Abstract
Current research highlights the use of natural products or phytochemicals as drugs and functional additives to treat obesity with few side effects. Sargassum horneri (SH) and Ulva australis (UA) are marine waste resources on Jeju Island, Republic of Korea. In this study, we analyzed their antioxidant and anti-obesity efficacies to confirm their potential as functional additives. We prepared SH and UA extracts using 80% ethanol and observed that free radical scavenging activity and total phenol content were high in SH extracts, and total flavonoid content was elevated in UA extracts. Additionally, 3T3-L1 cells were treated with SH and UA extracts, and the ability of the extracts to inhibit adipocyte differentiation was examined using Oil Red O staining and analysis of neutral fat content. We confirmed that the mRNA expression of the C/EBPα, PPARγ, and SREBP1c genes that act on adipocyte differentiation, and of FAS, a fatty acid synthase, was suppressed. Experiments in a mouse model of obesity showed that 12-week administration of a high-fat diet with 1% extract added to drinking water resulted in lower weight gain compared to the high-fat diet alone. These results suggest that SH and UA extracts have antioxidant properties and are effective in obesity prevention. Therefore, the two marine waste resources are potential functional additive candidates for preventing obesity.
1. Introduction
According to the definition by the World Health Organization (WHO), obesity is an unhealthy condition resulting from the abnormal or excessive accumulation of fat in adipose tissue [1,2]. The body mass index (BMI) is a person’s weight (kg) divided by the square of their height (m); individuals with BMI ranges between 25 and 30 are considered to be overweight and obese [1,2,3]. According to the WHO report on obesity, over 1.9 billion adults aged over 18 years old are overweight, and 600 million are reported to be obese, which makes obesity a problem in both developed and developing countries [4,5]. In Korea, according to the National Health and Nutrition Examination Survey, the prevalence of obesity among adults aged 19 years old and older is reported to be ~40% for men and ~26% for women. By 2030, the prevalence rate is expected to reach ~62% for men and 37% for women [6].
Among the factors that cause obesity are lack of exercise, stress, genetic factors, and endocrine disorders. Obesity is mainly caused by excessive body fat accumulation due to excessive calorie intake beyond consumption [7]. Adipocytes are endocrine organs that control several metabolic processes that regulate the body’s physiology. Adipocytes secrete various hormones that regulate metabolism, such as leptin and adiponectin, which are essential for maintaining homeostasis [8]. When excessive fat accumulates in adipocytes, functions such as intracellular endoplasmic reticulum stress and mitochondrial dysfunction are lost, and the risk of complications of obesity, such as hypertension, cardiovascular disease, cancer, and fatty liver, increases through insulin resistance and inflammatory responses [9,10]. Oxidative stress that affects obesity-related diseases increases the probability of various diseases by inducing chemical changes in the human body. Therefore, it is necessary to find antioxidant substances to protect cells in the human body from free radicals and prevent obesity.
Obesity-related studies have used mouse fibroblast 3T3-L1 preadipocytes to analyze the life cycle mechanisms of adipogenesis, lipogenesis, and lipolysis. The production and differentiation of adipocytes are regulated by transcription factors, such as CCATT/enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) [11]. C/EBPα levels increase in the late stage of differentiation along with those of PPARγ and induce the expression of genes that induce adipogenesis such as fatty acid synthase (FAS) and adiponectin (ADIPOQ). Their expression levels tend to markedly increase in differentiated adipocytes [12]. The regulation of genes related to adipocyte differentiation is one of the most important strategies for obesity prevention and treatment [13]. Therefore, therapeutic agents effective for adipocyte differentiation and the inhibition of adipogenesis are being released. The available drugs against obesity include orlistat, topiramate, sibutramine, rimonabant, and phenylpropanolamine; however, these drugs have side effects such as insomnia, anorexia, gastrointestinal disorders, increased blood pressure, and heart disease [14]. Overall, the only two drugs currently approved by the Food and Drug Administration (FDA) for long-term use are orlistat and sibutramine, while others are available only for short-term or limited use [15]. To address this problem, research on the development of treatments against obesity using safer natural products or phytochemicals that have fewer side effects is active [16].
Among such natural products, Sargassum horneri (SH) and Ulva australis (UA), used in the present study, are marine waste resources generated in large quantities on Jeju Island, Republic of Korea. SH, that belongs to the Sargassaceae algae, is a yellow-brown plant with thin, spatula-shaped leaves that grows mainly in winter. UA is a green alga in the Ulvaceae family and inhabits the middle and lower intertidal zones. Due to climate changes and rising water temperatures, the populations of these two plants are increasing and causing environmental and economic issues, including introduction to the coast, odor due to decomposition, the pollution of surrounding ecosystems, and damage to fishing boats [17].
SH, which causes various problems in Jeju, is a food rich in fucoidan and a known delicacy in East Asia, including China and Japan [18]. UA is known to enhance immune activity [19] and promote collagen biosynthesis [20]. Additionally, UA has been used in oriental medicine to treat hyperlipidemia, heat stroke, and urinary disorders [21]. To explore the use of seaweed as a valuable resource, several studies have analyzed the antioxidant [22], immune activity [23,24], whitening and anti-wrinkle [25], and anti-obesity effects [26] of SH. Similarly, some research has evaluated the antioxidant [27] and anti-inflammatory [28] effects of UA and its potential for bioethanol production [29]; yet, studies related to the effects of UA on obesity are scarce.
In this context, the present study compares the antioxidant effects of SH and UA extracts by analyzing their 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical activity and total polyphenol and flavonoid contents. In addition, the inhibitory effect of treatment with SH and UA extracts on 3T3-L1 adipocyte differentiation was analyzed. In an animal model of obesity, induced by a high-fat diet, the effect of extract administration on preventing obesity was investigated.
2. Results
2.1. Total Phenol and Flavonoid Contents
The total phenol content of the SH and UA ethanol extracts was analyzed using gallic acid as a reference material. The total phenol content of the SH extract was 95.5 ± 1.4 mg gallic acid equivalent (GAE)/g, and that of the UA extract was 62.4 ± 1.8 mg GAE/g. Thus, of the two materials, SH showed a higher phenol content than UA (Table 1).

Table 1.
Total phenol and flavonoid contents in S. horneri and U. australis ethanol extracts.
The total flavonoid contents of the SH and UA ethanol extracts were analyzed using naringin as a reference material. The results revealed that the total flavonoid contents of the SH and UA extracts amounted to 19.4 ± 1.0 mg quercetin equivalent (QE)/g and 20.9 ± 1.2 mg QE/g, respectively. Thus, regarding total flavonoid content, UA had a higher content than SH (Table 1).
2.2. DPPH Radical Scavenging Effects
The DPPH radical scavenging activity of the SH and UA ethanol extracts was evaluated. The SH and UA extracts were prepared at concentrations of 0.01, 0.1, 1, and 10 mg/mL, and the radical scavenging activity was measured. After that, the IC50 values were 0.64 ± 0.29 mg/mL for SH and 1.58 ± 0.17 mg/mL for UA. Both extracts showed lower activity than L-ascorbic acid, used as a positive control. However, when SH and UA were compared, the SH extract was found to have higher radical scavenging activity (Table 2).

Table 2.
