Abstract
Legume seeds, such as grass pea, yellow lupine, and narrow-leaf lupine, are highly nutritious and offer a wide range of health benefits. The objective of this research was to explore the possibility of partially replacing wheat flour (at levels of 10, 15, 20, and 25%) with flour derived from these legume seeds in sourdough wheat bread and examine its impact on the physicochemical and sensory properties of the bread. The physical properties of the dough were also assessed. The substitution of wheat flour with ground legume seeds resulted in increased water absorption (from 54.1 to 63.5%) and prolonged dough development time (from 2.0 to 13.5 min). Ground lupine seeds reduced the volume of the bread and increased its crumb density, consequently making the bread harder. The most significant increase in hardness was observed when narrow-leaf lupine flour was added to the wheat flour (from 8.4 to 22.5 N). Narrow-leaf lupine had the greatest impact on enhancing the protein content in the enriched bread (from 11.5 to 20%), while yellow lupine caused the highest increase in fiber content (from 1.9 to 6.9%). The proposed additives slightly but significantly (p < 0.05) increased the antioxidant activity and phenolic content in the bread samples. Importantly, for all legume seeds, replacing up to 15% of the wheat flour allowed the production of bread with high consumer acceptability.
1. Introduction
Bread, a widely consumed grain product worldwide, is predominantly made from wheat flour [1,2] which primarily contains starch (60–85%) and proteins (9–12%) [3,4]. The content of nutrients varies depending on the extraction rate [4]. The production of wheat bread typically involves the use of refined flours, also known as low-extraction flours, which are characterized by low levels of dietary fiber, proteins, mineral components, and bioactive compounds [2,3]. Furthermore, the proteins present in wheat flour are incomplete, lacking certain essential amino acids (mainly lysine, which limits their digestibility) [3,5,6].
Enhancing the nutritional quality of wheat bread can be achieved by incorporating natural plant-based ingredients into the recipe. Research has validated the positive nutritional impacts of adding herbs and spices to the bread [2,7,8,9,10], pomace [11,12,13,14,15], and pseudocereal flours [16,17]. Valuable recipe additions can also include flours made from legume seeds [4,18,19,20,21].
Leguminous plants belong to the family Leguminosae (or Fabaceae), which encompasses over 18,000 different species. Their cultivation accounts for approximately 15% of the world’s agricultural land [22]. In 2021, the production of legume seeds reached 88.97 million tons [23]. Legume seeds are a valuable source of food for people worldwide [24,25,26,27,28,29]. They are a good source of protein, dietary fiber, minerals, vitamins, and bioactive phenolic compounds [30,31,32,33,34]. Legume seeds contain anywhere from 17% to even 50% protein [34,35,36] that has a significantly higher biological value compared to grain protein [26,32,34]. They also have a lower glycemic index compared to grains [30,33,37,38]. Consuming legume seeds is recommended for the prevention of type 2 diabetes and can help better control glycemia in individuals with diabetes [39]. Regular consumption of legume seeds can also contribute to the prevention of other chronic diseases, such as obesity, cardiovascular diseases, and certain cancers [30,33,40]. Some of the most popular and commonly consumed legume seeds include peas, beans, broad beans, chickpeas, and lentils. Others, such as cowpeas, lupines, vetches, lentil vetches, or white lupines, are still underappreciated [27,29].
Grass pea, which has been grown since ancient times, is used in human nutrition and as animal feed is likely the oldest plant in Europe [41]. Currently, interest in this plant is limited to a few species, including Lathyrus sativus [42,43] and Lathyrus maritimus [44,45,46], mainly cultivated in India, Bangladesh, Pakistan, Nepal, Ethiopia, and China [47]. Grass pea contains 20–36% high-quality protein [48]. Its positive effects in treating cardiovascular diseases, hypoxia, and hypertension have been demonstrated [47,49,50,51].
Lupine has been cultivated for over 2000 years in Europe and South America [52,53]. Three main species of lupine are primarily grown: narrow-leafed lupine (Lupinus angustifolius), yellow lupine (Lupinus luteus), and white lupine (Lupinus albus). Lupine seeds have the highest protein content among all legume species (29–52% dry weight) [54]. The main protein groups in lupine are albumins and globulins, which are characterized by their high biological value due to a significant amount of essential amino acids, particularly lysine [1,54]. Lupine seeds are also a valuable source of carotenoids, tocopherols, and lecithin [4,55]. Consuming lupine seeds is recommended for the prevention of conditions such as hypercholesterolemia, diabetes, and arterial hypertension [4,19,56].
Due to their high nutritional value and sensory qualities, both grass pea and lupine seeds can be used as ingredients in functional food products [20,55,57,58]. This study aimed to assess the suitability of grass pea and lupine seed flour as a component in sourdough bread recipes using organic wheat flour as a base.
