Abstract
Cabo Ortegal, in Spain, was declared a UNESCO Global Geopark in 2023. An ultramafic complex makes it a geologically exciting unit, where many research works have been carried out. Serpentinites, formed by the weathering of peridotites, dunites, and other ultramafic rocks, have been described in detail in previous works. Their role as a construction material has been elucidated, although due to their complexity and heterogeneity, the results of using these materials for construction are not always successful. Talcose rocks, related to serpentinites, can also be found in the complex, although it is difficult to distinguish them. Talcose rocks may be a resource to be explored and exploited in an unpopulated area. The use of talc in pharmaceuticals, cosmetics, and handicrafts makes it a material of growing importance. However, the asbestos and heavy metal content should be severely limited to prevent health issues. The goal of this work is to start the characterization of these talcose rocks before promoting their potential use in different contexts to support the economy of an underpopulated area of Spain.
1. Introduction
Serpentinites are very attractive rocks that have been used in stone-built heritage for centuries. Today they can be found in contemporary buildings, both indoors and outdoors. These rocks are very difficult to characterize, as their mineralogy may change in one single outcrop and, therefore, their extraction is challenging for companies in the stone industry. The ultramafic complex of Cabo Ortegal has been studied in detail by many authors [1]. Serpentinite outcropping in the massif was subject to commercial exploitation during the last century [2]. However, the physical and mechanical characteristics as well as mineralogy of this rock made it difficult to obtain a stable return on their marketing. In Galicia, serpentinite outcrops are part of “emptied Spain”, areas that have been abandoned by the population due to the lack of job and economic prospects. The exploitation of serpentinite as a raw material for construction appears to no longer be attractive for entrepreneurs, but authors have found some talcose bodies in close contact with the serpentinite that may be worth a qualitative study. Talc (Mg3Si4O10(OH)2) is a trioctahedral phyllosilicate with a layered structure made of brucite-type (Mg(OH)2) layers sandwiched between two tetrahedral sheets of silicon-oxygen (T-O-T). The layers are held together by a weak bonding [3], yet there is no report in the literature describing the intercalation of water or any sort of organic molecular species between the talc layers. The layers of the structure have almost monoclinic symmetry but the nearly hexagonal rings of oxygen atoms on the surfaces of the layers, formed by the bases of the silica tetrahedral, are partly displaced so that the stack of layers forms a triclinic crystal [3]. Talc is taken as a standard for hardness, having the value 1 on the Mohs scale. Talc exhibits perfect cleavage along a (001) plane of symmetry, yielding flexible, slightly elastic lamellae. Although talc has a marked affinity for certain organic chemicals, it generally has very little chemical reactivity. It is infusible, inert to acids, and neither explosive nor flammable. Moreover, talc is a non-conductor of electricity and, thus, it is used in the manufacture of high frequency electrical insulators [3]. Regarding the mineral chemistry of talc, only minor differences from the end-member formula Mg3Si4O10(OH)2 are detectable. Small amounts of Al, Cr, or Ti may substitute Si and small amounts of Mn, Al, and Fe may substitute Mg in the octahedral sites. Very small amounts of Ca, Na, and K may also substitute Mg, but in several cases these elements may represent impurities [3]. Minor amounts of Ni are often found in talc associated with ultrabasic rocks, while ferrous iron was detected in hydrothermal talc. These heavy metal substitutions are of great interest for the scope of this paper. There are detailed descriptions of the igneous and metamorphic rocks that outcrop in the Cabo Ortegal complex, as well as of the petrogenesis of such rocks [1]. Serpentinites were also studied in an attempt to evaluate their quality as ornamental stone, considering that a large part of the heritage of the area was built with these serpentinites, which are transformed into talcose rocks that are locally known as pedra de toelo (and marketed as Verde Tuelo). Usually, talc crystallizes in lamellae and occurs mainly in metamorphosed ultrabasic rocks. However, it also crystallizes in high-grade kyanite-talc rocks and, by the replacement of olivine and tremolite, in siliceous dolomites [4]. It is the major component of economic deposits of steatite or soapstone. To the best of the authors’ knowledge, very little has been published on talcose rocks from Cabo Ortegal, probably because their distinction from serpentinite is not very clear and, in the field, it becomes very difficult to distinguish them (Figure 1). Furthermore, the chemical and mineralogical analysis are confusing due to the complicated distinction of mineral phases of the asbestos group using traditional techniques (e.g., petrography, X-ray diffraction). It is worth remembering that the term asbestos refers to a group of minerals including five fibrous amphiboles (amosite, i.e., the fibrous variety of cummingtonite–grunerite; crocidolite, i.e., is the fibrous variety of riebeckite; anthophyllite, actinolite and tremolite) and one fibrous serpentine mineral (chrysotile) [2,3].
