Abstract
Meeting our current energy demands requires a reliable and efficient renewable energy source that will bring balance between power generation and energy consumption. Organic photovoltaic cells (OPVs), perovskite solar cells and dye-sensitized solar cells (DSSCs) are among the next-generation technologies that are progressing as potential sustainable renewable energy sources. Since the discoveries of highly conductive organic charge-transfer compounds in the 1950s, organic semiconductors have captured attention. Organic photovoltaic solar cells possess key characteristics ideal for emerging next-generation technologies such as being nontoxic, abundant, an inexpensive nanomaterial with ease of production, including production under ambient conditions. In this review article, we discuss recent methods developed towards improving the stability and average efficiency of nanostructured materials in OPVs aimed at sustainable agriculture and improve land-use efficiency. A comprehensive overview on developing cost-effective and user-friendly organic solar cells to contribute towards improved environmental stability is provided. We also summarize recent advances in the synthetic methods used to produce nanostructured active absorber layers of OPVs with improved efficiencies to supply the energy required towards ending poverty and protecting the planet.
1. Introduction
One of the most pressing concerns for human living in the twenty-first century is energy [1]. With human population ever-increasing globally, the economic costs of energy are also shooting up dramatically and the demand for sustainable renewable energy rises drastically around the world. By 2040, it is expected that worldwide energy consumption would have increased by 50% [2]. According to Congcong and co., 80% of the world’s energy comes from fossil fuels [3]. This heavy dependence on fossil fuels does not only bring advantages but it is also associated with catastrophic disadvantages to the environment. Among these are greenhouse gases (GHGs) and chlorofluorocarbons (CFCs) which are the main sources of global warming. Figure 1 shows the smoke and steam that remains in the atmosphere in the early mornings after business days in urban areas.

Figure 1.
Combustion power plants and engines emitting smoke and steam to the atmosphere.
Renewable energy technology is currently one of the world’s fastest-growing sectors, with the goal of meeting future energy demands while also addressing environmental issues and helping to mitigate climate change [4,5]. Using nanostructured photovoltaics to harvest solar energy have a lot of promise, but large-scale implementation has run into a lot of roadblocks, the most significant ones are the high cost of technology [6], environmental instability [7], and low power conversion efficiencies [8]. Solar energy is the most plentiful, unlimited, and clean of all existing renewable energy sources, and because of that, scientists and researchers have designed solar cells to harvest this clean energy. Up until recently, solar cells are split into three primary kinds known as generations [9]. Solar cells of the first generation are highly expensive to develop (single crystal, multi-crystal solar cells, etc.) and have poor efficiency. The second generation includes solar cells with even lower efficiency but reduced production costs (such as tin-thin film and Cadmium Telluride (CdTe) solar cells), resulting in lower cost per watt than first-generation cells. Next-generation photovoltaic solar cells, popularly known as “third-generation PVs,” are low-cost but high-performance solar cells [10]. Simple production, minimal cost, and high performance are the major characteristics of these emerging solar cells. Additional advantages such as flexibility and light-weight may promote new applications including portable power, interior light harvesters, and solar wings for drones [11]. There are four primary kinds of emerging solar cells which seem to address the roadblocks towards large-scale implementation of solar cells, these emerging solar cells are organic photovoltaics (OPVs) [12], perovskite [13] dye-sensitized [14] and organic-inorganic hybrid solar cells [15]. Before these technologies could emerge, solid-state silicon solar cells have been the primary driving factor in photovoltaic technology for a few decades due to silicon being relatively abundant, environmentally benign, having good material quality and the widespread technological know-how [14].
However, because solid-state silicon solar cells are fragile and tight, they are not suitable for transportation. Another problem is that the components are still quite expensive when compared to some of the other solar technology alternatives. Nonetheless, technological advancements are gradually permitting the use of lower-cost but lower-quality silicon. As a result, silicon solar cells are becoming more inexpensive, particularly with government subsidies. Therefore, it is worth further investigation which could result in new breakthroughs for solid-state photovoltaic solar cells.
Moreover, another disadvantages of crystalline silicon is that its electronic band-gap is indirect in nature, making it a poor absorber of long wavelength sunlight [16]. Because of their high absorption coefficient, great carrier mobility, and easily adjustable band-gaps. Organic-inorganic hybrid solar cells (Figure 2) have arisen amongst the most promising light absorber layers essential for next-generation solar cells. They are multi-component molecules with at least one organic (typically the polymer) or inorganic material in the nanometre scale size domain, and they usually have substantially better performance than their non-hybrid counterparts. However, organic cations’ thermal and moisture sensitivity in the hybrid remains the major source of device instability [17]. The lateral charge transfer between the organic and inorganic layers is highly curtailed in a hybrid, but the active perpendicular layer orientation is thought to enable charge passage along the inorganic sheets to the substrates, as illustrated in Figure 2 [18]. Based on various favorable properties, organic solar devices have the capacity to overcome the difficulties faced by silicon-based and organic-inorganic hybrid solar cells [19]. The purpose of this review is to highlight some of the barriers behind high-cost traditional silicon-based photovoltaic solar cells and recent innovations in low-cost nanostructured organic photovoltaics.
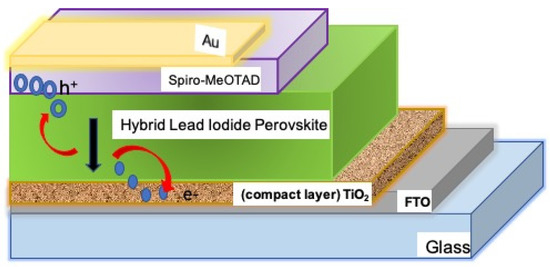
Figure 2.
Illustration of charge transfer process in a hybrid organic-inorganic solar cell.
Organic semiconductor materials primarily consist of conjugated small molecules and polymers. Organic photovoltaic devices based on semiconducting conjugated polymers have emerged as a viable alternative to traditional solid-state (silicon-based) solar systems, primarily due to their affordable production costs [20] for soluble organic molecules (produced via roll-to-roll processing techniques), abundant material (decreasing the supply and price constraints) as well as flexible substrates (permitting a wide variety of uses) [21]. They cleared the path for Tang to claim in 1986 that he had developed the first thin-film OSC that exceeded 1% efficiency. Despite the fact that they attracted a lot of interest for their ability to generate energy effectively even under dispersed light or interior illumination, solid-state dye-sensitized photovoltaic cells (DSSCs) still have low power conversion efficiency (11%) which still has to be improved [15]. Moreover, the light electrolyte causes a safety issue since the iodine and organic solvents can be volatized and leak; affecting the cell’s longevity [22]. The all-solid perovskite solar cell, which is based on DSSC but overcomes DSSC’s limitations, received a lot of interest from scientists as soon as it was disclosed. Despite the fact that the photoelectric conversion efficiency (PCE) of PSC has rapidly increased from 3.8 to 23.3% [23], device stability remains a challenge that has limited their applications. In general, each component of the technology has an impact on its performance by completing its assigned responsibilities. As a result, optimizing the device components is critical. As a result, OPVs provide an alternate solution to the problems that traditional cells confront, owing to their structural flexibility, ease of device manufacture, and quick energy recovery.
