The Heritability of Upper Airway Dimensions Using MRI Scans in Twins
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MR Imaging
2.3. Statistical Analysis
3. Results
3.1. Study Population
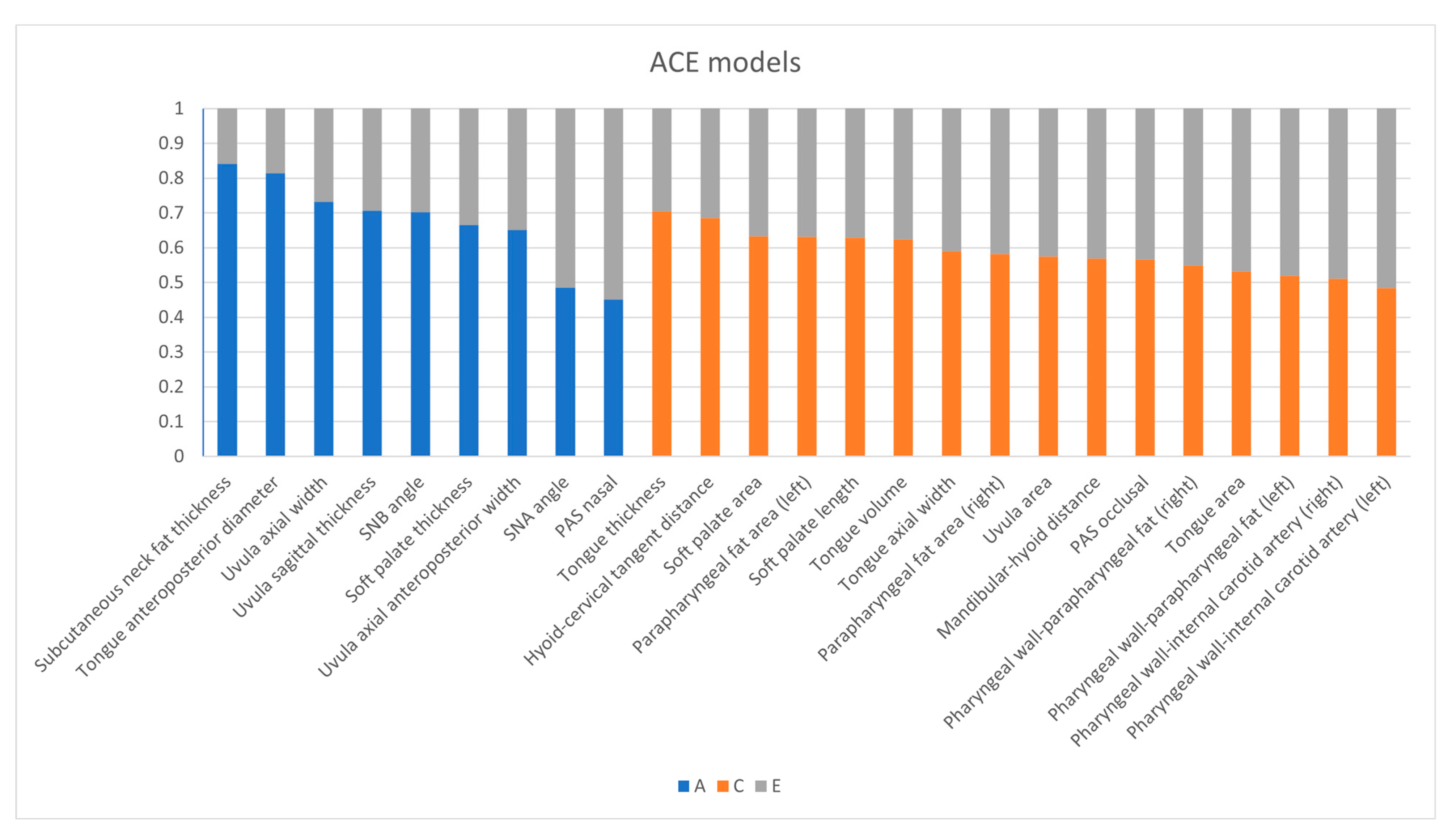
3.2. ACE Model
3.3. MZ-DZ Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Lin, N.; Ye, J.; Chang, Q.; Han, D.; Sperry, A. Upper Airway Fat Tissue Distribution in Subjects With Obstructive Sleep Apnea and Its Effect on Retropalatal Mechanical Loads. Respir. Care 2012, 57, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Villaneuva, A.T.C.; Buchanan, P.R.; Yee, B.J.; Grunstein, R.R. Ethnicity and obstructive sleep apnoea. Sleep Med. Rev. 2005, 9, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Redline, S.; Kump, K.; Tishler, P.V.; Browner, I.; Ferrette, V. Gender differences in sleep disordered breathing in a community-based sample. Am. J. Respir. Crit. Care Med. 1994, 149, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Madrid-Valero, J.J.; Barclay, N.L.; Rowe, R.; Perach, R.; Buysse, D.J.; Ordoñana, J.R.; Eley, T.C.; Gregory, A.M. Association between symptoms of sleep apnea and problem behaviors in young adult twins and siblings. Psychol. Med. 2020, 51, 1175–1182. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Javier Nieto, F.; O’CONNOR, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Małolepsza, A.; Kudrycka, A.; Karwowska, U.; Hoshino, T.; Wibowo, E.; Böjti, P.P.; Białas, A.; Kuczyński, W. The Role of Screening Questionnaires in the Assessment of Risk and Severity of Obstructive Sleep apnea—Polysomnography Versus Polygraphy. Adv. Respir. Med. 2021, 89, 188–196. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Syrico, A.; Kałdunska, A.; Pałka, Ł.; Sobolewska, E. The Usefulness of Modified Mallampati Score and CT Upper Airway Volume Measurements in Diagnosing OSA among Patients with Breathing-Related Sleep Disorders. Appl. Sci. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Lonia, L.; Scalese, M.; Rossato, G.; Bruno, G.; Zalunardo, F.; De Stefani, A.; Gracco, A. Validity of the STOP-Bang Questionnaire in Identifying OSA in a Dental Patient Cohort. Medicina 2020, 56, 324. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of Upper Airway Anatomic Risk Factors for Obstructive Sleep Apnea with Volumetric Magnetic Resonance Imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Whyte, A.; Gibson, D. Imaging of adult obstructive sleep apnoea. Eur. J. Radiol. 