DPPH radical scavenging activity of Sargassum horneri and Ulva australis ethanol extracts.
2.3. Cell Viability of 3T3-L1 Cells
To evaluate the potential cytotoxic effect of the SH and UA extracts on 3T3-L1 cells, the extracts were prepared at concentrations of 62.5, 125, 250, 500, and 1000 μg/mL and used to treat 3T3-L1 cells for 48 h. Treatments with either extract at a concentration of 1000 μg/mL resulted in a cell viability of 95%, whereas, at 500 μg/mL, cell viability was 100% (Figure 1). Thus, we confirmed that neither extract exhibited cytotoxicity at concentrations lower than 500 μg/mL. For subsequent analyses, the highest concentration used was 500 μg/mL.
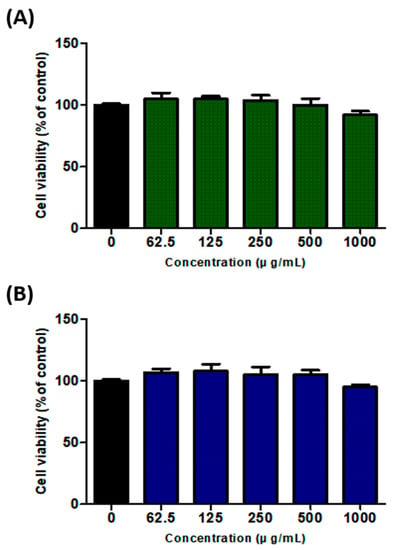
Figure 1.
Effects of S. horneri and U. australis extracts on 3T3-L1 cell viability. (A) S. horneri ethanol extract; (B) U. australis ethanol extract. The extracts were diluted to 0, 62.5, 125, 250, 500, and 1000 μg/mL and used to treat 3T3-L1 cells for 48 h. Cell viability was measured using the WST-1 assay. Data are expressed as the mean ± standard deviation (n = 3).
2.4. Inhibition of Adipogenesis in 3T3-L1 Cells
To confirm the inhibitory effects of SH and UA extracts on adipocyte differentiation in 3T3-L1 cells, the extracts were applied for 8 d at concentrations of 250 and 500 μg/mL, and Oil Red O staining was performed. In the cells treated with 250 and 500 μg/mL of SH extract, lipid accumulation was inhibited by 94.4% and 84.9%, respectively, compared to the control cells. In the case of the UA extract, 93.5% and 83.0% of the Oil Red O elution amounts were observed in each concentration. Importantly, treatment with the two extracts at a concentration of 500 μg/mL significantly inhibited the differentiation of 3T3-L1 cells compared to control cells (Figure 2).
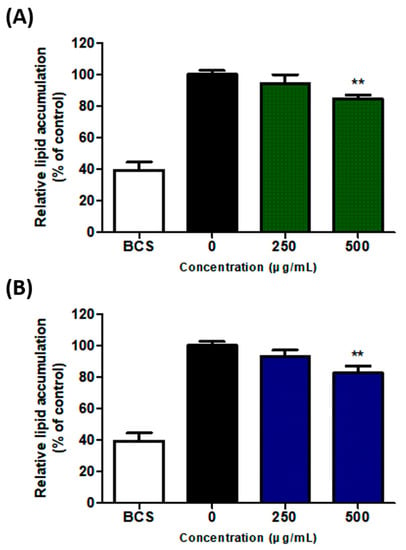
Figure 2.
Inhibition of adipocyte differentiation in 3T3-L1 cells treated with S. horneri and U. australis. (A) S. horneri ethanol extract; (B) U. australis ethanol extract. After an 8-day differentiation process, the Oil Red O stain was used; the dye was eluted using 100% isopropanol, and optical density (OD) at 500 nm was measured. Data are expressed as the mean ± standard deviation (n = 3). Significant differences: ** p < 0.01 vs. control cells.
2.5. Analysis of TG Content in 3T3-L1 Cells
To investigate the effect of SH and UA extracts on modifying TG content, which is a significant marker of adipogenesis, 3T3-L1 cells were treated with the extracts for 8 d and then analyzed. The TG content in the control cells was measured as 904.2 ± 35.4 mg/g of protein. However, in the cells treated with 250 and 500 μg/mL of SH extract, they amounted to 739.7 ± 26.7 and 688.4 ± 22.6 mg/g of protein, respectively; these differences were statistically significant (p < 0.01). Similarly, significant differences (p < 0.01) were noted in the case of the UA extract, with 705.9 ± 20.9 and 651.3 ± 29.0 mg/g of protein measured in the cells treated with 250 and 500 μg/mL of UA extract, respectively. Therefore, the results confirmed that the cells treated with both extracts showed inhibition of TG content in a concentration-dependent manner compared to the control cells (Figure 3).
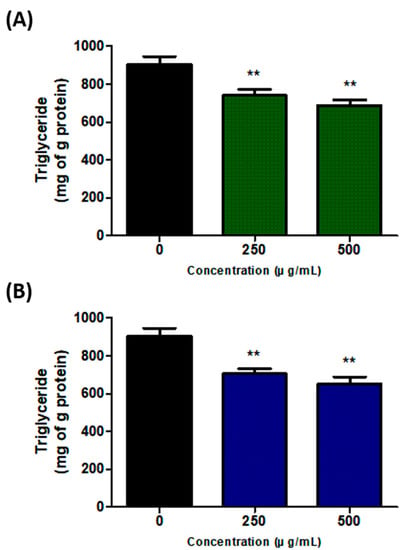
Figure 3.
Effects of S. horneri and U. australis treatment on triglyceride (TG) inhibition during adipocyte differentiation. (A) S. horneri ethanol extract; (B) U. australis ethanol extract. After 8 d of differentiation, the protein was extracted, and the intracellular TG content was determined using enzyme colorimetry. Data are expressed as the mean ± standard deviation (n = 3). Significant differences: ** p < 0.01 vs. control cells.
2.6. Effect on Adipogenic Gene Expression in 3T3-L1 Cells
To examine whether SH and UA inhibit the expression of adipogenic transcription factors, 3T3-L1 preadipocytes were treated with the extracts at various concentrations (250 and 500 μg/mL), cultured for 8 d, and analyzed. The SH and UA extracts inhibited the expressions of C/EBPα, PPARγ, SREBP1c, and FAS genes (Figure 4). Of the SH and UA extracts, the expression of adipogenic transcription factors was more highly inhibited at a concentration of 500 μg/mL of the UA extract.
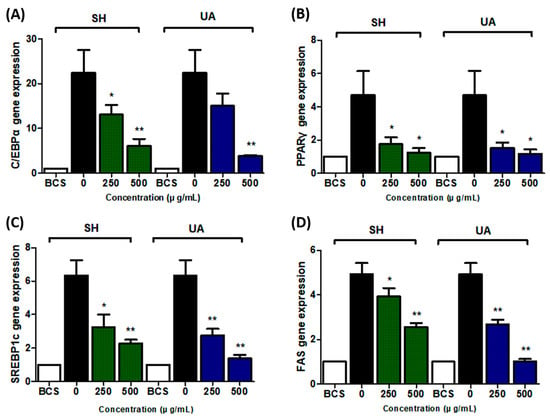
Figure 4.
