 dye from wastewater (calculated per one gram of removed dye for the demonstration of practical application) with the available literature [9,15,16,17,18,19], see Table 2. Only photocatalytic oxidation using a high dosage of a photocatalyst [16] or adsorption on expensive ZnO nanoparticles used in huge excess [19] reaches the removal efficiencies of the RB8 dye comparable with the method developed in this article. The combination of an alternative cheap sorbent such as BT with an available ionic liquid BAC offers effective, rapid and smooth removal of the RB8 dye from the model as well as from real wastewater.

Table 2.
Comparison of treatment methods applied for wastewater polluted with RB8 dye.
4. Conclusions
This study aimed to optimize the sorption procedure by applying alternative sorbents—biochar and bentonite—in the co-action of ionic liquids such as benzalkonium chloride or Aliquat 336 for the removal of the chromium complex of reactive azo dye Reactive Black 8 from wastewater.
The preliminary tests proved that alternative sorbents biochar (BC) and bentonite (BT) do not achieve the sorption capacities of virgin powdered active carbon (PAC) or montmorillonite (MTM), respectively. On the other hand, we proved that the co-action of BC or BT with ionic liquid BAC is the most promising method of RB8 separation from model wastewater. The amount of 2 g L−1 of BAC in co-action with 1 g L−1 of BT resulted in higher than 98% RB8 removal efficiency after 30 min of action. The comparable removal efficiencies were obtained using as high as 40 g L−1 of virgin PAC after at least 180 min of action.
The equilibrium sorption capacity of the BT in co-action with BAC reaches a value greater than 489 mg g−1. This high sorption capacity can be explained by the parallel effect of several proposed mechanisms: (i) sorption, (ii) the formation of less polar ion pairs and (iii) the separation of formed ion pairs using a BT sorbent.
However, the separation of RB8 (7 g L−1) from real wastewater established slightly worse results due to the complex matrix of the wastewater sample. For comparable removal efficiency of RB8 dye from real wastewater, it is required to apply an only 1.4 times higher dose of BAC and 4 times higher dose of BT than the optimized dosages applied within the RB8 removal from model wastewaters.
The application of alternative sorbents BC or BT decreases the costs of the developed separation process for RB8 dye removal from wastewater. Moreover, the addition of BAC to the used alternative sorbent BC or BT decreases the dosage of BC or BT and provides the required removal efficiencies of both the RB8 dye and/or residual BAC from wastewaters. Additionally, sorption with the proposed BC + BAC or BT + BAC systems proceeds smoothly from 30 to 60 min, in contrast with adsorption using conventional PAC (3–4 h is necessary for effective removal of RB8 dye using PAC). The application of BAC + B also saves energy and further reduces economic costs joined with waste treatment (compared with conventional sorption using active carbon). The optimized method of direct combination of BAC with BT for RB8 dye from wastewater is rapid, economically acceptable and almost as effective as that based on expensive PAC application.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app14020673/s1, File S1: Figure S1. Report from EDXRF analysis of commercial RB8 sample; Scheme S1. The experimental scope of RB8 separation using alternative sorbents with presence/absence of benzalkonium chloride; Figure S2. Absorption spectrum of RB8 in model and real wastewater (A) and calibration curve of model aq. solutions of RB8 (B); Figure S3. The experimental layout of the RB8 separation using alternative sorbents with presence/absence of benzalkonium chloride; Figure S4. The comparison of decolorization efficiency and COD removal of RB8 dye using PAC and MTM (40 g L−1) and alternative sorbents (40 g L−1) after 5 h of action; Figure S5. Report from EDXRF analysis of alternative BC sorbent saturated with RB8 dye; Figure S6. The comparison of octan-1-ol/water partition coefficients (log Pow) for RB8 and RB8-based ion pairs (R4N)4–RB8; Figure S7. The removal of AOX from model aq. solutions of RB8 (1 g L−1 aq. model solution) using optimal doses of BAC and BC or BT after 30 min of action; Figure S8. The dependencies of adsorption capacity on time within adsorption of RB8 (1 g L−1 aq. model solution) on (A) different sorbents (40 g L−1) or (B) co-action of BC (2 g L−1) with BAC (1.5 g L−1) or BT (2 g L−1) with BAC (2 g L−1); Figure S9. Report from EDXRF analysis of alternative BC sorbent saturated with (BAC)4RB8 ion pair; Table S1. A comparison of economic costs of tested separation methods of RB8 dye from model (1 g L−1) or real (7 g L−1) wastewater; Scheme S2. The proposed mechanisms of separation of RB8 dye from wastewaters. File S2: Measured data from chemical analysis and VIS spectroscopy.
