Featured Application
UV light exposure, which results in anti-aging ability and renewability of the bioactive surface of the implant, can improve clinical performance and offer a promising alternative to reverse the titanium aging process.
Abstract
Titanium is widely used as an implanted material in various clinical applications, especially in orthopedics and dental implantology. Following manufacturing and storage, titanium dental implants have the ability to undergo aging, which renders a reduction in osteoblast cellular activity during the healing process, so advancement of a surface treatment to recreate bioactive implant surfaces are required. Ultra-violet (UV) surface treatment has been introduced as a potential solution to reverse the aging process via removal of hydrocarbon contamination on the surface. This narrative review aimed to discuss the current understanding of the mechanism of titanium aging and provide insights into the mechanism that improves the biocompatibility of titanium implants following UV treatment. Additionally, the findings from preclinical and clinical studies is integratively presented. A reference search was performed through the PubMed, Embase, and Scopus databases based on the keywords titanium degradation, titanium aging, photofunctionalization, and UV treatment. Emerging data demonstrated the positive effect of UV light on osteoblast cells with enhanced alkaline phosphatase activity in vitro and increased bone-implant contact in animal studies. Despite limited human studies, the data reported here appear to support the benefit of UV light photofunctionalization on titanium surfaces as an alternative to reverse the titanium aging process. The direction of future research should focus on prospective randomized blinded clinical trials.
1. Introduction
In medicine, ultra-violet (UV) radiation is widely utilized in conjunction with various devices such as UV germicidal lamps and water purification as well as air and surface sterilization equipment. However, its use in dentistry is not as widely documented. In this field, UV light illumination is used mostly in the forensic identification of restorative materials, photo-polymerization of dental materials, and calculus detection in periodontal disease management. The recent plateau in the field of dental implantology has challenged many researchers to improve the implant success rate, which in turn has led to the identification of the ability of UV radiation to reverse titanium implant aging. Titanium implant aging, as a unique phenomenon, has been identified to reduce the osteoblastic activity essential for implant–bone integration [1,2]. The actual mechanism of aging in titanium is unknown. The surface of titanium is contaminated by various factors that can lead to aging. Thus, insights into how tissue integration between dental implants could be enhanced by understanding titanium aging and how this phenomenon can be reversed by photofunctionalization would be useful.
The criteria for successful implant therapy lie on a direct functional and structural connection between the living bone and the surface of a load-carrying implant without intervening soft tissue layers [3]. The most common material used for implantology is titanium. One of the current problems in titanium implant therapy is incomplete integration, in which bone–implant contact (BIC) was reported to be only up to 65% in clinical practice. This value is far below the ideal 100% shown in animal studies [4,5,6]. As the greying population has become a global phenomenon, partial or full edentulism needing implant therapy has also increased. This poses challenges in oral rehabilitation as reduced bone density may decrease the survival rate and more so, the success of implant treatment. The incomplete bone formation around a dental implant is thought to be the result of low cellular attraction to the implant surface. The reduced bone volume, or bone density as seen in aged patients [7,8] may also compromised the BIC as the cellular interaction reduced as a result of cell senescence [9,10].
Bone formation around an endo-osseous titanium dental implant depends on the chemical, physical, and topographical characteristics of its surface [11,12,13]. Many researchers have shown that physicochemical properties such as hydrophilicity enhance cell adhesion and proliferation [14,15,16] because of the improvement in cell function by high surface wettability [17,18,19]. Therefore, current trends in clinical dental implant therapy include modifications of surface treatment to improve the surface properties and surface energy, which in turn increase the wettability of the implant. Considering the ability of titanium dental implants to undergo aging following manufacturing [1,20] renders a reduction in osteoblast cellular [21,22] activity during the healing process, especially in reduced bone density of the elderly [7,8], UV treatment of titanium has been introduced as a potential solution to reverse the aging process via removal of hydrocarbon contamination on the surface and promote cell–titanium implant interaction [23,24,25]. Given the optical properties of bulk titanium [26], UV irradiation of its surface could enhance its hydrophilicity [27], thereby increasing protein adsorption [28]. Previous review papers have comprehensively addressed issues on the longevity of the titanium surface to remain biologically active following manufacturing [1,2], overview of chairside surface modifications to improve the biofunctionality of dental implants [29], and effect of UV exposure in in vitro and in vivo studies [20,30] up to the time point the reviews were published. To the authors’ knowledge, there has been no prior attempt to establish a focused question of whether UV light treatment of the implant surface improved the clinical performance of the implants. Thus, the purpose of this review was to gain an understanding of the mechanism of how bioactive surfaces renewed via UV light exposure on a titanium dental implant surface and to collectively integrate all preclinical and clinical evidence of the photofunctionalization of dental implants.
1.1. Titanium Passivation
Titanium is an extremely reactive metal that undergoes oxidation when exposed to water or air. It forms a tenacious oxide layer (titanium dioxide) that contributes to titanium electrochemical passivity. Titanium dioxide (TiO2), the formation of which is referred to as passivation, either in rutile or anatase form, is a dense, highly resistant passive oxide film that protects underlying metal from further oxidation and corrosion. Therefore, titanium exhibits high corrosion resistance because of the presence of this oxide film [31].
Theoretically, the proposed oxide layer formation starts with adsorbing oxygen on the surface of pure titanium to produce an oxide monolayer. Subsequently, an electron from the titanium will channel through the oxide layer to further adsorb oxygen, thereby producing oxygen ions [32]. An oxygen with a valence of only two electrons is relatively electronegative and will readily bind with lightly held valence electrons of titanium to further thicken the oxide layers until the activation energy for ion transport increases and eventually limits further oxide formation [33]. The TiO2 layers on the titanium surfaces remained susceptible to corrosive attack despite its high corrosion resistance [31], and is chemically stable only in the dark [34]. At any stage, if either anodization or cathodic potential is applied to titanium, the valance state of the titanium ions within the oxide film is disrupted, thereby leading to thickening and solubility/thinning of the oxide layer through reductive dissolution. The passivation of the implant alters surface composition. These changes can be associated with the changes in surface energy [35,36]. Likewise, the thickness and stability of the oxide film are relevant to implant performance because corrosion and ion release into the adjacent tissue are undesirable [37].
