Abstract
Different sources of nanomaterials on the adsorption of selenium (Se) in aqueous solutions were evaluated, including nanoscale municipal drinking water treatment residues (nWTRs) and agricultural waste pomegranate peels (PNPs), in comparison with commercial carbon nanoparticles (CNPs). Different Se(IV) treatments and application doses of each nanomaterial were evaluated. The Se adsorption kinetics were determined at different time intervals. The results showed that the Se sorption capacity of different nanomaterials and their mixtures varied significantly (p < 0.05). Se concentration, the application dosage of nanoparticles, and the interaction time of Se and nanoparticles in Se solutions significantly affect the efficiency of Se adsorption at pH 3.51. The sorption isotherm of Se varied amongst different nanomaterials. Se adsorption on CNPs, nWTRs-CNPs, nWTRs, PNPs-CNPs, nWTRs-PNPs, and PNPs at the 800 mg Se/L treatment was 79.93, 77.48, 76.00, 72.97, 70.49, and 68.16 mg Se/g sorbent, respectively. The H-type isotherm became dominant, indicating intensive interaction between Se and nanoparticles. With the Se treatment of 50 mg/L, the Se removal efficiency of CNPs, nWTRs-CNPs, nWTRs, PNPs-CNPs, nWTRs-PNPs, and PNPs was 100, 96, 93, 87, 85, and 80%, respectively, but became 100, 97, 95, 91, 88, and 85%, respectively, at a higher Se concentration of 800 mg/L. Increasing the application dosage of nanomaterials resulted in a significant increase in Se mass sorbed by the nanoparticles. Se adsorption was best predicted by the Langmuir isotherm model. The desorption rate of the Se mass sorbed by nanoparticles at 800 mg Se/L was 0.4% of the total Se adsorbed by CNPs, with 0.88% by nWTRs-CNPs and 1.69% by PNPs-CNPs, while higher Se desorption rates of 4.2, 7.3, and 17.6% were observed with nWTRs, nWTRs-PNPs, and PNPs, respectively. This study demonstrates that nanoscale municipal and agricultural solid waste materials can be effective in removing Se from contaminated water.
1. Introduction
Background concentrations of selenium (Se) in water under naturally occurring conditions are generally less than 0.5 μg/L [], while industrial wastewater contains Se up to 620 mg/L []. The permissible Se concentration in environmental waters varies significantly depending on water use. According to the U.S. Environmental Protection Agency (US EPA), the maximum contaminant level (MCL) for Se in public drinking water is 50 µg/L, while the permissible monthly average freshwater water column criterion of Se is 3.1 µg/L in flowing waters and 1.5 µg/L in impounded waters []. Selenium contamination can be a result from various industrial and agricultural sources, for example, 15 to 75 µg/L of Se in oil refinery wastewater, 3 to 12 µg/L in coal mining drainage, and 140 to 1400 µg/L in agricultural drainage [,,]. Selenium in the natural environment primarily presents in four different oxidation states: Se(VI), Se(IV), Se(0), and Se(-II). Selenate (SeO42−) and selenite (SeO32−) are the two most prevalent inorganic forms in natural water []. Of the four chemical states, selenate is the most water-soluble, while selenite can be easily adsorbed to water-borne particulates. Studies show that selenite is the dominant chemical species in fly ash discharge from coal-fired power plants, as well as in the effluent from oil refineries []. Thus, selenite is the most poisonous chemical form of Se to aquatic organisms [], being about 10 times more toxic than selenate [].
Environmental concerns of Se contamination in relation to wildlife and human health have sparked the scientific interest in developing effective remediation technologies for cleaning up Se-contaminated industrial wastewater. As an essential nutrient element to humans and animals, there is a very narrow margin between nutritionally required and toxic concentrations of Se. Long-term excessive Se exposure and uptake could cause different serious health problems []. Laclaustra et al. [] indicated that a higher risk of diabetes was correlated with high levels of Se exposure from pollution sources in the United States. The common symptoms of Se toxicity (or selenosis) in humans include skin rashes, neurological disorders, or hair and nail loss []. The Se toxic effects on human and animal health can also be influenced significantly by different environmental factors such as pH and redox potential. Thus, developing sustainable remediation technology for Se-contaminated wastewater is an essential need and of scientific importance to improve environmental water quality.
Different remediation techniques for Se-contaminated water have been developed and extensively evaluated in the past, including chemical precipitation, coagulation–flocculation, ion exchange, and membrane filtration. Those different techniques generally include four mechanistic approaches []: (1) chemical and physical separation, (2) adsorption, coagulation and precipitation, (3) redox processes like chemical reduction and catalysis, and (4) biological accumulation and transformation. Earlier studies showed that most of those remediation techniques often have some drawbacks, such as high operating costs, sludge/waste generation, and the limited capacity of Se removal from wastewater [,]. Therefore, there is growing interest in developing other alternative eco-friendly remediation techniques for the treatment of Se-contaminated water.
The Se adsorption process has previously received much research attention. The use of different adsorbing materials (or sorbents) for removing Se from wastewater has been evaluated in recent years []. The significant Se adsorption capacity has been observed with selected biological sorbents, such as chemically modified biomass, algal biomass, microbes, and agricultural wastes. These bio-substances contain polysaccharides that provide numerous reactive groups on their surfaces. Zhang et al. [] had investigated different agricultural wastes as the biosorbents, including moringa seed waste, woody sawdust, pomegranate, mango peels, eucalyptus, peanut shells, and rice husks. In recent years, emerging nanoscale materials have also been examined, showing promising results for the cleanup of Se-contaminated water. Unlike traditional remediation techniques, the new sustainable remediation technology used nanoscale sorbents generated from municipal solid waste products and naturally occurring biological materials, such as agricultural wastes. Nanoscale particles generally have a large surface area with extensively active adsorbing sites, as compared to bulk-sized adsorbing materials of the same chemical composition [,,]. Those adsorbing materials have been successfully used for the treatment of wastewater to meet the water Se regulation standards [,].
Even with recent substantial research progress, further investigation is still needed to fully determine the capacity of different nanomaterials that are generated from commonly available municipal and agricultural solid waste materials, such as drinking water treatment residues (WTRs) and pomegranate peels (PPs). To develop a sustainable remediation technique, this study examined the feasibility of using nanoparticles (NPs) that are generated from solid waste materials (WTRs and PPs) for adsorbing and retaining Se from contaminated water. Thus, the specific research objectives were (1) to examine the effects of the Se concentration, the application rate and the interacting duration of different adsorbing materials of nanomaterials on the effectiveness of Se sorption from wastewater; (2) to explore the mechanistic processes of Se sorption, including the kinetics of Se adsorption and desorption; (3) to evaluate the effectiveness of Se removal from Se-contaminated water through a remediation column containing different nano-sorbents.
