Identification of Fungal Community Associated with Deterioration of Optical Observation Instruments of Museums in Northern Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microscopical Investigations
2.2.2. Fungal Isolation
2.2.3. Identification and Analysis of Fungal Community
2.2.4. Screening of Fungi for Significant Growth and pH Reduction
2.2.5. Glass Biodeterioration Experiments
2.2.6. Assessment of Exopolysaccharide (EPS) Production
2.2.7. Statistical Analysis
3. Results
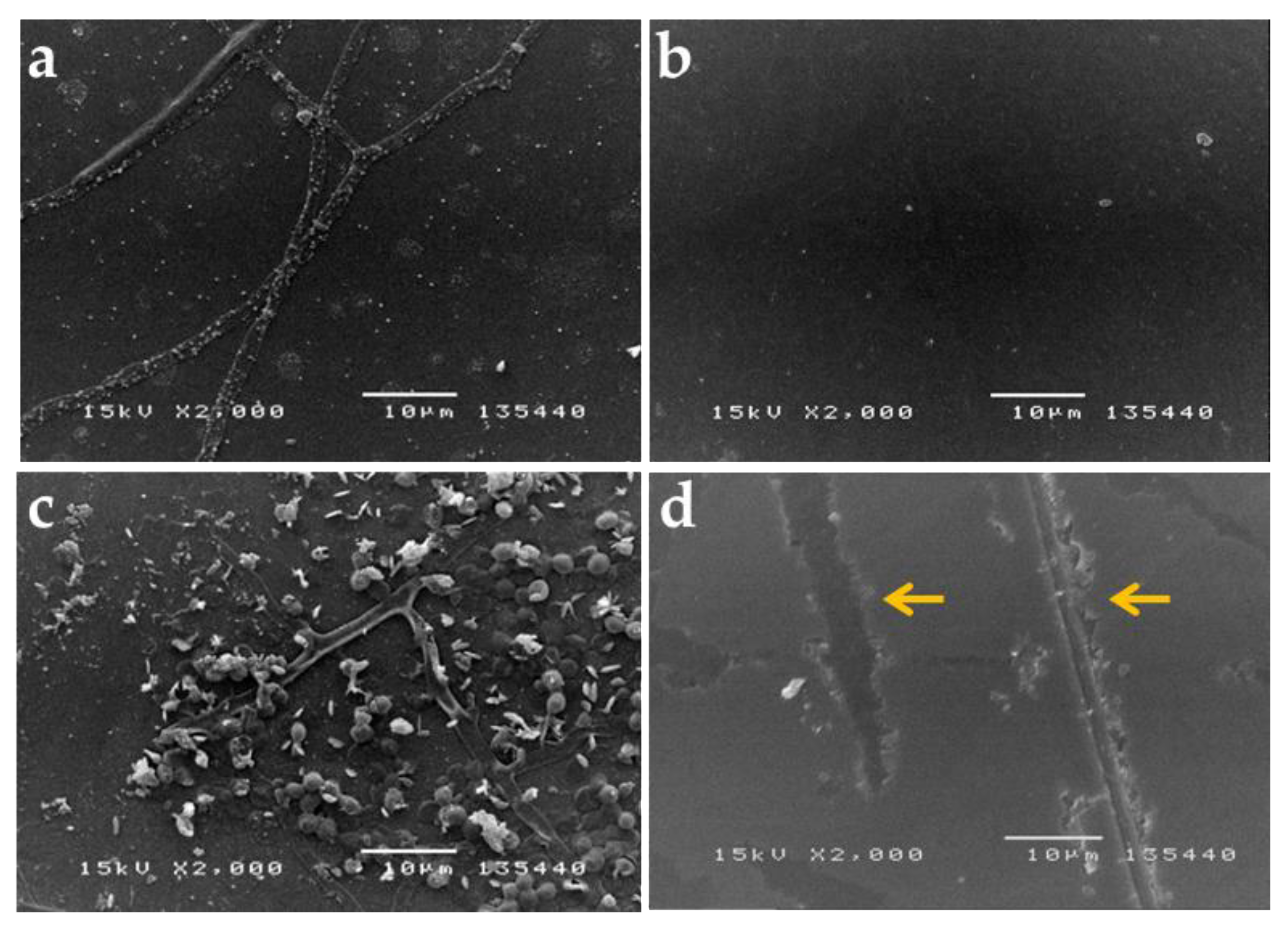
3.1. Visual Examination and Observation of Biodeterioration
3.2. Distribution and Identification of the Isolated Fungi
3.3. Evaluation of Growth, pH Changes, and Biodeterioration
3.4. EPS Production by Fungal Strains
3.5. Biodeterioration of the Glass Reproductions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schreiner, M. Glass of the past: The degradation and deterioration of medieval glass artifacts. Microchim. Acta 1991, 104, 255–264. [Google Scholar] [CrossRef]
- Drewello, R.; Weissmann, R. Microbially influenced corrosion of glass. Appl. Microbiol. Biotechnol. 1997, 47, 337–346. [Google Scholar] [CrossRef]
- Gorbushina, A.A.; Palinska, K.A. Biodeteriorative processes on glass: Experimental proof of the role of fungi and cyanobacteria. Aerobiologia 1999, 15, 183–192. [Google Scholar] [CrossRef]
- Rodrigues, A.; Gutierrez-Patricio, S.; Miller, A.Z.; Saiz-Jimenez, C.; Wiley, R.; Nunes, D.; Vilarigues, M.; Macedo, M.F. Fungal biodeterioration of stained-glass windows. Int. Biodeter. Biodegr. 2014, 90, 152–160. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art-tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Palm Bay, FL, USA, 2017. [Google Scholar]
- Müller, E.; Drewello, U.; Drewello, R.; Weißmann, R.; Wuertz, S. In situ analysis of biofilms on historic window glass using confocal laser scanning microscopy. J. Cult. Herit. 2001, 2, 31–42. [Google Scholar] [CrossRef]