2018, 102, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Mello Junior, C.F.D.; Guimarães Filho, H.A.; Gomes, C.A.D.B.; Paiva, C.C.D.A. Radiological findings in patients with obstructive sleep apnea. Jornal Brasileiro Pneumologia 2013, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Saxena, R.; Palmer, L. The genetics of obstructive sleep apnoea. Respirology 2017, 23, 18–27. [Google Scholar] [CrossRef]
- Patel, S.R.; Frame, J.M.; Larkin, E.K.; Redline, S. Heritability of upper airway dimensions derived using acoustic pharyngometry. Eur. Respir. J. 2008, 32, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Strohl, K.P.; Saunders, N.A.; Feldman, N.T.; Hallett, M. Obstructive Sleep Apnea in Family Members. N. Engl. J. Med. 1978, 299, 969–973. [Google Scholar] [CrossRef]
- Onodera, K.; Niikuni, N.; Chigono, T.; Nakajima, I.; Sakata, H.; Motizuki, H. Sleep disordered breathing in children with achondroplasia. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 453–461. [Google Scholar] [CrossRef]
- Rachmiel, A.; Emodi, O.; Aizenbud, D. Management of obstructive sleep apnea in pediatric craniofacial anomalies. Ann. Maxillofac. Surg. 2012, 2, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Keens, T.G.; Bautista, D.B.; Von Pechmann, W.S.; Ward, S.L. Obstructive sleep apnea in children with Down syndrome. Pediatrics 1991, 88, 132–139. [Google Scholar] [CrossRef]
- Driessen, C.; Joosten, K.F.M.; Bannink, N.; Bredero-Boelhouwer, H.H.; Hoeve, H.L.J.; Wolvius, E.B.; Rizopoulos, D.; Mathijssen, I.M.J. How does obstructive sleep apnoea evolve in syndromic craniosynostosis? A prospective cohort study. Arch. Dis. Child. 2013, 98, 538–543. [Google Scholar] [CrossRef]
- Chi, L.; Comyn, F.-L.; Keenan, B.T.; Cater, J.; Maislin, G.; Pack, A.I.; Schwab, R.J. Heritability of Craniofacial Structures in Normal Subjects and Patients with Sleep Apnea. Sleep 2014, 37, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Polderman, T.J.C.; Benyamin, B.; de Leeuw, C.A.; Sullivan, P.F.; Van Bochoven, A.; Visscher, P.M.; Posthuma, D. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet. 2015, 47, 702–709. [Google Scholar] [CrossRef]
- Verweij, K.J.H.; Mosing, M.A.; Zietsch, B.P.; Medland, S.E. Estimating Heritability from Twin Studies. Methods Mol. Biol. 2011, 850, 151–170. [Google Scholar] [CrossRef]
