Abstract
Smokers employing electronic nicotine delivery systems (ENDS) and heated tobacco products (HTP) are currently the most common types of smoking patients seen in the dental practice. Both types of smoking are currently viewed as less harmful than cigarette smoking. However, many studies already indicate that they could harm oral health. This systematic review and meta-analysis aimed to collect a comprehensive overview of the actual knowledge regarding ENDS and HTP from a clinical and a laboratory perspective. Publications available through PubMed, Embase, the Web of Science, Scopus, and Google Scholar were used to summarize the effects of ENDS and HTP on oral health. Six surveys on self-perceived gum disease (T2 = 9.47 I2 = 99.32%), three cross-sectional studies reporting the BOP score (T2 = 8.68 I2 = 99.13%), and four in vitro studies on apoptosis after vaping exposure in human oral fibroblasts (T 2 = 8.10 I2 = 91.50%) were separately analyzed. The risk of bias ranged from critical to low. Both ENDS and HTP seem to have detrimental effects on periodontal and peri-implant parameters, and laboratory tests confirmed the presence of carcinogenic and inflammatory biomarkers. flavored e-liquids may also be a caries risk factor. Comprehensive smoking counseling should be carried out with all types of smoking patients, investigating the type of habit in terms of duration, nicotine percentage, and additional flavorings. Additional research is necessary to assess the long-term effects of alternative tobacco products on oral health.
1. Introduction
Electronic cigarette smokers (ECS) are increasing all over the world [1,2], mainly because vaping electronic nicotine delivery system(s) (ENDS) are perceived to be safer than cigarette smoking and attracts young naïve subject, as well as adult smokers who want to quit or reduce tobacco consumption [3,4]. Nevertheless, the short- and long-term effects of ENDS within the oral cavity have been scarcely investigated and reported, considering the fact that ENDS entered the market between 2003 and 2004 [5,6].
ENDS consists of an LED battery-operated device that mimics the shape of a conventional cigarette; it comprises a metal heating element in a stainless-steel case, a cartridge that holds the e-liquid, and an atomizer. The e-liquid solutions can have different nicotine percentages and are available in different flavors [7]. The cartridge and solution chemicals are mainly diethylene glycol, glycerin, nitrosamines, and potentially harmful contaminants, such as heavy metals, aldehydes, and carbonyls [8]. Today’s available e-cigarettes are third-generation digitalized devices allowing for high consumer customization [9]. ENDS vaping is a “recreative style” of smoking that seems quite removed from conventional cigarettes and is common among teens and young adults for whom customization of any item is a popular marketing strategy [10]. Moreover, the way in which ENDS are switched on and off has changed “the smoking session”; ENDS can be turned off after one minute as well as after half an hour, while the cigarette, once lit, cannot be paused. Thus, vaping meets the needs of former cigarette smokers/quitters who are attracted to the possibility of controlling the nicotine concentration of the solution.
The effects of ENDS on general health are likely to be established with scientific evidence in the coming decades. Vaping side effects [11] have been related to:
- The respiratory system (cough, asthma and bronchitis);
- The cardiovascular system (heart rate and blood pressure increase);
- The oropharyngeal system (oral cavity and pharynx lesions);
- Skin and annexes (dermatitis);
- Second-hand smokers or passive smokers (increased cotinine levels);
- Other (headache, eye problems due to vapor and glycerol, burns and lacerations).
As alternative tobacco products, ENDS have not only been proposed on the market, but heated tobacco products (HTP) have also been developed. HTPs heat tobacco to a high enough temperature to release aerosol, without burning it or producing smoke. They differ from ENDS in that they heat a tobacco leaf/sheet and not a liquid [12]. As is the case for ENDS, HTPs have also been related to pulmonary [13] and cardiovascular diseases [14]. Moreover, it has been stated that they should not be recommended for smoking cessation [15].
Dental professionals are aware of the harmful effects of cigarette smoking, especially the increased risk of malignant lesions and the onset of periodontitis [16]. The onset and progression of periodontitis are directly related to the frequency of the habit. Smoking less than 9 cigarettes/per day is considered light smoking, while more than 31 cigarettes/per day is considered heavy smoking [17]. The use of electronic cigarettes has created doubts regarding the evaluation of the smoker/vaper profile, as a wide variety of ENDS and HTPs is available and dual smokers are quite frequent.
The design of the studies regarding the effects of ENDS and HTPs on the development and progression of periodontal and peri-implant diseases are mainly focused on comparisons between vapers, HTP smokers, cigarette smokers, and non-smokers. The clinical parameters evaluated are periodontal/peri-implant probing pocket depth (PPD), bleeding on probing (BOP), plaque index (PI), and clinical attachment loss (CAL). Along with the evaluation of these parameters, the collection of salivary and/or crevicular fluid samples was often performed, in which inflammatory biomarkers such as TNF-α, IL-1β, or IL-6 were measured. These inflammatory cytokines stimulate osteoclastic processes, increasing periodontal inflammation and bone loss [18]. Moreover, reactive aldehydes from ENDS aerosols may allow protein carbonylation that could lead to bone tissue injury in periodontitis [19]. These adverse effects require a systematic review employing a comprehensive overview of this topic, which poses a challenge for dentists who increasingly find themselves treating e-cigarette and HTP smokers.
Hence, the present systematic review aimed to evaluate the effect of ENDS and HTP on oral health variables or on human cells/oral bacteria by comparing vaping, non-smoking, dual smoking, and cigarette smoking. This review analyzed observational, interventional, and laboratory studies on human cells.
2. Materials and Methods
2.1. Study Registration
The review protocol was registered in PROSPERO (CRD42021276707) and followed the PRISMA guidelines [20].
2.2. Reporting Format
The PRISMA recommendations were adopted throughout the process of the present systematic review [21].
2.3. Population (P), Exposure (E), Comparison (C), Outcomes (O), and Study Design
The research question was formulated according to the following PECOs: P (population): electronic cigarette and heated tobacco smokers or human cells/oral bacteria exposed to ENDS; E (exposure): the use of vaping electronic cigarettes or heated tobacco products and/or vapor from electronic cigarettes or heated tobacco products; C (comparison): non-smokers (NS), former smokers (FS), cigarettes smokers (CS), dual smokers (DS), other types of smokers, and cigarette-to-ENDS or HTP switchers; Os (outcomes): changes in oral health parameters due to electronic cigarettes or heated tobacco products, both clinically assessed and self-reported by users, or the expression of apoptotic/necrosis biomarkers in cells.
2.4. Inclusion Criteria
The following inclusion criteria were adopted:
- Interventional and observational studies on ENDS and HTP and their effects on oral health;
- Studies including subjects of any age or sex;
- Full and/or pilot studies reporting data;
- For the laboratory studies, articles considering human oral cells or oral bacteria;
- Studies in English, without time limits.
2.5. Exclusion Criteria
The following exclusion criteria were adopted:
- Systematic or narrative reviews and meta-analyses;
- Theses and dissertations;
- Case reports/series;
- Studies reporting insufficient/unclear information, or not allowing data extraction;
- Papers not published in English;
- Papers focused on different aspects of health other than oral health;
- Studies for which the authors did not respond to the email requesting data clarification.
2.6. Search Strategies
A detailed search strategy was developed for each database, considering differences in controlled vocabulary and syntax rules (NC). The search strategy for each database is given in the Supplementary File S1.
2.7. Electronic Search
The electronic search was conducted by one author (NC) across five databases: PubMed (National Library of Medicine), the Web of Science (Clarivate Analytics), Embase (Elsevier), Scopus (Elsevier), and Google Scholar.
The search was performed in February 2023 and updated in August of the same year. All retrieved references were uploaded onto Endnote 20® software to check for duplicates and for study selection.
2.8. Manual Search
The reference lists of the studies included were used to identify additional records that were hand searched (NC).
2.9. Study Selection
After the exclusion of duplicates, two independent authors (NC and MGC) screened the papers by title and abstract; when in doubt, consensus was reached after consultation with a third author (GC). Agreement between the two screeners was assessed using Cohen’s Kappa score.
2.10. Data Extraction and Variable Analysis
Table 1, Table 2 and Table 3 display the summary of included articles divided by study type, such as observational studies in which the variables considered were age, sex, type of smoker, and type of records (clinical or from surveys). In the interventional studies, the variables considered were age, sex, type of smoker, and type of records (BOP, PD, and similar). In order to standardize the age of the samples of the included studies, the following classification was used: Early Adolescence (EA), for subjects aged 12 to 18 years; Young Adults (YA), for subjects aged 19 to 44 years; Middle Adults (MA), for subjects aged 45 to 65 years; and Older Adults (OA), for subjects aged 65 years and over [22].
Finally, in the laboratory studies, in addition to the type of smoker or cell, the type of exposure was also considered.
2.11. Risk of Bias
The quality of the randomized clinical trials (RCTs) was assessed using the ROB-2 tool [23]. For non-RCTs studies, the ROBIN-I (Risk Of Bias In Non-randomized Studies of Interventions) tool was used [24]. The biases evaluated for both tools were: confounding, selection of participants, classification of interventions, deviation from intended interventions, missing data, measurement of outcomes, and selection of reported results. The Risk-of-Bias Approach to Address Laboratory Studies [25] was used for articles with an exclusive ex vivo or in vitro design. Two reviewers (NC and TSC) conducted the assessments, and discussion resolved divergences. Details are reported in the Supplementary File S1.
2.12. Synthesis of the Results
Meta-analyses were conducted if at least three studies with similar comparisons reported the same outcomes. For dichotomous data (i.e., BOP), the primary measures of effect were risk ratios (RRs) and 95% confidence intervals (95% CI) [26]. The Stata 17.0 package® was used for the data analysis.
The estimate of variance between studies under the random-effects model requires better precision when the number of studies is very small. For this reason, the fixed-effect model and the inverse variance method were used to obtain pooled estimate rates. The I2 statistics were calculated to describe the percentage of variation across studies due to heterogeneity rather than chance. The heterogeneity was categorized as follows: <30%, not significant; 30–50%, moderate; 51–75%, substantial, and 76–100%, considerable. Clinical and methodological heterogeneity was assessed by examining the characteristics of the studies, for example, the similarity between the characteristics of the participants, interventions, and outcomes as specified in the inclusion criteria.
2.13. Subgroup Analyses
If there were sufficient data, subgroup analysis was performed to explore the influence of study characteristics such as age, sex, type of cells, and smoking/vaping sessions/experimental conditions.
2.14. Sensitivity Analysis
An analysis was also conducted to assess whether the stratification of studies by design or risk of bias (i.e., overall low risk vs. high risk) yielded similar or different results.
2.15. Unit-of-Analysis Issues
If some of the included studies possessed data from repeated or paired observations on participants, which could lead to unit-of-analysis errors, the advice given in Section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions was followed [27].

Table 1.
Characteristics of the observational studies.
Table 1.