- Carmona, N.; Laiz, L.; Gonzalez, J.M.; Garcia-Heras, M.; Villegas, M.A.; Saiz-Jimenez, C. Biodeterioration of historic stained glasses from the Cartuja de Miraflores (Spain). Int. Biodeter. Biodegr. 2006, 58, 155–161. [Google Scholar] [CrossRef]
- Piñar, G.; Garcia-Valles, M.; Gimeno-Torrente, D.; Fernandez-Turiel, J.L.; Ettenauer, J.; Sterflinger, K. Microscopic, chemical, and molecular-biological investigation of the decayed medieval stained window glasses of two Catalonian churches. Int. Biodeter. Biodegr. 2013, 84, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Corrêa Pinto, A.M.; Palomar, T.; Alves, L.C.; da Silva, S.H.M.; Monteiro, R.C.; Macedo, M.F.; Vilarigues, M.G. Fungal biodeterioration of stained-glass windows in monuments from Belém do Pará (Brazil). Int. Biodeter. Biodegr. 2019, 138, 106–113. [Google Scholar] [CrossRef]
- Krumbein, W.E.; Urzì, C.E.; Gehrmann, C. Biocorrosion and biodeterioration of antique and medieval glass. Geomicrobiol. J. 1991, 9, 139–160. [Google Scholar] [CrossRef]
- Marvasi, M.; Vedovato, E.; Balsamo, C.; Macherelli, A.; Dei, L.; Mastromei, G.; Perito, B. Bacterial community analysis on the Mediaeval stained glass window “Natività” in the Florence Cathedral. J. Cult. Herit. 2009, 10, 124–133. [Google Scholar] [CrossRef]
- Bindschedler, S.; Cailleau, G.; Verrecchia, E. Role of fungi in the biomineralization of calcite. Minerals 2016, 6, 41. [Google Scholar] [CrossRef]
- Weaver, J.L.; DePriest, P.T.; Plymale, A.E.; Pearce, C.I.; Arey, B.; Koestler, R.J. Microbial interactions with silicate glasses. NPJ Mater. Degrad. 2021, 5, 11. [Google Scholar] [CrossRef]
- Watkins, R.D. Mould in optical instruments. Community Eye Health 2003, 16, 28. [Google Scholar]
- Bartosik, M.; Zakowska, Z.; Cedzińska, K.; Rozniakowski, K. Biodeterioration of optical glass induced by lubricants used in optical instruments technology. Pol. J. Microbiol. 2010, 59, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1. [Google Scholar] [CrossRef]
