Featured Application
The application of this systematic review is a comprehensive study of food compounds, nutrition, food production, health and environmental sciences in improving female fertility.
Abstract
Pro-healthy behaviours, including the diet, are significant factors in maintaining women’s fertility health. However, to improve the patient’s nutrition management, it is important to seek food-derived bioactive compounds to support fertility treatment. This review analysed recent studies of food compounds related to fertility, using databases including PubMed, Web of Science and Science Direct as well as PRISMA (preferred reporting items for systematic reviews) to ensure complete and transparent reporting of systematic reviews. This review lists foods associated with a higher birth rate, using original papers from the last five years (2015). The analysis included the impact of food compounds such as caffeine, fatty acids, folates and vitamin D, as well as the intake of fish, whole grains, dairy and soya. In addition, dietary patterns and total diet composition supporting women’s fertility were also analysed. The results will encourage further research on the relationship between food components and fertility.
1. Introduction
Fertility, known as the ability to establish a clinical pregnancy, is dependent on multiple factors, including female age, environmental pollution, diet, tobacco use, alcohol intake, as well as diseases affecting endocrine function and the anatomy of the reproduction system [1,2]. In turn, infertility is medically defined as a failure to establish a clinical pregnancy after 12 months of regular and unprotected sexual intercourse. It is estimated that infertility in women of child-bearing age is 1 in 7 couples in developed countries and 1 in 4 couples in developing countries, which is increasing significantly [1]. The demand for infertility services is still growing and can be improved thanks to technological advances and the development of medicine.
Factors influencing fertility may be unmodifiable, such as age and environment, or could be medically treated, such as health status—including endocrine disorders. Furthermore, some factors could be modifiable, such as health behaviours, dietary patterns and micronutrient intake.
It has been confirmed that in-vitro fertilisation success rate seems to be highest during the summer months when the pollution of particulate matter (PM) is at its lowest [3]. Phthalates, which may negatively influence the fertility health of women [4], were found to affect the occupational health of hairdressers [5]. Some primary factors, such as excess body weight or underweight, also decrease the rate of fertility [6,7]. Body saturation with vitamin D was suggested to have a beneficial influence on fertility [8,9,10,11,12].
The recommendations by the Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility American Society for Reproductive Medicine, advise females to follow a healthy diet, avoid alcohol and decrease caffeine intake to a moderate level [13]. It is also advisable for women to supplement folate (400 µg/day) to decrease the chance of neural tube defects [13]. However, there is some evidence showing that some food ingredients and specific dietary patterns in women may be positively associated with pregnancy and live birth rates [14,15,16].
One of the food ingredients widely discussed in the context of fertility is sugar. It was shown that its presence in the diet reduces nutritional density and worsens its nutritional quality [17]. The possible mechanism between sugar-sweetened beverages and fertility was explained by increased insulin resistance, leading to oxidative stress. This relation may deleteriously affect semen quality and ovulatory function. Such a mechanism was hypothesised by Hatz, et al. [18], who studied a group of nearly five thousand women and found that fertility and the amount of sugar consumed in sugar-sweetened beverages (particularly sodas and energy drinks) was associated with lower fecundability [18].
A large group of compounds that are still being studied are bioactive compounds in food. Their roles in oxidative stress and fertility have been presented in many studies [19,20,21,22], including several studies on female animal models and bioactive food compounds [23,24,25]. Their role is hypothesised to diminish the effect on the endocrine system of disruptive chemicals. For example, there is some evidence that animals treated with BPA (Bisphenol A), after maternal supplementation of folate and a high phytoestrogen diet, influence oocyte growth and foetal methylation of DNA [26,27]. Other studies have highlighted that a low dose (but not a high dose) of ginger powder, improved the follicle counts of rats [23]. Therefore, it is not clear what dose will be as effective on humans. Additionally, human fertility is affected by complex and multiple factors which could be difficult to expose animals to.
The time of preconception may motivate couples to adopt healthier behaviours and to seek information on factors improving fertility. Even though medical consultation is still the most common source for seeking advice for fertility, social media and the internet also play significant roles [28]. The choice of supplement options is vast, as the fertility supplement market is continuously growing and it has been estimated to be worth USD 1.45 billion globally in 2018 [29]. The use of supplements is not always recommended by healthcare professionals, and their misuse may even pose a threat to health.
The literature related to food compounds and fertility has not been extensively collected, and there is no consensus on what the trends are in these studies or what groups of food ingredients should be considered as supportive or detrimental to fertility. In light of this evidence, an analysis of diet ingredients, food research (e.g., ginger, BPA) and fertility could lead to new supporting therapy strategies that affect the birth rate through the modulation of eating habits. Accordingly, this review provides an analysis of new food compound research influencing fertility and revises recent studies involving the impact of food bioactive compounds on women’s fertility.
