Abstract
The need to reduce anthropogenic greenhouse gas emissions drives the development of innovative carbon dioxide capture technologies. Microalgae-based biotechnologies represent a promising approach in this field. In this study, we evaluated the CO2 assimilation efficiency of two novel microalgae strains, Desmodesmus armatus ARC-06 and Tribonema minus ARC-10, under low (0.04%) and high (1.5%) CO2 conditions in a periodic cultivation system. The two strains exhibited distinct CO2 adaptation strategies. D. armatus demonstrated higher tolerance to low CO2 conditions, whereas T. minus showed superior performance under elevated CO2. Although elevated CO2 stimulated growth in both strains, their carbon dioxide sequestration efficiency (CDSE) differed markedly. The maximum CDSE was significantly higher in T. minus (30.0 ± 1.52%) compared to D. armatus (16.5 ± 1.12%). Similarly, the average CDSE over the cultivation period was greater in T. minus (19.1 ± 2.18%) than in D. armatus (11.8 ± 1.45%). These results underscore the importance of bioprospecting for novel microalgae strains, and the need for further research to develop efficient biological CO2 sequestration methods.
1. Introduction
Green biotechnology has become a crucial area of global technological development, fostering economic growth while reducing environmental risks. Among the various biological systems used in sustainable technologies, microalgae are of significant scientific and practical interest [1,2]. Microalgae represent one of the most productive photosynthetic systems. Their high growth rate enables efficient assimilation of CO2, transforming solar energy into the energy of chemical bonds in organic matter [3,4]. A unique feature of microalgae is their ability to fix carbon dioxide from various sources, including atmospheric and industrial-exhaust CO2, as well as dissolved bicarbonates [5,6]. The efficiency of photosynthetic CO2 assimilation in microalgae is 10–50 times higher than of terrestrial plants, and the synthesized organic carbon is a primary building block of their biomass [2,7]. Depending on the species, microalgae are capable of producing a diverse array of valuable biologically active compounds (BACs) [8,9]. These BACs find applications in various industries, including food, feed, nutraceuticals, and cosmetics [10,11]. Additionally, they can serve as key components in the production of sustainable plastics [12,13] and biofuels [14,15,16].
Due to their high efficiency in sequestering inorganic carbon, microalgae are of significant interest for developing biotechnologies to capture anthropogenic greenhouse gases (GHGs) [1,17,18]. First, producing 1 kg of dry microalgae requires between 1.3 and 2.4 kg (average 1.83 kg) of CO2 [3,19,20]. Thus, their cultivation can reduce anthropogenic CO2 emissions while simultaneously producing a wide range of BACs that are highly attractive for commercial investment [9,21]. Second, microalgae cultivation can utilize wastewater as a nutrient source. This approach provides an integrated solution to environmental challenges, addressing not only the reduction in anthropogenic CO2 emissions but also the development of water purification technologies [22,23]. Third, using various types of photobioreactors allows microalgae to be grown near sources of GHGs, and on land unsuitable for afforestation or traditional agriculture [24,25]. This eliminates competition for arable land with food crops, thus preserving land resources for agriculture and forest conservation [26,27]. Although current microalgae-based carbon capture technologies still require further refinement, significant progress has been made in this field [2,5,6]. Experts anticipate that these technologies could become cost-effective and environmentally sustainable solutions for shaping the future bioeconomy in the long term [17,28,29].
Current estimates indicate a high species diversity of algae. Approximately 50,500 species are listed in the online database AlgaeBase (https://www.algaebase.org, accessed on 1 September 2025) [30], but only about 4600 of these are available to researchers as pure cultures [31]. Only a few hundred species have been studied in detail for their biochemical composition and biotechnological potential, and industrial cultivation worldwide is limited to a few dozen strains [32]. Therefore, the full potential of microalgae has not yet been realized. Bioprospecting is a crucial process for the development of the microalgae industry. It involves the systematic search for new microalgae strains to be identified and evaluated for their potential biotechnological applications and commercial value [33,34].
The study aims to evaluate the efficiency of carbon dioxide bio-capture and organic carbon production using two new microalgae strains under low (0.04%) and high (1.5%) CO2 conditions under intensive cultivation conditions. The overall design of our project is illustrated in Figure S1. This paper presents the results corresponding to stage IV/IV(a).
2. Materials and Methods
2.1. Microalgae Strains and Cultivation Conditions
The studies were conducted on two new strains of microalgae: Desmodesmus armatus (ARC-06) and Tribonema minus (ARC-10). The strains were isolated in 2021 from a natural phytoplankton community in the Yenisei River (Siberia, Russia). Species identification was performed using both morphological and molecular genetic methods [35]. Pure cultures were maintained on Bold basal medium in Petri dishes at a temperature of 10.0 ± 0.5 °C and 24-h illumination of 50 μmol photons m−2 s−1 (white LED, 2700–3000 K) [36]. The cultures are stored at the Timiryazev Institute of Plant Physiology of the Russian Academy of Sciences.
All strains were grown using a modified Bold’s Basal medium (BBM 3N) containing 3-times nitrogen content and supplemented with vitamin B12. Composition (mg L−1) was as follows: NaNO3—750, KH2PO4—175, K2HPO4 × 3H2O—75, MgSO4 × 7H2O—75, Na2EDTA—45, CaCl2 × 2H2O—25, NaCl—25, FeCl3 × 6H2O—0.582, MnCl2 × 4H2O—0.246, ZnCl2—0.03, Na2MoO4 × 2H2O—0.024, CoCl2 × 6H2O—0.012, and 0.001 ng Cyanocobalamin made with deionized water Milli Q (18.2 MΩ cm). The initial media pH was 6.8, and the media was autoclaved at 125 °C degrees and 1.5 atm to sterilize.