- Garcia-Vallès, M.; Gimeno-Torrente, D.; Martínez-Manent, S.; Fernández-Turiel, J.L. Medieval stained glass in a Mediterranean climate: Typology, weathering and glass decay, and associated biomineralization processes and products. Am. Mineral. 2003, 88, 1996–2006. [Google Scholar] [CrossRef]
- Vasil’chenko, L.G.; Khromonygina, V.V.; Karapetyan, K.N.; Vasilenko, O.V.; Rabinovich, M.L. Cellobiose dehydrogenase formation by filamentous fungus Chaetomium sp. INBI 2-26(-). J. Biotechnol. 2005, 119, 44–59. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Nowak, A.; Komaniecka, I.; Choma, A.; Jarosz-Wilkołazka, A.; Osińska-Jaroszuk, M.; Tyśkiewicz, R.; Wiater, A.; Rogalski, J. Differences in production, composition, and antioxidant activities of exopolymeric substances (EPS) obtained from cultures of endophytic Fusarium culmorum strains with different effects on cereals. Molecules 2020, 25, 616. [Google Scholar] [CrossRef]
- Davis, S.; Chemello, C. Glass: Conservation and Preservation. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014; pp. 3047–3050. [Google Scholar]
- Stábile, F.M.; Volzone, C.; Ortiga, J. Thermal evolution of Na2O-K2O-CaO-SiO2-P2O5-Al2O3 glass system, and possible applications as biomedical devices. Procedia Manuf. Sci. 2015, 8, 332–337. [Google Scholar] [CrossRef]
- Partyka, J.; Gasek, K.; Pasiut, K.; Gajek, M. Effect of addition of BaO on sintering of glass–ceramic materials from SiO2–Al2O2–Na2O–K2O–CaO/MgO system. J. Therm. Anal. Calorim. 2016, 125, 1095–1103. [Google Scholar] [CrossRef]
- Song, H.S.; Yoo, Y.J.; Lee, G.J.; Chang, K.S.; Song, Y.M. Optical design of porous ZnO/TiO2 films for highly transparent glasses with broadband ultraviolet protection. J. Nanomater. 2017, 2017, 2738015. [Google Scholar] [CrossRef]