Effects of S. horneri and U. australis on adipogenic mRNA expression in 3T3-L1 cells. (A) C/EBPα, (B) PPARγ, (C) SREBP1c, and (D) FAS. 3T3-L1 preadipocytes were differentiated in the presence of different concentrations (250 and 500 μg/mL) of SH and UA for 8 d. The mRNA expression level was measured using qRT-PCR and normalized using that of the housekeeping gene GAPDH. Data are expressed as the mean ± standard deviation (n = 3). Significant differences: * p < 0.05, and ** p < 0.01 vs. control cells.
2.7. Changes in Body Weights and Food and Drink Intake of Mice
The anti-obesity effect was analyzed by administering SH and UA extracts in drinking water at 1% concentration together with a high-fat diet (HFD). In the group administered SH and UA in the drinking water, the rate of weight gain was lower than that in the HFD group (Figure 5A). The analysis of the weight gain showed that the SH and UA extract administration groups had a weight gain of 21.1 ± 2.1 and 19.6 ± 1.3 g, respectively, which were lower than the 24.9 ± 2.0 g in the HFD group. Significantly lower rates of weight gain were measured in all the groups that received the extracts, and of the two extracts, UA showed a lower rate of weight gain (Figure 5B).
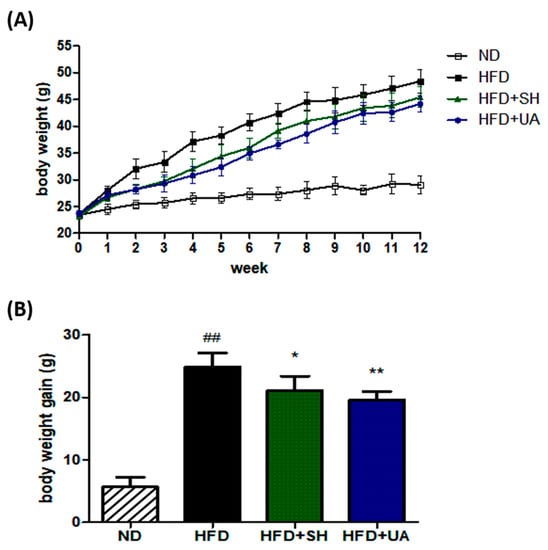
Figure 5.
Effects of S. horneri and U. australis on body weight in C57BL/6 mice fed a high-fat diet for 12 weeks. (A) Weekly changes in body weight; (B) body weight gain. ND, normal diet; HFD, high-fat diet; HFD+SH, administration of 1% S. horneri extract in the drinking water and HFD; HFD+UA, administration of 1% U. australis extract in the drinking water and HFD. Each value represents the mean ± standard deviation (n = 6). Significant differences: * p < 0.05, ** p < 0.01 vs. the HFD group, and ## p < 0.01 vs. the ND group.
Food and drink intake were analyzed to exclude the possibility that the extracts led to decreases in food or drinking water intake. Food and drink intake were measured at the same time every week and the results were analyzed. The results revealed no differences in food and drink intake between the HFD group and the two extract administration groups; we thus concluded that administration of the extracts had a weight loss effect (Table 3).

Table 3.
Changes in food and drink intake in C57BL/6 mice during 12 weeks of HFD-induced obesity.
2.8. Serum Biochemical Analysis
The results of the serum biochemical analysis showed that aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which are liver function test components, tended to increase in the HFD group compared to those in the normal group. However, AST and ALT levels in the group administered the two types of seaweed extracts significantly decreased compared to those in the HFD group. Gamma Glutamyl Transferase (GGT) levels did not identify significant differences in all groups. The level of total cholesterol (CHO) was high at 193.7 ± 11.3 mg/dL in the HFD group compared to that in the normal group. However, it significantly decreased to 142.4 ± 6.6 and 156.3 ± 9.7 mg/dL in the SH and UA extract administration groups, respectively. TG levels were 50.9 ± 6.0, 32.6 ± 6.1, and 39.0 ± 5.1 mg/dL, in the HFD, SH, and UA groups, respectively; TG levels significantly decreased in the groups treated with the extracts compared to those in the HFD group. HDL cholesterol (HDL-C) levels tended to increase in the high-fat diet group compared to the normal diet group. LDL cholesterol (LDL-C) levels, analyzed by total cholesterol, triglyceride, and HDL cholesterol levels, were higher in the HFD group at 71.9 ± 4.2 mg/dL compared with the normal group. However, in the SH and UA extract treatment groups, they were significantly reduced to 40.7 ± 4.4 and 40.8 ± 3.1 mg/dL, respectively. Blood glucose (GLU) levels, too, significantly decreased in the experimental groups compared to those in the HFD group (Table 4).

Table 4.
Changes in serum biochemical parameters in obese C57BL/6 mice induced by a high-fat diet.
2.9. Weight Analysis of Liver and White Adipose Tissue
The comparison of the weights of liver, epididymal fat, mesenteric fat, and retroperitoneal fat between the ND and HFD groups demonstrated that the weights of all tissues were significantly higher in the HFD group. The comparison of the epididymal fat mass between the HFD group and the extract-administered groups showed that the HFD SH and UA groups had significantly lower values than the ND group (3.01 ± 0.42, 2.25 ± 0.24, and 2.32 ± 0.17 g, respectively). Comparison of the weights of the liver, retroperitoneal fat, and mesenteric fat showed that tissue weights in the extract-administered groups were lower than those in the obese group; however, this difference did not reach statistical significance (Table 5). In the comparison between the two extract-administered groups, most of the tissue weights were lower in the UA-administered group than in the SH-administered group.

Table 5.
Changes in liver and white adipose tissue weight in obesity prevention test animals.
2.10. Histopathological Analysis of Liver and Adipose Tissues
Histological analysis of liver and adipose tissues was performed using hematoxylin and eosin (H&E) staining. Regarding the liver tissue, we observed that the number of fat droplets increased in the HFD group compared to those in the normal group. The number and size of lipid droplets in the group administered the SH and UA extracts tended to decrease compared to those in the HFD group (Figure 6). In addition, in the white adipose tissue, the size of adipocytes in the HFD group was enlarged compared to that in the normal group, and adipocyte size in the group administered the SH and UA extracts tended to decrease (Figure 7).
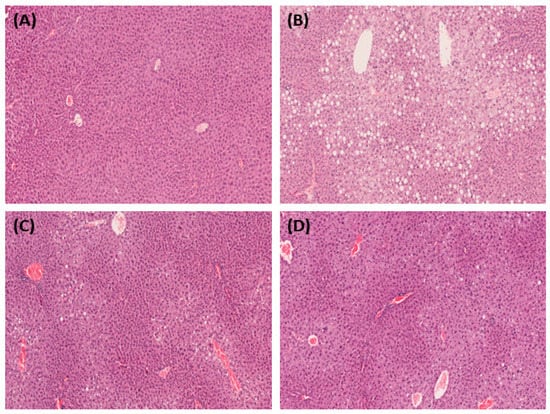
Figure 6.
Microphotographs of the liver in HFD-fed C57BL/6 mice showing the effect of treatment with S. horneri and U. australis. Representative liver sections stained for hematoxylin and eosin (H&E) (magnification × 200). (A) ND; (B) HFD; (C) HFD+SH; and (D) HFD+UA.