D. armatus and T. minus cells were pre-cultured for five days in 300 mL Erlenmeyer flasks containing 150 mL of sterile BBM 3N medium. The cultures were grown on an orbital shaker at 100 rpm, at a room temperature of 22.5 ± 0.5 °C, under illumination of 150 μmol photons m−2 s−1 (white LED, 2700–3000 K). The description of the LED module and the spectral composition of the light are provided in the Supplementary Materials (Figure S2). Exponentially growing cultures of each strain, each with a dry biomass concentration of approximately 0.1 g L−1, were used as the inoculum for the next experimental phase.
The experiment was conducted using a laboratory system for intensive cultivation (LSIC) whose design, technical specifications, and operating principles are described in detail elsewhere [37] and illustrated in Figures S3 and S4. Microalgae strains were grown in BBM 3N medium at 27.0 ± 0.6 °C under continuous 24-h illumination of 300 μmol photons m−2 s−1 (white LED, 2700–3000 K).
Two CO2 supply modes were applied. Cultures were continuously bubbled with either (1) air (0.04% CO2, classified as a low CO2 level) or (2) an air mixture enriched to 1.5% (v/v) CO2 (a high CO2 level), which served as the sole inorganic carbon source. In our work, the classification of “low” and “high” CO2 levels was based on the article by Wang et al. [38]. The gas flow rate was maintained at ~0.2 L min−1 for both treatments. The CO2 concentration in the gas mixture was monitored using a PKU-4 NMT gas analyzer (JSC EKSiS, Moscow, Russia) with an absolute error of ±(0.1 + 0.05°C), where C is the nominal CO2 concentration. Illumination levels within the working volume of the vial and at the surface of the LED modules were measured using a Li-189 quantum radiometer equipped with an LI-190SA sensor (LI-COR, Lincoln, NE, USA). The spectral composition of the light was determined using an LI-180 spectrometer (LI-COR, Lincoln, NE, USA). We calculated the average illumination on the surface of the culture vials from these data in accordance with a previously described method [37,39].
Water used for medium preparation was purified using a six-stage reverse osmosis system, AP-800DIR-400 (AquaPro Industrial Co., Ltd., Nanking E. Rd., Taipei, Taiwan). All containers, hoses, filters, and liquids were sterilized, either by autoclaving or by treatment with hot steam followed by 70% ethanol. D. armatus and T. minus strains were cultivated with three biological replicates per treatment. The experiment was conducted over a 9-day period under periodic cultivation conditions. Microalgae samples were collected on days 0, 3, 6, and 9 at the same time each day. In each sample, we measured the optical density, pH of the medium, microalgae biomass, and organic carbon concentration.
2.2. Growth and Productivity Measurements
Optical density (OD750) was measured using a Genesys 10S UV-Vis spectrophotometer at a wavelength of 750 nm (Thermo Fisher Scientific, Waltham, MA, USA). At the same time, the pH level of the samples was determined using a SevenEasy pH meter equipped with an InLab 413 electrode (Mettler-Toledo, Greifensee, Switzerland).
Microalgae biomass was gravimetrically determined. A 10 mL aliquot of the suspension was diluted in 100 mL of sterile nutrient medium and thoroughly mixed. The resulting sample was filtered through pre-weighed 47 mm, 0.45 μm pore-size Millipore membrane filters, followed by three stages of washing: twice with distilled water and once with Milli-Q. After drying at 105 °C to constant weight for 6 h, the filters were reweighed with an accuracy of ±0.001 g.
Biomass was calculated using the following formula:
where m1 is filter weight before filtration (g); m2 is filter weight after filtration (g); V, volume of filtered suspension (L). The results were expressed as g L−1 of dry weight (DW).
The culture productivity (P) was calculated as the change in biomass concentration over time, as follows:
where B is the biomass concentration (g DW L−1) and t is time (days). The results were expressed as (g L−1 d−1).
The specific growth rate (μ) was calculated using the following formula:
where B1 and B2 represent the biomass concentrations at times t1 and t2, respectively. The results were expressed as (d−1).
2.3. Organic Carbon
The organic carbon (OC) content was determined using high-temperature catalytic oxidation followed by IR detection. A 1 mL aliquot of the suspension was diluted in 100 mL of sterile nutrient medium and filtered through GF/F Whatman glass fiber filters with a 47 mm diameter and 0.7 μm pore size. The filters were successively washed with distilled water and Milli-Q, then dried at 60 °C for 12 h. They were stored in tight containers in the dark at room temperature until analysis.
Carbon analysis was performed in a TOC 5000-V-CPH analyzer with a module for solid samples (SSM 5000-A (Shimadzu, Kyoto, Japan)) in two modes:
- Total carbon (TC)—pyrolysis at 900 °C in oxygen;
- Inorganic carbon (IC)—acid extraction (1 mL 25% H3PO4) at 200 °C.
Calibration was performed using a glucose standard (Merck, Darmstadt, Germany). Total organic carbon (TOC) was calculated as follows:
TOC = TC − IC
The results were expressed as g C L−1 and/or percentage (%) of DW.
The membrane filters were pre-washed with a 1N HNO3 solution, dried at 105 °C until constant weight, and weighed. The glass fiber filters were pre-combusted in a muffle furnace at 400 °C for 8 h. These procedures are standard for filter preparation and ensure analytical purity.