2. Materials and Methods
2.1. Preparation and Characterization of Nanomaterials
This study included three different types of nanomaterials. (1) Drinking water treatment residual nanoparticles (nWTR): The original drinking water treatment residuals (WTRs) were collected from a drinking water treatment plant in Alexandria, Egypt. The chemical composition of the WTRs primarily includes amorphous metal oxides/hydroxides (such as Al, Fe, and Ca), along with minerals, humic substances, and various chemical compounds, originating from local canal water (a tributary of Nile). Concentrations of Se in the surface water were at the background level (<0.4 µg/L). The WTRs were dried and ground into nanoparticles using a mechanical grinding method that was previously reported by Elkhatib et al. []. (2) Pomegranate peel nanoparticles (PNPs): Pomegranate peels were collected from a fruit juice factory located in Borg Al-Arab city in Alexandria. Pomegranate peels (PPs) contain polyphenols, including tannins, flavonoids, and phenolic acids. PPs are also sources of fiber, alkaloids, vitamins, organic acids, as well as minerals containing Ca and K. After being oven-dried at 80 °C, PPs were mechanically processed into nanoparticles using the technique reported by Elkhatib et al. []. (3) Carbon nanoparticles (CNPs): CNPs are pure carbon graphite nanoparticles that are commercially available from ACS Material, LLC (Pasadena, CA, USA).
The particle size distribution and zeta potential were determined using Zetasizer (Malvern Panalytical) []. The specific surface area (SSA) and total pore volume were determined by the Brunauer–Emmett–Teller (BET) method reported in Elkhatib et al. [,]. The general physical and chemical properties of the three types of nanomaterials are compiled in Table 1, showing a diameter of <100 nm for studied nanoparticles. Their specific surface area (SSA) ranged from 99 to 155 m2/g. Their total pore volumes varied from 0.027 to 0.085 cm3/g. The zeta potential values (−0.31 to −5.82 mV) show the presence of negative charges on nanoparticle surfaces and relatively higher stability of commercially available CNPs in water solutions.

Table 1.
General properties of nanoscale drinking water treatment residues (nWTRs), nanoscale pomegranate peels (PNPs), and pure carbon graphite nanoparticles (CNPs).
The nanomaterials were further characterized by the Fourier transform infrared (FTIR) analysis [] with a Bruker Vertex 70/v spectrometer (Bruker Corp, Billerica, MA, USA), equipped with a horizontal ATR diamond crystal accessory (9 reflections; angle of incidence: 45) and a Mercury Cadmium Telluride (MCT) detector, so that the possible modifications of active sites on NPs as results of Se adsorption could be identified. The wavenumbers used for the mid-infrared light in FTIR analysis ranged from 400 to 4000 cm−1.
2.2. Optimizing Parameters for Maximum Se Adsorption by Different Nanomaterials
Sorption isotherms and kinetics were determined for the optimal parameters of the maximum adsorption of Se. Starting with a 1:100 ratio of the nanomaterial mass to the volume of Se-contaminated water, 0.2 g of each nano-sorbent was mixed with 20 mL of Se (Na2SeO3) solutions of different concentrations (50, 100, 200, 400, and 800 mg Se/L). This range of the Se treatment was selected in consideration of the high Se concentration of 620 mg/L observed in mining wastewater []. The NP-treated Se solution was shaken at 400 rpm at pH 3.5 (the initial solution pH) for two hours, a pre-determined equilibrium time from the kinetic experiment. The Se sorption kinetics were determined by taking aliquots at different time intervals of 0.5, 1, 2, 5, 10, 15, 20, 30, 40, 60, 90, and 120 min. Each measurement was carried out at 25 ± 3 °C (room temperature) in three replicates. Concentrations of total Se in the aliquot samples were analyzed using Inductively coupled Plasma–Mass Spectroscopy (PerkinElmer ICP-MS). The amount of Se ions adsorbed on different NPs at each sampling time (t) was calculated as follows:
where V is the volume of Se solution (L), m is the weight of NPs (g), and Ci and Ce are the initial and the equilibrium Se concentrations (mg/L), respectively.
q = [(Ci − Ce) × V]/m
The Se adsorption efficiency of NPs was calculated using the following equation:
where E is the sorption efficiency (%), while Ci and Ce are the initial and the equilibrium Se concentrations, respectively.
E (%) = [(Ci − Ce)/Ci] × 100
Assuming that the adsorption occurs on the homogeneous adsorbent surface for the Langmuir model and the multi-layer adsorption process can be described by the Freundlich model, this study determined the maximum sorption capacities (qmax) for Se sorption using these two models. In addition, these two commonly used kinetic models, including the first-order and the power function, were used to fit the experimental data. The best-fit model was selected based on the standard error of estimate (SE) and the determination coefficient (R2).
2.3. The Dosage of Different Nanomaterials
The effect of different doses of each nanomaterial type on Se sorption efficiency was examined at pH 3.5 and 25 °C. The application dosage was 0.5, 1, 1.5, or 2 g in 40 mL solution of 100 mg Se/L (as Na2SeO3). The mixtures of two different nanomaterials (i.e., nWTR-PNPs, nWTR-CNPs, and PNPs-CNPs) were prepared based on equal weight.
2.4. Desorption of the Se Adsorbed on Nanomaterials
The desorption of the Se adsorbed on NPs was evaluated by the following procedure: The Se-laden NPs in the Se solution of 800 mg Se/L as Na2SeO3 were filtered using 0.45 µm pore-size filters, and then 0.1 g of Se-laden NPs was mixed with 10 mL of double-deionized water. The mixture was shaken at 400 rpm at 25 °C for two hours and then centrifuged at 5000× g for 15 min at 25 °C. Concentrations of Se in the supernatant were determined using ICP-MS.
2.5. Selenium Removal from Simulated Wastewater Using Different NPs
Using a batch method at room temperature, the effectiveness of different NPs for Se removal from simulated wastewater was evaluated. For a high sorbent-to-solution ratio of 1:500, compared to 1:100 in previous experiments, 0.1 g (dry weight) NPs was added to 50 mL of wastewater containing 50 mg Se/L as Na2SeO3, and then well-mixed using a magnetic stirrer for 2 h at room temperature to reach the equilibrium. The mixture was centrifuged at 5000× g for 15 min at 25 °C and then filtered using 0.45 µm pore-size filters. Concentrations of Se in the supernatant were determined by ICP-MS, and the Se removal efficiencies by different nanomaterials were calculated.
In addition, a column experiment was conducted to determine the Se removal efficiency using NPs from wastewater. A glass column (2 cm diameter and 30 cm length) containing the layers of 5 g of fine sand, 10 g of coarse sand, and 1 g of studied nanomaterials (both as each type and mixtures of two) was prepared. The water-holding capacity of the substrate in each column was 20 mL. The flow rate of the wastewater containing 50 mg Se/L as Na2SeO3 was regulated at 0.5 mL/min. The effluent sample of 20 mL was collected consecutively 5 times, representing 5 times the water-holding capacity’s total volume of 100 mL. The effluent samples were analyzed for Se concentrations using ICP-MS.
