Featured Application
Understanding biofilm formation patterns of disinfectant-sensitive and -resistant strains under different concentrations of the disinfectant might help to evaluate the association between resistance to disinfectants and persistence in L. monocytogenes.
Abstract
Listeria monocytogenes is one of the main foodborne pathogens. The formation of biofilms by L. monocytogenes contributes to its resistance to disinfectants, which represents a serious risk for food production plants. The aim of this study was to compare the effect of sub-inhibitory concentrations of benzalkonium chloride (BAC) (1.25 or 2.5 mg/L) on biofilm production and on biofilm reduction after exposure to an inhibitory concentration of BAC (1280 mg/L) in two isogenic L. monocytogenes strains: the BAC-sensitive wild-type strain S2-1 and its BAC-resistant mutant derivative S2BAC, which presented a multidrug resistance phenotype. The biofilm-forming ability of the strains under different BAC concentrations was evaluated by the resazurin method using polystyrene microplates. The biofilm reduction after BAC exposure was evaluated by using stainless steel coupons (SSCs). When the resazurin method was used, S2BAC produced significantly more biofilm in the presence of a sub-inhibitory concentration of BAC compared to that in the culture medium without BAC (p < 0.05). When the SSC method was used, the presence of sub-inhibitory concentrations of BAC resulted in a higher resistance of the biofilm for S2BAC compared to that in the culture medium without BAC (p < 0.05). This was not observed with the sensitive S2-1 strain. These results suggest that biofilm behavior depends on the strain and sub-inhibitory concentrations of disinfectants and may explain the ability of certain isolates to persist in niches of food processing plants.
1. Introduction
Listeria monocytogenes is an opportunistic foodborne pathogen responsible for listeriosis, a disease associated with high mortality rates [1]. Listeriosis is mainly caused by the consumption of contaminated, predominantly ready-to-eat, food [2]. Disinfection is the most common method used to eliminate L. monocytogenes from the food-processing environment [3]. Quaternary ammonium compounds (QACs), such as benzalkonium chloride (BAC), are widely used as disinfectants in food-processing areas [4,5,6]. The presence of BAC in the environment is a growing public health concern, as BAC can select not only for biocide resistance but also for clinically relevant antibiotic resistance [7].
It is acknowledged that strains of L. monocytogenes with a BAC minimum inhibitory concentration (MIC) of ≥10 mg/L harbor genetic determinants, such as bcrABC [8] and qacH [9], that are known to contribute to BAC resistance. Both transferable resistance genes are prevalent in the food industry [10,11,12]. The efflux systems encoded by these genes belong to the small multidrug resistance (SMR) protein family [8,9]. These pumps are usually chromosomally encoded and affect a broad spectrum of antimicrobial compounds [13]. In some studies, the aforementioned acquired determinants are not detected in certain resistant strains [11,14]. In these cases, resistance may be due to the overexpression of endogenous efflux pumps due to mutations in regulatory elements. This can occur due to exposure to QACs or the stress induced by these compounds [13]. Efflux pumps, such as MdrL (multidrug resistance Listeria) and Lde (Listeria drug efflux), which belong to the major facilitator superfamily (MFS), and affect a different spectrum of antimicrobial compounds compared to SMR transporters, have been associated with L. monocytogenes adaptation and resistance to BAC [5,13,15].
Although the BAC resistance genotype and phenotype may be unrelated in L. monocytogenes-forming biofilms [16], QACs used without subsequent effective rinsing [6,17] may be present at residual sublethal concentrations after disinfection in industries, thereby providing a growth advantage to resistant bacteria. In fact, Møretrø et al. [18] have shown that BAC resistance provides a growth advantage at residual QAC concentrations present after disinfection. Thus, resistance might be responsible for the persistence of L. monocytogenes in the food industry [13].
Exposure of L. monocytogenes to sub-inhibitory concentrations of BAC can differently influence the biofilm formation capacity of resistant (BAC-R) and sensitive (BAC-S) strains [19]. Previous results, using the crystal violet staining method [19], have shown that resistant strains growing in polystyrene microplates produced significantly more biofilm in the presence of sub-inhibitory concentrations of BAC than in the same conditions but without BAC [19]. Crystal violet staining for biofilm quantification in microtiter plates has limitations, as it stains both live and dead cells as well as some components present in the biofilm matrix (total biofilm biomass) and is, therefore, not applicable to viability measurements. Nevertheless, viability staining followed by confocal laser microscopy [20] confirmed the results of biofilm stimulation by BAC obtained with the crystal violet method [19].