Characteristics of the observational studies.
| Authors, Year (Country) | Total N of Participants (% Male); N of Participants in Each Group | Outcome | Aim | Conclusion |
| Study Design | Age (Years) | |||
| Part A—Studies with Clinical Examination | ||||
| Al Aali et al., 2018 (Saudi Arabia) [28] Cross-sectional with clinical examination | 92 (100) EC: 47, NS: 45 YA | BOP, PPD, biomarkers, X-rays | Compare clinical and X-rays peri-implant parameters and levels of TNF-a and IL-1b levels among ECS and NS | Clinical, microbiological, and radiographic peri-implant parameters are compromised among ECS. |
| Alazmi et al., 2021 (Saudi Arabia) [29] Cross-sectional with clinical examination | 127 (72); ECS 63, NS 64 YA | Peri-implant CBL, PD, PI, BOP, X-rays, and self-reported OH status and practice | Assess peri-implant parameters at 8 years follow-up | Implants of ECS and NS exhibited clinical and radiographical status when at home OH practice was good. |
| Ali et al., 2022 (Kuwait) [30] Cross-sectional with clinical examination | 75 (21); ECS 18, CS 19, NS 38 MA | PPD, PI, GI, CAL, MBL, IL, whole saliva | Compare the periodontal status and saliva, IL-15, and -18 levels among CS, ECS, NS | Clinically, CS and NS demonstrate similar periodontal statuses; IL and salivary parameters are more elevated in smokers. |
| AlQahtani et al., 2018 (Saudi Arabia) [31] Cross-sectional with clinical examination | 160 (100); ECS: Age: 42 YA | BOP, PI, PPD, biomarkers | Compare clinical and radiographic peri-implant parameters and proinflammatory cytokine profile in the peri-implant sulcular fluid among the groups | ECS and OS (waterpipe) users may be at risk of poor peri-implant health. Tobacco smoking is associated with poor peri-implant health. |
| AlQahtani et al., 2019 (Saudi Arabia) [32] Cross-sectional with clinical examination | 137 (100); ECS: 34, CS: 35, OS: 33, NS: 35 YA | Cotinine levels at peri-implant BOP, PI, PD, biomarkers | Compare cotinine levels in the peri-implant sulcular fluid among the groups | Cotinine levels in the peri-implant sulcular fluid of cigarette and OS (waterpipe) smokers and electronic-cigarette users are comparable. |
| Alqahtani et al., 2022 (Saudi Arabia) [33] Cross-sectional with clinical examination | 150 (12); ECS 50, CS 50, NS 50 YA | Community periodontal index treatment need | Evaluate the periodontal treatment needs among CS, ECS, and NS | CS require more complicated periodontal treatment compared to ECS. |
| ArRejaie et al., 2019 (Saudi Arabia) [34] Cross-sectional with clinical examination | 95 (100); ECS: 31, CS: 32, NS: 32 YA | BOP, PI, PPD, CAL, MBL, biomarkers (MMP, IL), X-rays | Compare clinical and radiographic peri-implant parameters and biomarkers among CS, ECS, and NS | Peri-implant health was compromised among CS than ECS and NS. Increased levels of proinflammatory citokines were found in CS and ECS. |
| Bardellini et al., 2018 (Italy) [35] Prospective case-control | 90 (71); FS: 45, EC: 45 MA | Oral mucosal lesions | To evaluate the prevalence and characteristics of OMLs in FS compared to ECS | No statistically significant differences regarding total prevalence of OMLs between FS and ECS. Nicotine stomatitis, hairy tongue, and angular cheilitis were significantly more common among ECS. |
| Binshabaib et al., 2019 (USA) [36] Cross-sectional with clinical examination | 135 (92); CS: 46, ECS: 44, NS: 45 YA | BOP, PI, PPD, X-rays, biomarkers | Compare the clinical periodontal status and gingival crevicular fluid (GCF) cytokine profile among CS, ECS, and NS. | Periodontal status is poorer and GCF levels of proinflammatory cytokines are higher in CS compared with ECS and NS. |
| Dalrmyple et al., 2022 (Germany) [37] Pilot study—cross-sectional | 33 (45); ECS 11, CS 11, NS 11 | Breath odor | Determine differences in breath odor between ECS, CS, and NS | ECS breath has a reduced smoke odor and more pleasant aroma than CS, and is comparable to NS. |
| Ghazali et al., 2019 (Malasya) [38] Cross-sectional with clinical examination | 135 (99); CS: 45, ECS: 45, NS: 45 EA, YA, MA | DMFT | Evaluate caries experience among CS, ECS, and NS | CS and ECS have potential detrimental effect on caries development. |
| Herndon et al., 2022 (USA) [39] Cross-sectional questionnaire with clinical examination | 4544 (48); current ECS: 260, no current ECS: 4284 EA, YA, MA, OA | Self-reported OH | Recall of ECS for HPV test | E-cigarette use increases the persistence of HPV infection. |
| Ibraheem et al., 2020 (Saudi Arabia) [40] Cross-sectional with clinical examination | 120 (100); ECS: 30, CS:30, NS: 30, OS: 30 MA | BOP, PI, PPD, X-rays, NF-kappa B ligand | Compare the levels of receptor activator of NF-kappa B ligand (RANKL) and osteoprotegerin (OPG) in the GCF of the groups | CS and OS (waterpipe) and ECS usage is associated with an increased expression of RANKL and OPG in the GCF. |
| Javed et al., 2017 (USA) [41] Cross-sectional questionnaire and clinical examination | 94 (100); ECS: 31, CS: 33, NS: 30 YA | Self-reported OH status PI, GI, PPD, CAL, biomarkers | Assess periodontal parameters and self-perceived OH | Periodontal inflammation and self-perceived OH are exacerbated in CSS compared with ECS and NS. |
| Jeong et al., 2020 (South Korea) [42] Cross-sectional questionnaire and clinical examination | 13,551 (58); ECS: 222, NS: 8342, CS: 2330, FS: 2667 EA, YA, MA, OA | Self-reported OH status | Self-report periodontal status, community periodontal index | The results of the current study could motivate both ECS and CS to quit by highlighting the association of conventional cigarette smoking and electronic cigarette vaping with periodontal disease. |
| Karaaslan et al.2020 (Turkey) [43] Cross-sectional with clinical examination | 57 (68); ECS: 19, CS: 19, FS: 19 YA | PI, GI, PD, CAL, MBL, biomarkers | Effects of smoking on oxidative stress markers, proinflammatory cytokines levels, and periodontal clinical parameters in patients with periodontitis | Vaping ECS and CS had the same unfavorable effects on the markers of oxidative stress and inflammatory cytokines. |
| Mokeem et al., 2018 (Saudi Arabia) [44] Cross-sectional with clinical examination | 154 (100); CS: 39, OS: 40, ECS: 37, NS: 38 YA | PPD, PI, BOP, CAL, X-rays | Compare periodontal index and biomarkers among smokers and NS | Clinical and radiographic parameters of periodontal inflammation were poorer in CS and OS (waterpipe) than ECS and NS. |
| Tatullo et al., 2016 (Italy) [45] Cross-sectional questionnaire and clinical examination | 110 (81) ECS: 110 (all former CS) YA | Self-reported need to smoke PI, BI, PBI | Verify the clinical variations of periodontal health induced by EC and investigate the awareness of ECS about their health variations and need to turn back to CS | E-cigarette can be considered as a valuable alternative to tobacco cigarettes, with a positive impact on periodontal and general health status. |
| Vohra et al., 2019 (USA) [46] Cross-sectional questionnaire and clinical examination | 105 (100); CS: 28, ECS: 51, NS: 26 YA | Self-reported OH BOP, PI, PPD, CAL | Compare self-rated oral symptoms with periodontal status | Pain in teeth and gums is more often perceived by CS than ECS and NS. CS is more associated with increased PI and PD than is ECS. |
| Part B—without Clinical Examination | ||||
| Akinkugbe 2019 (USA) [47] | 13,650 (50); current ECS 418, current CS 634, Other 12,598 | Self-reported OH status and OH practice—PATH | Epidemiology of dental status of CS and ECS among adolescents | Dual users are associated with poor oral health outcomes. |
| Cross-sectional questionnaire | EA | |||
| Alade et al., 2022 (Nigeria) [48] | 2870 (51); CS 378, ECS 167, DS 401, NS 1916 | Self-reported oral lesions | Effects on OH for different smokers who had COVID-19 infection | ECS had 1.5 times higher odds of reporting oral lesions than NS. Those who had COVID-19 infection had higher odds of gingivitis. |
| Cross-sectional questionnaire | MC, EA, YA | |||
| Alhajj et al., 2022 (Yemen) [49] Cross-sectional questionnaire | 5676 (40); ECS 255, CS 596, DS 261, NS 4565 YA, MA | Self-reported OH status and OH practice | Assess self-reported OH practices and events in ECS | ECS reported more oral health-related conditions, particularly xerostomia and black tongue, and heart palpitation. |
| Alqobaly et al., 2022 (UK) [50] Cross-sectional questionnaire | 8129 (48); erce data not applicable MA | Self-reported periodontal status | Assess self-reported periodontal disease in ECS | ECS use is associated with self-reported periodontal disease. |
| Atuegwu et al., 2019 (USA) [51] Cross-sectional questionnaire | 32,320 (46), in 3 waves of survey; NS: 9632, regular ECS: 329, non regular ECS: 8298 EA, YA, MA, OA | New cases of gum disease in 12 months | Assess the association between ECS and PD | ECS may be harmful to OH. |
| Ho Cho et al., 2017 (South Korea) [52] Cross-sectional questionnaire | 65,528 (52); ECS: 1556, Former ECS: 3848, Never ECS: 60,124 EA | Oral symptoms (Gingival pain/bleeding, tongue or cheek pain, cracked or broken teeth) | Assess the relationship between EC use and OH | ECS among adolescents may be a risk factor for tongue and/or inside-cheek pain and cracked or broken teeth. |
| Huilgol et al., 2019 (USA) [53] Cross-sectional questionnaire | 456,343 (43); ECS: 15,019, Non ECS: 441,324 EA, YA, MA, OA | Self-reported poor OH symptoms | Assess the ECS use on OH | Daily use, but not intemittent use, of ECS was independently associated with poor OH. |
| Irusa el al; 2022 (USA) [54] Cross-sectional questionnaire | 13,080 (48); ECS 136, Other 12,944 EY, YA, MA, OA | Caries risk | CAMBRA tool (the caries management from risk assessment) | ECS had higher caries risk than non-ECS. |
| Abafalvi et al., 2018 (Hungary) [55] Cross-sectional questionnaire | 930 (83) ECS: 767, DS: 163 EA, YA, MA, OA | Self-reported oral hygiene practice | Assess self-reported oral hygiene practice among ECS and DS | Both groups showed inadequate oral hygiene practices. |
| Vemulapalli et al., 2021 (USA) [56] Cross-sectional questionnaire | 4618 (48); EC: 247, FS: 700, NS: 3671, DS:120, Former DS: 561 EA, YA, MA | Untreated caries | Examine the association between ECS and untreated caries | Both ECS and DS are associated with an increased occurrence of untreated caries. |
| Vora et al., 2019 (USA) [57] Cross-sectional questionnaire | TOT: 32,300 (48) ECS: 97, NS: 9076, CS: 4231, FS: 14,115, OS: 4748 EA, YA, MA | Self-reported OH status | Evaluate self-reported gum disease among ECS and other types of smokers | Numerous tobacco use patterns were associated with worse periodontal health compared to NS. |
| Yoshioka et al., 2022 (Japan) [58] Cross-sectional questionnaire | TOT 10,439 (54) 1034 CS, 437 heated tobacco products, FS 1853, 1049 DS, NS 5796 EA, YA, MA, OA | Self-reported history of PDis | Compare self-reported periodontal disease among smokers and NS | All the smokers were significantly associated with a higher prevalence of periodontal diseases compared to NS. |
EC: electronic cigarettes; ECS: electronic cigarettes smokers; CS: cigarettes smokers/cigarettes; DS: dual smokers; NS: non-smokers/never-smokers; FS: former smokers; OS: other type of smokers (waterpipe, cigars etc.); OH: oral health, PDis: periodontal disease; PPD: probing pocket depth; BOP: bleeding on probing; PI: plaque index; CAL: clinical attachment loss; GI: gingival index; SRP: scaling and root planning; GCF: gingival crevicular fluid; IL: inter leucine; CP: chronic periodontitis; NA: not available; FMUS: full-mouth ultrasonic scaling; PDT: photodynamic therapy; p-iM: implant mucositis; MD: mechanical debridement; MBL marginal bone loss; BI: bleeding index; PBI: papillary bleeding index; OMLs: oral mucosal lesions; MC = middle children; EA = early adolescents; YA = young adults; MA = middle adults; OA = older adults.

Table 2.
Characteristics of interventional studies.
Table 2.
