Abstract
Intake of a postbiotic product can support immunity depending on specific conditions of the consumer. The present study evaluates the potential impact of baseline values on the change of various immune factors (α-defensin, β-defensin, cathelicidin, and secretory IgA) after three months of consumption of a postbiotic based on cow’s milk fermented with Lactobacillus paracasei CBA L74 in a young population. For the analysis, raw data of three studies were used in a multivariate analysis applying confounding factors. One study in newborns demonstrated that intake of the postbiotic yielded an increase in the concentrations of α-defensin and secretory IgA (at least p < 0.02), while for all factors, except β-defensin, the higher the baseline values the lower the increase (at least p < 0.002). Two combined studies in young children (aged 1–4 years) showed an increase in the concentration of all factors after intake of the postbiotic (at least p < 0.003), but now showing the higher the baseline values the higher the increase after three months (at least p < 0.02) in only the postbiotic group. It is concluded that consumption of the postbiotic leads to a baseline- and age-dependent increase in the concentrations of the immune factors under study in both newborns and young children. It is hypothesized that maturation of the immune system leads to different effects on optimizing host defense factors via this postbiotic intake.
1. Introduction
Pre- and probiotics in food applications are well known for their beneficial effects for human health [1,2]. A relatively novel development in this area is the application of postbiotics [3]. The concept of postbiotics is defined as all gut microbiome (GM)-derived substances that confer a beneficial effect to the host and do not meet the pre-/probiotic definition. These GM-derived substances can comprise both microbial compounds and microbial metabolism (synthesis of metabolites and products from microbial fermentation of several foods) [4,5]. Microbial compounds include peptidoglycans, polysaccharides, lipoteichoic acids, enzymes, bacteriocins, proteins, while microbial metabolites include lactic acid, peptides/proteins, organic acids, and short chain fatty acids (SCFAs) [5]. The beneficial impact of postbiotics is based on the observation that the positive effects elicited by the gut microbiome (GM) are mainly mediated by the production of metabolites [5]. Postbiotics exert similar beneficial health effects to probiotics, but the absence of viable microorganisms abolishes the risks associated with their intake. Consumption of probiotics might result in the occurrence of health issues to the consumer, such as translocation of microbes over the gut epithelium, the cross-over of antibiotic resistance genes, and sepsis [6]. These are caused by the introduction of viable bacteria into the gastro-intestinal tract. These issues might be circumvented using postbiotics, not only for the adult consumer but also for the (very) young, especially when the immune system starts to develop.
Increasing preclinical and clinical evidence supports the efficacy of the postbiotic product derived from cow’s milk fermentation with the probiotic Lactobacillus paracasei CBA L74 (FM-CBA L74) in preventing pediatric infectious diseases and affecting the maturation of the host defense mechanism [7,8,9,10,11,12]. These effects are paralleled with a beneficial regulation of innate and adaptive immune response and gut microbiota composition and function. These observations led us to analyze the impact of consumption of FM-CBA L74 on the baseline dependency of the change in various immune factors in more depth, especially when the immune status is developing.
Within the present study three studies have been identified to address the impact of the intake of the postbiotic (FM-CBA L74) on various immune markers in newborns and young children. These markers include α- and β-defensin, cathelicidin, and secretory immunoglobulin-A (s-IgA). Of these factors, defensins and cathelicidin (LL-37) are small cationic innate immunity peptides that exhibit a pivotal role in killing pathogens, by membrane disruption, but also display other functionalities, such as activation of various cell types, chemotactic activity, and cytotoxicity [13,14]. The α- and β-defensins are produced by neutrophils, macrophages, epithelial and Paneth cells. Secretory Immunoglobulin A (s-IgA) plays an important role in the first line of adaptive defense in the gastrointestinal tract by blocking the adherence of pathogenic bacteria to epithelial cell receptors and by inhibition of the activity of bacterial toxins [15]. Moreover, its impact on the establishment of gut microbiota and the development of immunity shortly after birth is underlined as well [16]. It is evident that s-IgA has a major impact on the adaptive component of immunity, already at the start of life [17]. The distinction between the innate and adaptive component is not as strict as suggested [18,19], but it is evident that the above-mentioned factors are of extreme importance in the defense against unwanted entry of pathogenic microorganisms. This is particularly true at birth when immunity is naive while at the same time intestinal permeability represents a serious threat [20].
Both in vitro and in vivo data showed that intake of FM-CBA L74 affected the immune response substantially, such as downregulating the inflammatory reaction by decreasing levels of IL-12p70 and increasing those of IL-10 [9]. This results in protection against colitis development in mice. In vitro innate immunity peptides (human β-defensin and LL-37) were upregulated in a dose-dependent fashion after stimulation of human epithelial cells by the same postbiotic product [10].
