Abstract
The standard methods for sediment phosphorus (P) fractionation are impractical for use with suspended solids due to the inherent difficulties associated with collecting sufficient sample quantities for analysis. To allow the fractionation analysis of small quantities of suspended solids or sediment, we developed a P-microfractionation (P-MF) method and evaluated the minimum sample size threshold. The dry mass threshold is likely <1.0 g for Utah Lake suspended solids and between 0.35 and 0.99 g for Utah Lake sediments, though we recommend experimentation to refine these thresholds for other locations, as Utah Lake sediment P concentrations are high (~1000 mg kg−1). We estimated dry mass using duplicate samples, as drying a sample changes the P fractions. We show that Utah Lake suspended solids have a significantly higher P content across most P fractions compared to those in sediments, emphasizing the importance of considering suspended solids when managing water nutrient levels in eutrophic water bodies. P-MF has the potential to enable researchers to use reasonably sized water samples to assess the P sorption behavior of suspended solids, a measurement not typically performed.
1. Introduction
1.1. Background
Phosphorus (P) geochemistry is complex due to the wide variety of phases in which P occurs. For example, P forms mineral phases with calcium (Ca), iron (Fe), and aluminum (Al); it can also form organophosphorus salts through interactions with organic compounds or sorbs onto solid surfaces through surface or cation exchange reactions [1,2]. These solid phases, known as P fractions, offer insight into the bioavailability of P as a nutrient to support growth and sustain life, especially in aquatic systems. Most work on P fractionation has been performed to support agriculture [2,3,4,5], though there have been some applications for reservoir and lake sediments [2,6,7,8,9,10,11,12]. Acioly et al. [13] provided quantitative data on trace elements in water and sediments, including human health risk estimates. This study supports the relevance of physicochemical sediment characterization, central to the P fractionization framework.
A variety of fractionation procedures have been developed to quantify the P fractions present in a given system, such as the methods by Kovar and Pierzynski [2], Petersen and Corey [14], and those summarized by Kleinman et al. [3]. Although these methods use different processes and emphasize P storage across various fractions, they all follow a similar procedure: a soil or sediment sample is collected and then subjected to a series of physical or chemical treatments in a series of sequential extractions to isolate and measure P within the selected P fractions.
Existing methods for assessing P fractions in sediments and soils generally require a minimum of 1 to 2 g of a dried sample to perform an analysis [2], with some methods requiring as little as 0.5 g [15]. Sample size is not typically an issue because soils and sediments are generally abundant and are easily sampled. However, this is not the case for suspended solids, as most freshwater bodies have suspended solids concentrations on the order of a few tens to a few hundreds of mg L−1. This means that the number of liters needed to acquire a single gram of dry sediment from a typical water body could be in double or triple digits. This study presents the development and implementation of a P-MF method that uses significantly smaller sample sizes to improve the feasibility of measuring and analyzing the P-fractional content of suspended solids in aquatic systems. We use Utah Lake sediments and suspended solids as a case study, both because our current research focuses on Utah Lake and the fact that Utah Lake sediments have elevated P concentrations.
1.2. P Fractionation of Suspended Solids
Sequential extraction is often used to measure P fractions in soils and sediments, but is rarely applied to suspended solids because their low concentration in surface waters requires very large samples for analysis. Researchers have consequently resorted to conducting fractionation on bottom sediments as an analog for describing the P fractions of suspended solids [16]. Such estimates, however, are not necessarily representative of the actual composition and behavior of P fractions in suspended solids. This is because the biogeochemical and physical conditions between the bottom sediments and the water column can differ markedly, particularly in the photic zone; the suspended solids generally have a smaller particle distribution than sediments; and most sediments are anoxic while suspended solids exist in an aerobic environment [17]. Direct analysis of suspended solids is preferable over proxy analysis of lakebed or riverbed sediments when suspended solids are the primary subject of interest.
Challenges arise when performing a sequential extraction on small samples. For example, sediment heterogeneity can be magnified by small samples, in which a few particles of a given mineral can significantly change the results. Another issue is the amount of the target element, P, remaining in the fluids at the end of an extraction step, which, in small samples, can be significant compared to the quantities in the solid portion of the sample. Additionally, sample loss between fractionation steps can cause the extraction of already dilute extractants to become less accurate with each successive step and thereby produce skewed results that are not representative of their true fractions [18].
Parallel extraction procedures have been developed and used as an alternative method to address some of these issues [19]. Parallel extraction methods use the same leaching reagents as sequential extraction, but the methods are applied to separate subsamples rather than using a consecutive series of extractions on a single sample. This approach requires an understanding of the aggressivity of each reagent and extraction step, as stronger leaching reagents often extract the target phases of weaker reagents in addition to their own phases, or they may not extract the weaker phase at all, making data analysis difficult. Once a hierarchy of aggressivity is determined for a parallel extraction, a user can correct the results to quantify the extraction amounts of the more aggressive steps by considering the amounts likely extracted from the weaker phases or fractions [19].
Parallel extraction may appear to be a favorable method for evaluating suspended solids because of its ability to be applied to smaller samples. However, each parallel extraction step requires a separate subsample and therefore requires an initial sample that is multiple times larger than the amount required for sequential extraction. In addition to requiring significantly more material, parallel extraction is also less desirable because it generates results that are more difficult to interpret due to sample variation and the need for correction factors.
Researchers have published successful efforts to sequentially extract P from suspended solids by collecting between 2.5 and 5.0 L of sample water and then either centrifuging or settling the samples to obtain enough material to perform extractions. These samples, however, were taken from rivers or streams immediately following a storm event, and consequently had very high levels of suspended solids to ensure that enough sample was collected for analysis [7,8,20]. Applying these approaches to typical lake and reservoir waters is difficult as they would require water volumes that are 10 to 100 times as large as these river samples to collect the same amount of solid material.
