Abstract
Acute alcoholic liver injury is an important health problem worldwide. Apples are rich in many nutrients and have a variety of biological activities, including antioxidant, anti-inflammatory, and anti-tumor, and therefore have the potential to be a natural protective agent against acute alcoholic liver injury. This study evaluated the protective effect of apples (Malus pumila Mill) on acute alcoholic liver injury in rats. Male Wistar rats were randomly assigned to four groups: a control group (C), a control group that was fed fresh apples (CA), an ethanol-treated group (E), and an ethanol-treated group that was fed fresh apples (EA). Rats were treated with continuous forced gavage with 40° ethanol (4 mL/kg) for one week to simulate human alcoholism. Liver injury was assessed based on changes in the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), as well as histological analysis. The protective effect of apples on alcoholic liver injury was assessed in terms of alcohol metabolism, oxidative stress, inflammation, lipid synthesis, and tissue fibrosis. The results showed that apple consumption protected against alcoholic liver injury, as indicated by the decreased serum ALT and AST levels, reduced liver lipid peroxidation, and improved liver histopathology. Moreover, apple consumption increased antioxidant enzyme activity and reduced inflammatory cytokine levels in the liver. These findings suggest that apple consumption may have a protective effect against acute ethanol-induced liver injury in rats, possibly through its antioxidant and anti-inflammatory properties.
1. Introduction
The liver is a vital organ in the human body responsible for detoxification and metabolism. Alcohol abuse damages the liver, and the metabolic disorders caused by ethanol and its metabolites lead to alcoholic liver disease (ALD), including steatosis, hepatitis, fibrosis, and cirrhosis [1]. The pathogenesis of ALD is associated with oxidative stress (OS), lipid peroxidation, and inflammatory cytokine production caused by ethanol and its metabolites [2]. The liver metabolizes ethanol via three enzymatic pathways: alcohol dehydrogenase, cytochrome p450 (also known as the microsomal ethanol oxidizing system [MEOS]), and catalase (CAT) [3]. Alcohol abuse activates the cytochrome P450 family 2 (CYP2E1) in the MEOS, leading to the production and accumulation of reactive oxygen species (ROS) in the hepatocytes [4]. Excess ROS causes OS and lipid peroxidation, which induces hepatocyte steatosis and disrupts membrane permeability [5]. Exposure of hepatocytes to OS and lipid peroxidation causes hepatic inflammation, fatty liver, and fibrosis [6,7,8].
There is growing interest in identifying natural compounds that can protect the liver from ethanol-induced damage. Apples are readily available and contain various beneficial compounds, including sugars, organic acids, and polyphenols [9]. Apples improve overall health, and their wide range of pharmacological properties is indicated by the ancient proverb, “An apple a day keeps the doctor away” [10]. Apples have antioxidant and antimicrobial properties and regulate cholesterol metabolism [11,12]. Given the safety and accessibility of natural food products, the development of dietary therapies has attracted widespread interest in recent years, and many natural foods are alternatives to pharmacological therapies [13,14,15]. Components such as quercetin and phloretin in apples have hepatoprotective effects that can counteract the damaging effects of alcohol on the liver. Previous studies have shown that quercetin has potent antioxidant and anti-inflammatory properties, which may help protect the liver against OS and inflammation induced by alcohol consumption [16,17,18]. However, the effects of apple consumption on ALD are unknown.
The significance of this study lies in the potential for apple consumption to provide a simple and natural solution for protecting against liver injury caused by alcohol consumption. Apples have high antioxidant activity, which is a rich source of quercetin, and improve lipid metabolism [11,12], indicating the potential of apples in improving ALD. This study assessed the hepatoprotective effects of apples against acute alcohol-induced liver injury in rats by performing enzyme activity assays, a real-time polymerase chain reaction, and an enzyme-linked immunosorbent assay. While further research is needed to determine whether the protective effects seen in rats can be replicated in humans, given the significant health risks associated with alcohol consumption, finding simple and accessible ways to protect against its harmful effects is of great importance. If further research confirms these findings, the consumption of apples may provide a safe and natural way to protect against liver injury caused by alcohol consumption.
2. Materials and Methods
2.1. Animals and Experimental Design
Forty 6-week-old male Wistar rats (180–190 g) were purchased from SiPeiFu Biotechnology Co. The animals were housed in standard cages (CAT:YK-S1000, Youke Instrument Equipment Co., Ltd., Hefei, China) with a 12 h/12 h light/dark cycle and were acclimatized for 1 week.
The rats were randomly assigned to four groups (10 animals per group): a control group (C), a control group that was fed fresh apples (CA), an ethanol-treated group (E), and an ethanol-treated group that was fed fresh apples (EA). Rats in the four groups were given different treatments. Rats in groups CA and EA were force-fed food containing 10 g/kg of apples (Malus pumila Mill), while rats in groups C and E were given an equal mass of saline, simulating the daily intake of apples in humans. Rats in groups E and EA were orally given a single dose of 40° ethanol (4 mL/kg body weight), simulating alcoholism in humans, for one week. Rats in groups C and CA were given an equal mass of saline during this time. All groups were fed sterile standard chow (SiPeiFu Biotechnology Co., Ltd., Beijing, China) and sterile drinking water; and all rats were allowed ad libitum access to food and water. The study period was one week, and body weight was measured at the end of the experiment. The animals fasted for 24 h before being decapitated. The serum samples and liver tissues were collected for subsequent analysis.