2. Materials and Methods
2.1. Search Strategy
A systematic search of literature published before December 2019 was performed in PubMed (National Institiute of Health, USA)(https://www.ncbi.nlm.nih.gov/pubmed), Web of Science (Clarivate Analytics, USA) (https://www.webofknowledge.com), Scopus (Elsevier, RELX Group plc), (https://www.scopus.com) and Science Direct (Elsevier, RELX Group plc) (https://www.sciencedirect.com/) to identify studies describing the association between bioactive food compound intake and women’s fertility. The search strategy was restricted to English language original articles. The following types of documents were excluded: review, book and book chapters.
The search was based upon the following index terms, titles or abstracts listed below: ((bioactive OR nutrient OR food OR ingredient OR vitamin OR mineral OR antioxidant OR phytonutrient) AND (fertility)). The protocol was registered in the “PROSPERO International prospective register of systematic reviews” PROSPERO 2020: CRD42020160223 and is available on https://www.crd.york.ac.uk/prospero/display_record.php?ID = CRD42020160223.
2.2. Inclusion and Exclusion Criteria
Studies on the influence of food compounds on infertility, signs and symptom changes in patients affected by infertility were included. Studies using different food components concerning changes in the concentration of biomarkers for the assessment of infertility and changes in symptoms were analysed. The systematic search included a population of women in the reproductive age 21–50 with diagnosed infertility or healthy women trying to conceive. All studies conducted on animals and case reports were excluded. The studies included were both qualitative and quantitative. A quality assessment of questionable articles was performed with a checklist described by Kmet et al. [30]. Articles written in a language other than English were excluded. Since the search included new trends in food research, it only included articles within the last five years (2015).
2.3. Study Extraction Process
The study selection process includes an assessment of articles based upon titles, abstracts and full text, which were performed by two independent researchers in parallel in each database. At each step of the assessment, all disagreements between the researchers were resolved after consultation with the review coordinator. Only in the case of disagreement during the title assessment process was the paper included in the next step. Full-texts of all records that were selected in the abstract review phase were searched for through the library of Poznan University of Life Sciences.
3. Results
A total of 4609 studies were screened for inclusion in this systematic review. After the elimination process (Figure 1), a total of 25 qualitative studies and 4 quantitative studies were included. The studies were performed internationally and included the following countries USA (n = 20) [15,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45], Australia (n = 1) [46], New Zealand (n = 1) [46], Ireland (n = 1) [46], United Kingdom (n = 2) [46,47], China (n = 1) [48], Denmark (n = 4) [41,43,49,50], Greece (n = 1) [14], Iran (n = 2) [51,52], Brazil (n = 2) [53,54], Canada (n = 1) [43], Russia (n = 1) [55], Italy (n = 2) [56,57], Spain (n = 1) [58]. The articles concerned female fertility and the intake of a Mediterranean dietary pattern (n = 2) [51,57], fruit, vegetables and whole grain intake (n = 3) [38,46,53], fish (n = 1) [42], dairy (n = 3) [15,43,44], types of fatty acids (n = 4) [39,40,41], soy (n = 2) [32,33], caffeine (n = 3) [45,50,54], folate and B12 (n = 4) [34,35,59,60], melatonin (n = 1) [58], CoQ10 (n = 1) [48] as well as a combination of different compounds (n = 4) [37,47,52,55]. The women who participated in the studies were either planning pregnancy (n = 6) [36,40,41,45,46,55], infertile (n = 3) [49,51,55], undergoing or subjected to assistive reproductive technology (ART) therapy (n = 16) [14,15,31,34,35,39,42,44,50,52,53,54,57,58]. The main results of the studies have been summarized in Table 1 (qualitative studies) and Table 2 (quantitative studies).
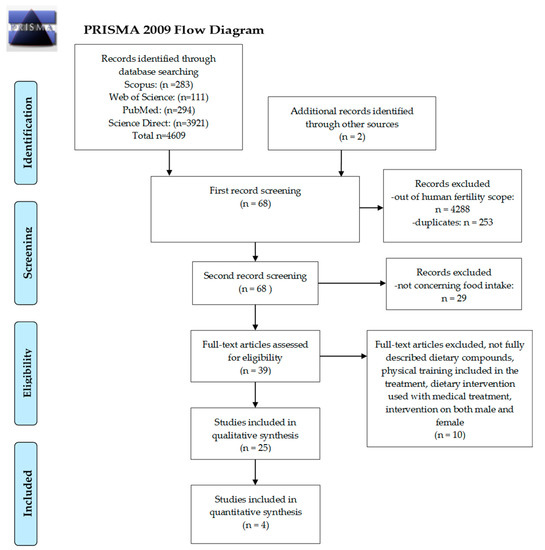
Figure 1.