2.6. Data and Statistical Analysis
Data were statistically analyzed using CoStat Software Program (6.45) []. The level of significance (α) was 0.05. The standard error of estimate (SE) and the determination coefficient (R2) were used to compare the fitness difference between models.
3. Results and Discussion
3.1. Kinetics and Sorption Isotherm of Selenite and Its Sorption Efficiency
3.1.1. Kinetics and Sorption Isotherm of Se on NPs
The kinetic test was conducted to determine the time needed for Se sorption equilibrium on different nanomaterials, as well as to evaluate the sorption capability of the nanomaterials. Figure 1 illustrates that the Se sorption is a function of interaction time. Evidently, as the contact time increases to 120 min, the Se (IV) adsorption by different nanomaterials increased steadily before reaching stabilization. About 95–99% of the Se was sorption-bound during the first 20 min with NPs, according to the sorption kinetics of Se on NPs. This was followed by sluggish sorption at 298 K (Figure 1). The rapid loading of sorption sites on NPs with Se ions in the initial phase caused the fast sorption of Se during the first 20 min, followed by a slow sorption process due to the lack of active sites.
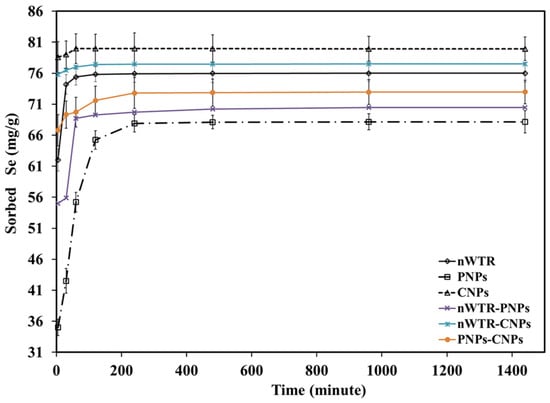
Figure 1.
Effect of contact time of different nanomaterials on selenium sorption in the Se solution of 50 to 800 mg/L at 25 °C.
With the starting Se concentration ranging from 50 to 800 mg Se/L in this study, the sorption isotherms were measured to ascertain the sorption capacity of different nanomaterials (Figure 2). The Se sorption isotherm varied significantly among different types of nanoparticles, showing different Se sorption capacities of nWTR, PNPs, CNPs, nWTR-PNPs, nWTR-CNPs, and PNPs-CNPs. The current research findings generally agree with previous studies, showing the relationship between the quantity of Se sorbed by the nanomaterial and the Se concentration at equilibrium []. The Se sorption to the NPs was strongly influenced by the initial Se concentration in the solution. The Se mass adsorbed on the NPs increased as the initial Se concentration increased from 50 to 800 mg/L at room temperature (25 °C). More Se ions would likely become available for adsorption on surfaces of nanomaterials with increasing Se concentrations in the solution. Therefore, the initial Se concentration significantly affects the Se sorption on NPs. The Se mass in the solution might be rather less in relation to the number of available sorbing sites at lower initial Se concentrations, and therefore, the sorbed Se at lower concentrations was very high and represented with a H-shape, referring to strong sorption [,,,].
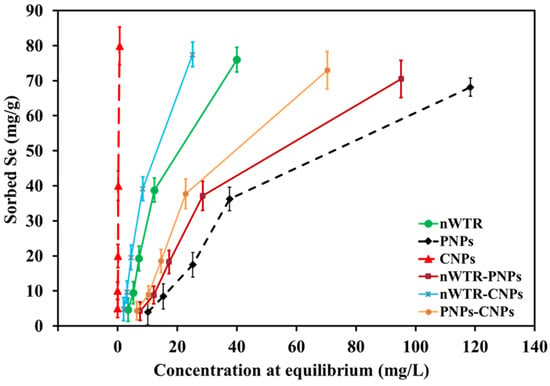
Figure 2.
Concentrations of selenium adsorbed on different nanomaterials in the selenium solution of 50 to 800 mg/L at 25 °C.
The Se sorption isotherms in different NPs treatments showed H-type (Figure 2). A “H-type” isotherm indicates a substantial interaction between Se and the NPs. The amount of Se adsorbed on different NPs was in the following descending order: CNPs > nWTR-CNPs > nWTR > PNPs-CNPs > nWTR-PNPs > PNPs (Figure 2). The amount of Se sorbed on CNPs, nWTR-CNPs, nWTR, PNPs-CNPs, nWTR-PNPs, and PNPs at the 800 mg Se/L treatment was 79.93, 77.48, 76.0, 72.97, 70.49, and 68.16 mg Se/g sorbent, respectively (Figure 1). Because these NPs are amorphous with high specific surface areas, they have great abilities of adsorbing Se from the Se solution. These research findings are consistent with other previous observations using similar sorbent materials [,,,,,,]. Water treatment residuals (WTRs) contain amorphous metal oxides/hydroxides (commonly Al, Fe, and Ca) and various clay mineral deposits that were produced during the flocculation process of drinking water treatments commonly involving the use of alum [Al2(SO4)3.14H2O]) []. Therefore, nanoscale WTRs can have a high aptitude for oxyanions adsorption in this study. Ippolito et al. [] also previously demonstrated that WTRs could adsorb SeO32− and SeO42− and, during the sorption process, SeO32− can be reduced to Se0 by minerals in WTRs. Because of the development of inner-sphere Se(IV) complexation with mineral oxides, the highest Se(IV) sorption was observed at pH 9 [].
According to Lu et al. [], graphene oxides (GOs) have a strong sorption ability for heavy metals because of their enormous surface area and the abundance of oxidized functional groups (e.g., amine, carboxylic, and hydroxyl). Other carbon nanomaterials have also been examined for superior sorption of heavy metals from industrial wastewaters [,,]. To develop new adsorbing materials with a high adsorption capacity and selectivity for specific pollutants, polymeric polymers have also been investigated. For example, for Se(IV) adsorption, Bandara et al. [] created nanocomposite beads containing graphene oxides, polyethylenimine, and chitosan, and the beads have been used for Se oxyanions removal by electrostatic attractions in wastewater. In addition, green-synthesized silver nanoparticles (AgNPs) and Cyperus laevigatus biomass showed a high sorption capacity for Se(IV) []. Urbanova et al. [] evaluated important variables that influence SeO32− removal using magnetite nanoparticles. They reported that there was a rapid initial removal process of selenite, followed by a slower phase, suggesting complicated mechanisms of ion sorption. The main sorptive interaction was thought to be chemisorption, with a small part of diffusion. For the effective sorption of Se anions at pH 7, Priya et al. [] produced LDH/rGO nanocomposites functionalized with β-Cyclodextrin (CD) and carboxymethylcellulose (CC). Their study examined the effectiveness of using CD- and CC-incorporated LDH/rGO bio-composites for removing Se ions. In a different study, Zhang et al. [] investigated the sorption behaviors of nano-TiO2 and discovered that the Se sorption in aqueous solution reached the equilibrium in about 5 min. The Se sorption was pH-dependent, and the highest sorption was observed at solution pH 2 to 6.