- Szily, M.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.T.; Forgo, B.; Lee, J.; Kim, E.; Sung, J.; Kunos, L.; Meszaros, M.; et al. Genetic influences on the onset of obstructive sleep apnoea and daytime sleepiness: A twin study. Respir. Res. 2019, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Littvay, L.; Métneki, J.; Tarnoki, A.D.; Tarnoki, D.L. The Hungarian Twin Registry. Twin Res. Hum. Genet. 2012, 16, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tarnoki, A.D.; Tarnoki, D.L.; Forgo, B.; Szabo, H.; Melicher, D.; Metneki, J.; Littvay, L. The Hungarian Twin Registry Update: Turning From a Voluntary to a Population-Based Registry. Twin Res. Hum. Genet. 2019, 22, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Comyn, F.-L.; Mitra, N.; Reilly, M.P.; Wan, F.; Maislin, G.; Chmiewski, L.; Thorne-FitzGerald, M.D.; Victor, U.N.; Pack, A.I.; et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur. Respir. J. 2011, 38, 348–358. [Google Scholar] [CrossRef]
- Liu, S.Y.C.; Huon, L.K.; Lo, M.T.; Chang, Y.C.; Capasso, R.; Chen, Y.J.; Shih, T.T.F.; Wang, P.C. Static craniofacial measurements and dynamic airway collapse patterns associated with severe obstructive sleep apnoea: A sleep MRI study. Clin. Otolaryngol. 2016, 41, 700–706. [Google Scholar] [CrossRef]
- Kim, A.M.; Keenan, B.T.; Jackson, N.; Chan, E.L.; Staley, B.; Poptani, H.; Torigian, D.A.; Pack, A.I.; Schwab, R.J. Tongue Fat and its Relationship to Obstructive Sleep Apnea. Sleep 2014, 37, 1639–1648. [Google Scholar] [CrossRef]
- Cosentini, T.; Le Donne, R.; Mancini, D.; Colavita, N. Magnetic resonance imaging of the upper airway in obstructive sleep apnea. Radiol. Med. 2004, 108, 404–416. [Google Scholar]
- Iida-Kondo, C.; Yoshino, N.; Kurabayashi, T.; Mataki, S.; Hasegawa, M.; Kurosaki, N. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imaging. J. Med. Dent. Sci. 2006, 53, 119–126. [Google Scholar] [PubMed]
- Shelton, K.E.; Woodson, H.; Gay, S.; Suratt, P.M. Pharyngeal Fat in Obstructive Sleep Apnea. Am. Rev. Respir. Dis. 1993, 148, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.D.; Wang, S.H.; Manuel, A.R.; Keenan, B.T.; McIntyre, A.G.; Schwab, R.J.; Stradling, J.R. Relationships between MRI fat distributions and sleep apnea and obesity hypoventilation syndrome in very obese patients. Sleep Breath. 2017, 22, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Neale, M. Mx: Statistical Modeling, 6th ed.; Virginia Commonwealth University: Richmond, VA, USA, 2004. [Google Scholar]