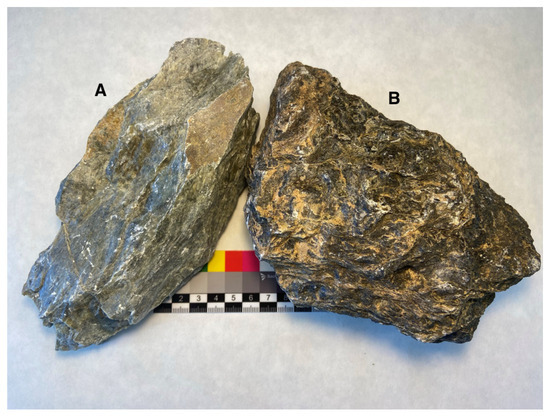
Figure 1.
This work is focused on two sampling areas with very similar characteristics in hand specimen and outcrop. (A) From Somozas, mostly made of talc, (B) from Ferraría, made of talc, but also containing serpentine and carbonate.
Due to the worldwide economic importance of talc as a raw material [5], the authors consider this publication to be an important step towards spreading knowledge of these rocks in the area of study.
This pilot study considers only a very limited number of samples (two, Figure 1) to evaluate whether it is worth extending the study to include more samples and other analytical techniques (e.g., stable isotopes) for a complete characterization. The main goal of this paper is to preliminarily characterize talcose rocks, in order to find an easy way to distinguish them from nearby serpentinite, and to promote the potential value of these rocks to boost the local economy of an already industrially inactive area that has recently been recognized as a UNESCO Global Geopark (216th Session of the UNESCO Executive Council; Paris, 23 July 2023), partly due to the importance of the geology of the area.
2. Geographical and Geological Setting
The samples for this study were collected in two quarries located at Somozas and Ferraría (municipality of Moeche, A Coruña) that formerly exploited ultramafic and serpentinized ultramafic rocks, mainly as refractory material, but also as ornamental stone (Figure 2a,b). Based on the geological cartography of Spain made by the Spanish Geological Survey [6], these rocks, made of olivine, pyroxene, amphibole, and iron minerals, outcrop in the form of stripes and lenses or segregations of irregular size throughout the central and southern domain of the complex of Cabo Ortegal (Figure 3). These bands or lenses are arranged between felsic rocks of gneissic nature and sets of mafic rocks (eclogites, granulites, amphibolites, and metabasites) that make this Cabo Ortegal complex a geologically unique one. Another study carried out by the same organization [7] confirms that the selected quarries for this research rocks were marketed for ornamental purposes under the names Verde Pirineos and Verde Tuelo. The ornamental potential of these rocks is due to their color (dark gray to green) and to a series of textural features originated in the two phases of deformation undergone by these rocks: one of cataclastic type and a later one causing the recrystallization of carbonate minerals filling fissures and generating a very characteristic anastomosed and nodular texture. The ornamental rock exploited in both quarries was mainly produced as pieces for cladding sheets, although, traditionally, it was used as a raw material for the construction of monuments of religious (especially cruceiros) and ethnographic significance in the region. Both quarries ceased their activity because of the depletion of exploitable levels of the rock due to an increase in the degree of jointing (in Ferraría) or in talc levels (in Somozas), thus reducing its mechanical resistance.

Figure 2.
Outcrops in sampled quarries. (a) Balbina quarry (Somozas sample) (b) Ferraría de Abaixo (Ferraría sample). Both quarries are inactive at present.
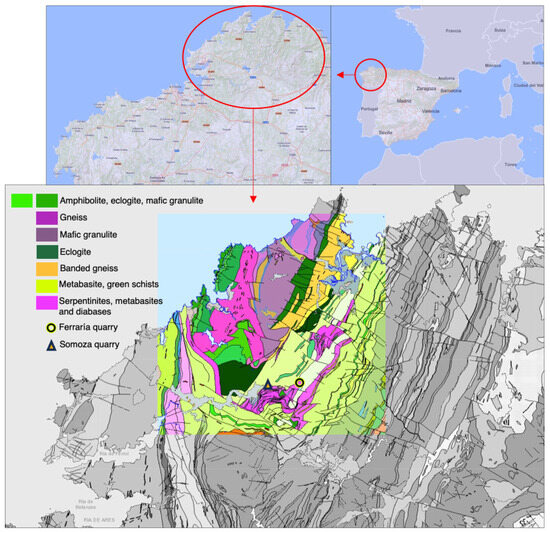
Figure 3.