- Johansson, W.; Peralta, A.; Jonson, B.; Anand, S.; Österlund, L.; Karlsson, S. Transparent TiO2 and ZnO thin films on glass for UV protection of PV modules. Front. Mater. Sci. 2019, 6, 259. [Google Scholar] [CrossRef]
- Frisvad, J.; Gravesen, S. Penicillium and Aspergillus from Danish homes and working places with indoor air problems: Identification and mycotoxin determination. Health Implic. Fungi Indoor Environ. 1994, 2, 281–290. [Google Scholar]
- Guiamet, P.; Borrego, S.; Lavin, P.; Perdomo, I.; de Saravia, S.G. Biofouling and biodeterioration in materials stored at the Historical Archive of the Museum of La Plata, Argentine and at the National Archive of the Republic of Cuba. Colloids Surf. B 2011, 85, 229–234. [Google Scholar] [CrossRef]
- Fog Nielsen, K. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Logan, B.E. Localized attraction correlates with bacterial adhesion to glass and metal oxide substrata. Environ. Sci. Technol. 2006, 40, 2983–2988. [Google Scholar] [CrossRef]
- Borrego, S.; Guiamet, P.; Gómez de Saravia, S.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The quality of air at archives and the biodeterioration of photographs. Int. Biodeter. Biodegrad. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Trovão, J.; Portugal, A. Current knowledge on the fungal degradation abilities profiled through biodeteriorative plate essays. Appl. Sci. 2021, 11, 4196. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic acid production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Tkacz, J.S., Lange, L., Eds.; Springer US: Boston, MA, USA, 2004; pp. 307–340. [Google Scholar]
- Goldberg, I.; Rokem, J.S. Organic and fatty acid production, microbial. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 358–382. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef]
- Little, B.; Staehle, R.; Davis, R. Fungal influenced corrosion of post-tensioned cables. Int. Biodeter. Biodegr. 2001, 47, 71–77. [Google Scholar] [CrossRef]