1.3. Utah Lake Target Study
Because traditional approaches for measuring P fractions from suspended solids are impractical for most surface waters, we developed a P-MF method that requires minimal solid material for analysis, with samples of sizes that can be applied to the moderately turbid waters often encountered in freshwater lakes. To demonstrate the effectiveness of our method, we applied it to Utah Lake, a large, shallow freshwater lake in north-central Utah with waters that are both eutrophic and turbid. Utah Lake’s suspended solids typically fall between 30 and 75 mg L−1 (Figure 1). While these levels are higher than is typically seen in most lakes and reservoirs, these levels are not unusual [21,22,23]. However, obtaining enough material for traditional sequential extraction methods (approximately 1 to 2 g samples at minimum), even at these concentrations, would still require a very large water sample [9,11].
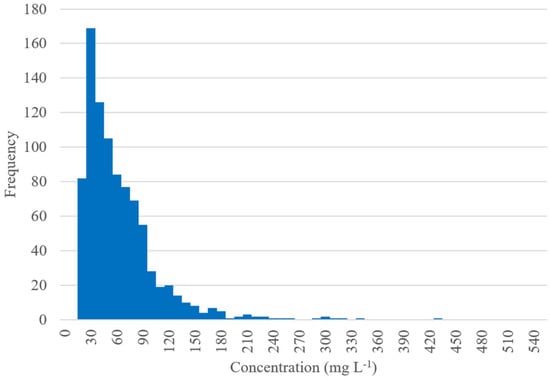
Figure 1.
Utah Lake total suspended solids from the Ambient Water Quality Management System (AWQMS) database provided by the Utah Department of Environmental Quality, depicting 936 lake water samples from 1989–2019. Utah Lake’s shallow depth, combined with wind-induced waves and lakebed bioturbation, causes the lake to experience elevated levels of suspended solids, falling mostly in the 30 to 75 mg L−1 range.
For example, lake water with suspended solids concentrations of 75 to 30 mg L−1, like that of Utah Lake, requires about 13 to 33 L of water, respectively, to acquire a 1 g sample for analysis, and double that, or 26 to 66 L, to acquire a 2 g sample. This means that it is impractical to perform P fractionation on suspended solids from Utah Lake using traditional methods even though the lake is relatively turbid, as collecting ~20 to 50 L of water for a single sample is unrealistic for most assessment purposes. The majority of water bodies have even lower concentrations of suspended solids, making the collection of 1 to 2 g samples even more difficult [23]. The development of a P-MF method using Utah Lake samples serves as a useful demonstration for adapting and applying P-MF to other surface waters.
1.4. Research Overview
This study developed a P-MF method that accurately measures P fractions from small quantities of suspended solids and sediment. P-fractionation methods are standardized and used extensively, particularly in agriculture. The method outlined in this study is based on previously published fractionation methods that are valid for inorganic solids under aerobic conditions, with our study focusing on determining the minimum sample size that can be reliably used for analysis.
In Section 2.1, we define the P fractions examined by our method, describe how those fractions are quantified, and explain why we selected those fractions for analysis. In Section 2.2, we discuss the use of lakebed sediments and suspended solids from Utah Lake as the primary study material for our method development. In Section 2.3, we explain how we adapted an existing P fractionation method to assess a variety of small sample sizes, and in Section 2.4, we summarize the steps of our P fractionation method along with the calculations required to convert the results into units of mass concentration.
In Section 3.1, we present our baseline results from performing our P-MF method on different quantities of lakebed sediment and suspended solids from Utah Lake. This includes subsections on sample detection limits, the dry mass of our samples, sample controls, and the extraction results for both total P and the P fractions measured. In Section 3.2, we use one-way ANOVA analysis to determine if the observed differences in sample size were statistically significant. In Section 3.3, we use post-hoc tests to determine which sample sizes produced anomalous results.
In Study Limitations and Suggestions for Future Research, we summarize the methods and results of this study and conclude by identifying some of the P-MF method and study limitations, along with suggestions for future research.
2. Methods
2.1. Fraction Descriptions
We based our P-MF method on the method used by Casbeer et al. [11], with additional details found in Casbeer [24]. We selected this method because it is designed for aerobic conditions (which should hold for suspended solids collected in most natural waters) and includes the assessment of the water-soluble fraction. This method does not separate Al-bound P from Fe- and Mg-bound P as separate fractions but rather groups them into a single fraction. These fractions are typically released under anaerobic conditions and can generally be treated as one phase when evaluating ecological and environmental impacts.
We quantified five fractions: water-soluble P (), loosely sorbed P (), Fe- and Al-bound P (), Ca-bound P (), and residual P (). The sum of these fractions is used to quantify total P (). As a check, Casbeer suggests that duplicate samples be digested to determine , which can then be compared to the integrated value to quantify potential errors within sample variability and act as a quality assurance check.
The Casbeer et al. [11] method uses a sample size of 10 g and performs a set of sequential extraction steps. In the first step, 20 mL of deionized (DI) water is mixed with the sediment sample, centrifuged, and then filtered to produce the supernatant for measuring . In the second step, 20 mL of 1 M KCl is mixed with the sediment and is shaken for 2 h before being centrifuged and filtered to produce the supernatant for measuring . In the third step, 20 mL of 0.1 M NaOH is mixed with the sediment and is shaken for 17 h before being centrifuged and filtered to produce the supernatant for measuring . In the fourth step, 20 mL of 0.5 M HCl is mixed with the sediment and is shaken for 24 h before being centrifuged and filtered to produce the supernatant for measuring . In the fifth step, the remaining sediment is subjected to persulfate digestion to produce the supernatant for measuring .
The fraction represents P sorbed to the solid surface as well as P contained in the pore water of the sample. is of significant interest to lake managers as P in this fraction is bioavailable, readily moves between solid and aquatic phases, and can strongly influence water column concentrations of dissolved P.