In dental implantology, a satisfactory biological response across the entire spectrum of interactions (water–proteins–cells), depending on the chemical and topographic properties of the surface, determines the amount of bone that will come into contact with the biomaterials [13,38]. Moreover, the hydrophilic status of the material surfaces is a representative marker for surface energy and seems to affect the capacity to adsorb proteins and attract cells for interaction [39]. Osteoblast migration and proliferation occur during the initial stage of healing and critically affect the outcomes of bone–titanium integration. Up to the present time, various modifications for improving the physicochemical and topographic characteristics of dental implant surfaces have been investigated. However, a detailed discussion of each surface modification method is beyond the scope of this review.
1.2. Titanium Degradation
Aged titanium impaired the migration, attachment, spread, and proliferation of osteoblasts, and such effect is unrelated to the surface topography or texture of implant-based biomaterials [22,40,41]. Biological aging or time-dependent degradation of titanium occurs due to surface contamination over time under ambient conditions upon storage and transfer before reaching the end-users. The mechanism of the degradation process is unknown because of the stability of the oxide layer of titanium, however, progressive accumulation of hydrogen and carbon compounds occurs over time on the surface exposed to ambient temperature [2,28]. When exposed to atmosphere, the TiO2 surface can bind to hydrocarbons in the air through interactions with carboxyl and amine groups, regardless of the type of surface treatment [42]. This progressive accumulation of organic molecules on the titanium surfaces cannot be avoided [25]. The deposition of hydrocarbons onto the titanium surface is inversely proportional with osteoblast activity [25,28]. In comparisons of UV-treated and untreated titanium surfaces, osteoblast attachment in the former was found to be more profound and was higher in number [43], demonstrating lamellipodia-like actin projections in multiple directions [21] and possessing mature cytoskeletal development on UV-treated surfaces. Osteoblasts on the untreated surface were found to be rounded, lacking cytoskeleton formation, and presented delayed cellular proliferation [41,44]. Time-dependent degradation also caused the implant surface to become more electronegative [45] and hydrophobic [25,40]. The two proposed mechanisms of time-dependent titanium degradation are discussed in this section. The first mechanism involves hydrocarbon compound contamination on the external layers of TiO2. The second mechanism involves changes in surface energy that resulted from alterations in the electrostatic status of the titanium surface.
1.2.1. Titanium Surface Contamination
The presence of impurities on the surface of an implant affects wettability as these impurities prevent the adhesion and adherence of water molecules. The hydrocarbon contamination of titanium dental implants could occur during machining or surface modifications, sterilization, packaging, and storage prior to clinical use. Even at small quantities, trace compounds such as polycarbonyls or hydrocarbons may alter the implant surface properties. The presence of trace organic impurities or adventitious contaminants on the surface of an implant is unavoidable and is thought to affect the response to protein absorption and cells adjacent to the implant. Hayashi et al. [25] evaluated the effect of carbon contamination on cell behaviors by regulating the amount of carbon deposited onto the titanium surface using machined oils prior to cell culture. As expected, the specimens treated with acetone and machine oil exhibited a high carbon content and reduced peaks for TiO2 on the surface [25].
Generally, machined surfaces contained significantly more carbon than the roughened surfaces [17] because the process of machining involves cutting and polishing, in which the implants directly make contact with the machining tools such as organic lubricating fluids [18]. In comparison, the plasma-spraying technique resulted in cleaner surfaces because of the nature of the finishing technique, where the implant surface does not come into contact with machining tools or lubricating fluids and organic contaminants are literally removed during the process due to the high temperature of the plasma spray. Conversely, acid etching either by hydrofluoric or hydrochloric/sulfuric acid dissolves the outermost TiO2 layers of the implant surface; hence, the hydrocarbon compounds are virtually eliminated alongside the outer layers [35,46]. Notably, traces of foreign materials such as metals, lubricants, detergents, or other specific chemical compounds attach to the implant surface during processing and act as contaminants [39]. Hydrocarbon contamination on the surface was found to alter the surface zeta potential of the titanium surface to become electronegative. This reaction led to the entrapment of air bubbles and the blocking of the protein receptor, thereby interfering with the interaction between the proteins and cells [1,47,48].
Following manufacture, sterilization is one of the final surface preparations performed before packaging to ensure that the implants prepared are free from bacterial contamination. Interestingly, one further issue highlighted by studying cell–surface interactions is the fact that cleaning and sterilization methods may affect the surface energy of implants [49,50,51]. Notwithstanding, our effort to reduce bacterial contamination inevitably contributes to non-biological surface contamination of the titanium implants. Thus, autoclaving or ethanol or butanol sterilization creates organic contamination [49,52]. Hence, sterilization via the hydrothermal method [53,54], gamma ray [55], or intense UV light exposure [50,51,56] is recommended to achieve titanium with high surface energy that can induce cell adhesion [41,44] as well as improve cellular activity [22,47] and osseointegration [57,58].
In addition to processing and cleaning, the surface properties of titanium implants are also affected by the storage medium used. To our knowledge, most commercially used titanium implants are provided in sterile, gas-permeable packaging so that they can be stored up to expiry dates of approximately four years following fabrication. Given the nature of the packaging, plastic casing, and absence of light, the chemisorbed hydroxyl groups on the titanium surface are replaced with oxygen and carbon from air. However, the level of hydrocarbon, not hydrophilicity level, was found to be inversely correlated with protein adsorption and cell attachment [25]. The hydrocarbon formation on the titanium surface can start as early as four weeks after the production. Therefore, the amount of hydrocarbon adsorbed on TiO2 from the time of manufacture to the time of implantation is crucial in determining the initial affinity level for osteoblasts. Choi et al. [59] observed larger spindle-shaped MC3T3-E1 with extended actin filaments on the UV-treated surface of titanium stored in distilled water prior to their experiment. To date, the dental implant system with a SLActive® surface (Institute Straumann AG, Basel, Switzerland) is the only implant system that is rinsed with nitrogen to prevent exposure to air and stored in a glass ampoule containing saline solution (0.9% sodium chloride) following manufacture. The implant is shown Figure 1a. This mode of storage not only improves the initial wetting conditions by lowering the hydrocarbon contaminations, but also maintains a chemically active surface by increasing its surface free energy [60]. This solution is known to protect the TiO2 surface layer and has an increasing effect after prolonged exposure while maintaining surface wettability. The wettability of the surface increased as the surface is soaked by blood in the clinical view of surgical implant placement as shown Figure 1b. The presence of both Na+ and Cl− electrolytes in aqueous solution rapidly repassivates the damaged TiO2 layers [60,61]. In a study comparing preferable storage media, Wennerberg et al. [62] suggested that, unlike that of pure water, wet storage in aqueous solution reorganized the TiO2 nanostructures. Kamo et al. [63] suggested that the use of gas-barrier (vacuum) packaging during shelf storage resulted in better wettability, and hence, greater protein adsorption compared with the use of a gas-permeable container.