As the explanation for the important problem of the persistence of L. monocytogenes is still poorly understood [21], two other methods were used in this work to better understand how the strain susceptibility and the presence of BAC affect the resistance of biofilms: (i) The resazurin (RSZ)-based viability staining, which is often used to quantify metabolically active biofilm cells grown in microtiter plates [22], and represents a very simple and fast method to be used for high throughput screening of antimicrobials [23], and (ii) biofilm cell enumeration and biofilm reduction after BAC exposure of biofilms produced on stainless steel coupons (SSC) [16]. Since most food plants have stainless steel growth niches in production and storage areas, and the efficiency of the sanitizers should ideally be tested in field conditions [24], this last assay is performed in conditions that closely resemble those of the food industry. Accordingly, the susceptibility of biofilms, formed by two isogenic L. monocytogenes strains, the BAC-S wild-type strain S2-1 and its BAC-R mutant derivative S2BAC, to inhibitory concentrations of BAC was compared. The results obtained support the association between the reduced susceptibility to disinfectants in food production environments and the widely recognized problem of the persistence of L. monocytogenes [21].
2. Materials and Methods
2.1. Bacterial Strains
Two L. monocytogenes strains of molecular serotype (or PCR serogroup) IIa [14] were tested: strain S2-1 was isolated from a pork processing plant, and S2BAC (a laboratory mutant of S2-1, obtained after a single contact with 10 mg/L of BAC, with a frequency of 5 × 10-9) [19]. Isolates were stored at −80 °C in tryptic soy yeast extract broth (TSB-YE) (Biokar Diagnostics, Beauvais, France) supplemented with 15% v/v glycerol. Bacterial strains from the −80 °C stock collection were streaked onto tryptone soy yeast extract agar (TSA-YE) and grown overnight at 37 °C. Thereafter, isolated colonies were picked from the plates to prepare a working collection in semi-solid TSA-YE that was kept at 4 °C until use.
2.2. Antibacterial Compounds
All compounds were purchased from Sigma–Aldrich (St. Louis, MO, USA). Stock solutions were prepared and stored according to the manufacturer’s recommendations.
2.3. MIC Determination
Strains S2-1 and S2BAC were analyzed to determine their MICs to BAC, cetyltrimethylammonium bromide (CTAB), ethidium bromide (EB) and ciprofloxacin (CIP), by using a dilution test on Mueller–Hinton agar (Biolife, Milano, Italy) containing doubling dilutions of antibacterial compounds [25]. For MIC determination, all compounds were used in the range of 0.07–40 mg/L, except in the case of EB (10–320 mg/L). Plates were inoculated with 104 CFU per spot and incubated aerobically for 24 h at 37 °C. The MIC was defined as the lowest concentration of compound that inhibited growth. MICs were determined in at least two separate assays, and each strain was assayed in duplicate. To determine efflux pump activity, the inhibitor reserpine (at a final concentration of 10 mg/L) was added to each Mueller–Hinton agar plate containing BAC [14]. Two susceptible strains of L. monocytogenes (ATCC 49594 (Scott A) and ATCC BAA-679 (EGD-e)) were included for quality control.
2.4. Biofilm Assay on Polystyrene Microplates
The biofilm production assay on polystyrene microtiter plates was used to evaluate the ability of BAC to prevent or reduce biofilm formation. BAC was added to individual wells of flat-bottomed, sterile 96-well polystyrene microtiter plates (Greiner Bio-One, Frickenhausen, Germany). BAC was tested at 1.25, 2.5 and 5.0 mg/L, the concentrations of BAC that showed different effects on biofilm formation, according to our previous results [19]. The concentrations of overnight cultures of S2-1 and S2BAC were adjusted with TSB-YE to approximately 5 × 107 colony-forming units (CFU)/mL. A volume of 0.1 mL of the diluted cell suspension was transferred to the wells of a microtiter plate. For each strain, eight wells (rows) of different BAC concentrations (columns) were inoculated. Two independent experiments were conducted (n = 16). Eight wells filled with sterile medium served as blank controls. Subsequently, the plates were incubated at 37 °C for 24 h, to allow for biofilm formation.