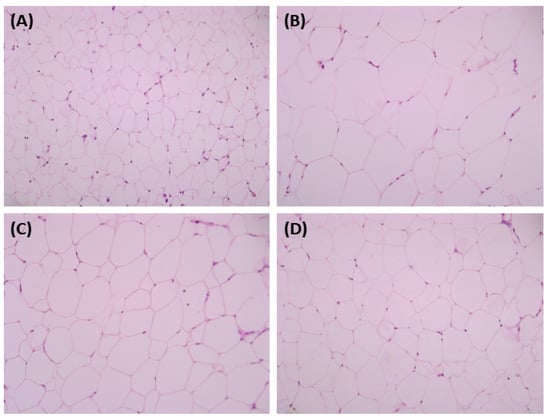
Figure 7.
Microphotographs of the adipose tissue in HFD-fed C57BL/6 mice showing the effect of treatment with S. horneri and U. australis. Representative sections of adipose tissue stained for H&E (magnification × 200). (A) ND; (B) HFD; (C) HFD+SH; and (D) HFD+UA.
2.11. Bioactive Compounds Present in the Extracts
The bioactive compounds present in the SH and UA extracts are shown in Table 6. The main compounds present in the SH extract were analyzed as Neophytadiene, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, and Hexadecanoic acid ethyl ester. The main compound present in the UA extract was analyzed as 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-,acetate, [R-[R*,R*-(E)]]-.

Table 6.
Compounds isolated from S. horneri and U. australis extracts.
3. Discussion
Obesity is a chronic metabolic disorder that threatens the health of people in developed and developing countries due to excessive body fat accumulation [30]. Obesity is related to various diseases, such as diabetes, hypertension, hyperlipidemia, stroke, and angina, and is known to be a major cause of aging [31]. Accordingly, prevention and treatment of obesity are important. To treat obesity, a combination of diet and drug approaches has been used. However, orlistat and rimonabant, commercially available drugs to treat obesity, have limitations in their use in children, patients with myocardial infarction, liver disease, and kidney disease, as well as various side effects, such as headache, abdominal pain, and panic disorder [32,33]. In this context, interest in food products based on natural substances, such as anti-obesity substances, oriental medicines, and medicinal plants, has been steadily growing [34].
Seaweed has been widely used as diet food due to its low fat and calorie content and the presence of various essential nutrients, such as protein, vitamins, and minerals whose deficiency is commonly noted during dieting [13]. Most seaweeds have been used as food and functional additives; however, SH and UA are not consumed in Korea and have been classified as marine organic waste resources causing coastal pollution on Jeju Island [35]. In the present study, extracts were prepared using 80% ethanol to upcycle these two seaweed waste resources into functional materials. Their antioxidant effect was then identified, and anti-obesity effects were confirmed in vitro and in vivo.
The antioxidant activities of SH and UA extracts were analyzed based on their DPPH radical scavenging activity. DPPH radical scavenging activity measures antioxidant activity via a reduction process in amines and aromatic compounds due to the electron-donating ability of antioxidants, and the degree of purple discoloration has previously been used as an indicator [36]. Using this method, we confirmed that the SH and UA extracts show free radical scavenging activity in a concentration-dependent manner. Additionally, the SH extract was found to have a higher scavenging activity than the UA extract. Phenolic compounds play an essential role as antioxidants, possess phenolic hydroxyl groups, which are collectively referred to as flavonoids, anthocyanins, tannins, and catechins, and are widely distributed in the plant kingdom [37]. Flavonoids are pigments in various plants that have been reported to have antioxidant, UV-blocking, anti-mutagenic, antiviral, and anti-inflammatory effects [38]. In the present study, the total polyphenol contents in the SH and UA extracts were compared, and SH was found to have a higher content than UA (95.5 and 62.4 mg GAE/g, respectively). This finding is consistent with previous research showing that brown algae have a higher polyphenol content than green and red algae [39]. In addition, when we compared the flavonoid contents in the two materials, we found that SH was 19.4 and UA 20.9 mg QE/g, indicating that the two materials were analyzed similarly.
To confirm whether the SH and UA extracts affect adipocyte formation, they were applied at concentrations equal to or lower than 500 μg/mL, which do not exhibit cytotoxicity during the differentiation process. The results confirmed that adipocyte differentiation was inhibited in a concentration-dependent manner. During the differentiation of preadipocyte 3T3-L1 cells, fat is accumulated in the cells, mostly as neutral fat [40]. The TG content in the cells decreased in a concentration-dependent manner. After treatment with SH and UA extracts at a concentration of 500 μg/mL, TG content decreased by more than 30%. A previous study on the inhibition of adipocyte differentiation using SH reported that TG content decreased by 23% in the experimental group treated with SH at 1 mg/mL for 8 d [41]. In contrast, in our results, TG content was considerably reduced when the extract was applied at a concentration of 500 μg/mL. This difference may be due to differences in the drying method, extraction solvent, and seaweed extraction method.
Preadipocytes were treated with the 3-isobutyl-1-methylxanthine (IBMX) complex, and specific genes were expressed during the differentiation process into adipocytes. Several transcription factors, including PPARγ, C/EBPα, and SREBP1c, play a pivotal role in regulating the differentiation and accumulation of adipocytes [42,43]. PPARγ is a predominantly liver-distributed transcription factor that, when activated, produces marked changes in fat morphology, fat accumulation, and insulin sensitivity [44]. C/EBPa plays an important role in adipogenesis. Expressions of PPARγ and C/EBPα are mutually regulated at the preadipocyte differentiation stage, and the two genes cooperate to promote adipogenesis and regulate the expression of SREBP1c and FAS [45,46]. The results of our analysis confirmed that the expression of transcription factors of adipogenesis was downregulated in a concentration-dependent manner. In addition, evidence suggests that differentiation into adipocytes is inhibited by the suppression of FAS expression [47], an important metabolic enzyme acting on adipogenesis and lipogenesis. Therefore, our results confirmed that the two seaweed extracts function as suppressors of the expression of key transcription factors that act during the fat differentiation process and that UA regulates the expression of transcription factors more effectively than SH.
The HFD mouse model has been widely used to investigate anti-obesity effects and for the development of anti-obesity drugs [48]. Obese mice fed an HFD are characterized by increased body weight and adipocyte size, fatty liver development, and increased plasma GLU and TG levels [47]. In the present study, to confirm the effect of preventing obesity in C57BL/6 mice induced by an HFD, the extract was added to drinking water at 1% concentration and administered for 12 weeks. In general, extracts have been administrated mainly via the oral route, but they may also be supplied through feed or water [26,49]. Since the extracts were added to the drinking water, we investigated potential differences between feed and drinking water intake. The results showed no differences in diet and water intake between the HFD and the extract administration groups, and no issues were observed regarding palatability. The weight gain rate significantly decreased in the group administered the SH and UA extracts compared to that in the HFD group. A previous obesity study using SH showed a protective effect against obesity when the substance was added to a high-fat diet at 2% and 6% and fed to mice [26]. However, this study found that adding the extract to drinking water at a concentration of 1% showed anti-obesity effects despite a lower treatment concentration than the previous results. Furthermore, the group that was administered the UA extract had a lower rate of weight gain than the SH group.
