Featured Application
This work provides a detailed description of the topological insulator behavior and the surface band structures of CsPbBrxI3−x (x = 0, 1, 2, and 3) perovskites.
Abstract
First-principles density functional theory was used to determine the surface band structures of CsPbBrxI3−x (x = 0, 1, 2, and 3) perovskites. The equilibrium lattice constants of CsPbBrxI3−x were obtained from the minimum of the total energy as a function of the iodine concentration. We discovered that the band gaps of CsPbBrxI3−x decreased monotonically under pressure. The phase change from a normal insulator to a topological insulator was found at approximately 2–4 GPa. The Pbp- and Brs-orbitals inverted at the R symmetric point with and without spin–orbit coupling. Nontrivial Z2 topological numbers were obtained, and the surface conduction bands were demonstrated theoretically using a 1 × 1 × 10 supercell. We ascertained that CsPbBr2I has the largest electric polarization 0.025 C/m2 under a compression strain of 10%. We also observed that in the normal insulation phase, the band gap increases with a small displacement of the central Pb atom in the z-direction, but in the topological insulator phase, the band gap decreases with the movement of the Pb atom in the z-direction. Additionally, in the supercell structure, CsPbBrxI3−x is a ferroelectric topological insulator because the Pb atom leaves its own equilibrium position.
1. Introduction
Topological insulators (TIs) have an insulating gap in the bulk and gapless surface states at the same time, and these insulators have been extensively studied in past decades [1,2] due to their potential applications in spintronic devices [3,4]. Therefore, TIs have attracted much attention in both the fundamental physics and experimental research fields. Studies on BiSe2 [5], half-Heusler alloys such as LnPtBi3 [6,7], and binary compounds such as HgTe [8,9] have demonstrated that those materials are normal insulators, but they may also be transformed into TIs by applying spin-orbit coupling (SOC), axial or uniaxial strain, and impurities. Topologically trivial to nontrivial transitions of TIs are identified by observing the band inversion in the Brillouin zone. Fu and Kane [10] introduced a useful and practical Chern number or topological number Z2, which is calculated by analyzing the bulk band structure and provides a physical meaning in the prediction of edge states at interfaces.
Perovskite crystals are of interest in the fields of high thermoelectric power, ferroelectricity, superconductivity, spin electronic devices, and colossal magnetoresistance [11,12,13]. Studies on perovskites have been aimed at, for example, energy-saving and cost efficiency as well as the manufacture of stable solar cells [14]. For example, the cesium lead halide perovskite (CsPbX3; X = Cl, Br, and I) has received considerable attention for its potential application in high-performance solar cells [15,16] and light-emitting diodes [17,18]. Furthermore, organic lead halide (CH3NH3PbX3) nanocrystals and bulk crystals are promising high-efficiency solar cells with power conversion efficiencies exceeding 20% [19]. The TI phase in perovskites and double perovskites has been studied theoretically [20,21,22]. The coexistence of the TI phase and other phases, such as those of superconductivity, magnetism, and ferroelectricity, are of particular interest. Liu et al. [23] reported that cesium lead halide perovskite (CsPbI3) has a TI phase under strain together with a ferroelectric phase.
Previous theoretical studies on CsPbX3 (X = Cl, Br, and I) have focused on its electronic [23,24,25,26] and optical [24,25] properties. For example, Afsari et al. [25] reported that CsPbI3 becomes a TI under very high pressure [23,26]. However, the reported band gap for the CsPbI3 in the fully relativistic scheme is significantly underestimated. The calculated band gap of CsPbI3 is 1.48 eV without SOC in the generalized gradient approximation (GGA) and becomes 0.29 eV when the SOC is considered, revealing that the SOC effect is strong in these cesium lead halide perovskites. Nonetheless, the optical band gap for CsPbI3 has been reported to be 1.73 eV experimentally [27,28].
In this study, we investigated the TI phase of CsPbBrxI3−x (x = 0, 1, 2, and 3) under hydrostatic strain. The paper is organized into the following sections: Section 2 describes the crystal structures and computational methods, initially addressing the structural and electronic properties with and without consideration of SOC. Section 3 depicts the bulk and surface band structures and describes the calculation of the electric polarization produced by Pb atom distortion. We confirm theoretically that the slight distortion will not disrupt the TI phase of cesium lead halide perovskite. In the final section, we draw conclusions.