To quantitatively measure the living cells present in the biofilm, it was stained with RSZ, which is a redox indicator that allows the metabolic activity of the cells to be monitored [23,26]. The RSZ sodium salt (Sigma) was dissolved in Milli-Q water at a concentration of 200 mg/L and sterilized by filtration. RSZ staining was performed as described by Paytubi et al. [23], with small changes. In summary, after removing the culture medium, the plates were washed twice with distilled water to remove non-adhering bacterial cells and dried with paper towels. Thereafter, 0.1 mL of TSB-YE, diluted 1:10 in distilled water [26] and containing RSZ at 40 mg/L [23] was distributed in each well, and the plate was incubated in the dark for 1–2 h at 37 °C, without stirring. The color of the wells was observed visually. The blue color reflects the absence of metabolic activity (without biofilm or with dead cells in the biofilm) and the pink color (presence of resorufin, formed by the reduction of RSZ) reflects the cellular metabolic activity. When an intense pink color of the growth control was observed, A570 and A600 was monitored using the Thermo Multiskan Plate Reader. The metabolic activities (A570–A600) of L. monocytogenes biofilms were quantified in the absence or presence of BAC. Results from RSZ staining are expressed as means ± standard deviation of the metabolic activity (A570–A600) of L. monocytogenes biofilm.
2.5. Biofilm Assays on SSC
2.5.1. Biofilm Cell Enumeration after Incubation under Different Sub-Inhibitory BAC Concentrations (1.25 or 2.5 mg/L, 48 h)
Stainless steel coupons (SSCs) with 1 mm thick, 1 × 1 cm, type 304, 2B finish (Metalurgica Quinacorte, Lda, Lousa, Portugal) were used to produce the biofilms. The procedures for cleaning, degreasing and sterilizing the coupons were described elsewhere [16,27]. Briefly, coupons were first cleaned in acetone to remove grease, rinsed in distilled water, and consecutively immersed into a phosphoric-acid-based cleaner (CIP 200; Steris Corp.) for 20 min. Then, the coupons were rinsed again and autoclaved in test tubes before use. The procedure for the preparation of the inocula, incubation and detaching of the biofilms were as described in Costa et al. [27]. Briefly, the cleaned and sterilized coupons were immersed in 1.5 mL of the appropriate inocula suspension (approximately 107 CFU/mL in TSB-YE or in TSB-YE with 1.25 or 2.5 mg/L of BAC), in Parafilm-sealed 24-well microplates (Orange Scientific). Incubation proceeded without agitation for 48 h at 25 °C (room temperature). Afterwards, coupons were removed and rinsed with 1 mL of Ringer’s solution (RS) on both faces in order to remove planktonic cells. The rinsed coupons were placed in a new 24-well microplate with 1 mL of RS with glass beads (Ø = 3 mm) under and above the coupons. The microplate was then exposed to vortex agitation (Tittertek DSG, Flowlabs, Germany) for 1 min at maximum speed. The resulting suspensions were decimal-diluted, inoculated onto TSA-YE plates, and incubated overnight at 37 °C, for CFU counting. The assays were replicated using two biological replicates, with two technical replicates each (four replicates).
2.5.2. Biofilm logarithmic reduction after BAC exposure (1280 mg/L, 5 min)
The procedures for the evaluation of the antibiofilm activity of BAC were as performed previously for other disinfectants [16,27]. Briefly, after rinsing, the coupons with the biofilms were exposed to BAC (1280 mg/L) for 5 min at room temperature. After the exposure period, coupons were transferred to a new microplate, where the disinfectant was immediately neutralized with Dey–Engley neutralizing broth solution (Difco Laboratories, New Jersey, USA). Subsequently, the biofilm was detached from the coupons as described previously, and 0.1 mL of the resulting suspension was used to inoculate TSA-YE plates. The remaining suspension was decimal-diluted, and 0.1 mL aliquots were spread onto TSA-YE plates. For calculating log reductions, a control exposed to sterile water was used. For each isolate, four replicates were performed as described previously.