The serological analysis revealed that the levels of AST, ALT, CHO, TG, and blood GLU, which are related to liver function, were significantly reduced in the extract-administered groups compared to those in the HFD group. According to previous research results, Sargassaceae are rich in fucoxanthin, which has anti-obesity and anti-diabetic effects, while UA contains polysaccharides and dietary fiber, which is known to help with hypercholesterolemia [21,50,51]. In our results, we reasoned that the reduction in serological values in the two experimental groups compared to those in the obese group was due to the components in the SH and UA extracts. In addition, the results confirmed that accumulation of fat droplets in liver tissue was suppressed after administration of SH and UA extracts; similarly, the size of adipocytes in white adipose tissue was also suppressed. These results confirmed that SH and UA have antioxidant properties and can prevent fatty liver, TG, and blood GLU increases in obesity. Based on this evidence, we conclude that SH and UA have obesity-preventive effects.
Phytochemicals with traditional medicinal properties have been analyzed using various chemical analysis methods. Of the three biologically active compounds analyzed in SH extracts, Neophytadiene is known to have antimicrobial, antioxidant, and anti-inflammatory effects [52], and Hexadecanoic acid ethyl ester is known to have antioxidant, hypocholesterolemic, nematicide, and pesticide effects [53]. Phytol and phytyl acetate were analyzed in SH and UA extracts, which are not only diterpene compounds [54] but have been shown in various studies to have nitric oxide inhibitory activity as well as plant-growth-regulating, hypolipidemic, antimicrobial, and antiviral properties, among others [55]. The antioxidant and anti-obesity effects of SH and UA extracts are thought to be due to the effects of the compounds analyzed and the components present in the seaweed, such as dietary fiber.
Therefore, the results of this study suggest that SH and UA, typically considered marine waste resources, can be effectively used as functional additive candidates for obesity prevention. In addition, our results can be considered primary data for the recycling of seaweed waste.
4. Materials and Methods
4.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM), bovine calf serum (BCS), fetal bovine serum (FBS), and penicillin–streptomycin (PS) were purchased from Welgene (Daegu, Republic of Korea). Folin & Ciocalteu phenol reagent, IBMX, insulin from bovine pancreas, gallic acid, Oil Red O, and isopropanol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dexamethasone was purchased from the Tokyo Chemical Industry (Tokyo, Japan). The Cellvia cell viability assay kit was obtained from AbFrontier (Seoul, Republic of Korea). The TG colorimetric assay kit was purchased from Asan pharmaceuticals (Seoul, Republic of Korea). The AccuPrep® Universal RNA Extraction Kit was supplied by Bioneer (Daejeon, Republic of Korea). The One-Step TB Green® PrimeScript™ reverse transcriptase polymerase chain reaction (RT-PCR) and Bradford Protein Assay kits were purchased from Takara (Tokyo, Japan). The Korean Cell Line Bank (KCLB; Seoul, Republic of Korea) provided the 3T3-L1 cells (KCLB: 10092.1). All chemicals and solvents used in the present study were of analytical grade.
4.2. Preparation of S. horneri and U. australis Extracts
SH and UA were collected from the coast of Jeju Island at the Jeju Techno Park Biodiversity Research Center, washed and dried, and then grinded into powder. After immersing 50 g of the dried sample in 1 L of 80% ethanol, the extract was prepared by being stirred at room temperature for 24 h. The extracted material was filtered using a 0.45 µm bottle-top vacuum filter (Corning Inc, Corning, NY, USA). The filtered material was lyophilized after the ethanol was volatilized using a rotary evaporator (LabSciTech, Corona, CA, USA) to prepare the extract powder. Sample extraction was performed by repeating the process twice. The prepared sample was stored at −20 °C and used after being suspended in a cell culture medium or sterile distilled water.
4.3. Analysis of Total Phenol and Flavonoid Content
Total phenol content was measured using the Folin–Denis method [56]. One milliliter of the extract at a concentration of 1 mg/mL was mixed with 1 mL of Folin–Ciocalteu reagent and allowed to stand for 3 min. Next, 1 mL of 10% Na2CO3 solution was added and reacted at room temperature for 1 h. The absorbance at 760 nm was measured using a microplate reader (Varioskan LUX). The procedure was repeated using the standard substance gallic acid to prepare the calibration curve (y = 0.0025x; R2 = 0.9933). Total phenol content was expressed as mg GAE/g of extract sample.
Total flavonoid content was quantified using the diethylene glycol colorimetric method [57]. One milliliter of diethylene glycol and 100 μL of 1 N NaOH were mixed with 100 μL of SH and UA extracts (1 mg/mL), blocked from light, and reacted at 37 °C for 1 h. The absorbance at 420 nm was measured using a microplate reader (Varioskan LUX). The procedure was repeated using the standard substance quercetin to prepare a calibration curve (y = 0.0018x − 0.0019; R2 = 0.9977). Total flavonoid content was expressed as mg QE/g of extract sample.
4.4. DPPH Radical Scavenging Assay
The antioxidant activity of SH and UA extracts was evaluated using the DPPH (1,1-diphenyl-2-picrylhydrazyl) method [58]. Each extract was prepared at concentrations of 0.01, 0.1, 1, and 10 mg/mL using ethanol. Next, 190 μL of a substance of 10 µL and a 0.2 mM DPPH solution were added to a 96-well plate and mixed. The reaction was performed at 37 °C for 30 min, and the absorbance at 550 nm was measured using a microplate reader (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA). DPPH radical scavenging ability was calculated according to the following equation:
DPPH free radical scavenging effect (%) = [1-(absorbance of experimental group/absorbance of control group)] × 100
Half the maximum inhibitory concentration (IC50) was calculated using linear regression analysis and expressed as the mean of three measurements.
4.5. Cell Culture and Differentiation of 3T3-L1 Adipocyte Cells
3T3-L1 cells, a white adipocyte-like cell line, were cultured at 37 °C and 5% CO2 by adding 1% PS and 10% BCS to DMEM. The cells were cultured to approximately 70% confluency and then periodically passaged and used for experiments. For differentiation, the 3T3-L1 cells were first cultured using DMEM supplemented with 10% BCS until they reached confluence. After 2 d of confluence, the differentiation induction medium containing 10% FBS, 1% PS, 0.5 μM IBMX, 1 μM dexamethasone, and 10 μg/mL insulin in DMEM was replaced, and differentiation was induced for 48 h. The cells were then cultured for 6 d using DMEM containing 10% FBS, 1% PS, and 10 µg/mL of insulin; the culture medium was replaced every 2 d. The extract samples were applied from the beginning of differentiation and were used for further analysis after 8 d of treatment.
4.6. Cell Viability Assay
A water-soluble tetrazolium-1 (WST-1) assay was used to measure the cytotoxicity of the extracts and determine the range of concentrations to be used in the subsequent experiment. 3T3-L1 preadipocytes were dispensed at 1 × 105 cells/mL in 96-well plates and cultured for 24 h at 37 °C and 5% CO2. After sample treatment, Cellvia solution (WST-1) was added at 1/10 volume of the medium, and the cells were incubated for 30 min at 37 °C in a 5% CO2 incubator. The absorbance at 450 nm was measured using a microplate reader (Varioskan LUX). Subsequent experiments were performed in a concentration range that did not induce toxicity.