Characteristics of interventional studies.
| Authors, Year Country, Study Design | Sample (%Male) Age Category | Outcome and Parameters | Aim | Conclusions |
|---|---|---|---|---|
| Akram et al., 2021 (Australia) [59] Longitudinal, three observations: 3 m, 6 m | TOT: 60 (100) ECS: 30, CS: 30 YA, MA | PPD, BOP, CAL periodontal disease, biomarkers | Evaluate the periodontal parameters and MMP-8 and CTX in ECS and CS | CS showed an increased periodontal worsening compared to ECS. |
| Al Deeb et al., 2020 (Saudi Arabia) [60] Randomized controlled clinical trial 3 observations: BL, 2 w 12 w | TOT: 71 (100) ECS: 21, CS: 25, NS: 25 Age: YA | BOP, PPD, PI, biomarkers | Effectiveness of PDT AAOAN adjunctive therapeutic modality in the treatment of peri-implant mucositis for ECS and CS | PDT with adjunctive mechanical debridement reduced PI and PD, while increasing BOP, in addition to reducing pro-inflammatory biomarkers CS. |
| Al Hamoudi et al., 2020 (Saudi Arabia) [61] Longitudinal, two observations, BL, 3 m | TOT: 71 (88) ECS: 36 Age: 47 YA, MA | GI, PPD, CAL, X-rays, GCF, IL | Periodontal parameters pre-post SRP | Levels of GCF IL-4, IL-9, IL-10, and IL-13 increased following SRP in ECS and NS with CP; the anti-inflammatory effect of SRP was more profound in NS. |
| Al Harti. 2019 (Saudi Arabia) [62] Prospective | TOT: 89 (100) ECS: 28, CS: 30, NS: 31 Age: 34 YA | BOP, PI Percentage of CS and EC on periodontal tissues after FMUS | After FMUS, gingival inflammation is worse in CS compared with ECS and NS. | |
| Alhumaidan et al., 2022 (Saudi Arabia) [63] Longitudinal, two observations BL, 3 m | TOT: 54 (67) ECS: 18, CS 18, NS 18 YA | CAL, Mt, PDis, PI, and MBL, percent vary cortisol | Evaluate salivary CL and IL-1β levels in light CS and ECS users before and after non-surgical periodontal therapy | In CS and ECS, users without Pdis, clinical periodontal parameters and whole-salivary CL and Il-1β levels remain unchanged after non-surgical periodontal therapy. |
| AlJasser et al., 2021 (Saudi Arabia) [64] Longitudinal, four observations BL, 3 m, 6 m, 12 m | TOT: 60 (52) ECS: 20, CS 20, NS 20 MA | BOP, PI, PD, IL | Compare changes in clinical periodontal parameters and changes in salivary IL between CS, ECS, NS after peri-implant treatment | Electronic cigarette smoking was found to be a mercentagelent risk indicator for peri-implantitis. |
| AlRifaiy et al., 2018 (Saudi Arabia) [65] Randomized controlled clinical trial 2 observations BL, 3 m | TOT: 38 (100) ECS with PDT: 20 ECS without PDT: 18 YA | BOP, PI, PrD, MBL | Effectiveness of antimicrobial therapy and PDT or erconly in ECS with p-iM | Antimicrobial PDT is more effective compared to MD alone in the treatment of p-iM in ECS. |
| Alshibani et al., 2022 (Saudi Arabia) [66] RCT | TOT: 23 ECS Age not reported | CAL, PI, BOP, PPD, | Assess the effect of non-surgical periodontal therapy with adjunct photodynamic treatment for the management of periodontal inflammation in ECS | Photodynamic treatment is as effective as non- surgical therapy for the management of periodontal inflammation in ECS. |
| Reuther et al., 2016 (UK) [67] Pilot clinical trial, two observations BL, 30 min | TOT: 10 volunteers who vaped EC specifically for the trial (70) YA | Blood flow in oral mucosa | Blood flow after vaping measured with Doppler laser | EC may have an effect on blood flow in the oral mucosa. |
| Wadia et al., 2016 (UK) [68] Pilot longitudinal, two observations BL, 2 w | TOT: 20 switchers from CS to ECS EA, YA, MA, OA | BOP and GCF parameters | Compare the gingival health of a group of switcherercentagementage of sites with BOP increased statistically significantly 2 weeks after the switch. | |
| Xu et al., 2021 (China) [69] Longitudinal, two observations BL, 6 m | TOT: 101 (71) ECS: 32, CS: 31, NS: 38 YA | PPD, BOP, CAL, saliva sample | Evaluate the adverse effects of vaping on periodontal health | Periodontal severity status after 6 months was significantly worse in CS and ECS than NS. |
| Pouly et al., 2022 (Switzerland) [70] Randomized controlled clinical trial 3 observations BL, 3 m, 6 m | TOT: 172 (81) CS: 84, DS: 17, HTP: 70, Other: 1 YA, MA, OA | PPD, BOP, CAL, GI, PI | PD after scaling and root planingin smokers who switched or did not to HTP. | Scaling and root planing improves the course of PD similarly in CS and HTP. The treatment may mask favorables Pdis changes in the switchers. |
EC: electronic cigarettes; ECS: Electronic cigarettes smokers; CS: Cigarettes smokers/cigarettes; DS: dual smokes; NS: non-smokers/never-smokers; FS: former smokers; OS: other type of smokers (waterpipe, cigars etc.); HTTP: hetated tobacco smokers, OH: oral health, PDis: Periodontal disease; PPD: probing pocket depth; BOP: bleeding on probing, PI: plaque index; CAL: clinical attachment loss; GI: gingival index; SRP: scaling and root planning; GCF: gingival crevicular fluid; IL: inter leucine; CP: Chronic periodontitis; NA: not available; FMUS: full mouth ultrasonic scaling, PDT: photodynamic therapy, p-iM: implant mucositis; MD: mechanical debridement, MBL marginal bone loss CL: cortisol, BL: baseline, M: months, W: weeks, EA = early adolescents, YA = young adults, MA = middle adults, OA= older adults.

Table 3.
Characteristics of laboratory studies.
Table 3.
Characteristics of laboratory studies.
| Authors, Year Country, Sub-Section | Cell line/Strain/Teeth/Sample | Outcome | Aim and Exposure | Conclusion |
|---|---|---|---|---|
| Alanazi et al., 2019 Canada [71] Oral Candida | Gingival epithelial cells | C. albicans activity | Impact on C. albicans growth and expression of different virulent genes Exp: ECS | EC may interact with C. albicans to promote their pathogenesis, which may increase the risk of oral candidiasis in e-cigarette users. |
| Alanzi et al., 2018 Canada [72] Periodontology | HGF | HGF proliferation, migration, and apoptosis | Effects on HGF Exp: ECS ± nicotine and CS | Exposure to CS and EC negatively modulates gingival fibroblast activities. |
| Aldakheel et al., 2020 Saudi Arabia [73] Periodontology | Subgingival oral biofilm sample from CS, ECS, NS | Quantity of pathogenic bacteria | Compare and quantify pathogenic bacteria from ECS, CS, NS, with and without periodontitis | Counts of periodontopathogen bacteria in the subgingival oral-biofilm are comparable among CS and ECS. |
| Alzoubi et al., 2020 Giordania [74] Oral microbioma | Nasal and oral swabs from ECS, CS, NS | Microbial profile from ECS, CS, NS | To examine the oral and nasal microbial profile and antibiotics susceptibility in the ECS, CS, NS | ECS might be less harmful to microbiota compared to CS. |
| Catala-Valentin et al., 2022 USA [75] Oral microbioma | S. sanguinis, S. gordonii, S. mutans | Bacterial growth | The effect on the growth of S. mutans, S. sanguis, S. gordonii the formation of biofilm, Exp: ECS | ECS exposure hinders S. sanguis and S. gordonii growth while enhancing biofilm formation, hydrophobicity, and attachment for S. mutans. |
| Catala-Valentin et al., 2022 USA [76] Oral microbioma | S. aureus | Bacterial growth and oral epithelial cells deregulation | S. aureus attachment to oral epithelial cells and bacterial biofilm formation Exp: ECS | ECS promote S. aureus colonization and modulate the oral inflammatory response, possibly promoting oral periodontitis and preneoplasia. |
| Chopyk et al., 2021 USA [77] Oral microbioma | Saliva, oral mucosa cells | Oral microbiome changes | Comparative analysis of the microbial community profiles Exp: ECS, NS | There are notable differences in the oral bacterial community composition and diversity in EC users as compared to the controls. |
| Cicho’nska et al., 2019 Poland [78] Other | Salivary sample from ECS, CS, NS | Chemical property of saliva | Asses if ECS have an influence on selected antibacterial properties of saliva | Saliva of ECS showed changes in antibacterial properties in comparison to the NS and CS. |
| Cicho’nska et al., 2021 Poland [79] Other | Salivary sample from ECS, CS, NS | Antioxidant capacity and nucleotide metabolites in saliva | Assess if ECS influence the antioxidant capacity of saliva | ECS affects antioxidant capacity of saliva to the same extent as CS, when comparing smokers to NS. |
| Cicho’nska et al., 2022 [80] Poland Other | Salivary sample from ECS, CS, NS | Physicochemical properties of saliva (pH, protein, calcium phosphates) | Assess the impact of ECS on selected physicochemical properties of saliva | Saliva of ECS presents changes in physicochemical composition in comparison to CS and NC; statistically significant differences were observed only in calcium concentration. |
| Cicho’nska et al., 2022 Poland [81] Oral microbioma | Buccal oral mucosa from ECS, CS, NS | Bacterial survival and growth | Observe if there were any changes in oral bacteria of ECS | ECS caused changes in oral bacteria compared to CS and NS, especially with respect to colonization of potentially pathogenic bacteria. |
| Cuadra et al., 2019 USA [82] Cariology | S. gordonii, S. intermedius, S. mitis, S. oralis | Bacterial survival and growth | Impact on survival and growth of OCS Exp: various ECs and CS aerosols | Flavorless EC aerosol (± nicotine) is less detrimental to the survival and growth of OCS than CS. |
| Fischman et al., 2020 USA [83] Cariology | S. gordonii, S. intermedius, S. mitis, S. oralis | Planktonic growth curves | Effect on the growth of OCS Exp: flavor and flavorless ECS | Flavored e-liquids are more detrimental to the growth of OCS than flavorless e-liquids. |
| Franco et al., 2016 Italy [84] Oral cancer | Cytologic exam from ECS, CS, NS | Oral cancer cytologic exam—scraping oral mucosa | Evaluate the safety of EC and to establish their role in the prevention of oral cancer | The use of ECS seems to be safe for oral cells and should be suggested as an aid to smoking cessation. |
| Ganesan et al., 2018 USA [85] Oral microbioma | Subgingival plaque | Biofilm architecture changes | Effects on the subgingival microbiome Exp: ECS | The study questions the safety of EC. |
| Guo et al., 2021 USA [86] Oral cancer | Buccal human cells | DNA damage | Evaluate the formation of apurinic/apyrimidinic (AP) sites EXP: ECS, CS, NS | Propylene glycol may inhibit bacteria in oral cells, resulting in reduced inflammation and related effects, and reduced AP site levels in ECS DNA. |
| Ji et al., 2019 USA [87] Other | Human oral keratinocytes | Gene changes | Impacts on the gene pathways of normal human oral keratinocytes Exp: ECS | EC aerosols upregulate the UPR pathway genes in human oral keratinocytes, as well as the induction of UPR response. |
| Kim et al., 2018 USA [88] Cariology | S. mutans | Microbial adhesion to enamel | Cariogenic potential Exp: EC aerosols with sweet flavors | Flavored EC products negatively affect teeth and pose a potential OH risk (similar properties of gelatinous sweets or acidic drinks). |
| Kamal et al., 2022 Egypt [89] Oral cancer | Saliva | IL, biomarkers | Evaluate the effect of vaping and cigarette smoking on IL and salivary growth factor compared to NS Exp: ECS, CS, NS | ECS have higher levels of inflammatory and cancer risk biomarkers than NS, but lower than CS. |
| Manyaga et al., 2021 USA [90] Oral cancer | Oral cancer cells | Cell viability | Effects on cisplatin resistance in head and neck cancer cells Exp: ECS | EC use might increase chemotherapy resistance. |
| Mokeem et al., 2018 Saudi Arabia [91] Oral Candida | Oral rinse from CS, ECS, waterpipe smokers, NS | Oral candida carriage from oral rinse culture | To compare oral Candida carriage among CS, ECS, waterpipe smokers, NS | Oral C. albicans carriage was significantly higher among smokers than NS. |
| Nelson et al., 2019 USA [92] Cariology | S. gordonii, S. mitis, S. oralis | Planktonic growth curves | Impact on growth of OCS Exp: ECS and CS | CS is more detrimental to the growth and biofilm formation of OCS than the use of flavorless EC aerosols or liquid ± nicotine. |