2.6. Data Analysis
The differences between the means of the results obtained with the RSZ were analyzed with the two-sample Student’s t-test and the calculations were performed using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA).
Data from the enumeration on SSC (log CFU/cm2) and from antibiofilm activity of BAC (reduction of log CFU/cm2) assays were checked for agreement to the normal distribution and for homogeneity of variance (Anderson–Darling test and Levene’s test, respectively) by using the MiniTab17 software (Minitab, Inc., Pennsylvania, USA). When normality and the homogeneity of variance were confirmed, a one-way analysis of variance (ANOVA) with Tukey ’s test was performed to calculate statistical differences between the average values. When the data did not comply with ANOVA assumptions, the non-parametric Kruskal–Wallis median test was used. In both cases, the software, Statistica version 7.0 (Statsoft Inc., Tulsa, USA), was used. The confidence level for significance was 95% (p < 0.05) in all cases.
3. Results and Discussion
3.1. MIC Determination
In our previous studies, we used an MIC of 10 mg/L of BAC to differentiate between BAC resistance and sensitivity phenotypes of isolates of L. monocytogenes [13,14]. According to this criterion, the S2BAC included in the present study would be considered as BAC-R (MIC 20 mg/L) and S2-1 as BAC-S (MIC 2.5 mg/L) (Table 1). Susceptibility testing also revealed that S2-1 and quality control strains were similar in terms of the MIC of BAC, and that the BAC-R isolate also showed increased MICs of different compounds, especially EB and CIP, two well-known substrates for different multidrug-resistant efflux pumps [13,14,15], when compared to its parent strain S2-1 (Table 1). Reduced susceptibility to BAC, EB and CIP should have the same molecular mechanism [8,13]. Small changes were also observed in the MICs of other quaternary ammoniums such as CTAB (Table 1). For S2BAC, the overexpression of Listeria efflux pumps similar to MdrL [13,15] may be related to its multidrug resistance phenotype.

Table 1.
Minimum inhibitory concentrations (MICs) of different compounds for BAC-S and BAC-R strains.
To determine efflux pump activity, changes in the MICs of BAC were observed both in the presence and absence of reserpine [14]. The MICs of BAC revealed an effect of reserpine on the MIC of BAC in both BAC-S and BAC-R isolates (Table 1). The effect of reserpine and the increased MICs of EB and CIP are not observed in BAC-R qacH-harboring strains [14]. The change observed in the MICs suggests that the resistance of S2BAC was related to endogenous efflux pumps of the MFS class [13,14,15].
In food production environments, L. monocytogenes efflux pumps do not confer resistance to disinfectants at the concentrations commonly used. In resistant strains, the MICs of BAC (20 mg/L) (Table 1) were much lower than the concentrations used in practice (200 mg/L or greater) [13]; thus, QACs are still considered an effective way to control BAC-R strains of L. monocytogenes [13]. Nevertheless, low-level BAC resistance in these L. monocytogenes strains may contribute to their adaptation and survival [13,18]; hence, the resistant strains may have the potential to persist in food ecosystems.
3.2. Biofilm Assay on Polystyrene Microplates
3.2.1. Biofilm Formation with no added BAC
The BAC-R strain showed a lower amount of biofilm compared to the BAC-S strain (Table 2). Therefore, the association between reduced biofilm formation and BAC resistance—previously noted by Rodríguez-Melcón et al. through the use of confocal microscopy with biofilms formed on polystyrene [20]—was confirmed in the present study through the use of RSZ-staining of biofilms formed on the same type of surface. However, Ortiz et al. [19], using the method of staining with crystal violet in the same type of polystyrene microtiter plates, indicated that the total biomass of biofilm formed by laboratory BAC-R mutant S2BAC was similar to that of its parent strain S2-1 and higher than that of natural BAC-R strains.

Table 2.
Effect of benzalkonium chloride (BAC) on the prevention of biofilm formation by two strains of Listeria monocytogenes. The metabolic activities (A570–A600) of biofilms were quantified after incubation for 24 h using the resazurin reduction assay.