2. Materials and Methods
2.1. Raw Materials
To prepare the bread dough, the following ingredients were utilized: commercially available white wheat flour (type 550, BIO from BioLife Sp. z o.o. located in Bielsk Podlaski, Poland); fresh pressed yeast sourced from Lesaffre Polska S.A. in Wołczyn, Poland; and salt supplied by Cenos Sp. z o.o. in Września, Poland. Additionally, flours obtained from grass pea (GP) of the Derek variety, narrow-leaved lupine (NL) of the Roland variety, and yellow lupine (YL) of the Salut variety were used. The legume seeds were obtained from an organic cultivation experiment carried out at the Institute of Soil Science and Plant Cultivation—State Research Institute in Puławy. These seeds were harvested in 2022, cleaned, and subsequently ground into particles measuring less than 1.0 mm using an A11 analytical mill (IKA Works GmbH & Co., Staufen, Germany).
2.2. Baking Properties of Wheat Flour and Physical Properties of Dough
The baking properties of the wheat flour such as the yield and quality of gluten (AACC, Method 38.12), the falling number (AACC, Method 56–81B), and the physical properties of the dough were determined using a Farinograph-E model 810114 (Brabender GmbH & Co. KG, Duisburg, Germany (AACC Method 54–21) according to the approved methods of the American Association of Cereal Chemistry (AACC) [59]. Prior to the analysis, blends were prepared by replacing wheat flour with legume seed flours (GP, NL, and YL) in amounts of 10, 15, 20, and 25% by weight of the flour. The control sample consisted of wheat flour dough.
2.3. Baking Procedure
The bread dough was prepared using a two-phase method with wheat sourdough. The sourdough was inoculated with cultures of Lactiplantibacillus plantarum and Levilactobacillus brevis bacteria obtained from the collection of pure cultures of the Department of Food Technology and Assessment, Warsaw University of Life Sciences, Warsaw, Poland. The sourdough had a yield of 200%. Fermentation of the sourdough took place for 7 days at a temperature of 25 °C. The sourdough was added in an amount equivalent to 10% of the total weight of the wheat flour. The basic recipe for the bread dough (control sample) included 700 g of wheat flour, 70 g of wheat sourdough, 21 g of fresh pressed yeast, 10.5 g of salt, and water added as necessary to achieve a dough consistency of 350 BU. The amount of water added was determined based on the calculated water absorption capacity of the farinographic flour/blends. In the blends, wheat flour was substituted with legume seed flours, GP, NL, and annual yellow lupine (YL) in varying proportions of 10, 15, 20, and 25%. The bread was prepared according to the protocol described by Cacak-Pietrzak et al. [2]. In Table 1, an explanation of the description of the abbreviations used for the bread sample analysis is presented.

Table 1.
List of codes for analyzed bread samples.
2.4. Basic Composition of Raw Materials
The basic chemical composition (moisture (Method 44–15.02), ash (Method 08–01.01), protein (Method 46–10.01), and fat (Method 30–10.01)) of the wheat flour (WF), GP, NL, YL, and bread was determined using AACC methods [2,59]. Additionally, the amount of digestible carbohydrates was computed according to the difference [2].
2.5. Bread Yield, Volume, and Density
The bread loaves were weighed, and the bread volume, yield, and density were calculated [14,60]. The volume of the bread was measured using a 3D scanner (NextEngine, West Los Angeles, CA, USA) and calculated using computer software (MeshlLab, ISTI-CNR Research Centre, Rome, Italy). It was then converted to represent 100 g of bread. Additionally, the density of the bread’s crumb was also determined along with the ratio of the bread sample’s mass to its volume. The yield of bread (Y) was calculated as follows:
where b represents the weight of the dough portion before baking, c is the mass of the bread after cooling (in grams), and w is the dough yield. In addition, the baking losses were calculated [2].
2.6. Crumb Texture
The texture parameters of the crumb were determined using a TA.XT2i texture analyzer (Stable Microsystem, Surrey, UK). The analysis was conducted according to the methodology described by Romankiewicz et al. [60]. Briefly, circular samples with a diameter of 30 mm were obtained by cutting bread slices that were 20 mm thick. These samples were then compressed using a 25 mm diameter probe. The compression was set at 40% penetration with a 45 s delay between the first and second compressions. The probe speed was set at 1 mm/s. The texture parameters of the bread crumb were determined by analyzing the resulting curve using the Texture Expert Exceed v. 1.00 computer software.
2.7. Color of Raw Materials and Bread Samples
The color parameters of the raw materials and crumb samples (L*—lightness, a*—redness or greenness, and b*—yellowness or blueness) were determined using the reflectance method in the CIE-Lab* color space. The absolute color difference (ΔE) was also calculated [61].
2.8. Total Phenolic Content and Antioxidant Capacity
2.8.1. Extract Preparation
The hydroalcoholic extracts were prepared to determine the total phenolic content and antioxidant capacity of both the raw materials and bread samples. A total of 1 g of each type of raw material and bread was mixed with 50 mL of 50% (v/v) methanol. The mixture was allowed to extract for 30 min at room temperature and subsequently subjected to centrifugation at 9000× g for 15 min. The procedure was repeated three times, and the supernatants were collected for the analysis and stored at −20 °C until further analyses.
2.8.2. Total Phenolic Content
The total polyphenol content was determined using the Folin–Ciocalteu spectrophotometric method following the procedure described by Romankiewicz et al. [60]. The concentration of phenolic compounds was read from the standard curve determined for gallic acid (the linearity range was 10 µg/mL to 2000 µg/mL; R2 = 0.999) and expressed as the gallic acid equivalent (GAE) in mg/g DW.