Although the different treatments require the addition of apples and alcohol to the diet, which may affect changes in the proportion of macronutrients such as fat and protein in the energy supply, resulting in differences in caloric intake, such differences are unimportant compared to the total daily caloric intake. We are more concerned with the functional effects provided by apples and alcohol than the calorie differences caused by apple and alcohol consumption.
2.2. Measurement of Serum Markers
Plasma was centrifuged at 6000 rpm for 10 min, and the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using an automatic biochemical analyzer (Chemray 800; Rayto Life and Analytical Sciences Co., Ltd., Shenzhen, China).
2.3. Measurement of Enzyme Activity Indicators
Liver tissue was added to sterile saline (g:mL = 1:9) and ground on ice to make a 10% tissue homogenate. The supernatant was obtained by centrifugation at 5000 rpm for 10 min. The enzyme activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), the levels of malondialdehyde (MDA), alcohol dehydrogenase (ADH), acetaldehyde dehydrogenase (ALDH), and catalase (CAT) were measured in supernatants. Protein concentration was quantified using a protein concentration assay kit (CAT:No. A045-4-2; Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China). SOD activity was determined using a SOD assay kit (CAT:No. A001-1; Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China) in 10 μL of each sample. GPx activity was measured using the GPx assay kit (CAT:No. A005-1; Jiancheng Biological Engineering Research Institute, Nanjing, China) in 200 μL of each sample. CAT activity was determined using the CAT assay kit (CAT:No. A007-1; Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China) in 50 μL of each sample. MDA concentrations were measured using the MDA assay kit (CAT:No. A003-1-2; Nanjing Jiancheng Biological Engineering Research Institute, Nanjing, China). ADH and ALDH activity was measured separately for each sample using the ADH assay kit (CAT:No. BC1085; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) and the ALDH assay kit (CAT:No. BC0755; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China).
2.4. Histopathology
Tissue specimens were collected from the tip of a liver lobe and fixed in 4% paraformaldehyde for 24 h. Tissue samples were dehydrated in an ethanol series (75% for 4 h, 85% for 2 h, 90% for 2 h, 95% for 1 h, anhydrous ethanol for 1 h), benzyl alcohol for 10 min, and xylene for 20 min. The samples were embedded in paraffin and cut into 4 μm sections. Then, the tissues were stained with a hematoxylin-eosin (HE) solution using the HE staining kit (CAT:G1005, Servicebio Technology Co., Ltd., Wuhan, China) and observed by light microscopy.
2.5. Enzymelinked Immunosorbent Assay (ELISA) for Immune Factor Levels
Each liver sample was weighed proportionally and an appropriate amount of saline (g:mL = 1:1) was added to prepare a 10% homogenate under ice water bath conditions, centrifuged at 3000 rpm for 10 min, and the supernatant was taken for determination. The level of interleukin 1 beta (IL-1β) was measured using a IL-1β ELISA Kit (CAT:GER0002, Servicebio Technology Co., Ltd., Wuhan, China), the level of tumor necrosis factor alpha (TNF-α) was measured using a TNF-α ELISA Kit (CAT:GER0004, Servicebio Technology Co., Ltd., Wuhan, China), and the level of interleukin 10 (IL-10) was measured using a IL-10 ELISA Kit (CAT:GER0003, Servicebio Technology Co., Ltd., Wuhan, China).
2.6. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using the RNAprep Pure Tissue Kit (CAT:DP431, Tiangen Biotech Co., Ltd., Beijing, China). The RNA concentration and quality were assessed using a NanoDrop2000. RNA (1.0 μg) was reverse-transcribed into cDNA using the Evo M-MLV RT Mix Kit (CAT:No. AG11728; Accurate Biotechnology Co., Ltd., Hunan, China). A real-time quantitative polymerase chain reaction (RT-qPCR) was performed using the SYBR Green Premix Pro Taq HS kit (CAT:No. AG11701; Accurate Biotechnology Co., Ltd., Wuhan, China) in a StepOnePlus Real-Time Fluorescence Quantitative PCR System (Thermo Fisher Scientific Co., Ltd., Shanghai, China). The relative expression of each target gene was normalized to the housekeeping gene actin beta (Actb) using the 2−ΔΔCT method. The target genes measured include alcohol dehydrogenase 1 (Adh1), aldehyde dehydrogenase 1 family, member A1 (Aldh2), cytochrome P450, family 2, subfamily e, polypeptide 1 (Cyp2e1), catalase (Cat), nuclear factor, erythroid-derived 2-like 2 (Nrf2), heme oxygenase 1 (Ho-1), interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), interleukin 10 (IL-10), fatty acid binding protein 2 (Fabp2), acetyl-CoA carboxylase alpha (Acac), carnitine palmitoyltransferase 1A (Cpt1a), collagen type I alpha 1 chain (Col1a1), and alpha-2 smooth muscle actin (Acta2). The primers used in RT-qPCR are shown in the Supplemental Table S1.