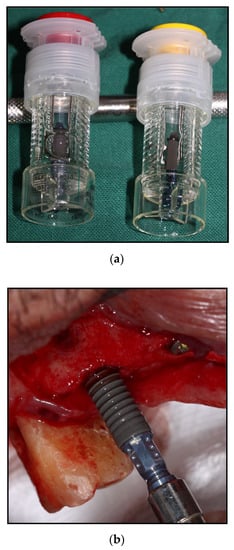
Figure 1.
Straumann SLActive® Implant system (Institute Straumann AG, Basel, Switzerland); (a) isotonic saline solution as novel storage to maintain surface bioactivity; (b) wettability of implant shown as blood drawn onto the implant surface.
1.2.2. Titanium Surface Energy Changes
As mentioned in the previous paragraph, the osseointegration of bone to implant is influenced by the implant’s surface characteristics such as surface chemistry, topography, and wettability, along with the presence of impurities. The passivation and thickening of TiO2 layers occur when an electron from titanium adsorbs oxygen from the air and produces oxygen ions. The ions on the surface are relatively electronegative and continuously maintain electronegative charges in the presence of air when stored in a gas-permeable container over time. Compared with the newly produced highly electropositive implant with high surface free energy [64], the positively charged new titanium surface directly interacts with the negatively charged biological cells. This interaction between the new titanium surface and osteoblasts occurs through electrostatic forces without cell–protein interaction. Att et al. [47] reported that the application of divalent cations such as calcium ions on a four-week-old titanium surface increased albumin adsorption, whereas application on a new TiO2 surface exhibited otherwise. Corresponding with the above-mentioned reaction, researchers [47] suggested that the divalent calcium cations deposited onto the old titanium surface act as bridges between the negative TiO2 surface and the protein molecules. This finding indicates that the electrostatic property of the titanium surface plays a role in protein adsorption and biological interaction and is a critical factor in determining titanium bioactivity.
1.3. Ultra-violet Photofunctionalization on Titanium Surface
UV photofunctionalization is defined as the phenomenon of titanium surface modification with intense UV treatment of specific wavelength and strength including the change in the physicochemical properties [43,45] and the improvement in biological features [21,22]. Two types of UV light were used for this phenomenon. The UVA light acts via the photocatalytic effects of crystalline TiO2, whereas UVC works via direct photolysis without acting as a photocatalyst. This part of the review aims to discuss how photo-induction of TiO2 superhydrophilicity occurs and how this can lead to an enhancement of the biological activity of the bone cells. The proposed mechanism of reactions thoroughly discussed below are classified based on how TiO2 reacted upon exposure to UV light.
1.3.1. Photocatalytic Degradation and Water Decomposition
TiO2 in any form, either in anatase, rutile, or brookite, exhibit excellent optical properties [26]. They possess powerful photocatalysts for various significant reactions due to their chemical stability and high reactivity. The original water decomposition reaction photocatalyzed by light was proposed by Fujishima and Honda [65]. They suggested that the water molecules decomposed into oxygen and hydrogen with TiO2 as a cathodic catalyst and in the presence of UV light, following the overall equations below:
(oxidation reaction) TiO2 + hν → e− + h+
(reduction reaction) 2H2O + 4h+ → O2 + 4H+
2H+ + 2e− → H2
The overall reaction is 2H2O + 4hν → O2 + 2H2
TiO2 surface has been reported to gradually increase in the water-contact angle induced by longer storage period, however, UV irradiation repeatedly regenerated surface amphiphilicity [22,27,28] from the water decomposition reaction. This is a photochemical reaction catalyzed by TiO2 upon exposure to UV light. Some molecules such as oxygen and water molecules were adsorbed on or desorbed from the titanium surfaces under UV light consisting of wavelengths shorter than its band gap, approximately 415 nm [66] or with an energy above the band gap energy [67]. The photo-induced superhydrophilicity of TiO2 surfaces was initially explained by an increase in the amount of hydroxyl groups formed through UV light irradiation [27]. During UV light exposure, photoexcited electrons are captured by oxygen molecules, creating holes and forming electron-deficient transition species, which later deprotonate water molecules to form two hydroxyl groups, each coordinated to different titanium cations [33]. This phenomenon creates surface oxygen vacancies at the bridging sites, thereby resulting in the conversion of relevant Ti4+ sites to Ti3+ sites [42]. The latter are favorable for dissociative water adsorption [57]. In the dark, the hydroxyl groups gradually desorb from the surface in the form of H2O2 or H2O + O2.
Photocatalytic decomposition of organic contaminants is a process different to that of photoinduced hydrophilic conversion. When TiO2 is irradiated with UV light (λ < 380 nm: energy greater than the band gap of TiO2 = 3.2 eV), an electron-hole pair is generated. In turn, adsorbed molecules such as oxygen and water molecules are reduced rapidly and oxidized to produce reactive oxygen species such as superoxide ions (•O2−) and hydroxyl radicals (•OH) [32]. These oxygen radicals react with the inorganic or organic surface impurities, thereby leading to their decomposition and removing the hydrocarbon compounds from the titanium surface [67]. The overall reaction is schematically presented in Figure 2. Greater carbon contamination was observed on non-UV-treated surfaces compared with that on UV-treated surfaces, with carbon content reducing upon UV treatment [25,42,68]. The removal of carbon increased the wettability and altered the surface charge from electronegative to electropositive.