2.8.3. Antiradical Activity against DPPH Free Radicals
The ability to quench free radicals of DPPH was determined using the spectrophotometric method [2,62]. The inhibition percentage of DPPH discoloration was calculated as in (1):
where Ac—the absorbance of control and Ap—the absorbance of extract.
AA = (Ac − Ap)/(Ac)·100%,
The antiradical activity was expressed as EC50 (efficient concentration), i.e., the concentration of sample (mg DW/mL) needed to obtain 50% of initial activity.
2.8.4. Antiradical Activity against ABTS•+ Free Radicals
The ability to quench cationic radicals ABTS•+ was determined according to the procedure described by Romankiewicz et al. [60] and Re et al. [63].
The results of antioxidant activity were expressed as the EC50 index (mg DM/mL) [2].
2.9. Sensory Evaluation of Bread
The sensory evaluation of the bread was conducted according to the methodology provided by Garcia-Gómez et al. [64] using a 9-point hedonic scale one day after baking. The evaluation team consisted of 52 panelists (employees and students of Warsaw University of Life Sciences, Warsaw, Poland) aged 20 to 58 years. The participants selected for the study were required to be regular bread consumers. Consumer evaluations were conducted in individual sensory booths within a sensory laboratory by utilizing a hedonic taste sheet. The bread samples were assessed at room temperature. Participants were instructed to rinse their mouths with water between samples to ensure a clean palate. The evaluations followed a sequential monadic test design employing a complete block design. The consumers were not provided any information about the samples and did not receive any monetary incentives to avoid bias in their participation.
2.10. Statistical Analysis of Results
The measurements were carried out at least three times, and the statistical analysis was conducted using Statistica 13.3 software from TIBCO Software (Palo Alto, CA, USA). The analysis involved performing an analysis of variance (ANOVA) and determining homogeneous groups using Tukey’s test with a significance level set at α = 0.05.
3. Results and Discussion
3.1. Water Absorption and Physical Properties of Dough
Conducting farinographic assessment is helpful in developing bread recipes and determining optimal parameters for the dough fermentation process [65]. The water absorption of the tested wheat flour was relatively low (54.1%) (Table 2) but typical for light wheat flours obtained from organic wheat grains [66]. The addition of flour made from legume seeds significantly increased the water absorption of the mixtures. The greatest changes in water absorption (increase up to 63.5%) occurred after adding YL at a 25% level (YLD25). This means that the dough with this additive could absorb more water, resulting in higher dough and bread yield in practice. The increased water absorption of the mixtures was probably due to the high protein and fiber content in the legume seed flours, as indicated by the research results of other authors [21,67,68]. The addition of flour made from legume seeds had a statistically significant effect on prolonging the dough development time from 2.0 min (CD) to 13.7 min (YLD15). The greatest increase in this parameter was observed with the addition of legume seed flour at a level of 10–15%, while further increasing the level of addition resulted in its decrease. Interestingly, the dough stability varied depending on the type and level of the additive, either increasing or decreasing. The dough stability of the control sample (CD) was 9.1 min. Significant prolongation of the dough stability was observed when adding YL at 10% and 15% levels (YLD10 and YLD15). On the other hand, a decrease in dough stability, indicating weakening of its structure, occurred when adding GP and NL at levels of 15% and above. The softening of the control dough (CD) was 35 BU. Changes in this parameter also depended on the type and level of the additive. The dough with GP and NL additives up to 15% (GPD10, GPD15, NLD10, and NLD15), as well as all dough with YL additives exhibited significantly less softening compared to the control sample (CD).

Table 2.
Water absorption and physical properties of dough and enriched dough samples.
3.2. Basic Properties of Bread Samples
During the baking and cooling process of bread, there is a loss in its mass caused by the evaporation of water and other volatile substances, such as carbon dioxide, alcohol, and volatile acids. As a result, in addition to the decrease in mass, there is also a loss in bread aroma [69]. Substituting a portion of wheat flour with YL and NL wheat flour resulted in a statistically significant reduction in bread baking loss from 11.6% (CB) to 10.1% (YLB25) (Table 3). However, the addition of GP increased the baking loss of the bread, but only when used in quantities of 15% and 20% (GPB15 and GPB20). The bread yield of the control (CB) was 139.0%. There was a statistically significant increase in the bread yield after adding YL at 15% and above, and at a 25% level of this additive, the bread yield increased up to 147.1% (YLB25). This can be attributed to the high content of fiber and protein in the added ingredient. These substances had a high capacity for absorbing and retaining water in the dough, resulting in better dough consistency and reduced water loss during the baking process [1,19]. The bread volume made from wheat flour (CB) was 365 cm3 per 100 g, and the crumb’s specific mass was 0.26 g·cm−3. The addition of legume flour significantly reduced the bread volume, which led to an increase in the crumb’s specific mass. The values of these parameters changed linearly with the increasing level of substitution of wheat flour with legume flours. The reduction in bread volume after introducing ingredients with high fiber content into the recipe can be explained by the phenomenon of interrupting the continuity of the gluten network by these components. Additionally, the formation of the gluten network may have been hindered due to the presence of additional non-gluten proteins [60,65,70]. The formation of a weaker gluten network resulted in the loss of part of the generated carbon dioxide during fermentation and, consequently, the production of bread with a smaller volume [71].