2.7. Semi-Quantitative Real-Time Polymerase Chain Reaction (sPCR)
To verify the accuracy of the RT-qPCR results in the E and EA groups, sPCR was used to validate the target genes (Adh1, Aldh2, Cyp2e1, Cat, Nrf2, Ho-1, IL-1β, TNF-α, IL-10, Fabp2, Acac, Cpt1a, Col1a1 and Acta2). Complementary DNA (cDNA) was amplified using the 2× Rapid Taq Master Mix kit (CAT:No. P222-01; Vazyme Biotech Co., Ltd., Nanjing, China). The primer sequences and amplification conditions are shown in the Supplemental Table S2. The PCR products were separated in 2% agarose gels. The gels were viewed using an imaging system.
2.8. Statistical Analysis
All values are expressed as the standard error of the mean and three technical replicates were performed for each sample. GraphPad Prism 9.4.1 software (GraphPad Software Inc., Boston, MA, USA) was used to plot and statistically analyze the data, and Adobe Photoshop 2020 (Adobe Systems Incorporated, Mountain View, CA, USA). was used for image typesetting. Analyses of differences between groups were performed using ANOVA, and p-values < 0.05 were considered statistically significant.
3. Results
3.1. Apples Help Slow Weight Growth and Improve the Serum Index in Rats after Alcohol Consumption
The aim of this study was to investigate the protective effect of apple consumption on ALD, using multiple aspects such as serum markers, liver tissue sections, alcohol metabolism indicators, OS indicators, and inflammatory factor indicators to assess liver damage and effects (Figure 1A).
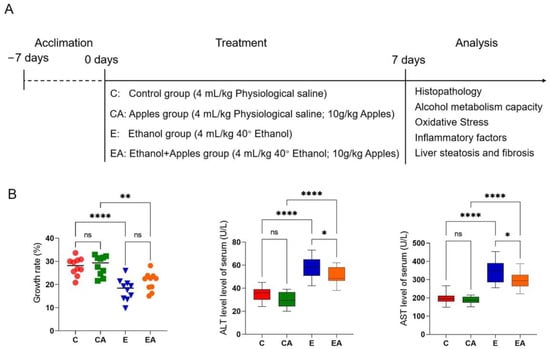
Figure 1.
Experimental design diagram and body weight gain rate and serum index of rats. (A) Experimental procedures. (B) Growth rate and serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). ns: p > 0.05, *: p < 0.05, **: p < 0.01, ****: p < 0.0001.
The results of the study showed that apple consumption did not have a significant effect on the body weight growth rate of rats after alcohol consumption, but had a positive effect on the serum index of the rats. Specifically, there was no significant difference in the body weight growth rate between rats consuming apples and those not consuming apples during the week-long drinking experiment. However, the serum ALT and AST levels are positively correlated with the degree of liver damage: the serum indices of ALT and AST were significantly improved in the group of rats that consumed apples, suggesting a potential protective effect of apple consumption against alcohol-induced liver damage. Overall, these results suggest that incorporating apples into the diet may be a beneficial strategy for mitigating the negative effects of alcohol consumption on the body (Figure 1B).
3.2. Apples Help Reduce the Effects of Ethanol on Liver Tissue
Studies have found that ethanol exposure leads to significant morphological changes in the liver, such as poorly defined hepatocyte boundaries, cytoplasmic vacuolization, and cellular degeneration. However, apple consumption can mitigate these effects to some extent and has a protective effect against alcoholic liver injury. Specifically, by observing the morphological changes of hepatocytes, we found that the degree of degeneration and disorganization of the cell arrangement were more severe and the intercellular spacing was larger in the E group compared with the EA group. Hepatocytes in the EA group were relatively more compact than those in the E group, which was also visually evident (Figure 2).
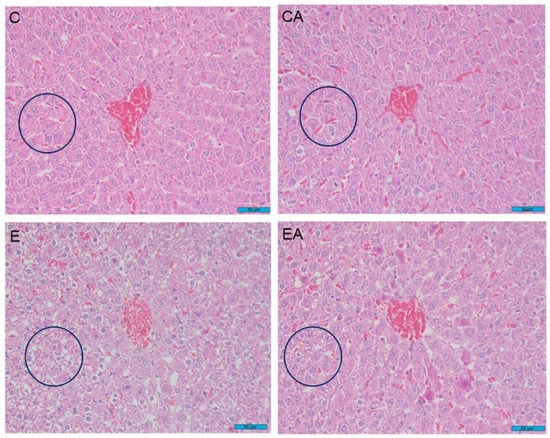
Figure 2.
Sections of rat liver tissue between different treatments. Hematoxylin-eosin-stained sections (800× magnification). Bar = 50 μm. Control group (C), control group fed fresh apples (CA), ethanol-treated group (E), and ethanol-treated group fed fresh apples (EA).