The fraction, while called “loosely sorbed P”, represents the P that can be extracted by ion exchange. This is P sorbed to the surface by electrostatic forces based on ion charge [25]. The and fractions are readily available to the water column and will release or sorb P easily with changing water chemistry and P concentrations. The concentration of P in the solids is a function of P concentration in the water column when sorption is at equilibrium [25]. For natural waters with high P concentrations in the sediments and suspended solids, this phenomenon can result in a sorption-dominated system that behaves as a buffered system, where water column P concentrations are relatively insensitive to P inflows as the sediments and suspended solids act as a large reservoir for both the uptake and release of P. This effect is strengthened if sediments are continually mixed and suspended in the water column, allowing the sorbed P to come to equilibrium. In these cases, the sediments act as a P reservoir with the ratio of the concentration in the solid phase to the concentration in the aquatic phase generally on the order of several hundred [26].
The fraction represents P that is co-precipitated with Fe and Al minerals. These minerals are relatively insoluble under oxic conditions but are significantly more soluble under anoxic conditions, such as those found within lakebed sediments. For example, the stability constant for Fe(OH)2 which is the dominant species under anoxic conditions, is several orders of magnitude higher than that of Fe(OH)3, which is the dominant species under aerobic conditions [27]. This higher solubility of iron hydroxide in anoxic conditions means that the fraction can be released to the water column if anoxic sediments are disturbed. If the aerobic water column is saturated with respect to Fe and Al minerals or other similar minerals, then these minerals will re-precipitate and potentially co-precipitate P [28]. This P fraction in the sediment can act as a P source for the water column if the water column is not saturated with respect to Fe or Al as most sediments are anoxic and P in the pore water can diffuse up into the water column. If the water column contains Fe or Al, the fraction can scavenge P from the water column when evaporation concentrates mineral species and likewise acts as a source of P when anoxic sediments are disturbed, or through the slower diffusion transport from anoxic sediments.
The fraction represents Ca minerals that contain P. Utah Lake is unique in that it is near saturation levels for calcite because of high levels of evaporation and because of tributaries that flow through carbonate-dominated geology. Studies have shown that over 90% of the P input to Utah Lake does not exit the lake, with various studies attributing this loss of P to coprecipitation with Ca minerals or sorption on solids [6,9,26]. The fraction is not available to the water column under most circumstances due to the lake’s high pH, though in periods when the water is not saturated with respect to calcite, such as from springtime inflows, these minerals can dissolve, releasing P.
The fraction represents the P that remains following the extraction of all other fractions including the P in residual mineral phases and organic materials, which are not generally available to the water column. While the fraction can sometimes be assumed to represent organically bound P, the quantification of specific organic P subfractions would require the use of alternative analytical methods. While it would be interesting to evaluate different types of organically bound P fractions associated with suspended solids (such as from different types of algae or biochar), doing so is beyond the scope of our method.
2.2. Study Materials
The primary materials for this study included suspended solids and lakebed sediment samples from Utah Lake, which we used to develop and test our method’s effectiveness on small quantities of both suspended and settled material. We made the decision to use Utah Lake sediment for several reasons. First, Utah Lake is a eutrophic lake with sediments that are known to have a high P content of between 600 and 1500 mg kg−1 [6,9,29], making them ideal for P fractionation experiments. Second, Utah Lake is a turbid lake with high levels of suspended solids (Figure 1), which are presumed to be composed primarily of lakebed sediments. The lake’s high turbidity allowed us to collect a solid sample with less water than would be needed for most other water bodies. Third, knowledge of the P fractions associated with the lake’s suspended solids would be helpful for lake management decisions, as the lake has been held under public scrutiny due to its recurrent harmful algal blooms [26,30,31].
2.3. Method Development
Our main objective was to create a P-MF method suitable for measuring P fractions in very small quantities of suspended solids and sediments. Suspended solids can be collected from a water sample by either (1) centrifuging the sample and then decanting the excess water or (2) allowing particulate matter to settle to the bottom of the sampling container and then siphoning off the excess water. Filtration is not recommended as suspended solids and extractants can be retained by the filter and thereby introduce complications introduced by their removal.
Centrifugation is generally considered the quickest method for separating suspended solids from water, but doing so requires a centrifuge that can process large quantities of water, and even then, centrifugation can take hours to produce enough material for analysis if the concentration of suspended solids is low. If a cold room or a sufficiently large refrigerator is available, then allowing suspended sediments to settle out is a viable option and can be used as a pre-concentration step prior to centrifugation. Regardless of the method used, the quantity of wet solids required depends on the target amount of dry material for analysis, as well as the desired number of replicate samples.
In small samples, the pore fluids can contain significant quantities of P compared to the amount of P in the sample, while these same amounts are insignificant with larger samples. To address this issue, we added wash steps to address the issue of P that is left behind in the pore fluids in smaller samples. For small sample sizes, the amounts of pore fluid and extractant fluid are similar, and thus, the amount of P in the pore fluid remaining after an extraction step could be significant in mass calculations. This is not the case in the published methods, where extractant volumes are significantly larger than pore volumes [11]. The addition of this wash step helps ensure that extracted P is attributed to the correct fraction and reduces the likelihood of P sorbing back onto the sediments between fractionation steps.
We experimentally determined a recommended minimum sample size threshold by subjecting an array of increasingly smaller quantities of P-rich sediments to our P-MF method. The wet sample sizes we evaluated included 1.4 g, 0.5 g, 0.4 g, 0.3 g, and 0.2 g samples, with the 1.4 g samples acting as a standard, given that many fractionation methods recommend between 1 and 2 g samples for analysis.
Besides sample size, the primary modifications we made to the method by Casbeer et al. [11] included adjusting the ratio of the extractant to the sample size, the addition of washes after collecting the supernatant from each sequential extraction, and replacing the persulfate digestion step with microwave-assisted acid digestion.