2. Structures and Computational Methods
The perovskite ABZ3 is a cubic structure with a space group of Pm3m. The B-site cations correspond to six anions to form cuboctahedra, which are chemically inert ions arranged in a cuboctahedron. The B-site and Z-site refer to the atomic arrangement in the unit cell of cations and anions, respectively. The Wyckoff position for the A-site cation is located at the corner (0, 0, 0), that of the B-site cation is located at the center (1/2, 1/2, 1/2), and those of the Z-site anions are located at face-center (1/2, 1/2, 0), (1/2, 0, 1/2), and (0, 1/2, 1/2). Studies have reported that, under experimental conditions, lead halide perovskites have a simple cubic phase at high temperatures. In this study, the structural and electronic properties of CsPbBrxI3−x (x = 0, 1, 2, and 3) perovskites were calculated using density functional theory as implemented in the Vienna ab initio simulation package (VASP) [29,30] along with the Perdew-Burke-Ernzerhof (PBE) GGA [31] for the exchange-correlation functional. The Γ-centered Monkhorst-Pack scheme with a k-mesh of 12 × 12 × 12 was used for the first Brillouin zone integration. The self-consistent total energy criterion was set to 1.0 × 10−6 eV. The plane wave cutoff energy was set to 350 eV. The equilibrium lattice constant of the cubic perovskite structure was obtained from the minimum of the total energy as a function of the lattice constant. The SOC effect was considered after the equilibrium lattice constant was found. To identify the surface conduction band of CsPbBrxI3−x perovskites, we prepared a supercell containing 10 formula units with a vacuum spacing of approximately 20 Å, which is wide enough to decouple the interlayer interaction. The surface atoms were fixed (i.e., with no further structural optimization possible) to correspond with the one in the bulk form. The atomic structure of CsPbX3 (X = Br and I) bulk is shown in Figure 1a, and schematics of two initial surface structure models—specifically with one PbX2- and CsX- and two PbX2-terminated slabs as shown in Figure 1b,c, respectively—were constructed from known bulk geometries to help clarify which plane contributes to the surface conduction bands. The nonpolar supercells contained 10 formula units with a vacuum of 20 Å, which is large enough to help obtain the surface conduction bands.
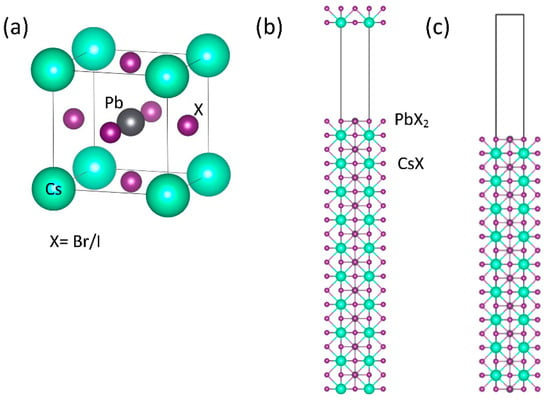
Figure 1.
Schematic of the CsPbX3 (X = Br or I) crystal structure (a), one PbX2- and CsX- (b) and two PbX2- (c) terminated slabs. The atoms are represented by spheres: Cs (green, large), Pb (dark blue, medium), and X (purple, small).
We studied the electric polarization caused by the movement of the Pb atom in the z-direction. Displacement of Pb atom in the z-direction breaks the inverse space symmetric of the CsPbBrxI3−x, resulting in a finite electric polarization. In addition to using the band inversion, the so-called Chern number or topological number Z2 can be used to prove the characteristics of TIs. We calculated Z2 by considering the parity of the wave function at specific k points reported by Fu and Kane [10].