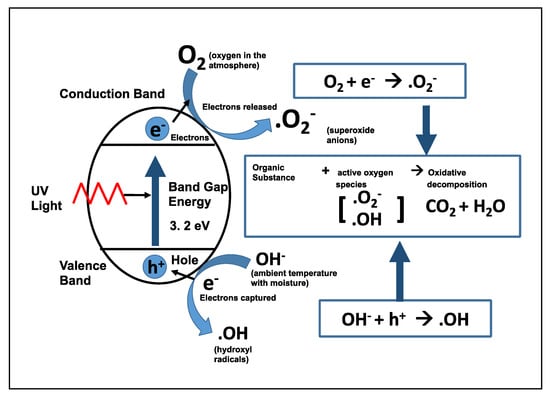
Figure 2.
Schematic photoexcitation of an electron on the TiO2 surface and the creation of holes, which attract water molecules to generate hydroxyl radicals and superoxide ions. (Adapted from [67]).
1.3.2. Direct Electrostatic Interactions
Schneider et al. [66] mentioned that changes of interfacial energies between solid surfaces and liquid play an important role in the photoinduction phenomenon. The irradiation of TiO2 to UV light results in the excitation of an electron from the valence band to the conduction band of TiO2, thereby producing negative-electron (e−) and positive-hole (h+) pairs. The positive hole on the superficial layer of TiO2 increases the surface free energy to become more electropositive. The divalent cations following UV-treated titanium surfaces act as direct attractants for cells, and the positively charged TiO2 surface can attach directly to negatively charged proteins and cells without requiring ionic bridges such as calcium ions (Ca2+) to attract proteins and cells. The electropositive titanium surfaces exhibit a regulatory role by determining their bioactivity and attracting negatively charged proteins, blood, and cells on the titanium surfaces.
Notably, naturally occurring sunlight consists of both UVA and UVC. Although UVC does not penetrate the ozone layer, it can be produced by a germicidal UV lamp. A UV light energy greater than 3.2 eV is required to induce TiO2 photocatalytic activity [67]. Aita et al. [21] demonstrated that UVA and UVC treatments both produced titanium surfaces with high wettability (contact angle <5°) under different underlying mechanisms. UVA serves as an energy source for the photocatalytic reaction of TiO2, where the electron-hole pair is generated. Thus, hydrocarbons on the TiO2 surface are removed via reaction with reactive oxygen species (as presented in Figure 2). UVC induces the photolysis of hydrocarbon compounds from the TiO2 surface [43,69], thereby causing the latter to decompose directly, which can lead to superhydrophilicity. In the study by Att et al. [41], initial cell osteoblast attachment and proliferation were enhanced on the UVC-treated surface, but not UVA-treated surface, despite comparable increased wettability. Similar cell reactions were also reported by Aita et al. [21] and Gao et al. [70]. In a dog animal model, Hirakawa et al. [71] showed a significant increase in BIC after two weeks of healing with the implant treated with UVA.
2. Search Strategy
The scope of the manuscript covers the mechanism of reaction of both titanium degradation and reversal processes by photofunctionalization, and the clinical efficacy of UV pre-treatment of titanium implants. The inclusion criteria involve original research or review articles published in any language and no restriction with regard to the publication year was applied including ‘Online-early’, ‘Ahead of print’, and ‘In-press’ articles. Given that the knowledge gap and the literature may be diffuse due to the robustness of available evidence, we considered adopting a scoping review approach. The following questions: “how does titanium aged?” and “what are the mechanisms of reversing the aging of titanium?” and “how effective the UV light treatment of implant surface improved the clinical performance of the implants?” were considered in the search strategy. The search was made on the MEDLINE (via PubMed), Embase (via Ovid), and Scopus databases for relevant articles. The strategy used a combination of MeSh terms and free text words of the following keywords: ‘photofunctionalization’, ‘titanium degradation’, ‘titanium surface’, ‘ultraviolet’ and ‘UVA’, ‘UVC’ and ‘ultraviolet light’ (different key words were connected with OR and AND). Example of the search was: ‘photofunctionalization’ (All Fields) AND ’dental implants (MeSH terms), (“ultraviolet rays”[MeSH Terms] OR (“ultraviolet”[All Fields] AND (“dental implants”[MeSH Terms] OR (“titanium”[All Fields] AND “implants”[All Fields]) OR “dental implants”[All Fields]) OR (“titanium”[All Fields] AND “photofunctionalization”[All Fields]) AND “animals”[All Fields]). Other relevant references were obtained from the citation in the selected articles. Since this is a narrative review, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was not strictly followed.
3. Results
A single examiner (MR) performed the data extraction. The aforementioned data were collected and organized into tables. As this review focused on the clinical performance of the UV pretreatment, the in vitro studies utilizing monolayer cell culture approaches were not all included. The selected papers were those that focused on the mono-layer cell culture studies involving osteoblast cells of varying origin (tabulated in Table 1). In most monolayer studies, the specimen used were disc-shaped titanium or titanium alloys [44,72]. Common cells used were bone marrow-derived osteoblasts of Sprague-Dawley rats [44,72]. The osteoblasts showed profound actin projection and lamellipodia-like processes on UV treated titanium or titanium alloy surfaces.

Table 1.
Studies comparing the effect of UV photofunctionalization of titanium surfaces on osteoblast cells of varying origins
Using the term ‘animal studies’ or ‘animal models’ in combination with the above keywords, the search yielded twenty-five (25) articles relating to photofunctionalization of the titanium surface. There was no expert discussion and consensus in selecting articles reporting the effect of photofunctionalization or UV light treatment in animal models. The articles selected were categorized based on the size of the animals used (Table 2 for rats, Table 3 for rabbits, and Table 4), in which either BIC, bone volume, or bone mineral density data are available. In rats, implants were specifically placed in the femur (Table 2), whereas in rabbits, the implants were either placed in the femur or in the tibia (Table 3). In canines and minipigs, the implants were placed either in the maxilla or mandible (Table 3). All implants placed in tibias and femurs were unloaded. In detail, most authors reported significantly increased BIC [57,71,73,74,75,76,77,78,79], push-in values [40,44,80,81,82,83], bone volume [72,84], or bone mineral [85,86] were noted in all animal models studied for photofunctionalized groups disregarding implant surface modifications/topography. The differences were insignificant for both UV-treated and non-UV-treated reported in four animal studies [87,88,89,90].

Table 2.
Effect of UV photofunctionalization on rat models.