Preferred reporting items for systematic reviews (PRISMA) Study selection process diagram.

Table 1.
Qualitative studies on bioactive food components and women’s fertility included in the review process.

Table 2.
Quantitative studies concerning bioactive food components and female fertility qualified for the review.
4. Discussion
This systematic review aimed to identify emergent trends in food compounds studies which influence women’s fertility. We qualified a total of 29 studies from the past five years among women planning to conceive either naturally or with assisted reproductive technologies.
4.1. Dietary Patterns, Intake of Fruits, Vegetables and Whole Grains
The modern diet and nutrition analysis based on dietary patterns also assessed the nutrition behaviours as a whole, rather than looking at a single nutrient. Dietary patterns are defined as the quantity, variety, or combination of different foods and beverages in a diet and the frequency in which they are habitually consumed. The frequency reflects food compounds consumed in the diet directly. The most common dietary patterns are pro-healthy, Mediterranean, western and dairy-related [61,62]. The results of this systematic review (presented in Table 1) showed that a high intake of fruits and vegetables and adherence to a pro-healthy dietary pattern is associated with a higher average number of oocytes [51] and embryo quality [53]. The Mediterranean dietary pattern has been associated with supporting fertility health in women. Karayannis et al. found that this dietary pattern is only related to the live birth rate in women under the age of 35 [14]. Another study showed that it was related only to higher oocyte number and clinical pregnancy in women over 35 [57]. The Mediterranean diet is characterised by a high intake of extra virgin olive oil, vegetables, fruits, cereals, nuts and legumes, a moderate intake of fish and other meat, dairy products and red wine and low intakes of eggs and sweets [63]. Another dietary pattern used in the studies was a fertility diet dietary pattern characterised by the intake of supplemental folate and B12, low-pesticide residue produce, high intake of whole grains, seafood, dairy and soy foods. This dietary pattern was positively related to the likelihood of live birth [31].
4.2. Fatty Acids and Fish Intake
The current review has shown that there is no conclusive evidence about the impact of polyunsaturated fatty acids, including the intake of omega-3 on human fertility [13]. The results of the review are inconclusive: two studies found an association between omega-3 fatty acids and fertility [40,41], while another study found no impact of these fatty acids on fertility [39]. However, all of the studies analysing fatty acid intake found that the amount of trans fatty acid consumed is negatively related to the live birth ratio [39,41]. These results also support Grieger et al., who found that a high consumption of fast foods (a rich source of trans fatty acids) influences fertility [46].
4.3. Dairy
For many years, the topic of high dairy intake has been controversial and linked with both a positive and negative impact on the health of women [16,64,65]. Nevertheless, to date, most of the studies concerning female reproductive health have been skewed towards a positive association. Moreover, all of the studies in this systematic search, including the period 2015–2019 concerning dairy intake and fertility, have found at least a small positive relationship between these variables [15,43,44]. It should be noted that dairy foods are generally perceived as a pro-healthy dietary attribute. We studied this group of foods previously and found that a more significant effect on dairy consumption by women was the family environment than health-related protective factors [66].
4.4. Caffeine
Decreasing the caffeine intake to moderate during the time of preconception has been recommended to couples who plan pregnancy [13]. A high intake of caffeine, especially black tea [45] and coffee with added sugar and diet soft drinks [54] has been related to a lower live birth rate. However, coffee intake of 1–5 cups daily has been associated with higher chances of a live birth than none [50]. The intake of caffeine and coffee may be associated with more favourable dietary patterns and health behaviours which could influence fertility [67]. This could be the reason why different types of caffeinated beverages bring contrasting results.
4.5. Soy
The use of nutrients as factor diminishing adverse health effects of environmental pollutants was found in research concerning soy. Soy, as the food product containing phytosterols, could alleviate the effect of endocrine disruptor bisphenol A. This hypothesis is supported by a study concerning women undergoing ART, urinary bisphenol A and soy intake and their influence on live birth rate [32]. Intake of soya, regardless of environmental pollutants, was also related to a higher probability of life birth [33]. High soy intake has also been related to weight loss in women with polycystic ovary syndrome (PCOS), which may improve health and disease results [68]. The results of the above studies show that food ingredients may not always have a direct impact on fertility and their indirect impact is equally important. However, more studies concerning soy intake on fertility and women’s health are needed. Many studies suggest that high soy intake may cause interference with ovarian function because of its high phytoestrogen content [69,70]. More studies are needed to determine the appropriate intake of soy of women trying to conceive, since an excessive intake of soy may not be safe.