3. Results and Discussion
The calculated equilibrium lattice constants, scalar relativistic band gap , and fully relativistic band gap (eV) of the CsPbBrxI3−x perovskites are listed in Table 1. For the CsPbBr3, we obtained a relaxed lattice constant of a = 5.99 Å in the equilibrium structure, which was consistent with the theoretical value of 5.99 Å [24]. The calculated lattice constant for the CsPbI3 was 6.39 Å, in excellent agreement with its theoretical values of 6.40 [25], 6.39 [24], and 6.05 Å [32]. The equilibrium lattice constants increased monotonically with iodine concentration. The Wigner–Seitz radii for the Cs, Pb, Br, and I atoms are 1.323, 1.725, 1.164, and 1.487 Å, respectively. Therefore, the calculated lattice constants of the CsPbBrxI3−x perovskites were increased due to the larger Wigner–Seitz radius of iodine. The fully relativistic band gap was significantly lower than the scalar relativistic band gap of the CsPbBrxI3−x perovskites due to the strong SOC effect. When SOC is considered, the band gaps of the CsPbBrxI3−x perovskites lead to a substantial underestimation of calculated band gaps. The calculated band gaps of the CsPbBrxI3−x perovskites induced by SOC were 0.67, 0.49, 0.39, and 0.33 eV for the CsPbBr3, CsPbBr2I, CsPbBrI2, and CsPbI3, respectively. This underestimation of the band gap can be corrected by applying, for example, the G0W0 or PBE0 approximations, but the tendency of the band gap to decrease under strain will remain unchanged. The reduction in the band gap caused by the SOC effect commonly appears in not only bulk TIs but also two-dimensional transition metal dichalcogenides (TMDCs) [33]. A useful and practical empirical formula of TMDCs in terms of the PBE band gap and G0W0 band gap was introduced by Garcia et al. [34] who introduced the form = 1.358 × + 0.904, where the G0W0 is an approximation with Hartree-Fock (HF) Green function G and the screened interaction W without further iterations. Here we adopt the G0W0 approximation because it is the most physically grounded method to accurately predict the energy band bap, as presented in Table 1 and Figure 2. To compare our calculated band gaps, we also employed the PBE0 hybrid functional which combines the PBE exchange energy and HF exchange energy in a set 3:1 ratio, along with the full PBE correlation energy, as also summarized in brackets in Table 1. The CsPbBrxI3−x perovskite obtained from the G0W0 empirical approach has the largest band gap. The PBE0 band gaps with SOC are between the PBE and G0W0 band gaps. For the experimental results of CsPbBr3 and CsPbI3 perovskites, the G0W0 band gaps with SOC are underestimated by approximately 23% and 22%, respectively. Analysis of the fully relativistic band gaps suggests that more external pressure may be required to produce a normal TI phase transition under the empirical correction with a constant of 0.904 eV.

Table 1.
Calculated equilibrium lattice constant a, scalar relativistic band gap , and fully relativistic band gap of the CsPbBr3, CsPbBr2I, CsPbBrI2, and CsPbI3 perovskites.
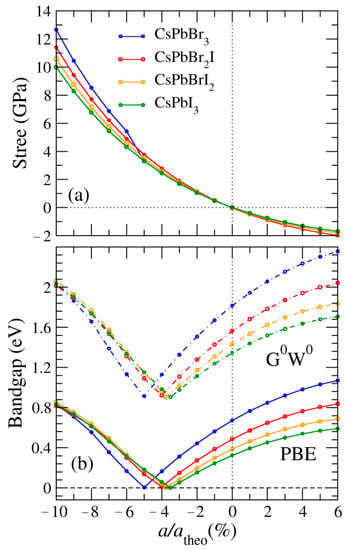
Figure 2.
Top and bottom panels illustrating external stress (a) and calculated band gaps (b), respectively, for CsPbBr3, CsPbBr2I, CsPbBrI2 and CsPbI3 perovskites as a function of the lattice constant and its equilibrium lattice constant, a/atheo, as a percentage.
3.1. Band Structures
To estimate the strain effect on the trivial topology feature, we illustrate in Figure 2 the stress-ratio a/atheo and band gap-ratio a/atheo relationships for hydrostatically strained CsPbBrxI3−x over the wide a/atheo range from −10% to 6%. At the hydrostatically compressed strain value of about 4% to 5%, our calculations indicate that the band gaps of CsPbBrxI3−x become zero, indicating that the CsPbBrxI3−x perovskites become TIs. In addition, the phase transition from normal insulators to TIs occurs at approximately 2–4 GPa. The critical external pressure of the CsPbBrxI3−x phase transition from the normal insulators to TIs decreases monotonically with iodine concentration. On the other hand, the TI regime of the CsPbBrxI3−x perovskite occurs at a/atheo from −3% to −5%. To observe how external pressure and SOC affect the band structure, the band structures of CsPbI3 under different lattice constants and with and without SOC are represented in Figure 3. We discovered that the calculated band gaps of CsPbI3 were direct without SOC and decreased monotonically as external pressure increased, as shown in Figure 3a–c. When SOC was considered, the calculated band gap of CsPbI3 at the equilibrium lattice constant was only a quarter of the corresponding one without SOC, as illustrated in Figure 3a,d. Figure 3e indicates that the calculated band gap of CsPbI3 is reduced to zero under an a/atheo ratio of −3.5%. To further clarify the TI behavior described in Figure 3f, we determined how the calculated band gap of CsPbI3 increases under an a/atheo ratio of −8%, demonstrating that the Dirac corn appears at the high symmetric R point. The so-called band inversion also occurred at the point R where the Pbp- and Is- orbitals are inverted due to SOC. This energy band reversal illustrates a theory of material topology. We also noted that as stress increased, the energy of the highest occupied band at point M also increased.