The main limitations of the crystal violet method are the lack of reproducibility and lack of sensitivity [19,28]. As the crystal violet method stains total biofilm biomass, a poor correlation between crystal violet measurements and cell enumeration might be observed, while the significance of the positive correlation may vary with strains [19,28]. Hence, reduced biofilm of BAC-R strains as observed by RSZ-staining might be more realistic than total biomass measured by crystal violet.
3.2.2. Biofilm Formation at Different Doses of BAC
Only two concentrations of BAC (1.25, and 2.5 mg/L) affected the biofilm formation capacity of the two strains of L. monocytogenes differently. Table 2 shows the metabolic activity of the biofilm formed in the presence of BAC in relation to biofilms formed without BAC, by the two strains. In the presence of 1.25 mg/L of BAC, strain S2BAC showed significantly reduced biofilm levels in relation to the control, without BAC (p < 0.05, Student’s t-test) (Table 2). In the presence of 2.5 mg/L of BAC, i.e., the MIC of BAC-S strain S2-1 (Table 2), S2BAC significantly increased biofilm metabolic activity, compared with the control, without BAC (p < 0.05, Student’s t-test). Therefore, the resistance of S2BAC resulted in the stimulation of biofilm formation at a concentration of BAC that caused a reduction of the biofilm level in the parent strain S2-1 (Table 2). At 5 mg/L of BAC, the complete reduction of metabolic activity of the biofilm was observed in both strains (Table 2).
RSZ-based viability staining is often used to quantify metabolically active biofilm cells grown in microtiter plates [22]. This approach is less expensive and less laborious than conventional plating techniques. The oxidation-reduction indicator, RSZ, undergoes a colorimetric change in response to cellular metabolic reduction. Thus, the effect of disinfectants on biofilm formation, by resistant and susceptible strains, can be quantified using RSZ, and RSZ-staining might improve the results obtained with crystal violet staining. For example, with low biofilm-forming prfA mutants [19] and viable but non-culturable (VBNC) cells present in biofilms [29]. Ortiz et al. [19] reported that biofilm stimulation by BAC was not observed with BAC-R prfA mutant strains due to the low biofilm formed by prfA mutants, while our unpublished results reveal a better inter-strain reproducibility and sensitivity for RSZ-staining than for crystal violet staining with prfA mutant strains. These results support the association between reduced susceptibility to disinfectants in food-production environments and the widely recognized problem of the persistence of L. monocytogenes, including long-term persistent prfA mutant strains ([14], unpublished results).
The most widely used technique to estimate biofilm cell viability is the determination of colony-forming units (CFU) on agar media. However, a subpopulation of biofilm cells can be VBNC and would not be detected by the CFU approach. It is known that L. monocytogenes and other pathogens are able to survive environmental stresses such as disinfectants by entering into a VBNC state [29]. As there is a good correlation between results obtained with RSZ-based quantification and CFU counts [22], and the lower limit of quantification with the RSZ method can reach 103 CFU/biofilm [22], RSZ can detect metabolic activity in VBNC bacteria present in biofilms if the number of cells is above that figure.
3.3. Biofilm Assays on SSC
Furthermore, S2BAC and S2-1 were used to produce biofilms on stainless steel coupons (SSC). When the SSC method was used to evaluate the antibiofilm activity of BAC, two approaches were used to test the effect of the presence of sub-inhibitory BAC: biofilm cell enumeration after incubation under different sub-inhibitory BAC concentrations (1.25 or 2.5 mg/L, 48 h), and biofilm logarithmic reduction after BAC exposure (1280 mg/L, 5 min).
3.3.1. Biofilm Cell Enumeration after Incubation under Different Sub-Inhibitory BAC Concentrations (1.25 or 2.5 mg/L, 48 h)
Biofilm-forming ability was determined by enumerating viable cells on culture plates. The results show that the presence of sub-inhibitory concentrations of BAC did not have a significant effect (p > 0.05) on the number of viable cells in the biofilm (Figure 1a).