Table 3.
Effect of UV photofunctionalization on rabbit models.

Table 4.
Effect of UV photofunctionalization on big animal models.
The literature search did not yield any randomized controlled clinical trials or prospective studies relevant to photofunctionalization other than seven (7) retrospective studies including a case series and case report. There was one (1) clinical trial on the photofunctionalization of titanium in orthopedic patients [91]. No direct data analysis was attempted, as clinical case reports do not provide an adequate source of evidence. The reported data of clinical retrospective studies and case reports are seen and summarized in Table 5.

Table 5.
Effect of UV photofunctionalization in humans.
The literature search found that several methods have been applied as the source of ultraviolet light with various wavelengths. The source of UVA light was mainly from a mercury lamp (6–15 W) with exposure time ranging from 2 h to 24 h [92]. Meanwhile, the UVC light source was from a 15 W bactericidal lamp and exposure time ranging from 2 h to 48 h [72]. Some studies used photo-generated devices (Figure 3), which are available commercially to treat the implant surfaces at chairside, with an exposure time of only around 12–15 min [82,83,90].

Figure 3.
Therabeam® SuperOsseo, (Ushio Inc., Tokyo, Japan).
4. Discussion
The concept of surface finish or topography on the biological response to an implant was studied by Albrektsson et al. [102]. The chemical and physical surface properties [103] such as surface topography and roughness, surface chemistry, and surface energy affect the initial cell response at the cell–material interface, enhance cell proliferation and differentiation, and eventually affect the rate and quality of new tissue formation. Surfaces with high wettability can influence the bonding strength, promote protein adsorption, and enhance cell adhesion compared with hydrophobic surfaces. However, surface modification techniques appear to affect the wettability, hydrophobicity, and surface charge of certain implants and alter the extent of protein adsorption [18,19]. For the past years, UV light treatment has been applied to enhance the biological properties of the titanium surface by altering its surface chemistry without altering the surface topography. The use of UV light to condition the implant surface has emerged from the knowledge of the photocatalytic degradation properties of TiO2 based on the photo-induced hydrophilicity and decomposition reaction described above [32]. The effect of photofunctionalization on different surfaces was evaluated by many researchers, and the production of superhydrophilic surfaces with increased and accelerated bone-implant integration was demonstrated in in vitro [21,22,93] and in vivo [58,73,87,96,98] studies.
The following paragraphs aim to provide a brief and crucial overview of the adhesion behaviors of osteoblast cells on superhydrophilic surfaces following UV photofunctionalization. The effects of UV photofunctionalization on osteoblasts are summarized in Table 1. The wettability values are also highlighted in this table. These studies (although not an exhaustive list) suggest that the UV-light exposure of biomaterials (titanium) enhances the migration, attachment, and proliferation of osteoblasts and its lineage. From this table, it can be seen that different types of osteoblasts may show a similar positive response toward the UV-enhanced surface. In contrast, however, Altmann et al. [93] found that primary human alveolar osteoblast morphology and initial attachment were not affected by bioactivation via UV photofunctionalization, but were influenced by surface topography. Similar results were also reported by Hayashi et al. [104]. As seen in their experiments, no differences were observed in the morphology of osteoblasts or in their induction and activity when primary human osteoblast cells were subjected to implants with UV light surface activation. As they assumed that the indifferent morphology was due to the lack of stimulatory factors in the growth media used to culture the already differentiated osteoblasts, the negligible effect of UV light could be related to the dissimilarities of TiUnite surface treatment. The TiUnite by Nobel Biocare Implant System has an anodized coating of TiO2 layers with Sa and Sdr values of 1.1 µm and 37%, respectively. Hence, their study supported the findings of Mustafa et al. [38], who demonstrated that the proliferation and differentiation of cells derived from human mandibular bone were enhanced by the surface roughness of the titanium implant. Furthermore, Cochran [13] reported that implants with rough surfaces offered more significant advantages than those with smoother surfaces, especially in compromised bone. In addition, Henningsen et al. [105] showed that the argon-plasma-treated surface was superior to the UV-treated surface of the same topographical specimens. These findings could encourage future research on the utilization of a surface conditioning tool other than UV technology without altering the surface topography.
The success of early- and late-stage osseointegration in association with surface conditioning with UV light was also established via various animal experiments. The evidence from animal research provided us with information on where histological sections could be obtained and where bone volumes could be measured. Animal research has indicated that photofunctionalization of implants prior to insertion into the bone has a positive impact on BIC [57]. In addition, micro-computed tomography (micro-CT) was used to evaluate the bone formation and volume, and the biomechanical strength of the bone–implant integration assessed with the push-in test was higher compared with those of non-treated implants. Direct association between photofunctionalization and osseointegration was found. Table 2, Table 3 and Table 4 summarize some selected studies utilizing different animal models of varying sizes, in which BIC or bone volume (BV) were evaluated. Given the circumstances, systematic reviews of preclinical studies in this subject matter could be initiated. Notably, non-human primates such as monkeys have anatomies that are more similar to the human anatomy and histology than any other animal. Thus, they may offer a high degree of relevance to humans, both in specific physiologic and biochemical similarities. Most studies have utilized small animals [57,73,85], which are clinically substandard, so the influence of UV irradiation on functionally loaded implants is not possible to evaluate. Similar studies on the photofunctionalization of dental implants utilizing non-human primate models are yet to be found in the literature. Thus, this animal model could be a direction of research prior to future clinical studies on humans. To date, studies on the effect of photofunctionalization in humans, especially randomized controlled trials, are yet to be reported. Currently, only retrospective case controls [100] and case series [58,96] have been reported. Table 5 summarizes the findings from the literature that pertain to the effect of UV light treatment on osseointegration in humans. Therefore, to validate the current findings, future research should focus on prospective randomized blinded clinical trials.
5. Conclusions
This review explores the extent of the current knowledge and significant publications in the field of TiO2 photocatalysis, especially from the perspective of the reversal of time-dependent degradation of titanium dental implants via photofunctionalization. Titanium dental implants age in an inevitable manner during their inventory and distribution as well as during storage before use. Therefore, the clinical performance based on BIC is below the ideal value of 100%. Addressing this nature of titanium, the anti-aging effect and renewability of its bioactive surface upon exposure to UV light provide clinical and scientific significance for the use of titanium as a dental implant material. Although there is increasing evidence of the positive impact of UV light treatment on osteoblast activity, limited human trials and the relevant in vivo studies do not allow us to make robust conclusions.
