Pasture Floristic Composition as an Indicator of Soil pH Correction and Sheep Stocking Rate in Montado Ecosystem
Abstract
1. Introduction
2. Materials and Methods
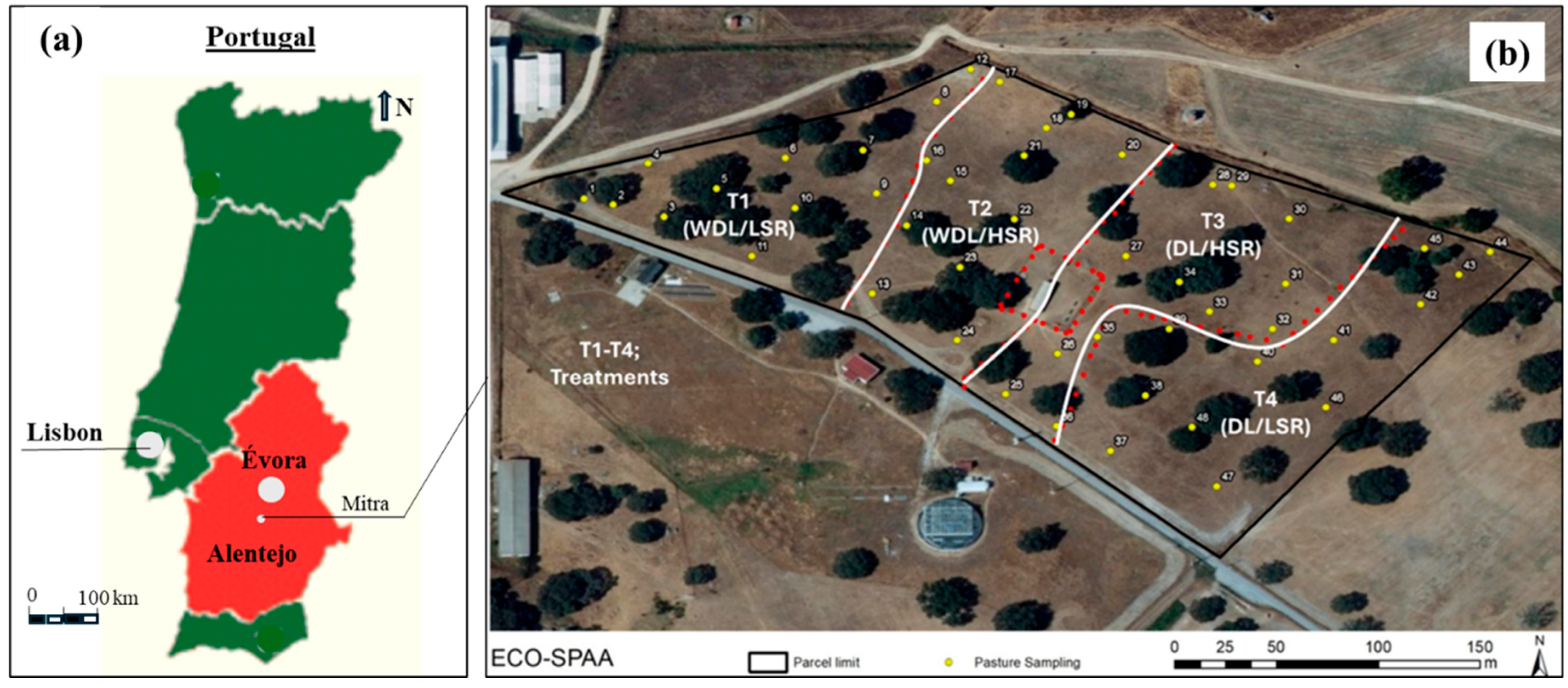
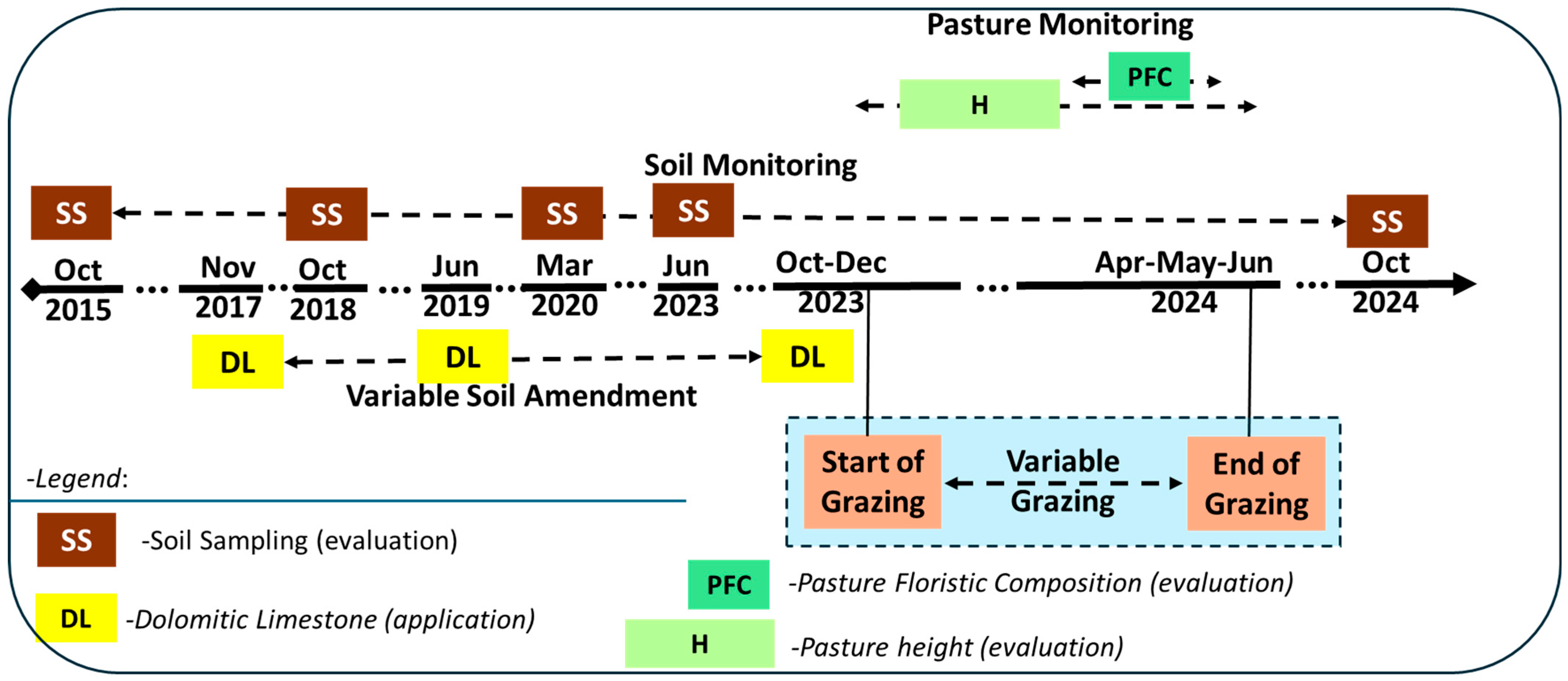
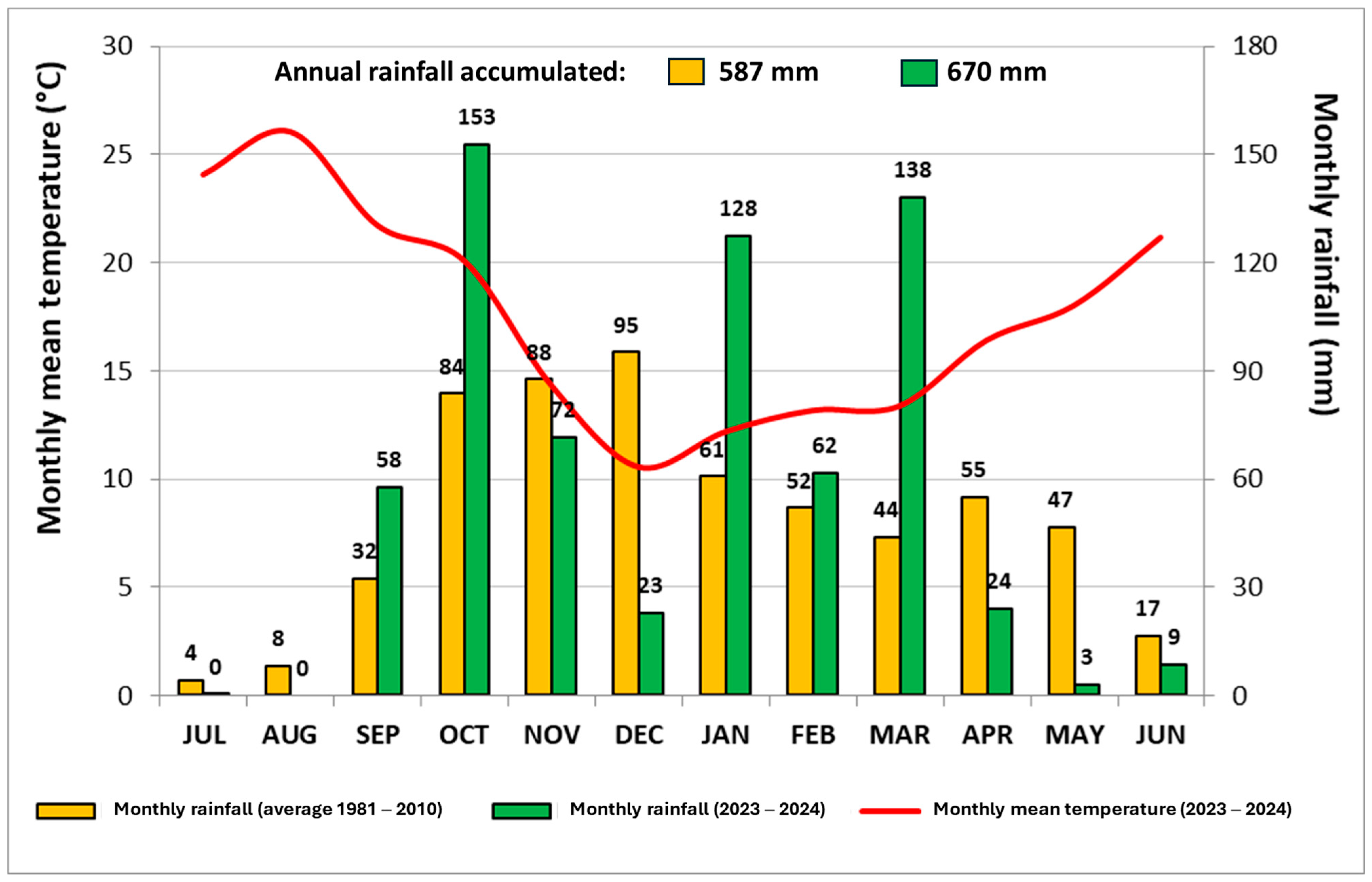