Author Contributions
Conceptualization, B.K. and T.W.; methodology, T.W.; validation, B.K. and M.P.; formal analysis, B.K.; investigation, B.K., T.W. and K.M.; resources, B.K. and K.M.; data curation, B.K., M.P. and K.M.; writing—original draft preparation, B.K.; writing—review and editing, T.W.; visualization, B.K.; supervision, T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Cho, J.; Cho, J.K.; Lee, J.; Lee, D.; Park, C.; Kim, S. Optimization of salting-out crystallization for an efficient in situ separation of synthetic anthraquinone-and azo-type reactive dyes. Sep. Purif. Technol. 2009, 68, 138–144. [Google Scholar] [CrossRef]
- Sarasa, J.; Roche, M.P.; Ormad, M.P.; Gimeno, E.; Puig, A.; Ovelleiro, J.L. Treatment of a wastewater resulting from dyes manufacturing with ozone and chemical coagulation. Water Res. 1998, 32, 2721–2727. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ma, L.M.; Li, T.; Zhang, Y.L.; Gao, H.W. Preparation and characterization of silver thiocyanate–tetrabromo-tetrachlorofluorescein inclusion material and its adsorption to synthetic dye. Colloids Surf. A Physicochem. Eng. 2009, 333, 126–132. [Google Scholar] [CrossRef]
- Šimek, M.; Mikulášek, P.; Kalenda, P.; Weidlich, T. Possibilities for removal of chlorinated dye Mordant Blue 9 from model waste. Chem. Pap. 2016, 70, 470–476. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential technologies for elimination from (waste) water. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Jha, P.; Modi, N.; Jobby, R.; Desai, N. Differential Expression of Antioxidant Enzymes During Degradation of Azo Dye Reactive black 8 in Hairy roots of Physalis minima L. Int. J. Phytoremediation 2015, 17, 305–312. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Kuchtová, G.; Hojová, L.; Staňová, A.V.; Marton, M.; Vrška, M.; Behúl, M.; Dušek, L. The influence of micro-/macro-structure of a boron-doped diamond electrode on the degradation of azo dye Direct Red 80. Electrochim. Acta 2023, 464, 142924. [Google Scholar] [CrossRef]
- Weidlich, T.; Opršal, J.; Krejčová, A.; Jašúrek, B. Effect of glucose on lowering Al–Ni alloy consumption in dehalogenation of halogenoanilines. Monatsh. Chem. 2015, 146, 613–620. [Google Scholar] [CrossRef]
- Bendová, H.; Kamenická, B.; Weidlich, T.; Beneš, L.; Vlček, M.; Lacina, P.; Švec, P. Application of Raney Al-Ni alloy for simple hydrodehalogenation of Diclofenac and other halogenated biocidal contaminants in alkaline aqueous solution under ambient conditions. Materials 2022, 15, 3939. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, T.; Kamenicka, B. Recycling of Spent Hydrodehalogenation Catalysts–Problems Dealing with Separation of Aluminium. Inz. Miner. 2019, 21, 177–182. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Zhang, D. Decolorisation and mineralisation of CI Reactive Black 8 by the Fenton and ultrasound/Fenton methods. Color. Technol. 2007, 123, 101–105. [Google Scholar] [CrossRef]
- Edrissi, M.; Samadanian-Isfahani, S.; Soleymani, M. Preparation of cobalt molybdate nanoparticles; Taguchi optimization and photocatalytic oxidation of Reactive Black 8 dye. Powder Technol. 2013, 249, 378–385. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Zhang, H.; Zhang, J.; Li, L. Decolorization of CI Reactive Black 8 by electrochemical process with/without ultrasonic irradiation. Desalin. Water Treat. 2012, 44, 36–43. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Wang, Z.; Wu, F. Decolorisation of CI Reactive Black 8 by zero-valent iron powder with/without ultrasonic irradiation. Color. Technol. 2007, 123, 203–208. [Google Scholar] [CrossRef]