In summary, we evaluated different sediment sample sizes, added washes between extractions, and adjusted extractant and wash volumes. We then followed these modified fractionation procedures to determine the minimum sample size that could be used to reliably ascertain P fractions in small samples, such as those obtained from suspended sediments. In the following sections, we present details on the P-MF method and recommend sample sizes.
2.4. Method Description
The Supplementary Materials contain a detailed description of the final method. We summarize and describe the method in this section, but do not provide all the required details.
Our P-MF procedure quantified five P fractions: , , , , and . The required extractants for our method are DI water for , 1.0 M KCl for , 0.1 M NaOH for , and 0.5 M HCl for . The remaining sample is then microwave digested to measure . We used triplicate measurements, blanks, standard sample sizes, and as quality control checks.
We measured the first four fractions by sequentially applying extractants to a sediment sample and collecting and analyzing the resulting supernatants to determine the mass of P extracted. These are the same extractants used by Casbeer et al. [11], though with different volume ratios. While Casbeer et al. used an extractant-to-sample ratio of 2 mL to 1 g of sediment, we opted to use a ratio of 10 mL of extractant per 1 g of sample material for our P-MF method. This constant ratio helped provide comparability among the different sample sizes during the method development.
We also washed each sample between extraction steps in ratios of 10 mL of the wash solution per 1 g of wet sample material. After a wash, we collected the resulting supernatants and combined the volume of wash solution with the supernatant of the P extractant for that step. We used washes of 1.0 M KCl for each fraction except the , for which we used DI water as the wash. We included the wash volumes in mass computations to determine the mass of P extracted from the solids at each step.
We analyzed P concentrations in the extracted supernatant using a Thermo Scientific iCAP 7400 ICP-OES (Themo Fisher Scientific, Bremen, Germany). We added deionized water to the sample when the combined supernatant volume for a given fraction was insufficient for analysis. This additional liquid volume was accounted for in P mass computations.
constituted the final fraction and required digestion of any remaining solids, which are often organic in nature but can also be comprised of other mineral forms. We found that the persulfate digestion used by Casbeer et al. [11] was insufficient to completely digest the remaining solids, and we therefore chose to use microwave-assisted acid digestion (US EPA Method 3051A) to perform this step. However, there were several instances when there was not enough remaining sediment to perform a digestion for the final residual fraction. Microwave digestion requires a minimum of 0.1 g of material, and the analysis of lesser amounts may yield inaccurate results.
ICP-OES provides measurements of elemental P in units of ppm, which is essentially equivalent to mg L−1 for the density of our aquatic and supernatant samples. These measurements include both the extractant volume and any water used for dilution when the concentration is too high or for augmentation if the sample volume is too small. Based on the Casbeer et al. [11] method, we converted ICP-OES measurements to sample P solids concentrations in mg kg−1 using the equation:
where:
= the calculated sample P concentration (mg kg−1).
= the measured aqueous P concentration (mg L−1).
= the final volume of supernatant/solution (mL).
= the dilution factor (the undiluted volume divided by the diluted volume).
= the estimated mass of dry sample subjected to P extraction (g).
The numerator on the right side of the equation is the mass of P in the analyzed liquid sample in mg, and the denominator is the original mass of the suspended solids or sediments, not the mass at the beginning of the extraction step. Care should be taken to distinguish between wet and dry sediment masses. Saturated samples, such as suspended solids and lakebed sediments, must be analyzed while wet in order to preserve their chemical integrity as drying the sample can change the chemical form of P and thus alter the P fractions. However, the dry sediment mass of the original sample is required to calculate the sediment P concentrations of the measured fractions. To measure the dry mass, we collected sample duplicates to serve as proxies for measuring the water content and dry masses of the original samples. For this study, we will refer to samples used in the analysis by their dry masses and report the resulting P fractions in mg kg−1.
The dilution factor in this equation () is included for instances when the concentration of P in the supernatant or solution is above the analytical range of the instrument (i.e., is overrange) and therefore requires dilution for P to become measurable or when samples require augmentation for analysis. When dilution is not used, the dilution factor has a value of 1, thus becoming negligible for the purposes of this equation.
3. Results and Discussion
3.1. Baseline Results
3.1.1. Detection Limits
Of the 156 samples we ran for P-MF analysis, 151 of them (97%) produced results that were within detection limits for the ICP-OES. The five exceptions were all under-range values representing water-soluble P or loosely sorbed P, both of which tended to be less prevalent fractions for Utah Lake sediments.
3.1.2. Dry Mass of Samples
For P-MF analysis, we used wet sample sizes of 1.4 g, 0.5 g, 0.4 g, 0.3 g, and 0.2 g for both our lakebed sediment and suspended solids samples. To estimate the dry mass for the P-MF samples, we dried duplicates, and we found that lakebed sediments were 70% dry mass, while our suspended solids were 32% dry mass. This resulted in dry sample sizes of 0.99 g, 0.35 g, 0.28 g, 0.21 g, and 0.14 g for our lakebed sediment samples and 0.44 g, 0.16 g, 0.13 g, 0.09 g, and 0.06 g for our suspended solids samples. Each sample size for both sediment types was tested in triplicate as a quality control measure.
3.1.3. Sample Controls
Given that most P-fractionation methods for sediment require a minimum of 1 to 2 g of dried sample for analysis [2], we aimed to have the largest sample size for both our lakebed sediment and suspended solids samples be within that range so that these samples could act as a control or ground truth for their respective solid type, i.e., sediments or suspended solids. While we achieved this for our largest lakebed sediment samples, each having a dry mass of ~1 g per sample, the water content of our suspended solids samples was much higher than expected, causing the largest samples to each have a dry mass of only 0.44 g. This sample size is consequently not large enough to act as a true control, but its inclusion still provides helpful insight for P-MF analysis.