The present study aimed at performing a multivariate analysis of the effect of the intake of FM-CBA L74 on the change in fecal concentrations, and the baseline dependency in this, of both innate (α-defensin, β-defensin, and cathelicidin) and adaptive (secretory IgA (s-IgA)) components of the host immune system in newborns and in children (aged 1–4 years), as based on the raw data of the three studies mentioned above [7,8,12]. In this analysis, the impact of the baseline value of the respective immune factors on the development of these factors was specifically addressed, with baseline value defined as the actual value of the respective immune factor per person at the start of the consumption. A distinction of datasets between newborns and young children was made in order to check a potential effect of maturation of the immune system. It was postulated that this association was age-dependent with a first phase for immune initiation in the newborn and a second phase of immune maturation in the latter. Finally, the relationship between the changes in the various fecal immune factors was also investigated to determine to which extent an overall reaction by the above consumption could be detected.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
We performed a systematic review of Randomized Controlled Trials (RCTs) and observational studies (cohort studies, case series) evaluating the effects of cow’s milk fermented with Lactobacillus paracasei CBA L74 on immunological factors in newborns and young children. A comprehensive literature search was conducted in PubMed and Web of Science. Search strategies were adjusted for each engine using the following combination of keywords: L. paracasei CBA L74 AND α-defensin, secretory IgA, β-defensin and cathelicidin AND placebo, double-blind, randomized controlled trials AND postbiotic with no limitations for time or language. We excluded studies published only as meeting abstracts, pre-marketing clinical trials, or duplicates.
2.2. Studies Involved
Three studies were identified and included in the present evaluation: Corsello et al., 2017 [7], Nocerino et al. [8], and Roggero et al., 2020 [12], all of them being placebo, double-blind, randomized controlled trials with a parallel design. The main aim of the newborn study [12] was to address the impact of the investigational product (FM-CBA L74) on immune defense mechanisms (antimicrobial peptides, s-IgA), the microbiota and its metabolome, while that in the two young infant studies [7,8] the focus was on intake of the investigational product and reducing common infectious diseases in children attending daycare or preschool. In the newborn study three various groups were defined: breastfed, standard formula fed (based on cow’s milk), and fermented formula fed. In the present study, the standard formula fed was taken as reference and evaluated against the fermented formula fed. As a reference group in the young children study a maltodextrin control was applied.
Please find the various details listed in Table 1.

Table 1.
Main features of the studies considered in the analysis.
The following exclusion criteria were used in the respective studies:
Corsello [7] and Nocerino [8]:
- -
- age below 12 months or above 48 months.
- -
- concomitant chronic infections, chronic systemic diseases, chronic inflammatory bowel diseases, autoimmune diseases, immunodeficiency, malignancy, metabolic diseases, chronic respiratory tract diseases including respiratory allergies and cystic fibrosis, malformations of gastrointestinal or urinary or respiratory tract, history of respiratory or gastrointestinal or urinary tract surgery, congenital cardiac defects, functional bowel disorders, suspected or challenge-proved food allergy, food intolerances, severe malnutrition (z-score for weight-for-height) and use of antibiotics or pre/pro/synbiotics or immune stimulating products in the 2 weeks before the enrolment.
Roggero [12]:
- -
- the presence of congenital diseases, chromosomal abnormalities, and/or conditions that could interfere with growth, such as brain, metabolic, cardiac and gastrointestinal diseases, perinatal infections, being born to a mother affected by endocrine and/or metabolic diseases or having a family history of allergic disease.
Preparation of the investigated product.
The preparation of the product was already described in various papers. In short: the basis of the product was skimmed milk fermented with L. paracasei CBA L74, which was isolated from the feces of healthy infants. The fermentation was initiated with appr. 1.0 × 106 colony-forming units (CFU) and stopped when reaching appr. 5.9 × 109 CFU/g (after a 15 h incubation at 37 °C). Live bacteria were then inactivated by heating at 85 °C for 20 s, subsequently, the formula was spray dried.
2.3. Ethics
Because the present investigation deals with the raw data of three already published studies, no patients were involved in setting the research question or the outcome measures of the present manuscript; therefore, no ethics approval was required. Permission of the responsible author of the three published papers to use the raw data was obtained.
2.4. Data
Raw data of all studies were obtained from one of the respective responsible authors and used in the various analyses. Since the baseline values of the various immune factors were substantially different between newborns and young children they were separately analyzed and presented. The data of both studies on young children were combined into one dataset.