Geographical location of the Somozas and Ferraría quarries and geological contextualization, indicating the most relevant rock formations in the Cabo Ortegal complex. Modified from [6].
In spite of the fact that both samples come from the same geological formation, important differences can be observed after a detailed analysis, while, at hand scale, the differences are minor (Figure 1). The Somozas sample seems softer and has a slightly lighter green color; the Ferraría sample is darker in comparison (Figure 1). Both samples have an unctuous touch, more apparent in the sample from Somozas.
3. Materials and Methods
Ten kilos of rocks were sampled from the quarries and were prepared for mineralogical and geochemical study. Thermal analysis was carried out to confirm the mineralogical composition.
3.1. Petrographic Analyses
The petrographic study was carried out on the thin sections, prepared after mortising them in epoxy resin to avoid further fragmentation. A complete petrographic examination followed [8], describing the mineralogy and textures. A Leica DM2500P petrographic microscope (PM) (Leica Microsistemas, Barcelona, Spain) under transmitted light was used for this purpose in the petrographic laboratories of the University of Salamanca.
3.2. Mineralogical Analyses
X-ray powder diffractometry (XRPD) was used for the characterization of the samples with a Bruker D8 Advance (Bruker, Billerica, MA, USA) X-ray diffractometer at 40 kV and 40 mA. The instrument was equipped with a copper tube and a curved graphite monochromator. Scans were recorded in the range of 3–66° 2θ, with a step interval of 0.02° 2θ and a step-counting time of 3 s/step. EVA software Ver. 3.0 (DIFFRACplus EVA) was used to identify the mineral phases and experimental peaks upon comparison with the PDF2 reference patterns. This analysis was carried out in the X-ray laboratory of the University of Salamanca.
3.3. Thermal Analysis
Thermal analysis (differential scanning calorimetry DSC and derivative thermogravimetry DTG) has been carried out with a Netzsch STA 449 C Jupiter instrument (Netzsch-Gerätebau GmbH, Selb, Germany). Heating was performed in alumina crucibles under an air flow of 30 cm3min−1 and a heating rate of 10 °C/min. Samples were ground in an agate mortar and about 15 mg were heated in the range of 25–1200 °C. Thermal analysis accuracy was checked with four repeated collections on kaolinite reference samples, revealing good reproducibility (instrumental T precision of ±1.2 °C), DSC detection limit < 1 µW, and theoretical weight sensitivity of 0.10. Netzsch proteus thermal analysis software (https://analyzing-testing.netzsch.com/en/products/software/proteus, accessed on 2 November 2023) (Netzsch-Gerätebau GmbH, Selb, Germany) was used to obtain: (i) endothermic peaks, (ii) exothermic peaks, and (iii) DTG (derivative thermogravimetry). The experiments were carried out at the University of Calabria.
3.4. Geochemistry of Major and Trace Elements
The chemical composition (whole rock major and trace elements) was obtained by means of inductively coupled plasma mass spectrometry (ICP-MS) using an ICP-MS AGILENT 7800 (GenTech Scientific LLC, Arcade, NY, USA). For the analysis, 0.1 g of powder from each sample was digested with HNO3 + HF under pressure, in high-pressure vessels, in a Milestone microwave. Equipment from the University of Salamanca was used for this purpose.
4. Results
Talcose rocks are intertwined with the serpentinites, making it difficult to separate lithologies. Although, to the naked eye, the differences between the specimens of the studied samples were not conspicuous (almost the same color, fragile aspect, unctuosity to the touch, etc.) (Figure 1), the mineralogy (petrography and X-ray) (Figure 4, Figure 5, Figure 6 and Figure 7) and the geochemistry revealed many differences through detailed observations.
4.1. Petrography
The sample from Somozas is made almost exclusively of talc (Figure 4). It is possible to see some remnants of serpentine minerals, although they are so scarce that they cannot be detected by XRD (see below).

Figure 4.
Thin section of the Somozas sample. This sample is very homogeneous, and is made of a matrix of talc, with very few remains of fibrous minerals (the grey fibers in the picture). Cross-polarized image.