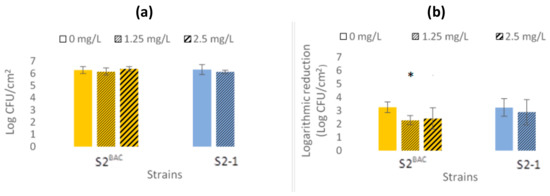
Figure 1.
Antibiofilm activity of benzalkonium chloride (BAC). (a) Biofilm cell enumeration: comparison of the biofilms formed by cell enumeration on stainless steel coupons, after incubation for 48 h at 25 °C, under different sub-inhibitory BAC concentrations. (b) Biofilm logarithmic reduction: comparison of the log reduction, after BAC exposure (1280 mg/L, 5 min), of the biofilms formed under different sub-inhibitory BAC concentrations. The presence of an asterisk in the bar indicates significant differences from the other conditions (p < 0.05). The values shown are averages of four replicates. Standard deviations are indicated.
The results concerning the biofilm-forming ability of the isolates on SSC suggested that biofilm growth tends to be more unaffected by sub-inhibitory concentrations of BAC than that on polystyrene. L. monocytogenes strains may have diverse responses during their contact to abiotic surfaces. The type of contact surface may determine the biofilms’ structure, therefore affecting the exposure of attached cells to stressful conditions [28].
3.3.2. Biofilm Logarithmic Reduction after BAC Exposure (1280 mg/L, 5 min)
The susceptibility of biofilms formed under different BAC sub-inhibitory concentrations was determined by logarithmic reduction (the difference between Log CFU/cm2 values of cellular enumeration—biofilm formation—and the corresponding Log CFU/cm2 values obtained after exposure to BAC, equaling 1280 mg/L, 5 min). For strain S2-1, the presence of sub-inhibitory concentrations of BAC did not have a significant effect on biofilm susceptibility (p > 0.05). However, for the BAC-R strain S2BAC, the presence of a sub-inhibitory concentration of BAC (1.25 mg/L) resulted in higher resistance of the biofilm (p < 0.05) (Figure 1b).
Biofilm formation in microtiter plates has many well-known advantages and is certainly the most commonly used method for biofilm quantification. However, major drawbacks of 96-well microtiter plates are the nutrient depletion during the incubation period, and the fact that biofilm biomass is not solely formed as the result of a biofilm-forming process but also from cells sedimented to the bottom of the wells [23,28]. In the case of the crystal violet assay, the dye binds to negatively charged surface molecules and polysaccharides in the matrix, and the assay may be influenced by the amount of exocellular polymer and by cell sedimentation, which increases with planktonic growth [30].
Biofilms developed on stainless steel were reported to be more resistant to BAC than its planktonic cell’s counterparts [16,28]. This methodology is closer to the conditions in the food industry than methods based on surface materials other than stainless steel. Biofilms formed on SSC showed a susceptibility that depended on the sensitivity of the strain and on the sub-inhibitory concentrations of BAC used to grow the biofilms. The results in this study for the strain S2BAC, when compared to using the results of crystal violet staining [19], suggest that the increase in resistance may be related to a greater number of extracellular polymers in the biofilm matrix of this strain [31]. In fact, when biofilms were examined through confocal laser scanning microscopy, it was found that exposure to sub-MICs of BAC increased biofilm development by S2BAC and reduced biofilm by S2-1 [20]. It is therefore important to understand how the strain susceptibility and growth conditions differently regulate the biofilm-forming ability and the biofilm resistance of L. monocytogenes.
4. Conclusions
The study of foodborne pathogens that have biofilm formation capacity, such as L. monocytogenes, is important for food safety and consumer health. Failure in following sanitation procedures may result in biofilm formation on food preparation equipment and surfaces, in the presence of sub-inhibitory concentrations of disinfectants, such as BAC, thus leading to the contamination and/or recontamination of food products. Resistance to biocides caused by the application of disinfectants may be an important factor for the persistence of L. monocytogenes in food production environments [13,18].