Before performing the various statistical analyses, the distribution in the data was checked via Shapiro–Wilks (gamma-3 and gamma-4). The data of the Intention-to-Treat (ITT) population of both studies was analyzed in the present manuscript.
2.5. Analysis of the Outcome Using the Data of the Three Studies
Differences in baseline concentrations of the various immune factors between placebo and investigational product as well as between newborns and young children was performed via Student’s t-test. A general Estimating Equations (GEE) analysis or a multiple regression analysis, both techniques in a linear as well as in quadratic fashion, was applied with respect to the change in the concentration of the various immunological parameters considering various potentially confounding factors, as follows: gender, age, breastfeeding, duration, number of siblings, passive smoking, age at school, and BMI. Both multivariate analyses allowed to distinguish an overall difference in outcome (all values higher in one group over the other, independent of the baseline concentration) between both groups versus a changing difference (in- or decreasing difference in outcome dependent on the actual baseline concentrations). This was achieved via the inclusion of interaction terms in the multivariate analyses. All analyses were performed in a stepwise and dummy approach, fit of the model was completed via F-test or Wald Chi-square evaluation.
2.6. Association between Parameters
Association was calculated via regression analysis, either parametric or Poisson, depending on the distribution of the data. Normality in the residual fraction was checked.
Throughout the present study, a p-value of 0.05 was considered to identify significance applying two-sided testing. Statistical analysis was performed via Stata, version 12 (Statacorp, College Station, TX, USA) and GraphPad, version 6 (GraphPad Prism, San Diego, CA, USA), the latter was also used for graphical representation of the data.
3. Results
3.1. Start-Values
The baseline levels of the various parameters of the evaluated studies are listed in Table 2, enabling a comparison of concentrations between newborns and young children.

Table 2.
Descriptive values of the baseline levels of the various immunological factors in the stool samples collected from newborns and from young children.
No differences were encountered in the concentration of the various baseline values between placebo and postbiotic per newborns or young children. However, comparison of the baseline values of the various factors between newborns and young children as presented in Table 2 revealed significant differences for α-defensin (p < 0.001) and β-defensin (p < 0.00001), both being higher in the newborns than in the young children, and cathelicidin (p < 0.0000001), being higher in the young children than in the newborns. The somewhat higher values of s-IgA in the newborns as compared to those in the young children are not of statistical significance (p > 0.25), giving the relatively large standard deviation.
3.2. The Roggero Study: Newborns
A significant (p < 0.002) association between the fecal concentration of α-defensin at the baseline and the change in these concentrations after 3 months of consumption was observed in both groups (Figure 1), being inverse (the higher the baseline concentration the lower the increase). Moreover, throughout the range of baseline values, the changes were significantly (p < 0.02) larger (more positive) for the postbiotic than for the control product. The method of delivery (spontaneous versus caesarian section) had an impact as well (p < 0.02) with larger changes in the former.
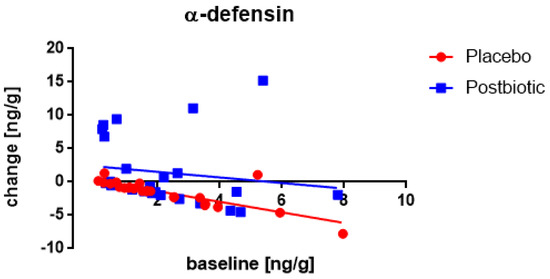
Figure 1.
Newborns: the association between the baseline concentration of fecal α-defensin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
No significant association between the (squared) fecal concentration of β-defensin at the baseline and the change in these concentrations after 3 months of consumption was observed (Figure 2). Only at higher baseline values the increase became significantly (p < 0.003) larger (more positive) for the postbiotic than for the control product, acknowledging the parabolic fit in both groups. The bodyweight at the start showed a significant (p < 0.03) effect on the change in β-defensin as well demonstrating the higher the weight the lower the change.
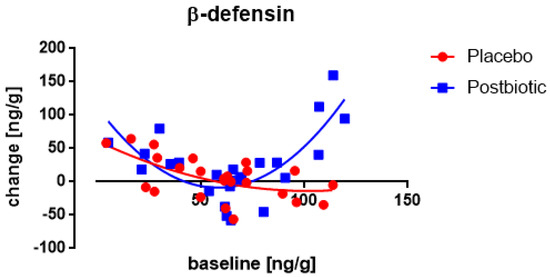
Figure 2.
Newborns: the association between the baseline concentration of fecal β-defensin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
A significant (p < 0.001) association between the fecal concentration of cathelicidin at the baseline and the change in these concentrations after 3 months of consumption was observed in both groups (Figure 3), being inverse (the higher the baseline concentration the lower the increase). There were no differences in these changes between the postbiotic and the control product established. No impact of any confounder on the change in concentrations was noted.