Table 3.
Basic properties of control and enriched bread samples.
3.3. Crumb Texture
The addition of flour made from legume seeds had a statistically significant impact on the increase in the hardness of bread compared to the control sample. The hardness of the control sample (CB) was 8.37 N (Table 4). As the level of legume flour addition increased, the values of this parameter increased linearly. The addition of GP at a level of 25% resulted in an almost twofold increase in bread hardness (13.04 N) compared to the control sample, while in the case of a 25% addition of YL and NL, the hardness of the bread increased almost threefold (21.19 N and 22.54 N, respectively). These changes were due to a decrease in loaf volume and an increase in crumb density, which made the crumb more compact and dense. Other texture parameters such as elasticity, springiness, and cohesiveness of the crumb gradually decreased with increasing levels of legume flour addition. These changes were generally statistically significant compared to the control sample except for bread enriched with up to 20% GP. Many studies [1,10,12,71] indicated that the addition of plant-based ingredients such as legume flour negatively affected the texture of wheat bread. These changes resulted from a weaker gluten network structure and reduced retention of carbon dioxide generated during dough fermentation.

Table 4.
Crumb texture of control and enriched bread samples.
3.4. Color of Raw Materials and Bread
Color is one of the key indicators of bread quality and plays a significant role in consumer acceptance [14,60]. The lightness values (L*) were significantly highest for the control sample (CB) and bread with 10% YL (YLB10) (Table 5). As the level of legume flour addition increased, the lightness of the bread crumb decreased linearly, which was due to the darker color of these added ingredients compared to wheat flour. Similarly, the values of the color parameters a* and b* changed with the increasing inclusion of legume flour in the bread formulation. Bread with a YL addition particularly exhibited high intensities of red and yellow colors, which corresponded to the color of this ingredient. The absolute color difference (ΔE) between the control bread and the bread enriched with legume flour ranged from 8.0 to 21.3. This indicated that even a 10% addition of legume flour had a significant impact on the color of the bread crumb, and the observed changes in terms of darkening were noticeable even to an inexperienced observer. Many studies [1,7,9,10,12,14,65] indicated changes in bread color resulting from enriching the composition with natural plant-based additives. These changes were caused by the presence of natural pigments in plant-based raw materials. Legume seeds, for example, contain carotenoids characterized by intense yellow-orange color. Lupine seeds, in particular, are rich in these compounds [4,55].

Table 5.
Color of raw materials, control, and enriched bread samples.
3.5. Basic Chemical Composition of Raw Materials and Bread
The parameters of wheat flour (WF) were as follows: total protein content—11.13% dry matter (DM), total ash content—0.59% DM, fiber content—1.83% DM, fat content—1.22% DM, and carbohydrate content—85.24% DM (Table 6). The wheat flour exhibited low enzymatic activity of amylolytic enzymes (falling number—310 s) and a low wet gluten yield of 23.3%, typical for light wheat flours obtained from organic grain milling [66]. It should be emphasized that the gluten showed good quality (gluten index 93). Flour from legume seeds contained significantly more total protein than wheat flour. The content of this component in the GP flour was 31.99% DM, while in the YL and NL flours, it was 34.01% and 48.0% DM, respectively. The high protein content in legume seeds has been indicated by the results of studies by many authors [18,21,22,41,58,68]. Flours from legume seeds also proved to be much better sources of fiber and, in the case of lupine flours, fat as well. The highest amounts of these components were found in the YL flour, namely 19.31% and 6.42% DM, respectively. The total ash content in the legume seed flours ranged from 3.31% to 3.83% DM, which means they contained 5.6 to 6.5 times more mineral components compared to the wheat flour.

Table 6.
Basic chemical composition of raw materials, control, and enriched bread samples.
The chemical composition of the control bread (CB) was as follows: total protein content of 11.45% DM, total ash content of 0.87% DM, fiber content of 1.90% DM, fat content of 1.29% DM, and carbohydrate content of 84.49% DM (Table 5). The moisture of the bread samples was between 37.1 and 38.2%. With an increase in the level of addition of flours from legume seeds, the total protein content, total ash content, crude fiber, and fat content increased significantly, while the carbohydrate content decreased compared to the control sample. This resulted in an increase in the nutritional value of the bread. Bread with the addition of flours from legume seeds can be a good source of complete protein mainly composed of albumins and globulins [41,54]. The protein content in the bread with 25% levels of GP, YL, and NL was as follows: 16.04% DM (GPB25), 16.89% DM (YLB25), and 20.00% DM (NLB25). Importantly, protein from lentils and lupines contains significant amounts of lysine [1,41,54], which is a limiting amino acid for the biological value of wheat protein [6]. Additionally, it is a good source of amino acids such as leucine and arginine [1,41,54]. The fiber content and mineral components increased when compared to the CB sample. The bread with 25% legume flour contained about twice as much dietary fiber (LSB25) or three times as much dietary fiber (LAB25 and LLB25) and about twice as many minerals.