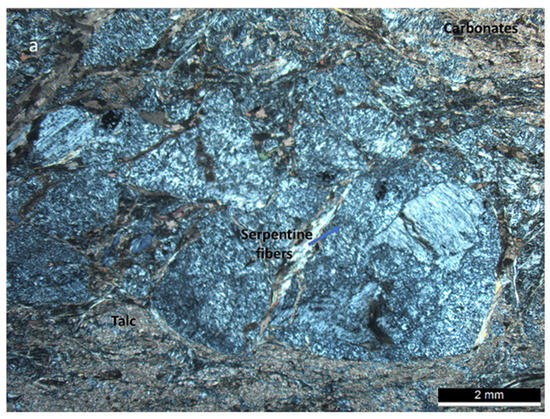

Figure 5.
(a,b) Thin section of the Ferraría sample. The sample shows a brechoid texture, with areas made of serpentine minerals (grey fibers) surrounded by carbonate (brownish areas) that also enclosed talc (brightly colored minerals). Two views of the same thin section are included. Cross-polarized view.
Under the microscope, the mineralogy observed in the sample from Ferraría consists of serpentine phases distributed inside nodulous textures, surrounded by talc and carbonate (Figure 5a), that show a deformed texture from mineral alignment (Figure 5b).
From the texture in the sample of Somozas (Figure 4), it can be seen that the generation of talc is produced at the expenses of the fibrous phases (i.e., phyllosilicates and serpentine minerals).
Microscopic observations are ratified against the X-ray diffractograms.
4.2. X-Ray Diffraction
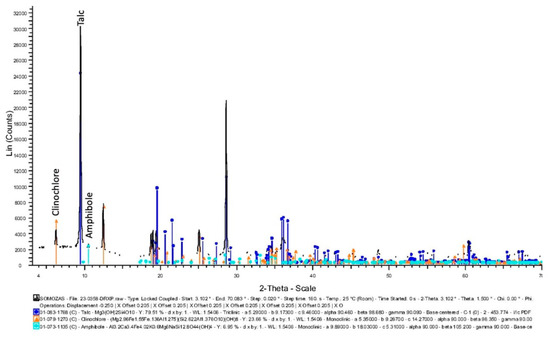
Figure 6.
XRPD of the Somozas sample, in which peaks of a talc (dark blue color) are preeminent, also containing some clinochlore (orange color) and amphibole (i.e., tremolite, light blue color).
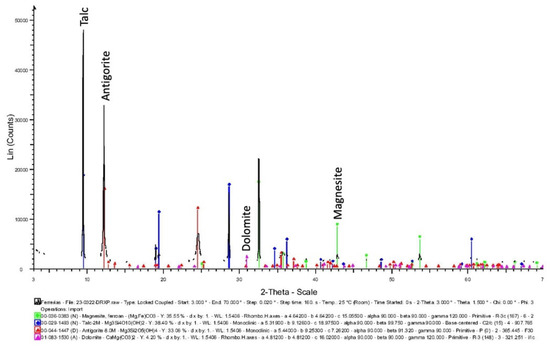
Figure 7.
XRPD of the sample from Ferraría, in which peaks of talc are preeminent (dark blue color), also containing some serpentine (antigorite) (red color), dolomite (magenta color), and magnesite (green color).
4.3. Thermal Analysis
The results from thermal analyses allowed a more accurate mineralogical characterization of the samples to be conducted, enabling the distinguishing of the polymorphs from the serpentine (e.g., chrysotile, antigorite, and lizardite) (Figure 8 and Figure 9).
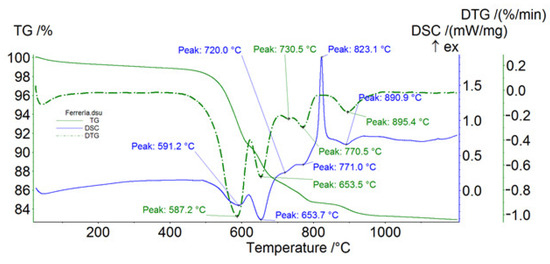
Figure 8.
Thermal analysis of the Ferraría sample. Blue solid line, DSC; green dashed line, DTG; green solid line, TG; ex = exothermic.
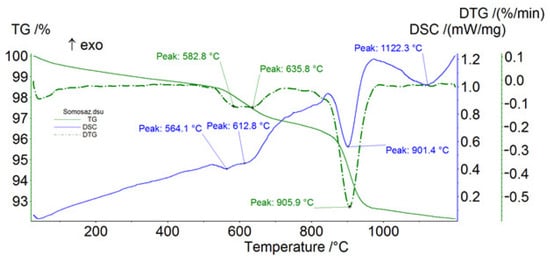
Figure 9.