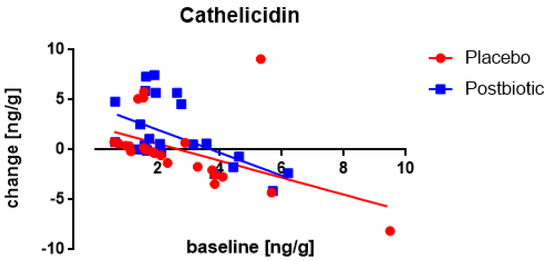
Figure 3.
Newborns: the association between the baseline concentration of fecal cathelicidin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
In both groups, a significant (p < 0.001) association between the fecal concentration of s-IgA at the baseline and the change in the concentration after 3 months of consumption was observed (Figure 4), being inverse (the higher the baseline concentration the lower the increase). Moreover, throughout the range of baseline values, the changes were significantly (p < 0.001) larger (more positive) for the postbiotic than for the control product. No impact of any confounder on the change in concentrations was noted.
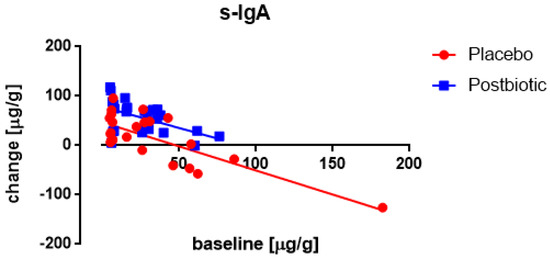
Figure 4.
Newborns: the association between the baseline concentration of fecal s-IgA and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
Overall, after consumption for 3 months the changes in all biomarkers, except that of β-defensin, were inversely associated with the baseline value of the respective biomarker while the increase in values of α-defensin and s-IgA was significantly larger after consumption of the postbiotic than after that of the placebo.
3.3. Nocerino and Corsello Studies: Children Aged 1–4 Years
A significant (p < 0.003) association between the fecal concentration of α-defensin at the baseline and the change in these concentrations after 3 months of consumption was only observed for the postbiotic group (Figure 5), being positive (the higher the baseline concentration the larger the increase). Moreover, throughout the range of baseline values, the increases were significantly (p < 0.002) larger for the postbiotic than for the control product and obviously, the difference was increasing at higher baseline values (p < 0.001). No impact of any confounder on the change in concentrations was noted.
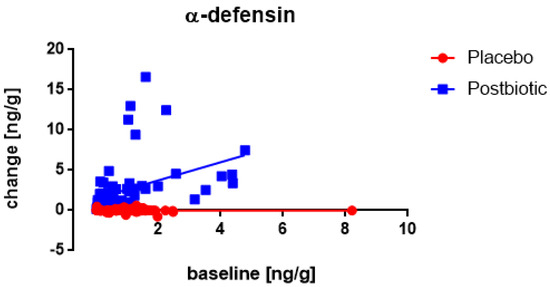
Figure 5.
Young children: the association between the baseline concentration of fecal α-defensin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
Only for the experimental postbiotic a significant (p < 0.02) association between the fecal concentration of β-defensin at the baseline and the change in these concentrations after 3 months of consumption was observed (Figure 6), being positive (the higher the baseline concentration the larger the increase). Moreover, throughout the range of baseline values, the increases were significantly (p < 0.003) larger for the postbiotic than for the control group and obviously, this difference increased at higher baseline values (p < 0.001). Two other factors were relevant: the age and the body weight. The older children presented a larger increase in β-defensin (p < 0.005) than younger children and children with a higher body weight showed a lower increase in β-defensin (p < 0.005).
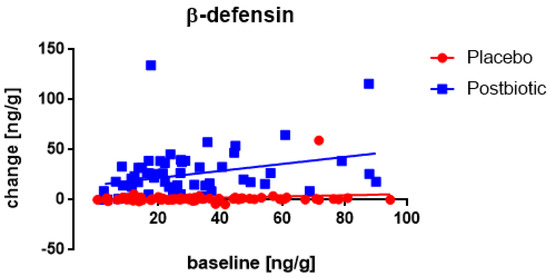
Figure 6.
Young children: the association between the baseline concentration of fecal β-defensin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
A significant (p < 0.002) association between the fecal concentration of cathelicidin at the baseline and the change in the concentration after the 3-month consumption period was only observed for the postbiotic group (Figure 7), being positive (the higher the baseline concentration the larger the increase). Moreover, throughout the range of baseline values, the increases were significantly (p < 0.001) larger for the postbiotic group than for the control product and obviously, this difference was found to increase at higher baseline values (p < 0.002). The increase in cathelicidin was larger in males than in females (p < 0.03).