- Khoshhesab, M.Z.; Gonbadi, K.; Rezaei Behbehani, G. Removal of reactive black 8 dye from aqueous solutions using zinc oxide nanoparticles: Investigation of adsorption parameters. Desalin. Water Treat. 2015, 56, 1558–1565. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Soares, S.F.; Órfão, J.J.; Figueiredo, J.L. Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon 2003, 41, 811–821. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.; Summers, R.S.; Cook, S.M. Environmental comparison of biochar and activated carbon for tertiary wastewater treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef] [PubMed]
- Praveen, S.; Jegan, J.; Bhagavathi Pushpa, T.; Gokulan, R.; Bulgariu, L. Biochar for removal of dyes in contaminated water: An overview. Biochar 2022, 4, 10. [Google Scholar] [CrossRef]
- Brynda, J.; Skoblia, S.; Pohořelý, M.; Beňo, Z.; Soukup, K.; Jeremiáš, M.; Svoboda, K. Wood chips gasification in a fixed-bed multi-stage gasifier for decentralized high-efficiency CHP and biochar production: Long-term commercial operation. Fuel 2020, 281, 118637. [Google Scholar] [CrossRef]
- Seyedsadr, S.; Šípek, V.; Jačka, L.; Sněhota, M.; Beesley, L.; Pohořelý, M.; Trakal, L. Biochar considerably increases the easily available water and nutrient content in low-organic soils amended with compost and manure. Chemosphere 2022, 293, 133586. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Chia, C.H.; Downie, A.; Munroe, P. Characteristics of Biochar: Physical and Structural Properties. In Biochar for Environmental Management; Routledge: London, UK, 2015; Volume 89. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Prot. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Murray, H.H. Bentonite applications. Dev. Clay Sci. 2006, 2, 111–130. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Waanders, F.; Fourie, C.L. Adsorption of Congo Red by surfactant-impregnated bentonite clay. Desalin. Water Treat. 2016, 57, 27663–27671. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Bendou, S.; Amrani, M. Effect of hydrochloric acid on the structural of sodic-bentonite clay. JMMCE 2014, 2, 10. [Google Scholar] [CrossRef]
- Kamenická, B.; Matějíček, P.; Weidlich, T.; Pohořelý, M. Application of Biochar for Treating the Water Contaminated with Polar Halogenated Organic Pollutants. In Applications of Biochar for Environmental Safety; InTech Open: Rijeka, Croatia, 2020; Volume 241. [Google Scholar] [CrossRef]
- Kosaiyakanon, C.; Kungsanant, S. Adsorption of reactive dyes from wastewater using cationic surfactant-modified coffee husk biochar. Environ. Nat. Resour. J. 2020, 18, 21–32. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Adsorption removal of Methylene Blue (MB) dye from aqueous solution by biochar prepared from Eucalyptus sheathiana bark: Kinetic, equilibrium, mechanism, thermodynamic and process design. Desalin. Water Treat 2016, 57, 28964–28980. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Kafle, S.R.; Kim, B.S. Surface modification of biochar for dye removal from wastewater. Catalysts 2022, 12, 817. [Google Scholar] [CrossRef]
- Çelik, A.; Yildiz, N.; Çalimli, A. Adsorption of some organic compounds by hexadecyltrimethylammonium-bentonite. Rev. Chem. Eng. 2000, 16, 301–312. [Google Scholar] [CrossRef]
- Özcan, A.; Ömeroğlu, Ç.; Erdoğan, Y.; Özcan, A.S. Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19. J. Hazard. Mater. 2007, 140, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ma, J. Simultaneous removal of acid dye and cationic surfactant from water by bentonite in one-step process. J. Chem. Eng. 2008, 139, 503–509. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Vedaraman, N.; Surianarayanan, M.; Macfarlane, D.R. Extraction and recovery of azo dyes into an ionic liquid. Talanta 2006, 69, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.C.; Wang, J.J.; Xuan, X.P.; Fan, J.; Fan, M. Factors affecting ionic liquids-based removal of anionic dyes from water. Environ. Sci. Technol. 2007, 41, 5090–5095. [Google Scholar] [CrossRef]