4.6. Folate
The Center for Disease Control and Prevention (CDC) in the United States, recommends that healthy women with a low risk of birth defects should supplement 400 µg a day of folic acid at least 12 weeks before conception and early pregnancy to avoid neural tube defects [71]. However, it is unknown whether folate intake is related to female fertility. Recent studies have turned to methylenetetrahydrofolate reductase (MTHFR) gene mutations as the cause of recurrent miscarriages [72]. It seems that supplementation of vitamins B6, B12 and supraphysiologic methylfolate could help women with MTHFR gene mutations to conceive [73]. A high intake of folate and B12 was associated with an increased birth rate in women undergoing ART [59]. A high folate-to-homocysteine ratio was related to a lower risk of anovulation in regularly menstruating females [60]. Interesting retrospective studies were also conducted on supplemental folate intake and pollutant exposure among women undergoing ART. The subjects with high pollutant exposure had a lower rate of live birth [35] or implantation ratio [34]; however, folate supplementation positively modified these results in both studies. These results agree with the animal studies mentioned previously [26,27].
4.7. Vitamin D
During the current study, a number of studies were found which analysed the effect of vitamin D [8,9,10,11,36,49,74,75,76,77,78]. Nine of them showed a positive, statistically significant association with female fertility [8,9,11,36,49,74,76,77,78]. However, since only possible interactions with food compounds were searched for, and vitamin D is mostly formed under sun exposure, two of them concerning vitamin D dietary intake were included in the review. Summing up the issues of vitamin D and fertility, it should be emphasised that food products fortified with vitamin D could be advisable [49] for some populations, not only because of the fertility support but also overall health [79].
4.8. Antioxidants
The search also resulted in study findings involved in other single micronutrients. In a Russian study concerning serum metal concentration in the blood of women, it was found that females with infertility and miscarriage had a lower concentration of copper than pregnant women [55]. Moreover, there was no difference between the copper levels of pregnant women and a healthy control group, which supports the hypothesis that copper may play a role in female fertility.
A diet rich in antioxidants, such as the Mediterranean dietary pattern could improve fertility, although the only recent study concerning the intake of antioxidants did not support this hypothesis. Moreover, the intake of foods with a high concentration of β-carotene, lutein and zeaxanthin was inversely associated with live birth rates [37]. The reasoning of these results may be variable, starting from the accuracy of the food frequency questionnaire used in the study to the dietary pattern which leads to these results. The most important is the fact that supplementing antioxidants as fertility support or for other health conditions could be dangerous and should not be advised to the patients.
4.9. Other Food Compounds
Most of the studies found in the systematic search were qualitative and retrospective, although four prospective randomised trials published between 2015 and 2019 were also included in the review (Table 2). The studies concerned coenzyme Q10 [48], melatonin [58] and multiple nutrient supplementation of vitamin E and D [52] as well as numerous micronutrient or only folate supplement [47]. All of the included study interventions had a positive effect on the pregnancy rate and should be further studied to support fertility treatment nutritionally.
5. Conclusions
In conclusion, it should be noted that reproductive performance is influenced by food and type of nutrition. The findings of the current study suggest that the importance of food production. In particular, the availability and intake of pro-healthy food compounds is a significant factor supporting fertility in females. Women planning pregnancy should especially ensure an intake of fruits and vegetables, folate and vitamin D. A high intake of sweetened beverages and trans-fatty acids appears to decrease the chance of pregnancy and live birth rate. Soy food intake needs to be further analysed because of its endocrine-disruptive properties. Environmental pollution influences fertility around the world, and the appropriate intake of bioactive food compounds could diminish this effect. However, the excessive intake of specific micronutrients, such as antioxidants, may decrease fertility. More randomised prospective studies are needed to analyse the impact of micronutrients on fertility, taking into consideration the patient environment and environmental pollution.