3.3. Apple Intake Has a Positive Effect on the Ability to Metabolize Alcohol
Excessive alcohol consumption decreases the ability of the liver to metabolize alcohol, thereby exacerbating the negative effects of alcohol and its metabolites [19]. Key enzymes of alcohol metabolism include ADH, ALDH, cytochromeP450 (CYP2E1), and catalase (CAT). Ethanol treatment resulted in the downregulation of Adh1, Aldh2, and Cat expression, and apple consumption significantly alleviated the downregulation of these genes. However, the elevation of Cyp2e1 by ethanol treatment may be related to the function of the OS (Figure 3A). The sPCR results verified the differences in gene expression related to alcohol metabolism induced by apple consumption under ethanol treatment, confirming the accuracy of RT-qPCR (Figure 3B). Interestingly, the activity of the alcohol-metabolizing enzyme ADH in the liver was consistent with the trend in gene expression, and consumption of apples significantly increased the ADH activity in the liver after alcohol consumption (Figure 3C). These results suggest that apple consumption helps restore the ability of the liver to metabolize alcohol that has been inhibited by excessive alcohol consumption.
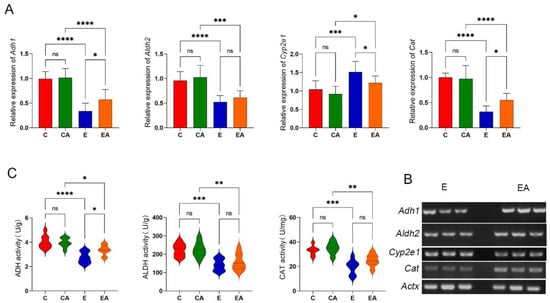
Figure 3.
Gene expression and enzyme activity of alcohol metabolism in rat liver. (A) Analysis of the gene expression levels of alcohol dehydrogenase 1 (Adh1), aldehyde dehydrogenase 1 family, member A1 (Aldh2), cytochrome P450, family 2, subfamily e, polypeptide 1 (Cyp2e1), and catalase (Cat) in liver tissues by real-time quantitative polymerase chain reaction (RT-qPCR). (B) Semi-quantitative real-time polymerase chain reaction (sPCR) to verify differential expression of Adh1, Aldh2, Cyp2e1, and Cat in the E and EA groups. Actx was used as a housekeeping gene. (C) Enzymatic activities of alcohol dehydrogenase (ADH), acetaldehyde dehydrogenase (ALDH), and catalase (CAT) in rat liver tissues. ns: p > 0.05, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
3.4. Apples Slow down the Oxidative Stress Caused by Ethanol
ALD-associated OS is characterized by a decrease in the activity of SOD, GPx, and CAT in the liver and the increased production of lipid peroxidation products, including MDA [20]. In the present study, alcohol decreased the enzymatic activities of SOD, GPx, and CAT and increased the level of MDA in the liver of rats, and apple consumption reversed these effects to some extent (Figure 4A). In conclusion, apple consumption reduces the inhibitory effect of alcohol on antioxidant enzymes in the liver, reduces the extent of lipid peroxidation, and to some extent reduces alcohol-induced oxidative stress.
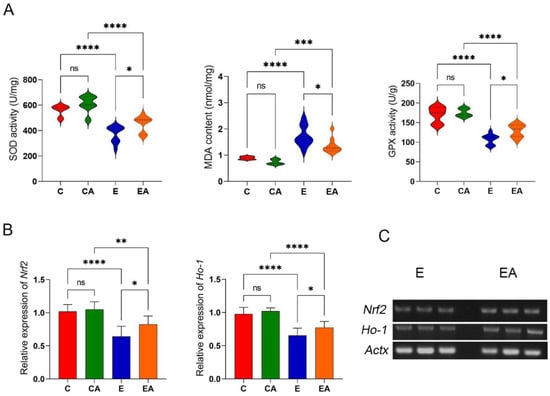
Figure 4.
Genes expression and enzyme activity of oxidative stress in rat liver. (A) Superoxide dismutase (SOD) activity, glutathione peroxidase (GPx) activity, and the levels of malondialdehyde (MDA). (B) Analysis of the gene expression levels of nuclear factor erythroid-derived 2-like 2 (Nrf2) and heme oxygenase 1 (Ho-1) in liver tissues by RT-qPCR. (C) sPCR to verify differential expression of Nrf2 and Ho-1 in the E and EA groups. ns: p > 0.05, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
Cyp2e1 is implicated in the production and accumulation of ROS in ALD. The proteins encoded by Nrf2 and Ho-1 decrease OS by scavenging ROS [21]. Ethanol exposure upregulated Cyp2e1 and downregulated Nrf2 and Ho-1, while apple consumption reversed these effects to some extent (Figure 4B,C). These results suggest that ethanol disrupted the oxidative balance in the liver, and apple consumption reversed this effect.
3.5. Apples Reduce the Inflammatory Response Induced by Alcohol
The production of inflammatory factors is a pathological hallmark of ALD [22]. Ethanol treatment increased the mRNA and protein expression of proinflammatory factors IL-1β and TNF-α, whereas apple consumption had a significant effect on reducing the expression levels of these pro-inflammatory factors. Although the expression of the inflammation suppressor interleukin 10 (IL-10) also increased with alcohol treatment, apple consumption did not reverse this effect, but instead significantly increased the mRNA and protein expression of IL-10 (Figure 5). Therefore, it can be concluded that apples can partially reduce the production of inflammatory factors induced by alcohol treatment and alleviate the degree of inflammation in ALD.