Thermal analysis of Somozas sample. Blue solid line, DSC; green dashed line, DTG; green solid line, TG; exo = exothermic.
The thermal data (DSC and DTG peaks) confirm the presence of the phases detected by XRPD. Furthermore, chrysotile was identified [9] in both samples, but in the Somozas sample it was detected in very low quantities (Table 1). Lizardite and antigorite were only detected in the sample from Ferraría (Table 1). Tremolite was identified in the Somozas sample [9,10] and the data obtained using thermal analysis reduced the ambiguities regarding the distinction between asbestos tremolite from non-asbestos tremolite [10]. Based on the breakdown temperature of the tremolite samples observed in this study (i.e., 1123 °C) (Figure 8; Table 2), we conclude that tremolite was non-asbestiform, having a prismatic morphology [11]. Crossing the data from the thermal analyses and those from the XRPD, talc turns out to be predominant in the Somozas sample.

Table 1.
Semi-quantitative mineralogical composition of the samples in order of declining relative quantities, detected by XRPD, DSC/DTG, and PM. Tcl = talc, Ctl = chrysotile, Liz = lizardite, Ant = antigorite, Tr = tremolite, Mag = Magnetite, Dol = dolomite, Chl = Chlorite. It is only possible to distinguish a brechoid texture in this sample under the microscope.

Table 2.
Peak temperatures in DSC and DTG curves. w = weak, s = strong, endo = endothermic, exo = exothermic, Tcl = talc, Ctl = chrysotile, Liz = lizardite, Ant = antigorite, Tr = tremolite, Mag = Magnetite, Dol = dolomite, Chl = chlorite.
4.4. Geochemistry
From the results of major elements (Figure 4 and Table 3), the sample from Ferraría resembles the composition of a serpentinite and the sample from Somozas resembles the composition of a talcocite. However, the Ferraría sample diverges somewhat from the serpentinite standard composition. In fact, the composition of the major element is very similar to the composition of the talcocite from Somozas, except for some elements. SiO2, TiO2, and Al2O3 are higher in the Somozas sample compared to the Ferraría sample. In this comparison, Somozas is lower in CaO and LOI (Loss on Ignition), all being characteristic of highly carbonatized serpentinites (See Table 3 and Figure 10). Ultramafic rocks have poor trace element chemistry, except for some heavy metals such as Cr and Ni. This geochemistry may be inherited from its precursors (e.g., harzburgite), which is an important issue when discussing the potential uses of talcose rocks as pharmaceutical or cosmetic products. In our samples, Somozas is slightly enriched in Li, Zn, Y, Zr, Nb, Th, and U, and lower in Cu, Sr, and Pb. The other elements are very similar in both samples (See Table 3 and Figure 11).

Table 3.
Content of major (%, expressed as oxide) and minor elements (ppm of elements) of Ferraría and Somozas samples. LOI = Loss on Ignition.
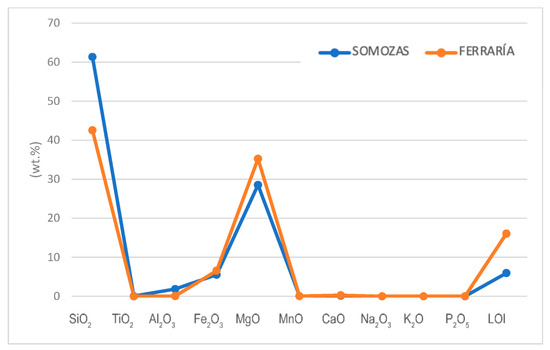
Figure 10.
Major elements in the study samples. Concentrations are in wt.%.
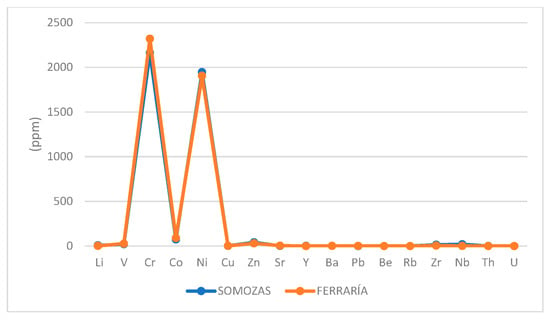
Figure 11.
Trace elements in the study samples. Concentrations are in ppm.