| Park et al., 2023 USA [93] Oral Microbioma | Sub-gengival plaque and saliva | Bacterial composition/diversity | Evaluate the microbiome and gingival inflammation Exp: ECS, NS | ECS can increase microbial dysbiosis that may lead to periodontal disease. |
| Rouabhia et al., 2020 Canada [94] Cariology | S. mutans | Bacterial growth and expression virulence genes | The effect on the growth of S. mutans, the formation of biofilm, and the expression of virulence genes Exp: ECS | EC increased the growth of S. mutans and the expression of virulent genes and promoted the adhesion and formation of biofilms on teeth surfaces. |
| Rouabhia et al., 2016 Canada [95] Periodontology | Human gingival ephitelial cells | Cell modification and apoptotic activity | Effects on human gingival epithelial cells Exp: ECS | Exposure to e-cigarette vapor induced cell shape modification and increased LDH activity and mediated cell activity by promoting apoptosis (caspase-3). |
| Sancilio et al., 2016 Italy [96] Periodontology | HGF | HGF and ROS production | Effects on HGF Exp: ECS | There is a role for EC fluids in the pathogenesis of oral diseases, such as periodontitis. |
| Sancilio et al., 2017 Italy [97] Periodontology | HGF | HGF citotoxicity markers | Effects on HGF Exp: EC liquids (with and without nicotine) | EC liquids (with and without nicotine) trigger molecular and morphologic responses in oral fibroblasts. |
| Schwarzmeier et al., 2021 Brasil [98] Other | Exfoliative cytology of the tongue and the mouth from ECS, CS, NS, FS | Oral cells anomalies | To investigate cytogenetic and cytotoxic damage through the evaluation of micronuclei in the oral mucosa of ECS | The use of ECS and alcohol by former smokers can cause more damage to the cells of the oral mucosa compared to those who have not used ECS. |
| Shaikh et al., 2019 UK [99] Periodontology | Human gengival mucosa | Cell morphology alterations, healing process | Effects on the proliferation of normal and cancerous monolayer of human oral mucosa and oral wound healing Exp: EC liquid after short-term and medium-term exposure | Medium-term exposure to high concentrations of the EC liquid had cytotoxic effects on normal human oral fibroblasts and keratinocytes. The exposure prolonged the wound healing of NOF and OKF6 oral mucosa cells. |
| Sundar et al., 2016 USA [100] Periodontology | Human periodontal fibroblast | ROS presence | Mechanism of gingival epithelial inflammation and pro-senescence in human oral epithelial cells and periodontal ligament fibroblasts Exp: EC aerosols with flavorings | There is a pathologic role of EC aerosol and its flavoring to cells and tissues of the oral cavity. |
| Thomas et al., 2022 USA [101] Oral Microbioma | Subgengival plaque | Bacterial composition/diversity | Evaluate the microbiome in subjects with mild periodontitis Exp: ECS, CS, NS | ECS have a unique microbiome that seems healthier than CS, but not compared with NS. |
| Tishchenko et al., 2022 Ukraine [102] Oral Microbioma | Plaque from cervical region | Bacterial growth | Evaluate the changes of dental microbiocenosis among adolescents who use devices for heating tobacco products and vaping | ECS promotes opportunistic transient streptococci, while hindering resident plaque microflora. |
| Tommasi et al., 2019 USA [103] Oral cancer | Oral ephitelium | Gene transcript deregulation | Regulation of genes and associated molecular pathways, genome-wide, in oral cells Exp: ECS, CS, NS | There is a deregulation of critically important genes and associated molecular pathways in the oral epithelium of vapers that bears both resemblances and differences with that of smokers. |
| Tsai et al., 2020 USA [104] Oral cancer | Gingival and tongue squamous cell | Cell invasion and gene expression | impact on gingival squamous cell carcinoma invasion, RAGE expression, and the elaboration of pro-inflammatory molecules. Exp: ECS with flavor and nicotine | Electronic cigarette flavoring and nicotine orchestrate the differential regulation of oral squamous cell carcinoma (OSCC) cell invasion and inflammatory effects. |
| Vermehren et al., 2020 Germany [105] Periodontology | HGF | Metabolic activity of HGF | Compare the effects on HGF in terms of proliferation, metabolic activity, cell death, and formation of ROS. Exp: ECS and CS | Exposure of HGF to ECS does not seem to be as harmful as traditional CS. |
| Willershausen et al., 2014 Germany [106] Periodontology | HF | HFs proliferation | Influence on the viability and proliferation of human periodontal ligament fibroblasts Exp: different EC liquids | The proliferation rates of the cells incubated with nicotine or the various flavored liquids were reduced in comparison to those of the untreated control cells (not all reductions were statistically significant). |
| Zhao et al., 2019 USA [107] Other | Human premolars | Tooth discoloration | Effects on the color of enamel, dentin, and composite resin restorations, as well as the effects of whitening treatments Exp: ECS, CS, red wine, coffee, and soy sauce | Tooth discoloration associated with EC aerosol is minimal. |
| Morishita et al., 2022 Japan [108] Oral cancer | Oral mucosal cells | Gene mutations | Regulation of genes and associated molecular pathways, genome-wide, in oral cells Exp: HTP, CS | Heated tobacco products and CS had similar cytotoxic effects. |
| Uehara et al., 2023 Japan [109] Oral cancer | Human gingival cells | Gene deregulation | Gene mutation in human cells Exp: heated tobacco products, non- heated tobacco products | Long-term HTP stimulation affected the epithelial differentiation and keratinization of gingival epithelial cells. |
| Pagano et al., 2021 Italy [110] Other | HGF and keratinocytes | Cells alterations/biological effects | Effect on cell viability, morphology, migration, apoptosis, and cell cycle Exp: heated tobacco products | HTP extracts increased both cell viability and migration. No morphological alterations were observed. HTP may have clinical effects on oral cell populations. |
| Marinucci et al., 2022 Italy [111] Other | HGF and keratinocytes | Cells alterations/biological effects | Effect on cell viability, morphology, migration, apoptosis, cell cycle, and epithelial–mesenchymal transition Exp: heated tobacco products, CS, and EC | CS induced significant damage, EC did not result in morphological and functional alterations in vitro, and HTP mainly modified oral cell function. |
ECS: electronic cigarette smoker/electronic cigarette smoke; CS: cigarettes smokers/cigarettes smoke; NS: non smoke aerosol; OH: oral health; OCS: oral commensal streptococci; HGF: human gingival fibroblasts; HF: human fibroblasts; ROS: reactive species oxygen; LDH: cytotoxicity markers; NOF: normal oral fibroblasts; HTP: heated tobacco products.
3. Results
3.1. Search
A total of 1104 articles were retrieved. Five additional articles were found via cross-referencing. After the removal of duplicates (n = 420), 689 papers were screened by title and abstract; the agreement between the two screeners was 95.72%, with a Cohen’s Kappa score of 0.65 (SE = 0.05; p < 0.01). Finally, 96 papers were obtained in the full-text format. After full-text reading, 12 articles were excluded, as the inclusion criteria were unmet (Figure 1). All the included studies were published between 2014 and 2023. Every effort was made to obtain original data from the authors, when needed.
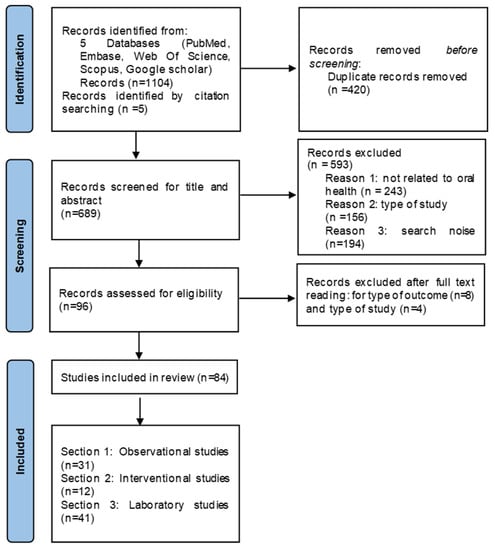
Figure 1.
Prisma Flow Chart.
3.2. Observational Studies
3.2.1. Data Synthesis
A total of 31 studies were observational (Table 1): 10 were cross-sectional surveys using questionnaires (7 were self-administered, 2 were in-person/audio computer-assisted, and 1 was an interview) [41,45,46,47,48,49,50,53,57,58,112,113]; 6 studies included a cross-sectional observation with periodontal clinical examination (mainly BOP and other periodontal indicators) [30,33,36,40,43,44]; 5 studies focused on the previously mentioned parameters, but were related to implants [28,29,32,34,46]; 5 studies included both a questionnaire and a clinical examination [38,42,56,57], and in 1 of these, the examination consisted of an oral HPV test [39]; 3 studies analyzed caries variables [43,51,54]; 1 study examined oral mucosal lesions, and the last evaluated breath odor [37].
All participants were adults (age > 18 years), except for five studies that included younger patients [39,47,48,54,114]. Age varied among the studies conducted using surveys, where children, early adolescents, young adults, middle adults, and older adults were questioned, and those using cross-section studies with clinical examinations, which were performed mainly on young adults and middle adults. Four studies included participants with systemic diseases [35,47,48,50], while all the others included only healthy participants. Additionally, in 13 studies, participants with recent dental treatments or pharmacological therapies were excluded (from 1 to 6 months before the clinical assessment).
Ten surveys included questions on socio-demographic status, self-perceived oral health and/or oral health practice [31,39,42,47,49,50,52,53,57,114]. Of these, six studies analyzed data from national surveys on smoking habits, with a sample size ranging from 4618 [51] to 456,343 subjects [45]. Another study was a national questionnaire survey about self-reported oral lesions that differentiated the type of smoking and even the differences among subjects infected by COVID-19 [48]. Different characteristics of ECS were described in two papers, which were both derived from the same questionnaire performed by the NIH in the USA between 2016 and 2018, called PATH (Population Assessment of Tobacco and Health). The first [112] focused on self-perceived oral health in the first wave of the PATH survey, while the second one [40] focused on new gingivitis cases before the third wave. A third study used the PATH survey from the 2013–2014 database and focused on adolescents’ oral health and smoking status. Another study recalled ECS from the NHANES (National Health and Nutrition Examination Survey) performed in 2015–2016 and collected oral cells for HPV testing [39]. One study from a national Japanese survey considered HTP and periodontitis using two questions: if periodontal disease is present, and if it has been treated [58]. Several studies described data regarding the “smoking session”, including duration and daily frequency. The effect of ENDS on periodontal parameters (i.e., BOP, PI, PPD, CAL) was examined in nine cross-sectional studies, while peri-implant parameters (i.e., BOP, PPD) were exanimated in five studies. From these 14 studies, 9 studies further analyzed salivary inflammatory biomarkers or receptors of crevicular fluid. The sample size ranged from 57 [61] to 160 subjects [31].
3.2.2. Risk of Bias across Studies
Most observational studies (87%) were rated with a moderate risk of bias (Supplementary File S1). The participant selection procedure was rated at a moderate risk of bias (68%) because a detailed history of smoking habits was not considered, which could have influenced the results.
3.2.3. Main Results of Included Studies
Vaping has been indicated to harm oral health, with a general decrease in self-perceived oral health status. In one study [45], the daily use of e-cigarettes was reported to be more detrimental than intermittent use. In contrast, in another study [56], its use was described as a valuable option for CS quitters, although it may be a risk factor for pain in the cheek and broken teeth [52]. Non-smokers, CS, and ECS with higher education level showed more knowledge and awareness regarding the potential negative effects of smoking on oral health [62].