- Maes, H.H. ACE Model. In Encyclopedia of Statistics in Behavioral Science; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Dziak, J.J.; Coffman, D.L.; Lanza, S.T.; Li, R.; Jermiin, L.S. Sensitivity and specificity of information criteria. Briefings Bioinform. 2019, 21, 553–565. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; He, M.; Zhang, H. Statistical Inference in Mixed Models and Analysis of Twin and Family Data. Biometrics 2011, 67, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Yang, H.C.; Lim, S.C. Anatomy and Pathophysiology of Upper Airway Obstructive Sleep Apnoea: Review of the Current Literature. Sleep Med. Res. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Johal, A.; Patel, S.I.; Battagel, J.M. The relationship between craniofacial anatomy and obstructive sleep apnoea: A case-controlled study. J. Sleep Res. 2007, 16, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Battagel, J.M.; Johal, A.; Kotecha, B. A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur. J. Orthod. 2000, 22, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Tsuiki, S.; Isono, S.; Ishikawa, T.; Yamashiro, Y.; Tatsumi, K.; Nishino, T. Anatomical Balance of the Upper Airway and Obstructive Sleep Apnea. Anesthesiology 2008, 108, 1009–1015. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Ho, C.-Y.; Lee, P.-L.; Tan, C.-T. Cephalometric Analysis of Nonobese Snorers Either with or Without Obstructive Sleep Apnea Syndrome. Angle Orthod. 2007, 77, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Riha, R.L.; Brander, P.; Vennelle, M.; Douglas, N.J. A Cephalometric Comparison of Patients With the Sleep Apnea/Hypopnea Syndrome and Their Siblings. Sleep 2005, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.B.; Maislin, G.; Schwab, R.J. Physical Findings and the Risk for Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2000, 162, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Reiner, D.J.; Jan, T.A.; Boughter, J.J.D.; Li, C.-X.; Lu, L.; Williams, R.W.; Waters, R.S. Genetic Analysis of Tongue Size and Taste Papillae Number and Size in Recombinant Inbred Strains of Mice. Chem. Senses 2008, 33, 693–707. [Google Scholar] [CrossRef]
- Pinto, G.N.D.S.; Filho, L.I.; Previdelli, I.T.D.S.; Ramos, A.L.; Yamashita, A.L.; Stabile, G.A.V.; Stabile, C.L.P.; Iwaki, L.C.V. Three-dimensional alterations in pharyngeal airspace, soft palate, and hyoid bone of class II and class III patients submitted to bimaxillary orthognathic surgery: A retrospective study. J. Cranio-Maxillofacial Surg. 2019, 47, 883–894. [Google Scholar] [CrossRef]