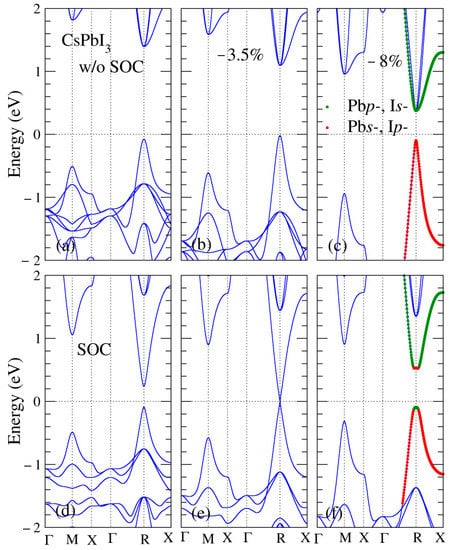
Figure 3.
CsPbI3 band structures corresponding to (a) the equilibrium lattice constant, (b) the a/atheo ratio of −3.5% with spin–orbit coupling (SOC) and (c) the a/atheo ratio of −8% without SOC; (d) the equilibrium lattice constant, (e) the a/atheo ratio of −3.5% with SOC and (f) the a/atheo ratio of −8% without SOC, respectively. The dashed line is the Fermi level. The green line represents Pbp- and Is- orbitals. The red line depicts Pbs- and Ip- orbitals.
A detailed comparison of the calculated band gaps of CsPbBrI2 perovskites, which are virtually identical to doping iodine atoms in CsPbBr3, is also warranted. The band structures of CsPbBrI2 perovskites under different lattice constants with and without SOC are shown in Figure 4. The calculated band gaps of CsPbBrI2 were also similar in behavior to those of CsPbI3, which show that the calculated band gaps of CsPbBrI2 are direct without SOC and decrease monotonically as external pressure increases, as illustrated in Figure 4a–c. Figure 4c clearly indicates that Pbp- and Brs-orbitals are degenerate or close together in energy (0.2 eV), whereas Pbp- and Is-orbitals are degenerate in energy, which was 0.6 eV above the Fermi level under the a/atheo ratio of −8% at the R point. When SOC was considered, the calculated band gap of CsPbBrI2 at the equilibrium lattice constant was approximately one-third of the corresponding one without SOC, as shown in Figure 4a,d. Figure 4e shows the calculated band gap of CsPbBrI2 approaching zero under the a/atheo ratio of −3.8%. When SOC was considered, band reversal still occurred at the R point, as shown in Figure 4f. Notably, the energy of the highest occupied band at the M point became higher than the energy band at the R point at the a/atheo ratio of −8%, showing that the transition may not be useful for optical applications.
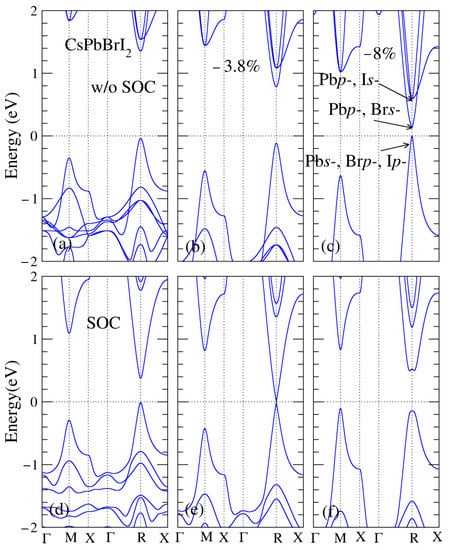
Figure 4.
CsPbBrI2 band structures corresponding to (a) equilibrium lattice constant, as well as (b) the ratio a/atheo of −3.8% with and (c) −8% without the spin–orbit coupling; (d) equilibrium lattice constant, (e) the ratio a/atheo of −3.8% with and (f) −8% without the spin–orbit coupling. The dashed line is the Fermi level.