3.1.2. Se Adsorption Efficiencies of Different Nanomaterials
The adsorption efficiencies of Se by different NPs are presented in Figure 3, showing that the Se adsorption efficiency of CNPs, nWTR-CNPs, nWTR, PNPs- CNPs, nWTR-PNPs, and PNPs with the 50 mg Se/L treatment was 100, 96, 93, 87, 85, and 80%, respectively, but at the 800 mg Se/L treatment, became 100, 97, 95, 91, 88, and 85%, respectively. When the sorption sites of NPs became nearly saturated, the Se adsorption efficiencies by different nanomaterials did not increase significantly when the Se treatment increased from 50 mg/L to 800 mg/L.
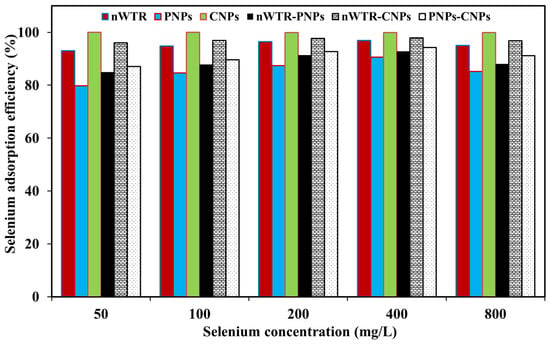
Figure 3.
The selenium adsorption efficiency of different nanomaterials in the selenium solution of 50 to 800 mg/L at 25 °C.
The results of this study were comparable with other studies that used different plant materials for Se adsorption, such as the adsorption of Se(IV) by red fungus (Ganoderma lucidum) [], by Saccharomyces cerevisiae [], and by green alga (Cladophora hutchinsiae) []. In addition to those raw plant materials, specific chemically treated plant materials, such as hot sulfuric acid-treated peanut shell and rice husk, contain more active sorbing sites [,]. The alkaline heat treatment has also been utilized to prepare nano-hydroxyapatite from fish scale []. For practical application, the cost of those chemical treatments may need to be evaluated.
3.1.3. Se Sorption vs. Sorbent Dosage
The effect of the application dosage of different nanomaterials on the amount of Se adsorption was determined in 100 mL Se solution of 800 mg/L. Increasing the sorbent dosage from 0.5 to 2 g resulted in a significant increase in the amounts of Se sorbed by NPs (Figure 4). When the dosage of CNPs increased from 0.5 to 2 g in the Se solution, the Se mass sorbed on CNPs increased from 126 to 151 mg/g, while the amount of Se sorbed by PNPs increased from 25 to 61.51 mg/g. The mass of Se sorbed by different nanomaterials in the Se solutions followed a descending order of CNPs > nWTR-CNPs > nWTR > PNPs-CNPs > nWTR-PNPs > PNPs (Figure 4).
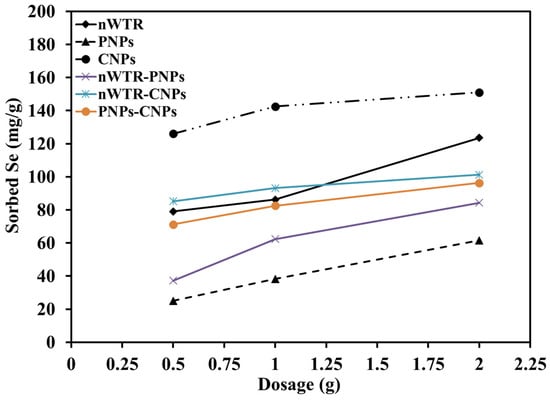
Figure 4.
Effect of the dosage of different nanomaterials on selenium adsorption in 40 mL selenium solution of 800 mg/L at 25 °C.
The increase in sorbent dosage significantly affected the sorption of Se oxyanion in wastewater because higher application dosages can provide more available functional groups as well as more active adsorbing sites on the surfaces of NPs [,,]. However, it is important to realize that increasing the dosage will not always result in higher Se adsorption and Se removal rates because excessive adsorbing materials might not increase the number of Se adsorption sites [].
Dev et al. [] recently investigated the possibility of using food waste as adsorbing materials for wastewater treatments. They developed composite beads as low-cost biosorbents, including bare citrus peels, Ca-alginate gel, and Ca-alginate–citrus peel, to treat selenite-contaminated water. The qmax was 116.2 mg/g for citrus peels, followed by 72.1 mg/g for Ca-alginate, and 111.9 mg/g for Ca-alginate–citrus peel. Citrus peels, both naked and immobilized, displayed the highest selenite adsorption rate []. Further, the effect of the adsorbent structure in Ca-alginate beads on the adsorption of selenite using orange peels was also examined, showing that HSeO3− continued to be the most common chemical form of Se(IV) with pH from 2 to 8, while SeO32− became predominant when increasing pH to 12 []. Similar research findings have also been reported by Ghazizadeh et al. []. The capacity of citrus peels in biosorbing Se(IV) increased substantially when pH increased from 2 to 8, with a biosorption ability of 28.2 mg/g at pH 8.
3.1.4. Desorption of the Se Adsorbed by Different Nanomaterials
The desorption of the Se mass sorbed by different nanomaterials in the 800 mg/L Se treatment was determined, and the results are shown in Figure 5. The desorption from CNPs only accounted for 0.4% of the total Se mass sorbed by CNPs from the solution, followed by 0.88% from nWTR-CNP and 1.69% from PNPs-CNPs. The strong sorption capacity of CNPs (with H-shape isotherm) and the greater stability of the Se bound with CNPs alone or mixed with nWTR or PNPs led to a very low rate of Se desorption. Conversely, the Se sorbed by other NPs showed moderate-to-high levels of desorption, with 4.21, 7.26, and 17.61% Se desorption rates from nWTR, nWTR-PNPs, and PNPs, respectively (Figure 5). Tuzen and Sari [] previously investigated the biosorption of Se(IV) by a green algal species (Cladophora hutchinsiae) and found that only 20% of the adsorbed Se desorbed/released after having 10 biosorption–desorption cycles. Further, this marine algal species also demonstrated its remarkable stability of the adsorbed Se during the regeneration process.
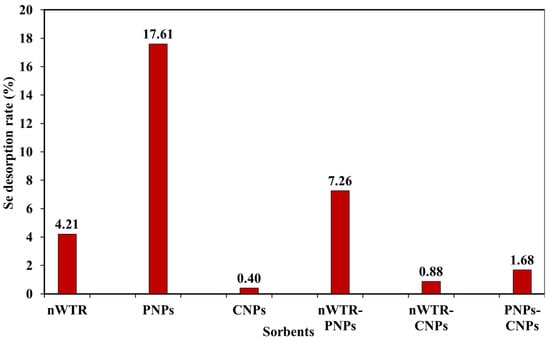
Figure 5.
The desorption rate of the selenium mass sorbed by different nanomaterials in 100 mL solution of 800 mg Se/L at 25 °C.