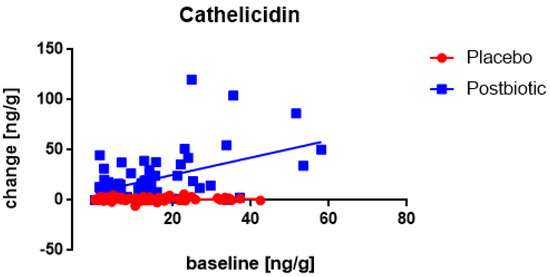
Figure 7.
Young children: the association between the baseline concentration of fecal cathelicidin and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
A significant (p < 0.006) association between the fecal concentration of secretory IgA at the baseline and the change in these concentrations after 3 months of consumption was only observed in the postbiotic cow’s milk drink (Figure 8), being positive (the higher the baseline concentration the larger the increase). Moreover, throughout the range of baseline values, the increases were significantly (p < 0.001) larger for the postbiotic group than for the control group, no increase in this difference at higher baseline values was noted. No impact of any confounder on the change in concentrations was noted.
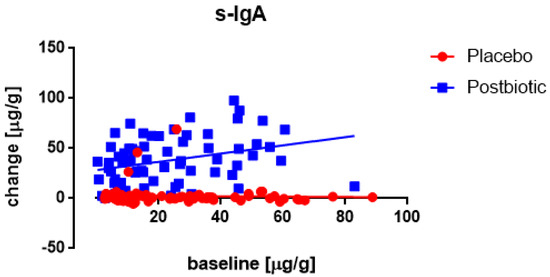
Figure 8.
Young children: the association between the baseline concentration of fecal s-IgA and the change in these concentrations after 3 months of consumption. Each value represents one single observation.
Overall, the values of all biomarkers yielded a significantly larger increase at the end of the 3-month consumption period in the postbiotic group if compared with the placebo group. This difference increased further in subjects with higher baseline values, except for s-IgA.
3.4. Associations between the Changes in the Various Immunological Factors
The studies in young children demonstrated significant positive interrelationships for all biomarkers, the lowest being that between α-defensin and s-IgA with a p-value of 0.018 (for all others p < 0.002): an increase in one factor was associated with that in another. The study in newborns showed positive associations only for the combinations α-defensin and β-defensin (p < 0.001) and s-IgA and β-defensin (p < 0.05), all others exerted no statistical significance.
3.5. Butyrate: Only One Study Nocerino et al., 2017: Young Children Aged 1–4 Years
No significant association between the fecal concentration of butyrate at the baseline and the change in these concentrations after 3 months of consumption was observed (Figure 9). Throughout the range of baseline values, the increases were significantly (p < 0.002) larger for the postbiotic group than for the control group. Both gender and age yielded a significant association with the increases in concentrations: females showed a larger (p < 0.03) increase than males, and older children a larger (p < 0.02) increase than younger children.
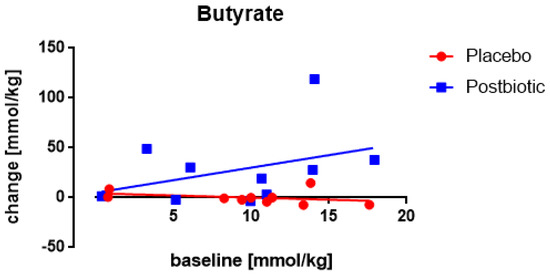
Figure 9.
Young children: the association between the baseline concentration of fecal butyrate and the change in these concentrations after the 3-months study period. Each value represents one single observation.
A negative association between the increase in butyrate and the number of common infectious diseases (CID: both respiratory and gastro-intestinal tract) was observed (p < 0.03) (Figure 10): the higher the increase in butyrate the lower the number of CID (Figure 10). The observed association showed an exponential relationship: N total CID = 2.28.e (−0.029.increase in butyrate), showing an asymptotic level at the theoretical value of no total CID at relatively high levels of butyrate.
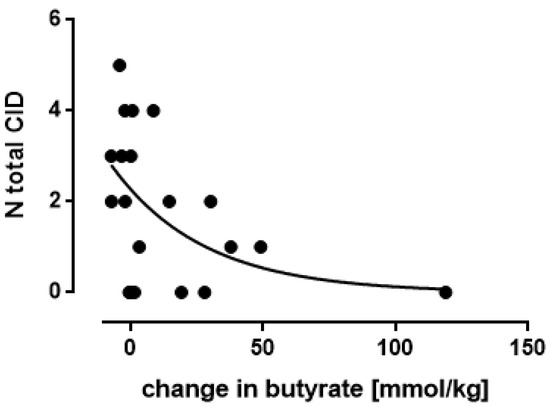
Figure 10.