Also, the geochemistry of serpentinites may vary depending on the geodynamic setting in which they are formed, owing to differences in the composition and temperature of the hydration fluids. Serpentinites are a sink for B, Cl, and other volatiles, which can influence in the final geochemistry of these rocks [12]. The concentration of these volatile phases may have helped in the transformation into talcose rocks. The chemistry of volatiles and stable isotopes will be a useful tool to distinguish between the serpentinite and the talcose transformation.
5. Discussion
Talcose rocks (or talcocites/soapstone) consist largely of talc. They have a soapy feel and are soft enough to be carved with a knife [13]. They are very dense, non-porous rocks, in contrast with their serpentinite precursor. Testing on different soapstones around the world [14,15] provides data that are compatible with the requirements of ASTM C97 for water absorption (0.1%, much lower than the one obtained for the Galicia local serpentinite (≈0.70%, [2]). The compressive and flexural strength of some soapstone has shown values of 21 and 14 MPa. The former mechanical value is too low to accomplish the ASTM standards [14] to be used as dimension stone, but the flexural strength is quite high [15,16]. We were not able to test our samples for the physical and mechanical behavior, as they were so fragile, but we intend to increase the sampling and implement other testing in future works.
Talc has been used in pharmaceuticals for a long time as part of traditional Chinese medicine. Talcose rocks can be easily cut and carved, if necessary, for industrial applications, which should be further explored if other uses are not possible for these rocks. The analyses carried out on the samples from the two quarries in this pilot study, both mineralogical and geochemical, indicate a different composition, even though they come from rock outcrops mapped under the same nature (basic and ultrabasic rocks). This fact confirms the existence of significant variability within these rocks at almost the outcrop scale, contributing to reaffirming the geologic complexity of the Cabo Ortegal complex. The differences between both samples reside fundamentally in the talc content (more abundant in the Somozas sample) and in the presence of minerals from the serpentine group (more abundant in the Ferraría sample). The presence of both minerals could justify the discussion regarding the exploitation of these rocks for industrial use.
Moreover, talc crystals, due to their relatively low cost and good heat resistance properties, as well as their hydrophobic characteristics, electrical insulation, lubrication, and acid resistance, are ideal as a surface for laboratory countertops and electrical switchboards. It is also an important filler, and it is widely used in many industrial fields, such as ceramics, paper, cosmetics, paints, food, ceramics, polymers, rubber, and insecticides [17,18,19]. For these reasons, in recent years, continuing interest has been expressed regarding the study of the deposits and characteristics of talc.
However, talc is subject to contamination from other surrounding materials (i.e., serpentinites), such as heavy metals or even asbestos [9,20], which is known to be a carcinogenic material. For example, talc ore extracted in the state of New York is heavily contaminated from tremolite asbestos, which results in a high mortality rate among workers [21,22,23]. In this scenario, very recently, a multinational pharmaceutical company was forced to pay billions in a settlement to resolve claims involving asbestos in its talc products, although it was not clearly demonstrated [24].
In the interest of finding some industry related to the ultramafic complex of Cabo Ortegal, an attempt to characterize the talcose rocks that outcrop among the serpentinites has been carried out. The high content of Cr, Ni, and other heavy metals (contents of Y, Zr, Nb, Th, and U are even higher than in the serpentinite) preclude its use in the pharmaceutical industry. Indeed, in agreement with [19], the common presence of these foreign ions (i.e., Cr, Ni, etc.) in talc may prevent the high performance of this mineral. Nonetheless, based on thermal data, the tremolite contaminating the talc in the Somozas sample is not asbestos tremolite, which is a hazardous component. This gives us some hope of continuing with further characterization studies, because there might be a way of removing the impurities. To make the Cabo Ortegal ultramafic complex economically exploitable for industrial uses, the mineral impurities and foreign ions present in talc must be removed, and many papers have proposed different purification techniques for the treatment of talc ore, which are cited in different scientific sources [25,26,27,28]. Acid leaching, wet attrition, magnetic separation, and the flotation technique been reported to obtain suitably pure talc for industrial applications. These treatments may be considered if talcose rocks are sufficiently abundant and interesting for industrial use, which should be contemplated by the industry’s stakeholders.
6. Conclusions
From the results for this pilot research, it seems that the talc from the area of study is not very appropriate for use in pharmaceuticals and the cosmetic industry [18], but talcose rocks may be used for carving and in ceramics (e.g., [5,29]). Furthermore, talc has been shown to be an excellent substitute for feldspar (albite) in porcelain manufacture [30] or in blocks for electric and thermal insulation [3]. It should be noted that talcose rocks in general have distinct and useful applications compared to serpentinites. For these reasons, the exploitation of talc as an alternative raw material could trigger an artisanal activity in the area, linked to the local economy that surely will flourish with the recognition of the area as a UNESCO Global Geopark in 2023 (see above).