A useful and practical topological number of Z2 in terms of the Green function and corresponding to the charge density derived from VASP and the Wannier90 package [37] was used to determine the characteristics of TIs. As shown in Figure 5, the Wannier functions of the CsPbI3 perovskite were applied in the range of −6 to 3 eV, which closely matched the band structure of the CsPbI3 perovskite derived from VASP. Here, we followed the work by Fu et al. [10] and calculated the Z2 invariants from six time-reversal invariant planes in the Brillouin zone [38], which have four independent Z2 integers, to obtain the four indices (ν0; ν1, ν2, ν3). Note that ν0 is called the strong insulator index and ν1, ν2, and ν3 are called weak TI indices. In addition, Z2 = 1 and Z2 = 0 correspond to topological and trivial insulators, respectively. Our calculations indicated that all indices of CsPbBrxI3−x (x = 0, 1, 2, and 3) with hydrostatic strain were (1;0,0,0), demonstrating that CsPbBrxI3−x are strong TIs.
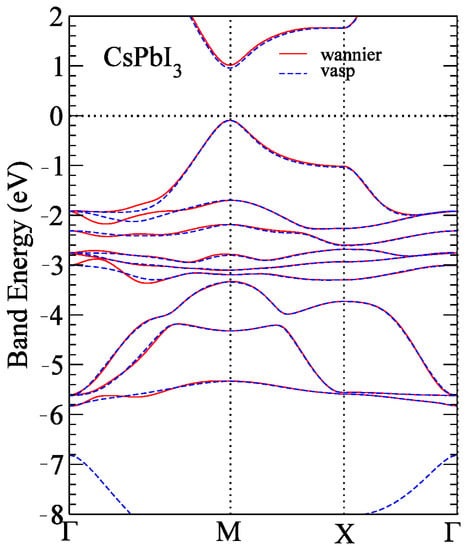
Figure 5.
CsPbI3 band structures derived from VASP (dashed blue line) and Wannier function (red line).
3.2. Electric Polarization
The preference for inversion symmetry with respect to CsPbBrxI3−x causes band structure changes. To clarify this, we applied a small displacement of the central Pb atom in the z-direction in CsPbBrxI3−x to break the inversion symmetry. Figure 6 shows the calculated band gap and electric polarization as a function of the Pb displacement in the z-direction of the CsPbBrxI3−x cell, indicating that the all band gaps of CsPbBrxI3−x at the equilibrium lattice constants increased markedly with the Pb displacement in the z-direction as shown in Figure 6a–d. Notably, the band gaps of CsPbBrxI3−x at the equilibrium lattice constants decreased with the iodine concentration, showing that the movement of Pb around the iodine atoms significantly narrows the energy band gap, as shown in Figure 6a–d. The largest electric polarizations Pele of CsPbBr3, CsPbBr2I, CsPbBrI2, and CsPbI3 at the equilibrium lattice constants were 0.003, 0.02, 0.015, and 0.013 C/m2, respectively. The electric polarizations Pele increased monotonically with the Pb displacement in CsPbBr2I, CsPbBrI2, and CsPbI3 at the equilibrium lattice constants, but the CsPbBr3 was close to the highest Pele on the Pb displacement of 0.0275 Å in the z-direction. The aforementioned data indicate that the behavior of TIs is intimately related to their respective electronic band structures and can be enhanced by increasing the iodine concentration or the Pb displacement of CsPbBrxI3−x. By contrast, all band gaps of CsPbBrxI3−x compressed by 10% relative to their equilibrium bulk lattice parameters; the lattice constants used for CsPbBr3, CsPbBr2I, CsPbBrI2, and CsPbI3 were 5.39, 5.53, 5.64, and 5.75 Å, respectively, which decreased substantially as shown in Figure 6e–h, suggesting that more external pressure may be required to approach TI behavior. With the displacement of Pb in the z-direction, the band gaps of the hydrostatically compressed CsPbBrxI3−x decreased significantly as the polarizations increase. The largest electric polarization of 0.025 C/m2 was found in CsPbBr2I under compression strain of 10% when Pb was moved along the I–Pb–I direction, as shown in Figure 6f. A theoretical study [23] reported that the electric polarization of CsPbI3 induced by a Pb displacement of 0.04 Å was approximately 0.030 C/m2, which was about two times greater than but still consistent with our result.