3.6. Phenolic Content and Antioxidant Capacity
The total content of phenolic compounds in the raw materials ranged from 0.86 mg GAE g DM−1 in the wheat flour to 2.15 mg GAE g DM−1 in the YL flour. The extracts from these raw materials also exhibited the lowest and highest antioxidant activity, respectively (Table 7). Enriching bread with flour from legume seeds resulted in a slight but statistically significant increase in the phenolic content in the bread. The highest increase was observed in the bread enriched with NL (from 0.72 mg GAE g DM−1 (CB) to 1.12 mg GAE g DM−1 (NLB25)), while the smallest increase was observed in the bread with GP (from 0.72 mg GAE g DM−1 (CB) to 0.99 mg GAE g DM−1 (GLB25)). The values of the EC50 index were also significantly lower for the bread enriched with legume seed flour. This indicated higher antioxidant activity of the enriched bread. These relationships were observed for both antioxidant activities against DPPH and ABTS. The bread enriched with NL flour exhibited the highest activity against DPPH and consequently the lowest EC50 values, while the bread with GP flour showed the highest activity against ABTS. On the other hand, the bread enriched with YL flour had the lowest antioxidant activity against ABTS, and the bread with GP flour had the lowest antioxidant capacity against DPPH. The increase in the content of phenolic compounds and antioxidant activity in the enriched bread resulted from the use of whole grain legume seed flour as an additive. Most of the bioactive substances were present in the fruit-seed coat of seeds [31]. Many authors observed that flour enrichment with different additives plant additives increased the antioxidant activity of bread. This effect was especially visible when raw materials that were rich in fiber were incorporated into wheat flour [10,13,14].

Table 7.
Phenolic content and antioxidant capacity of raw materials, control, and enriched bread samples.
3.7. Sensory Evaluation Results
Currently, customers are increasingly seeking bakery products with enhanced nutritional value that retain sensory appeal. The nutritional value and sensory properties of bread depend on the type and quality of ingredients used as well as the applied technological process [71,72]. In our research, we used flour from seeds of selected legume species as an additional ingredient in the recipes. Additionally, the bread dough was prepared using a two-phase sourdough method, which is rarely used in industrial wheat bread production. When assessing the overall appearance of the loaf, the panelists paid attention to its shape, the degree of rising, and the appearance of the crust surface. The evaluators awarded the highest scores for these characteristics (8.5 points) to the control bread (CB) (Table 8), which had the most significant rise (Figure 1). The bread with a 10% addition of GP, YL, or NL (GPB10, YLB10, and NLB10) as well as a 15% addition of YL or NL (YLB15 and NLB15) obtained comparable scores to the control sample. In general, the addition of legume seed flour primarily resulted in a reduction in the degree of rising of the loaf, which was particularly noticeable at the highest level of addition (25%). However, the evaluators had no major concerns regarding the loaves’ shapes and the appearance of the crust surface. Therefore, all bread samples received ratings above 5 points on a 9-point hedonic scale for overall appearance, indicating consumer acceptability. The bread was also highly rated in terms of aroma and taste. The control bread received the highest ratings in these aspects (8.6 and 8.7 points, respectively), as it was exceptionally aromatic and had a delicate taste with a slightly perceptible sour note. According to the evaluators, the bread with a 10% addition of GP, YL, or NL (GPB10, YLB10, and NLB10) as well as a 15% addition of GP or NL (GPB15 and NLB15) had comparable aromas to the control sample. Similarly, bread with a 10% addition of GP, YL, or NL (GPB10, YLB10, and NLB10) as well as a 15% addition of GP or NL (GPB15 and NLB15) received scores comparable to the control sample in terms of taste. With a higher proportion of legume seed flour, a characteristic aroma and an aftertaste described as “bean-like” or “pea-like,” were noticeable. According to some panelists, the bread with the highest proportion of legume seed flour (25%) had a slightly bitter aftertaste. Based on the scores given for the taste of the bread, the consumer acceptability threshold was set at a level of 15% inclusion of GP and NL and 20% YL. Klupsaite et al. [71] obtained higher scores for the taste and aroma of bread with the addition of lupine sourdough at levels of 3% and 6% compared to the control sample. According to the panelists, this bread was also characterized by a more pronounced acidic taste.

Table 8.
Results of sensory evaluation of control and enriched bread samples (9-point hedonic scale).

Figure 1.
Appearance of obtained bread loaves. CB—control bread; GPB10-GPB25—bread with 10, 15, 20, and 25% of grass pea flour; NLB10-NLB25—bread with 10, 15, 20, and 25% of narrow-leaf lupine flour; YLB10-YLB25—bread with 10, 15, 20, and 25% of yellow lupine flour.
In terms of texture, the control bread received the highest rating (8.7 points) and was characterized by a uniform fine-pored structure of the crumb (Figure 2). The bread with a 10% addition of GP, YL, or NL (GPB10, YLB10, and NLB10) as well as a 15% addition of GP or YL (GPB15 and YLB15) obtained comparable scores for this characteristic. As the level of legume seed flour addition increased, the crumb became increasingly compact, and larger pores were also visible in the cross section. Therefore, the breads with a 20% proportion of GP or NL (GPB20 and NLB20) and a 25% proportion of YL (YLB25) were rated below the consumer acceptability threshold in terms of texture. The inclusion of legume seed flours in the recipe affected the color of both the crust and the crumb of the bread. The control bread had a light brown golden crust and a beige-colored crumb. As the proportion of legume seed flours increased, both the color of the crust and the crumb gradually darkened, with the crumb becoming more yellow in color. The consumer acceptability threshold for color was set at 15% for GP and YL and 20% for NL. A similar darkening of the crumb color was also observed by Klupsaite et al. [71] and Bartkiene et al. [58] when using lupine flour as an additive. In summary, the overall sensory evaluation scores indicated that the addition of GP and NL should not exceed 15%, while for YL, its maximum inclusion in the bread recipe could be 20%. On the other hand, Hall and Johnson [18] determined the maximum level of lupine flour addition to wheat bread accepted by consumers to be 10%.