2.4. Carbon Dioxide Fixation
The CO2 fixation rate (FCO2) was calculated by two methods:
- (1)
- Direct measurement of the OC content photosynthesized in the LSIC vials (dFCO2):
- (2)
- Stoichiometric composition of microalgae cells (sFCO2):
Calculations were performed for three intervals: 0–3, 3–6, and 6–9 days of the experiment. For the total cultivation period (0–9 days), the values of dFCO2 and sFCO2 were considered as the average rate of CO2 fixation for the entire experiment. The results (dFCO2 and sFCO2) were expressed as g CO2 L−1 d−1.
2.5. Carbon Dioxide Sequestration Efficiency (CDSE)
The experimental conditions and gas laws were used to calculate the CO2 flow (g L−1 d−1) entering the culture during the experiment. The estimated carbon dioxide sequestration efficiency (CDSE) of the culture was then calculated as follows:
where is the mass of CO2 assimilated for the production of organic matter; and is the mass of CO2 supplied into the LSIC. was calculated as the total volume of CO2 multiplied by ρ CO2 = 1.82 g L−1 (20 °C, 105 Pa) [39]. The results were expressed in percentage (%) form.
2.6. Statistics
All experiments were conducted in triplicate (n = 3). Results were presented as mean values and their errors (x ± mx). Statistical analyses were performed using the Statistica 12.6 software package. Two types of comparisons were conducted:
- Temporal dynamics analysis: To assess the changes in each parameter over time within a single strain and CO2 condition, we used a one-way analysis of variance (ANOVA). For statistically significant ANOVA results, post hoc pairwise comparisons between different days of cultivation were performed using Tukey’s honestly significant difference (HSD) test.
- Inter-strain comparison: To assess the differences between the two strains (D. armatus and T. minus) on the same day of cultivation and under the same CO2 condition, an independent samples Student’s t-test was used.
A p-value of less than 0.05 was considered statistically significant for all analyses.
3. Results
3.1. Growth Characteristics of Microalgae Strains
Under low CO2 levels, the optical density (OD750) of the D. armatus suspension increased from an initial value of 0.12 ± 0.01 to 3.13 ± 0.05 by day 9 (F = 352.8, p < 0.001). When cultivated under high CO2 concentrations, the OD increased to 5.73 ± 0.05 (F = 1258.8, p < 0.001). A similar trend was observed for T. minus; its OD750 reached 2.57 ± 0.06 (F = 362.7, p < 0.001) under low CO2 and 8.15 ± 0.04 (F = 1715.5, p < 0.001) under high CO2 conditions. These results indicate a stimulatory effect of elevated carbon dioxide concentration on cell proliferation (Table 1).

Table 1.
Growth characteristics of the D. armatus ARC-06 and T. minus ARC-10 strains under different CO2 conditions.
Changes in medium pH reflect physiological differences between the strains. When aerated with air, the pH increased to 10.75 ± 0.08 (F = 172.1, p < 0.001) for D. armatus and 9.08 ± 0.09 (F = 113.2, p < 0.001) for T. minus. This increase is attributed to active photosynthesis and absorption of dissolved CO2, which induces a shift in the carbonate equilibrium. Under 1.5% CO2 supplementation, the pH did not exceed 8.0 on day 9 for either of the two strains ARC-06 and ARC-10, demonstrating the buffering effect of the supplied carbon dioxide (Table 1).
Biomass accumulation correlated well with optical density. The maximum dry biomass of D. armatus was 2.82 ± 0.07 g L−1 under low CO2 and 4.47 ± 0.18 g L−1 under high CO2, while that of T. minus was 1.83 ± 0.10 g L−1 and 6.81 ± 0.13 g L−1, respectively (Figure 1a,c). The OC content increased proportionally with biomass. In D. armatus, the OC content reached 1.44 ± 0.12 g C L−1 under low CO2 and 2.33 ± 0.16 g C L−1 under high CO2. Similarly, in T. minus, the OC content increased to 0.88 ± 0.08 g C L−1 and 3.67 ± 0.22 g C L−1 under low and high CO2 conditions, respectively (Figure 1b,d).
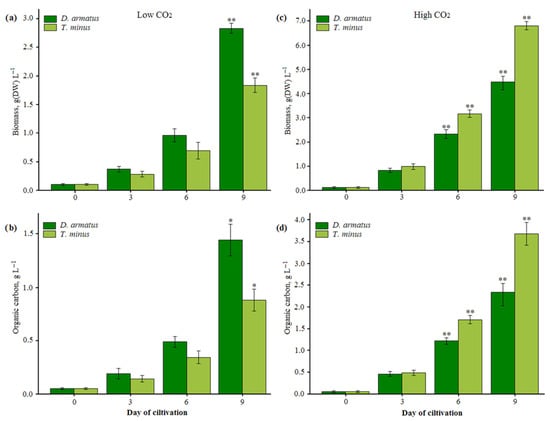
Figure 1.
Biomass and organic carbon concentration in two strains of D. armatus and T. minus under low (a,b) and high (c,d) CO2 conditions. *—differences are significant at p < 0.05, ** at p < 0.01.
The observed differences in the response of the two species to the composition of the gas phase were of particular interest. Under low CO2 conditions, the T. minus showed lower productivity (P) compared to D. armatus. The average P values for T. minus and D. armatus for the entire experimental period were 0.19 ± 0.01 and 0.31 ± 0.01 g L−1 d−1, respectively. This indicates a more efficient adaptation of D. armatus to carbon-limiting conditions.
However, a contrasting pattern emerged when the strains were grown with 1.5% CO2. Under these conditions, T. minus exhibited markedly higher productivity, reaching an average of 0.75 ± 0.01 g L−1 d−1. This value was 1.5 times greater than that of D. armatus (0.48 ± 0.02 g L−1 d−1) (Figure 2a,c). This pronounced response of T. minus to elevated CO2 concentrations demonstrates its superior physiological adaptation to a carbon-enriched environment.