Author Contributions
Conceptualization, M.R. and W.L.C.; Methodology, M.R.; Investigation, M.R.; Resources, M.R.; Data curation, M.R.; Writing—original draft preparation, M.R.; Writing—review and editing, M.R., W.C.N., R.A.O., and W.L.C.; Visualization, M.R., W.C.N., and R.A.O.; Supervision, W.C.N., R.A.O., and W.L.C.; Project administration, M.R. and W.L.C.; Funding acquisition, W.C.N. and W.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This review was part of the project funded by the Ministry of Higher Education High Impact Research Grant (UM.C/625/HIR/MOHE/DENT/05) of Malaysia.
Conflicts of Interest
The authors declare no conflicts of interest. Ethical Approval was not necessary for the review paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Att, W.; Ogawa, T. Biological aging of implant surface and their restoration with ultraviolet treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implants 2012, 27, 753–761. [Google Scholar]
- Lee, J.H.; Ogawa, T. The biological aging of titanium implants. Implant Dent. 2012, 21, 415–421. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, Å. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implants 2003, 18, 200–210. [Google Scholar]
- Ericsson, I.; Johansson, C.B.; Bystedt, H.; Norton, M.R. A histomorphometric evaluation of bone-to-implant contact on machine-prepared and roughened titanium dental implants. A pilot study in the dog. Clin. Oral Implants Res. 1994, 5, 202–206. [Google Scholar] [CrossRef]
- Weinlaender, M.; Kenney, E.B.; Lekovic, V.; Beumer, J., 3rd; Moy, P.K.; Lewis, S. Histomorphometry of bone apposition around three types of endosseous dental implants. Int. J. Oral Maxillofac. Implants 1992, 7, 491–496. [Google Scholar]
- Compton, S.; Clark, D.; Chan, S.; Kuc, I.; Wubie, B.; Levin, L. Dental implants in the elderly population: A long-term follow-up. Int. J. Oral Maxillofac. Implants 2017, 32, 164–170. [Google Scholar] [CrossRef]
- Dudley, J. Implants for the ageing population. Aust. Dent. J. 2015, 60 (Suppl. 1), 28–43. [Google Scholar] [CrossRef]
- Marie, P.J. Bone cell senescence: Mechanisms and perspectives. J. Bone Miner. Res. 2014, 29, 1311–1321. [Google Scholar] [CrossRef]
- Boskey, A.L.; Coleman, R. Aging and bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: surface characteristics, interface biology and clinical outcome. J. Royal Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, J.; Liu, X.; Zheng, M.; Yang, J.; Tan, J. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: the potential surface modification developed from implant to abutment. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Zizzari, V.L.; Iaculli, F.; Mortellaro, C.; Tete, S.; Piattelli, A. Relationship between the surface energy and the histologic results of different titanium surfaces. J. Craniofac. Surg. 2014, 25, 863–867. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Bruzzone, G.; Carpi, A.; Di Santi, G.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 1. Surface analysis. Int. J. Oral Maxillofac. Implants 2003, 18, 40–45. [Google Scholar]
- Cassinelli, C.; Morra, M.; Bruzzone, G.; Carpi, A.; Di Santi, G.; Giardino, R.; Fini, M. Surface chemistry effects of topographic modification of titanium dental implant surfaces: 2. In vitro experiments. Int. J. Oral Maxillofac. Implants 2003, 18, 46–52. [Google Scholar]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet photofunctionalization of titanium implants. Int. J. Oral Maxillofac. Implants 2014, 29, e95–e102. [Google Scholar] [CrossRef]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Suzuki, T.; Iwasa, F.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; et al. Ultraviolet light treatment for the restoration of age-related degradation of titanium bioactivity. Int. J. Oral Maxillofac. Implants 2010, 25, 49–62. [Google Scholar] [PubMed]
- McIntyre, N.S.; Davidson, R.D.; Walzak, T.L.; Williston, R.; Westcott, M.; Pekarsky, A. Uses of ultraviolet/ozone for hydrocarbon removal: Applications to surfaces of complex composition or geometry. J. Vac. Sci. Technol. A 1991, 9, 1355–1359. [Google Scholar] [CrossRef]
- Ohtsu, N.; Masahashi, N.; Mizukoshi, Y.; Wagatsuma, K. Hydrocarbon decomposition on a hydrophilic TiO2 surface by UV irradiation: Spectral and quantitative analysis using in-situ XPS technique. Langmuir 2009, 25, 11586–11591. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon deposition attenuates osteoblast activity on titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile? - Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Kim, B.G.; Seo, S.-J.; Lee, J.-H.; Kim, H.-W. On-site surface functionalization for titanium dental implant with nanotopography: Review and outlook. J. Nanomater. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Flanagan, D. Photofunctionalization of dental implants. J. Oral Implantol. 2016, 42, 445–450. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. C R Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Weimer, J.J.; Lemons, J.E. Effect of passivation and dry heat-sterilization on surface energy and topography of unalloyed titanium implants. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 89–101. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Raikar, G.N.; Liu, J.; Lemons, J.E.; Vohra, Y.; Gregory, J.C. Effect of surface treatment on unalloyed titanium implants: Spectroscopic analyses. J. Biomed. Mater. Res. 1998, 40, 646–659. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91. [Google Scholar] [CrossRef]