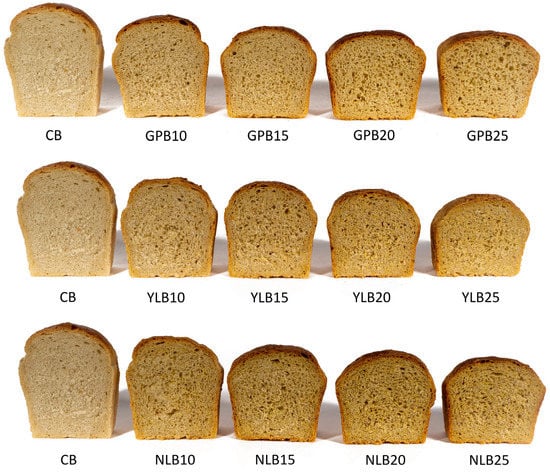
Figure 2.
Crumb of obtained bread samples. CB—control bread; GPB10-GPB25—bread with 10, 15, 20, and 25% of grass pea flour; NLB10-NLB25—bread with 10, 15, 20, and 25% of narrow-leaf lupine flour; YLB10-YLB25—bread with 10, 15, 20, and 25% of yellow lupine flour.
4. Conclusions
The partial substitution of wheat flour with legume flour in the analyzed raw materials had several effects. It led to an increase in water absorption, the development time of the dough, and the bread yield. The highest flour water absorption and bread yield were found for bread with yellow lupine flour, whereas the lowest was for the grass pea flour bread. Legume flours positively influenced the bread composition, including higher contents of protein, fiber minerals, and phenolic compounds. These improvements were most pronounced when narrow-leaf lupine flour was added to the wheat flour. Moreover, the lightness of crumb decreased, while redness and yellowness increased as a result of bread enrichment with lupine and grass pea flour. Additionally, all the additives used increased the antioxidant activity of the bread against ABTS and DPPH radicals. However, there were some negative consequences of the bread enrichment as well. The volume of the bread decreased, and the bread crumb became harder. Furthermore, sensory properties like smell, taste, and texture were negatively affected, resulting in reduced consumer acceptance of the bread, especially when the legume raw materials were added in amounts exceeding 15% of the total flour weight.
Author Contributions
Conceptualization, G.C.-P., K.S., J.K., J.B. and D.D.; methodology, G.C.-P., K.S., J.K., J.B. and D.D.; software, G.C.-P., K.S. and D.D.; validation, G.C.-P., K.S. and D.D.; formal analysis, G.C.-P., K.S. and D.D.; investigation, G.C.-P., K.S. and D.D.; resources, G.C.-P., K.S., J.K., J.B. and D.D.; data curation, G.C.-P. and D.D.; writing—original draft preparation, G.C.-P., K.S. and D.D.; writing—review and editing, G.C.-P., K.S. and D.D.; visualization, G.C.-P.; supervision, K.S. and D.D.; project administration, J.K. and J.B.; funding acquisition, J.K. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture and Rural Development under the topic “Research on the optimization of the selection of varieties in organic cultivation of agricultural plants, recommended for commercial field production, with particular emphasis on unfavorable climatic and soil conditions, especially related to water shortage, Determination of good practices of protection against pests in these crops, with particular emphasis on drought. Evaluation of the usefulness of seeds of selected leguminous plant species to improve the quality of bread” (Project No. DEJ.re.027.5202/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Research for this publication was conducted using research equipment purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CZiZ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Conflicts of Interest
The authors declare no conflict of interest.