1.1. Bulk Heterojunction (BHJ) Nanomaterial in OPVs
In OPV, a BHJ is basically a multilayer nanostructure with each layer in the device design formed using a different manufacturing method. To begin with, it is made up of an absorbing (photoactive) layer, that consists of two components, a donor material is usually a conjugated polymer [1], conjugated pigments [24] or oligomers, and for an acceptor material it is often fullerene derivatives but recently BHJ have been replaced by their counterparts, non-fullerene derivatives. As shown in Figure 3, the absorbing layer is located between the anode and the top relatively low function (WF) cathode. Interfacial layers such as a hole transport layer (HTL) and an electron transport layer (ETL) [3] are typically put between the anode-photoactive and cathode-photoactive interfaces to improve the performance and stability of BHJ OPVs [25]. Congcong Wu et al. proposed that a nanoscopic BHJ shape of the donor-acceptor network with continuous interpenetrating phase characteristics is critical for highly efficient OPVs. They attributed this to BHJ materials’ low dielectric constant, in which a photon produced by an electron-hole pair (exciton) had an unusually high exciton binding energy and a short diffusion length [3].
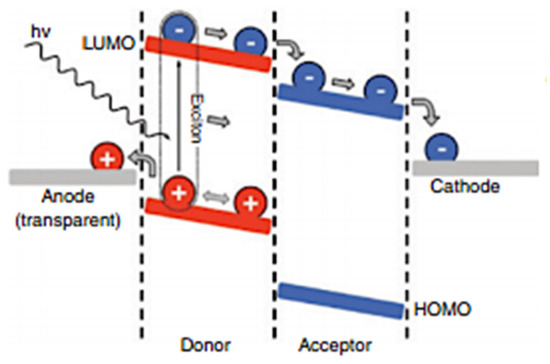
Figure 3.
Stepwise operation of a BHJ OPV device (Reproduced from reference [25] with copyrights permission from Elsevier Science and Technology Journals).
They suggested that the key to achieving high PCE is to enhance dissociation efficiency. Because of the strong bonding of the exciton, efficient separation of the electron-hole pair requires the application of an additional field, which may be done by utilizing a large chemical potential difference at the donor-acceptor interface and depending on fast electron transfer. A p-n junction, similar to those seen in silicon solar cells, can be formed via the donor-acceptor contact. As a result, improving the charge separation ratio against recombination by raising the PCE by increasing the donor-acceptor interfacial area is a viable option. An example of an OPV device that employs BHJ is an inverted OPV device. In an inverted device, the bottom transparent electrode acts as the cathode, while the top electrode serves as the anode. Inverted solar cells offer equivalent performance to regular solar cells and have a higher level of environmental stability [26].
1.2. The Operatzion Principle of OPVs
Saqib Rafique et al. [25] proposed a simpler operating principle for the BHJ OSC device, consisting of four basic steps: (i) photon absorption and exciton generation, (ii) exciton dissociation, (iii) charge transfer, and (iv) charge collection. Light was absorbed by the donor material, which is a conjugated polymer, in their BHJ OPV device. An electron is accelerated from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) when photons are absorbed (LUMO) [27]. The excitons diffused to the donor-acceptor interface, where there was enough potential energy drop to divide them into free charge carriers, such as electrons and holes [28]. After splitting into free charge carriers, each carrier was delivered to the appropriate electrode through a bicontinuous interpenetrating channel that prevented recombination and charge trapping. Some limitations and losses could occur during these steps such as absorption loss [29] due to spectral mismatch, thermalization loss [30], the insufficient energy required for exciton splitting, and charge recombination, etc. Below is a full description of each of the steps involved in the process, from light absorption to charge carrier collection.
1.2.1. Light Absorption and Exciton Generation
Increased efficiency is a near-linear driver for lowering the cost of PV power per kilowatt-hour since efficiency is a fundamental parameter in the development of photovoltaic (PV) systems. Shockley and Queisser (S-Q) proposed a theoretical framework for calculating the limiting efficiency of a single junction solar cell in 1961, based on the notion of detailed balancing, which equates the incoming and outgoing photon fluxes for a device under open-circuit circumstances [31]. This Shockley–Queisser limit defines the maximum practicable solar energy conversion efficiency for applied active materials. The photoactive layer must absorb the maximum amount of incoming sunlight as this is the standard by which new photovoltaic technologies are compared (see Figure 4a). The limit is that the maximum solar conversion efficiency is around 33.7% for a single p-n junction photovoltaic cell, assuming typical sunlight conditions (unconcentrated, AM 1.5 solar spectrum), at a band-gap of 1.34 eV. The multi-junction concept is the most relevant approach to overcome the Shockley–Queisser limit for single-junction photovoltaic cells.
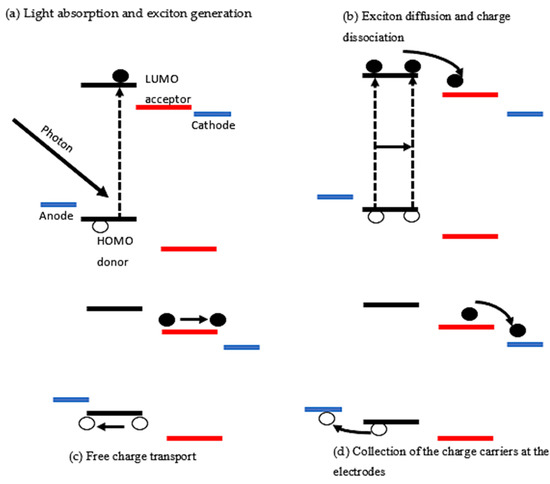
Figure 4.
Illustration of the working principles of an organic photovoltaic solar cell displaying the processes of (a) light absorption and exciton generation, (b) exciton diffusion and charge dissociation, (c) free charge transportation and lastly, (d) collection of charge carriers at the electrodes.
Questions have been raised about whether nanostructured organic photovoltaic devices can exceed the Shockley-Queisser limit. As previously stated, for high efficiency, light is absorbed by both donor and acceptor component of the BHJ photoactive layer [25], and light with high sufficient energy levels is absorbed by the OPV. Unlike in the inorganic material where the exciton binding energy (Eb) is small enough to reduce the attraction between the electron and hole pairs, organic excitons have large binding energy (typically 0.2–1.0 eV) that drives the electron and hole to recombine after photoexcitation. A solution to this problem was proposed by Tang et al. in 1986 [32] which has been working ever since. It involves combining two organic semiconductors with slightly offset HOMO and LUMO energies, such that the photoexcited electron in the first material (normally the donor) is transferred to the second material (acceptor), allowing for the charges to be separated at the junction between these two materials.