2.1. Study Area, Treatments, and Chronological Approach
- T1 (WDL/LSR)—Without dolomitic limestone; Low stocking rate (7 sheep/ha);
- T2 (WDL/HSR)—Without dolomitic limestone; High stocking rate (18 sheep/ha);
- T3 (DL/HSR)—With dolomitic limestone; High stocking rate;
- T4 (DL/LSR)—With dolomitic limestone; Low stocking rate.
2.2. Soil Monitoring
2.3. Pasture Monitoring
- (i)
- Composition—coverage of species and families (%);
- (ii)
- Vegetation structural parameters—total cover (%), green cover (%), bare soil (%) and tree leaf litter (%);
- (iii)
- Floristic community metrics—species and family richness (number) and diversity (Shannon–Wiener index).
2.4. Data Processing and Analysis
3. Results
3.1. Soil Amendment
3.2. Pasture Structural Parameters
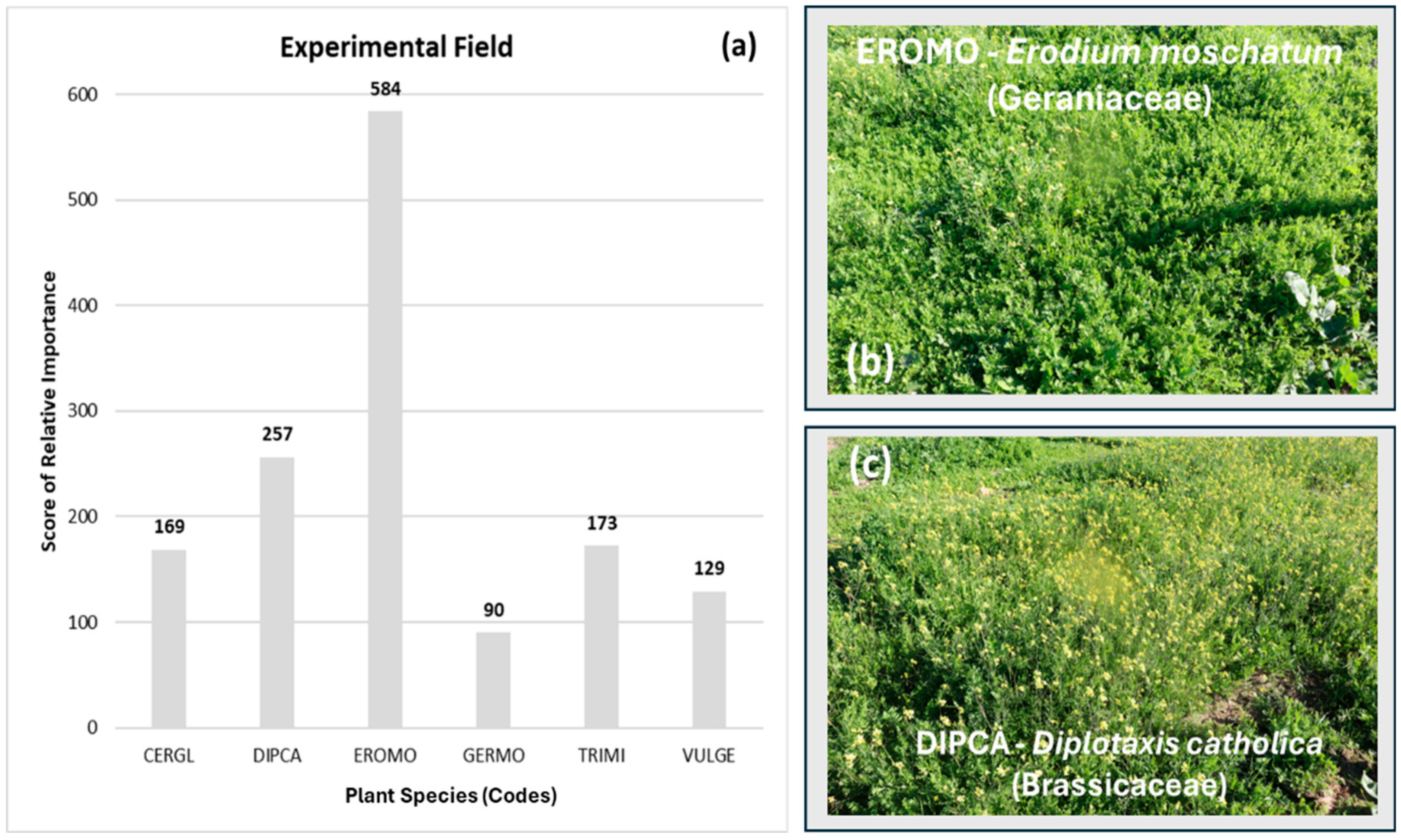
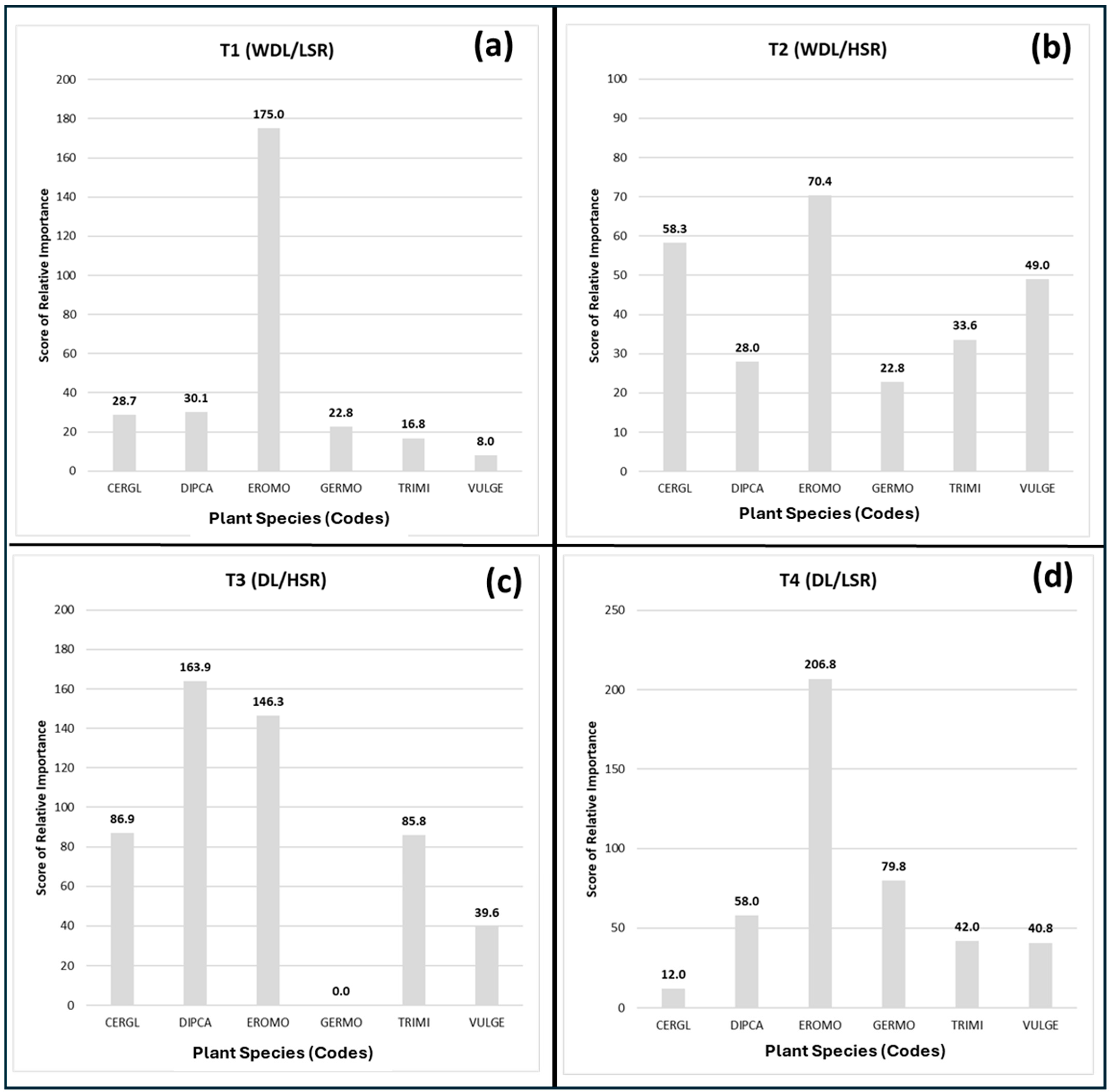
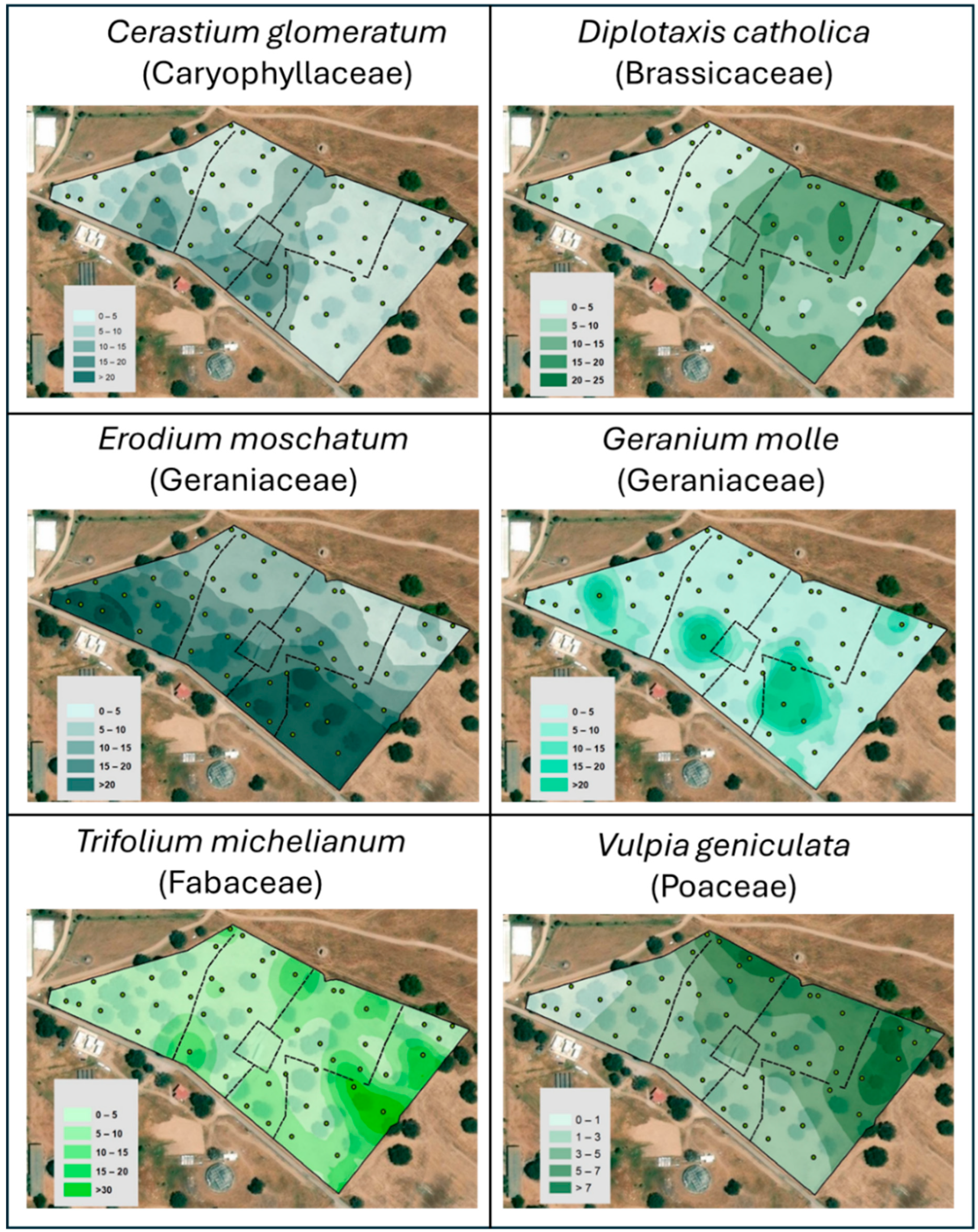
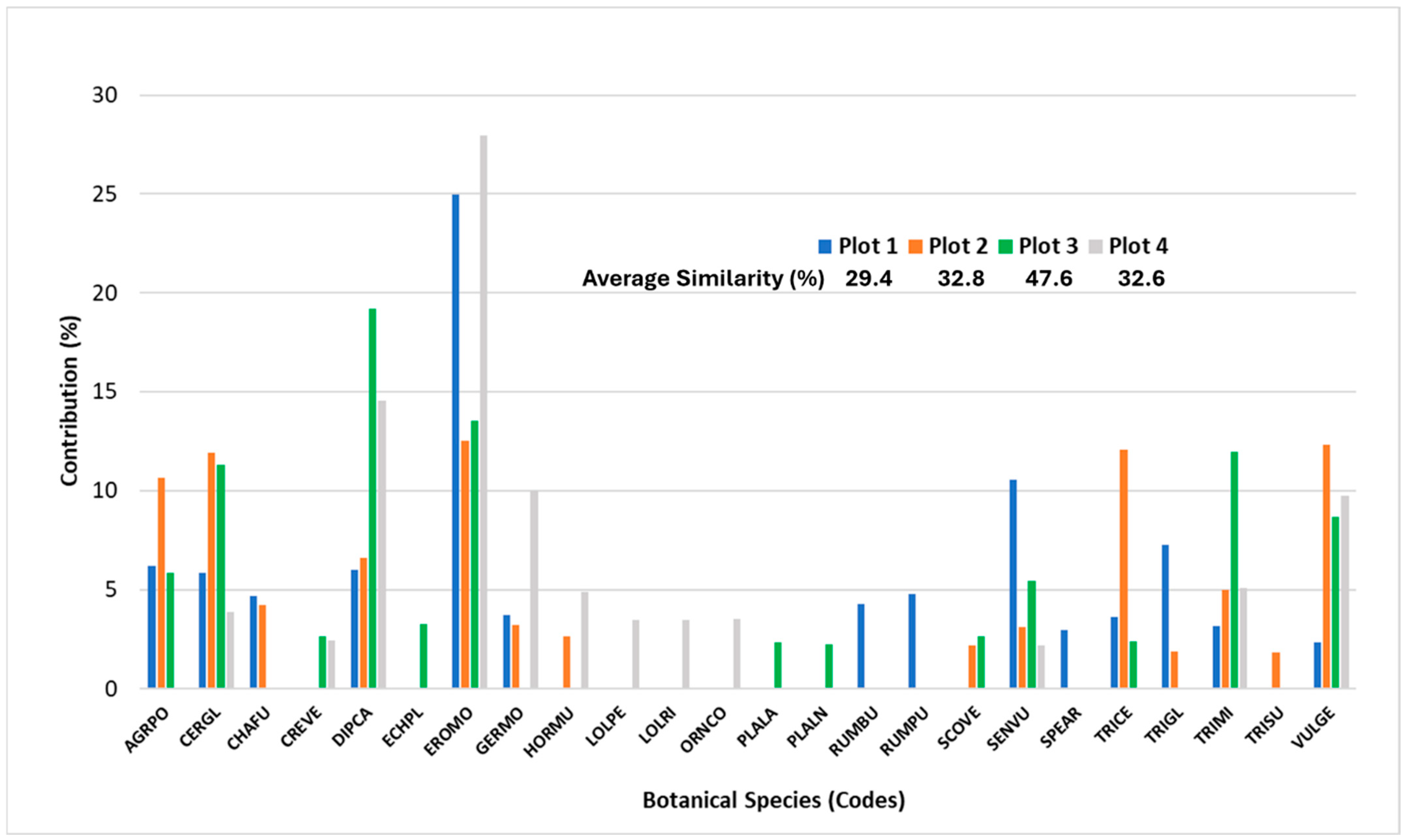