References
- López, E.P.; Goldner, M.C. Influence of storage time for the acceptability of bread formulated with lupine protein isolate and added brea gum. LWT—Food Sci. Technol. 2015, 64, 1171–1178. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Wójcik, M.; Krajewska, A. Dandelion Flowers as an Additive to Wheat Bread: Physical Properties of Dough and Bread Quality. Appl. Sci. 2023, 13, 477. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S. Nutritional, Health, and Technological Functionality of Lupin Flour Additionto Bread and Other Baked Products: Benefits and Challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Varshney, A.; Rai, M.; Chikara, A.; Pohty, A.L.; Joshi, A.; Binjola, A.; Singh, C.P.; Rawat, K.; Rather, M.A.; et al. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. J. Agric. Food Res. 2023, 12, 100619. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.; Nieróbca, A.; Grabiński, J.; Studnicki, M.; Sujka, K.; Dziki, D. Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain. Plants 2023, 12, 364. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Biernacka, B. Cistus incanus L. as an Innovative Functional Additive to Wheat Bread. Foods 2019, 8, 349. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Kalisz, S.; Sujka, K. Effect of the Addition of Dried Dandelion Roots (Taraxacum officinale F. H. Wigg.) on Wheat Dough and Bread Properties. Molecules 2021, 26, 7564. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Hassonn, W.H.; Gawlik-Dziki, U.; Sułek, A.; Różyło, R.; Suger, D. The fruit of sumac (Rhus coriaria L.) as a functional additive and salt replacement to wheat bread. LWT—Food Sci. Technol. 2021, 136, 110346. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Łysiak, G.; Kulig, R.; Cacak-Pietrzak, G. Textural and sensory properties of wheat bread fortified with nettle (Urtica dioica L.) produced by scalded flour method. J. Food Process. Preserv. 2021, 45, e15851. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat bread fortification by grape pomace powder: Nutritional, technological, antioxidant, and sensory properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Cantero, L.; Salmerón, J.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.d.P.; Bustamante, M.Á.; Matias, S.; Navarro, V.; Simón, E.; Martínez, O. Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Appl. Sci. 2022, 12, 5934. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabríny, L.; Kačániová, M. Selected Physico-Chemical, Nutritional, Antioxidant and Sensory Properties of Wheat Bread Supplemented with Apple Pomace Powder as a By-Product from Juice Production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Parol-Nadłonek, N.; Kalisz, S.; Krajewska, A.; Stępniewska, S. Wheat Bread Enriched with Black Chokeberry (Aronia melanocarpa L.) Pomace: Physicochemical Properties and Sensory Evaluation. Appl. Sci. 2023, 13, 6936. [Google Scholar] [CrossRef]
- Stanciu, I.; Ungureanu, E.L.; Popa, E.E.; Geicu-Cristea, M.; Draghici, M.; Mitelut, A.C.; Mustatea, G.; Popa, M.E. The Experimental Development of Bread with Enriched Nutritional Properties Using Organic Sea Buckthorn Pomace. Appl. Sci. 2023, 13, 6513. [Google Scholar] [CrossRef]
- Derkanosova, N.M.; Stakhurlova, A.A.; Pshenichnaya, I.A.; Ponomareva, I.N.; Peregonchaya, O.V.; Sokolova, S.A. Amaranth as a bread enriching ingredient. Foods Raw Mater. 2020, 8, 223–231. [Google Scholar] [CrossRef]
- Cotovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.S.; Johnson, S.K. Sensory acceptability of foods containing Australian sweet lupin (Lupinus angustifolius) flour. J. Food Sci. 2004, 69, 92–97. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S. Optimization of formulation and process of Australian sweet lupin (ASL)—Wheat bread. LWT—Food Sci. Technol. 2015, 61, 359–367. [Google Scholar] [CrossRef]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carboni, D.A.; Salinas, V.M.; Puppo, C.M. Production of legume-wheat dough of optimum quality for breadmaking: Essential analyses required. Curr. Opin. Food Sci. 2023, 49, 100970. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 May 2023).
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Rajhi, I.; Baccouri, B.; Rajhi, F.; Mhadhbi, H.; Flamini, G. Monitoring the volatile compounds status of whole seeds and flours of legume cultivars. Food Biosci. 2021, 41, 101105. [Google Scholar] [CrossRef]
- Tas, A.A.; Shah, A.U. The replacement of cereals by legumes in extruded snack foods: Science, technology and challenges. Trends Food Sci. Technol. 2021, 116, 701–711. [Google Scholar] [CrossRef]
- Das, G.; Sharma, A.; Sarkar, P.K. Conventional and emerging processing techniques for the post-harvest reduction of antinutrients in edible legumes. Appl. Food Res. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Ulrike, M. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Akin-Ajani, O.D.; Ajala, T.O.; Ogunniyi, Q.A.; Fettke, J.; Odeku, O.A. Nutritional and pharmacological potentials of orphan legumes: Subfamily faboideae. Heliyon 2023, 9, e15493. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole grains and pulses: A comparison of the nutritional and health benefits. J. Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef] [PubMed]
- Temba, M.C.; Njobeh, P.B.; Adebo, O.A.; Olugbile, A.O.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- de Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Oyeyinka, A.S.; Singh, S.; Amonsou, E.O. A review on structural, digestibility and physicochemical properties of legume starch-lipid complexes. Food Chem. 2021, 349, 129165. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Mejia, S.B.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Antioxidant activity of seed polyphenols in fifteen wild Lathyrus species from South Spain. LWT—Food Sci. Technol. 2009, 42, 705–709. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Campbell, C.G. Genotype variation in BOAA, condensed tannins, phenolics and enzyme-inhibitors of grass pea (Lathyrus sativus). Can. J. Plant Sci. 1992, 72, 1037–1047. [Google Scholar] [CrossRef]