The maximum possible efficiency is achieved when non-radiative recombination is absent and all generated carriers are either collected as current in the leads or recombine, emitting a single photon per electron-hole pair. Conjugated polymers, in particular, with extremely thin photoactive layers (up to 100 nm), may absorb light at the maximum of their absorption spectrum with a high absorption coefficient, unlike their inorganic silicon-based equivalents, which need hundreds of micrometres. It is important to produce donor polymers that absorb light in the longer wavelength area for next-generation organic photovoltaics, that is, the absorption border should fall at wavelengths larger than 700 nm. The band-gap of such polymers should be smaller than 2 eV [33].
1.2.2. Exciton Diffusion and Charge Dissociation
The second step involves the energy offset in LUMO between donor and acceptor materials which breaks the Coulomb attraction that ultimately causes the excitons to dissociate [34]. As most of the conjugated polymers exhibit a shorter lifetime of the excitons, the diffusion lengths are limited to a few nanometres, less than 20 nm, which is much shorter than the optical absorption pass length that is approximately 100–200 nm. It is, therefore, a prerequisite that excitons must be generated within their diffusion length (LD) for efficient charge generation [35]. Exciton diffusion length is defined as the distance travelled by an exciton before recombination (see Figure 4b) [36]. The reported excitons diffusion length for various conjugated polymers (of free carriers) significantly varies from 5 to 20 nm [25]. Thus, the thickness of the photoactive layer is very much critical for an efficient charge generation. Nong V. Hoang and colleagues have recently released their work which demonstrates that single-material OPVs are being revived, not only because of their conceptual simplicity and considerably increased stability, but also because they may be used to learn about exciton dissociation and charge separation mechanisms. The production of intermediate inter-molecular charge-transfer (CT) excitons at the homojunction of a single p- and n-type doped organic semiconductor or at the interface of undoped domains with differing molecular orientation and packing has been shown to aid exciton dissociation in such devices [37].
1.2.3. Free Charge Carriers Transport
After the exciton dissociation into free charge carriers’ step, the charges are transported towards the respective electrodes as shown in Figure 4c above. The transportation of charge carriers in organic semiconductors mostly takes place by hopping from one localized state to the next [38]. Separated holes and electrons are dispersed inside the donor and acceptor phases, respectively, after charge transfer occurs at the D/A contact. The transport of holes and electrons to their respective electrodes is then aided by an internal electric field derived from the Fermi level difference between the electrodes, with efficiency varying depending on their mobilities during the hopping process [39]. Concomitantly, nongeminate recombination (NGR) is typically in competition with the collection of photogenerated charge carriers to each electrode not only at the short-circuit but also at the open-circuit conditions, and therefore impacts on both external quantum efficiency (EQE) and fill factor (FF). Therefore, optimised active layers should, in some cases, be kept as thin as possible (typically ∼100 nm) to avoid NGR loss. This, in return, may decrease its effectiveness of solar light absorption. As such, a key to efficient EQE and FF is suppressing NGR beyond limitations arising from the Langevin recombination.
1.2.4. Collection of the Charge Carriers at the Electrodes
Finally, charge carriers created by photons that do not recombine are removed from the photoactive layer and sent to the electrodes (see Figure 4d). There is a potential barrier at the photoactive layer/electrodes interface that is reduced to maximize the extraction of charges [27]. The barrier inhibits charge transport, resulting in significant charge accumulation at the interfaces. Charge accumulation increases the likelihood of recombination and reduces device performance. As a result, it’s critical to boost charge separation and transfer efficiency. An interface buffer layer, which includes the anode and cathode buffer layers, is added in OPVs to overcome this problem. These buffer layers are frequently used to provide an ohmic contact between the active layer and the electrode, allowing for effective charge extraction and separation [40]. Therefore, the WF of the anode should match with the HOMO of the donor material, while the WF of the cathode must match with the LUMO of the acceptor material [41]. If the WFs match well as described, then the contacts are said to be Ohmic contacts. Contrary to this, if there is a mismatch between the anode and cathode with that of donor HOMO or acceptor LUMO, respectively, then no Ohmic contacts would be established. Ultimately, the performance of the solar cells will reduce [42]. The charge collection at the respective electrodes concludes the steps from absorption of light to generation of photocurrent.
2. Types of Organic Solar Cells
Organic photovoltaic cells are classified into two types based on the molecular framework, namely; small molecule-based and polymer-based OPVs [19]. Small donors have a well-defined molecular weight, are simple to synthesize and purify, and have small batch-to-batch variability [43]. Improved crystallization, as well as the capacity to achieve high phase purity and modify crystal alignments, enables the development of small-molecule solar cells with high charge mobility and low energy losses [44]. Polymer-based solar cells (PB-OSCs), on the other hand, have quickly emerged as one of the most promising low-cost solar cell alternatives, due to the ability for large-scale and flexible solar cells fabrication via solution processing [45]. Small molecule-based solar cells have increased performance in terms of efficiency from 0.001% in the 1990s [19] to more than 14% in 2018 [46] which is a significantly higher efficiency than any OPV (before these findings the maximum PCE of OPVs achieved by PB-OSCs, was approximately 13%). Below, we compare these two classes which are also divided into two subclasses namely, fullerene and nonfullerene. We are focusing specifically on recent advances made in (i) nanostructures, (ii) synthetic pathway, and (iii) operating mechanism towards improved performance efficiencies for innovative applications.
2.1. Small-Molecule OPV Cells
Small-molecule OPVs feature well-defined molecular architectures that give outstanding mechanical stability and charge carrier flexibility, allowing for greater efficiency gains [47]. Most small molecule OPVs are based on electron-rich thiophene units, supporting the importance of this heterocyclic core in materials science. Guangchao Han and Yuanping Yi [48] conducted a study in 2018 to investigate the impact of nanomolecular architectures of electron donors on interfacial arrangements and intermolecular charge-transfer (CT) processes for small-molecule OPVs by means of multiscale theoretical simulations. They synthesized three oligothiophene [49] nanomaterial derivatives namely, D–π– A–π–D, π–A–D– A–π and A–π–D–π–A which served as donors (D) in combination through a pie-bond (π) with the fullerene acceptor (A) (6,6)-phenyl C71-butyric acid methyl ester (PC71BM).
To acquire the mesoscopic morphologies of the two colleagues’ materials, the two colleagues used the melt-quenched [50] approach to perform atomistic molecular dynamics (MD) simulations, which were successfully implemented to elucidate the interfacial geometries for the donor/acceptor blends of each derivative named above. They were able to attain PCEs of 7–9% while assembling their devices based on the three mixtures, which is not as awful for fullerene-based devices. Their findings also revealed that regardless of the varying push-pull configurations of electron donors, HOMO levels tend to be more concentrated on the material’s core unit. Non-fullerene equivalents have made greater development in recent years, inspired by the success of fullerene-based small molecule OPVs.