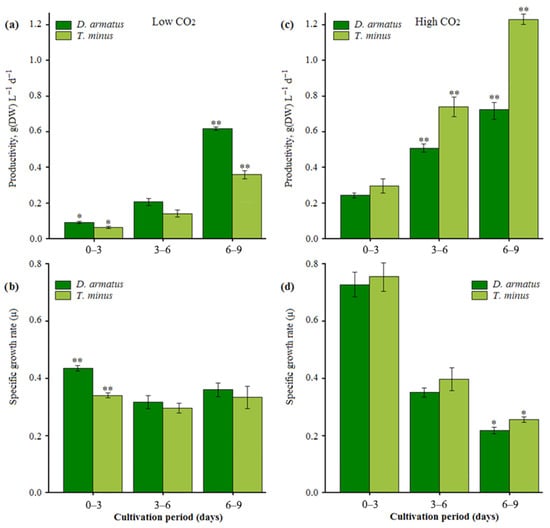
Figure 2.
Productivity and specific growth rates of two strains of D. armatus and T. minus under low (a,b) and high (c,d) CO2 conditions. *—differences are significant at p < 0.05, ** at p < 0.01.
The values and dynamics of the specific growth rate (SGR, μ) showed similar trends between the two species. At low CO2 levels, the SGR was higher in D. armatus, whereas at high CO2 levels it was higher in T. minus. The average SGR over the entire experimental period was 0.37 ± 0.03 d−1 for D. armatus and 0.32 ± 0.01 d−1 for T. minus under low CO2 conditions. Under high CO2 conditions, the average SGR was 0.42 ± 0.15 d−1 and 0.47 ± 0.14 d−1 for D. armatus and T. minus, respectively. However, the differences between the average SGRs were not statistically significant, except for the 0–3 day interval at the low CO2 level and the 6–9 day interval at the high CO2 level (Figure 2b,d).
Analysis of the OC content relative to DW revealed distinct carbon metabolism characteristics in the studied strains. Under low CO2 conditions, the OC content of D. armatus remained stable throughout the experiment, ranging from 52.38 ± 0.92% on day 3 to 52.79 ± 0.85% on day 9 (F = 1.67, p = 0.264), with an average of 52.48 ± 0.82%. In contrast, under high CO2 conditions, the OC dynamics showed a different pattern: a maximum value of 55.56 ± 0.94% was reached on day 3, followed by a decrease to 52.10 ± 0.87% by day 9 (F = 21.8, p < 0.0023), resulting in an average of 53.36 ± 1.01%.
T. minus exhibited different dynamics. Under low CO2 conditions, the relative OC content was minimal (average 48.63 ± 0.43%) and remained virtually unchanged throughout cultivation, ranging from 48.05 ± 0.85% to 49.42 ± 0.61% (F = 22.1, p = 0.0075). However, under high CO2 supply, the OC content gradually increased from 49.50 ± 1.15% on day 3 to 53.83 ± 1.04% by day 9 (F = 98.6, p < 0.001), with an average value of 52.35 ± 0.82%.
The overall average OC content relative to DW across both strains and both CO2 conditions was 51.42 ± 0.75%.
3.2. Carbon Dioxide Capture and CDSE
Significant differences in metabolic activity were observed between the two studied strains. Under low CO2 conditions, D. armatus exhibited an average CO2 fixation rate of 0.57 ± 0.04 g CO2 L−1 d−1, which was 1.7 times higher than that of T. minus (0.34 ± 0.03 g CO2 L−1 d−1). This higher fixation rate is consistent with the greater OC productivity of D. armatus, which reached 1.44 ± 0.12 g C L−1, compared to 0.88 ± 0.08 g C L−1 in T. minus. However, this trend reversed under high CO2 levels. The T. minus strain demonstrated better adaptation, increasing its fixation rate by ~4.5-fold to 1.50 ± 0.09 g CO2 L−1 d−1. In contrast, D. armatus displayed only a ~1.6-fold increase, reaching 0.93 ± 0.06 g CO2 L−1 d−1 (Table 2).

Table 2.
Rates of carbon dioxide fixation (g CO2 L−1 d−1) by the strains D. armatus ARC-06 and T. minus ARC-10 under different cultivation conditions. In the numerator is dFCO2, in the denominator is sFCO2.
T. minus achieved the highest fixation rates during the final cultivation period (days 6–9), reaching a maximum of 2.41 ± 0.07 g CO2 L−1 d−1. This maximum rate was 70–75% higher than that of D. armatus (1.30 ± 0.05 g CO2 L−1 d−1). The ability of T. minus to assimilate CO2 more efficiently is directly related to its growth characteristics (Figure 1 and Figure 2).
Similar trends in the carbon dioxide sequestration efficiency (CDSE) of D. armatus and T. minus were observed under high CO2 conditions (Figure 3).
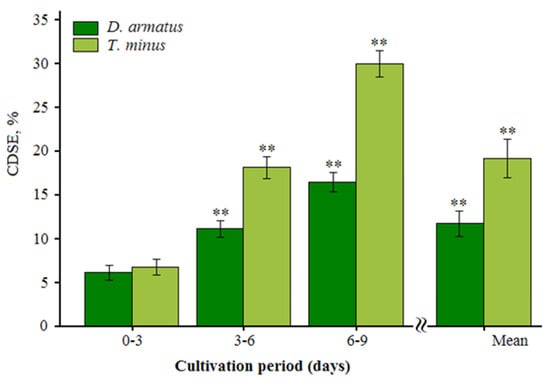
Figure 3.
Carbon dioxide fixation efficiency of the two strains D. armatus (ARC-06) and T. minus (ARC-10) under high CO2 conditions. **—differences are significant at p < 0.01.