3.1.4. Total Phosphorus Results
After measuring the dry mass of our duplicate samples, we measured the (total P) of these duplicates and found them to be in the range of 310–320 mg kg−1 for the lakebed sediments and 550–600 mg kg−1 for the suspended solids. These raw values represent the actual levels of in the lakebed sediments and suspended solids samples, as these were dried samples that had not been subjected to other extraction steps. Estimated , in the sequential samples, which is determined by the sum of the P fractions of each sample size, covered a much wider range than the raw measurements, being 180–390 mg kg−1 for the lakebed sediments and 550–1450 mg kg−1 for the suspended solids (Figure 2; Table 1).
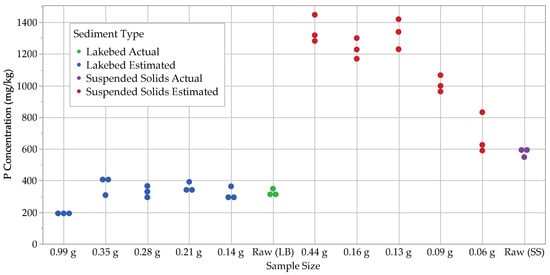
Figure 2.
Results for both actual (raw) and estimated total P.

Table 1.
P-MF results for total phosphorus (mg kg−1) with each sample size including three replicates. The controls, which had original wet masses of 1.4 g, are shaded in gray, and the raw (actual ) measurements are shown in blue numbering.
The raw measurements are used primarily to check the accuracy of the estimated values, with similar results indicating a more accurate fractionation run. Some dissimilarity should be allowed, however, as sediment is heterogenous by nature, and small sample sizes are more prone to be influenced by heterogeneity. Interestingly, the smallest sample sizes from both the lakebed sediments and suspended solids were the most similar to the raw measurements. The lakebed sediments overall were the most similar to the raw measurements, while the suspended solids varied quite markedly, particularly as sample size increased. Greater variation among suspended solids is to be expected, though, as their source materials are typically more diverse than those of lakebed sediments.
3.1.5. Results for P Fractions
Using the P-MF method described in Section 2.4, we performed a total of 150 P-fraction measurements for our lakebed sediment and suspended solids samples (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7; Table 1). As a reminder, the individual fractions included in our analysis were water-soluble P (), loosely sorbed P (), Fe- and Al-bound P (), Ca-bound P (), and residual P ().
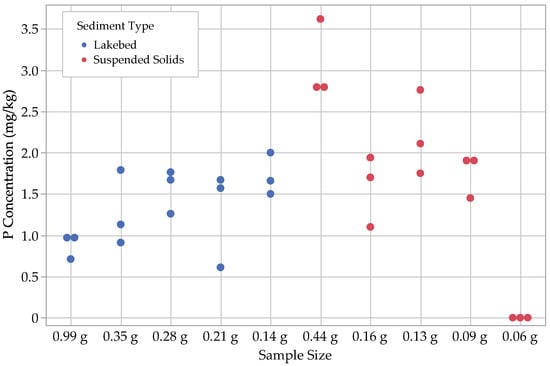
Figure 3.
Results for water-soluble P (. The 0.06 g results for suspended solids were below detection limits and are therefore set to zero.
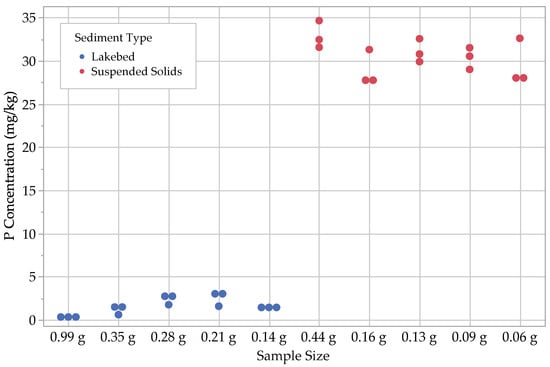
Figure 4.
Results for loosely sorbed P (.
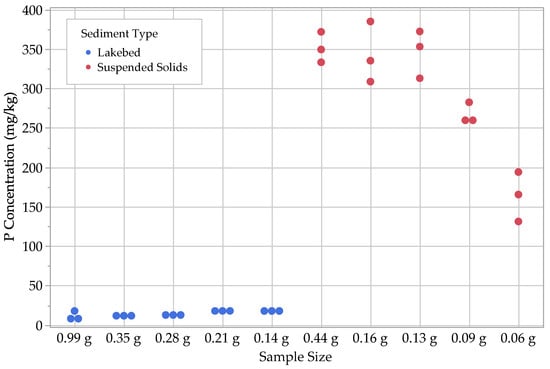
Figure 5.
Results for Fe- and Al-bound P (.
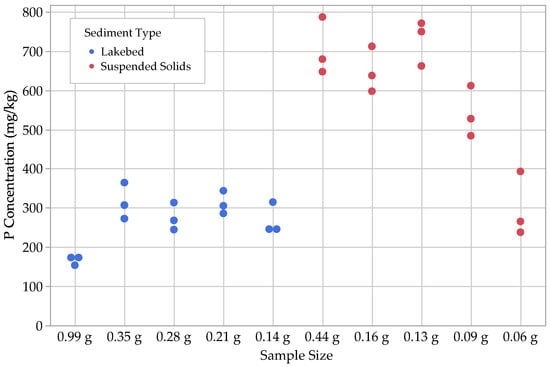
Figure 6.
Results for Ca-bound P, (.
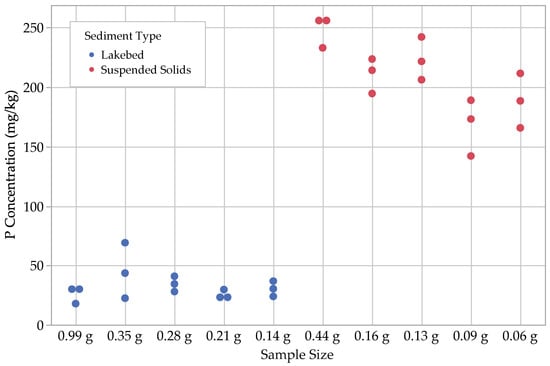
Figure 7.