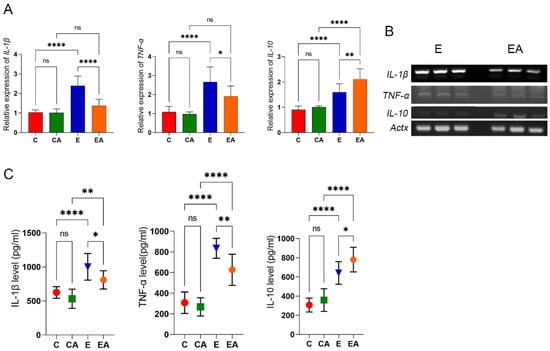
Figure 5.
Gene expression and protein levels of inflammation-related to rat liver tissue. (A) Analysis of the gene expression levels of interleukin 1 (IL-1β), tumor necrosis factor (TNF-α), and interleukin 10 (IL-10) in liver tissues by RT-qPCR. (B) sPCR to verify differential expression of IL-1β, TNF-α, and IL-10 in the E and EA groups. (C) Protein levels of interleukin 1 (IL-1β), tumor necrosis factor (TNF-α), and interleukin 10 (IL-10) in rat liver. ns: p > 0.05, *: p < 0.05, **: p < 0.01, ****: p < 0.0001.
3.6. Apple Improves the Expression of Genes Related to Lipid Metabolism and Liver Fibrosis
Ethanol abuse causes hepatic steatosis and lipid accumulation. Ethanol increases the mRNA expression of hepatic lipid synthesis genes Fabp2 and Acac [23] and downregulates the lipolytic gene Cpt1a [24]. Consistent with these data, ethanol upregulated Fabp2 and Acac and downregulated Cpt1a, while apple consumption reduced these effects (Figure 6). Ethanol abuse causes liver fibrosis. Collagen type I alpha 1 (COL1A1) and alpha-smooth muscle actin (α-SMA, encoded by Acta2) are typical fibrosis markers [25]. Ethanol increased the relative expression of Col1a1 and Acta2, while apple consumption reduced these effects (Figure 6). The above results suggest that apple intake improved the amount of gene expression associated with alcohol-induced hepatic steatosis and liver fibrosis. Apples, as a natural food with potential protective effects, could be used as one of the strategies to prevent and treat liver disease caused by ALD.
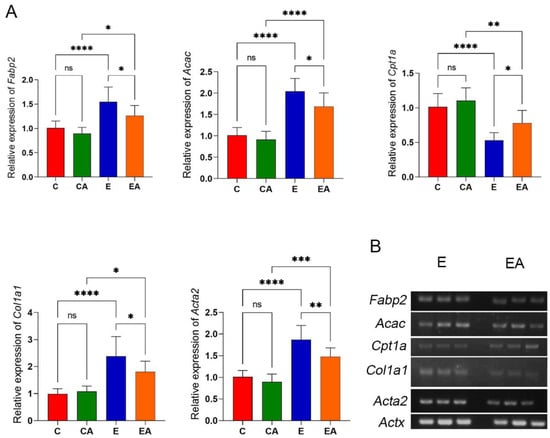
Figure 6.
Gene expression levels of involved in lipid metabolism and liver fibrosis. (A) Analysis of the gene expression levels of fatty acid-binding protein 2 (Fabp2), acetyl-CoA carboxylase alpha (Acac), carnitine palmitoyltransferase 1A (Cpt1a), collagen type I alpha 1 chain (Col1a1), and alpha-2 smooth muscle actin (Acta2) in liver tissues by RT-qPCR. (B) sPCR to verify differential expression of Fabp2, Acac, Cpt1a, Col1a1, and Acta2 in the E and EA groups. ns: p > 0.05, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.
4. Discussion
Alcohol abuse can cause severe damage to the liver and ALD due to OS, lipid peroxidation, and inflammation induced by ethanol and its metabolites [26]. Some components of apples have hepatoprotective effects, including antioxidant activity and modulation of lipid metabolism [18,27,28]. In addition, apple polyphenols ameliorate alcohol-induced hepatocyte and neuronal injury [29,30]. Therefore, we hypothesized that apples protected against ALD.
ALT and AST are traditional markers of liver injury since they are released into the circulation when xenobiotics impair the structural integrity of hepatocytes [31]. In our study, ethanol treatment for 10 days increased in rats. HE staining showed that ethanol caused significant pathological changes in the liver, including edema and the impairment of hepatocyte morphogenesis, consistent with the previous studies [32,33,34], indicating that we successfully established a rat model of ALD. Apple intake improved ALD by decreasing the serum levels of ALT and AST, reducing edema, and improving hepatocyte morphogenesis. Some compounds in apples are known to alleviate ALD [16,35].