Cross-sectional studies, including clinical examinations, compared ECS with CS, NS, and OS. Vaping is associated with unfavorable effects on periodontal and peri-implant parameters, causing an increase in inflammatory biomarkers/receptors, especially if compared with NS. The use of ENDS was associated with an increased expression of NF-kappa B ligand receptor activator and osteoprotegerin in the gingival crevicular fluid [69]. Both vapers and smokers exhibited unfavorable effects on oxidative stress markers and inflammatory cytokines, such as GSHPx and 8-OHdG [61], even if worse results were reported in CS [89]. Moreover, one study reported a significant association between e-cigarette use and the presence of oral HPV-16 [39].
Two studies [43,51] concluded that both CS and ECS reflect a higher occurrence of untreated caries and a higher caries risk than do non-ECS [54]. In one study, statistically significant differences were found in oral mucosal lesion prevalence comparing ECS and FS [35], and in another study, ECS showed a 1.5 higher odds of reporting oral lesions than NS [48].
Female subjects accounted for 51% of the sample of survey-based cross-sectional studies, 17% of surveys that included a clinical evaluation, and only 7% of cross-sectional studies based on clinical evaluation.
3.2.4. Meta-Analysis
Meta-analyses of six studies [31,41,46,49,57,114] on self-perceived gingivitis were performed (Figure 2). The total number of subjects considered was 562.837. High heterogeneity was observed (T2 = 9.47 I2 = 99.32%), and three papers described an association between self-reported gingivitis and ENDS use.
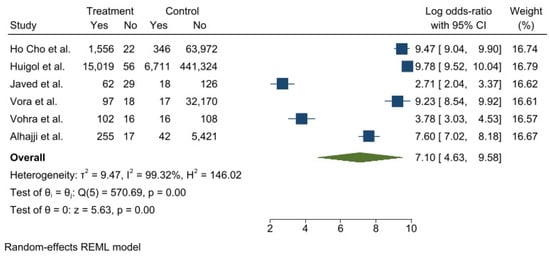
Figure 2.
Forest plot of the included observational studies reporting self-perceived gingivitis (ECS = treatment, non-ECS = control) [31,41,46,49,57,114].
BOP was registered in three studies [40,41,57] including a total of 319 subjects; bleeding on probing was associated with ENDS (Figure 3), with a gain in high heterogeneity of T2 = 8.68 I2 = 99.13%.
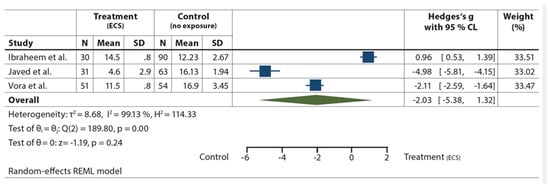
Figure 3.
Forest plot of the included observational studies on BOP (ECS = treatment, Non-ECS = control) [40,41,57].
3.3. Interventional Studies
3.3.1. Data Synthesis
Twelve studies were included in this category [59,60,62,63,65,66,67,68,70]. All subjects enrolled were adults; sample sizes ranged from 10 to 172 (Table 2). The results of the age categories were as follows: 54% of the studies were conducted on young adults, 9% on average adults and young adults, 28% on both young and average adults, and finally, 9% included all age categories.
Five studies evaluated the effects of ENDS on periodontal parameters, investigating inflammatory markers [59,60,63,64,65]. Seven studies re-evaluated the periodontal or peri-implant parameters after a specific treatment [60,63,64,66,68,69,100]. One study [67] assessed the blood flow after vaping using a Doppler laser. The last study evaluated periodontal parameters in subjects that switched or did not switch from cigarettes to HTP [70]. Of the 772 subjects (the sum of participants in all these studies), 83% were male. In one study, including 20 subjects, the participant’s sex was not specified [105].
3.3.2. Risk of Bias across Studies
Four studies [66,68,70,100] were RCTs and were rated with an overall “some concern” risk of bias, according to the ROB-2 tool (Supplementary File S1). The remaining eight studies [59,60,63,64,65,67,68] were assessed using ROBINS-I and evaluated with a moderate bias risk (Supplementary File S1).
3.3.3. Main Results of Included Studies
A worsening of the periodontal condition was observed, mainly in CS compared to ECS and NS. However, one study found that the severity of periodontal disease was significantly greater after 6 months of using both CS and ECS compared to NS [106]. In a sample of 20 former CS who switched to electronic cigarettes, the percentage of sites with BOP increased statistically after 2 weeks [105]. In the studies comparing different parameters after periodontal treatments, the results were as follows: scaling and root planning produced an anti-inflammatory effect more pronounced in NS than in ECS and CS [61]; photodynamic treatment is as effective as non-surgical therapy in the management of periodontal inflammation in ECS [66]; another study [68], which evaluated peri-implant sites, affirmed that photodynamic therapy with adjunctive mechanical debridement reduced PI and PPD, but an increase in BOP was still observed in both ECS and CS; ECS was considered a risk indicator for peri-implantitis [64]; finally, an antimicrobial treatment added to photodynamic therapy was more effective in ECS with peri-implantitis compared to the photodynamic therapy alone [100]. One study [70] compared switchers and non-switchers from CS to HTP using a multicenter design, and all the participants received a scaling and root planning treatment that had a positive effect on periodontal health, but possibly obscured the beneficial effect of quitting CS.
Due to the heterogeneity of the studies, a meta-analysis could not be performed.
3.4. Laboratory Studies
3.4.1. Data Synthesis
Of the 41 included studies (Table 3), 8 evaluated the effect of ENDS on commensal bacteria growth or Streptococcus mutans adhesion to enamel [75,76,82,83,88,92,94,102]; 3 evaluated bacterial composition/diversity [87,95,97], 9 considered metabolic or morphological changes of gingival fibroblasts or gingival endothelial cells after ENDS exposure [71,87,88,95,96,97,99,100,105]; 6 studied the effect of ENDS on oral cancer cells or gene deregulation in oral epithelial cells [84,86,90,103,104]; and 11 evaluated the effects of vaping on heterogeneous outcomes: C. albicans growth, microbial community changes, gene pathways of normal human oral keratinocytes, and whitening effect on extracted teeth [44,71,73,78,79,80,85,98,103,104,107]. Four studies [108,109,110,111] evaluated the effect of HTP on human cells for biological and genetical alterations.
3.4.2. Risk of Bias across Studies
All the studies resulted in an overall “probably low” risk of bias.
3.4.3. Main Results of the Included Studies
Articles regarding the effects of ENDS on cariogenic bacteria [82,92,95] concluded that flavored liquids from e-cigarette are detrimental to oral health, and an effect on enamel similar to that of gelatinous sweets or acidic drinks has been speculated [72]. Studies concerning periodontal issues have considered human gingival fibroblasts, oral mucosa cells, or periodontium cells exposed to aerosols derived from electronic or conventional cigarettes, using unexposed cells as the control. ENDS with a longer exposure time and higher nicotine concentration induced a harmful modulation of cellular activities and promoted the expression of apoptotic and cytotoxic pathways. Exposure to e-vapors is not as harmful as exposure to cigarette smoking [44]. Studies investigating ENDS and oral cancer have been conducted on oral epithelial cells and concluded the following: ENDS increases the resistance to chemotherapy [90]; the use of e-cigarettes seems to be safe for oral cells and should be suggested as an aid to smoking cessation [84]; ENDS-exposed cells exhibit deregulation of critically important genes that could enhance cell invasion and inflammatory effects [74,85,89]; propylene glycol contained in EC liquid could inhibit bacterial-induced inflammation in the oral cavity and mask the reduced formation of apurinic/apyrimidinic (AP) sites, indicating DNA damage [86].
Similarly, two studies on HTP confirmed gene modifications when oral cells were exposed to HTP [108,109]. When compared to ECS and CS, HTP seems to modify oral cell function [111]. With respect to CS exposure, HTP was not associated with the apoptotic pathway, although clinical effects on oral cells could not be excluded [110]. Two studies analyzed the effects of vaping on oral candida, concluding that C. albicans carriage would be higher in ECS and CS than in NS [77,114]. No firm conclusions could be reached regarding oral microbial changes after vaping or smoking exposure: one study questioned the proper safety of electronic cigarettes [115], and another concluded that ENDS might be less harmful to the oral microbiota than conventional cigarettes because they did not reduce the carriage of methicillin-susceptible Staphylococcus aureus [32]; a similar conclusion was drawn from another study that affirmed that S. aureus is promoted by EC vapors, enhancing periodontitis [76]. One study [116] stated that there are significant differences in the composition and diversity of the oral bacterial community in ECS and NS, with a significant increase in Veillonella and Haemophilus species in ECS, while another study pointed out that the microbiome of ECS with mild periodontitis seems healthier, but not compared to NS [101]; the possible dysbiosis of ECS may lead to periodontal disease [93]. Two other studies on oral bacterial growth in ECS stated that steam promotes streptococci in the oral biofilm [75,102].
3.4.4. Meta-Analysis
The meta-analysis was performed by combining data from four studies reporting the apoptosis rate of human gingival fibroblast after 24 h of exposure to e-cigarette vapor or air (i.e., no exposure), as shown in Figure 4 [72,95,97,105]. The number of independent repetitions under the same conditions was considered as a sample size. A high heterogeneity was observed (T2 = 8.10 I2 = 91.50%).
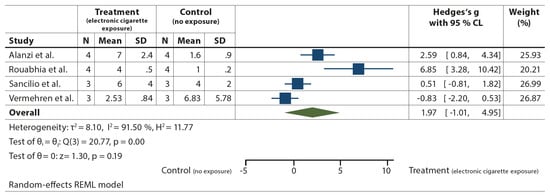
Figure 4.
Apoptosis of human gingival fibroblasts after 24 h exposure to EC versus no exposure [72,95,97,105].
4. Discussion
The present review explored vaping and heat-related products concerning oral health, investigating the comprehensive effects of ENDS and HTP on different clinical and cellular variables and offering an overview of the current literature through observational, interventional, and laboratory studies. Other recent reviews [42,116,117] have focused only on periodontal parameters and revealed data consistent with those reported in the present study, underlying that the results must be considered with caution because of the heterogeneity of the articles and the scarcity of RCTs.
The cross-sectional studies based on surveys highlighted that the responders often need to be aware of the potentially harmful effects of alternative tobacco products [36,42,45,59]. This outcome underlines need for dental professionals to provide complete smoking counseling, which should become a routine practice. Nevertheless, these surveys were judged as having a high-moderate risk of bias. While the national surveys could investigate the younger population, the same cannot be said for clinical examinations, in which mainly adults were evaluated. In regards to waterpipe (the term includes narghile, hookah, shisha, and hubble-bubble) smokers, similar detrimental effects on oral health as to those noted for ENDS were found [31]. This last category seems quite challenging to study, as waterpipes are often used in social contexts, and daily use is rare, even if a substantial use, especially in Saudia Arabia, is reported [31]. Dual smokers and former smokers were studied only in observational studies, but no clear conclusions could be made regarding these categories, as the definition of how long a subject is considered a dual smoker or how much time should pass before being labeled as a former smoker are not uniform among studies. Few studies have been found dealing with HTP and oral health; this could have a country-specific explanation, as HTP is more widespread in Japan and the USA, but less so in Europe, where its use has recently increased [116].
Another finding of the review is that there is ahigh variability observed in laboratory studies: different results, cell lines, bacterial strains, and oral cancer cells were considered. In any case, most of the cell line studies focused on human gingival fibroblasts, showing that e-liquids increased apoptosis and the appearance of necrosis biomarkers compared with those in unexposed cells [95,96,103]. Studies based on bacterial strains focused on commensal oral streptococci such as S. mutans [75,94]. It has been found that flavored e-liquids are often sweeter and stickier than unflavored ones, promoting bacterial adhesion and reducing normal commensal flora, with dysbiosis of the oral microbiome [82,83,94]. Regarding studies on oral cancer cells [108], conclusive hypotheses could not be drawn, since some papers suggested that ENDS and HTP might be possible alternatives to cigarette smoking. In contrast, other studies concluded that its effects are similar to those of conventional smoking [109,110,111].