By using the RSZ method in polystyrene microplates, the L.monocytogenes BAC-R mutant (S2BAC) was shown to produce significantly more biofilm in the presence of sub-inhibitory BAC concentrations compared to that in the culture medium without BAC. This effect was not observed in its isogenic BAC-S wild-type strain (S2-1). When the enumeration SSC method was used, the presence of sub-inhibitory BAC did not have a significant effect on the number of viable cells in the biofilms for both strains. However, the presence of sub-inhibitory BAC resulted in the higher resistance of the biofilms of S2BAC. Although this strain did not show a significant difference in the number of viable cells present in the biofilm produced with and without BAC (SSC method), it showed more metabolic activity under sub-inhibitory BAC concentrations (RSZ method).
In summary, sub-inhibitory BAC can affect certain resistant strains by stimulating biofilm formation on polystyrene, while on stainless steel, a higher resistance of the biofilm can be observed. Thus, growth conditions on different surfaces may regulate resistance of biofilms of strains with different susceptibilities. More research on biofilm’s susceptibility under conditions closer to the food industry environment are welcomed, including but not limited to assays with biofilms formed in different types of surfaces.
Contact of microorganisms with sublethal concentrations of disinfectants present in the environment can increase their ability to survive and persist in the niches or reservoirs of food-processing plants. Therefore, our study supports the association between selective pressure exerted by biocides in food-production environments and the persistence of L. monocytogenes.
Author Contributions
L.B. (Lourenço Bonneville): Executed the experiments, and interpreted the results; S.O.: Executed the experiments, and interpreted the results; V.M.: Executed the experiments, and interpreted the results; L.B. (Luisa Brito): Designed the experiments, interpreted the results, and wrote the manuscript; J.V.M.-S.: Designed the experiments, interpreted the results, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Project grant RTI2018-098267-R-C31 from the Spanish Ministry of Science, Innovation and Universities and by project PORBIOTA-Portuguese E-Infrastructure for Information and Research on Biodiversity (POCI-01-0145-FEDER-022127), funded by Operational Thematic Program for Competitiveness and Internationalization (POCI), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER).
Acknowledgments
We thank Pilar López (INIA, Madrid, Spain) and Ana Carla Silva (Laboratory of Microbiology, ISA-ULisboa) for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whitinge, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Langsrud, S.; Lunestad, B.T.; Scheie, A.A.; Sunde, M.; Yazdankhah, S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Elhanafi, D.; Dutta, V.; Kathariou, S. Genetic characterization of plasmid-associated benzalkonium chloride resistance determinants in a Listeria monocytogenes strain from the 1998-1999 outbreak. Appl. Environ. Microbiol. 2010, 76, 8231–8238. [Google Scholar] [CrossRef]
- Müller, A.; Rychli, K.; Muhterem-Uyar, M.; Zaiser, A.; Stessl, B.; Guinane, C.M.; Cotter, P.D.; Wagner, M.; Schmitz-Esser, S. Tn6188-a novel transposon in Listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS ONE 2013, 8, e76835. [Google Scholar] [CrossRef]
- Cherifi, T.; Carrillo, C.; Lambert, D.; Miniaï, I.; Quessy, S.; Larivière-Gauthier, G.; Blais, B.; Fravalo, P. Genomic characterization of Listeria monocytogenes isolates reveals that their persistence in a pig slaughterhouse is linked to the presence of benzalkonium chloride resistance genes. BMC Microbiol. 2018, 18, 220. [Google Scholar] [CrossRef]
- Ebner, R.; Stephan, R.; Althaus, D.; Brisse, S.; Maury, M.; Tasara, T. Phenotypic and genotypic characteristics of Listeria monocytogenes strains isolated during 2011-2014 from different food matrices in Switzerland. Food Control 2015, 57, 321–326. [Google Scholar] [CrossRef]
- Meier, A.B.; Guldimann, C.; Markkula, A.; Pöntinen, A.; Korkeala, H.; Tasara, T. Comparative phenotypic and genotypic analysis of swiss and finnish Listeria monocytogenes isolates with respect to benzalkonium chloride resistance. Front. Microbiol. 2017, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential impact of the resistance to quaternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; López-Alonso, V.; Rodríguez, P.; Martínez-Suárez, J.V. The connection between persistent, disinfectant-resistant Listeria monocytogenes strains from two geographically separate Iberian pork processing plants: Evidence from comparative genome analysis. Appl. Environ. Microbiol. 2016, 82, 308–317. [Google Scholar] [CrossRef]
- Romanova, N.A.; Wolffs, P.F.; Brovko, L.Y.; Griffiths, M.W. Role of efflux pumps in adaptation and resistance of Listeria monocytogenes to benzalkonium chloride. Appl. Environ. Microbiol. 2006, 72, 3498–3503. [Google Scholar] [CrossRef]
- Barroso, I.; Maia, V.; Cabrita, P.; Martínez-Suárez, J.V.; Brito, L. The benzalkonium chloride resistant or sensitive phenotype of Listeria monocytogenes planktonic cells did not dictate the susceptibility of its biofilm counterparts. Food Res. Int. 2019, 123, 373–382. [Google Scholar] [CrossRef]
- U. S. Environmental Protection Agency. 2011; CFR-2011-title40-vol24-sec180-940, Tolerance Exemptions for Active and Inert Ingredients for use in Antimicrobial Formulations [Food Contact Surface Sanitising Solutions]. Available online: https://www.epa.gov/pesticide-registration/inert-ingredients-overview-and-guidance (accessed on 4 November 2019).