ALD affects the mRNA and protein expression of enzymes involved in alcohol metabolism, and OS and proinflammatory cytokines reduce the mRNA and protein expression of ADH and ALDH [19]. ADH and ALDH play a vital role in alcohol catabolism. The impairment of alcohol metabolism increases alcohol toxicity and stimulates feedback pathways that exacerbate liver injury in vivo [36]. ADH metabolizes ethanol to toxic acetaldehydes [37]. Mammalian ADH has six isoforms (ADH1-ADH6), and ADH1 and ADH4 are involved in ethanol metabolism [38]. Acetaldehydes are metabolized to acetate by ALDHs, including ALDH1A1 [36,39]. Alcohols are further metabolized by ALDH to carbon dioxide and water [3]. In the present study, we found that apple consumption partially restored the mRNA expression and enzyme activity of ADH and ALDH, suggesting that apples may reduce the effects of ALD by improving the ability to metabolize alcohol. However, the underlying mechanism has not been evaluated.
Ethanol abuse disrupts the oxidant-antioxidant balance. Ethanol increases the activity of CYP2E1, which is involved in alcohol metabolism, and the production of superoxide radicals, hydrogen peroxide, nitric oxide, and hydroxyl radicals [40]. Moreover, ethanol reduces the activity of antioxidant enzymes (SOD and GPx) and the expression of antioxidant genes (Nrf2, Ho-1) in the liver, leading to lipid peroxidation and inflammation [41]. Apple phlorizin exerts antioxidant and hepatoprotective activity by increasing Nrf2 expression and the activity of antioxidant enzymes [42]. Further, apple consumption increases the serum activity of SOD and GPx [43]. Flavonols (quercetin-3-glucoside, quercetin-3-D-galactoside, and quercetin-3-rhamnoside) from ‘Golden Delicious’ apples reduced OS in obese rats [12]. These results demonstrate that apples have an excellent antioxidant capacity and protect against ALD by reducing OS.
Alcohol abuse induces inflammatory responses in the liver by activating inflammatory genes and pathways, thereby inducing the expression of proinflammatory cytokines. IL-1β and TNF-α play important roles in ALD [44]. Ethanol ingestion increases intestinal epithelial permeability, releasing bacteria and lipopolysaccharide (LPS) into the circulation, and LPS induces the secretion of proinflammatory cytokines by Kupffer cells in the liver [22,45]. Conversely, apple polyphenols upregulate the expression of intestinal tight junction proteins, reducing intestinal permeability and inflammation [46,47].
Ethanol intake impairs hepatic lipid metabolism [48]. Further, ethanol disrupts lipid homeostasis by increasing triglyceride and free fatty acid synthesis and decreasing the elimination of free fatty acids from hepatocytes, leading to lipid deposition in the liver [49]. Fabp2 promotes the production of n-3 polyunsaturated fatty acids in the intestine by regulating the genes involved in fatty acid absorption and the synthesis of triacylglycerol and cholesterol [50]. Acetyl-CoA carboxylase 1 (ACC1), encoded by the Acac gene, is a rate-limiting enzyme involved in the ab initio synthesis of fatty acids and catalyzes the conversion of acetyl-CoA to malonyl-CoA [51]. Cpt1a is implicated in hepatic fatty acid oxidation [52]. In our study, apple intake significantly downregulated Fabp2 and Acac and upregulated Cpt1a in ethanol-treated rats. Apples improve lipid metabolism and reduce lipid accumulation in the liver, and these effects may be due to dietary fiber and polyphenols [53,54,55].
Liver fibrosis is a chronic inflammatory process triggered by several factors, including alcohol abuse. Fibrosis is characterized by the deposition of extracellular matrix components, especially fibrillar collagens, by activated hepatic stellate cells and the increased expression of markers of liver fibrosis, including COL1A1 and α-SMA [56]. In this study, apple intake reduced alcohol-induced liver inflammation and decreased the expression of Col1a1 and Acta2. Polyphenols improve fibrosis by decreasing inflammation, OS, and the levels of fibrosis markers [57]. Dietary fiber reduces liver fibrosis in mice [58]. Nonetheless, the effects of other compounds in apples and mechanisms of action need to be further studied.
5. Conclusions
The consumption of apple (Malus pumila Mill) has a protective effect against ethanol-induced liver injury in rats. This effect may be achieved by improving the hepatic alcohol metabolism capacity and antioxidant capacity, reducing the production of pro-inflammatory cytokines, and improving the expression of genes associated with fatty liver and liver fibrosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13085112/s1, Table S1: Primer sequences for real-time quantitative PCR. Table S2. Primer sequences for real-time semi-quantitative PCR.
Author Contributions
D.-G.H.: Conceptualization, Validation, Formal analysis, Supervision, Funding acquisition. C.W.: Conceptualization, Methodology, Software, Investigation, Data curation, Writing-original draft, Visualization. C.-N.M.: Methodology, Software X.-L.L., Q.S., Q.Z., Y.-Y.L. and C.-Y.Y.: Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by grants from the National Key Research and Development Program of China (2022YFD2100102, 2018YFD1000200); the National Natural Science Foundation of China (32122080, 31972375); and Shandong Province (ZR2020YQ25).
Institutional Review Board Statement
The study species is not listed in the List of Protected Animals in China and experimental research on this species is legal in China, no institutional permission was required for the collection of animals.