The risk of bias assessment resulted in low risk. At the same time, the meta-analysis, although conducted on only a few studies, confirmed that electronic cigarette smoking may have a detrimental role for oral commensal bacteria.
A major limitation of this systematic review is the high heterogeneity among the studies, reflecting the lack of standardized study designs. Most studies compare ENDS or HTP with traditional cigarettes to determine whether vaping is safer/less harmful for oral health than is cigarette smoking [30,32,33,40,41,57,63]. However, the comparisons are often simplistic and the conclusions uncertain, as the content of e-liquids (nicotine percentage, flavorings, etc.) is profoundly different from that of conventional cigarettes. Moreover, the time of use of ENDS is very different from that of cigarettes, making it challenging to compare with the precise definitions of heavy and light smokers established for conventional cigarette smokers. A further limitation is the sex of subjects enrolled in the available studies. The surveys included samples of both sexes, but most of the enrolled e-cigarette users were female. In contrast, the studies that included clinical assessments and interventional studies were conducted almost entirely on males [59,60,61,62,65]. The question then arises regarding how the sex variable might have influenced the results and how the results obtained on male subjects may be extended to young women, who represent the majority of e-cigarette users in Western countries. In addition, many clinical studies performed on men were conducted in Saudi Arabia [31,33,48,49,50,59,60,63,64,66,89], where other smoking habits, such as the use of shisha and water pipes, are common and may have affected the results. Finally, the appearance of ENDS in the market has increased the number of dual smokers, which is a confounding factor that should be evaluated.
To overcome the above limitations and draw conclusions about the role of ENDS in oral health, further studies using standardized methodologies and taking into account all the specific details related to ENDS, such as the type of electronic cigarette, e-liquid composition, and time of use, are needed.
The main strength of this review is that it is the first, to the knowledge of the authors, to provide a broad overview of the effects of different e-cigarettes on oral health, including both clinical and self-assessment health studies, as well as in vitro studies, providing the reader with a complete picture of current knowledge.
5. Conclusions
ENDS vaping is a relatively recently introduced activity, and current investigations cannot provide sufficient evidence to confirm its effect on oral health; in fact, the findings from this review can only offer hypotheses on the harms of ENDS use. The self-perceived appearance of gingivitis and BOP noted by e-smokers cannot provide conclusive findings of ENDS use. In vitro studies show that electronic cigarettes containing nicotine appear to promote detrimental cellular pathways in human gingival fibroblast. Higher nicotine percentages and flavored e-liquids seem to have a detrimental effect on periodontal and peri-implant tissues through pathways similar to those of conventional cigarette smoke; these e-liquids may additionally represent a caries risk factor.
As a consequence of these findings, comprehensive vaping counseling should be provided to all smoking patients, investigating the type of habit in terms of duration, nicotine percentage, and additional flavorings accessed. Particular attention should be paid to dual smokers.
In conclusion, both ENDS and HTP have a potential detrimental effect on periodontal and peri-implant parameters, and laboratory tests confirmed the presence of carcinogenic and inflammatory biomarkers. Flavored e-liquids may also be a caries risk factor. Research is necessary to assess the long-term effects of alternative tobacco products on oral health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13179654/s1, Supplementary Files for “Electronic Cigarettes: A Systematic Review for Dental Practitioners”.
Author Contributions
Conceptualization, N.C. And G.C. (Guglielmo Campus).; methodology, G.C. (Guglielmo Campus); software, A.R.; validation, M.G.C. and T.S.C.; formal analysis, N.C., M.E.-O. and M.G.C.; investigation, A.R.; resources, G.C. (Guglielmo Campus); data curation, N.C.; writing—original draft preparation, N.C. and. M.E.-O.; writing—review and editing, N.C.; visualization, G.C. (Guglielmo Campus); supervision, N.C.; project administration, G.C. (Giulio Conti); funding acquisition, G.C. (Giulio Conti). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Acronyms | Terms |
| ENDS | electronic nicotine delivery systems |
| ECS | electronic cigarette smokers |
| HTP | heated tobacco products |
| CS | cigarettes smokers |
| NS | non-smokers |
| DS | dual smokers |
| OS | other smokers (waterpipe, shisha, etc.) |
| FS | former smokers |
References
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L585–L595. [Google Scholar] [CrossRef]
- Orellana-Barrios, M.A.; Payne, D.; Mulkey, Z.; Nugent, K. Electronic Cigarettes-A Narrative Review for Clinicians. Am. J. Med. 2015, 128, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.D.; Rice, T.R. Youth vaping: A review and update on global epidemiology, physical and behavioral health risks, and clinical considerations. Eur. J. Pediatr. 2021, 181, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ralho, A.; Coelho, A.; Ribeiro, M.; Paula, A.; Amaro, I.; Sousa, J.; Marto, C.; Ferreira, M.; Carrilho, E. Effects of Electronic Cigarettes on Oral Cavity: A Systematic Review. J. Evid. Based Dent. Pract. 2019, 19, 101318. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef]
- Holliday, R.; Chaffee, B.W.; Jakubovics, N.S.; Kist, R.; Preshaw, P.M. Electronic Cigarettes and Oral Health. J. Dent. Res. 2021, 100, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qiu, Z.; Chen, J.; Shang, C. Synthetic nicotine e-liquids sold in US online vape shops. Prev. Med. Rep. 2023, 33, 102222. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Health Effects of Trace Metals in Electronic Cigarette Aerosols—A Systematic Review. Biol. Trace Elem. Res. 2019, 188, 295–315. [Google Scholar] [CrossRef]
- Sood, A.K.; Kesic, M.J.; Hernandez, M.L. Electronic cigarettes: One size does not fit all. J. Allergy Clin. Immunol. 2018, 141, 1973–1982. [Google Scholar] [CrossRef]
- Arora, N.; Dreze, X.; Ghose, A.; Hess, J.D.; Iyengar, R.; Jing, B.; Joshi, Y.; Kumar, V.; Lurie, N.; Neslin, S.; et al. Putting one-to-one marketing to work: Personalization, customization, and choice. Mark. Lett. 2008, 19, 305–321. [Google Scholar] [CrossRef]
- Amato, L.; Cruciani, F.; Solimini, R.; Barca, A.; Pacifici, R.; Davoli, M. Effects of electronic cigarettes on health: A systematic review of the available evidence. Recenti Prog. Med. 2020, 111, 30–43. [Google Scholar] [CrossRef]
- Tattan-Birch, H.; Hartmann-Boyce, J.; Kock, L.; Simonavicius, E.; Brose, L.; Jackson, S.; Shahab, L.; Brown, J. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database Syst. Rev. 2022, 1, CD013790. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Gutierrez, O.A.; Falfan-Valencia, R.; Ramirez-Venegas, A.; Sansores, R.H.; Ponciano-Rodriguez, G.; Perez-Rubio, G. Lung Damage Caused by Heated Tobacco Products and Electronic Nicotine Delivery Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4079. [Google Scholar] [CrossRef] [PubMed]
- Znyk, M.; Jurewicz, J.; Kaleta, D. Exposure to Heated Tobacco Products and Adverse Health Effects, a Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6651. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Kanai, O.; Tabuchi, T.; Mio, T. Association of heated tobacco product use with tobacco use cessation in a Japanese workplace: A prospective study. Thorax 2021, 76, 615–617. [Google Scholar] [CrossRef] [PubMed]
- WHO; FDI. Tobacco or Oral Health An Advocacy Guide for Oral Health Professionals; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Figueredo, C.A.; Abdelhay, N.; Figueredo, C.M.; Catunda, R.; Gibson, M.P. The impact of vaping on periodontitis: A systematic review. Clin. Exp. Dent. Res. 2021, 7, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Li, J.; Cochran, D.L. Inflammation and uncoupling as mechanisms of periodontal bone loss. J. Dent. Res. 2011, 90, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Baltacioglu, E.; Akalin, F.A.; Alver, A.; Deger, O.; Karabulut, E. Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Arch. Oral Biol. 2008, 53, 716–722. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Lindemann, E.A.; Chen, E.S.; Wang, Y.; Skube, S.J.; Melton, G.B. Representation of Social History Factors Across Age Groups: A Topic Analysis of Free-Text Social Documentation. AMIA Annu. Symp. Proc. 2017, 2017, 1169–1178. [Google Scholar] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Rooney, A.A.; Boyles, A.L.; Wolfe, M.S.; Bucher, J.R.; Thayer, K.A. Systematic review and evidence integration for literature-based environmental health science assessments. Environ. Health Perspect. 2014, 122, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Al-Aali, K.A.; Alrabiah, M.; ArRejaie, A.S.; Abduljabbar, T.; Vohra, F.; Akram, Z. Peri-implant parameters, tumor necrosis factor-alpha, and interleukin-1 beta levels in vaping individuals. Clin. Implant. Dent. Relat. Res. 2018, 20, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Alazmi, S.O.; Almutairi, F.J.; Alresheedi, B.A. Comparison of Peri-Implant Clinicoradiographic Parameters among Non-Smokers and Individuals Using Electronic Nicotine Delivery Systems at 8 Years of Follow-up. Oral Health Prev. Dent. 2021, 19, 511–516. [Google Scholar] [CrossRef]
- Ali, D.; Kuyunov, I.; Baskaradoss, J.K.; Mikami, T. Comparison of periodontal status and salivary IL-15 and -18 levels in cigarette-smokers and individuals using electronic nicotine delivery systems. BMC Oral Health 2022, 22, 655. [Google Scholar] [CrossRef]
- AlQahtani, M.A.; Alayad, A.S.; Alshihri, A.; Correa, F.O.B.; Akram, Z. Clinical peri-implant parameters and inflammatory cytokine profile among smokers of cigarette, e-cigarette, and waterpipe. Clin. Implant. Dent. Relat. Res. 2018, 20, 1016–1021. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alqahtani, M.; Albaqawi, A.H.; Al-Kheraif, A.A.; Javed, F. Comparison of cotinine levels in the peri-implant sulcular fluid among cigarette and waterpipe smokers, electronic-cigarette users, and nonsmokers. Clin. Implant. Dent. Relat. Res. 2019, 21, 702–707. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Alqhtani, N.R.; Gufran, K.; Alsakr, A.M.; Alshehri, A.; Binaljadm, T.M.; Alzamil, F.F.; Alqwiri, A.S.; Alotaibi, N.M.; Harun, H.M.W. Comparative assessment of periodontal treatment needs among the electronic cigarette users and traditional smokers. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2676–2682. [Google Scholar] [CrossRef]