- Cordero, I. Fungus: How to prevent growth and remove it from optical components. Community Eye Health 2013, 26, 57. [Google Scholar]
- Sand, W.; Bock, E. Biodeterioration of mineral materials by microorganisms—biogenic sulfuric and nitric acid corrosion of concrete and natural stone. Geomicrobiol. J. 1991, 9, 129–138. [Google Scholar] [CrossRef]
- Gomoiu, I.; Catley, B.J. Properties of a kaolin-flocculating polymer elaborated by Byssochlamys nivea. Enzyme Microb. Technol. 1996, 19, 45–49. [Google Scholar] [CrossRef]
- Kogan, G.; Matulová, M.; Michalková, E. Extracellular polysaccharides of Penicillium vermiculatum. Z. Naturforsch. C. J. Biosci. 2002, 57, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ruperez, P.; Leal, J.A. Extracellular galactosaminogalactan from Aspergillus parasiticus. Trans. Brit. Mycol. Soc. 1981, 77, 621–625. [Google Scholar] [CrossRef]
- Sutherland, I.W. Biotechnology of Microbial Exopolysaccharides; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Lindberg, B. Structural studies of varianose. Carbohydr. Res. 1980, 82, 97–102. [Google Scholar] [CrossRef]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
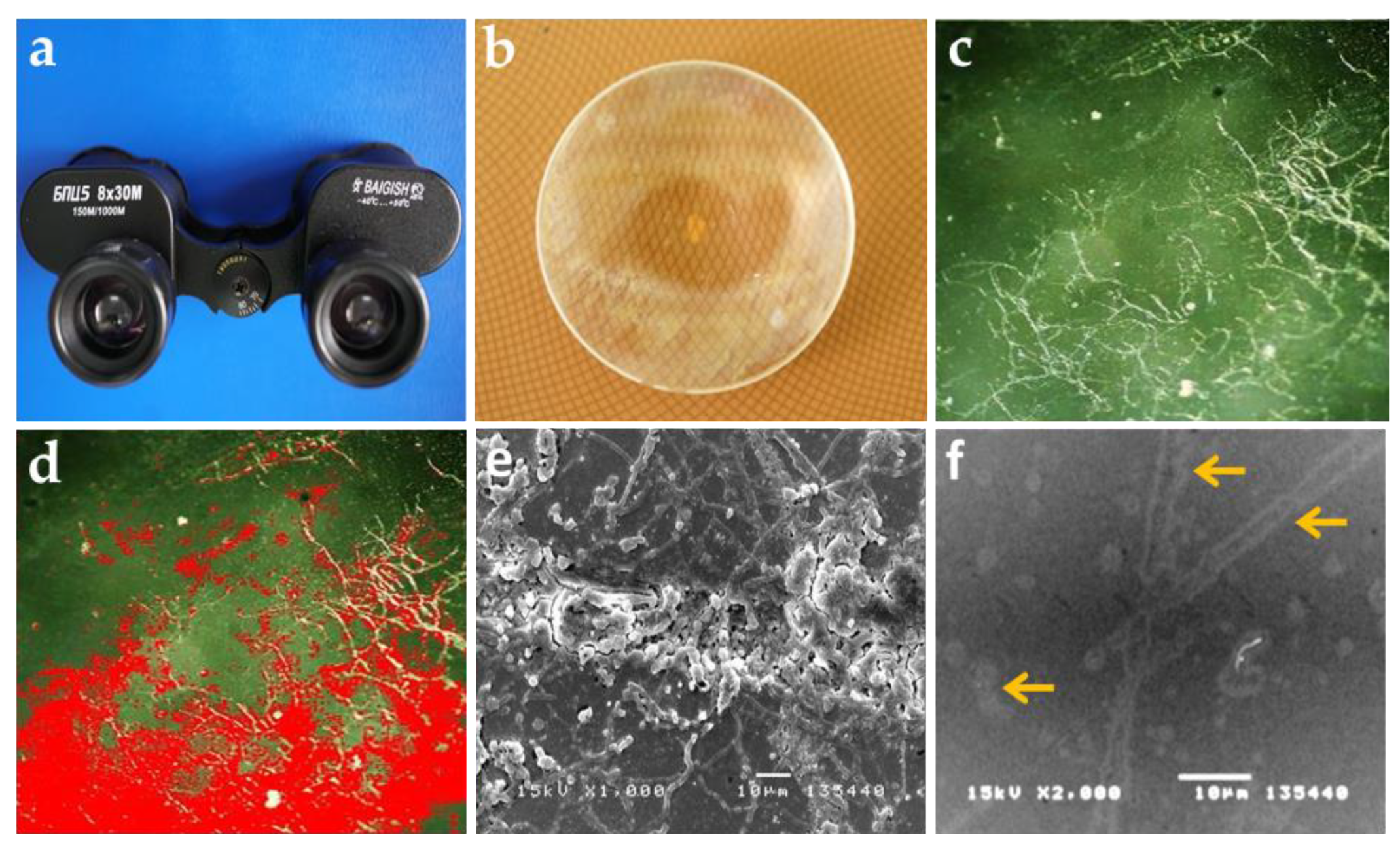
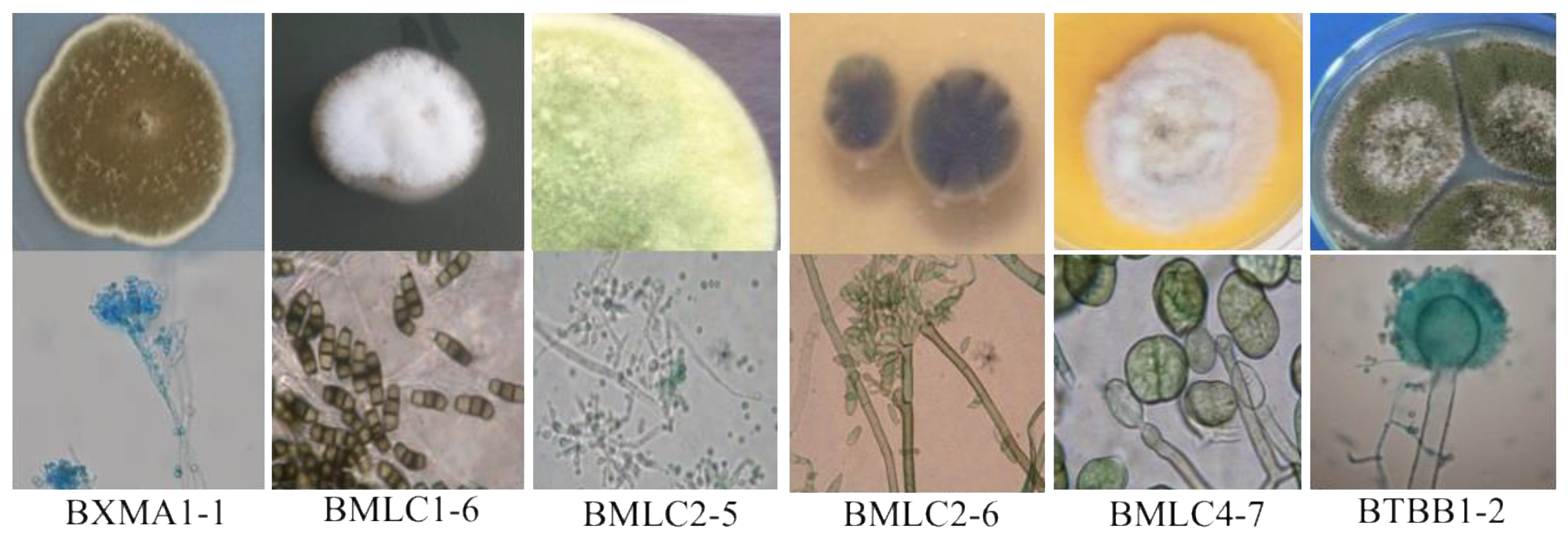
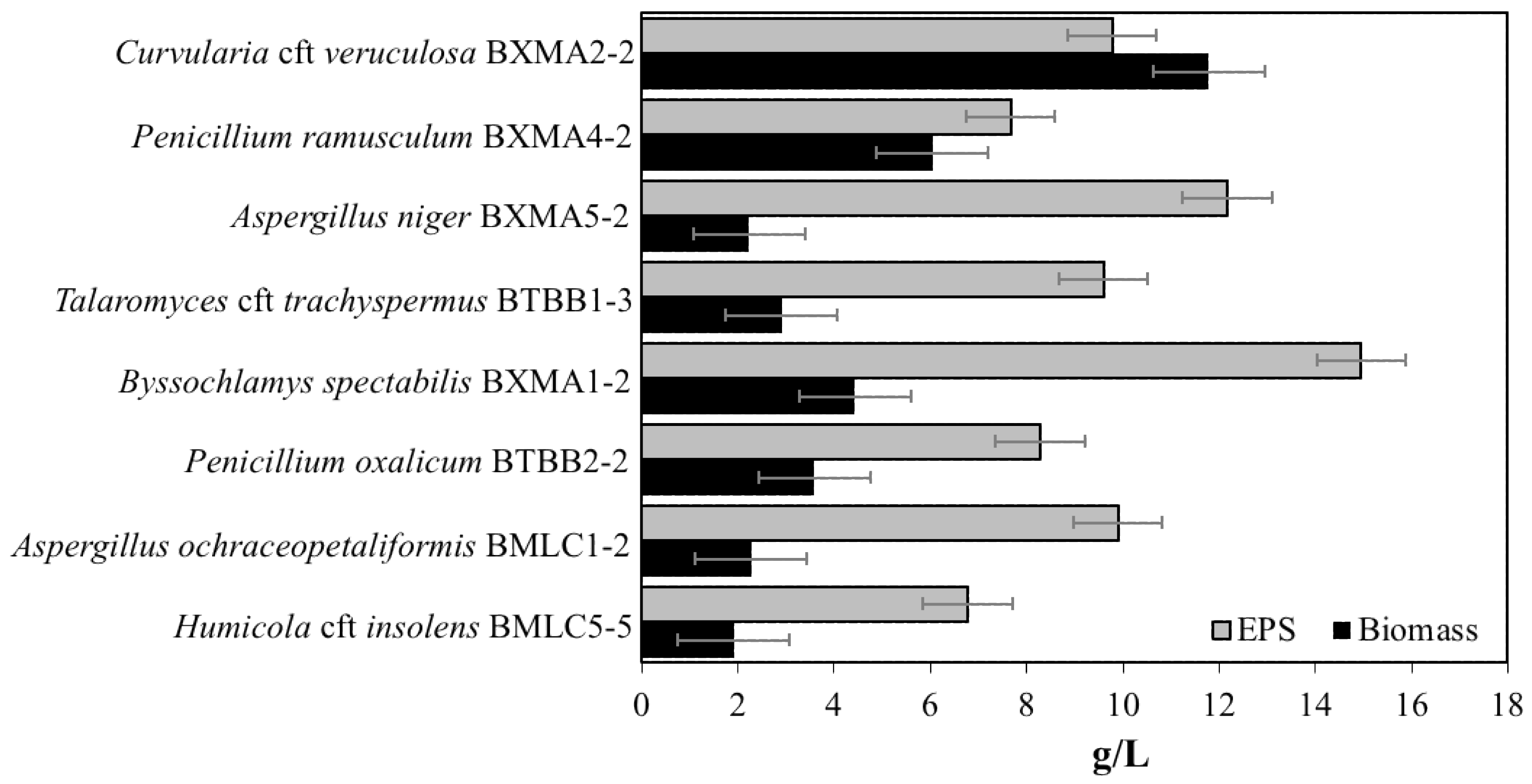
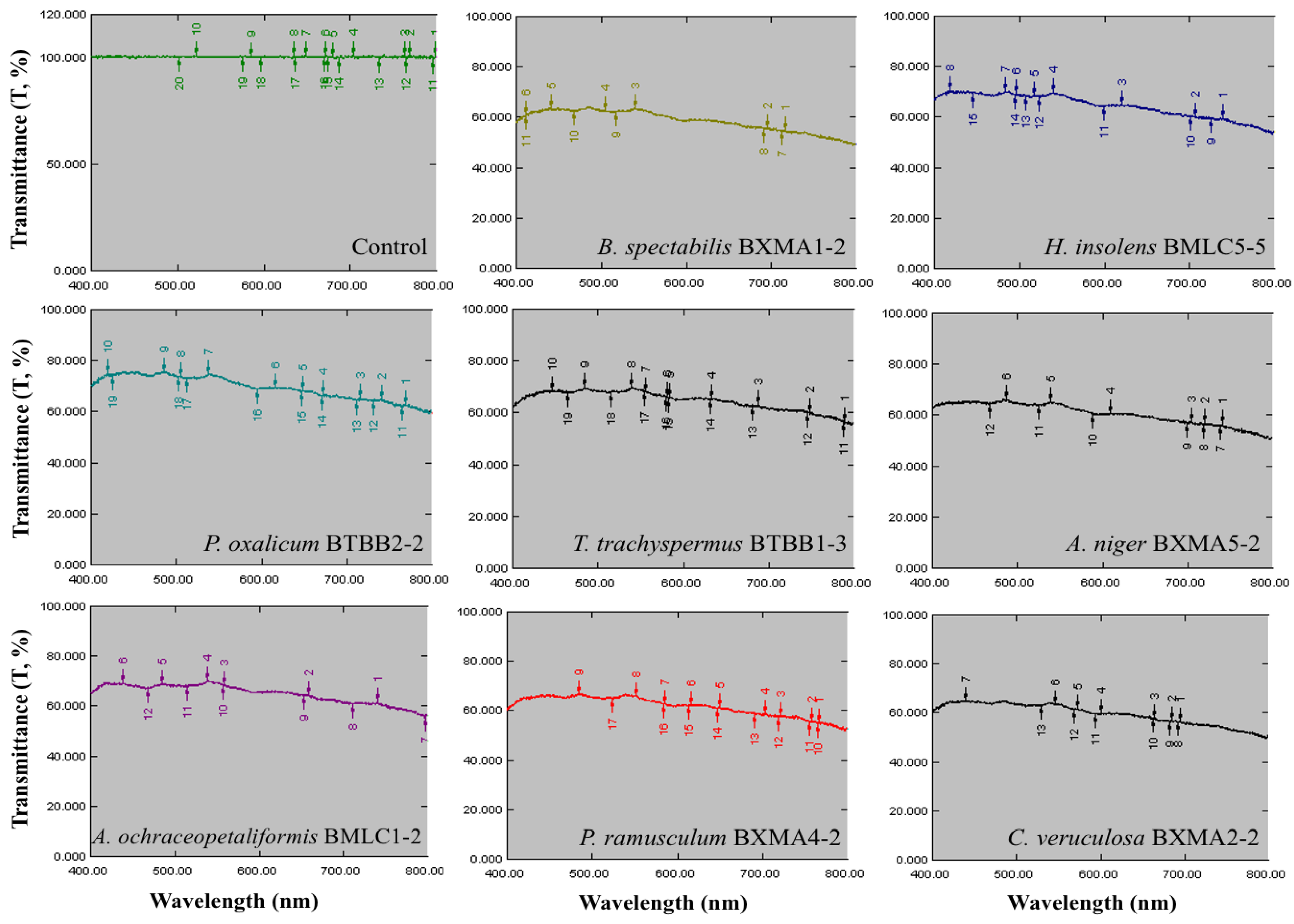
| Place | Glass Sample | Evaluation of the Extent of Fungal Growth * | |
|---|---|---|---|
| Hyphal Surface Coverage (%) | Harmful Grade | ||
| Muong Cultural Space Museum, Hanoi | BXMA1 | 29 ± 1.2 | 2 |
| BXMA2 | 35 ± 1.5 | 3 | |
| BXMA3 | 26 ± 1.3 | 2 | |
| BXMA4 | 21 ± 1.2 | 2 | |
| BXMA5 | 21 ± 1.5 | 2 | |
| Museum of Biology, Phu Tho | BTBB1 | 28 ± 1.5 | 2 |
| BTBB2 | 41 ± 1.7 | 3 | |
| BTBB3 | 27 ± 1.6 | 2 | |
| BTBB4 | 43 ± 2.1 | 3 | |
| BTBB5 | 48 ± 1.4 | 3 | |
| Thu Museum, Vinh Phuc | BMLC1 | 41 ± 1.6 | 3 |
| BMLC2 | 36 ± 1.8 | 3 | |
| BMLC3 | 34 ± 1.6 | 3 | |