Author Contributions
Conceptualisation, M.C.-M. and A.B.-D.; methodology, M.C.-M., M.K. and A.B.-D.; investigation, A.B.-D. and E.K.; resources, M.C.-M.; data curation, A.B.-D. and E.K.; writing-original draft preparation, A.B.-D.; writing-review and editing, A.B.-D., E.K., M.K., M.C.-M.; supervision, M.C.-M.; project administration, M.C.-M.; funding acquisition, M.C.-M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.Y.; Song, H.; Shin, S.J.; Kim, J. Association between in vitro fertilization success rate and ambient air pollution: A possible explanation of within-year variation of in vitro fertilization success rate. Obs. Gynecol. Sci. 2020, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef]
- Kolena, B.; Petrovicova, I.; Sidlovska, M.; Hlisnikova, H.; Tomasovova, E.; Zoldakova, V.; Trajtelova, H.; Rybansky, L.; Wimmerova, S.; Trnovec, T. Phthalates exposure and occupational symptoms among Slovakian hairdressing apprentices. Appl. Sci. 2019, 9, 3321. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Kudesia, R.; Wu, H.; Hunter Cohn, K.; Tan, L.; Lee, J.A.; Copperman, A.B.; Yurttas Beim, P. The Effect of Female Body Mass Index on in Vitro Fertilization Cycle Outcomes: A Multi-Center Analysis. J. Assist. Reprod. Genet. 2018, 35, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Czerwińska, A.; Olszak-Wąsik, K.; Olejek, A.; Czerwiński, M.; Tukiendorf, A. Vitamin D and anti-müllerian hormone levels in infertility treatment: The change-point problem. Nutrients 2019, 11, 1053. [Google Scholar] [CrossRef]
- Kokanall, D.; Karaca, M.; Ozakśit, G.; Elmas, B.; Üstün, Y.E. Serum Vitamin D Levels in Fertile and Infertile Women with Polycystic Ovary Syndrome. Geburtshilfe Frauenheilkd. 2019, 79, 510–516. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ersoy, E.; Tokmak, A.; Sargin, A.; Ozgu-Erdinc, A.S.; Erkaya, S.; Ibrahim Yakut, H. Do serum vitamin D levels have any effect on intrauterine insemination success? Int. J. Fertil. Steril. 2018, 12, 164–168. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; ten Boekel, E.; ter Wee, M.M.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): A cross-sectional study. PLoS One 2018, 13, e0204748. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.Z.; Baird, D.D.; Wilcox, A.J.; Weinberg, C.R.; Steiner, A.Z. 25-Hydroxyvitamin D (25(OH)D) and biomarkers of ovarian reserve. Menopause 2018, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, S.; Butts, S.; Fossum, G.; Gracia, C.; La Barbera, A.; Mersereau, J.; Odem, R.; Paulson, R.; Penzias, A.; Pisarska, M.; et al. Optimizing natural fertility: A committee opinion. Fertil. Steril. 2017, 107, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Mastrominas, M.; Yiannakouris, N. Adherence to the Mediterranean diet and IVF success rate among non-obese women attempting fertility. Hum. Reprod. 2018, 33, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Afeiche, M.C.; Chiu, Y.H.; Gaskins, A.J.; Williams, P.L.; Souter, I.; Wright, D.L.; Hauser, R.; Chavarro, J.E. Dairy intake in relation to in vitro fertilization outcomes among women from a fertility clinic. Hum. Reprod. 2016, 31, 563–571. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chavarro, J.E. Diet and fertility: A review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef]
- Czlapka-Matyasik, M.; Lonnie, M.; Wadolowska, L.; Frelich, A. “Cutting down on sugar” by non-dieting young women: An impact on diet quality on weekdays and the weekend. Nutrients 2018, 10, 1463. [Google Scholar] [CrossRef]
- Hatch, E.E.; Wesselink, A.K.; Hahn, K.A.; Michiel, J.J.; Mikkelsen, E.M.; Sorensen, H.T.; Rothman, K.J.; Wise, L.A. Intake of Sugar-sweetened Beverages and Fecundability in a North American Preconception Cohort. Epidemiology 2018, 29, 369–378. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Update 2008. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Smits, R.M.; Mackenzie-Proctor, R.; Fleischer, K.; Showell, M.G. Antioxidants in fertility: Impact on male and female reproductive outcomes. Fertil. Steril. 2018, 110, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Seven, B.; Timur, H.; Yorganci, A.; Inal, H.A.; Kalem, M.N.; Kalem, Z.; Han, O.; Bilezikci, B. Ginger (zingiber officinale) might improve female fertility: A rat model. J. Chin. Med. Assoc. 2018, 81, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Luo, L.-L.; Xu, J.-J.; Zhuang, X.-L.; Kong, X.-X.; Fu, Y.-C. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 2010, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, J.; Wang, X.; Sun, J.; Li, Z. Icariin exerts a protective effect against D-galactose induced premature ovarian failure via promoting DNA damage repair. Biomed. Pharmacother. 2019, 118, 109218. [Google Scholar] [CrossRef]
- Muhlhauser, A.; Susiarjo, M.; Rubio, C.; Griswold, J.; Gorence, G.; Hassold, T.; Hunt, P.A. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol. Reprod. 2009, 80, 1066–1071. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Walker, R.; Blumfield, M.; Truby, H. Beliefs and advice-seeking behaviours for fertility and pregnancy: A cross-sectional study of a global sample. J. Hum. Nutr. Diet. 2018, 31, 486–495. [Google Scholar] [CrossRef]
- Grand View Research Fertility Supplements Market Size, Share & Trends Analysis Report By Ingredient (Natural, Synthetic/Blend), By Product (Capsules, Tablets, Soft gels), By End Use, By Distribution Channel, And Segment Forecasts, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/fertility-supplements-market (accessed on 19 December 2019).