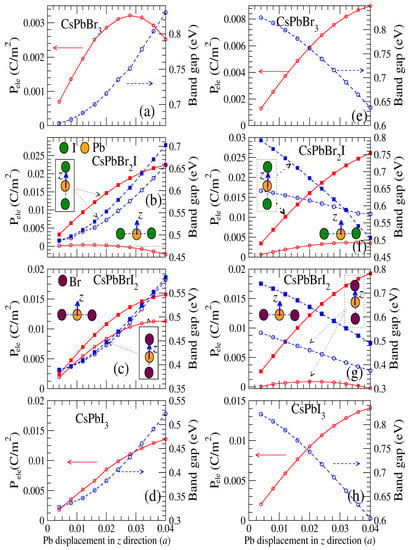
Figure 6.
Plots on the left (a–d) and right (e–h) depict the calculated electric polarization Pele (C/m2) (red solid lines) and band gaps (eV) (blue dashed lines), respectively, for the CsPbBr3 (a,e), CsPbBr2I (b,f), CsPbBrI2 (c,g), and CsPbI3 (d,h) perovskites as a function of Pb displacement in equilibrium (compressive strain of 10%).
3.3. Surface Band Structure
The topological surface band structures of CsPbBr3 and CsPbI3 from the supercell structure illustrated in Figure 1b, namely one PbBr2- (PbI2-) and CsBr- (CsI-) terminated slabs, are depicted in Figure 7a,e, respectively, and they exhibited a Dirac cone near the Fermi level at the M points of the surface Brillouin zones with the respect to the natural behavior. The polar surface band structures of CsPbBr3 and CsPbI3 come from the center Pb atom moved out-of-plane at approximately 0.08 Å, as shown in Figure 7b,f, respectively. This indicates that the surface conduction band is separated into positive and negative surfaces in terms of energy or achieves a ferroelectric TI. The Dirac cone split because of the break in the time-reversal symmetry, suggesting the presence of a Rashba band-splitting mechanism caused by the electric polarization from the Pb displacement. The topological surface band structures of CsPbBr3 and CsPbI3 from the supercell structure are illustrated in Figure 1c and two PbBr2- and PbI2- terminated slabs are depicted in Figure 7c,g, respectively. The PbBr2 (PbI2) surface did not appear to contribute to Dirac corn-like energy dispersion, but the CsBr (CsI) surface did. Furthermore, the polar surface band structures of CsPbBr3 and CsPbI3 with PbBr2- andPbI2- terminated surfaces on both sides are depicted in Figure 7d,h, respectively, showing that PbBr2 (PbI2) surfaces change from not contributing topological surface conduction bands to contributing such bands under the effect of polarization.
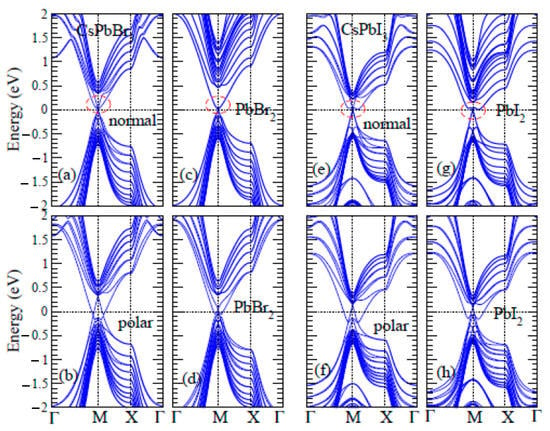
Figure 7.
Comparison of the topological surface band structures of CsPbBr3 in (a–d) and CsPbI3 in (e–h) with surface normal (a,e), polarization in the z-direction (b,f), and two PbBr2- and PbI2-surface normals (c,g), and polarization in the z-direction (d,h), respectively.
4. Conclusions
The electronic band structures of the CsPbBrxI3−x perovskites were studied theoretically using density functional theory with the GGA. The CsPbBrxI3−x perovskite was found to become a TI when the external pressure exceeded its critical stress. The band inversion of the CsPbBrxI3−x perovskite occurred at the high symmetric R point with and without SOC. All indices of CsPbBrxI3−x (x = 0, 1, 2, and 3) determined from a Z2 topological invariant with hydrostatic strain were (1;0,0,0), further confirming that CsPbBrxI3−x perovskites are strong TIs. The largest electric polarization of 0.025 C/m2 was found in the electric polarization of CsPbBr2I induced by Pb displacement along the I–Pb–I direction. The Dirac-cone-like energy dispersion of CsPbBrxI3−x at the M point was enhanced in regions where electric polarization was increased, leading to ferroelectric TIs.