Young children: the association between the change in fecal butyrate and the absolute number of common infectious diseases (CID), both respiratory and gastro-intestinal, at the end of the experimental interval (day 90), as observed in one study (Nocerino, 2017). Each symbol represents one observation, the curve shows the exponential regression curve.
4. Discussion
The main aim of the present study was to investigate a potential difference in the impact of consumption of the postbiotic FM-CBA74 on the concentration of various immune markers between newborns and young children, more specifically regarding a potential baseline dependent effect. The underlying thought was that the immune system is considered to be in a different phase between both age groups: in the newborns it is starting to develop whereas in young children the system is already in a more matured state. This perspective could affect the relationship between baseline value and the potential of an increase in the various factors. The results of the present study show that the 3-month consumption with CBA L74 exerts a beneficial stimulatory activity on the various immune biomarkers in newborns (α-defensin and s-IgA) and in young children (all factors) of which three represent the innate (α-defensin, β-defensin, and cathelicidin) and one (s-IgA) the adaptive component s-IgA. The baseline levels of the immune biomarkers had a significant influence on the increase after the 3-month consumption period: those in the newborns being negative (the higher the value at the start the lower the increase after 3 months) except for β-defensin, whereas those in the young children being positive for only the postbiotic group (the higher the value at the start the larger the increase after 3-month consumption) whereas in the placebo group no impact was observed at all. Acknowledging the fact that the number of participants in the newborn study was substantially lower than that in the studies on young children, clear associations of the increases in the biomarkers were demonstrated for most of the parameters in the latter while in the former only between α-defensin and β-defensin, and between s-IgA and β-defensin. Overall, consumption of the postbiotic yields higher levels of all immune biomarkers studied in both newborns and young children. Moreover, the effect of the starting levels is found shortly after birth irrespective of the drink and in children 1–4 years of age only after consumption of the postbiotic.
To our knowledge, the differentiating influence of the baseline value on the increase in the various immune markers between newborns and young children as observed in the present manuscript is presented for the first time. This phenomenon might be explained by a different maturation stage of the immune system between newborns (initiation stage) and young infants (maturation stage). One of the important characteristics during maturation is the decrease in intestinal permeability [21] leading to a difference in uptake of macromolecules and immunoglobulins between or via the intestinal epithelial cells. Importantly, a direct effect of feeding patterns of breast versus formula milk on tight junction functionality via zonulin has not been established [22]. Another striking feature is the enhanced endocytosis capacity of immature intestinal epithelial cells shortly after birth [23], which might give rise to a sensitization of the appropriate immune cells leading to an upregulation of immune markers. With respect to a difference in development between the innate and the adaptive component of immunity, the observation that the development of the latter starts at birth whereas that of innate immunity already during pregnancy [24] might be of importance here. This timing of the innate component enables the newborn to combat potential pathogens effectively from birth onwards. The results of the present study show that the baseline concentration of cathelicidin is higher in young infants than in newborns in contrast to those of alpha- and beta-defensin, suggesting that not all innate immunity components develop in the same time-dependent way. This phenomenon might be explained by a different phase in the immunological development of the child as mentioned above. No such significant differences were observed for s-IgA levels, representing the adaptive component. At birth, as demonstrated [12], consumption of the postbiotic FM-CBA L74 led to an increase in s-IgA levels to similar values as those of breastfed newborns, in contrast to that observed in the placebo. Compensation in this might take place: newborns with high levels of immune factors at the start should avoid even higher levels to minimize unwanted side effects, resulting in a negative association with starting levels. At 1–4 years old the immune system is readily developed, especially in the humoral part of immunity [25], leaving no room for further improvement in factors, except when stimulated, as in the present study by consumption of the postbiotic cow’s milk drink: the positive association between the increases and the start levels is only observed in this group and not in the placebo.
Another factor of importance for the baseline concentrations of immune markers is the development of the microbiome, which depends, if applicable, on the selection of the infant formula [26]. The diet of the mother during pregnancy is known to affect this composition [27] as well as the general status of the mother [28]. Overall, the composition of the microbiome is affected by many other factors, such as method of delivery, maternal diet, introduction to solid food, etc. [29]. The relationship between microbiome and the concentration of immune factors at any age during development needs further attention.