- Kmet, L.M.; Lee, R.C.; Cook, L.S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. HTA Initiative # 13 Series; 2004; ISBN 1-896956-71-XX. Available online: https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e (accessed on 28 June 2020).
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E. Dietary patterns and outcomes of assisted reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Mínguez-Alarcón, L.; Chiu, Y.H.; Gaskins, A.J.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R. Soy intake modifies the relation between urinary bisphenol a concentrations and pregnancy outcomes among women undergoing assisted reproduction. J. Clin. Endocrinol. Metab. 2016, 101, 1082–1090. [Google Scholar] [CrossRef]
- Vanegas, J.C.; Afeiche, M.C.; Gaskins, A.J.; Mínguez-Alarcón, L.; Williams, P.L.; Wright, D.L.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil. Steril. 2015, 103, 749–755.e2. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Mínguez-Alarcón, L.; Fong, K.C.; Awad, Y.A.; Di, Q.; Chavarro, J.E.; Ford, J.B.; Coull, B.A.; Schwartz, J.; Kloog, I.; et al. Supplemental Folate and the Relationship Between Traffic-Related Air Pollution and Livebirth Among Women Undergoing Assisted Reproduction. Am. J. Epidemiol. 2019, 188, 1595–1604. [Google Scholar] [CrossRef]
- Minguez-Alarcon, L.; Gaskins, A.J.; Chiu, Y.-H.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R.; Chavarro, J.E. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod. Toxicol. 2016, 65, 104–112. [Google Scholar] [CrossRef]
- Fung, J.L.; Hartman, T.J.; Schleicher, R.L.; Goldman, M.B. Association of vitamin D intake and serum levels with fertility: Results from the Lifestyle and Fertility Study. Fertil. Steril. 2017, 108, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.; Nassan, F.L.; Chiu, Y.H.; Mínguez-Alarcón, L.; Williams, P.L.; Souter, I.; Hauser, R.; Chavarro, J.E. Intake of Antioxidants in Relation to Infertility Treatment Outcomes with Assisted Reproductive Technologies. Epidemiology 2019, 30, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Keller, M.G.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil. Steril. 2016, 105, 1503–1510.e4. [Google Scholar] [CrossRef] [PubMed]
- Eskew, A.M.; Wormer, K.C.; Matthews, M.L.; Norton, H.J.; Papadakis, M.A.; Hurst, B.S. The association between fatty acid index and in vitro fertilization outcomes. J. Assist. Reprod. Genet. 2017, 34, 1627–1632. [Google Scholar] [CrossRef]
- Mumford, S.L.; Chavarro, J.E.; Zhang, C.; Perkins, N.J.; Sjaarda, L.A.; Pollack, A.Z.; Schliep, K.C.; Michels, K.A.; Zarek, S.M.; Plowden, T.C.; et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am. J. Clin. Nutr. 2016, 103, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Wesselink, A.K.; Tucker, K.L.; Saklani, S.; Mikkelsen, E.M.; Cueto, H.; Riis, A.H.; Trolle, E.; Mckinnon, C.J.; Hahn, K.A.; et al. Original Contribution Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am. J. Epidemiol. 2018, 187, 60–74. [Google Scholar] [CrossRef]
- Nassan, F.L.; Chiu, Y.-H.; Vanegas, J.C.; Gaskins, A.J.; Williams, P.L.; Ford, J.B.; Attaman, J.; Hauser, R.; Chavarro, J.E. Intake of protein-rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am. J. Clin. Nutr. 2018, 108, 1104–1112. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Mikkelsen, E.M.; Cueto, H.; Hahn, K.A.; Rothman, K.J.; Tucker, K.L.; Sorensen, H.T.; Hatch, E.E. Dairy intake and fecundability in 2 preconception cohort studies. Am. J. Clin. Nutr. 2017, 105, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Souter, I.; Chiu, Y.H.; Batsis, M.; Afeiche, M.C.; Williams, P.L.; Hauser, R.; Chavarro, J.E. The association of protein intake (amount and type) with ovarian antral follicle counts among infertile women: Results from the EARTH prospective study cohort. BJOG An Int. J. Obstet. Gynaecol. 2017, 124, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Wise, L.A.; Rothman, K.J.; Hahn, K.A.; Mikkelsen, E.M.; Mahalingaiah, S.; Hatch, E.E. Caffeine and caffeinated beverage consumption and fecundability in a preconception cohort. Reprod. Toxicol. 