Author Contributions
Conceptualization, J.-C.T. and P.-L.L.; methodology; validation, Y.-H.H.; formal analysis, J.-C.T., Y.-H.H. and P.-L.L.; investigation, Y.-H.H.; writing—original draft preparation, J.-C.T. and P.-L.L.; writing—review and editing, J.-C.T. and P.-L.L.; supervision, P.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology (MOST), Taiwan, grant numbers 109-2221-E-005 -042 and 108-2221-E-005 -001.
Acknowledgments
Computational studies were performed using the resources of the National Center for High Performance Computing, Taiwan. This manuscript was edited by Wallace Academic Editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wen, X.-G. Topological orders and edge excitations in fractional quantum Hall states. Adv. Phys. 1995, 44, 405. [Google Scholar] [CrossRef]
- Thouless, D.-J.; Kohmoto, M.; Nightingale, M.-P.; Nijs, M.-D. Quantized Hall Conductance in a Two-Dimensional Periodic Potential. Phys. Rev. Lett. 1982, 49, 405. [Google Scholar] [CrossRef]
- Moore, J.-E. The birth of topological insulators. Nature 2010, 464, 194. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wen, J.; Zhou, J.; Xiao, D.; Yao, Y. First-principles calculation of Z2 topological invariants within the FP-LAPW formalism. Comput. Phys. Commun. 2012, 183, 1849. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.-X.; Qi, X.-L.; Dai, X.; Fang, Z.; Zhang, S.-C. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat. Phys. 2009, 5, 438. [Google Scholar] [CrossRef]
- Lin, H.; Wray, L.-A.; Xia, Y.; Xu, S.; Jia, S.; Cava, R.-J.; Bansil, A.; Hasan, M.-Z. Half-Heusler ternary compounds as new multifunctional experimental platforms for topological quantum phenomena. Nat. Mater. 2010, 7, 546. [Google Scholar] [CrossRef]
- Chadov, S.; Qi, X.; Kübler, J.; Fecher, G.-H.; Felser, C.; Zhang, S.C. Tunable multifunctional topological insulators in ternary Heusler compounds. Nat. Mater. 2010, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Xiao, D.; Zhang, Y.; Yao, Y. Half-Heusler topological insulators: A first-principles study with the Tran-Blaha modified Becke-Johnson density functional. Phys. Rev. B 2010, 82, 235121. [Google Scholar] [CrossRef]
- Bernevig, B.-A.; Hughes, T.-L.; Chang, S.-C. Quantum Spin Hall Effect and Topological Phase Transition in HgTe Quantum Wells. Science 2006, 314, 1757. [Google Scholar] [CrossRef]
- Fu, L.; Kane, C.-L. Topological Insulators in Three Dimensions. Phys. Rev. B 2007, 98, 106803. [Google Scholar] [CrossRef]
- Moskvin, S.; Makhnev, A.-A.; Nomerovannaya, L.-N.; Loshkareva, N.-N.; Balbashov, A.-M. Interplay of p−d and d−d charge transfer transitions in rare-earth perovskite manganites. Phys. Rev. B 2010, 82, 035106. [Google Scholar] [CrossRef]
- Weeks, C.; Franz, M. Topological insulators on the Lieb and perovskite lattices. Phys. Rev. B 2010, 82, 085310. [Google Scholar] [CrossRef]
- Mathur, N.; Littlewood, P. Mesoscopic Texture in Manganites. Phys. Today 2003, 56, 25. [Google Scholar] [CrossRef]
- Heo, J.-H.; Im, S.-H.; Noh, J.-H.; Mandal, T.-N.; Lim, C.-S.; Chang, J.-A.; Lee, Y.-H.; Kim, H.-J.; Sarkar, A.; Nazeeruddin, M.-K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photon. 2013, 7, 486. [Google Scholar] [CrossRef]
- Green, M.-A.; Baillie, A.-H.; Snaith, H.-J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506514. [Google Scholar] [CrossRef]
- Sum, T.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162. [Google Scholar] [CrossRef]
- Park, D.-H.; Han, J.-S.; Kim, W.; Jang, H.-S. Facile synthesis of thermally stable CsPbBr3 perovskite quantum dot-inorganic SiO2 composites and their application to white light-emitting diodes with wide color gamut. Dyes Pigment. 2018, 149, 246. [Google Scholar] [CrossRef]