3.2. Mechanisms of Se Removal in Wastewater
The mechanisms of Se removal from wastewater primarily include adsorption, redox precipitation, surface complexation, ligand exchange, and electrostatic attraction, although these mechanistic processes vary significantly depending on the characteristics of wastewater. Clearly, Se removal by sorbing materials may entail multiple mechanistic approaches, rather than just via adsorption. Indeed, the application of nanomaterials shows the most important Se(IV) adsorption to surfaces of NPs []. To select an appropriate nanomaterial with high Se adsorption capacity for the wastewater treatment, the mechanistic processes of Se adsorption and the change in surface chemistry of sorbing nanomaterials, such as elemental composition and functional groups of different NPs, have been evaluated using FTIR analysis in this study (Table 2). The shifting of some functional groups between bands as resulted from Se sorption on NPs demonstrates the complexation between Se and the function groups on the sorbent surface. Those changes in counter ions associated with carboxylate and hydroxylate anions may have resulted in the shifts, indicating that acidic groups (carboxyl and hydroxyl) are the major contributors to the complexation of Se ions and ion exchange processes, and that may occur between Se and counter ions linked to carboxylate and hydroxylate anions.

Table 2.
The FTIR spectral characteristics of different nanomaterials before and after Se(IV) sorption.
The new bond creation of Se–O linkage at 1630 cm−1 in the FTIR spectrum of different nanomaterials can provide the second ceiling of the adsorption. The cationic adsorbent surface contacts with Se anions via electrostatic interaction, which was demonstrated by significant chemical reductions in metal–oxide lattice vibration peaks and the shifting of expanded hydroxyl groups to lower wavenumbers. In general, the broad band between 1700 cm−1 and 500 cm−1 shows the majority of the changes that occur on the NPs following the sorption of Se. This band change or shifting can be attributed to the complexation of Se ions with carboxylic group (-COOH) and bound O-H bands of carboxylic acids []. Ion exchange between protons of symmetric or asymmetric C-H was previously illustrated by the changes in band 2921 prior to and during Se sorption []. C-H stretching brought on by hydrogen bonding between carboxylic acids or their esters and Se ions was observed by the elimination of peaks at 1737 following Se sorption []. The electrostatic process may be responsible for the variation in peaks between 1500 cm−1 and 1300 cm−1, which are brought on by the C-O stretching of COOH functional groups [].
3.3. Sorption Isotherm and Kinetic Modeling
The quantity of sorbate molecules that might be sorbed on active sites and the sorption process between active sites on the sorbing material both are described by the Freundlich and Langmuir sorption isotherm models (Table 3, Figure 6). There is no inter-molecular interaction, and the Langmuir sorption isotherm model is used to describe one layer of sorption [].

Table 3.
Parameters of the Freundlich and Langmuir isotherm model for Se sorption on different nanomaterials.
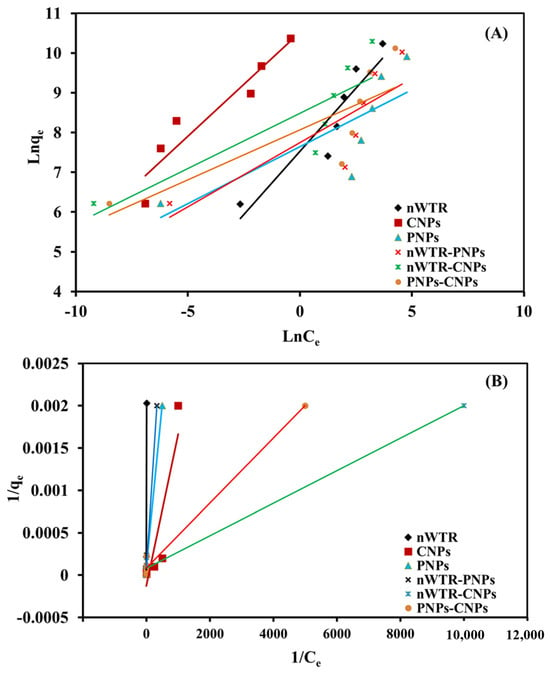
Figure 6.
The Freundlich (A) and Langmuir (B) isotherm models for the selenium sorbed on different nanomaterials in the selenium solution of 50 to 800 mg/L at 25 °C.
The Langmuir isotherm model matches the adsorption data very well (R2 = 0.91–0.99, SE = 0.00009–0.0004). The SE values of the Langmuir model for Se sorption on the selected nanomaterials were relatively low, and as a result, the Langmuir model can best describe the experimental sorption data, suggesting that the monolayer mode of adsorption was responsible for Se sorption on the surfaces of NPs. This study determined the highest Se adsorption capacity (qmax) of different nanomaterials, and the qmax value of CNPs, nWTR- CNPs, nWTR, PNPs-CNPs, nWTR-PNPs, and PNPs was 100, 12.5, 16.67, 11.10, 11.10, and 11.10 mg Se/g, respectively, because the specific surface area of each nano-sorbent increases the number of sorption sites on the nanoparticle surface. The adsorption capability and the surface reactivity of NPs were also improved significantly by their high specific surface area []. The KL constant values that are ascribed to the affinity between the NPs and Se ions indeed support the research finding in this study (Table 3, Figure 6). Ippolito et al. [] reported that the adsorption capacity of drinking water treatment residuals (WTRs) for Se(IV) was 1.50 mg/g at 25 °C and pH 9, and the proposed sorption mechanism also involves redox, precipitation, and surface complexation. Zhou and Haynes [] revealed that the adsorption capacity of WTRs was 0.28 mg/g at pH 5 and 25 °C using the Langmuir model for estimating the adsorption capacity, and the proposed sorption mechanism also includes redox, precipitation, and surface complexation. Bandara et al. [] used the Langmuir model to estimate the adsorption capacity of grapheme oxide hydrogel beads for Se(IV) at 25 °C. With the concentration range of 5 to 250 mg/L, the adsorption capacity was 1.62 mg/g. In addition, Abdelhadi et al. [] reported that the adsorption capacity of olive mill solid waste biochar to Se(IV) was 20 mg/g at the Se concentration of 50 mg/L at 25 °C and pH 7. Conversely, the sorption process via multi-molecular layer adsorption, where the active sites on the sorbent are not evenly distributed with inter-molecular interaction, can be described by the Freundlich sorption isotherm model []. As a result, when the sorbate concentration increases, so will the quantity of adsorbed Se molecules (Table 3, Figure 6A).
For the treatments in this study, Se sorption was fitted to the Freundlich equation, with R2 varying between 0.63 and 0.89 (Table 3, Figure 6B). The slope and intercept of the linear Freundlich equation were used to construct the Freundlich isotherm parameters shown in Table 3. Higher KF values at CNPs or in combination with nWTR/PNPs indicate their strong sorption capacity for Se []. For the NPs in this study, the 1/n values of the Freundlich isotherm varied within a small range of 0.20–0.60, and they are consistent with the general shape of the Se isotherms, as shown in Figure 6A. Selenium sorption at concentrations between 50 and 800 mg Se/L was well-represented by the Freundlich model. However, the SE values of the Freundlich model for Se sorption on different nanomaterials were significantly greater than those of the Langmuir model, indicating the Langmuir model’s superiority over the Freundlich model in explaining Se sorption by the NPs [].