- Møretrø, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2016, 241, 215–224. [Google Scholar] [CrossRef]
- Ortiz, S.; López, V.; Martínez-Suárez, J.V. The influence of subminimal inhibitory concentrations of benzalkonium chloride on biofilm formation by Listeria monocytogenes. Int. J. Food Microbiol. 2014, 189, 106–112. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Capita, R.; Rodríguez-Jerez, J.J.; Martínez-Suárez, J.V.; Alonso-Calleja, C. Effect of low doses of disinfectants on the biofilm-forming abilityof Listeria monocytogenes. Foodborne Pathog. Dis. 2019, 16, 262–268. [Google Scholar] [CrossRef]
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Paytubi, S.; de La Cruz, M.; Tormo, J.R.; Martín, J.; González, I.; González-Menendez, V.; Genilloud, O.; Reyes, F.; Vicente, F.; Madrid, C.; et al. A high-throughput screening platform of microbial natural products for the discovery of molecules with antibiofilm properties against Salmonella. Front Microbiol. 2017, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.; Machado, H.; Brito, L. Biofilms of Listeria monocytogenes produced at 12 °C either in pure culture or in co-culture with Pseudomonas aeruginosa showed reduced susceptibility to sanitizers. J. Food Sci. 2011, 76, M142–M148. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI supplement VET08; CLSI: Wayne, NJ, USA, 2018. [Google Scholar]
- Mariscal, A.; Lopez-Gigosos, R.M.; Carnero-Varo, M.; Fernandez-Crehuet, J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl. Microbiol. Biotechnol. 2009, 82, 773–783. [Google Scholar] [CrossRef]
- Costa, A.; Bertolotti, L.; Brito, L.; Civera, T. Biofilm formation and disinfectant susceptibility of persistent and nonpersistent Listeria monocytogenes isolates from Gorgonzola cheese processing plants. Foodborne Pathog. Dis. 2016, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Poimenidou, S.V.; Chrysadakou, M.; Tzakoniati, A.; Bikouli, V.C.; Nychas, G.-J.; Skandamis, P.N. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int. J. Food Microbiol. 2016, 237, 164–171. [Google Scholar] [CrossRef]
- Brauge, T.; Faille, C.; Sadovskaya, I.; Charbit, A.; Benezech, T.; Shen, Y.; Loessner, M.J.; Bautista, J.R.; Midelet-Bourdin, G. The absence of N-acetylglucosamine in wall teichoic acids of Listeria monocytogenes modifies biofilm architecture and tolerance to rinsing and cleaning procedures. PLoS ONE. 2018, 13, e0190879. [Google Scholar] [CrossRef]
- Lourenço, A.; Rego, F.; Brito, L.; Frank, J. Evaluation of methods toassess the biofilm forming ability of Listeria monocytogenes. J. Food Prot. 2012, 75, 1411–1417. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Di Ciccio, P.; Zanardi, E.; Ghidini, S.; Ianieri, A. A look inside the Listeria monocytogenes biofilms extracellular matrix. Microorganisms 2016, 4, 22. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).