- Kurt, G.; Sisman, C.; Akin, E.; Akcam, T. Cephalometric Comparison of Pharyngeal Airway in Snoring and Non-Snoring Patients. Eur. J. Dent. 2011, 05, 084–088. [Google Scholar] [CrossRef][Green Version]
- Avci, S.; Lakadamyali, H.; Lakadamyali, H.; Aydin, E.; Tekindal, M.A. Relationships among retropalatal airway, pharyngeal length, and craniofacial structures determined by magnetic resonance imaging in patients with obstructive sleep apnea. Sleep Breath. 2018, 23, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ono, T. Tongue and upper airway function in subjects with and without obstructive sleep apnea. Jpn. Dent. Sci. Rev. 2012, 48, 71–80. [Google Scholar] [CrossRef][Green Version]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining Phenotypic Causes of Obstructive Sleep Apnea. Identification of Novel Therapeutic Targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Schwab, R.J.; Gupta, K.B.; Gefter, W.B.; Metzger, L.J.; Hoffman, E.; Pack, A. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am. J. Respir. Crit. Care Med. 1995, 152, 1673–1689. [Google Scholar] [CrossRef]
- Soares, D.; Sinawe, H.; Folbe, A.J.; Yoo, G.; Badr, S.; Rowley, J.A.; Lin, H.-S. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope 2012, 122, 473–479. [Google Scholar] [CrossRef]
- Jang, M.-S.; Kim, H.Y.; Dhong, H.-J.; Chung, S.-K.; Hong, S.D.; Cho, H.-J.; Jung, T.-Y. Effect of Parapharyngeal Fat on Dynamic Obstruction of the Upper Airway in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2014, 190, 1318–1321. [Google Scholar] [CrossRef]
- Isono, S. Obesity and obstructive sleep apnoea: Mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology 2011, 17, 32–42. [Google Scholar] [CrossRef]
- Sacks, H.; Symonds, M.E. Anatomical locations of human brown adipose tissue: Functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013, 62, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Schleinitz, D.; Böttcher, Y.; Blüher, M.; Kovacs, P. The genetics of fat distribution. Diabetologia 2014, 57, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Pravenec, M.; Saba, L.M.; Zídek, V.; Landa, V.; Mlejnek, P.; Šilhavý, J.; Simakova, M.; Strnad, H.; Trnovská, J.; Skop, V.; et al. Systems genetic analysis of brown adipose tissue function. Physiol. Genom. 2018, 50, 52–66. [Google Scholar] [CrossRef]