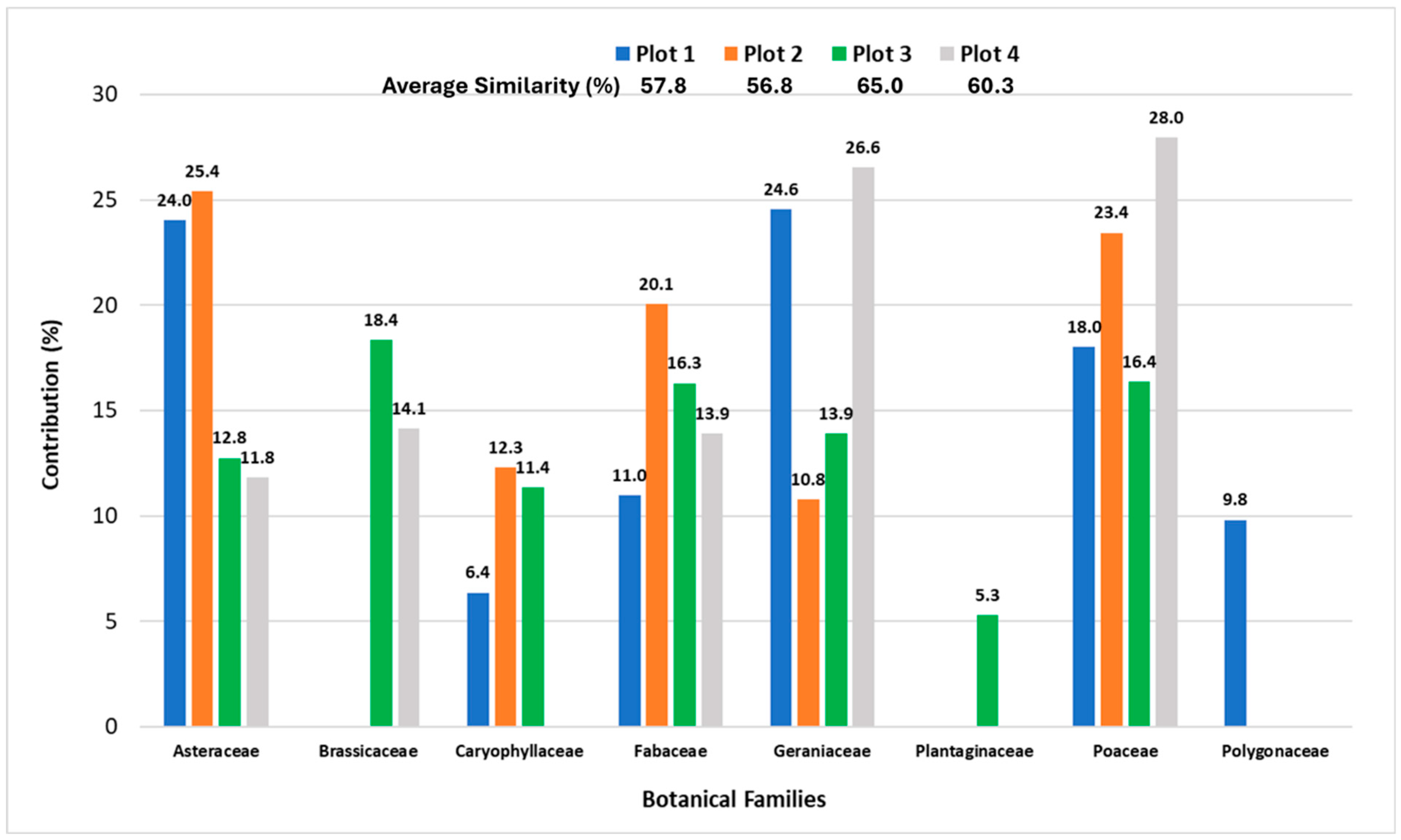
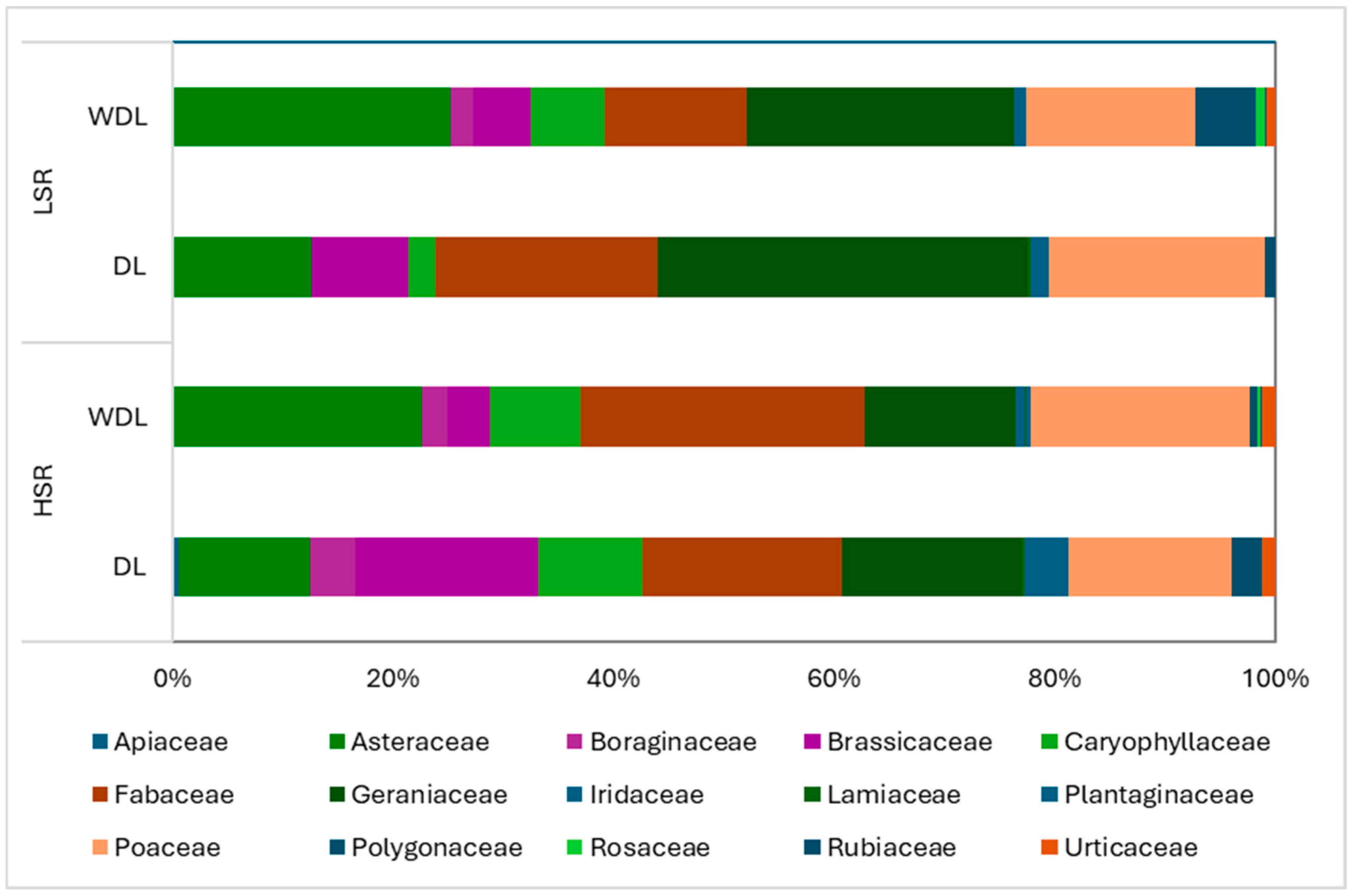
3.3. Pasture Floristic Composition and Diversity
3.4. Changes in Pasture Plant Community Driven by Lime Application and Stocking Rate
4. Discussion
4.1. Changes in Pasture Plant Community Driven by Lime Application and Stocking Rate
- (i)
- Spergula arvensis as an indicator of untreated soils (T1 + T2) and Plantago lagopus as an indicator of treated soils (T3 + T4);
- (ii)
- Scorpiurus vermiculatus as an indicator of high stocking rate (T2 + T3) and Lolium perenne as an indicator of low stocking rate (T1 + T4);
- (iii)
- Agrotis pourreti and Trifolium glomeratum as indicator species that respond negatively to treated soils and low stocking rate (T1 + T2 + T3);
- (iv)
- Vulpia geniculata an indicator species that responds negatively to untreated soils and low stocking rate (T2 + T3 + T4).