| BMLC4 | 25 ± 1.3 | 2 | |
| BMLC5 | 43 ± 2.2 | 3 | |
| Fungal Strain | GenBank Accession Number | The Changes in the pH of Media after Fungal Growth | ||||
|---|---|---|---|---|---|---|
| MT1 (Initial pH 7.2) | MT2 (Initial pH 6.5) | Hyphal Surface Coverage (%) | ||||
| pH | Growth | pH | Growth | |||
| Aspergillus fumigatus BMLC1-1 | MW911781 | 2.53 ± 0.4 | +++ | 3.17 ± 0.1 | +++++ | 4.64 ± 1.4 |
| Aspergillus ochraceopetaliformis BMLC1-2 | MN394129 | 3.15 ± 0.1 | ++++ | 5.26 ± 0.6 | +++ | 33.61 ± 3.7 |
| Aspergillus asperescens BMLC1-3 | MZ292395 | 4.28 ± 0.3 | ++++ | 5.68 ± 0.1 | ++++ | 2.26 ± 1.1 |
| Aspergillus sclerotiorum BMLC1-4 | MN394130 | 4.34 ± 0.1 | ++++ | 5.66 ± 0.3 | ++++ | 3.87 ± 1.3 |
| Penicillium lanoso BMLC1-5 | MZ292396 | 2.78 ± 0.3 | ++++ | 6.26 ± 0.2 | ++++ | 15.28 ± 2.4 |
| Pithomyces chartarum BMLC1-6 | MN394131 | 3.18 ± 0.4 | ++++ | 6.46 ± 0.8 | ++++ | 32.33 ± 3.5 |
| Neopestalotiopsis sp. BMLC1-7 | MN394132 | 5.43 ± 0.5 | ++++ | 4.87 ± 0.6 | +++ | 23.14 ± 2.8 |
| Penicillium chermesinum BMLC2-1 | MN394134 | 2.91 ± 0.1 | ++++ | 8.93 ± 0.5 | +++++ | 20.28 ± 2.2 |
| Penicillium roqueforti BMLC2-3 | MZ292397 | 3.47 ± 0.2 | +++ | 7.21 ± 0.9 | ++++ | 19.21 ± 2.4 |
| Trichoderma koningiopsis BMLC2-5 | MN394133 | 4.15 ± 0.4 | ++++ | 4.27 ± 0.6 | +++ | 18.90 ± 2.3 |
| Cladosporium tenuissimum BMLC2-6 | MN394135 | 5.34 ± 0.6 | +++ | 8.24 ± 0.7 | ++ | 36.43 ± 3.4 |
| Cladosporium tenuissimum BMLC2-7 | MN394136 | 2.22 ± 0.3 | +++ | 2.68 ± 0.7 | ++++ | 27.65 ± 2.6 |
| Penicillium toxicarium BMLC2-8 | MN394137 | 4.19 ± 0.2 | +++ | 6.91 ± 0.5 | +++ | 11.18 ± 2.3 |
| Aspergillus niger BMLC3-4 | MW911782 | 2.56 ± 0.8 | +++ | 6.23 ± 0.4 | ++++ | 21.53 ± 2.2 |
| Pithomyces maydicus BMLC3-6 | MN394138 | 5.95 ± 0.4 | ++++ | 5.35 ± 0.5 | +++ | 12.15 ± 2.1 |
| Byssochlamys cft spectabilis BMLC4-6 | MN394139 | 4.28 ± 0.8 | ++++ | 5.68 ± 0.5 | ++++ | 14.99 ± 2.5 |