- Yang, W.-S.; Noh, J.-H.; Jeon, N.-J.; Kim, Y.-C.; Ryu, S.; Seo, J.; Seok, S.-I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Im, J.; Freeman, A.-J. Topological insulator phase in halide perovskite structures. Phys. Rev. B 2012, 86, 121102(R). [Google Scholar] [CrossRef]
- Jin, H.; Rhim, S.-H.; Im, J.; Freeman, A.-J. Topological Oxide Insulator in Cubic Perovskite Structure. Sci. Rep. 2013, 3, 1651. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.-T.; Wang, H.; Kim, J.; Wu, R.; Wang, Y.-K.; Lu, C.-K. Synthesis, structural characterization and reactivity of metallacarboranes of lanthanides and early transition metals. J. Phys. Chem. Lett. 2017, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kim, Y.; Tan, L.-Z.; Rappe, A.-M. Strain-Induced Ferroelectric Topological Insulator. Nano Lett. 2016, 16, 1663. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Yang, J.-H.; Liu, H.-R.; Xiang, H.-J.; Gong, X.-G. First-principles study on the electronic and optical properties of cubic ABX3 halide perovskites. Phys. Lett. A 2014, 378, 290. [Google Scholar] [CrossRef]
- Afsari, M.; Boochani, A.; Hantezadeh, M. Electronic, optical and elastic properties of cubic perovskite CsPbI3: Using first principles study. Optik 2016, 127, 11433. [Google Scholar] [CrossRef]
- Afsari, M.; Boochani, A.; Hantezadeh, M.; Elahi, S.-M. Topological nature in cubic phase of perovskite CsPbI3: By DFT. Solid State Commun. 2017, 259, 10. [Google Scholar] [CrossRef]
- Yin, Y.-H.; Luan, W.-L.; Zhang, C.-X.; Yang, F.-Q. A high quality and quantity hybrid perovskite quantum dots (CsPbX3, X= Cl, Br and I) powders synthesis via ionic displacement. Science 2017, 100, 012057. [Google Scholar] [CrossRef]
- Eperon, G.-E.; Stranks, S.-D.; Menelaou, C.; Johnston, M.-B.; Herz, L.-M.; Snaith, H.-J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüllerr, J. Ultrasoft pseudopotentials applied to magnetic Fe, Co, and Ni: From atoms to solids. Comput. Mater. Sci. 1996, 6, 15. [Google Scholar] [CrossRef]
- Perdew, J.-P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Park, C.-H. First-Principles Study of the Structural and the Electronic Properties of the Lead-Halide-Based Inorganic-Organic Perovskites (CH3NH3) PbX3 and CsPbX3 (X = Cl, Br, I). J. Korean Phys. Soc. 2004, 44, 889. [Google Scholar]
- Garcia, A.M.; Corro, E.D.; Kalbac, M.; Frank, O. Tuning the electronic properties of monolayer and bilayer transition metal dichalcogenide compounds under direct out-of-plane compression. Phys. Chem. Chem. Phys. 2017, 19, 13333. [Google Scholar] [CrossRef]
- Garcia, A.M.; Valero, R.; Illas, F. An empirical, yet practical way to predict the band gap in solids by using density functional band structure calculations. J. Phys. Chem. C 2017, 121, 18862. [Google Scholar] [CrossRef]
- Hoffman, J.-B.; Schleper, A.-L.; Kamat, P.-V. Ransformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3–x through Halide Exchange. J. Am. Chem. Soc. 2016, 138, 8603. [Google Scholar] [CrossRef]
- Marina, R.; Filip, E.-G.; Eperon, J.-H.; Snaith, H.-J.; Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar]
- Pizzi, G.; Vitale, V.; Arita, R.; Blüge, S.; Freimuth, F.; Géranton, G.; Gibertini, M.; Gresch, D.; Johnson, C.; Koretsune, T.; et al. Wannier90 as a community code: New features and applications. J. Phys. Condens. Matter 2020, 32, 165902. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-S.; Zhang, S.-N.; Song, H.-F.; Troyer, M.; Soluyanov, A.-A. Wannier Tools: An open-source software package for novel topological materials. Comput. Phys. Commun. 2018, 224, 405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).