4.7. Oil Red O Staining of 3T3-L1 Adipocytes
Adipogenesis in 3T3-L1 cells was measured using Oil Red O staining. Differentiated 3T3-L1 cells were washed thrice with 1×phosphate-buffered saline (PBS, pH 7.4) and then fixed with 10% formaldehyde for 1 h at room temperature. After the cells were washed twice with distilled water (DW), they were treated with 60% isopropanol for 5 min at room temperature. Next, the isopropanol was removed, and the cells were allowed to dry at room temperature. A solution of 500 mg Oil Red O dissolved in 100 mL of 2-propanol was mixed with distilled water at a ratio of 6:4 and filtered through a 0.45 μm filter. The Oil Red O solution was added to the plates, and the cells were incubated for 1 h at room temperature. After staining, the cells were washed 4 times with DW, and the dye was eluted using 100% isopropanol. The optical density (OD) at 500 nm was then measured using a spectrophotometer (Milton Roy Company, New York, NY, USA).
4.8. Measurement of Intracellular Triglyceride Levels
After adipocyte differentiation, 3T3-L1 cells were washed with cold 1×PBS. After adding DW, the cells were collected using a cell scraper, and chloroform and methanol were added to lyse the cells. The cell lysate was incubated in a shaking incubator at 37 °C for 1 h and then centrifuged at 3000 rpm for 5 min. After transferring the bottom layer to an Eppendorf tube, the lid was opened and left for more than 24 h; the samples for analysis were prepared by adding 20 μL of 100% ethanol. Intracellular TG content was measured using the TG colorimetric assay kit. The cellular protein concentration was measured using the Bradford Protein Assay Kit, and the TG content was normalized to that of cellular protein.
4.9. Total RNA Extraction and Quantitative (q)RT-PCR
Differentiated preadipocytes were washed with 1×PBS, and total RNA was extracted using an Accuprep® universal RNA extraction kit (Bioneer). The extracted RNA was quantified using NanoDrop 2000 (Thermo Fisher Scientific). The qRT-PCR was performed using a One-Step TB Green® PrimeScript™ RT-PCR Kit (Takara) with 1 µg of RNA on a 7500 Fast Real-time PCR system (Applied Biosystems, Waltham, MA, USA). The conditions were 42 °C for 50 s, 95 °C for 10 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 30 s. The primers used in this laboratory are listed in Table 7 [1,2]. The expression of target genes was normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Changes in gene expression were calculated using the 2−ΔΔCT method, and the relative mRNA amount was quantified.

Table 7.
Target genes and their primer sequences used for qRT-PCR.
4.10. Animal Experiment
Five-week-old male C57BL/6 mice were purchased from KOSA BIO Inc. (Sungnam, Republic of Korea) and were housed in specific-pathogen-free conditions. During the experiment, the mice were maintained in a stable breeding environment with a light/dark cycle of 12 h at a temperature of 23 ± 3 °C, relative humidity of 50 ± 10%, and 150–300 Lux. A standard diet (Samtako Bio Korea, Seoul, Republic of Korea) and water were freely consumed by the mice for one week before the experiment, while the animals were allowed to adapt to the environment. The C57BL/6 mice were divided into the following four groups (six animals per group): (1) control group fed a normal diet; (2) HFD+DW group; (3) HFD + 1% SH drinking administration group; (4) HFD + 1% UA drinking administration group. The experiment was conducted for 12 weeks, and the mice in all HFD groups were fed a diet that contained 60% kcal from fat purchased from Research Diets (catalog number D12492: protein 20% kcal, fat 60% kcal, carbohydrate 20% kcal, energy density 5.21 kcal/g; New Brunswick, NJ, USA). This study was approved by the Animal Experimentation Ethics Committee of the Catholic University of Pusan (CUP AEC 2021-001).
4.11. Analysis of Body Weight Change and Food and Drink Intake
To analyze the changes in the weight of the experimental animals, weight was measured using an electronic balance once a week at a fixed time (10:00 A.M.) during the experimental period. Body weight gain was expressed as a percentage by comparing the weight measured at the beginning and end of the experimental period. Food and drink intake were also measured once a week at a fixed time. The daily dietary and liquid intake per animal were calculated by measuring the serving and remaining amounts of food and water.
4.12. Serum Biochemical Analysis
The experimental animals were fasted for 12 h before necropsy, and a mixture of 25 mg/kg of alfaxalone (Alfaxan®; Careside, Seongnam-si, Republic of Korea) and 5 mg/kg of xylazine (Rompun®; Bayer Korea, Seoul, Republic of Korea) was used as anesthetic. After anesthesia, blood was collected from the heart and coagulated at room temperature for serum biochemical tests. The serum obtained after centrifugation at 2500 rpm for 30 min was analyzed using a blood biochemical analyzer (BT 1500; Biotecnica Instruments SpA, Roma, Italy). The levels of the following components were analyzed: AST, ALT, GGT, CHO, TG, HDL-C, and GLU. LDL cholesterol was calculated using the Friedewald equation: LDL-cholesterol = total cholesterol—HDL cholesterol—(triglycerides/5).
4.13. Organ Weight and Histopathological Analysis
After deep anesthesia was achieved, the liver, epididymal fat, retroperitoneal fat, and mesenteric fat of the mice were extracted, weighed, and analyzed as absolute organ weights against body weight. The excised organs were washed with physiological saline, and, after removing moisture with a filter paper, a part of the excised tissue was fixed in 10% formalin and embedded in a paraffin block for sectioning (3 μm thickness) and subsequent histopathological examination. Tissue sections were stained using H&E and evaluated using a microscope (Olympus BX51; Olympus Optical Co., Tokyo, Japan).
4.14. Gas Chromatography–Mass Spectroscopy Analysis
After GC–MS analysis was performed by sending a request to the Korea Polymer Testing and Research Institute (Koptri, Seoul, Republic of Korea), analysis was performed using Agilent Technologies GC-MS (GC-7890A, MS 5975C) and Agilent J&W DB-5MS Ultra Inert column (30 m × 0.25 mm × 0.25 μM). The GC oven was programmed to heat as follows: An initial temperature of 40 °C was set and held at this temperature for 5 min; then, the oven temperature was increased to 280 °C at a rate of 10 °C /min and held for 5 min. The injector temperature was 250 °C, the flow rate of the carrier gas, helium, was 1.0 mL/min, and the split ratio was 1:10. The mass spectrum scan range was set to 30–500 (m/z). The analyzed compounds were compared to the spectra of the samples using the Koptri mass spectral library to identify the compound name, molecular weight, and structure of the test material.
4.15. Statistical Analysis
GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA) was used for data analysis. Values were expressed as the mean ± standard deviation and tested using one-way ANOVA for significant differences; p values < 0.05 were considered to indicate significant differences.