Is the increase in the concentration of the various factors as established by the consumption of the intervention potentially harmful to the host? The actual concentration of the fecal parameter 3 months after the start of consumption of the intervention product is far below the levels observed during inflammation/infection, such as β-defensin in children with inflammatory bowel diseases [30]: in children suffering from ulcerative colitis a median level of 356 ng/g was obtained. The same holds true in newborns. A study in preterm neonates fed with a combination of breast milk or formula, or combinations hereof, from complete breast milk to complete formula feed, β-defensin concentrations of about 340 ng/mL in neonates on predominant formula milk were encountered [31]. Similar observations were found for s-IgA. A study on the impact of various formulas on fecal s-IgA levels in healthy infants [32] showed that in the control group (after 26 wks of intervention) concentrations of 377 μg/g versus 729 μg/g in the GOS/FOS group was observed. The above shows that concentrations as yielded in the present study due to the intervention with the postbiotic cow’s milk drink, were substantially lower than documented for the various conditions as mentioned in the various studies above. It is hypothesized that the levels in the present study are representative for a state of vigilance optimizing the immune system in newborns and young infants, not to be compared with those during a state of infection/inflammation with unwanted side effects.
Is the increase in concentrations as observed in the present study associated with a beneficial physiological effect? In the study on newborns [12], consumption for 3 months of the fermented cow’s milk yielded s-IgA levels similar to those observed in newborns on breast milk whereas those in the control group remained significantly lower: consumption of the intervention clearly led to an improvement in the immune status in these newborns. In the child study [7,8], the increase in immunological parameters was paralleled by a reduction in the incidence of common infectious diseases (CID), although a direct causal relationship was not substantiated. It seems that the focus seems to be to optimize the immune system into a state of vigilance to eradicate pathogens without leading to a complete activation with all unwanted side effects. It remains to be established to which extent certain threshold levels differ between newborns and young children which might explain the difference in baseline effect between newborns and young children as observed in the present study. Again, it is stressed that in the present study the number of newborns is relatively low.
In the study of Nocerino [8], butyrate determinations in feces were also performed but unfortunately only in a limited number of children. The observation is of importance since the increases in fecal concentrations of this factor turned out to be associated with the number of total infections in these children: the higher the concentration of butyrate the lower the number. At high concentrations this number theoretically decreases to no infections at all.These results fit well within the antimicrobial activity of this molecule produced by specific gut bacteria [33]. Another functionality of butyrate is to fuel the epithelial lining cells leading to a proposed optimized barrier function against pathogenic organisms [34].
Unfortunately, there are various drawbacks attached to the study as laid down in the present manuscript. The selection of members of the innate and especially of the adaptive component of the immune system might be too limited to generate overall conclusions on the impact of the consumption of the investigated postbiotic on immune status. Moreover, only humoral factors have been considered, leaving the cellular part blank. However, the investigated factors belong to the very early phase of immune response followed by subsequent reactions. The target groups in the various studies were addressing the early phases in life. To which extent the same conclusions hold during adolescence and adulthood remain to be detected. Moreover, the number of observations in the newborns is relatively low which potentially might lead to a lack of sensitivity and specificity; an increase in numbers to confirm the present results is certainly encouraged.
In conclusion, the results of the present study reveal a stimulatory effect of consumption of FM-CBA L74 on the fecal concentrations of various immune factors representing both the innate and adaptive component in both newborns and young children. The main difference between both age groups is that in newborns, both treated and control, the starting concentration of the various factors demonstrate a negative impact on the magnitude of increase (the higher at the start the lower the increase) whereas in the young children a positive effect was shown but then only in the treated group (the higher the baseline concentration the larger the increase). It is hypothesized that this is caused by a different maturation phase of the immune system between both age groups. It is obvious that these results should be investigated in older persons as well to establish a general overview of the impact of FM-CBA L74 on immunity.
Author Contributions
Conceptualization, W.C. and A.B.; methodology, W.C.; software, W.C.; validation, W.C., V.C. and A.B.; formal analysis, W.C.; investigation, W.C., D.v.O., V.C., L.B., L.P., C.B., L.C., L.V., S.C., L.C. and A.B.; resources, V.C. and A.B.; data curation, W.C. and V.C.; writing—original draft preparation, W.C.; writing—review and editing, W.C.; visualization, W.C.; supervision, W.C. and A.B.; project administration, W.C. and V.C.; funding acquisition, V.C. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by KraftHeinz, Nijmegen, the Netherlands: WC received financial support for the research as described and the writing of the present manuscript.
Institutional Review Board Statement
Because the present investigation deals with three already published studies, no patients were involved in setting the research question or the outcome measures; therefore, no ethics approval was required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the various studies.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality reasons.
Acknowledgments
The authors would like to dedicate this manuscript to the memory of Dick van Olderen, who sadly passed away on 7 July 2021. They would also like to express their gratitude to Roberto Berni Canani and Rita Nocerino for sharing their data as well as providing critical remarks during the writing of the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, or in the writing of the manuscript.