- Redline, S.; Tosteson, T.; Tishler, P.V.; Carskadon, M.A.; Milliman, R.P. Studies in the Genetics of Obstructive Sleep Apnea: Familial Aggregation of Symptoms Associated with Sleep-related Breathing Disturbances. Am. Rev. Respir. Dis. 1992, 145, 440–444. [Google Scholar] [CrossRef]
- Roosenboom, J.; Hens, G.; Mattern, B.C.; Shriver, M.D.; Claes, P. Exploring the Underlying Genetics of Craniofacial Morphology through Various Sources of Knowledge. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wing, Y.; Zhang, J.; Lam, S.; Li, S.; Tang, N.; Lai, K.; Li, A. Familial aggregation and heritability of insomnia in a community-based study. Sleep Med. 2012, 13, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Larkin, E.K.; Redline, S. Shared genetic basis for obstructive sleep apnea and adiposity measures. Int. J. Obes. 2008, 32, 795–800. [Google Scholar] [CrossRef]
- Desai, A.V.; Cherkas, L.F.; Spector, T.D.; Williams, A.J. Genetic influences in self-reported symptoms of obstructive sleep apnoea and restless legs: A twin study. Twin Res. 2004, 7, 589–595. [Google Scholar] [CrossRef]
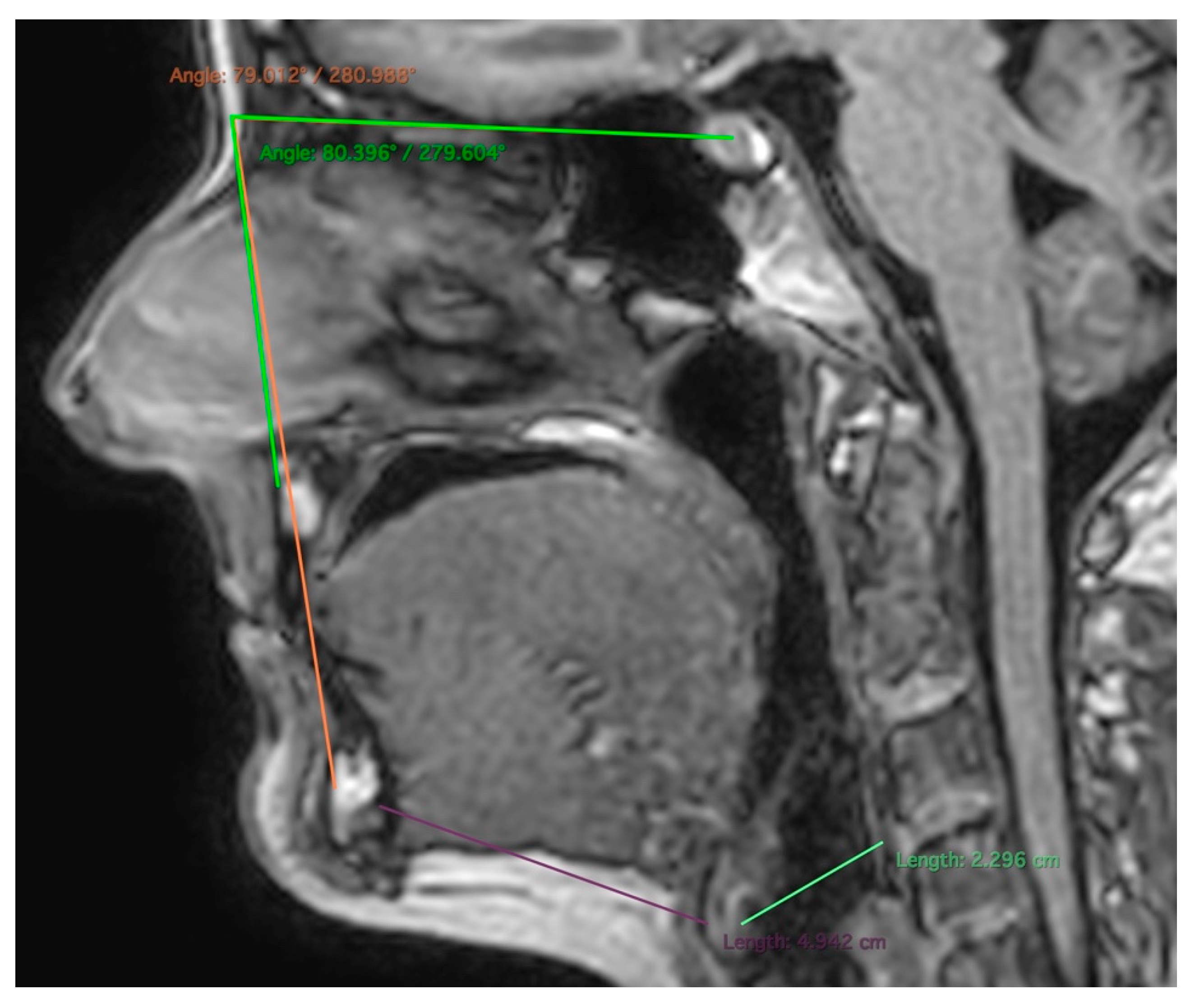
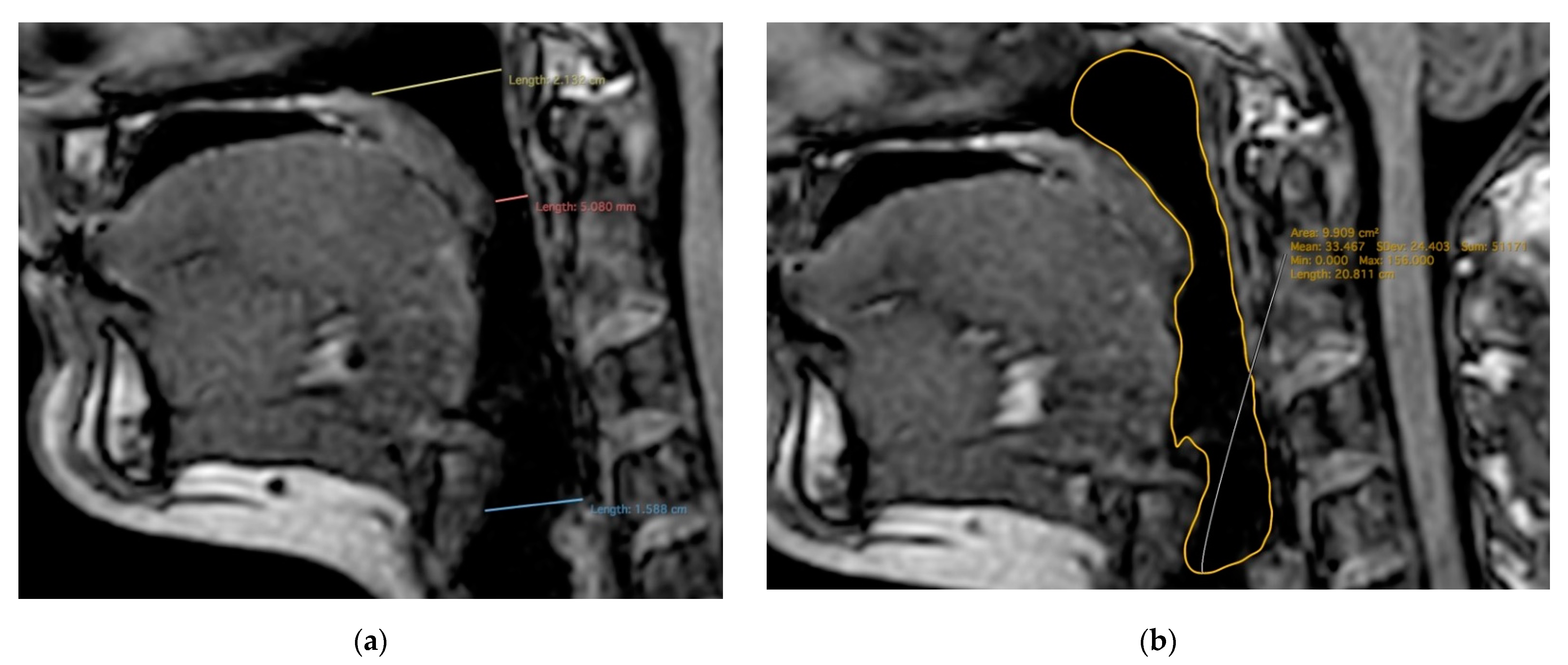
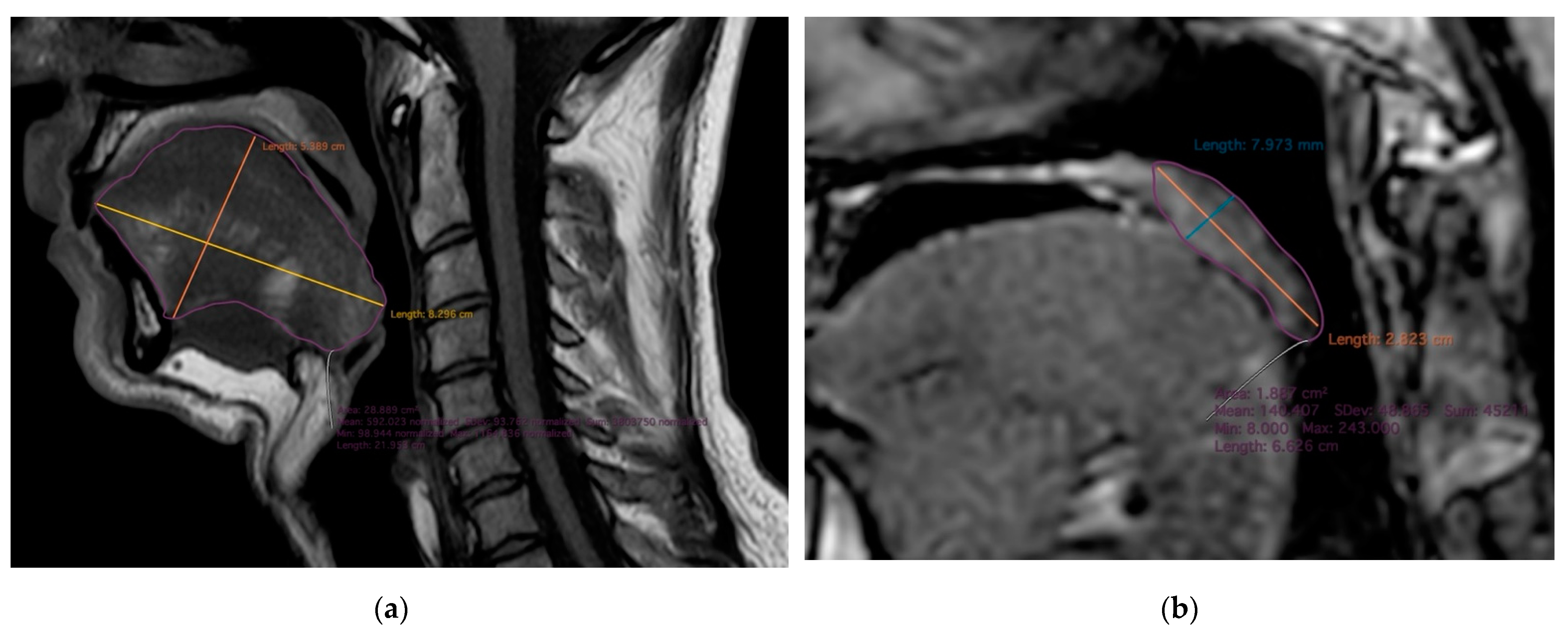
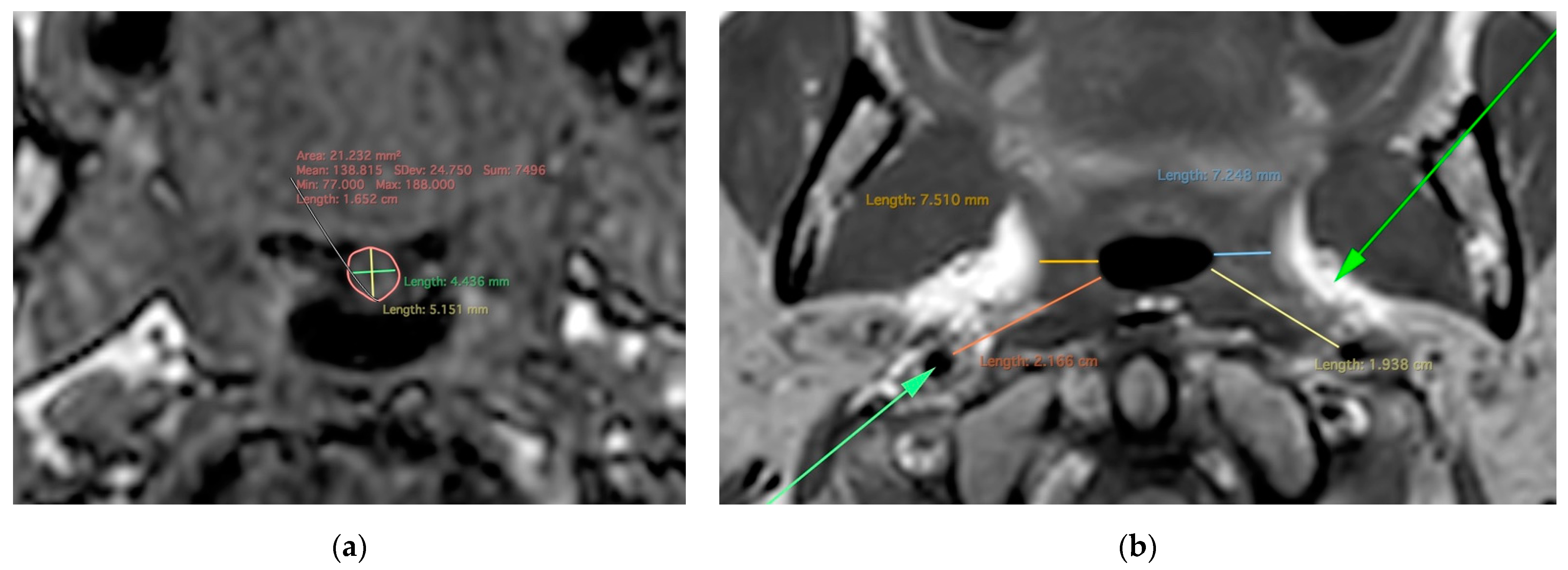
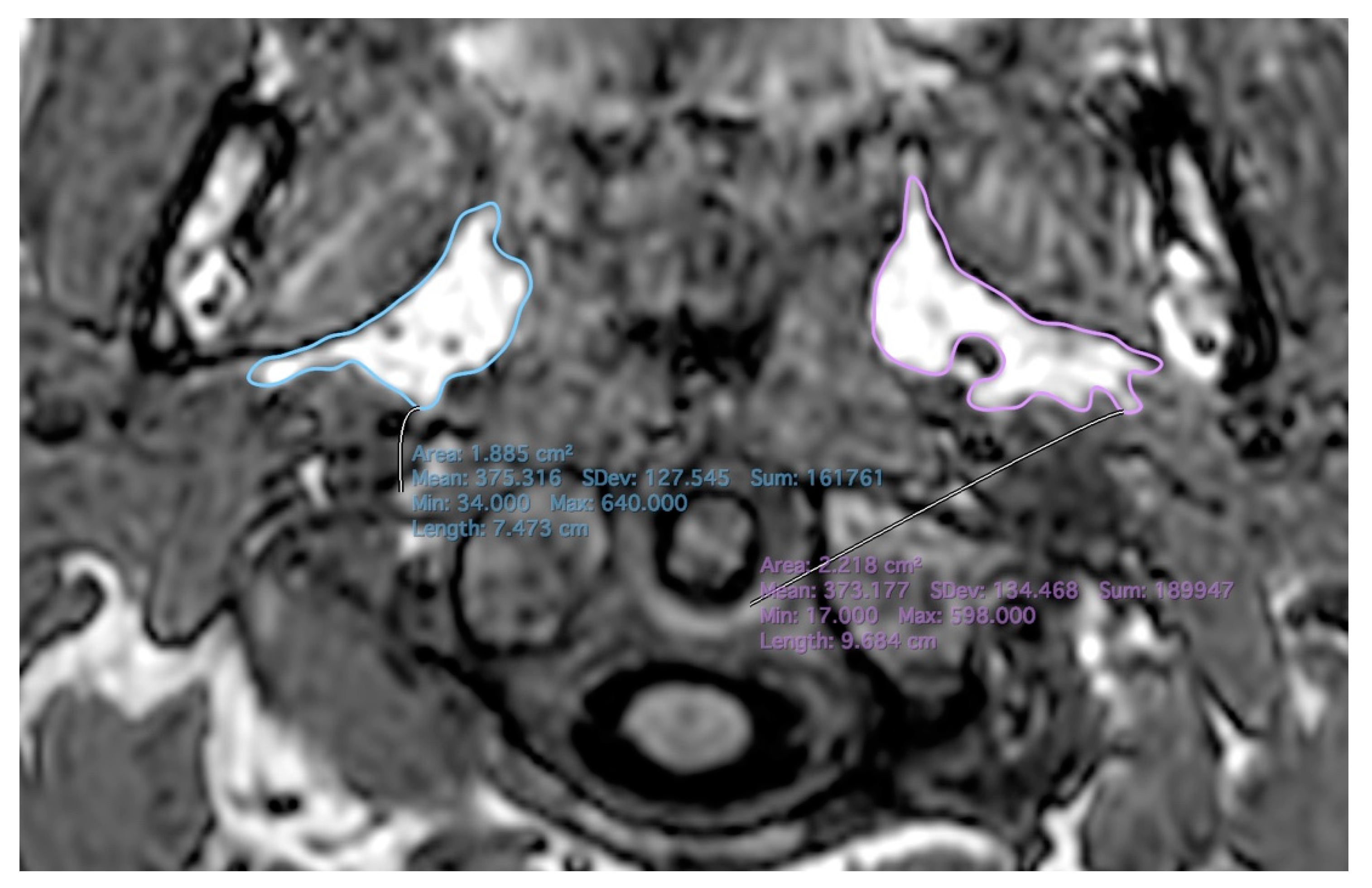
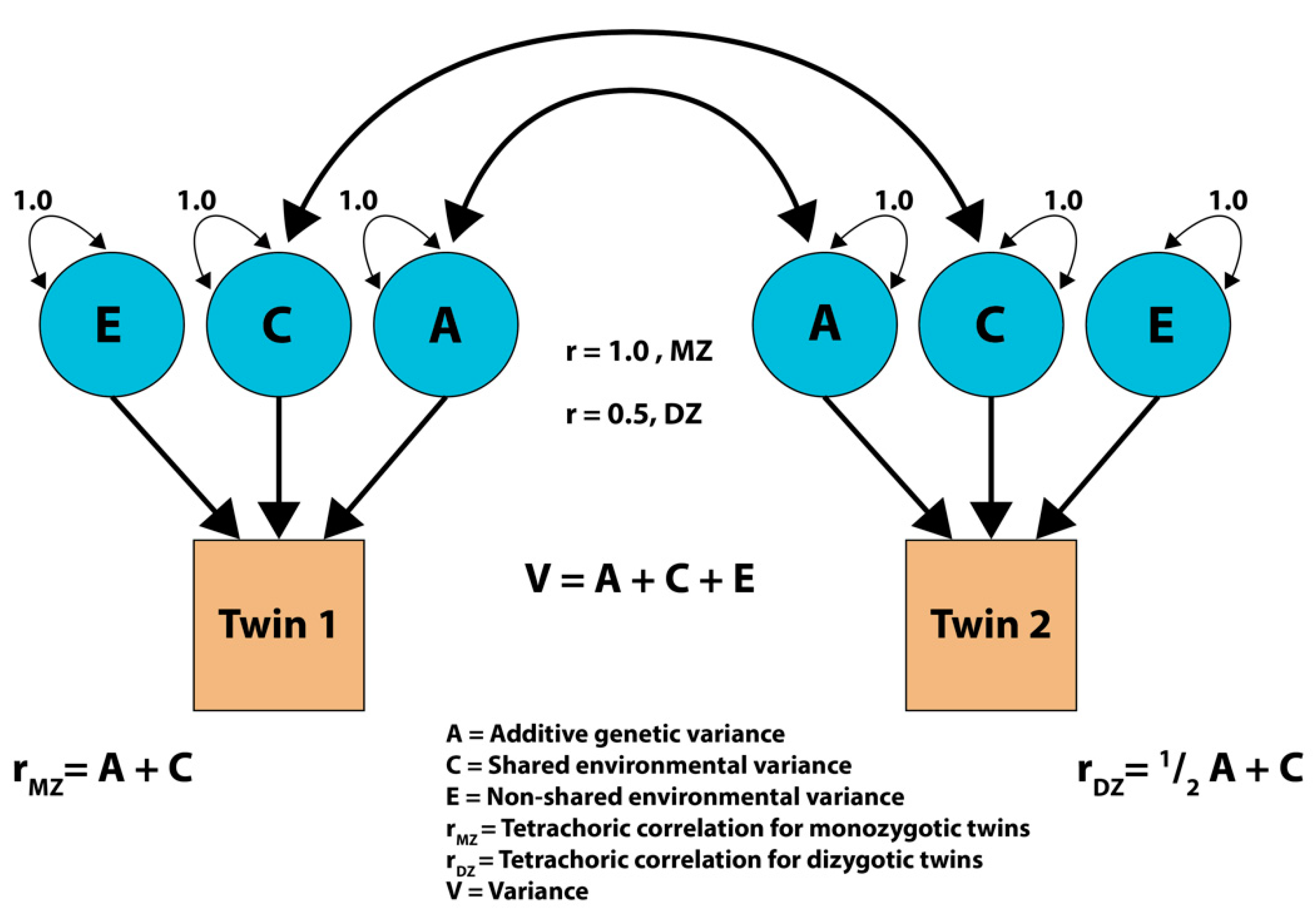
| Total | MZ | DZ | p-Values | |
|---|---|---|---|---|
| Number of participants, N | 110 | 66 | 44 | |
| Age, (years) median (Q1–Q3) | 53 (44–64) | 50 (42–56) | 59 (48–69) | 0.002 |
| Sex, N (%) | Males: 34 (30.9) | Males: 24 (36.4) | Males: 10 (22.7) | 0.146 |
| Females: 76 (69.1) | Females: 42 (63.6) | Females: 34 (77.3) | ||
| BMI, median (Q1–Q3) | 24.8 (22.4–27.3) | 24.3 (22.2–27.2) | 25.2 (23.1–27.7) | 0.803 |
| Smoking, N (%) | 16 (14.5) | 13 (19.7) | 3 (6.8) | 0.096 |
| Hypertension, N (%) | 24 (21.8) | 12 (18.2) | 12 (27.3) | 0.346 |
| Diabetes Mellitus, N (%) | 7 (6.4) | 3 (4.5) | 4 (9.1) | 0.434 |
| Dyslipidemia, N (%) | 20 (18.2) | 9 (13.6) | 11 (25) | 0.140 |
| BMI, body mass index |
| Measurements | A | C | E |
|---|---|---|---|
| SNA angle | A: 0.485 (0.142, 0.713) | C: 0 | E: 0.515 (0.287, 0.858) |
| SNB angle | A: 0.702 (0.422, 0.849) | C: 0 | E: 0.298 (0.151, 0.578) |
| Mandibular–hyoid distance | A: 0 | C: 0.569 (0.312, 0.746) | E: 0.431 (0.254, 0.688) |
| Hyoid–cervical tangent distance | A: 0 | C: 0.685 (0.510, 0.805) | E: 0.315 (0.195, 0.490) |
| Soft palate length | A: 0 | C: 0.629 (0.437, 0.765) | E: 0.371 (0.235, 0.563) |