Data Availability Statement
Data will not be made public due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, Y.S.; Sim, J.; Seo, S.; Seo, W. Alcoholic liver disease: A new insight into the pathogenesis of liver disease. Arch. Pharmacal Res. 2022, 45, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef]
- Angireddy, R.; Chowdhury, A.R.; Zielonka, J.; Ruthel, G.; Kalyanaraman, B.; Avadhani, N.G. Alcohol-induced CYP2E1, mitochondrial dynamics and retrograde signaling in human hepatic 3D organoids. Free. Radic. Biol. Med. 2020, 159, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Lach, T.; Cichoż-Lach, H. Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. J. Clin. Med. 2021, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Coppens, V.; Morrens, M.; Destoop, M.; Dom, G. The Interplay of Inflammatory Processes and Cognition in Alcohol Use Disorders—A Systematic Review. Front. Psychiatry 2019, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, C.; Thomes, P.G.; Kharbanda, K.K.; Casey, C.A.; McNiven, M.A.; Donohue, T.M. Lipophagy and Alcohol-Induced Fatty Liver. Front. Pharmacol. 2019, 10, 495. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv. Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, I.S.; Otles, S. Effects of Green Apple (Golden Delicious) and Its Three Major Flavonols Consumption on Obesity, Lipids, and Oxidative Stress in Obese Rats. Molecules 2022, 27, 1243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Valero-Vello, M.; Peris-Martínez, C.; García-Medina, J.J.; Sanz-González, S.M.; Ramírez, A.I.; Fernández-Albarral, J.A.; Galarreta-Mira, D.; Zanón-Moreno, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the Antioxidant, Anti-Inflammatory, and Neuroprotective Potential of Natural Food and Nutritional Supplements for Ocular Health in the Mediterranean Population. Foods 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Mariadoss, V.A.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini-Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Jia, M.; Ren, D.; Nie, Y.; Yang, X. Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct. 2017, 8, 1282–1292. [Google Scholar] [CrossRef]
- Ren, T.; Mackowiak, B.; Lin, Y.; Gao, Y.; Niu, J.; Gao, B. Hepatic injury and inflammation alter ethanol metabolism and drinking behavior. Food Chem. Toxicol. 2020, 136, 111070. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, X.; Yi, R.; Zhao, X. Antioxidative and Anti-Inflammatory Effects of Lactobacillus plantarum ZS62 on Alcohol-Induced Subacute Hepatic Damage. Oxidative Med. Cell. Longev. 2021, 2021, 7337988. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free. Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Qi, B.; Hao, S.; Ji, R. Camel milk ameliorates inflammatory mechanisms in an alcohol-induced liver injury mouse model. Sci. Rep. 2021, 11, 22811. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Wang, P.; Xia, Y.; Yan, N.; Takahashi, S.; Krausz, K.W.; Hao, H.; Yan, T.; Gonzalez, F.J. Withaferin A alleviates ethanol-induced liver injury by inhibiting hepatic lipogenesis. Food Chem. Toxicol. 2022, 160, 112807. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Woo, M.J.; Ham, J.R.; Lee, M.K. Anti-steatotic and anti-inflammatory effects of Hovenia dulcis Thunb. extracts in chronic alcohol-fed rats. Biomed. Pharmacother. 2017, 90, 393–401. [Google Scholar] [CrossRef]
- Bingül, İ.; Başaran-Küçükgergin, C.; Aydın, A.F.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Nunes, P.C.; Barbosa, F.K.S.; de Araújo Silva, A.K.C.; Lima, M.D.S.; Alves, A.F.; de Magalhães Cordeiro, A.M.T.; Alcântara, M.A.; de Albuquerque Meireles, B.R.L.; Melo, N.F.C.B.; de Souza Aquino, J.; et al. Malay apple (Syzygium malaccense) promotes changes in lipid metabolism and a hepatoprotective effect in rats fed a high-fat diet. Food Res. Int. 2022, 155, 110994. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Yang, J.; Lin, F.; Sun, Y.; Li, T.; Wu, C. Hepatoprotective effect of apple polyphenols against concanavalin A-induced immunological liver injury in mice. Chem. Biol. Interact. 2016, 258, 159–165. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Li, L.; Gao, Y.; Wang, F.; Zhang, Y.; Fan, Y.; Wu, C. Protective effect of apple polyphenols on chronic ethanol exposure-induced neural injury in rats. Chem. Biol. Interact. 2020, 326, 109113. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Zhou, J.; Sun, P.; Zhao, L.; Zhou, F. Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods 2022, 11, 3014. [Google Scholar] [CrossRef]