5. Conclusions
The results of this study confirmed the antioxidant effects of SH and UA extracts and the inhibition of transcription factors involved in lipid accumulation and adipogenesis in 3T3-L1 cells. They have also been shown to improve anti-obesity effects and serum biochemical markers in mice given a high-fat diet and a 1% concentration of the extract in drinking water. Based on these results, we have obtained basic data suggesting the possibility of their use as a functional additive candidate for obesity prevention that can be applied not only to humans but also to pets. However, further research is necessary to analyze the bioactive compounds of SH and UA, their molecular mechanisms, and the synergistic effects of mixed administration of the two extracts in preventing obesity. However, there is a lack of data analyzing the bioactive compounds and mechanisms of action of SH and UA extracts for their anti-obesity effects. Therefore, it is considered necessary to study the analysis of the bioactive compounds, the mechanisms of action, and the synergistic effects of the mixed administration of the two extracts when studying their anti-obesity effect by preparing fractions of the two extracts.
Author Contributions
Conceptualization, K.-S.C., H.-S.K. and H.-R.K.; methodology, Y.-H.L. and M.-H.Y.; software, S.C.K., H.-B.H. and Y.-M.H.; validation, Y.-H.J. and M.-H.Y.; formal analysis, Y.-H.L. and H.-S.K.; investigation, H.-R.K.; resources, Y.-H.J.; data curation, Y.-H.L. and K.-S.C.; writing—original draft preparation, Y.-H.L., H.-R.K. and K.-S.C.; writing—review and editing, S.C.K., H.-B.H., Y.-M.H. and Y.-H.J.; funding acquisition, H.-R.K. and Y.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (RS-2022-00165637 to H.-R.K.) and the Industrialization of Organic Resources in Jeju Special Self-Governing Province.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Experimentation Ethics Committee of the Catholic University of Pusan (CUP AEC 2021-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, H.M.; Jo, J.; Park, C.; Choi, B.J.; Lee, H.G.; Kim, K.Y. Epibionts associated with floating Sargassum horneri in the Korea Strait. Algae 2019, 34, 303–313. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, B.K.; Yeo, M.H.; Yoon, W.J.; Chang, K.S. Inhibition of lipid accumulation by the ethyl acetate fraction of Distylium racemosum in vitro and in vivo. Toxicol. Rep. 2019, 6, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, M.Y.; Chan, K.C.; Chung, P.J.; Ou, T.T.; Wang, C.J. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, J.H.; Kim, Y.G.; Lee, C.H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Park, J.H.; Guo, L.; Kang, H.M.; Son, B.G.; Kang, J.S.; Lee, Y.J.; Park, Y.H.; Je, B.I.; Choi, Y.W. Leaves of Cudrania tricuspidata on the shoot positional sequence show different inhibition of adipogenesis activity in 3T3-L1 cells. J. Life Sci. 2021, 31, 209–218. [Google Scholar]
- Lee, Y.S.; Seo, Y.H.; Kim, J.Y. Antiobesity effect of radish leaf extracts on high fat diet-induced obesity in mice. Korean J. Food Sci. Technol. 2022, 54, 297–305. [Google Scholar]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.J.; Jee, Y.; Kang, S.; Jang, J.; Jang, J.; et al. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, O.H.; Zheng, Y.; Kang, I.J. Erigeron annuus (L.) Pers. extract inhibits reactive oxygen species (ROS) production and fat accumulation in 3T3-L1 cells by activating an AMP-dependent kinase signaling pathway. Antioxidants 2019, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.N.; Choi, J.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2013, 93, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Lee, H.I.; Yun, K.W.; Ham, J.R.; Lee, M.K. Inhibitory Effects of Aralia elata Sprout Hot-Water Extract on Adipocyte Differentiation and Triglyceride Synthesis in 3T3-L1 cells. J. Korean Soc. Food Sci. Nutr. 2020, 49, 631–637. [Google Scholar] [CrossRef]
- Cha, J.Y.; Nepali, S.; Lee, H.Y.; Hwang, S.W.; Choi, S.Y.; Yeon, J.M.; Song, B.J.; Kim, D.K.; Lee, Y.M. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp. Ther. Med. 2018, 15, 5070–5076. [Google Scholar] [CrossRef]
- Derosa, G.; Cicero, A.F.; Murdolo, G.; Piccinni, M.N.; Fogari, E.; Bertone, G.; Ciccarelli, L.; Fogari, R. Efficacy and safety comparative evaluation of orlistat and sibutramine treatment in hypertensive obese patients. Diabetes Obes. Metab. 2005, 7, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent advances in nanoencapsulation of phytochemicals to combat obesity and its comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Oh, H.J.; Kim, S.; Yun, S.H.; Kang, J.H.; Park, S.R.; Lee, H.J. The origin and population genetic structure of the “golden tide” seaweeds, Sargassum horneri Korean Waters. Sci. Rep. 2019, 9, 7757. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.; Fernando, I.P.S.; Ahn, G. (−)-Loliolide Isolated from Sargassum horneri Protects against Fine Dust-Induced Oxidative Stress in Human Keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef]
- Han, J.G.; Ha, J.H.; Choi, Y.B.; Go, J.L.; Kang, D.H.; Lee, H.Y. The comparison of extraction process for enhancement of immunomodulating activities of Ulva pertusa kjellman. Korean J. Food Sci. Technol. 2009, 41, 380–385. [Google Scholar]
- Ko, H.J.; Kim, G.B.; Lee, D.H.; Lee, G.S.; Pyo, H.B. The effect of hydrolyzed Jeju Ulva pertusa on the proliferation and type I collagen synthesis in replicative senescent fibroblasts. J. Soc Cosmet. Sci. Korea. 2013, 39, 177–186. [Google Scholar]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Boo, H.J.; Moon, H.S.; Jee, Y.; Jeon, Y.J.; Chun, J.Y. Appearance characteristics and antioxidant activity of Jeju coast Sargassum horneri according to collecting time. J. Korean Soc. Food Sci. Nutr. 2020, 49, 485–492. [Google Scholar] [CrossRef]
- Kim, D.S.; Sung, N.Y.; Park, S.Y.; Kim, G.; Eom, J.; Yoo, J.G.; Seo, I.; Han, I.; Cho, Y.; Kim, K. Immunomodulating activity of Sargassum horneri extracts in RAW 264.7 macrophages. J. Nutr. Health 2018, 51, 507–514. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.G.; Kwak, H.S. Simultaneous application of chemicals and temperature for the effective control of trouble seaweed Ulva australis. Weed Turf Sci. 2018, 7, 35–45. [Google Scholar]
- Gam, D.; Hong, J.; Jeon, S.; Baek, D.; Kim, J. Development of ultrasound-assisted extraction for production of bioactive compounds with whitening and antiwrinkle effects from Sargassum horneri. Korean Soc. Bioeng. J. 2020, 35, 294–302. [Google Scholar]