| Soft palate thickness | A: 0.666 (0.442, 0.804) | C: 0 | E: 0.334 (0.196, 0.558) |
| Soft palate area | A: 0 | C: 0.633 (0.444, 0.768) | E: 0.367 (0.232, 0.556) |
| Uvula sagittal thickness | A: 0.707 (0.507, 0.829) | C: 0 | E: 0.293 (0.171, 0.493) |
| Uvula axial anteroposterior width | A: 0.651 (0.308, 0.827) | C: 0 | E: 0.349 (0.173, 0.692) |
| Uvula axial width | A: 0.732 (0.469, 0.863) | C: 0 | E: 0.268 (0.137, 0.531) |
| Uvula area | A: 0 | C: 0.575 (0.324, 0.750) | E: 0.425 (0.250, 0.676) |
| PAS nasal | A: 0.452 (0.174, 0.657) | C: 0 | E: 0.548 (0.343, 0.826) |
| PAS occlusal | A: 0 | C: 0.567 (0.357, 0.723) | E: 0.433 (0.277, 0.643) |
| Tongue anteroposterior diameter | A: 0.815 (0.633, 0.904) | C: 0 | E: 0.185 (0.096, 0.367) |
| Tongue thickness | A: 0 | C: 0.705 (0.490, 0.836) | E: 0.295 (0.164, 0.510) |
| Tongue axial width | A: 0 | C: 0.591 (0.334, 0.763) | E: 0.409 (0.237, 0.666) |
| Tongue volume | A: 0 | C: 0.625 (0.376, 0.786) | E: 0.375 (0.214, 0.624) |
| Tongue area | A: 0 | C: 0.532 (0.291, 0.698) | E: 0.468 (0.302, 0.709) |
| Subcutaneous neck fat thickness | A: 0.841 (0.719, 0.908) | C: 0 | E: 0.159 (0.092, 0.281) |
| Parapharyngeal fat area (right) | A: 0 | C: 0.582 (0.328, 0.756) | E: 0.418 (0.244, 0.672) |
| Parapharyngeal fat area (left) | A: 0 | C: 0.631 (0.388, 0.789) | E: 0.369 (0.211, 0.612) |
| Pharyngeal wall-parapharyngeal fat (right) | A: 0 | C: 0.549 (0.281, 0.735) | E: 0.451 (0.265, 0.719) |
| Pharyngeal wall-parapharyngeal fat (left) | A: 0 | C: 0.52 (0.247, 0.714) | E: 0.48 (0.286, 0.753) |
| Pharyngeal wall-internal carotid artery (right) | A: 0 | C: 0.511 (0.234, 0.709) | E: 0.489 (0.291, 0.766) |
| Pharyngeal wall-internal carotid artery (left) | A: 0 | C: 0.484 (0.206, 0.689) | E: 0.516 (0.311, 0.794) |
| Measurements | RMZ | RDZ |
|---|---|---|
| SNA angle | 0.511 (0.120, 0.764) | 0.297 (−0.267, 0.710) |
| SNB angle | 0.716 (0.445, 0.866) | 0.129 (−0.433, 0.616) |
| Mandibular–hyoid distance | 0.538 (0.156, 0.782) | 0.623 (0.181, 0.856) |
| Hyoid–cervical tangent distance | 0.550 (0.236, 0.760) | 0.811 (0.595, 0.918) |
| Soft palate length | 0.724 (0.504 0.854) | 0.429 (0.017, 0.715) |
| Soft palate thickness | 0.640 (0.376, 0.809) | 0.465 (0.060, 0.740) |
| Soft palate area | 0.673 (0.431, 0.825) | 0.614 (0.271, 0.819) |
| Uvula sagittal thickness | 0.707 (0.484, 0.843) | 0.412 (0.01, 0.699) |
| Uvula axial anteroposterior width | 0.568 (0.179, 0.799) | 0.428 (−0.186, 0.781) |