- ArRejaie, A.S.; Al-Aali, K.A.; Alrabiah, M.; Vohra, F.; Mokeem, S.A.; Basunbul, G.; Alrahlah, A.; Abduljabbar, T. Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers. J. Periodontol. 2019, 90, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Bardellini, E.; Amadori, F.; Conti, G.; Majorana, A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol. Scand. 2018, 76, 226–228. [Google Scholar] [CrossRef]
- BinShabaib, M.; Alharthi, S.S.; Akram, Z.; Khan, J.; Rahman, I.; Romanos, G.E.; Javed, F. Clinical periodontal status and gingival crevicular fluid cytokine profile among cigarette-smokers, electronic-cigarette users and never-smokers. Arch. Oral Biol. 2019, 102, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, A.; Coburn, S.; Brandt, M.; Hardie, G.; Murphy, J. Pilot study to determine differences in breath odour between cigarette and e-cigarette consumers. Sci. Rep. 2022, 12, 2204. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, A.I.A.; Ismail, A.F.; Daud, A. Caries Experience among Cigarette and E-Cigarette Users: A 6-Month Prospective Study. J. Pharm. Sci. Res. 2019, 11, 2566–2569. [Google Scholar]
- Herndon, P.; Jassal, J.S.; Cramer, J.D. Association between E-cigarette use and oral HPV-16 infection. Oral Oncol. 2022, 125, 105676. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, W.I.; Fageeh, H.I.; Preethanath, R.S.; Alzahrani, F.A.; Al-Zawawi, A.S.; Divakar, D.D.; Al-Kheraif, A.A. Comparison of RANKL and osteoprotegerin levels in the gingival crevicular fluid of young cigarette- and waterpipe-smokers and individuals using electronic nicotine delivery systems. Arch. Oral Biol. 2020, 115, 104714. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Rahman, I.; Romanos, G.E. Comparison of Periodontal Parameters and Self-Perceived Oral Symptoms Among Cigarette Smokers, Individuals Vaping Electronic Cigarettes, and Never-Smokers. J. Periodontol. 2017, 88, 1059–1065. [Google Scholar] [CrossRef]
- Jeong, W.; Choi, D.W.; Kim, Y.K.; Lee, H.J.; Lee, S.A.; Park, E.C.; Jang, S.I. Associations of electronic and conventional cigarette use with periodontal disease in South Korean adults. J. Periodontol. 2020, 91, 55–64. [Google Scholar] [CrossRef]
- Karaaslan, F.; Dikilitaş, A.; Yiğit, U. The effects of vaping electronic cigarettes on periodontitis. Aust. Dent. J. 2020, 65, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, S.A.; Alasqah, M.N.; Michelogiannakis, D.; Al-Kheraif, A.A.; Romanos, G.E.; Javed, F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette- and waterpipe-smokers and E-cig users. Environ. Toxicol. Pharmacol. 2018, 61, 38–43. [Google Scholar] [CrossRef]
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine 2016, 95, e5589. Available online: https://journals.lww.com/00005792-201612060-201600074 (accessed on 17 August 2023). [CrossRef] [PubMed]
- Vohra, F.; Bukhari, I.A.; Sheikh, S.A.; Albaijan, R.; Naseem, M. Comparison of self-rated oral symptoms and periodontal status among cigarette smokers and individuals using electronic nicotine delivery systems. J. Am. Coll. Health 2020, 68, 788–793. [Google Scholar] [CrossRef]
- Akinkugbe, A.A. Cigarettes, E-cigarettes, and Adolescents’ Oral Health: Findings from the Population Assessment of Tobacco and Health (PATH) Study. JDR Clin. Trans. Res. 2019, 4, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Alade, O.; Folayan, M.O.; Adeniyi, A.; Adeyemo, Y.I.; Oyapero, A.; Olatosi, O.O.; Nzomiwu, C.; Popoola, B.O.; Eigbobo, J.; Oziegbe, E.; et al. Differences in Oral Lesions Associated with Tobacco Smoking, E-Cigarette Use and COVID-19 Infection among Adolescents and Young People in Nigeria. Int. J. Environ. Res. Public Health 2022, 19, 10509. [Google Scholar] [CrossRef]
- Alhajj, M.N.; Al-Maweri, S.A.; Folayan, M.O.; Halboub, E.; Khader, Y.; Omar, R.; Amran, A.G.; Al-Batayneh, O.B.; Celebić, A.; Persic, S.; et al. Oral health practices and self-reported adverse effects of E-cigarette use among dental students in 11 countries: An online survey. BMC Oral Health 2022, 22, 18. [Google Scholar] [CrossRef]
- AlQobaly, L.; Abed, H.; Alsahafi, Y.; Sabbah, W.; Hakeem, F.F. Does smoking explain the association between use of e-cigarettes and self-reported periodontal disease? J. Dent. 2022, 122, 104164. [Google Scholar] [CrossRef]
- Atuegwu, N.; Perez, M.; Oncken, C.; Thacker, S.; Mead, E.; Mortensen, E. Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey. Int. J. Environ. Res. Public Health 2019, 16, 1263. [Google Scholar] [CrossRef]
- Cho, J.H. The association between electronic-cigarette use and self-reported oral symptoms including cracked or broken teeth and tongue and/or inside-cheek pain among adolescents: A cross-sectional study. PLoS ONE 2017, 12, e0180506. [Google Scholar] [CrossRef] [PubMed]
- Huilgol, P.; Bhatt, S.P.; Biligowda, N.; Wright, N.C.; Wells, J.M. Association of e-cigarette use with oral health: A population-based cross-sectional questionnaire study. J. Public Health 2019, 41, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Irusa, K.F.; Finkelman, M.; Magnuson, B.; Donovan, T.; Eisen, S.E. A comparison of the caries risk between patients who use vapes or electronic cigarettes and those who do not: A cross-sectional study. J. Am. Dent. Assoc. 2022, 153, 1179–1183. [Google Scholar] [CrossRef]
- Abafalvi, L.; Penzes, M.; Urban, R.; Foley, K.L.; Kaan, R.; Kispelyi, B.; Hermann, P. Perceived health effects of vaping among Hungarian adult e-cigarette-only and dual users: A cross-sectional internet survey. BMC Public Health 2019, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Vemulapalli, A.; Mandapati, S.R.; Kotha, A.; Aryal, S. Association between vaping and untreated caries. J. Am. Dent. Assoc. 2021, 152, 720–729. [Google Scholar] [CrossRef]
- Vora, M.V.; Chaffee, B.W. Tobacco-use patterns and self-reported oral health outcomes. J. Am. Dent. Assoc. 2019, 150, 332–344.e2. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Tabuchi, T. Combustible cigarettes, heated tobacco products, combined product use, and periodontal disease: A cross-sectional JASTIS study. PLoS ONE 2021, 16, e0248989. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Aati, S.; Alrahlah, A.; Vohra, F.; Fawzy, A. Longitudinal evaluation of clinical, spectral and tissue degradation biomarkers in progression of periodontitis among cigarette and electronic cigarette smokers. J. Dent. 2021, 109, 103678. [Google Scholar] [CrossRef]
- Al Deeb, M.; Alresayes, S.; A Mokeem, S.; Alhenaki, A.M.; AlHelal, A.; Shafqat, S.S.; Vohra, F.; Abduljabbar, T. Clinical and immunological peri-implant parameters among cigarette and electronic smoking patients treated with photochemotherapy: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 31, 101800. [Google Scholar] [CrossRef]
- Al-Hamoudi, N.; Alsahhaf, A.; Al Deeb, M.; Alrabiah, M.; Vohra, F.; Abduljabbar, T. Effect of scaling and root planing on the expression of anti-inflammatory cytokines (IL-4, IL-9, IL-10, and IL-13) in the gingival crevicular fluid of electronic cigarette users and non-smokers with moderate chronic periodontitis. J. Periodontal Implant. Sci. 2020, 50, 74. [Google Scholar] [CrossRef]
- Alharthi, S.S.; BinShabaib, M.; Akram, Z.; Rahman, I.; Romanos, G.E.; Javed, F. Impact of cigarette smoking and vaping on the outcome of full-mouth ultrasonic scaling among patients with gingival inflammation: A prospective study. Clin. Oral Investig. 2019, 23, 2751–2758. [Google Scholar] [CrossRef]
- Alhumaidan, A.A.; Al-Aali, K.A.; Vohra, F.; Javed, F.; Abduljabbar, T. Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. Int. J. Environ. Res. Public Health 2022, 19, 11290. [Google Scholar] [CrossRef] [PubMed]
- AlJasser, R.; Zahid, M.; AlSarhan, M.; AlOtaibi, D.; AlOraini, S. The effect of conventional versus electronic cigarette use on treatment outcomes of peri-implant disease. BMC Oral Health 2021, 21, 480. [Google Scholar] [CrossRef]
- Al Rifaiy, M.Q.; Qutub, O.A.; Alasqah, M.N.; Al-Sowygh, Z.H.; Mokeem, S.A.; Alrahlah, A. Effectiveness of adjunctive antimicrobial photodynamic therapy in reducing peri-implant inflammatory response in individuals vaping electronic cigarettes: A randomized controlled clinical trial. Photodiagn. Photodyn. Ther. 2018, 22, 132–136. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1572100018300711 (accessed on 17 August 2023). [CrossRef]
- Alshibani, N.; Alssum, L.; Basudan, A.; Shaheen, M.; Alqutub, M.N.; Dahash, F.A.; Alkattan, R. Non-surgical periodontal therapy with adjunct photodynamic therapy for the management of periodontal inflammation in adults using nicotine-free electronic-cigarette: A randomized control trial. Photodiagn. Photodyn. Ther. 2022, 38, 102820. [Google Scholar] [CrossRef]
- Reuther, W.J.; Hale, B.; Matharu, J.; Blythe, J.N.; Brennan, P.A. Do you mind if I vape? Immediate effects of electronic cigarettes on perfusion in buccal mucosal tissue—A pilot study. Br. J. Oral Maxillofac. Surg. 2016, 54, 338–341. [Google Scholar] [CrossRef]
- Wadia, R.; Booth, V.; Yap, H.F.; Moyes, D.L. A pilot study of the gingival response when smokers switch from smoking to vaping. Br. Dent. J. 2016, 221, 722–726. [Google Scholar] [CrossRef]
- Xu, F.; Pushalkar, S.; Lin, Z.; Thomas, S.C.; Persaud, J.K.; Sierra, M.A.; Vardhan, M.; Vasconcelos, R.; Akapo, A.; Guo, Y.; et al. Electronic cigarette use enriches periodontal pathogens. Mol. Oral Microbiol. 2022, 37, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Pouly, S.; Ng, W.T.; Blanc, N.; Hession, P.; Zanetti, F.; Battey, J.N.D.; de La Bourdonnaye, G.; Heremans, A.; Haziza, C. Effect of switching from cigarette smoking to the use of the tobacco heating system on periodontitis treatment outcome: Periodontal parameter results from a multicenter Japanese study. Front. Dent. Med. 2022, 3, 915079. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-Cigarettes Increase Candida albicans Growth and Modulate its Interaction with Gingival Epithelial Cells. Int. J. Environ. Res. Public Health 2019, 16, 294. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Alduraywish, S.A.; Jhugroo, P.; Jhugroo, C.; Divakar, D.D. Quantification of pathogenic bacteria in the subgingival oral biofilm samples collected from cigarette-smokers, individuals using electronic nicotine delivery systems and non-smokers with and without periodontitis. Arch. Oral Biol. 2020, 117, 104793. [Google Scholar] [CrossRef]
- Alzoubi, H.; Abu-Lubad, M.; Al-Mnayyis, A.a.; Satari, A.; Alzobi, M.; Ramadneh, M.A.; Jarajreh, D.a. Effect of Electronic Cigarettes on the Carriage of Selected Organisms in the Nasal and Oral Cavity in Comparison to Tobacco Smokers and Non-smokers. J. Clin. Diagn. Res. 2020, 14, DC11–DC15. Available online: https://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2020&volume=14&issue=7&page=DC11&issn=0973-709x&id=13852 (accessed on 17 August 2023). [CrossRef]