- Murakami, S.; Hirazawa, C.; Ohya, T.; Yoshikawa, R.; Mizutani, T.; Ma, N.; Moriyama, M.; Ito, T.; Matsuzaki, C. The edible brown seaweed Sargassum horneri (Turner) C. Agardh ameliorates high-fat diet-induced obesity, diabetes, and hepatic steatosis in mice. Nutrients 2021, 13, 551. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, K.H.; Yook, H.S. Antioxidant activities of fermented Ulva pertusa Kjellman. J. Korean Soc. Food Sci. Nutr. 2020, 49, 940–945. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, M.J.; Kim, K.B.W.R.; Park, S.H.; Choi, H.D.; Park, S.Y.; Jang, M.R.; Im, M.H.; Ahn, D.H. Anti-inflammatory effects of ethanol extracts from Ulva pertusa Kjellman on LPS-induced RAW 264.7 cells and mouse model. Microbiol. Biotechnol. Lett. 2016, 44, 479–487. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, C.G.; Ahn, J.H.; Seo, Y.C.; Lee, S.E.; Jung, K.H.; Kang, D.; Cho, J.; Choi, G.; Lee, H. Enhancement of saccharification yield of Ulva pertusa Kjellman by high pressure homogenization process for bioethanol production. KSBB J. 2011, 26, 400–406. [Google Scholar] [CrossRef]
- Kang, I.S.; Hwang, K.Y.; Choi, A.; Roh, K.; Choi, J.H.; Sim, Y.; Park, Y.; Oh, M.S. Anti-obesity effects of herbal extract YY312 in C57BL/6 mice fed a high-fat diet and 3T3-L1 cells. Korea J. Herbol. 2013, 28, 23–31. [Google Scholar] [CrossRef]
- Chai, H.Y.; Sin, J.S.; Kwon, W.; Choi, E.K.; Cho, Y.M.; Zhang, H.S.; Hwang, S.Y.; Song, H.B.; Kim, Y.B.; Kang, J.K. Effect of BNs-3 and BNs-7, Extracts of Citrous Orange Peel, on the Obesity Induced by ad libitum Feeding a cholesterol-Containing Diet in Rats. J. Toxicol. Pub. Health 2003, 19, 189–195. [Google Scholar]
- Lee, J.S.; Kang, Y.H.; Kim, K.K.; Yun, Y.K.; Lim, J.G.; Kim, T.W.; Kim, D.J.; Won, S.Y.; Bae, M.H.; Choi, H.S.; et al. Exploration of optimum conditions for production of saccharogenic mixed grain beverages and assessment of anti-diabetic activity. J. Nutr. Health 2014, 47, 12–22. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, W.I.; Kang, K.H. Inhibitory effects of Bojungchiseub-tang on adipocyte differentiation and adipogenesis in 3T3-L1 preadipocytes. Korean J. Orient. Physiol. Pathol. 2014, 28, 288–295. [Google Scholar] [CrossRef]
- Jeon, H.J.; Yu, S.N.; Kim, S.H.; Park, S.K.; Choi, H.D.; Kim, K.Y.; Lee, S.Y.; Chun, S.S.; Ahn, S.C. Antiobesity effect of citrus peel extract fermented with Aspergillus oryzae. J. Life Sci. 2014, 24, 827–836. [Google Scholar] [CrossRef][Green Version]
- Jun, J.Y.; Lee, S.Y.; Kim, B.M.; Jeong, I.H. Effect of lactic acid extracts of Sargassum horneri on bone formation in female sprage-dawley rats. Korean J. Fish Aquat. Sci. 2011, 44, 25–30. [Google Scholar]
- Lee, S.H.; Jang, M.; Kim, G.H. Antioxidative effects of extracts from different parts of Epimedium koreanum Nakai. J. Korean Soc. Food Sci. Nutr. 2016, 45, 188–193. [Google Scholar] [CrossRef]
- Kim, E.J.; Choi, J.Y.; Yu, M.R.; Kim, M.Y.; Lee, S.H.; Lee, B.H. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, B.H.; An, X.; Jeong, H.R.; Kim, Y.E.; Lee, I.; Lee, H.; Kim, D. Total phenolics, total flavonoids, and antioxidant capacity in the leaves, bulbs, and roots of Allium hookeri. Korean J. Food Sci. Technol. 2015, 47, 261–266. [Google Scholar] [CrossRef]
- Ahn, S.M.; Hong, Y.K.; Kwon, G.S.; Sohn, H.Y. Evaluation of in-vitro anticoagulation activity of 35 different seaweed extracts. J. Life Sci. 2010, 20, 1640–1647. [Google Scholar] [CrossRef]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 875–888. [Google Scholar] [CrossRef]
- Kwon, D.H.; Choi, Y.H.; Kim, B.W.; Hwang, H.J. Effects of ethanol extract of Sargassum horneri on adipocyte differentiation and adipogenesis in 3T3-L1 preadipocytes. J. Life Sci. 2019, 29, 209–214. [Google Scholar]
- Lee, H.Y.; Kang, R.H.; Yoon, Y.S. SH21B, an anti-obesity herbal composition, inhibits fat accumulation in 3T3-L1 adipocytes and high fat diet-induced obese mice through the modulation of the adipogenesis pathway. J. Ethnopharmacol. 2010, 127, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Park, S.W.; Choe, S.S.; Jeong, H.W.; Park, J.Y.; Choi, E.W.; Seen, D.S.; Jeong, J.Y.; Lee, T.G. Foenumoside B from Lysimachia foenum-Graecum inhibits adipocyte differentiation and obesity induced by high-fat diet. Biochem. Biophys. Res. Commun. 2012, 417, 800–806. [Google Scholar] [CrossRef]
- Maruyama, H.; Kiyono, S.; Kondo, T.; Sekimoto, T.; Yokosuka, O. Palmitate-induced regulation of PPARγ via PGC1α: A mechanism for lipid accumulation in the liver in nonalcoholic fatty liver disease. Int. J. Med. Sci. 2016, 13, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Han, G.; Ferelli, M.; Kallichanda, N.; Lane, R.H. Programmed upregulation of adipogenic transcription factors in intrauterine growth-restricted offspring. Reprod. Sci. 2008, 15, 785–796. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Shon, M.S.; Shin, Y.J.; Lee, S.C.; Kim, G.N. Anti-obese activities of kaki-tannins prepared from various persimmons through inhibition of 3T3-L1 adipogenesis. Kor J. Aesthet. Cosmetol. 2014, 12, 539–546. [Google Scholar]
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef]
- Samuel Wu, Y.H.S.; Chiu, C.H.; Yang, D.J.; Lin, Y.L.; Tseng, J.K.; Chen, Y.C. Inhibitory effects of litchi (Litchi chinensis Sonn.) flower-water extracts on lipase activity and diet-induced obesity. J. Funct. Foods 2013, 5, 923–929. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivera, M.L.; Barragan-Galvez, J.C.; Gasca-Martínez, D.; Hidalgo-Figueroa, S.; Isiordia-Espinoza, M.; Alonso-Castro, A.J. In Vivo Neuropharmacological Effects of Neophytadiene. Molecules 2023, 28, 3457. [Google Scholar] [CrossRef] [PubMed]
- Siswadi, S.; Saragih, G.S. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar]
- Olofinsan, K.A.; Salau, V.F.; Erukainure, O.L.; Islam, M.S. Senna petersiana (Bolle) leaf extract modulates glycemic homeostasis and improves dysregulated enzyme activities in fructose-fed streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2023, 303, 115998. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, I.P.; Sousa Teixeira, M.V.; Jacometti Cardoso Furtado, N.A. An overview of biotransformation and toxicity of diterpenes. Molecules 2018, 23, 1387. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Um, H.J.; Kim, G.H. Studies on the flavonoid compositions of Elsholtzia spp. Korean. J. Food Nutr. 2007, 20, 103–107. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).