| Uvula axial width | 0.612 (0.270, 0.815) | 0.521 (0.018, 0.811) |
| Uvula area | 0.602 (0.263, 0.81) | 0.577 (0.078, 0.843) |
| PAS nasal | 0.489 (0.181, 0.709) | 0.283 (−0.145, 0.620) |
| PAS occlusal | 0.595 (0.313, 0.782) | 0.569 (0.196, 0.8000) |
| Tongue anteroposterior diameter | 0.856 (0.694, 0.934) | 0.079 (−0.416, 0.536) |
| Tongue thickness | 0.793 (0.563, 0.905) | 0.446 (−0.120, 0.782) |
| Tongue axial width | 0.639 (0.315, 0.825) | 0.515 (−0.055, 0.818) |
| Tongue volume | 0.666 (0.36 0.84) | 0.525 (−0.008, 0.815) |
| Tongue area | 0.588 (0.282, 0.761) | 0.45 (−0.015, 0.721) |
| Subcutaneous neck fat thickness | 0.834 (0.681, 0.917) | 0.598 (0.238, 0.815) |
| Parapharyngeal fat area (right) | 0.603 (0.269, 0.805) | 0.624 (0.174, 0.853) |
| Parapharyngeal fat area (left) | 0.656 (0.343, 0.836) | 0.597 (0.101, 0.846) |
| Pharyngeal wall-parapharyngeal fat (right) | 0.510 (0.128, 0.757) | 0.616 (0.168, 0.85) |
| Pharyngeal wall-parapharyngeal fat (left) | 0.510 (0.135, 0.756) | 0.561 (0.099, 0.823) |
| Pharyngeal wall-internal carotid artery (right) | 0.493 (0.115, 0.746) | 0.568 (0.102, 0.827) |
| Pharyngeal wall-internal carotid artery (left) | 0.493 (0.120, 0.744) | 0.518 (0.036, 0.802) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokkel, Z.; Szily, M.; Sipos, B.; Oluk, E.; Piroska, M.; Kalina, I.; Maurovich-Horvat, P.; Bikov, A.; Tarnoki, D.L.; Tarnoki, A.D. The Heritability of Upper Airway Dimensions Using MRI Scans in Twins. Appl. Sci. 2022, 12, 7646. https://doi.org/10.3390/app12157646
Jokkel Z, Szily M, Sipos B, Oluk E, Piroska M, Kalina I, Maurovich-Horvat P, Bikov A, Tarnoki DL, Tarnoki AD. The Heritability of Upper Airway Dimensions Using MRI Scans in Twins. Applied Sciences. 2022; 12(15):7646. https://doi.org/10.3390/app12157646
Chicago/Turabian StyleJokkel, Zsofia, Marcell Szily, Boldizsar Sipos, Ezgisu Oluk, Marton Piroska, Ildikó Kalina, Pál Maurovich-Horvat, Andras Bikov, David Laszlo Tarnoki, and Adam Domonkos Tarnoki. 2022. "The Heritability of Upper Airway Dimensions Using MRI Scans in Twins" Applied Sciences 12, no. 15: 7646. https://doi.org/10.3390/app12157646
APA StyleJokkel, Z., Szily, M., Sipos, B., Oluk, E., Piroska, M., Kalina, I., Maurovich-Horvat, P., Bikov, A., Tarnoki, D. L., & Tarnoki, A. D. (2022). The Heritability of Upper Airway Dimensions Using MRI Scans in Twins. Applied Sciences, 12(15), 7646. https://doi.org/10.3390/app12157646