DSSCs have been developed throughout the last three decades, according to the latest data of verified efficiency tests from the National Renewable Energy Laboratory (NREL), however, their efficiency has achieved saturation of about 12%. (See Figure 5). Congcong Wu et al. [3] ascribed this to the use of nanostructured photoanode components notably mesoporous Titanium dioxide (TiO2) with complex core-shell nanocomposite and face-oriented nanocrystal designs, as well as counter electrode adjustment. The OPVs have a shorter background than the DSSCs, but they show promise because their efficiency has increased by nearly 17%. (See Figure 5). Nanomaterials may play a significant part in OPVs in a variety of ways. Surface states passivation and morphological modification, organic layer protection, energy barrier reduction, charge transport selection, and optical spacer are all possible with nanomaterial at the interfaces. All of these characteristics will help to reduce energy loss at the OPV cell’s interface. Finally, the PSCs have a higher efficiency than most commercial polycrystalline silicon photovoltaic cells (Figure 5), which is around 25%. Nanomaterials are commonly employed in PSC electrodes, charge transport layers, and perovskite photoactive layers, much as they are in OPVs. The performance of PSCs has been improved using the knowledge learned from OPVs and DSSCs.
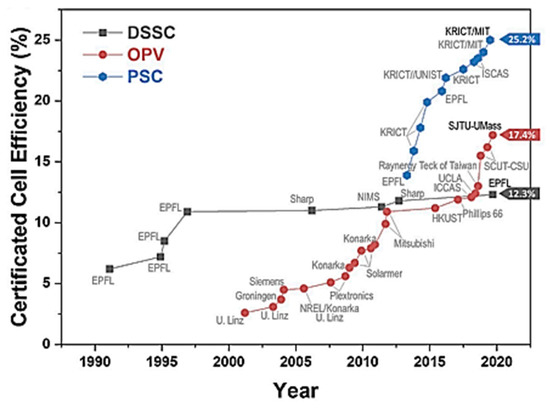
Figure 5.
Summary of the new-generation solar cells presenting their historically developed efficiency (adapted from reference [3] NREL efficiency table).
To explore the potentiality of non-fullerene-based nanomaterial as next generation for sustainable organic photovoltaics, Hao Zhang and colleagues [46] lately in 2019 designed and synthesized two nanomaterial non-fulleferene acceptors, 3,9-Bis(2-methylene-((3-(1,1-dicyanomethylene)-6/7-chloro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (IT-2Cl) and 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-dichloro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (IT-4Cl) from a typical synthetic route of 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-difluoro)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene (IT-4F) as shown in Figure 6A below. However, the precursors for the two acceptors ITIC-2Cl and ITIC-4Cl do not undergo halogen exchange reaction and that was attributed to the increased dipole moments of the end groups from 2.26 of IT-4F to 2.77 D of IT-xCl (where x is either 2 or 4), and thus enhanced the intramolecular charge-transfer (ICT) effects in IT-xCl. When the two end groups (from reaction A) are blended with IDTT-CHO they give the desired two non-fullerene nanomaterial acceptors as shown in Figure 6B, the chemical structures of the two acceptors are shown in Figure 6C.
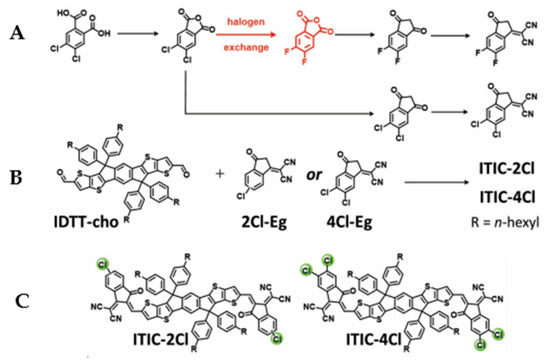
Figure 6.
Formation of non-fullerene precursors (A) the synthesis of ITIC-2Cl and ITIC-4Cl (B) chemical structures of ITIC-2Cl and ITIC-4Cl acceptors (C) (Reproduced from Reference [46] with permission, copyright 2018 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim, Germany).
2.2. Polymer-Based OPV Cells
To investigate the performance of as-synthesized nanomaterial, Poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione)] (PBDB-T) was employed as a donor to fabricate an OPV device for each of the two acceptors and the OPV device delivered PCE of 14.18% and 13.45% for ITIC-2Cl and ITIC-4Cl acceptor, respectively. The improved performances of the two single-junction devices, due to the variation in wavelengths for the two acceptors is shown in Table 1.

Table 1.
Photovoltaic performance of small-molecule non-fullerene-based acceptors.
To further improve the performance of non-fullerene-based OPVs Qishi Liu et al. [51] developed a copolymer donor, Poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-5,5′-(5,8-bis(4-(2-butyloctyl)thiophen-2-yl)dithieno[3′,2′:3,4;2″,3″:5,6]benzo[1,2-c][1,2,5]thiadiazole)] (D18) using a fused-ring acceptor unit, dithieno[3′,2′:3,4;2″,3″:5,6]benzo[1,2-c][1,2,5]thiadiazole (DTBT) that has a larger molecular plane [52] and thus, gifted D18 a higher hole mobility. When blended with BTP-4F acceptor, the device demonstrated a PCE of 18.22%, which is the highest efficiency for organic solar cells to date.
The second class of OPVs which are conjugated polymer-based OPVs, on the other hand, have also gotten a lot of attention from researchers because of their benefits over silicon-based solar cells, they possess medium or low band-gap; with high carrier mobility and matched energy levels with good solubility. Same as their counterparts, polymer-based OPVs are also divided into two well-known subclasses namely, fullerene and nonfullerene-based polymers [53]. Yong Cui et al. [54]. have recently reported a nonf-ullerene chlorinated low band-gap nanomaterial acceptor 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-dichloro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (BTP-4Cl) [55] where the halogen atoms of the fluorinated non-fullerene acceptor 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (BTP-4F) [56] were replaced with chlorine atoms. When the OPV cells were fabricated using the polymer donor PBDB-TF, the BTP-4Cl-based device yielded a higher VOC of 0.867 V compared with that of the BTP-4F-based device which was 0.834 V. That difference was ascribed on the blend films which indicated that the BTP-4Cl-containing film had a higher electro-luminescence quantum efficiency (EQEEL) of 3.47 × 10−4 compared to its BTP-4F counterpart (1.40 × 10−4). The consensus which was reached is that the different VOC values indicated that there was a reduced non-radiative energy loss (Eloss,nr) of approximately 24 meV that contributed to the improved VOC of the BTP-4Cl nanomaterial acceptor. As a result, high efficiencies of 16.5% and 15.3% were achieved for BTP-4Cl and BTP-4F respectively.