To further investigate the sorption mechanism of Se(IV) by different nanomaterials, two popular kinetic models, first-order [] and power function [], were selected to fit the experimental data:
where q: the quantity of sorbate (mg) sorbed per (kg) of sorbent; qe: the equilibrium quantity of sorbate sorbed; ka (μg/g min): the apparent sorption diffusion rate coefficient; Ci: starting concentration of sorbate (mg/L); t: reaction time (min); 1/m: constant.
First-order model: Ln (qe − q) = a − ka t
Power function model: q = ka Ci t 1/m
The agreement between the experimental and predicted data was evaluated using R2 and SE values (Figure 7). Higher R2 and lower SE values suggest that the two models can properly represent the kinetics of Se sorption on different nanomaterials. Each model used in this study has a specific function. The “Ln q” versus “t” relationship in the first-order model is commonly linear when the sorption–desorption process follows the first-order equation. Furthermore, the parameters ka, Ci, and 1/m in the power function model were ascertained using the intercept and slope of the linear plot.
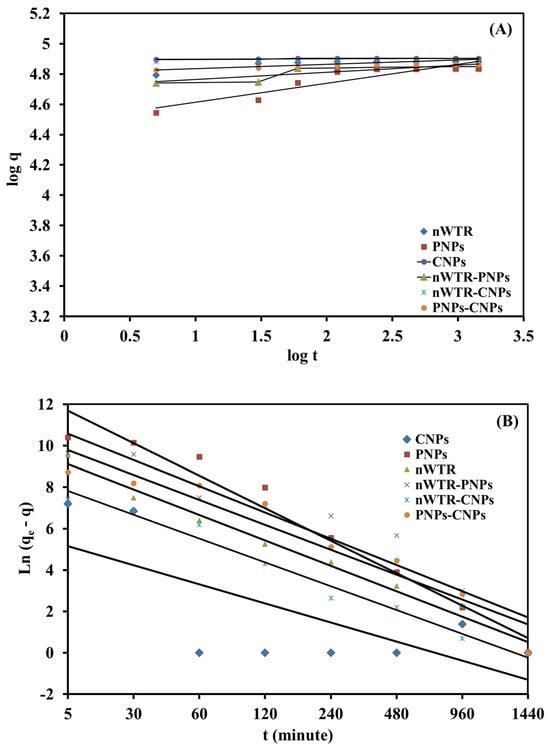
Figure 7.
The power function (A) and first-order (B) kinetic models of the selenium sorbed on different nanomaterials in the Se solution of 50 to 800 mg/L at 25 °C.
3.4. Se Removal from Wastewater Through the NPs Treatment Column
The Se-laden wastewater (50 mg/L) flew through a packed treatment column containing different nanomaterials. Concentrations of Se in the treated water from the columns containing nWTR, PNPs, CNPs, nWTR-PNPs, nWTR-CNPs, and PNPs-CNPs were 3.5, 10.12, 0.12, 7.5, 1.99, and 6.44 mg/L, respectively (Figure 8). The Se removal efficiencies of nWTR, PNPs, CNPs, nWTR-PNPs, nWTR-CNPs, and PNPs-CNPs treatment columns were 98.6, 96, 100, 97, 99.2, and 97.4%, respectively.
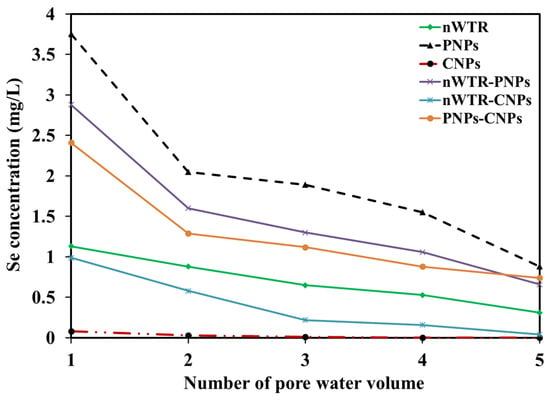
Figure 8.
Effect of different nanomaterials on the selenium removal in 100 mL Se solution of 800 mg/L at 25 °C.
The flow rate of Se-contaminated wastewater is an important parameter for assessing the Se sorption process []. In this study, the flow rate was fixed during Se sorption by different nanomaterials. Strong Se sorption by CNPs was likely related to the low levels of Se present in the solution after a total of 100 mL water passed through the CNPs-packed column. Clearly, CNPs are the most effective sorbent materials for removing Se from wastewater [,,,]. In addition to the sorption of Se, Ekwonna et al. [] indicated that Bacillus cereus, a bacterial strain isolated from wheat straw and biosolid–WTR–sand substrates showed a significant biotransformation ability of reducing selenate and selenite to elemental Se, and further, forming volatile Se organic compounds in Se-contaminated wastewater. Adsorption presents a widely favorable, cost-effective, and efficient method for Se contamination remediation due to its simplicity, non-toxicity, and high contaminant removal capacity []. Recently, surface modification of adsorbents has garnered attention for enhanced porosity and surface area, leading to improved adsorption capacity towards Se oxyanions. The surface complexation modeling represents recent advancement in the study of adsorption mechanisms, by offering a mechanistic and chemically accurate pathway to predict heavy metal adsorption in considering adsorption speciation, active sites, and complexation constants, which overcomes the limitations of traditional isotherm models [].
4. Conclusions
The Se sorption to different nanoscale solid waste materials was significantly affected by the initial Se concentration and the application rate of nanoparticles. The H-type isotherm observed with the nanomaterials indicates substantial interaction between Se and the nanoparticles. Carbon nanoparticles (CPNs) showed higher Se adsorption efficiency than nanoscale drinking water treatment residues (nWTRs) and nanoscale pomegranate peels (PNPs). The desorption rate of the Se mass sorbed on surfaces of different nanomaterials was generally low due to the high stability of Se bound to nanoparticles. The Se removal from wastewater by nanoparticles mainly results from rapid and significant adsorption of Se(IV) to nanoparticle surfaces. Amongst different nanomaterials, CPNs were the most effective sorbent for removing Se from contaminated water, followed by nWTRs and PNPs. For developing a sustainable remediation technique, this study demonstrates that nanoparticles generated from different industrial and agricultural waste materials could be used to effectively clean up Se-contaminated wastewater.