Both strains showed a gradual increase in CDSE over time, although significant differences were observed in the final values. During the initial cultivation phase (0–3 days), CDSE values were relatively low and did not differ significantly between the strains, with averages of 6.24 ± 0.76% for D. armatus and 6.71 ± 0.88% for T. minus.
However, during the 3–6 day period, T. minus exhibited significantly higher CO2 sequestration efficiency than D. armatus, and this difference became more pronounced by the end of the experiment. Overall, T. minus demonstrated a significantly higher average CDSE (19.1 ± 2.18%) compared to D. armatus (11.8 ± 1.45%) under high CO2 conditions. These dynamics in carbon dioxide sequestration are consistent with the previously identified growth characteristics and CO2 fixation rates of these strains (Figure 1 and Figure 2).
4. Discussion
Carbon dioxide bio-capture relies on the ability of microalgae to utilize CO2 as their sole carbon source for photosynthesis, making them an attractive option for carbon capture and utilization and/or storage (CCUS) [17,18,40]. Consequently, rates of CO2 fixation and productivity are critical parameters for screening microalgae strains suitable for biotechnological applications in biosequestration [4,41,42]. In our study, we observed that D. armatus and T. minus exhibit distinct growth characteristics and metabolic activity under low and high CO2 conditions. These differences in metabolic activity reflect species-specific adaptation strategies to varying CO2 levels [43], which are likely influenced by both their natural habitat conditions and their unique enzymatic systems. These systems have enabled green (D. armatus) and yellow-green (T. minus) algae to evolve adaptive responses to environmental fluctuations over their evolution [3,44].
In natural ecosystems, microalgae inhabit diverse ecosystems with highly variable CO2 concentrations. In such ecosystems, inorganic carbon exists in three primary forms (CO2, HCO3−, or CO32−) whose relative proportions are dictated by environmental pH [43,45]. This ecological variability has driven the evolution of multiple CO2 acclimation states in microalgae, mirroring their natural habitat adaptations. At least three distinct CO2 adaptation responses have been identified: “high CO2” (0.5–5%), “low CO2” (0.03–0.4%), and “very low CO2” (<0.02%), classified based on differences in photosynthetic CO2 affinity and physiological traits [38,43]. Our findings indicate that D. armatus displays notable tolerance to low CO2 conditions, whereas T. minus exhibits a pronounced adaptability to high CO2 environments. At low and very-low CO2 levels, microalgae use various strategies centered on the direct “pumping” of inorganic carbon into the cells. These strategies are known as “CO2 concentrating mechanisms” (CCMs), in which the active cellular uptake of inorganic carbon against the concentration gradient is an energy-dependent process [45,46].
Although the concept of using algae for carbon capture, utilization, and storage (CCUS) has existed for some time, the commercialization of this promising technology still faces a number of challenges [47]. Previous research has predominantly focused on algae-based CCUS for biofuel production, leading to extensive knowledge of lipid and biomass yields under CO2 enrichment. However, data on net carbon reduction efficiency remains limited, and uncertainties persist regarding the capacity of algae to extract CO2 from gas streams. Indeed, studies have prioritized lipid production for biofuels and high-value compounds over quantifying the cellular carbon capture potential [48].
One of the key challenges in assessing efficiency of CO2 capture from gas flow, and particularly in comparing metrics across different microalgae strains, is a lack of methodological consistency across studies. Some studies rely on direct measurement of changes in CO2 concentration at the cultivation system’s inlet and outlet, while others use computational methods based on biomass accumulation, assuming a fixed 50% organic carbon content in algae [49]. However, methods that rely on directly measuring CO2 concentration at the bioreactor inlet and outlet have a significant limitation: they do not account for the amount of gas dissolved in the nutrient medium that is not assimilated by microalgae during cultivation [50,51,52]. In our study, we directly measured the synthesized organic carbon. The sole source of inorganic carbon for synthesis was the CO2 from the gas flow. Rates of CO2 fixation calculated by direct measurement of the OC (dFCO2) and via biomass stoichiometric composition (sFCO2), were in close agreement, with an average difference within ± 10% (Table 2). This close agreement is important for further research.
Our results are consistent with data from studies on Chlorella sorokiniana IPPAS C-1, Neochlorella semenenkoi IPPAS C-1210, and Desmodesmus armatus ARC-06 cultivated in a PBR at an elevated CO2 levels, which showed similar patterns [53,54]. A comparable trend was reported for Synechococcus PCC 7002 and Synechocystis PCC 6803 strains, with maximum CO2 uptake being achieved at the exponential growth phase followed by a decrease [55]. However, significant differences in CO2 sequestration efficiency between different species have been reported. For instance, [53] found that the average CDSE value was 25.4% for C. sorokiniana, 22.7% for N. semenenkoi, and 12.8% for D. armatus grown under the same cultivation conditions in a PBR.
Despite extensive studies on the carbon dioxide sequestration efficiency (CDSE) of microalgae, direct comparison of resultant data across the literature remains challenging. This difficulty arises from a multitude of factors that directly influence CO2 uptake efficiency, in addition to species-specific characteristics. These factors include the following: gas flow rate [56]; CO2 concentration and the presence of impurities in the gas stream [57]; the CO2: O2 ratio during photosynthesis in photobioreactors (PBRs) [5,39]; illumination parameters (light source type, regime, intensity, and spectrum) [58,59]; medium pH and mixing method for maintaining interphase contact; cultivation temperature and bioreactor design [41,54]; nutrient composition of the growth medium [60].