- Mustafa, K.; Wroblewski, J.; Lopez, B.S.; Wennerberg, A.; Hultenby, K.; Arvidson, K. Determining optimal surface roughness of TiO2 blasted titanium implant material for attachment, proliferation and differentiation of cells derived from human mandibular alveolar bone. Clin. Oral Implants Res. 2001, 12, 515–525. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implant. Part 1: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet treatment overcomes time-related degrading bioactivity of titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium–cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Pompella, A.; Kubacki, J.; Szade, J.; Roy, R.A.; Hedzelek, W. Photofunctionalization of titanium: An alternative explanation of its chemical-physical mechanism. PLoS ONE 2016, 11, e0157481. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Ueno, T.; Minamikawa, H.; Iwasa, F.; Yoshino, F.; Kimoto, K.; Lee, M.C.; Ogawa, T. Electrostatic control of protein adsorption on UV-photofunctionalized titanium. Acta Biomater. 2010, 6, 4175–4180. [Google Scholar] [CrossRef] [PubMed]
- Kilpadi, D.V.; Lemons, J.E. Surface energy characterization of unalloyed titanium implants. J. Biomed. Mater. Res. 1994, 28, 1419–1425. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lemons, J.E.; Liu, J.; Raikar, G.N.; Weimer, J.J.; Vohra, Y. Cleaning and heat-treatment effects on unalloyed titanium implant surfaces. Int. J. Oral Maxillofac. Implants 2000, 15, 219–230. [Google Scholar]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- Doundoulakis, J.H. Surface analysis of titanium after sterilization: Role in implant tissue interface and bioadhesion. J. Prosthet. Dent. 1987, 58, 471–478. [Google Scholar] [CrossRef]
- Vezeau, P.J.; Koorbusch, G.F.; Draughn, R.A.; Keller, J.C. Effects of multiple sterilization on surface characteristics and in vitro biologic responses to titanium. J. Oral Maxillofac. Surg. 1996, 54, 738–746. [Google Scholar] [CrossRef]
- Shi, X.; Xu, L.; Violin, K.B.; Lu, S. Improved osseointegration of long-term stored SLA implant by hydrothermal sterilization. J. Mech. Behav. Biomed. Mater. 2016, 53, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Takebe, J.; Itoh, S.; Okada, J.; Ishibashi, K. Anodic oxidation and hydrothermal treatment of titanium results in a surface that causes increased attachment and altered cytoskeletal morphology of rat bone marrow stromal cells in vitro. J. Biomed. Mater. Res. 2000, 51, 398–407. [Google Scholar] [CrossRef]
- Ueno, T.; Takeuchi, M.; Hori, N.; Iwasa, F.; Minamikawa, H.; Igarashi, Y.; Anpo, M.; Ogawa, T. Gamma ray treatment enhances bioactivity and osseointegration capability of titanium. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.J.; Bavastrello, V.; Covani, U.; Barone, A.; Nicolini, C. An in-vitro study of the sterilization of titanium dental implants using low intensity UV-radiation. Dent. Mater. 2005, 21, 756–760. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Funato, A.; Yamada, M.; Ogawa, T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int. J. Oral Maxillofac. Implants 2013, 28, 1261–1271. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, W.S.; Cha, J.Y.; Lee, J.H.; Lee, K.J.; Yu, H.S.; Choi, E.H.; Kim, K.M.; Hwang, C.J. Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment. Sci. Rep. 2017, 7, 3833. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Svanborg, L.M.; Berner, S.; Andersson, M. Spontaneously formed nanostructures on titanium surfaces. Clin. Oral Implants Res. 2013, 24, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kamo, M.; Kyomoto, M.; Miyaji, F. Time course of surface characteristics of alkali- and heat-treated titanium dental implants during vacuum storage. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1453–1460. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Yates, J.T. Photochemistry on TiO2: Mechanisms behind the surface chemistry. Surf. Sci. 2009, 603, 1605–1612. [Google Scholar] [CrossRef]
- Yasuda, K.; Okazaki, Y.; Abe, Y.; Tsuga, K. Effective UV/Ozone irradiation method for decontamination of hydroxyapatite surfaces. Heliyon 2017, 3, e00372. [Google Scholar] [CrossRef]
- Al Qahtani, M.S.A.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephan, I.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, e157–e167. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Zhou, L.; Guo, Z.; Rong, M.; Liu, X.; Lai, C.; Ding, X. The effects of different wavelength UV photofunctionalization on micro-arc oxidized titanium. PLoS ONE 2013, 8, e68086. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Jimbo, R.; Shibata, Y.; Watanabe, I.; Wennerberg, A.; Sawase, T. Accelerated bone formation on photo-induced hydrophilic titanium implants: An experimental study in the dog mandible. Clin. Oral Implants Res. 2013, 24, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef]
- Yamauchi, R.; Itabashi, T.; Wada, K.; Tanaka, T.; Kumagai, G.; Ishibashi, Y. Photofunctionalised Ti6Al4V implants enhance early phase osseointegration. Bone Joint Res. 2017, 6, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Ono, D.; Hirakawa, Y.; Odatsu, T.; Tanaka, T.; Sawase, T. Accelerated photo-induced hydrophilicity promotes osseointegration: An animal study. Clin. Implant Dent. Relat. Res. 2011, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Koak, J.Y.; Kim, S.K.; Han, C.H.; Heo, S.J. The effect of ultraviolet-C irradiation via a bactericidal ultraviolet sterilizer on an anodized titanium implant: a study in rabbits. Int. J. Oral Maxillofac. Implants 2013, 28, 57–66. [Google Scholar] [CrossRef][Green Version]
- Pyo, S.-W.; Park, Y.B.; Moon, H.S.; Lee, J.-H.; Ogawa, T. Photofunctionalization enhances bone-implant contact, dynamics of interfacial osteogenesis, marginal bone seal, and removal torque value of implants. Implant Dent. 2013, 22, 666–675. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Chen, X.; Wang, X.; He, F.; Wang, H. The in vivo bone response of ultraviolet-irradiated titanium implants modified with spontaneously formed nanostructures: An experimental study in rabbits. Int. J. Oral Maxillofac. Implants 2016, 31, 776–784. [Google Scholar] [CrossRef]
- Kim, M.Y.; Choi, H.; Lee, J.H.; Kim, J.H.; Jung, H.S.; Kim, J.H.; Park, Y.B.; Moon, H.S. UV Photofunctionalization Effect on Bone Graft in Critical One-Wall Defect around Implant: A Pilot Study in Beagle Dogs. Biomed. Res. Int. 2016, 2016, 4385279. [Google Scholar] [CrossRef]