Author Contributions
Conceptualization, A.M.M., N.O.F. and Z.-Q.L.; methodology, A.M.M. and Z.-Q.L.; software, A.M.M. and N.O.F.; validation, A.M.M., N.O.F. and Z.-Q.L.; formal analysis, A.M.M.; investigation, A.M.M. and Z.-Q.L.; resources, A.M.M., N.O.F. and Z.-Q.L.; data curation, A.M.M. and Z.-Q.L.; writing—original draft preparation, A.M.M.; writing—review and editing, A.M.M., N.O.F. and Z.-Q.L.; visualization, A.M.M. and Z.-Q.L.; supervision, Z.-Q.L. All authors have read and agreed to the published version of the manuscript research.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge Alexandria University, Ministry of Agriculture, and Southern Illinois University at Edwardsville for administrative, financial and technical support and availability of materials used for experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janz, D.M. 7—Selenium. Fish Physiol. 2011, 31 Pt A, 327–374. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Gebretsadik, G.G.; Gebru, S.B. Review on environmental selenium: Occurrence, public health implications and biological treatment strategies. Environ. Chall. 2023, 11, 100698. [Google Scholar] [CrossRef]
- US EPA. Aquatic Life Criterion—Selenium. 2025. Available online: https://www.epa.gov/wqc/aquatic-life-criterion-selenium (accessed on 11 November 2025).
- Naga Jyothi, M.S.V.; Ramaiah, B.J.; Maliyekkal, S.M. Occurrence, Contamination, Speciation and Analysis of Selenium in the Environment. In Measurement, Analysis and Remediation of Environmental Pollutants. Energy, Environment, and Sustainability; Gupta, T., Singh, S., Rajput, P., Agarwal, A., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-related selenium contamination Alaska, and the state of current knowledge. Minerals 2017, 7, 46. [Google Scholar] [CrossRef]
- Inam, M.A.; Usman, M.; Iftikhar, R.; Velizarov, S.; Ernst, M. Recent progress in selenium remediation from aqueous systems: State-of-the-art technologies, challenges, and prospects. Water. 2025, 17, 2241. [Google Scholar] [CrossRef]
- Torres, J.; Pintos, V.; Domínguez, S.; Kremer, C.; Kremer, E. Selenite and selenate speciation in natural waters: Interaction with divalent metal ions. J. Solut. Chem. 2010, 39, 1–10. [Google Scholar] [CrossRef]
- Gebreeyessus, G.D.; Zewge, F. A review on environmental selenium issues. SN Appl. Sci. 2019, 1, 55. [Google Scholar] [CrossRef]
- Liang, L.; Yang, W.; Guan, X.; Li, J.; Xu, Z.; Wu, J.; Huang, Y.; Zhang, X. Kinetics and mechanisms of pH-dependent selenite removal by zero valent iron. Water Res. 2013, 47, 5846–5855. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.M.; Guallar, E. Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ. Health Perspect. 2009, 117, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Bano, I.; Zare, H. A comprehensive review on selenium and its effects on human health and distribution in middle eastern countries. Biol. Trace Elem. Research. 2022, 200, 971–987. [Google Scholar] [CrossRef]
- Li, T.; Xu, H.; Zhang, Y.; Zhang, H.; Hu, X.; Sun, Y.; Gu, X.; Luo, J.; Zhou, B.; Gao, B. Treatment technologies for selenium contaminated water: A critical review. Environ. Pollut. 2022, 299, 118858. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A.J.C.O.I.E. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Ezugbe, O.E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ekwonna, T.; Akindeju, O.; Amos, B.; Lin, Z.Q. Selenium Removal from Wastewater by Microbial Transformation and Volatilization. In Mixed Cultures in Industrial Bioprocesses; Springer Nature: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.C.; Guan, Y. Efficient removal of selenate in water by cationic poly (allyltrimethylammonium) grafted chitosan and biochar composite. Environ. Res. 2021, 194, 110667. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Visht, S.; Sharma, P.; Kumar, N. Formulation, characterization and application on nanoparticle: A review. Rev. Pharm. Sin. 2011, 2, 17–26. [Google Scholar]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of NPs: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001–013026. [Google Scholar] [CrossRef]
- Prabhakar, R.; Samadder, S.R. Low cost and easy synthesis of aluminum oxide nanoparticles for arsenite removal from groundwater: A complete batch study. J. Mol. Liq. 2018, 250, 192–201. [Google Scholar] [CrossRef]
- Kidgell, J.T.; de Nys, R.; Hu, Y.; Paul, N.A.; Roberts, D.A. Bioremediation of a complex industrial effluent by biosorbents derived from freshwater macroalgae. PLoS ONE 2014, 9, e94706. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Bradu, C.; Boussouga, Y.A.; Aliaskari, M.; Schäfer, A.I.; Das, S.; Wilson, L.D.; Ike, M.; Inoue, D.; et al. Methods for selenium removal from contaminated waters: A review. Environ. Chem. Lett. 2022, 20, 2019–2041. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Mahdy, A.M.; Salama, K.A. Green synthesis of nanoparticles by milling residues of water treatment. Environ. Chem. Lett. 2015, 13, 333–339. [Google Scholar] [CrossRef]
- Mayara, A.R.C.; Danielle, G.; Wander, G.B.; Luciana, C.O. A systematic literature review on adsorption of potentially toxic elements from aquatic systems by sugarcane and corn residues. Adsorption 2025, 31, 80. [Google Scholar] [CrossRef]
- CoStat Software Program. For the Design and Analysis of Agronomic Research Experiments; CoStat Software Program: Berkeley, CA, USA, 1986; pp. 3–30. [Google Scholar]
- Binupriya, A.R.; Sathishkumar, M.; Jung, S.H.; Song, S.H.; Yun, S.I. A novel method in utilization of bokbunja seed wastes from wineries in liquid-phase sequestration of reactive blue 4. Int. J. Environ. Res. 2009, 3, 1–12. [Google Scholar]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified adsorbents for removal of heavy metals from aqueous environment: A review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Priya, V.N.; Rajkumar, M.; Rajendran, V.; Mobika, J.S.L.; Veena, B.; Ahila, P. Sustainable selenium ions adsorption of cyclodextrin and cellulose functionalized layered double hydroxide/reduced graphene oxide nanocomposites. J. Water Process Eng. 2025, 69, 106580. [Google Scholar] [CrossRef]
- Badr, N.B.; Al-Qahtani, K.M.; Mahmoud, A.E.D. Factorial experimental design for optimizing selenium sorption on Cyperus laevigatus biomass and green-synthesized nano- silver. Alex. Eng. J. 2020, 59, 5219–5229. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, N.; Yang, L.; Lin, Q. Sorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solution. J. Hazard. Mater. 2009, 170, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Scheckel, K.G.; Barbarick, K.A. Selenium adsorption to aluminum-based water treatment residuals. J. Colloid Interface Sci. 2009, 338, 48–55. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, J.; Zeng, H.; Liu, Q. Polyamine-modified magnetic graphene oxide nanocomposite for enhanced selenium removal. Sep. Purif. Technol. 2017, 183, 249–257. [Google Scholar] [CrossRef]