- Wang, X.F.; Warkentin, T.D.; Briggs, C.J.; Oomah, B.D.; Campbell, C.G.; Woods, S. Total phenolics and condensed tannins in field pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.). Euphytica 1998, 101, 97–102. [Google Scholar] [CrossRef]
- Chavan, U.D.; Amarowicz, R.; Shahidi, F. Antioxidant activity of phenolic fractions of beach pea (Lathyrus maritimus L.). J. Food Lipids 1999, 6, 1–11. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Amarowicz, R.; Shahidi, F. Phytochemical components of beach pea (Lathyrus maritimus L.). Food Chem. 2003, 81, 61–71. [Google Scholar] [CrossRef]
- Shahidi, F.; Chavan, U.D.; Naczk, M.; Amarowicz, R. Nutrient distribution and phenolic antioxidants in air-classified fractions of beach pea (Lathyrus maritimus L.). J. Agric. Food Chem. 2001, 49, 926–933. [Google Scholar] [CrossRef]
- Dixit, G.P.; Parihar, A.K.; Abhishek Bohra, A.; Singh, N.P. Achievements and prospects of grass pea (Lathyrus sativus L.) improvement for sustainable food production. Crop J. 2006, 4, 407–416. [Google Scholar] [CrossRef]
- Łabuda, S.; Chwil, S. Rhythm of biomass and macroelements accumulation in grass pea (Lathyrus sativus L.). Rocz. Glebozn. 1996, 47, 79–87. (In Polish) [Google Scholar]
- Rao, S.L. A look at the brighter facets of β-N-oxalyl-L-α,β-diaminopropionic acid, homoarginine and the grass pea. Food Chem. Toxicol. 2011, 49, 620–622. [Google Scholar] [CrossRef]
- Singh, S.S.; Rao, S.L.N. Lessons from neurolathyrism: A disease of the past & the future of Lathyrus sativus (Khesari dal). Indian J. Med. Res. 2013, 138, 32–37. [Google Scholar]
- Khandare, A.L.; Babu, J.J.; Ankulu, M.; Aparna, N.; Shirfule, A.; Rao, G.S. Grass pea consumption & present scenario of neurolathyrism in Maharashtra state of India. Indian J. Med. Res. 2014, 140, 96–101. [Google Scholar] [PubMed]
- Cowling, W.A.; Buirchell, B.J.; Tapia, M.E. Lupin. Promoting the Conservation and Use of Underutilized and Neglected Crops 23; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1998; pp. 1–100. [Google Scholar]
- Gladstones, J.S. Distribution, origin, taxonomy, history and importance. In Lupins as Crop Plants: Biology, Production and Utilisation; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Wallingford, UK, 1998; pp. 1–37. [Google Scholar]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Zill-e-Huma; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Jamróz, M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem. 2008, 109, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Juodeikiene, G.; Vidmantiene, D.; Viskelis, P.; Urbonaviciene, D. Nutritional and quality aspects of wheat sourdough bread using L. luteus and L. angustifolius flours fermented by Pedioccocus acidilactici. Int. J. Food Sci. Technol. 2011, 46, 1724–1733. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemistry Approved Methods, 10th ed.; AACC: St. Paul, MN, USA, 2000; Available online: http://methods.aaccnet.org/toc.aspx (accessed on 10 May 2023).
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvia hispanica L.) addition on quality and nutritional value of wheat bread. J. Food Qual. 2017, 2017, 7352631. [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophys. 2018, 33, 217–225. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.; Fernández-Canto, N.; Vázquez-Odériz, M.L.; Quiroga-García, M.; Muñoz-Ferreiro, N.; Romero-Rodríguez, M.Á. Sensory descriptive analysis and hedonic consumer test for Galician type breads. Food Control 2022, 134, 108765. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Gawlik-Dziki, U.; Sułek, A.; Kocira, S.; Biernacka, B. Effect of Moldavian dragonhead (Dracocephalum moldavica L.) leaves on the baking properties of wheat flour and quality of bread. CyTA—J. Food 2019, 17, 536–543. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Gromadzka, K.; Dziki, D. Milling and Baking Quality of Spring Wheat (Triticum aestivum L.) from Organic Farming. Agriculture 2021, 11, 765. [Google Scholar] [CrossRef]
- Rehman, S.; Alistair Paterson, A.; Hussain, S.; Murtaza, M.A.; Mehmood, S. Influence of partial substitution of wheat flour with vetch (Lathyrus sativus L.) flour on quality characteristics of doughnuts. LWT—Food Sci. Technol. 2007, 40, 73–82. [Google Scholar] [CrossRef]
- Wandersleben, T.; Morales, E.; Burgos-Díaz, C.; Barahona, T.; Labra, E.; Rubilar, M.; Salvo-Garrido, H. Enhancement of functional and nutritional properties of bread using a mix of natural ingredients from novel varieties of flaxseed and lupine. LWT—Food Sci. Technol. 2018, 91, 48–54. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Gałȩzewski, L.; Knapowski, T.; Kozera, W.; Wacławowicz, R. Mineral Composition and Baking Value of the Winter Wheat Grain under Varied Environmental and Agronomic Conditions. J. Chem. 2018, 2018, 5013825. [Google Scholar] [CrossRef]
- Steffolani, E.; Martinez, M.M.; León, A.E.; Gómez, M. Effect of pre-hydration of chia (Salvia hispanica L.) seed and flour on the quality of wheat flour breads. LWT—Food Sci. Technol. 2015, 61, 401–406. [Google Scholar] [CrossRef]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT—Food Sci. Technol. 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Cappelli, A.; Lupori, L.; Cini, E. Baking technology: A systematic review of machines and plants and their effect on final products, including improvement strategies. Trends Food Sci. Technol. 2021, 115, 275–284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).