- Xie, K.; Chen, C.H.; Tsai, S.P.; Lu, P.-J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Cai, Z.; Ge, H.; Ma, S.; Yu, Y.; Liu, J.; Zhang, T. Transcriptome analysis reveals the hepatoprotective mechanism of soybean meal peptides against alcohol-induced acute liver injury mice. Food Chem. Toxicol. 2021, 154, 112353. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Jaganath, I.B.; Satharasinghe, D.; Yong, C.Y.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y.; et al. The in vivo hepato-recovery effects of the polyphenol-rich fermented food XenijiTM on ethanol-induced liver damage. RSC Adv. 2017, 7, 38287–38299. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Wang, X.; Luo, X.; Yang, L.; Zhang, R.; Yin, H.; Xie, D.; Pan, Y.; Chen, Y. Hepatic deletion of Smad7 in mouse leads to spontaneous liver dysfunction and aggravates alcoholic liver injury. PLoS ONE 2011, 6, e17415. [Google Scholar] [CrossRef]
- Thomas, M.; Peters, T.J. Acetaldehyde: Its Role in Alcoholic Toxicity and Dependence. Br. J. Addict. 1981, 76, 375–378. [Google Scholar] [CrossRef]
- Höög, J.O.; Hedberg, J.J.; Strömberg, P.; Svensson, S. Mammalian alcohol dehydrogenase—Functional and structural implications. J. Biomed. Sci. 2001, 8, 71–76. [Google Scholar] [CrossRef]
- Hwang, P.H.; Lian, L.; Zavras, A.I. Alcohol intake and folate antagonism via CYP2E1 and ALDH1: Effects on oral carcinogenesis. Med. Hypotheses 2012, 78, 197–202. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, I.A. Cytochrome P450S and Alcoholic Liver Disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Shi, L.G.; Hu, G.Y.; Chen, S. Saponins from Panax japonicus Protect Against Alcohol-Induced Hepatic Injury in Mice by Up-regulating the Expression of GPX3, SOD1 and SOD3. Alcohol Alcohol. 2010, 45, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Z.; Liu, D.; Li, X.; Rehman, R.U.; Wang, H.; Wu, Z. Apple phlorizin attenuates oxidative stress in Drosophila melanogaster. J. Food Biochem. 2019, 43, e12744. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, A.G.; Schulz, M.; Silveira, T.T.; de Oliveira, M.V.; Patrício, M.J.; Gonzaga, L.V.; Fett, R.; da Silva, E.L.; Wazlawik, E. Apple intake improves antioxidant parameters in hemodialysis patients without affecting serum potassium levels. Nutr. Res. 2019, 64, 56–63. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef]
- Vassallo, G.; Mirijello, A.; Ferrulli, A.; Antonelli, M.; Landolfi, R.; Gasbarrini, A.; Addolorato, G. Review article: Alcohol and gut microbiota—The possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment. Pharmacol. Ther. 2015, 41, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; et al. Apple Polyphenols Improve Intestinal Antioxidant Capacity and Barrier Function by Activating the Nrf2/Keap1 Signaling Pathway in a Pig Model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Yao, Y.L.; Han, X.; Li, Z.M.; Lian, L.H.; Nan, J.X.; Wu, Y.L. Acanthoic Acid Can Partially Prevent Alcohol Exposure-Induced Liver Lipid Deposition and Inflammation. Front. Pharmacol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Fu, L.; Gao, J. n-3 PUFA reduction caused by fabp2 deletion interferes with triacylglycerol metabolism and cholesterolhomeostasis in fish. Appl. Microbiol. Biotechnol. 2020, 104, 2149–2161. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yuan, M.H.; Zhang, C.Y.; Liu, H.M.; Liu, J.R.; Wei, A.L.; Ye, Q.; Zeng, B.; Li, M.F.; Guo, Y.P.; et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Parnell, L.D.; Smith, C.E.; Guo, T.; Sayols-Baixeras, S.; Aslibekyan, S.; Tiwari, H.K.; Irvin, M.R.; Bender, C.; Fei, D.; et al. Carbohydrate and fat intake associated with risk of metabolic diseases through epigenetics of CPT1A. Am. J. Clin. Nutr. 2020, 112, 1200–1211. [Google Scholar] [CrossRef]
- Skinner, R.C.; Warren, D.C.; Lateef, S.N.; Benedito, V.A.; Tou, J.C. Apple Pomace Consumption Favorably Alters Hepatic Lipid Metabolism in Young Female Sprague-Dawley Rats Fed a Western Diet. Nutrients 2018, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Han, H.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Zeng, Q. Dietary fibre alleviates hepatic fat deposition via inhibiting lipogenic gene expression in meat ducks. J. Anim. Physiol. Anim. Nutr. 2018, 102, e736–e745. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, D.; Liu, F.; Wang, X.; Cui, Y.; Li, S.; Li, X. The Ameliorating Effects of Apple Polyphenol Extract on High-Fat-Diet-Induced Hepatic Steatosis Are SIRT1-Dependent: Evidence from Hepatic-Specific SIRT1 Heterozygous Mutant C57BL/6 Mice. J. Agric. Food Chem. 2022, 70, 5579–5594. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, S.; Stradiot, L.; Verhulst, S.; Thoen, L.; Mannaerts, I.; van Grunsven, L.A. Inhibitory effect of dietary capsaicin on liver fibrosis in mice. Mol. Nutr. Food Res. 2015, 59, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, W.Y.; Li, L.L.; Hu, C.; Tang, Q.Z. Therapeutic Potential of Polyphenols in Cardiac Fibrosis. Front. Pharmacol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Li, M.M.; Zhou, Y.; Zuo, L.; Nie, D.; Li, X.A. Dietary fiber regulates intestinal flora and suppresses liver and systemic inflammation to alleviate liver fibrosis in mice. Nutrition 2021, 81, 110959. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).