The CDSE reported for D. armatus cultivated in a PBR [53] is comparable to the values we obtained in an LSIC. This similarity in CDSE values between flat-panel PBRs and LSICs highlights the predictability of the ARC-06 strain’s performance, representing a key advantage for further exploration of its biotechnological potential across different cultivation systems.
5. Conclusions
The study reveals key differences in the production characteristics of Desmodesmus armatus ARC-06 and Tribonema minus ARC-10 strains under varying CO2 concentrations. While D. armatus exhibited high tolerance to CO2 deficiency, T. minus demonstrated a pronounced ability to accelerate carbon dioxide assimilation at elevated levels, as evidenced by its significantly higher biomass and organic carbon content compared to D. armatus.
The high CO2 sequestration efficiency of T. minus, which is almost twofold higher than that of D. armatus, makes it a promising candidate for carbon capture technologies. Conversely, D. armatus appears better suited for systems with natural aeration. This species demonstrates robust and predictable growth under standard conditions, maintaining balanced productivity across a wide range of CO2 levels.
Calculations of CO2 fixation rates based on both direct measurements of organic carbon (dFCO2) and the stoichiometric chemical composition of biomass (sFCO2) closely agreed, thereby facilitating further applied research in this area.
The identified characteristics of the two strains are fully consistent with the concept of bioprospecting, whereby primary biotechnological screening facilitates the planning of subsequent studies. This study expands our understanding of the species-specific characteristics of the studied microalgae in the context of CO2 bio-capture, and paves the way for optimizing technologies for their large-scale cultivation. The results demonstrate the need for a differentiated strain selection based on specific biotechnological objectives.
The distinct reactions of D. armatus and T. minus to 1.5% CO2 highlight their divergent potential for CCUS. Although this study provides a clear comparison between two physiologically relevant concentrations (low and high), future work should focus on a range of higher concentrations (5–15%) which are typical of industrial flue gases. Such an approach would allow for the precise determination of inorganic carbon fixation thresholds in T. minus and facilitate the investigation of the underlying biochemical adaptation mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12090319/s1, Figure S1: The overall design of our project; Figure S2: LED parameters and the spectral composition of the light; Figure S3: General scheme of the cultivation setup—laboratory system for intensive cultivation; Figure S4: Laboratory system for intensive cultivation (bubbling element).
Author Contributions
Conceptualization, methodology, N.V.L. and M.A.S.; validation, formal analysis, visualization, supervision, D.A.G., M.A.S. and N.V.L.; investigation, D.A.G., G.A.S., E.V.Z., M.A.G., B.Y.B., E.A.F. and N.V.L.; writing—original draft preparation, D.A.G., M.A.S., E.V.Z. and N.V.L.; writing—review and editing, D.A.G., M.A.S. and N.V.L.; project administration, funding acquisition, N.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out at the expense of a grant from the Russian Science Foundation no. 24-27-20026, https://rscf.ru/project/24-27-20026/ (accessed on 1 September 2025).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to their colleagues who are passionate about Arctic research and for their invaluable assistance in the collection of Arctic phytoplankton samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CO2 | Carbon dioxide |
| OC | Organic carbon |
| CDSE | Carbon dioxide sequestration efficiency |
| GAM | Gas–air mixture |
| FCO2 | Carbon dioxide fixation |
| LSIC | Laboratory system for intensive cultivation |
| DW | Dry weight |
References
- Udaypal; Goswami, R.K.; Mehariya, S.; Verma, P. Advances in microalgae-based carbon sequestration: Current status and future perspectives. Environ. Res. 2024, 249, 118397. [Google Scholar] [CrossRef]
- Shah, M.A.; Shibiru, A.L.; Kumar, V.; Srivastava, V.C. Carbon dioxide conversion to value-added products and fuels: Opportunities and challenges: A critical review. Int. J. Green Energy 2025, 22, 1532–1551. [Google Scholar] [CrossRef]
- Lobus, N.V.; Kulikovskiy, M.S. The Co-Evolution Aspects of the Biogeochemical Role of Phytoplankton in Aquatic Ecosystems: A Review. Biology 2023, 12, 92. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Abdur Razzak, S.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Zhu, T. Research progress and application of carbon sequestration in industrial flue gas by microalgae: A review. J. Environ. Sci. 2025, 152, 14–28. [Google Scholar] [CrossRef]
- Lobus, N.V.; Knyazeva, M.A.; Popova, A.F.; Kulikovskiy, M.S. Carbon Footprint Reduction and Climate Change Mitigation: A Review of the Approaches, Technologies, and Implementation Challenges. C 2023, 9, 120. [Google Scholar] [CrossRef]
- Santos Ballardo, D.U.; Rossi, S. (Eds.) Microalgae as Promising Source of Commercial Bioproducts; Developments in Applied Phycology; Springer Nature: Cham, Switzerland, 2025; Volume 14, ISBN 978-3-031-86432-2. [Google Scholar]