Results for residual P, (.
Several trends emerged among the P fraction results, most notably that while the water-soluble P concentrations, (, were similar between the lakebed sediments and the suspended solids, all other P fractions were significantly higher in the suspended solids. This is expected as previous work has shown that smaller particles sorb P at higher concentrations. This suggests that the P contained in the suspended solids in Utah Lake may have a higher P bioavailability than lakebed sediments and may have more influence on dissolved water column P concentration from sediment–water equilibrium sorption interactions.
Another noticeable trend among the results is that the sample sizes used as controls—0.99 g for lakebed sediments and 0.44 g for suspended solids—differ visually from the other sample sizes for each fraction. The trends are different between sediment types, as the control for lakebed sediments is consistently lower than other samples, while the standard for suspended solids is consistently higher.
With regard to the lakebed sediments, we found that the concentrations of and tended to be small, ranging from 1–3 mg kg−1, with the loosely sorbed fraction being the larger of the two. The next largest fractions were , with concentrations ranging from 8–18 mg kg−1, followed by , with concentrations ranging from 18–70 mg kg−1. Ca-bound P () was by far the largest fraction for the lakebed samples, with concentrations ranging from 150–360 mg kg−1.
For suspended solids, we found that, similar to the lakebed sediments, most concentrations ranged between 1 and 3 mg kg−1. The other P fractions in the suspended solids were noticeably different from the lakebed sediments, with ranging from 28–35 mg kg−1 and ranging from 140–260 mg kg−1. The largest P fractions for the suspended solids were the Fe- and Al-bound P, , with a range of 130–390 mg kg−1 and with a range of 240–790 mg kg−1 (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7; Table 2).

Table 2.
P-MF results for individual fractions (mg kg−1) with each sample size including three replicates. The controls, which had original wet masses of 1.4 g, are shaded in gray, and under-range measurements are shown in red numbering.
It should be noted that due to unavoidable sample loss during the fractionation process, all suspended solids samples, except the 0.44 g control, yielded less than the 0.1 g needed for the residual P measurement step. While mathematical corrections were made to account for the smaller sample masses used for this step, it is possible that these results for residual P may not be accurate. This oversight would have been avoided if we had not overestimated the dry mass content of our suspended sediment samples and used larger wet samples. We therefore suggest that the dry mass be measured in duplicate sediment samples before conducting P-MF analysis.
3.2. One-Way ANOVAs
One primary objective of this study was to determine the threshold for when sample size becomes the limiting factor for measuring P fractions in small samples. Our approach for determining this threshold involved comparing the results of our standard samples to smaller sample sizes using one-way ANOVAs. This allowed us to determine if statistical differences were present in the data
We found that most of the P fractions for both lakebed sediment and suspended solids had p-values that were well below our alpha value of 0.05, indicating that the differences were statistically significant and require a need for further analyses, as it is not clear if the difference is due to sample size or sample heterogeneity. On the other hand, the fractions with no statistically significant differences between sample sizes included water-soluble P and residual P for the lakebed sediments and loosely sorbed P for the suspended solids, having p-values of 0.15, 0.26, and 0.20, respectively. These P fractions are the most important when evaluating nutrient loads as they are readily bioavailable. Sample size consequently does not appear to affect the outcome of these fractions when analyzed using our P-MF method.
3.3. Post-Hoc Tests
While one-way ANOVAs are useful for detecting whether the differences in sample size are significant, they are insufficient for determining which sample sizes are associated with significantly different P concentrations. To determine which sample sizes were significantly different from each other, we applied two post hoc tests to the data, including Student’s t test and the Tukey–Kramer HSD test (Table 3).

Table 3.
Post hoc connected letters report for P-MF samples. Rows marked in green signify fractions with large ANOVA p-values (>0.05), indicating no statistically significant differences among the sample sizes for the given sediment type.
For the lakebed sediment samples, the one-way ANOVA tests indicated that the fractions for loosely sorbed P, Fe- and Al-bound P, and Ca-bound P had statistically significant differences based on their sample sizes. Both the Student’s t and Tukey–Kramer HSD tests grouped the two largest sample sizes together (0.99 g and 0.35 g) for loosely sorbed P, suggesting that this fraction can yield consistent results down to a dry sample mass of 0.35 g. This is not the case, however, for Fe- and Al-bound P and Ca-bound P, as the largest sample size (0.99 g) was grouped by itself, while the remaining sample sizes were all grouped together. With the second largest sample size being 0.35 g, it is possible that the threshold for minimum sample size is an intermediate value between 0.35 and 0.99 g. Further experimentation is necessary to determine the value for that threshold.
For the suspended solids samples, the one-way ANOVA tests indicated that all P fractions, with the exception of loosely sorbed P, contained statistically significant differences among the results for different sample sizes. In most cases, the largest and third-largest sample sizes (0.44 g and 0.13 g) were grouped together for all P fractions. The second largest was included with these groupings for both post hoc tests on the Fe- and Al-bound P and Ca-bound P fractions, as well as for the Tukey–Kramer HSD test on the residual P fraction. This suggests that consistent results could be obtained for these fractions down to a dry sample mass of 0.13 g. Interestingly, the second-largest sample size (0.16 g) does not group with the largest and third-largest sample sizes (0.44 g and 0.13 g) for the water-soluble P fraction, which suggests that the minimum sample size threshold is >0.16 g. Again, further experimentation is necessary to determine the value for that threshold.