- Gharehbaghi, M.; Shemirani, F. A Novel Method for Dye Removal: Ionic Liquid-Based Dispersive Liquid–Liquid Extraction (IL-DLLE). Clean 2012, 40, 290–297. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Asumana, C.; Yu, G. Extraction of soluble dyes from aqueous solutions with quaternary ammonium-based ionic liquids. Sep. Purif. Technol. 2013, 106, 105–109. [Google Scholar] [CrossRef]
- Dalari, B.L.S.K.; Giroletti, C.L.; Malaret, F.J.; Skoronski, E.; Hallett, J.P.; Matias, W.G.; Nagel-Hassemer, M.E. Application of a phosphonium-based ionic liquid for reactive textile dye removal: Extraction study and toxicological evaluation. J. Environ. Manag. 2002, 304, 114322. [Google Scholar] [CrossRef]
- Kamenická, B.; Weidlich, T.; Pouzar, M. Sorption of Halogenated Anti-Inflammatory Pharmaceuticals from Polluted Aqueous Streams on Activated Carbon: Lifetime Extension of Sorbent Caused by Benzalkonium Chloride Action. Water 2023, 15, 3178. [Google Scholar] [CrossRef]
- Kamenická, B.; Švec, P.; Weidlich, T. Separation of Anionic Chlorinated Dyes from Polluted Aqueous Streams Using Ionic Liquids and Their Subsequent Recycling. Int. J. Mol. Sci. 2023, 24, 12235. [Google Scholar] [CrossRef] [PubMed]
- Pohořelý, M.; Picek, I.; Skoblia, S. Apparatus for Multistage Gasification of Carbonaceous Fuels. Patent No. 306239/PV, 9 July 2015. [Google Scholar]
- Deischter, J.; Wolter, N.; Palkovits, R. Tailoring Activated carbons for efficient downstream processing: Selective liquid-phase adsorption of lysine. ChemSusChem 2020, 13, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Silcarbon Aktivkohle, SilCarbon CW20. Available online: http://www.silcarbon.eu/englisch/products/activated-carbon/powder-activated-carbon/index.html (accessed on 15 July 2023).
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Oliverio, M.; Procopio, A. Montmorillonite K10-catalyzed solvent-free conversion of furfural into cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef]
- Arij Global Trading, Bentonite Specifications. Available online: https://arijco.com/bentonite-specification/ (accessed on 16 November 2023).
- Hach Lange, COD Determination—LCK 1014 Method. Available online: https://aquaanalytics-tekhnika.ru/assets/products/178/hach-lck1014-khpk-metodika.pdf (accessed on 15 July 2023).
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6581159/ (accessed on 6 January 2024). [CrossRef]
- Mikkola, J.P.; Virtanen, P.; Sjöholm, R. Aliquat 336®—A versatile and affordable cation source for an entirely new family of hydrophobic ionic liquids. Green Chem. 2006, 8, 250–255. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arh. Hig. Rada. Toksikol. 2010, 61, 95–109. [Google Scholar] [CrossRef]
- Lavorgna, M.; Russo, C.; D’Abrosca, B.; Parrella, A.; Isidori, M. Toxicity and genotoxicity of the quaternary ammonium compound benzalkonium chloride (BAC) using Daphnia magna and Ceriodaphnia dubia as model systems. Environ. Pollut. 2016, 210, 34–39. [Google Scholar] [CrossRef]
- Texas Bentonite, Bentonite Prices. Available online: https://www.texasbentonite.com/how-much-does-sodium-bentonite-cost.html (accessed on 16 November 2023).
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, Y.; Gao, B.; Qiao, H.; Li, Y.; Wang, E.; Bao, C. Effective removal of ionic liquid using modified biochar and its biological effects. J. Taiwan Inst. Chem. 2016, 67, 318–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).