2016, 62, 39–45. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Bianco-Miotto, T.; Jankovic-Karasoulos, T.; Moran, L.J.; Wilson, R.L.; Leemaqz, S.Y.; Poston, L.; Mccowan, L.; Kenny, L.C.; et al. Pre-pregnancy fast food and fruit intake is associated with time to pregnancy. Hum. Reprod. 2018, 33, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Burt, E.; Gallagher, A.M.; Butler, L.; Venkatakrishnan, R.; Peitsidis, P. Prospective randomized trial of multiple micronutrients in subfertile women undergoing ovulation induction: A pilot study. Reprod. Biomed. Online 2012, 24, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Jensen, A.; Nielsen, M.L.; Guleria, S.; Kjaer, S.K.; Heitmann, B.L.; Kesmodel, U.S. Chances of live birth after exposure to vitamin D–fortified margarine in women with fertility problems: Results from a Danish population-based cohort study. Fertil. Steril. 2020, 1–8. [Google Scholar] [CrossRef]
- Lyngsø, J.; Kesmodel, U.S.; Bay, B.; Ingerslev, H.J.; Nybo Andersen, A.M.; Ramlau-Hansen, C.H. Impact of female daily coffee consumption on successful fertility treatment: A Danish cohort study. Fertil. Steril. 2019, 112, 120–129.e2. [Google Scholar] [CrossRef]
- Jahangirifar, M.; Taebi, M.; Nasr-Esfahani, M.H.; Askari, G.; Fung, J.L.; Hartman, T.J.; Schleicher, R.L.; Goldman, M.B.; Nassan, F.L.; Chiu, Y.Y.; et al. Dietary patterns and the outcomes of assisted reproductive techniques in women with primary infertility: A prospective cohort study. Int. J. Fertil. Steril. 2017, 12, 316–323. [Google Scholar] [CrossRef]
- Fatemi, F.; Mohammadzadeh, A.; Sadeghi, M.R.; Akhondi, M.M.; Mohammadmoradi, S.; Kamali, K.; Lackpour, N.; Jouhari, S.; Zafadoust, S.; Mokhtar, S.; et al. Role of vitamin E and D3 supplementation in Intra-Cytoplasmic Sperm Injection outcomes of women with polycystic ovarian syndrome: A double blinded randomized placebo-controlled trial. Clin. Nutr. ESPEN 2017, 18, 23–30. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Halpern, G.; Setti, A.S.; Figueira, R.C.S.; Iaconelli, A.; Borges, E. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod. Biomed. Online 2015, 31, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Setti, A.S.; Braga, D.P.d.A.F.; Halpern, G.; Figueira, R.d.C.S.; Iaconelli, A.; Borges, E. Is there an association between artificial sweetener consumption and assisted reproduction outcomes? Reprod. Biomed. Online 2018, 36, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Skalnaya, M.G.; Tinkov, A.A.; Lobanova, Y.N.; Chang, J.S.; Skalny, A.V. Serum levels of copper, iron, and manganese in women with pregnancy, miscarriage, and primary infertility. J. Trace Elem. Med. Biol. 2019, 56, 124–130. [Google Scholar] [CrossRef]
- Ricci, E.; Noli, S.; Cipriani, S.; La Vecchia, I.; Chiaffarino, F.; Ferrari, S.; Mauri, P.A.; Reschini, M.; Fedele, L.; Parazzini, F. Maternal and paternal caffeine intake and art outcomes in couples referring to an Italian fertility clinic: A prospective cohort. Nutrients 2018, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Bravi, F.; Noli, S.; Somigliana, E.; Cipriani, S.; Castiglioni, M.; Chiaffarino, F.; Vignali, M.; Gallotti, B.; Parazzini, F. Mediterranean diet and outcomes of assisted reproduction: An Italian cohort study. Am. J. Obstet. Gynecol. 2019, 221, 627.e1–627.e14. [Google Scholar] [CrossRef]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, Á.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Ford, J.B.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Association between serum folate and Vitamin B-12 and outcomes of assisted reproductive technologies. Am. J. Clin. Nutr. 2015, 102, 943–950. [Google Scholar] [CrossRef]
- Michels, K.A.; Wactawski-Wende, J.; Mills, J.L.; Schliep, K.C.; Gaskins, A.J.; Yeung, E.H.; Kim, K.; Plowden, T.C.; Sjaarda, L.A.; Chaljub, E.N.; et al. Folate, homocysteine and the ovarian cycle among healthy regularly menstruating women. Hum. Reprod. 2017, 32, 1743–1750. [Google Scholar] [CrossRef]