In this scenario, the Somozas sample is of better quality than the Ferraría sample, which is slightly converted into talc (see Figure 5), only slightly contaminated by asbestos such as chrysotile in very low amounts. Being so close to the talcose samples, this characteristic lowers the risk for health issues among workers. However, more specific analysis and testing are needed both to draw conclusions regarding the economic viability of the extraction and the potential environmental issues related to it.
A more complete study is in preparation, increasing the number of samples, but also the analytical techniques to clearly distinguish talcose rocks from serpentinites and to characterize the content of fibrous minerals in them (e.g., asbestos minerals), as well as any other characteristics to avoid any harm if the rocks are exploited in the future. We maintain the goal of finding a potential use for these rocks to improve the local economy of an increasingly underpopulated area in Galicia, Spain, without risking workers’ health.
Author Contributions
Conceptualization, D.P. and A.B.; methodology, D.P., A.J.L., T.R., A.R. and A.B.; formal analysis, D.P. and A.B.; investigation, D.P., A.J.L., A.R. and A.B.; resources, D.P., A.J.L., T.R., A.R. and A.B.; writing—original draft preparation, D.P., A.J.L., T.R. and A.B.; writing—review and editing, D.P., A.J.L., T.R., A.R. and A.B.; funding acquisition, D.P., A.J.L., T.R., A.R. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The regional government of A Coruña (Deputación da Coruña), in the interest of promoting the new Global Geopark and the potential use of the raw materials in the area, has funded the analytical work carried out at the University of Salamanca. Grant PID2021-123948OB-I00 funded by MCIN/AEI/10.13039/501100011033 and by ERDF “A way of making Europe”, by the European Union.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Girardeau, J.; Gil Ibarguchi, J.I.; Ben Jamaa, N. Evidence for a Heterogeneous Upper Mantle in the Cabo Ortegal Complex, Spain. Science 1989, 245, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Peinado, M.; Blanco, J.A.; Yenes, M. Geochemical characterization of a serpentinization process at Cabo Ortegal (NW Spain). Can. Mineral. 2008, 46, 317–327. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals, 2nd ed.; 3B; The Geological Society: London, UK, 2009. [Google Scholar]
- Eggert, R.G.; Kerrick, D.M. Metamorphic equilibria in the siliceous dolomite system: 6 kbar experimental data and geologic implications. Geochim. Cosmochim. Acta 1981, 45, 1039–1049. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries; US Geological Survey: Tallahassee, FL, USA, 2018. [Google Scholar]
- IGME. Mapa Geológico de España; Escala 1:50.000; Serie Magna; Hoja 7 Cedeira; Servicio de Publicaciones del Ministerio de Obras Públicas, Gobierno de España: Madrid, Spain, 1981. [Google Scholar]
- IGME. Mapa de rocas y Minerales Industriales de Galicia. E 1:250.000. 2008. Available online: https://info.igme.es/SidPDF/166000/585/166585_0000001.pdf (accessed on 30 August 2023). (In Spanish).
- ASTM Designation: C1721–15; Standard Guide for Petrographic Examination of Dimension Stone. ASTM International: West Conshohocken, PA, USA, 2018.
- Bloise, A.; Ricchiuti, C.; Navarro, R.; Punturo, R.; Lanzafame, G.; Pereira, D. Natural occurrence of asbestos in serpentinite quarries from Southern Spain. Environ. Geochem. Health 2021, 1–19. [Google Scholar] [CrossRef]
- Bloise, A. On the thermal breakdown of tremolite: A new method for distinguishing between asbestos and non-asbestos tremolite samples. J. Mater. Sci. 2023, 58, 8779–8795. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Gualtieri, A.F. The effect of grinding on tremolite asbestos and anthophyllite asbestos. Minerals 2018, 8, 274. [Google Scholar] [CrossRef]
- Pereira, M.D.; Shaw, D.M.; Acosta, A. Mobile trace elements and fluid-dominated processes in the Ronda peridotite, southern Spain. Can. Mineral. 2003, 41, 617–625. [Google Scholar] [CrossRef]
- BS EN 12670:2002; Natural Stone—Terminology. Bechtel Ltd.: London, UK, 2004.
- ASTM C1526-19; Standard Specification for Serpentine Dimension Stone. ASTM International: West Conshohocken, PA, USA, 2019.