- Silva, M.; Geada, P.; Pereira, R.N.; Teixeira, J.A. Microalgae biomass–A source of sustainable dietary bioactive compounds towards improved health and well-being. Food Chem. Adv. 2025, 6, 100926. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Siddhnath; Surasani, V.K.R.; Singh, A.; Singh, S.M.; Hauzoukim; Murthy, L.N.; Baraiya, K.G. Bioactive compounds from micro-algae and its application in foods: A review. Discov. Food 2024, 4, 27. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Neelakantan, N.; Tessman, M.; Sherman, S.D.; Griffin, G.; Pomeroy, R.; Mayfield, S.P.; Burkart, M.D. Flexible polyurethanes, renewable fuels, and flavorings from a microalgae oil waste stream. Green Chem. 2020, 22, 3088–3094. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Dutta, S.; Kataki, S.; Banerjee, I.; Pohrmen, C.B.; Jaiswal, K.K.; Jaiswal, A.K. Microalgal biorefineries in sustainable biofuel production and other high-value products. New Biotechnol. 2025, 87, 39–59. [Google Scholar] [CrossRef]
- Sharma, A.K.; Jaryal, S.; Sharma, S.; Dhyani, A.; Tewari, B.S.; Mahato, N. Biofuels from Microalgae: A Review on Microalgae Cultivation, Biodiesel Production Techniques and Storage Stability. Processes 2025, 13, 488. [Google Scholar] [CrossRef]
- Rashad, M.A.; Jamil, F.; Hussain, M.; Inayat, A.; Akhter, P.; Hamayun, M.H.; Ahsan, A.; Park, Y.-K. Zero-carbon solution: Microalgae as a low-cost feedstock for fuel production and carbon sequestration. Crit. Rev. Environ. Sci. Technol. 2025, 55, 1249–1272. [Google Scholar] [CrossRef]
- Tiwari, T.; Kaur, G.A.; Singh, P.K.; Balayan, S.; Mishra, A.; Tiwari, A. Emerging bio-capture strategies for greenhouse gas reduction: Navigating challenges towards carbon neutrality. Sci. Total Environ. 2024, 929, 172433. [Google Scholar] [CrossRef]
- Cruz, T.J.T.; Calixto, G.Q.; Câmara, F.R.d.A.; Teixeira, D.I.A.; Braga, R.M.; Pergher, S.B.C. Cultivation of Chlorella sp. in a Closed System Using Mining Wastewater and Simulated Flue Gas: Biomass Production and CO2 Fixation Potential. Sustain. Chem. 2025, 6, 11. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Cui, H.; Yang, Z.; Lu, Z.; Wang, Q.; Liu, J.; Song, L. Combination of utilization of CO2 from flue gas of biomass power plant and medium recycling to enhance cost-effective Spirulina production. J. Appl. Phycol. 2019, 31, 2175–2185. [Google Scholar] [CrossRef]
- Li, G.; Xiao, W.; Yang, T.; Lyu, T. Optimization and Process Effect for Microalgae Carbon Dioxide Fixation Technology Applications Based on Carbon Capture: A Comprehensive Review. J. Carbon Res. 2023, 9, 35. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Yang, L.; Huang, K.-X.; Chen, D.-Z.; Gao, F. Mechanisms and application of microalgae on removing emerging contaminants from wastewater: A review. Bioresour. Technol. 2022, 364, 128049. [Google Scholar] [CrossRef]
- Ethiraj, S.; Samuel, M.S.; Indumathi, S.M. A comprehensive review of the challenges and opportunities in microalgae-based wastewater treatment for eliminating organic, inorganic, and emerging pollutants. Biocatal. Agric. Biotechnol. 2024, 60, 103316. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Shi, W.; Deng, C.; Chen, C.; Feng, S. Global evaluation of carbon neutrality and peak carbon dioxide emissions: Current challenges and future outlook. Environ. Sci. Pollut. Res. 2022, 30, 81725–81744. [Google Scholar] [CrossRef]
- Ma, Z.; Cheah, W.Y.; Ng, I.-S.; Chang, J.-S.; Zhao, M.; Show, P.L. Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.; Nguyen, T.H.P. Sustainability of the four generations of biofuels—A review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Scapini, T.; Woiciechowski, A.L.; Manzoki, M.C.; Molina-Aulestia, D.T.; Martinez-Burgos, W.J.; Fanka, L.S.; Duda, L.J.; da Silva Vale, A.; de Carvalho, J.C.; Soccol, C.R. Microalgae-mediated biofixation as an innovative technology for flue gases towards carbon neutrality: A comprehensive review. J. Environ. Manag. 2024, 363, 121329. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Msigwa, G.; Tang, K.H.D.; Yap, P.-S. Implications of COVID-19 on global environmental pollution and carbon emissions with strategies for sustainability in the COVID-19 era. Sci. Total Environ. 2022, 809, 151657. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Levine, I.A. Algae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. [Google Scholar]
- Badary, A.; Hidasi, N.; Ferrari, S.; Mayfield, S.P. Isolation and characterization of microalgae strains able to grow on complex biomass hydrolysate for industrial application. Algal Res. 2024, 78, 103381. [Google Scholar] [CrossRef]
- Labara Tirado, J.; Herdean, A.; Ralph, P.J. The need for smart microalgal bioprospecting. Nat. Prod. Bioprospect. 2025, 15, 7. [Google Scholar] [CrossRef]
- Lobus, N.V.; Glushchenko, A.M.; Osadchiev, A.A.; Maltsev, Y.I.; Kapustin, D.A.; Konovalova, O.P.; Kulikovskiy, M.S.; Krylov, I.N.; Drozdova, A.N. Production of Fluorescent Dissolved Organic Matter by Microalgae Strains from the Ob and Yenisei Gulfs (Siberia). Plants 2022, 11, 3361. [Google Scholar] [CrossRef]