| Pleospora herbarum BMLC4-7 | MZ292398 | 6.21 ± 0.7 | ++++ | 6.43 ± 0.8 | ++++ | 10.91 ± 2.3 |
| Humicola cft insolens BMLC5-5 | MZ292399 | 3.35 ± 0.7 | +++ | 5.35 ± 0.7 | +++ | 35.26 ± 3.8 |
| Aspergillus sydowii BTBB1-1 | MN396671 | 5.15 ± 0.6 | ++ | 5.53 ± 0.3 | ++ | 10.40 ± 2.7 |
| Aspergillus flavus BTBB1-2 | MN396672 | 5.21 ± 0.3 | +++ | 5.85 ± 0.4 | +++ | 4.25 ± 1.5 |
| Talaromyces cft trachyspermus BTBB1-3 | MW911783 | 3.78 ± 0.6 | ++++ | 5.79 ± 0.8 | +++ | 36.95 ± 3.4 |
| Penicillium oxalicum BTBB2-2 | MN396673 | 2.24 ± 0.1 | ++++ | 7.02 ± 0.9 | +++++ | 35.95 ± 3.2 |
| Aspergillus cft salwaensis BTBB5-1 | MN396674 | 5.47 ± 0.6 | +++ | 6.85 ± 0.4 | +++ | 34.07 ± 3.8 |
| Penicillium decumbens BTBB5-2 | MN396675 | 2.17 ± 0.5 | ++++ | 3.39 ± 0.9 | +++++ | 21.58 ± 2.5 |
| Penicillium brevissimum BXMA1-1 | MH634479 | 4.9 ± 0.9 | + | 6.77 ± 0.3 | + | 24.18 ± 2.5 |
| Byssochlamys spectabilis BXMA1-2 | MH634480 | 2.68 ± 0.1 | +++ | 6.23 ± 0.7 | ++++ | 46.24 ± 3.3 |
| Aspergillus sydowii BXMA1-3 | MH634481 | 3.37 ± 0.2 | +++ | 7.2 ± 0.2 | ++++ | 3.79 ± 1.2 |
| Aspergillus tritici BXMA1-4 | MH634482 | 3.18 ± 0.5 | +++ | 7.11 ± 0.6 | +++ | 20.63 ± 2.6 |
| Aspergillus sydowii BXMA1-5 | MH634483 | 2.6 ± 0.4 | +++ | 7.47 ± 0.6 | +++++ | 13.97 ± 2.9 |
| Byssochlamys spectabilis BXMA2-1 | MH634484 | 2.7 ± 0.1 | ++++ | 6.05 ± 0.2 | ++++ | 19.41 ± 2.2 |
| Curvularia cft veruculosa BXMA2-2 | MH634485 | 3.2 ± 0.6 | ++++ | 5.32 ± 0.3 | ++++ | 41.04 ± 3.1 |
| Phomopsis cft tuberivora BXMA2-3 | MH634486 | 3.12 ± 0.4 | ++++ | 8.96 ± 0.2 | +++++ | 7.86 ± 1.5 |
| Coprinellus radians BXMA2-4 | MH634487 | 3.63 ± 0.2 | +++ | 8.32 ± 0.6 | +++++ | 7.74 ± 1.6 |
| Aspergillus flavus BXMA3-1 | MH634488 | 3.12 ± 0.7 | ++ | 7.98 ± 0.6 | ++++ | 31.82 ± 3.6 |
| Penicillium oxalicum BXMA3-2 | MH634489 | 3.77 ± 0.2 | ++ | 7.4 ± 0.2 | ++++ | 20.22 ± 2.4 |
| Curvularia lunata BXMA3-5 | MH634490 | 2.91 ± 0.2 | ++++ | 6.32 ± 0.4 | ++++ | 27.30 ± 2.3 |