- Vavro, M.; Gajda, J.; Přikryl, R.; Siegl, P. Soapstone as a locally used and limited sculptural material in remote area of Northern Moravia (Czech Republic). Environ. Earth Sci. 2015, 73, 4557–4571. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Mastali, M.; Kinnunen, P.; Illikainen, M. Alkali-activated soapstone waste—Mechanical properties, durability, and economic prospects. Sustain. Mater. Technol. 2019, 22, e00118. [Google Scholar] [CrossRef]
- Ferrage, E.; Martin, F.; Boudet, A.; Petit, S.; Fourty, G.; Jouffret, F.; Micoud, P.; de Parseval, P.; Salvi, S.; Bourgerette, C.; et al. Talc as nucleating agent of polypropylene: Morphology induced by lamellar particles addition and interface mineral-matrix modelization. J. Mater. Sci. 2022, 37, 1561–1573. [Google Scholar] [CrossRef]
- Ferrage, E.; Martin, F.; Petit, S.; Pejo-Soucaille, S.; Micoud, P.; Fourty, G.; Ferret, S.; Salvi, S.; De Parseval, P.; Fortune, P. Evaluation of talc morphology using FTIR and H/D substitution. Clay Miner. 2003, 38, 141–150. [Google Scholar] [CrossRef]
- Marzbani, P.; Resalati, H.; Ghasemian, A.; Shakeri, A. Talc, a multi-purpose filler: A review of talc’s features and improvement methods of its efficiency. Ann. Biol. Res. 2013, 4, 159–162. [Google Scholar]
- Lescano, L.; Locati, F.; Sfragulla, J.; Marfil, S.; Bonalumi, A.; Maiza, P. Asbestiform and non-asbestiform morphologies in a talc and vermiculite mine from the province of Córdoba (Argentina): A case study. Environ. Earth Sci. 2017, 76, 1–20. [Google Scholar] [CrossRef]
- Ross, M.; Smith, W.L.; Ashton, W.H. Triclinic talc and associated amphiboles from Gouverneur mining district New York. Am. Mineral. 1968, 53, 751–769. [Google Scholar]
- Kleinfeld, M.M.D.; Messite, J.M.D.; Zaki, M.H.M.D. Mortality experiences among talc workers: A follow-up study. J. Occup. Med. 1974, 16, 345–349. [Google Scholar] [PubMed]
- Virta, R.L. The Talc Industry-An Overview; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1989; p. 30. [Google Scholar]
- O’Brien, K.M.; Tworoger, S.S.; Harris, H.R.; Anderson, G.L.; Weinberg, C.R.; Trabert, B.; Kaunitz, A.M.; D’Aloisio, A.A.; Sandler, D.P.; Wentzensen, N. Association of Powder Use in the Genital Area with Risk of Ovarian Cancer. JAMA 2020, 323, 49–59. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Arifov, P.; Tadjiev, K.; Yunhua, X.U. Characterization and processing of talc-magnesite from the Zinelbulak deposit. Min. Sci. Technol. (China) 2010, 20, 415–420. [Google Scholar] [CrossRef]
- Baba, A.A.; Ibrahim, A.S.; Bale, R.B.; Adekola, F.A.; Alabi, A.G. Purification of a Nigerian talc ore by acid leaching. Appl. Clay Sci. 2015, 114, 476–483. [Google Scholar] [CrossRef]
- Sachanbiński, M.; Piórewicz, R.; Michalik, R. Heavy minerals in the serpentinite weathering cover of the Szklary massif. Geol. Sudet. 2020, 33, 131–141. [Google Scholar]
- Yehia, A.; Al-Wakeel, M.I. Talc separation from talc-carbonate ore to be suitable for different industrial applications. Miner. Eng. 2000, 13, 111–116. [Google Scholar] [CrossRef]
- Chandra, N.; Agnihotri, N.; Bhasin, S.; Khan, A.F. Effect of addition of talc on the sintering characteristics of fly ash based ceramic tiles. J. Eur. Ceram. Soc. 2005, 25, 81–88. [Google Scholar] [CrossRef]
- Da Silva, R.C.; La Porta, F.D.A.; Silva, G.A.D.S.; Tebcherani, R.M.; Kubaski, E.T.; Bonfante, E.A.; Tebcherani, S.M. Application of factorial model for an optimization of partial replacement of feldspar by talc on the sintering process. Int. J. Appl. Ceram. Technol. 2023, 20, 1576–1590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).