4. Conclusions
Study Limitations and Suggestions for Future Research
For this study, we created a P-MF method designed to accommodate small samples of sediment and suspended solids, and we sought to determine the smallest sample size that still provides accurate results. While our method proves that microfractionation is possible, more work is needed in order to more clearly define the threshold for minimum sample size. With respect to Utah Lake, we expect the threshold to be between 0.35 and 0.99 g for lakebed sediments and >0.16 g for Utah Lake suspended solids. We expect these limits to be somewhat location-specific, as different sediments and suspended solids will exhibit different behaviors.
We would like to highlight three limitations affecting this study, with the first limitation regarding the dry mass of our samples. A critical part of performing P-MF analysis is knowing the dry mass being tested. We conducted our experiments based on estimates for dry mass, and while this worked for our lakebed sediments, we overestimated the dry mass of our suspended solids samples. This caused the suspended solids samples to be smaller than expected, which consequently meant that we did not have a true control group for the suspended solids samples. Dry mass cannot be measured directly from samples undergoing analysis, as doing so can jeopardize the integrity of the samples’ P fractions. Duplicate samples must be used as a proxy for determining dry mass. While we measured the dry mass of duplicate samples, we did so alongside the P-MF process, and so the true dry mass of our samples was only determined after we selected the sample sizes partway through the experiment. To avoid this issue, we recommend waiting to perform P-MF analysis until after the samples’ dry mass has been determined to better understand the wet mass required for the test.
A second limitation of this study was the number of sample sizes being tested. For each sediment type, we sought to have a sample size that acted as a control representing a standard P-fractionation sample, along with four successively smaller sample sizes. While these sizes helped narrow the potential range for microfractionation samples from Utah Lake, the sample sizes tested were not exhaustive, and future iterations of this study could more clearly define the lower sample size thresholds for sediments and suspended solids.
A third limitation of this study was that we only evaluated lakebed sediment and suspended solids from Utah Lake. Our results show that the lake’s suspended solids have a significantly larger P content than the lakebed sediments, suggesting that the lake’s suspended solids are a greater source of bioavailable P. While we would assume that sediment and suspended solids from other water bodies would behave similarly, additional experimentation is needed to confirm this, especially since the composition of sediments and suspended solids from other locations will vary and thereby impact the manner and degree to which they store and release P. Future studies on P-MF processes should test sediments and suspended solids of diverse compositions from various locations.
Through the development of our P-MF method, we demonstrated that suspended sediments in Utah Lake have significantly higher P concentrations, especially for the fractions that interact readily with the water column. These concentrations can be on the order of 10 to 100× for loosely sorbed P, to several hundred times for Fe- and Al-bound P. This highlights the need for an acceptable method to measure P fractions in small samples, as measuring P fractions in lake sediments is not a good analog for concentrations in suspended solids.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12070218/s1. The standard operating procedure “Phosphorus Microfractionation (P-MF) Method for Total Suspended Solids”.
Author Contributions
Conceptualization, G.P.W. and J.B.T.; methodology, G.P.W. and J.B.T.; validation, A.W.M. and T.G.M.; formal analysis, G.P.W. and J.B.T.; investigation, G.P.W. and J.B.T.; resources, G.P.W. and J.B.T.; data curation, G.P.W. and J.B.T.; writing—original draft preparation, G.P.W. and J.B.T.; writing—review and editing, G.P.W., J.B.T., R.L.R., A.W.M., and T.G.M.; visualization, J.B.T.; supervision, G.P.W.; project administration, G.P.W.; funding acquisition, T.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Wasatch Front Water Quality Council.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Wasatch Front Water Quality Council for funding this project and the Brigham Young University Environmental Analytical Laboratory for facilitating the analysis of our samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lü, C.; Wang, B.; He, J.; Vogt, R.D.; Zhou, B.; Guan, R.; Zuo, L.; Wang, W.; Xie, Z.; Wang, J.; et al. Responses of Organic Phosphorus Fractionation to Environmental Conditions and Lake Evolution. Environ. Sci. Technol. 2016, 50, 4893–5422. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, 2nd ed.; North Carolina State University Raleigh: Raleigh, NC, USA, 2009. [Google Scholar]
- Kleinman, P.J.A.; Sharpley, A.N.; Gartley, K.; Jarrell, W.M.; Kuo, S.; Menon, R.G.; Myers, R.; Reddy, K.R.; Skogley, E.O. Interlaboratory comparison of soil phosphorus extracted by various soil test methods. Commun. Soil Sci. Plant Anal. 2001, 32, 2325–2345. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Yang, J.; Abdi, D.; Cade-Menun, B.J. Investigation of soil legacy phosphorus transformation in long-term agricultural fields using sequential fractionation, P K-edge XANES and solution P NMR spectroscopy. Environ. Sci. Technol. 2015, 49, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Leytem, A.B. Phosphorus compounds in sequential extracts of animal manures: Chemical speciation and a novel fractionation procedure. Environ. Sci. Technol. 2004, 38, 6101–6108. [Google Scholar] [CrossRef]