3. The Performance of Nanostructured Organic Devices
In the energy conversion processes, the use of nanostructured electrode materials have great potential in improving the overall electrochemical performance of renewable energy sources such as perovskites [57], OPVs and fuel cells [58]. Nanostructured materials demonstrated enhanced mechanical, electrical, and optical performance as a result of decreased dimensions at the nanoscale, which increase charge species mobility [59]. OPVs also employ nanostructured material in their fabrication, which is made up of a crucial component such as bulk heterojunction (BHJ) which was first introduced by Heeger et al. in 1995 [3]. Congcong Wu et al. [3] also made their input where nanoparticles (NP), ultrathin nanolayers, and graphene oxide were among the nanostructured materials they studied. Their research revealed that there are 3 main types of semiconducting nanocrystals for charge transport layers in OPVs: transition metal oxides (TMOs), alkali-metal compounds, and organic monolayers. Because of their high optical transparency and electrical conductivity, transition metal oxide (TMO) nanoparticles are one of the most popular charge transfer layers in OPVs [60].
Different TMO NPs can be employed as the electron or hole transfer layer depending on their functionality. To decrease energy loss during transportation inside the charge transfer layer, efficient charge transfer layers require strong electronic characteristics such as superior electrical conductivity or low series resistance, as well as high charge carrier mobility. Masahito Takakuwa et al. have produced ultraflexible OPVs with low incident light angle dependency and a PCE of 10.5% using ultrathin nanograting polymer substrates [61]. They point out that the optimized PCE of the OPV with an ultrathin nanograting polymer substrate was 71.3% at a 50° incident light angle, whereas the PCE of the OPV with a nonpatterned substrate was 65.6%. The nanograting-patterned fluorinated polymer enabled the development of periodic nanograting frameworks onto the back surface of a 1 m thick polymer substrate with a pitch of 760 nm and a height of 100 nm, while the reverse surface remained flat after the configuration of the planarization layer, according to their explanation. As a result, as compared to conventional active layer patterning methods, the proposed patterning approach is successful in preventing yield loss owing to active layer contamination.
3.1. Polymer-Based Nanostructures in the OPVs
Another method to improve light trapping is to utilize textured substrates, noble metal nanoparticles, tandem solar cells, and microlens arrays, in addition to creating novel materials. Another way to boost light absorption inside the active layer of PSCs is to use a microlens array (MLA) as an extra structure on the side of the transparent substrate opposite the active layer. Because MLA is on the opposite side of the substrate (see Figure 7), this method is non-intrusive and has no influence on the design process of the device or the active layer’s internal morphology. It has also been shown to be a universal means of increasing light absorption in various organic photovoltaic systems [62]. In the case of textured substrates, the aspect-ratio of textures must be optimized to guarantee that the active layers are coated in a conformal manner. Shunts and recombination will occur because of the irrational design [63]. The noble metal nanoparticles doped active layers employ plasmonic near-field enhancement effects to collect more light. On the other hand, sufficient metallic and surface stabilizer on metal might function as recombination sites, limiting charge transfer. Tandem polymer solar cells have a folded form that traps light at high angles and produces a high photo-current density. In addition, tandem polymer solar cells may be used to link several band-gap solar cells in series or parallel. Tvingstedt et al. produced a tandem cell by folding two planar but different cells together, and single cells reflect non-absorbed light onto another cell to accomplish spectrum broadening and light trapping [62]. The device’s overall performance was improved. The improved device performance can be ascribed to the active layer’s ordered structure, which can increase electron-hole pair separation and carrier mobilities even further. By combining nanoimprinting and lamination methods, Wiedemann et al. built comparable PCBM/P3HT bilayer devices with controlled nanostructured interfaces, which have a higher PCE than typical bilayer structured devices [64]. This method is also suitable for any other polymer combinations.
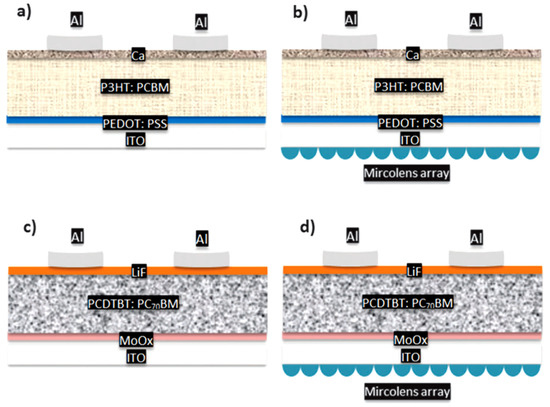
Figure 7.
Illustration of different structures of PSC device fabricated using: (a) P3HT: PCBM device without MLA and (b) with MLA; (c) PCDTBT: PC70BM device without MLA and (d) with MLA. (regenerated from reference [62] with copyrights from RSC Publishing, the Owner Societies 2013).
3.2. Organic Solar Cells Based on Small Molecule Donor and Polymer Acceptor
Polymer donors and small molecule acceptors are often used in organic solar cells. According to research by Zang et al. after 7 days of thermal annealing, the efficiency and thermal stability of the organic solar cell with combination of small molecule donor and polymer acceptor retained 89% of its initial efficiency [65]. Their device has a power conversion efficiency of 8.0% and small-size phase separation in the active layer. Because of large-size phase separation, most small-molecule donors have strong intermolecular interactions and high crystallinity, and so do not match polymer acceptors. By providing substantial steric hindrance, a suppressed-stacking between molecular backbones may be accomplished utilizing a mix of polymer donor and small molecule acceptor, SD/PA-type OPV device. In another study, a polymer acceptor based on the double B ← N bridged bipyridyl (BNBP) unit was employed to develop SD/PA-type small-molecule solar cell. With poly[(N,N′-bis(2-hexyldecyl)-diamine-bis(difluoro-borane)-2,2-bipyridine)-alt-(2,5-dithiophene)] as the acceptor and 7,7′-(4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b′]dithiophene-2,6-diyl)bis(6-fluoro-4-(5′-hexyl-[2,2′-bithiophen]-5-yl)benzo[c][1,2,5]thiadiazole) as the donor. The OPV device achieved a high open-circuit voltage (VOC) of 1.08 V and a PCE of 3.50% [66]. Although SD/PA systems have made significant progress in recent years, with a PCE increase of 9.51%, they still trail behind other types of OPVs, owing to the absence of high-performance polymer acceptors and the non-ideal shape of the SA/PA blend. Future research on SD/PA-type OPVs should focus on developing novel polymer acceptors with a narrow band-gap, broad absorption, and high electron mobility, as well as finding the right small molecule donors to match the polymer acceptors’ energy level, absorption spectrum, and hole/electron mobility. Predictably, the gap between SD/PA-type OPVs and other OPVs discussed in this review can be narrowed through material innovations and process optimization.