- Yuorieva, N.; Sinetova, M.; Messineva, E.; Kulichenko, I.; Fomenkov, A.; Vysotskaya, O.; Osipova, E.; Baikalova, A.; Prudnikova, O.; Titova, M.; et al. Plants, Cells, Algae, and Cyanobacteria In Vitro and Cryobank Collections at the Institute of Plant Physiology, Russian Academy of Sciences—A Platform for Research and Production Center. Biology 2023, 12, 838. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabrielyan, A.K.; Bobrovnikova, L.A.; Bedbenov, V.S.; Starikov, A.Y.; Zorina, A.A.; Gabel, B.V.; Los, D.A. Laboratory System for Intensive Cultivation of Microalgae and Cyanobacteria. Russ. J. Plant Physiol. 2023, 70, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, D.A.; Gabel, B.V.; Sinetova, M.A.; Gabrielian, A.K.; Markelova, A.G.; Shcherbakova, N.V.; Los, D.A. Optimization of CO2 Supply for the Intensive Cultivation of Chlorella sorokiniana IPPAS C-1 in the Laboratory and Pilot-Scale Flat-Panel Photobioreactors. Life 2022, 12, 1469. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Aghaalipour, E.; Akbulut, A.; Güllü, G. Carbon dioxide capture with microalgae species in continuous gas-supplied closed cultivation systems. Biochem. Eng. J. 2020, 163, 107741. [Google Scholar] [CrossRef]
- Tréguer, P.J.; Sutton, J.N.; Brzezinski, M.; Charette, M.A.; Devries, T.; Dutkiewicz, S.; Ehlert, C.; Hawkings, J.; Leynaert, A.; Liu, S.M.; et al. Reviews and syntheses: The biogeochemical cycle of silicon in the modern ocean. Biogeosciences 2021, 18, 1269–1289. [Google Scholar] [CrossRef]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Adapting from Low to High: An Update to CO2-Concentrating Mechanisms of Cyanobacteria and Microalgae. Plants 2023, 12, 1569. [Google Scholar] [CrossRef]
- Iñiguez, C.; Capó-Bauçà, S.; Niinemets, Ü.; Stoll, H.; Aguiló-Nicolau, P.; Galmés, J. Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 2020, 101, 897–918. [Google Scholar] [CrossRef]
- Catherall, E.; Musial, S.; Atkinson, N.; Walker, C.E.; Mackinder, L.C.M.; McCormick, A.J. From algae to plants: Understanding pyrenoid-based CO2-concentrating mechanisms. Trends Biochem. Sci. 2025, 50, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Ubando, A.T.; Anderson, S.; Ng, E.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life cycle assessment of microalgal biorefinery: A state-of-the-art review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef]
- Leflay, H.; Pandhal, J.; Brown, S. Direct measurements of CO2 capture are essential to assess the technical and economic potential of algal-CCUS. J. CO2 Util. 2021, 52, 101657. [Google Scholar] [CrossRef]
- de Godos, I.; Mendoza, J.L.; Acién, F.G.; Molina, E.; Banks, C.J.; Heaven, S.; Rogalla, F. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour. Technol. 2014, 153, 307–314. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.-F.; Sun, Z.-L. Mass transfer characteristics and effect of flue gas used in microalgae culture. Appl. Microbiol. Biotechnol. 2022, 106, 7013–7025. [Google Scholar] [CrossRef]
- Hajinajaf, N.; Fallahi, A.; Eustance, E.; Sarnaik, A.; Askari, A.; Najafi, M.; Davis, R.W.; Rittmann, B.E.; Varman, A.M. Managing carbon dioxide mass transfer in photobioreactors for enhancing microalgal biomass productivity. Algal Res. 2024, 80, 103506. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Savinykh, G.A.; Zadneprovskaya, E.V.; Goncharova, M.A.; Markelova, A.G.; Gabrielian, A.K.; Gabel, B.V.; Lobus, N.V. Productivity and Carbon Utilization of Three Green Microalgae Strains with High Biotechnological Potential Cultivated in Flat-Panel Photobioreactors. Phycology 2025, 5, 43. [Google Scholar] [CrossRef]
- Gabrielyan, D.A.; Sinetova, M.A.; Gabel, B.V.; Gabrielian, A.K.; Markelova, A.G.; Rodionova, M.V.; Bedbenov, V.S.; Shcherbakova, N.V.; Los, D.A. Cultivation of Chlorella sorokiniana IPPAS C-1 in Flat-Panel Photobioreactors: From a Laboratory to a Pilot Scale. Life 2022, 12, 1309. [Google Scholar] [CrossRef]
- Jones, C.M.; Innes, S.; Holland, S.; Burch, T.; Parrish, S.; Nielsen, D.R. In Situ, High-Resolution Quantification of CO2 Uptake Rates via Automated Off-Gas Analysis Illuminates Carbon Uptake Dynamics in Cyanobacterial Cultures. Biotechnol. Bioeng. 2025, 122, 594–605. [Google Scholar] [CrossRef]
- Banerjee, S.; Dasgupta, S.; Atta, A.; Das, D.; Dayal, D.; Malik, S.; Kumar, H.; Kishore, S.; Rustagi, S.; Almutary, A.G. Flow Rate Optimization in a Flat-Panel Photobioreactor for the Cultivation of Microalgae for Mitigating Waste Gas. Water 2023, 15, 2824. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Ryndin, K.G.; Kumar, V.; Vlaskin, M.S. The Influence of Elevated CO2 Concentrations on the Growth of Various Microalgae Strains. Plants 2023, 12, 2470. [Google Scholar] [CrossRef] [PubMed]
- Aljabory, M.N.; Alhaboubi, N.A. Green Solutions for CO2 Mitigation: Exploring Microalgae-Based Carbon Capture and Utilization Technologies. J. Biotechnol. Res. Cent. 2025, 19, 52–64. [Google Scholar] [CrossRef]
- Morais, M.G.; Rosa, G.M.; Moraes, L.; Lopes, L.C.; Costa, J.A.V. Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization. Membranes 2025, 15, 205. [Google Scholar] [CrossRef]
- Yadav, D.K.; Yadav, M.; Rani, P.; Yadav, A.; Bhardwaj, N.; Bishnoi, N.R.; Singh, A. Screening of best growth media for Chlorella vulgaris cultivation and biodiesel production. Biofuels 2024, 15, 271–277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).