- Shahdadian, F.; Ghiasvand, R.; Abbasi, B.; Feizi, A.; Saneei, P.; Shahshahan, Z. Association between Major Dietary Patterns and Polycystic Ovary Syndrome: Evidence from a case-control study. Appl. Physiol. Nutr. Metab. 2018, 44, 52–58. [Google Scholar] [CrossRef]
- Wadolowska, L.; Ulewicz, N.; Sobas, K.; Wuenstel, J.W.; Slowinska, M.A.; Niedzwiedzka, E.; Czlapka-Matyasik, M. Dairy-related dietary patterns, dietary calcium, body weight and composition: A study of obesity in polish mothers and daughters, the MODAF project. Nutrients 2018, 10, 90. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Rich-Edwards, J.W.; Rosner, B.; Willett, W.C. A prospective study of dairy foods intake and anovulatory infertility. Hum. Reprod. 2007, 22, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Al Sarakbi, W.; Salhab, M.; Mokbel, K. Dairy products and breast cancer risk: A review of the literature. Int. J. Fertil. Womens Med. 2005, 50, 244–249. [Google Scholar] [PubMed]
- Sobas, K.; Wadolowska, L.; Slowinska, M.A.; Czlapka-Matyasik, M.; Wuenstel, J.; Niedzwiedzka, E. Like mother, like daughter? Dietary and non-dietary bone fracture risk factors in mothers and their daughters. Iran. J. Public Health 2015, 44, 939–952. [Google Scholar]
- Salas-Huetos, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Karamali, M.; Kashanian, M.; Alaeinasab, S.; Asemi, Z. The effect of dietary soy intake on weight loss, glycaemic control, lipid profiles and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: A randomised clinical trial. J. Hum. Nutr. Diet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N. Adult ovarian function can be affected by high levels of soy. J. Nutr. 2010, 140, 2322S–2325S. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef]
- Williams, J.; Mai, C.T.; Mulinare, J.; Isenburg, J.; Flood, T.J.; Ethen, M.; Frohnert, B.; Kirby, R.S. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification—United States, 1995–2011. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1–5. [Google Scholar]
- Zhu, Y.; Wu, T.; Ye, L.; Li, G.; Zeng, Y.; Zhang, Y. Prevalent genotypes of methylenetetrahydrofolate reductase (MTHFR) in recurrent miscarriage and recurrent implantation failure. J. Assist. Reprod. Genet. 2018, 35, 1437–1442. [Google Scholar] [CrossRef]
- Serapinas, D.; Boreikaite, E.; Bartkeviciute, A.; Bandzeviciene, R.; Silkunas, M.; Bartkeviciene, D. The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reprod. Toxicol. 2017, 72, 159–163. [Google Scholar] [CrossRef]
- Mumford, S.L.; Garbose, R.A.; Kim, K.; Kissell, K.; Kuhr, D.L.; Omosigho, U.R.; Perkins, N.J.; Galai, N.; Silver, R.M.; Sjaarda, L.A.; et al. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: A prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 725–732. [Google Scholar] [CrossRef]
- Neville, G.; Martyn, F.; Kilbane, M.; O’Riordan, M.; Wingfield, M.; McKenna, M.; McAuliffe, F.M. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int. J. Gynecol. Obstet. 2016, 135, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Abadia, L.; Gaskins, A.J.; Chiu, Y.H.; Williams, P.L.; Keller, M.; Wright, D.L.; Souter, I.; Hauser, R. Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am. J. Clin. Nutr. 2016, 104, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Xu, Y.; Chu, Y.; Wang, X.; Li, Q.; Ma, Z.; Liu, Z.; Wan, Y. Effect of vitamin D status on normal fertilization rate following in vitro fertilization. Reprod. Biol. Endocrinol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallos, I.; Tobias, A.; Robinson, L.; Kirkman-Brown, J.; Dhillon-Smith, R.; Harb, H.; Eapen, A.; Rajkhowa, M.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A prospective cohort study. Reprod. Health 2019, 16, 1–10. [Google Scholar] [CrossRef]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).