- Catala-Valentin, A.; Bernard, J.N.; Caldwell, M.; Maxson, J.; Moore, S.D.; Andl, C.D. E-Cigarette Aerosol Exposure Favors the Growth and Colonization of Oral Streptococcus mutans Compared to Commensal Streptococci. Microbiol. Spectr. 2022, 10, e0242121. [Google Scholar] [CrossRef]
- Cátala-Valentín, A.R.; Almeda, J.; Bernard, J.N.; Cole, A.M.; Cole, A.L.; Moore, S.D.; Andl, C.D. E-Cigarette Aerosols Promote Oral S. aureus Colonization by Delaying an Immune Response and Bacterial Clearing. Cells 2022, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, J.; Bojanowski, C.M.; Shin, J.; Moshensky, A.; Fuentes, A.L.; Bonde, S.S.; Chuki, D.; Pride, D.T.; Crotty Alexander, L.E. Compositional Differences in the Oral Microbiome of E-cigarette Users. Front. Microbiol. 2021, 12, 599664. [Google Scholar] [CrossRef]
- Cichonska, D.; Kusiak, A.; Kochanska, B.; Ochocinska, J.; Swietlik, D. Influence of Electronic Cigarettes on Selected Antibacterial Properties of Saliva. Int. J. Environ. Res. Public Health 2019, 16, 4433. [Google Scholar] [CrossRef]
- Cichonska, D.; Krol, O.; Slominska, E.M.; Kochanska, B.; Swietlik, D.; Ochocinska, J.; Kusiak, A. Influence of Electronic Cigarettes on Antioxidant Capacity and Nucleotide Metabolites in Saliva. Toxics 2021, 9, 263. [Google Scholar] [CrossRef]
- Cichonska, D.; Kusiak, A.; Kochanska, B.; Ochocinska, J.; Swietlik, D. Influence of Electronic Cigarettes on Selected Physicochemical Properties of Saliva. Int. J. Environ. Res. Public Health 2022, 19, 3314. [Google Scholar] [CrossRef] [PubMed]
- Cichonska, D.; Kusiak, A.; Piechowicz, L.; Swietlik, D. A pilot investigation into the influence of electronic cigarettes on oral bacteria. Postepy Dermatol. Alergol. 2021, 38, 1092–1098. [Google Scholar] [CrossRef]
- Cuadra, G.A.; Smith, M.T.; Nelson, J.M.; Loh, E.K.; Palazzolo, D.L. A Comparison of Flavorless Electronic Cigarette-Generated Aerosol and Conventional Cigarette Smoke on the Survival and Growth of Common Oral Commensal Streptococci. Int. J. Environ. Res. Public Health 2019, 16, 1669. [Google Scholar] [CrossRef] [PubMed]
- Fischman, J.S.; Sista, S.; Lee, D.; Cuadra, G.A.; Palazzolo, D.L. Flavorless vs. Flavored Electronic Cigarette-Generated Aerosol and E-Liquid on the Growth of Common Oral Commensal Streptococci. Front. Physiol. 2020, 11, 585416. [Google Scholar] [CrossRef]
- Franco, T.; Trapasso, S.; Puzzo, L.; Allegra, E. Electronic Cigarette: Role in the Primary Prevention of Oral Cavity Cancer. Clin. Med. Insights Ear Nose Throat 2016, 9, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.M.; Dabdoub, S.M.; Nagaraja, H.N.; Scott, M.L.; Pamulapati, S.; Berman, M.L.; Shields, P.G.; Wewers, M.E.; Kumar, P.S. Adverse effects of electronic cigarettes on the disease-naive oral microbiome. Sci. Adv. 2020, 6, eaaz0108. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hecht, S.S. DNA damage in human oral cells induced by use of e-cigarettes. Drug Test. Anal. 2022, in press. [Google Scholar] [CrossRef]
- Ji, E.H.; Elzakra, N.; Chen, W.; Cui, L.; Lee, E.S.; Sun, B.; Messadi, D.; Xia, T.; Zhu, Y.; Hu, S. E-cigarette aerosols induce unfolded protein response in normal human oral keratinocytes. J. Cancer 2019, 10, 6915–6924. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Smith, S.; Beauchamp, C.; Song, Y.; Chiang, M.; Giuseppetti, A.; Frukhtbeyn, S.; Shaffer, I.; Wilhide, J.; Routkevitch, D.; et al. Cariogenic potential of sweet flavors in electronic-cigarette liquids. PLoS ONE 2018, 13, e0203717. [Google Scholar] [CrossRef]
- Kamal, N.M.; Shams, N.S. The impact of tobacco smoking and electronic cigarette vaping on salivary biomarkers. A comparative study. Saudi Dent. J. 2022, 34, 404–409. [Google Scholar] [CrossRef]
- Manyanga, J.; Ganapathy, V.; Bouharati, C.; Mehta, T.; Sadhasivam, B.; Acharya, P.; Zhao, D.; Queimado, L. Electronic cigarette aerosols alter the expression of cisplatin transporters and increase drug resistance in oral cancer cells. Sci. Rep. 2021, 11, 1821. [Google Scholar] [CrossRef]
- Mokeem, S.A.; Abduljabbar, T.; Al-Kheraif, A.A.; Alasqah, M.N.; Michelogiannakis, D.; Samaranayake, L.P.; Javed, F. Oral Candida carriage among cigarette- and waterpipe-smokers, and electronic cigarette users. Oral Dis. 2019, 25, 319–326. [Google Scholar] [CrossRef]
- Nelson, J.M.; Cuadra, G.A.; Palazzolo, D.L. A Comparison of Flavorless Electronic Cigarette-Generated Aerosol and Conventional Cigarette Smoke on the Planktonic Growth of Common Oral Commensal Streptococci. Int. J. Environ. Res. Public Health 2019, 16, 5004. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Koh, H.; Patatanian, M.; Reyes-Caballero, H.; Zhao, N.; Meinert, J.; Holbrook, J.T.; Leinbach, L.I.; Biswal, S. The mediating roles of the oral microbiome in saliva and subgingival sites between e-cigarette smoking and gingival inflammation. BMC Microbiol. 2023, 23, 35. [Google Scholar] [CrossRef]
- Rouabhia, M.; Semlali, A. Electronic cigarette vapor increases Streptococcus mutans growth, adhesion, biofilm formation, and expression of the biofilm-associated genes. Oral Dis. 2021, 27, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Semlali, A.; Zakrzewski, A.; Chmielewski, W.; Chakir, J. E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway: Effect of E-Cigarette on Epithelial Cells. J. Cell. Physiol. 2017, 232, 1539–1547. [Google Scholar] [CrossRef]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; di Giacomo, V. Cytotoxicity and apoptosis induction by e-cigarette fluids in human gingival fibroblasts. Clin. Oral Investig. 2016, 20, 477–483. Available online: http://link.springer.com/410.1007/s00784-00015-01537-x (accessed on 17 August 2023). [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Cataldi, A.; Sancillo, L.; Rana, R.A.; di Giacomo, V. Modifications in Human Oral Fibroblast Ultrastructure, Collagen Production, and Lysosomal Compartment in Response to Electronic Cigarette Fluids. J. Periodontol. 2017, 88, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Schwarzmeier, L.A.T.; da Cruz, B.S.; Ferreira, C.C.P.; Carvalho, B.; Alves, M.G.O.; Lima Carta, C.F.; Scholz, J.R.; Almeida, J.D. E-cig might cause cell damage of oral mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 435–443. [Google Scholar] [CrossRef]
- Shaikh, Z.N.; Alqahtani, A.; Almela, T.; Franklin, K.; Tayebi, L.; Moharamzadeh, K. Effects of electronic cigarette liquid on monolayer and 3D tissue-engineered models of human gingival mucosa. J. Adv. Periodontol. Implant. Dent. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef]
- Thomas, S.C.; Xu, F.; Pushalkar, S.; Lin, Z.; Thakor, N.; Vardhan, M.; Flaminio, Z.; Khodadadi-Jamayran, A.; Vasconcelos, R.; Akapo, A.; et al. Electronic Cigarette Use Promotes a Unique Periodontal Microbiome. mBio 2022, 13, e0007522. [Google Scholar] [CrossRef]
- Tishchenko, O.V.; Kryvenko, L.S.; Gargina, V.V. Influence of smoking heating up tobacco products and e-cigarettes on the microbiota of dental plaque. Pol. Merkur. Lekarski 2022, 50, 16–20. [Google Scholar]
- Tommasi, S.; Caliri, A.; Caceres, A.; Moreno, D.; Li, M.; Chen, Y.; Siegmund, K.; Besaratinia, A. Deregulation of Biologically Significant Genes and Associated Molecular Pathways in the Oral Epithelium of Electronic Cigarette Users. Int. J. Mol. Sci. 2019, 20, 738. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.Y.F.; Hirschi Budge, K.M.; Lepre, A.P.; Rhees, M.S.; Ajdaharian, J.; Geiler, J.; Epperson, D.G.; Astle, K.J.; Winden, D.R.; Arroyo, J.A.; et al. Cell invasion, RAGE expression, and inflammation in oral squamous cell carcinoma (OSCC) cells exposed to e-cigarette flavoring. Clin. Exp. Dent. Res. 2020, 6, 618–625. [Google Scholar] [CrossRef]
- Vermehren, M.F.; Wiesmann, N.; Deschner, J.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Comparative analysis of the impact of e-cigarette vapor and cigarette smoke on human gingival fibroblasts. Toxicol. In Vitro 2020, 69, 105005. [Google Scholar] [CrossRef] [PubMed]
- Willershausen, I.; Wolf, T.; Weyer, V.; Sader, R.; Ghanaati, S.; Willershausen, B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head Face Med. 2014, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zanetti, F.; Wang, L.; Pan, J.; Majeed, S.; Malmstrom, H.; Peitsch, M.C.; Hoeng, J.; Ren, Y. Effects of different discoloration challenges and whitening treatments on dental hard tissues and composite resin restorations. J. Dent. 2019, 89, 103182. [Google Scholar] [CrossRef]
- Morishita, Y.; Hasegawa, S.; Koie, S.; Ueda, S.; Miyabe, S.; Watanabe, S.; Goto, M.; Miyachi, H.; Nomoto, S.; Nagao, T. Cytotoxic, genotoxic, and toxicogenomic effects of heated tobacco products and cigarette smoke in human primary keratinocytes. Tob. Induc. Dis. 2022, 20, 82. [Google Scholar] [CrossRef]
- Uehara, O.; Nakamoto, N.; Hiraki, D.; Paudel, D.; Sugiyama, N.; Morikawa, T.; Yoshida, K.; Kawano, Y.; Shimo, T.; Furuichi, Y.; et al. Effects of prolonged stimulation with heated tobacco products (Ploom TECH+) on gingival epithelial cells. J. Periodontal Res. 2023, 58, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Negri, P.; Coniglio, M.; Bruscoli, S.; Di Michele, A.; Marchetti, M.C.; Valenti, C.; Gambelunghe, A.; Fanasca, L.; Billi, M.; et al. Heat-not-burn tobacco (IQOS), oral fibroblasts and keratinocytes: Cytotoxicity, morphological analysis, apoptosis and cellular cycle. An in vitro study. J. Periodontal Res. 2021, 56, 917–928. [Google Scholar] [CrossRef]
- Marinucci, L.; Coniglio, M.; Valenti, C.; Massari, S.; Di Michele, A.; Billi, M.; Bruscoli, S.; Negri, P.; Lombardo, G.; Cianetti, S.; et al. In Vitro effects of alternative smoking devices on oral cells: Electronic cigarette and heated tobacco product versus tobacco smoke. Arch. Oral Biol. 2022, 144, 105550. [Google Scholar] [CrossRef]
- Abdul, N.S.; Alshehri, N.M.; Bindawis, H.M.; Alzahrani, H.K.; Alanezi, A.F. Awareness of the Effects of Shisha and Electronic Cigarette Smoking on Oral Health In Saudi Population. Ann. Dent. Spec. 2020, 8, 41. [Google Scholar]
- Kaán, R.; Pénzes, M.; Abafalvi, L.; Hermann, P.; Kispélyi, B. Oral Hygiene Practices of Hungarian Adult E-Cigarette-Only and Dual Users. Oral Health Prev. Dent. 2020, 18, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Isik Andrikopoulos, G.; Farsalinos, K.; Poulas, K. Electronic Nicotine Delivery Systems (ENDS) and Their Relevance in Oral Health. Toxics 2019, 7, 61. [Google Scholar] [CrossRef]
- Ramôa, C.P.; Eissenberg, T.; Sahingur, S.E. Increasing popularity of waterpipe tobacco smoking and electronic cigarette use: Implications for oral healthcare. J. Periodontal Res. 2017, 52, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Laverty, A.A.; Vardavas, C.I.; Filippidis, F.T. Prevalence and reasons for use of Heated Tobacco Products (HTP) in Europe: An analysis of Eurobarometer data in 28 countries. Lancet Reg. Health Eur. 2021, 8, 100159. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Jessri, M.; Farah, C.S. Electronic nicotine delivery systems: Oral health implications and oral cancer risk. J. Oral Pathol. Med. 2021, 50, 316–322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).