- Abu-Hmeidan, H.Y.; Williams, G.P.; Miller, A.W. Characterizing total phosphorus in current and geologic utah lake sediments: Implications for water quality management issues. Hydrology 2018, 5, 8. [Google Scholar] [CrossRef]
- Dorich, R.A.; Nelson, D.W.; Sommers, L.E. Algal availability of sediment phosphorus in drainage water of the Black Creek Watershed. J. Environ. Qual. 1980, 9, 557–563. [Google Scholar] [CrossRef]
- Dorich, R.A.; Nelson, D.W.; Sommers, L.E. Estimating algal available phosphorus in suspended sediments by chemical extraction. J. Environ. Qual. 1985, 14, 400–405. [Google Scholar] [CrossRef]
- Randall, M.C.; Carling, G.T.; Dastrup, D.B.; Miller, T.; Nelson, S.T.; Rey, K.A.; Hansen, N.C.; Bickmore, B.R.; Aanderud, Z.T. Sediment potentially controls in-lake phosphorus cycling and harmful cyanobacteria in shallow, eutrophic Utah Lake. PLoS ONE 2019, 14, e0212238. [Google Scholar] [CrossRef]
- Hupfer, M.; Zak, D.; Roßberg, R.; Herzog, C.; Pöthig, R. Evaluation of a well-established sequential phosphorus fractionation technique for use in calcite-rich lake sediments: Identification and prevention of artifacts due to apatite formation. Limnol. Oceanogr. Methods 2009, 7, 399–410. [Google Scholar] [CrossRef]
- Casbeer, W.; Williams, G.P.; Borup, M.B. Phosphorus distribution in delta sediments: A unique data set from deer creek reservoir. Hydrology 2018, 5, 58. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; He, Z.; Guo, J.; Qu, X.; Xie, F.; Giesy, J.P.; Liao, H.; Guo, F. Characterization of organic phosphorus in lake sediments by sequential fractionation and enzymatic hydrolysis. Environ. Sci. Technol. 2013, 47, 7679–7687. [Google Scholar] [CrossRef] [PubMed]
- Acioly, T.M.d.S.; da Silva, M.F.; Barbosa, L.A.; Iannacone, J.; Viana, D.C. Levels of potentially toxic and essential elements in water and estimation of human health risks in a river located at the interface of brazilian savanna and amazon biomes (Tocantins River). Toxics 2024, 12, 444. [Google Scholar] [CrossRef]
- Petersen, G.W.; Corey, R.B. A modified Chang and Jackson procedure for routine fractionation of inorganic soil phosphates. Soil Sci. Soc. Am. J. 1966, 30, 563–565. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Pan, G.; Krom, M.D.; Zhang, M.; Zhang, X.; Wang, L.; Dai, L.; Sheng, Y.; Mortimer, R.J.G. Impact of Suspended Inorganic Particles on Phosphorus Cycling in the Yellow River (China). Environ. Sci. Technol. 2013, 47, 9559–10094. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Xie, Q.; Yang, R.; Guan, T.; Wu, D. Immobilization and Release Behavior of Phosphorus on Phoslock-Inactivated Sediment under Conditions Simulating the Photic Zone in Eutrophic Shallow Lakes. Environ. Sci. Technol. 2019, 53, 12151–12960. [Google Scholar] [CrossRef]
- Wilkinson, K.J.; Lead, J.R. Environmental Colloids and Particles Behaviour, Separation and Characterisation; John Wiley & Sons Ltd.: Chichester, UK, 2007; Volume 10. [Google Scholar]
- Kauppila, P.M. Sequential Extraction Procedure. Available online: https://mineclosure.gtk.fi/sequential-extraction-procedure/ (accessed on 26 April 2022).
- Chakrapani, G.J.; Subramanian, V. Fractionation of Heavy Metals and Phosphorus in Suspended Sediments of the Yamuna River, India. Environ. Monit. Assess. 1996, 43, 117–124. [Google Scholar] [CrossRef]
- Ellison, C.A.; Savage, B.E.; Johnson, G.D. Suspended-Sediment Concentrations, Loads, Total Suspended Solids, Turbidity, and Particle-Size Fractions for Selected Rivers in Minnesota, 2007 Through 2011; 2013–5205; US Geological Survey: Reston, VA, USA, 2014. [Google Scholar]
- Shen, F.; Verhoef, W.; Zhou, Y.; Salama, M.S.; Liu, X. Satellite estimates of wide-range suspended sediment concentrations in Changjiang (Yangtze) estuary using MERIS data. Estuaries Coasts 2010, 33, 1420–1429. [Google Scholar] [CrossRef]
- Meybeck, M.; Laroche, L.; Dürr, H.; Syvitski, J. Global variability of daily total suspended solids and their fluxes in rivers. Glob. Planet. Change 2003, 39, 65–93. [Google Scholar] [CrossRef]
- Casbeer, W.C. Phosphorus Fractionation and Distribution Across Delta of Deer Creek Reservoir. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2009. [Google Scholar]
- Domenico, P.A.; Schwartz, F.W. Physical and Chemical Hydrogeology; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Taggart, J.B.; Ryan, R.L.; Williams, G.P.; Miller, A.W.; Valek, R.A.; Tanner, K.B.; Cardall, A.C. Historical Phosphorus Mass and Concentrations in Utah Lake: A Case Study with Implications for Nutrient Load Management in a Sorption-Dominated Shallow Lake. Water 2024, 16, 933. [Google Scholar] [CrossRef]
- Millero, F.J.; Yao, W.; Aicher, J. The speciation of Fe(II) and Fe(III) in natural waters. Mar. Chem. 1995, 50, 21–39. [Google Scholar] [CrossRef]
- Kopáček, J.; Borovec, J.; Hejzlar, J.; Ulrich, K.-U.; Norton, S.A.; Amirbahman, A. Aluminum Control of Phosphorus Sorption by Lake Sediments. Environ. Sci. Technol. 2005, 39, 8784–8789. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.C. Characterizing the Fate and Mobility of Phosphorus in Utah Lake Sediments. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2017. [Google Scholar]
- Valek, R.A.; Tanner, K.B.; Taggart, J.B.; Ryan, R.L.; Cardall, A.C.; Woodland, L.M.; Oxborrow, M.J.; Williams, G.P.; Miller, A.W.; Sowby, R.B. Regulated Inductively Coupled Plasma–Optical Emission Spectrometry Detectible Elements in Utah Lake: Characterization and Discussion. Water 2024, 16, 2170. [Google Scholar] [CrossRef]
- Tanner, K.B.; Cardall, A.C.; Williams, G.P. A spatial long-term trend analysis of estimated chlorophyll-a concentrations in Utah Lake using Earth observation data. Remote Sens. 2022, 14, 3664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).