- Lee, J.B.; Jo, Y.H.; Choi, J.Y.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Yeo, I.L. The effect of ultraviolet photofunctionalization on a titanium dental implant with machined surface: An in vitro and in vivo study. Materials 2019, 12, 2078. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Hori, N.; Suzuki, T.; Ogawa, T. Effect of ultraviolet photoactivation of titanium on osseointegration in a rat model. Int. J. Oral Maxillofac. Implants 2010, 25, 287–294. [Google Scholar]
- Ishijima, M.; Ghassemi, A.; Soltanzadeh, P.; Tanaka, M.; Nakhaei, K.; Park, W.; Hirota, M.; Tsukimura, N.; Ogawa, T. Effect of UV photofunctionalization on osseointegration in aged rats. Implant Dent. 2016, 25, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, P.; Ghassemi, A.; Ishijima, M.; Tanaka, M.; Park, W.; Iwasaki, C.; Hirota, M.; Ogawa, T. Success rate and strength of osseointegration of immediately loaded UV-photofunctionalized implants in a rat model. J. Prosthet. Dent. 2017, 118, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Sugita, Y.; Honda, Y.; Kato, I.; Kubo, K.; Maeda, H.; Ogawa, T. Role of photofunctionalization in mitigating impaired osseointegration associated with Type 2 diabetes in rats. Int. J. Oral Maxillofac. Implants 2014, 29, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Tanaka, M.; Ishijima, M.; Iwasaki, C.; Park, W.; Ogawa, T. Effect of photofunctionalization on Ti6Al4V screw stability placed in segmental bone defects in rat femurs. J. Oral Maxillofac. Surg. 2016, 74, 861.e861. [Google Scholar] [CrossRef] [PubMed]
- Sawase, T.; Jimbo, R.; Baba, K.; Shibata, Y.; Ikeda, T.; Atsuta, M. Photo-induced hydrophilicity enhances initial cell behavior and early bone apposition. Clin. Oral Implants Res. 2008, 19, 491–496. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.I.; Yang, S.S.; Kim, B.S.; Kim, B.C.; Lee, J. The effect of alendronate soaking and ultraviolet treatment on bone-implant interface. Clin. Oral Implants Res. 2017, 28, 1164–1172. [Google Scholar] [CrossRef]
- Hayashi, M.; Jimbo, R.; Xue, Y.; Mustafa, K.; Andersson, M.; Wennerberg, A. Photocatalytically induced hydrophilicity influences bone remodelling at longer healing periods: a rabbit study. Clin. Oral Implants Res. 2014, 25, 749–754. [Google Scholar] [CrossRef]
- Miki, T.; Matsuno, T.; Hashimoto, Y.; Miyake, A.; Satomi, T. In vitro and in vivo evaluation of titanium surface modification for biological aging by electrolytic reducing ionic water. Appl. Sci. 2019, 9, 713. [Google Scholar] [CrossRef]
- Sanchez-Perez, A.; Cachazo-Jiménez, C.; Sanchez-Matas, C.; Martín-de-Llano, J.J.; Davis, S.; Carda-Batalla, C. Effects of ultraviolet photoactivation on osseointegration of commercial pure titanium dental implant after 8 weeks in a rabbit model. J. Oral Implantol. 2020. Epub a head of print. [Google Scholar] [CrossRef]
- Mehl, C.; Kern, M.; Neumann, F.; Bahr, T.; Wiltfang, J.; Gassling, V. Effect of ultraviolet photofunctionalization of dental titanium implants on osseointegration. J. Zhejiang Univ. Sci. B 2018, 19, 525–534. [Google Scholar] [CrossRef]
- Tominaga, H.; Matsuyama, K.; Morimoto, Y.; Yamamoto, T.; Komiya, S.; Ishidou, Y. The effect of ultraviolet photofunctionalization of titanium instrumentation in lumbar fusion: a non-randomized controlled trial. BMC Musculoskeletal Disorders 2019, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, D.; Sun, J.; Zhang, Y.; Xu, K. UV-enhanced bioactivity and cell response of micro-arc oxidized titania coatings. Acta Biomater. 2008, 4, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Bachle-Haas, M.; Wennerberg, A.; Att, W. Distinct cell functions of osteoblasts on UV-functionalized titanium- and zirconia-based implant materials are modulated by surface topography. Tissue Eng. Part C Methods 2013, 19, 850–863. [Google Scholar] [CrossRef]
- Yamazaki, M.; Yamada, M.; Ishizaki, K.; Sakurai, K. Ultraviolet-C irradiation to titanium implants increases peri-implant bone formation without impeding mineralization in a rabbit femur model. Acta Odontol. Scand. 2015, 73, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Matsuo, M.; Hoshi, N.; Takahashi, S.-s.; Kawamata, R.; Kimoto, K. Effect of Ultraviolet Irradiation of the Implant Surface on Progression of Periimplantitis—A Pilot Study in Dogs. Implant Dent. 2016, 25, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Funato, A.; Ogawa, T. Photofunctionalized dental implants: a case series in compromised bone. Int. J. Oral Maxillofac. Implants 2013, 28, 1589–1601. [Google Scholar] [CrossRef]
- Suzuki, S.; Kobayashi, H.; Ogawa, T. Implant stability change and osseointegration speed of immediately loaded photofunctionalized implants. Implant Dent. 2013, 22, 481–490. [Google Scholar] [CrossRef]
- Funato, A.; Tonotsuka, R.; Murabe, H.; Hirota, M.; Ogawa, T. A novel strategy for bone integration and regeneration: Case studies. J. Cosmet. Dent. 2014, 26, 75–86. [Google Scholar]
- Kitajima, H.; Ogawa, T. The use of photofunctionalized implants for low or extremely low primary stability cases. Int. J. Oral Maxillofac. Implants 2016, 439–447. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Implant stability development of photofunctionalized implants placed in pegular and complex cases: A case-control study. Int. J. Oral Maxillofac. Implants 2016, 31, 676–686. [Google Scholar] [CrossRef]
- Hirota, M.; Ozawa, T.; Iwai, T.; Ogawa, T.; Tohnai, I. Effect of photofunctionalization on early implant failure. Int. J. Oral Maxillofac. Implants 2018, 33, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. Biomed. Res. Int. 2015, 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Jimbo, R.; Lindh, L.; Sotres, J.; Sawase, T.; Mustafa, K.; Andersson, M.; Wennerberg, A. In vitro characterization and osteoblast responses to nanostructured photocatalytic TiO2 coated surfaces. Acta Biomater. 2012, 8, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and non-thermal plasma activation of titanium surfaces. Clin. Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).