| Penicillium brevissimum BXMA4-1 | MH634491 | 2.68 ± 0.1 | +++ | 7.28 ± 0.3 | ++++ | 31.533.6 |
| Penicillium ramusculum BXMA4-2 | MH634492 | 3.26 ± 0.7 | +++ | 5.15 ± 0.9 | ++++ | 36.20 ± 3.2 |
| Perenniporia cft tephropora BXMA4-4 | MH634493 | 2.87 ± 0.5 | ++++ | 4.5 ± 0.4 | ++ | 11.51 ± 2.8 |
| Aspergillus niger BXMA5-2 | MH634494 | 2.65 ± 0.9 | +++ | 4.24 ± 0.7 | +++++ | 35.66 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, C.C.; Nguyen, Q.H.; Nguyen, T.H.; Quach, N.T.; Dudhagara, P.; Vu, T.H.N.; Le, T.T.X.; Le, T.T.H.; Do, T.T.H.; Nguyen, V.D.; et al. Identification of Fungal Community Associated with Deterioration of Optical Observation Instruments of Museums in Northern Vietnam. Appl. Sci. 2021, 11, 5351. https://doi.org/10.3390/app11125351
Ngo CC, Nguyen QH, Nguyen TH, Quach NT, Dudhagara P, Vu THN, Le TTX, Le TTH, Do TTH, Nguyen VD, et al. Identification of Fungal Community Associated with Deterioration of Optical Observation Instruments of Museums in Northern Vietnam. Applied Sciences. 2021; 11(12):5351. https://doi.org/10.3390/app11125351
Chicago/Turabian StyleNgo, Cao Cuong, Quang Huy Nguyen, Thu Hoai Nguyen, Ngoc Tung Quach, Pravin Dudhagara, Thi Hanh Nguyen Vu, Thi Thanh Xuan Le, Thi Thu Hang Le, Thi Thu Hong Do, Van Duc Nguyen, and et al. 2021. "Identification of Fungal Community Associated with Deterioration of Optical Observation Instruments of Museums in Northern Vietnam" Applied Sciences 11, no. 12: 5351. https://doi.org/10.3390/app11125351
APA StyleNgo, C. C., Nguyen, Q. H., Nguyen, T. H., Quach, N. T., Dudhagara, P., Vu, T. H. N., Le, T. T. X., Le, T. T. H., Do, T. T. H., Nguyen, V. D., Nguyen, N. T., & Phi, Q.-T. (2021). Identification of Fungal Community Associated with Deterioration of Optical Observation Instruments of Museums in Northern Vietnam. Applied Sciences, 11(12), 5351. https://doi.org/10.3390/app11125351