- Benis, K.Z.; Damuchali, A.M.; Soltan, J.; McPhedran, K.N. Treatment of aqueous arsenic–A review of biochar modification methods. Sci. Total Environ. 2020, 739, 139750. [Google Scholar] [CrossRef]
- Benis, K.Z.; Damuchali, A.M.; McPhedran, K.N.; Soltan, J. Treatment of aqueous arsenic—A review of biosorbent preparation methods. J. Environ. Manag. 2020, 273, 111126. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Wang, S.; Wang, Y.; Song, H.; Lv, S.; Li, C. Adsorption performance and mechanism of antibiotics from aqueous solutions on porous boron nitride–carbon nanosheets. Environ. Sci. Water Res. Technol. 2020, 6, 1568–1575. [Google Scholar] [CrossRef]
- Mahdy, A.M.; Elkhatib, E.A.; Fathi, N.O.; Lin, Z.Q. Effects of co-application of biosolids and drinking water treatment residuals (DWTRs) on corn growth and bioavailable phosphorus and aluminum in alkaline soils in Egypt. J. Environ. Qual. 2009, 38, 1501–1510. [Google Scholar] [CrossRef]
- Bandara, P.C.; Perez, J.V.D.; Nadres, E.T.; Nannapaneni, R.G.; Krakowiak, K.J.; Rodrigues, D.F. Graphene ox-ide nanocomposite hydrogel beads for removal of selenium in contaminated water. ACS Appl. Polym. Mater. 2019, 1, 2668–2679. [Google Scholar] [CrossRef]
- Urbánová, L.; Bujdoš, M.; Matulová, M.; Miglierini, M.B.; Vyhnáleková, S.; Orovčík, Ľ.; Urík, M. Investigating the sorption behavior of selenite on commercial partially oxidized magnetite nanopowder under aerobic conditions: Characterization and mechanisms. Sep. Purif. Technol. 2024, 348, 127688. [Google Scholar] [CrossRef]
- Nettem, K.; Almusallam, A.S. Equilibrium, kinetic, and thermodynamic studies on the biosorption of selenium (IV) ions onto Ganoderma lucidum biomass. Sep. Sci. Technol. 2013, 48, 2293–2301. [Google Scholar] [CrossRef]
- Khakpour, H.; Younesi, H.; Mohammadhosseini, M. Two-stage biosorption of selenium from aqueous solution using dried biomass of the baker’s yeast Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2014, 2, 532–542. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A. Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chem. Eng. J. 2010, 158, 200–206. [Google Scholar] [CrossRef]
- El-Shafey, E.I. Removal of Se (IV) from aqueous solution using sulphuric acid-treated peanut shell. J. Environ. Manag. 2007, 84, 620–627. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, E.I. Sorption of Cd (II) and Se (IV) from aqueous solution using modified rice husk. J. Hazard. Mater. 2007, 147, 546–555. [Google Scholar] [CrossRef]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215, 522–532. [Google Scholar] [CrossRef]
- Luo, S.; Lu, T.; Peng, L.; Shao, J.; Zeng, Q.; Gu, J.D. Synthesis of nanoscale zero-valent iron immobilized in alginate microcapsules for removal of Pb (II) from aqueous solution. J. Mater. Chem. A 2014, 2, 15463–15472. [Google Scholar] [CrossRef]
- Manoko, M.C.; Chirwa, E.M.; Makgopa, K. Non-demineralized paper waste sludge derived magnetic biochar as sorbs for removal of methylene blue, phosphorus, and selenate in wastewater. Clean. Chem. Eng. 2022, 3, 100048. [Google Scholar] [CrossRef]
- Dev, S.; Khamkhash, A.; Ghosh, T.; Aggarwal, S. Adsorptive removal of Se (IV) by Citrus peels: Effect of ad-sorbent entrapment in calcium alginate beads. ACS Omega 2020, 5, 17215–17222. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, C.; Liang, J.; Tong, M. Efficient adsorption of selenium (IV) from water by hematite modified magnetic nanoparticles. Chemosphere 2018, 193, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, M.; Abbasloo, A.; Bivar, F. Speciation and removal of selenium (IV, VI) from water and wastewaters based on dried activated sludge before determination by flame atomic absorption spectrometry. Anal. Methods Environ. Chem. J. 2021, 4, 36–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Amrhein, C.; Frankenberger, W.T., Jr. Removal of selenate from water by zerovalent iron. J. Environ. Qual. 2005, 34, 487–495. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A.J.F.C. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Li, N.; Gao, Z.; Luo, D.; Tang, X.; Chen, D.; Hu, Y. Selenium level in the environment and the population of Zhoukoudian area, Beijing, China. Sci. Total Environ. 2007, 381, 105–111. [Google Scholar] [CrossRef]
- Farinella, N.V.; Matos, G.D.; Arruda, M.A.Z. Grape bagasse as a potential biosorbent of metals in effluent treatments. Bioresour. Technol. 2007, 98, 1940–1946. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Xu, Q.; Siracusa, G.; Di Gregorio, S.; Yuan, Q.C. COD removal from biologically stabilized landfill leachate using advanced oxidation processes (AOPs). Process Saf. Environ. Prot. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Haynes, R.J. A Comparison of water treatment sludge and red mud as adsorbents of As and Se in aqueous solution and their capacity for desorption and regeneration. Water Air Soil Pollut. 2012, 223, 5563–5573. [Google Scholar] [CrossRef]
- Abdelhadi, S.O.; Dosoretz, C.G.; Rytwo, G.; Gerchman, Y.; Azaizeh, H. Production of biochar from olive mill solid waste for heavy metal removal. Bioresour. Technol. 2017, 244, 759–767. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Christensen, T.H. Cadmium soil sorption at low concentrations: VIII. correlation with soil parameters. Water Air Soil Pollut. 1989, 44, 71–82. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Hern, J.L. Kinetics of potassium desorption from Appalachian soils. Soil Sci. 1988, 145, 11–19. [Google Scholar] [CrossRef]
- Kuo, S.; Lotse, E.G. Kinetics of phosphate adsorption and desorption by hematite and gibbsite1. Soil Sci. 1973, 116, 400–406. [Google Scholar] [CrossRef]
- Saha, P.D.; Chowdhury, S.; Mondal, M.; Sinha, K. Biosorption of Direct Red 28 (Congo Red) from Aqueous Solutions by Eggshells: Batch and Column Studies. Sep. Sci. Technol. 2012, 47, 112–123. [Google Scholar] [CrossRef]
- He, B.; Zhang, W.; Diao, Y.; Sun, S.; Zhang, Y.; Zhao, W.; Wen, F.; Yang, G. Mechanistic study of the adsorption capabilities of heavy metals on the surface of ferrihydrite: Batch sorption, modeling, and density functional theory. RSC Adv. 2025, 15, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, M.; Masoomi, S.R.; Magala, M.U.; Fathollahi-Fard, A.M.; Ghazikhani, A.; Behzadian, K. The application of artificial intelligence (AI) in adsorption process of heavy metals: A systematic review. Environ. Ind. Lett. 2024, 2, 57–78. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).