4. Next Generation Applications of OPVs
The use of solar energy commercially is still relatively low mainly because of poor efficiency of current solar systems, the relative high costs of maintenance as well as the environmental safety precautionary measures [58]. The GreenCape Sector Development Agency conducted a study on greater solar energy uptake for agricultural processing (agri-processing) sector, they specifically focused on solar photovoltaic systems for packhouses and solar thermal for heat processing [42]. Their findings highlighted opportunities for the agri-processing and solar industry, the barriers that the policy-makers need to address in order to unlock the economic development potential that could be enabled by a greater uptake of energy [67]. They also focused on potential areas in which researchers should look at unlocking the solar energy opportunities in food value chains. In this review, we probe various fields in which organic photovoltaic systems were employed to either improve the infrastructure or to provide renewable energy in the form of heat for warming and cooling processes. Some of the applications of OPVs include but are not limited to use in agriculture for greenhouses [68], water irrigation, purification of generated wastewater as well as breeding for farming [59].
4.1. Organic Photovoltaics Spearheading Sustainable Agricultural Processes
One of the fields where OPVs are used is in agriculture tailor-make greenhouses for environmental control of crops. Environmental control in greenhouses [63] is meant to achieve indoor temperatures, relative humidity, light and CO2, which are as close as possible to optimal growth conditions for plants by using heating, cooling, ventilation, variable shading, and CO2 enrichment and lighting systems [68]. A greenhouse structure covered with transparent materials can be made up of polymer (plastic), glass, or fiberglass, which can allow the short waves of solar irradiation to enter the greenhouse [64]. Researchers have conducted studies on the use of polymer-based nanomaterial in trying to promote green chemistry, water and food security as well as dynamic and energy prediction modules to reduce energy consumption [66]. The impact of semi-transparent flexible and lightweight OPVs based on P3HT: PCBM photoactive layer on pepper plant development was presented in a study by C. Zisis el al. They installed OPVs on the roof of a Mediterranean greenhouse, covering 22% of the total surface. The photosynthetic process of pepper plant vegetative development and fruit production was enabled by the construction of a sustainable eco-system in an OPV solar Mediterranean greenhouse.
According to their findings, semi-transparent OPV modules based on the P3HT: PCBM photoactive layer with transparency of up to 19.4% in the Photosynthetically Active Radiation (PAR) region are suitable for utilizing a given land area for both agriculture and energy generation [69]. Specifically, the OPV shading effect on plant growth is found to be beneficial for pepper plant cultivation [70]. When the absorption range of such active layer nanomaterials is properly aligned with that of the plants, the scenario shifts from competition to synergism, as part of the energy provided by the photovoltaic system can be used to better control humidity and temperature levels inside greenhouses and polytunnels. Finally, such simple agrivoltaic devices might help to sustain development in dry rural regions by supplying clean energy to the surrounding populations, in addition to contributing to food production. Plants require as much light as possible for photosynthesis, thus the characteristics are primarily governed by this requirement. This necessitates excellent photovoltaic module transparency in the range of 1.77 to 3.55 eV, as this is the area of the sun spectrum where photosynthetic action occurs [71].
4.2. Future Design of Nanomaterial-Based Photovoltaic Systems for Land and Agricultural Use
The physiochemical reaction of plants to the use of nanomaterials to boost food production output is now the subject of research. Results from a study by Jin et al. [72] shows that the size, chemical components, and functional groups present on a nanomaterial’s surface, as well as the kind of coating, all have a role in its absorption by plants. Plants’ general physiology is affected by nanomaterial contact and absorption, which causes molecular alterations [73]. Another application of nanomaterials in plants is in pesticide control, which has a significant impact on production yield. These are polymer-based nanocarriers that operate as protective storage for insecticides, comprising polysaccharides and polyesters. Furthermore, they tend to improve dispersion in aqueous conditions, making pesticide to be released more gradual and regulated. Polymer-based nanomaterials for pesticides have attracted a lot of attention due to their design flexibility, biocompatibility, and biodegradability (corn oil, beeswax, and cashew gum) [74].
5. Future Insights
What is typically thought of as a disadvantage for achieving high-level power-conversion efficiencies, notably imperfect absorption over a large wavelength range severely restricting photocurrent production, can really be addressed by developing more nanostructured materials of lower costs but producing high performance. The DTBT derivatives are recent nanomaterials which display promising features to achieve higher efficiencies. There is still room for improvement in the application of the OPVs more especially in areas such as marine where there is plenty of space to install panels without fear or doubt of the lives of animals living in that area because fishes and other marine animals can survive well without sunlight.
6. Conclusions
Organic semiconductors have developed as a desirable and financially feasible substitute to inorganic semiconductors. The advantages of employing organic molecules instead of conventional inorganic materials are discussed in this article, which include structural flexibility, simplicity of device production, and cost savings. Increased technological capabilities for vast industrial roll-to-roll manufacturing of thin-film organic semiconductor devices have evolved in the previous decade, which can be a significant cost-saver in both homes and commercial agrivoltaic systems. Thus, mass production of organic semiconductor devices can be produced cheap enough for practical use where flexibility, light-weight, and low cost are much more important than performance, not to mention in those cases where organic semiconductors have comparable or even better property/performance than inorganic materials. Therefore, we aimed to highlight this performance enhancement brought about by innovative organic photovoltaic developments. In so doing, the exciting prospects of meeting the sustainable development energy and food security needs of the future. The review provides a background on the conventional solid-state (silicon-based) photovoltaics systems and concomitantly discussing the importance of progressing towards innovative organic photovoltaic systems (OPVs); covering the different classes, their synthetic routes and operating principles. However, there is no doubt that room for improvement still exists because the main challenge for commercialization of these OPVs is in the development of large-scale modules with extended operational lifespan, high production yields, low manufacturing costs, and high efficiency.
Author Contributions
N.R. and R.M. conceived the presented idea and drafted the original version of manuscript. K.T. assisted with developing the theory and interpretation of data. L.M. verified the analytical methods and revised it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Food and Beverages Manufacturing Sector Education and Training Authority (FoodBev SETA). It is only a student bursary with no grant number provided. Only the name of the fund is required.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is sponsored by Food and Beverages Manufacturing Sector Education and Training Authority (FoodBev SETA). Would also like to thank the SensorLab of Chemistry department at the University of the Western Cape for providing resources to make this work a success.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, A.; Pathak, D.; Wagner, T. Organic photovoltaic materials: A review on synthesis, structure and properties. J. Optoelectron. Adv. Mater. 2014, 16, 1257–1268. [Google Scholar]
- Snoke, D.; Denev, S.; Liu, Y.; Pfeiffer, L.N.; West, K. Letters To Nature. Nature 2002, 418, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, K.; Batmunkh, M.; Bati, A.S.R.; Yang, D.; Jiang, Y.; Hou, Y.; Shapter, J.G.; Priya, S. Multifunctional nanostructured materials for next generation photovoltaics. Nano Energy 2020, 70. [Google Scholar] [CrossRef]
- Phillip, D.J. Fossil Fuels are Bad for Your Health and Harmful in Many Ways besides Climate Change; The Conversation: Melbourne, Australia, 2019; Volume 1. [Google Scholar]
- Zhang, T.; Yang, H. High efficiency plants and building integrated renewable energy systems: Building-integrated photovoltaics (BIPV). In Handbook of Energy Efficiency in Buildings; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128128176. [Google Scholar]
- Mehreen, G.; Yash, K.; Tariq, M. Review on recent trend of solar photovoltaic technology. Coast. Estuar. Process. 2016. [Google Scholar] [CrossRef] [Green Version]
- Speller, E.M.; Clarke, A.J.; Aristidou, N.; Wyatt, M.F.; Francàs, L.; Fish, G.; Cha, H.; Lee, H.K.H.; Luke, J.; Wadsworth, A.; et al. Toward improved environmental stability of polymer: Fullerene and polymer:Non-fullerene organic solar cells: A common energetic origin of light—A nd oxygen-induced degradation. ACS Energy Lett. 2019, 4, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GreenMatch. Organic Solar Cells—Compare Prices Here. 2021. Available online: https://www.greenmatch.co.uk/solar-energy/solar-panels/photovoltaic-cells/organic (accessed on 29 July 2021).