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. Expert consensus statement: The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document. The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Toalá, J.E.; Arioli, S.; Behare, P.; Belzer, C.; Berni Canani, R.; Chatel, J.M.; D’Auria, E.; de Freitas, M.Q.; Elinav, E.; Esmerino, E.A.; et al. Postbiotics—When simplification fails to clarify. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 825–826. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl 2), S129–S134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; de Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P. Preventive effect of cow’s milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano., F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef]
- Paparo, L.; Aitoro, R.; Nocerino, R.; Fierro, C.; Bruno, C.; Berni Canani, R. Direct effects of fermented cow’s milk product with Lactobacillus paracasei CBA L74 on human enterocytes. Benef. Microbes 2018, 9, 165–172. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Laiola, M.; Paparo, L.; Calignano, A.; De Caro, C.; Coretti, L.; Chiariotti, L.; Gilbert, J.A.; et al. Specific Signatures of the Gut Microbiota and Increased Levels of Butyrate in Children Treated with Fermented Cow’s Milk Containing Heat-Killed Lactobacillus paracasei CBA L74. Appl. Environ. Microbiol. 2017, 83, e01206-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Berni Canani, R.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Lehrer, R.I. Defensins. Eur. J. Haematol. 1990, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Eckmann, L.; Leopard, J.D.; Varki, N.; Kagnoff, M.F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Inf. Immun. 2002, 70, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, C.; Han, X.; Huang, W.; You, Y.; Zhan, J. Role of IgA in the early-life establishment of the gut microbiota and immunity: Implications for constructing a healthy start. Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Bridgman, S.L.; Konya, T.; Azad, M.B.; Sears, M.R.; Becker, A.B.; Turvey, S.E.; Mandhane, P.J.; Subbarao, P.; CHILD Study Investigators; Scott, J.A.; et al. Infant gut immunity: A preliminary study of IgA associations with breastfeeding. J. Dev. Orig. Health Dis. 2016, 7, 68–72. [Google Scholar] [CrossRef]
- Raj, P.A.; Dentino, A.R. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol. Lett. 2002, 206, 9–18. [Google Scholar] [CrossRef]
- Van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.M.; Van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 direct macrophage differentiation towards macrophages with a proinflammatory signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Campeotto, F.; Baldasarre, M.; Laforgia, N.; Viallon, V.; Kalach, N.; Amati, L.; Butel, M.J.; Dupont, C.; Kapel, N. Fecal expression of human β-defensin-2 following birth. Neonatology 2010, 98, 365–369. [Google Scholar] [CrossRef]
- Molès, J.P.; Tuaillon, E.; Kankasa, C.h.; Bedin, A.S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kolyva, S.; Triga, M.; Kritikou, D.; Chrysis, D. The effect of feeding patterns on serum zonulin levels in infants at 3-4 months of age. Eur. J. Pediatr. 2021, 180, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.; Sureda, E.A.; Pierzynowska, K.; Pierzynowski, S.; Pérez-Cano, F.J. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatter, C.; Trigo, N.F.; Christensen, S.; Ganal-Vonarburg, S.S. Maternal microbiota, early life colonization and breast milk drive immune development in the newborn. Front. Immunol. 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71, S112–S120. [Google Scholar] [CrossRef]
- Fabiano, V.; Indrio, F.; Verducci, E.; Calacaterra, V.; Pop, T.L.; Mari, A.; Zuccotti, G.V.; Cokugras, F.C.; Pettoello-Mantovani, M.; Goulet, O. Term infant formulas influencing gut microbiota, an overview. Nutrients 2021, 13, 4200. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Garcia-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442. [Google Scholar] [CrossRef]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef]
- Verducci, E.; DÁuria, E.; Bosetti, A.; DI Profio, E.; Vizzuso, S.; Milanta, C.; Pendezza, E.; Borsani, B.; Zuccotti, G.V. Immunomodulatory diet in pedriatic age. Minerva Pediatr. 2021, 73, 128–149. [Google Scholar]
- Kapel, N.; Benahmed, N.; Morali, A.; Svahn, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. Fecal beta-defensin-2 in children with inflammatory bowel diseases. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 117–120. [Google Scholar] [CrossRef]
- Corebima, B.I.R.V.; Rohsiswatmo, R.; Gavatri, P.; Patole, S. Fecal human β-defensin-2 (hBD-2) levels and gut microbiota patterns in preterm neonates with different feeding patterns. Iran J. Microbiol. 2019, 11, 151–159. [Google Scholar] [PubMed]
- Scholtens, P.A.M.J.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.J.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SFCAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).