- Kibria, M.T.; Ahammed, A.; Sony, S.M.; Hossain, F. A Review: Comparative studies on different generation solar cells technology. In Proceedings of the International Conference on Environmental Aspects of Bangladesh, Dhaka, Bangladesh, 23–24 May 2014; pp. 51–53. [Google Scholar]
- Chandler, D.L. Researchers improve efficiency of next-generation solar cell material. MIT News, 24 February 2021. [Google Scholar]
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P. Organic solar cells: Their developments and potentials. Renew. Sustain. Energy Rev. 2013, 21, 421–431. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Ji, S.; Hua, H.; Ma, Y.; Hu, R.; Zhang, J.; Chu, L.; Li, X.; Huang, W. Perfection of Perovskite Grain Boundary Passivation by Rhodium Incorporation for Efficient and Stable Solar Cells. Nano Micro Lett. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- De Angelis, F. Modeling materials and processes in hybrid/organic photovoltaics: From dye-sensitized to perovskite solar cells. Acc. Chem. Res. 2014, 47, 3349–3360. [Google Scholar] [CrossRef]
- Bhattacharya, S.; John, S. Beyond 30% Conversion Efficiency in Silicon Solar Cells: A Numerical Demonstration. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Archer, M.D.; Hill, R.; Firm, K. Clean Electricity from Photovoltaics; Imperial College Press: London, UK, 2001; Volume 1. [Google Scholar]
- Gan, X.; Wang, O.; Liu, K.; Du, X.; Guo, L.; Liu, H. 2D homologous organic-inorganic hybrids as light-absorbers for planer and nanorod-based perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 162, 93–102. [Google Scholar] [CrossRef]
- Ilmi, R.; Haque, A.; Khan, M.S. High efficiency small molecule-based donor materials for organic solar cells. Org. Electron. 2018, 58, 53–62. [Google Scholar] [CrossRef]
- Kalowekamo, J.; Baker, E. Estimating the manufacturing cost of purely organic solar cells. Sol. Energy 2009, 83, 1224–1231. [Google Scholar] [CrossRef]
- Mari-Louise, J.W.; Cameron, M. “Renewable Energy State of”, Dep. Energy, Matimba House, 192 Visag. Str, Corner Paul Kruger Visag. Str, Pretoria, South Africa, 2017. Available online: www.energy.gov.za (accessed on 5 July 2021).
- Complete Solid-State Dye-Sensitized Solar Cell. Global | Ricoh. Available online: https://www.ricoh.com/technology/tech/066_dssc (accessed on 5 July 2021).
- Xie, F.; Chen, C.C.; Wu, Y.; Li, X.; Cai, M.; Liu, X.; Yang, X.; Han, L. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- 1.10: Pi Conjugation—Chemistry LibreTexts. Available online: https://chem.libretexts.org/Courses/Purdue/Purdue%3A_Chem_26505%3A_Organic_Chemistry_I_(Lipton)/Chapter_1._Electronic_Structure_and_Chemical_Bonding/1.10%3A_Pi_Conjugation (accessed on 12 July 2021).
- Rafique, S.; Abdullah, S.M.; Sulaiman, K.; Iwamoto, M. Fundamentals of bulk heterojunction organic solar cells: An overview of stability/degradation issues and strategies for improvement. Renew. Sustain. Energy Rev. 2018, 84, 43–53. [Google Scholar] [CrossRef]
- Facchetti, A. Polymer donor-polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Deibel, C.; Dyakonov, V. Polymer-fullerene bulk heterojunction solar cells. Rep. Prog. Phys. 2010, 73, 096401. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; McGehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Siddiki, M.K.; Li, J.; Galipeau, D.; Qiao, Q. A review of polymer multijunction solar cells. Energy Environ. Sci. 2010, 3, 867–883. [Google Scholar] [CrossRef]
- Dhankhar, M.; Om Pal Singh, V.N.S.N. Power Plant Emitin Smoke Steam Stock Photo (Edit Now) 1555865300. Available online: https://www.shutterstock.com/image-photo/power-plant-emitin-smoke-steam-1555865300 (accessed on 29 June 2021).
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Ke, L.; Tan, L.; Lin, T.; Kietzke, T.; Chen, Z.K. Conjugated copolymers based on fluorene-thieno[3,2-b]thiophene for light-emitting diodes and photovoltaic cells. Macromolecules 2007, 40, 6164–6171. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhou, Y.; Liu, H.; Xue, Q.; Li, X.; Chueh, C.C.; Yip, H.L.; Zhu, Z.; Jen, A.K.Y. Highly efficient all-inorganic perovskite solar cells with suppressed non-radiative recombination by a Lewis base. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Mazzio, K.A.; Luscombe, C.K. The future of organic photovoltaics. Chem. Soc. Rev. 2015, 44, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.; Barkhouse, D.; Gunawan, O.; Gokmen, T.; Todorov, T.; Mitzi, D. Solar cell efficiency tables (version 40). IEEE Trans Fuzzy Syst 2012, 20, 1114–1129. [Google Scholar] [CrossRef]
- Zhou, Y.; Eck, M.; Krüger, M. Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ. Sci. 2010, 3, 1851–1864. [Google Scholar] [CrossRef]
- Hoang, N.V.; Nikolis, V.C.; Baisinger, L.; Vandewal, K.; Pshenichnikov, M.S. Diffusion-enhanced exciton dissociation in single-material organic solar cells. Phys. Chem. Chem. Phys. 2021, 23, 20848–20853. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Deng, W. Introduction to organic solar cells. Org. Hybrid Sol. Cells 2014, 9783319108, 1–18. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device physics of polymer:Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Primrose Magama, C.I.; Lebotsa, S. Renewable Energy and Physics; Agrucultural Research Council—Institute for Agri: Pretoria, South Africa, 2017. [Google Scholar]
- Van Vuuren, P.J.; Pineo, C.; Basson, L. Solar Energy in Agri-Processing. In Proceedings of the Southern African Solar Energy Conference, Stellenbosch, South Africa, 31 Octotber–2 November 2016. [Google Scholar]
- Zhang, H.; Liu, Y.; Sun, Y.; Li, M.; Kan, B.; Ke, X.; Zhang, Q.; Wan, X.; Chen, Y. Developing high-performance small molecule organic solar cells via a large planar structure and an electron-withdrawing central unit. Chem. Commun. 2017, 53, 451–454. [Google Scholar] [CrossRef]
- Collins, S.D.; Ran, N.A.; Heiber, M.C.; Nguyen, T.Q. Small is Powerful: Recent Progress in Solution-Processed Small Molecule Solar Cells. Adv. Energy Mater. 2017, 7, 1602242. [Google Scholar] [CrossRef]
- Chem, J.M.; Duan, C.; Huang, F.; Cao, Y. Recent development of push—Pull conjugated polymers for bulk-heterojunction photovoltaics: Rational design and fine tailoring of molecular structures. J. Mater. Chem. 2012, 22, 10416–10434. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Hou, J.; Zhu, J.; Zhang, J.; Li, W.; Yu, R. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated-Small-Molecule Acceptors. Adv. Mater. 2018, 1800613, 1–7. [Google Scholar] [CrossRef] [PubMed]
- There Are Grounds for Concern about Solar Power; Climate Change; Al Jazeera. Available online: https://www.aljazeera.com/opinions/2021/4/7/there-are-grounds-for-concern-about-solar-power (accessed on 29 June 2021).
- Han, G.; Yi, Y. Rationalizing Small-Molecule Donor Design toward High-Performance Organic Solar Cells: Perspective from Molecular Architectures. Adv. Theory Simul. 2018, 1, 1800091. [Google Scholar] [CrossRef]
- Pandolfi, F.; Rocco, D.; Mattiello, L. Synthesis and characterization of new D-π-A and A-π-D-π-A type oligothiophene derivatives. Org. Biomol. Chem. 2019, 17, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Yue, Y.; Li, P.; Qiao, A.; Tao, H.; Greaves, N.G.; Richards, T.; Lampronti, G.I.; Redfern, S.A.T.; Blanc, F.; et al. Melt-Quenched Glasses of Metal-Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 3484–3492. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J. Rational Design of High Performance Conjugated; University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2011. [Google Scholar]
- Nakabayashi, K.; Mori, H. Donor-acceptor block copolymers: Synthesis and solar cell applications. Materials 2014, 7, 3274–3290. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Ossila Group. Y7 (BTP-4Cl); Non-Fullerene Electron Acceptor (NFA); Ossila Ltd.: Sheffield, UK, 2019; Available online: https://www.ossila.com/products/y7 (accessed on 13 August 2021).
- Ossila Group. Y6 (BTP-4F); Non-Fullerene Electron Acceptor, 2304444-49-1; Ossila Ltd.: Sheffield, UK, 2019; Available online: https://www.ossila.com/products/y6 (accessed on 13 August 2021).
- Wang, M.; Wang, W.; Ma, B.; Shen, W.; Liu, L.; Cao, K.; Chen, S.; Huang, W. Lead-Free Perovskite Materials for Solar Cells; Springer: Singapore, 2021; Volume 13, ISBN 0123456789. [Google Scholar]
- Banerjee, J.; Dutta, K.; Rana, D. Carbon Nanomaterials in Renewable Energy Production and Storage Applications; Springer: Berlin, Germany, 2019; ISBN 9783030044749. [Google Scholar]
- Salame, P.H.; Pawade, V.B.; Bhanvase, B.A. Engineered Nanomaterials for Renewable Energy; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128133514. [Google Scholar]
- Intertek. Optical Properties of Polymers. 2020. Available online: https://www.intertek.com/polymers/optical-properties/ (accessed on 7 October 2021).
- Takakuwa, M.; Heo, S.W.; Fukuda, K.; Tajima, K.; Park, S.; Umezu, S.; Someya, T. Nanograting Structured Ultrathin Substrate for Ultraflexible Organic Photovoltaics. Small Methods 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Elshobaki, M.; Ye, Z.; Park, J.M.; Noack, M.A.; Ho, K.M.; Chaudhary, S. Microlens array induced light absorption enhancement in polymer solar cells. Phys. Chem. Chem. Phys. 2013, 15, 4297–4302. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, K.S.; Park, J.M.; Ho, K.M.; Chaudhary, S. On realizing higher efficiency polymer solar cells using a textured substrate platform. Adv. Mater. 2011, 23, 112–116. [Google Scholar] [CrossRef]
- He, X.; Gao, F.; Tu, G.; Hasko, D.; Hüttner, S.; Steiner, U.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Formation of nanopatterned polymer blends in photovoltaic devices. Nano Lett. 2010, 10, 1302–1307. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Miao, J.; Ding, Z.; Kan, B.; Lin, B.; Wan, X.; Ma, W.; Chen, Y.; Long, X.; Dou, C.; et al. Efficient and thermally stable organic solar cells based on small molecule donor and polymer acceptor. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ding, Z.; Long, X.; Dou, C.; Liu, J.; Wang, L. Organic solar cells based on a polymer acceptor and a small molecule donor with a high open-circuit voltage. J. Mater. Chem. C 2017, 5, 6812–6819. [Google Scholar] [CrossRef]
- Efficiency for Access Coalition(IFC); International Renewable Energy Agency (IRENA). Power for All: Fact Sheet; Power for All: San Francisco, CA, USA, 2020; pp. 32–33. [Google Scholar]
- Hassanien, R.H.E.; Li, M.; Dong Lin, W. Advanced applications of solar energy in agricultural greenhouses. Renew. Sustain. Energy Rev. 2016, 54, 989–1001. [Google Scholar] [CrossRef]
- Sulev, M.; Observatory, T.; Frederic, B. Photosynthetically Active Radiation: Measurement and Modeling. In Encyclopedia of Sustainability Science and Technology; Springer: Berlin, Germany, 2012; pp. 7970–8000. [Google Scholar] [CrossRef]
- Zisis, C.; Pechlivani, E.M.; Tsimikli, S.; Mekeridis, E.; Laskarakis, A.; Logothetidis, S. Organic photovoltaics on greenhouse rooftops: Effects on plant growth. Mater. Today Proc. 2020, 21, 65–72. [Google Scholar] [CrossRef]
- Meitzner, R.; Schubert, U.S.; Hoppe, H. Agrivoltaics—The Perfect Fit for the Future of Organic Photovoltaics. Adv. Energy Mater. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, X.; Li, X.; Zhang, Z.; Sun, L.; Fu, Z.; Lavoie, M.; Pan, X.; Qian, H. Distinct physiological and molecular responses in Arabidopsis thaliana exposed to aluminum oxide nanoparticles and ionic aluminum. Environ. Pollut. 2017, 228, 517–527. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).