4.2. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Specie | Code | Family | T1 | T2 | T3 | T4 | IV | Stat. | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Agrostis pourretti | AGRPO | Poaceae | 1 | 1 | 1 | 0 | 12 | 0.788 | 0.029 |
| Anagallis arvensis | ANAAR | Primulaceae | 0 | 1 | 0 | 0 | 2 | 0.289 | 1.000 |
| Anchusa azurea | ANCAZ | Boraginaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Andryala integrifolia | ANDIN | Asteraceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Aphanes arvensis | APHAR | Rosaceae | 1 | 0 | 0 | 0 | 1 | 0.369 | 0.241 |
| Arum italicum | ARUIT | Araceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Astragalus pelecinus | ASTPE | Fabaceae | 0 | 0 | 0 | 1 | 3 | 0.289 | 1.000 |
| Avena barbata | AVEBA | Poaceae | 0 | 1 | 0 | 1 | 8 | 0.477 | 0.322 |
| Bromus diandrus | BRODI | Poaceae | 0 | 0 | 0 | 1 | 3 | 0.289 | 1.000 |
| Bromus hordeaceus | BROHO | Poaceae | 0 | 1 | 0 | 1 | 8 | 0.335 | 0.604 |
| Bromus rubens | BRORU | Poaceae | 0 | 0 | 1 | 1 | 10 | 0.289 | 1.000 |
| Calendula arvensis | CALAR | Asteraceae | 1 | 0 | 0 | 0 | 1 | 0.452 | 0.091 |
| Callitriche stagnalis | CALST | Plantaginaceae | 0 | 0 | 1 | 0 | 4 | 0.289 | 1.000 |
| Carduus tenuiflorus | CARTE | Asteraceae | 1 | 1 | 1 | 1 | 15 | 0.323 | NA |
| Cerastium glomeratum | CERGL | Caryophyllaceae | 1 | 1 | 1 | 1 | 15 | 0.854 | NA |
| Chamaemelum fuscatum | CHAFU | Asteraceae | 1 | 1 | 1 | 0 | 12 | 0.642 | 0.193 |
| Chamaemelum mixtum | CHAMI | Asteraceae | 0 | 1 | 0 | 0 | 2 | 0.426 | 0.138 |
| Crepis capillaris | CRECA | Asteraceae | 1 | 0 | 0 | 1 | 6 | 0.448 | 0.430 |
| Crepis vesicaria | CREVE | Asteraceae | 1 | 1 | 1 | 1 | 15 | 0.540 | NA |
| Cynodon dactylon | CYNDA | Poaceae | 0 | 0 | 1 | 0 | 4 | 0.289 | 1.000 |
| Diplotaxis catholica | DIPCA | Brassicaceae | 1 | 1 | 1 | 1 | 15 | 0.866 | NA |
| Echium plantagineum | ECHPL | Boraginaceae | 1 | 1 | 1 | 0 | 12 | 0.553 | 0.144 |
| Erodium moschatum | EROMO | Geraniaceae | 1 | 1 | 1 | 1 | 15 | 0.913 | NA |
| Galium aparine | GALAP | Rubiaceae | 0 | 1 | 0 | 0 | 2 | 0.289 | 1.000 |
| Geranium dissectum | GERDI | Geraniaceae | 1 | 0 | 0 | 0 | 1 | 0.408 | 0.237 |
| Geranium molle | GERMO | Geraniaceae | 1 | 1 | 1 | 1 | 15 | 0.722 | NA |
| Gynandriris sisyrinchium | GYNSI | Iridaceae | 1 | 1 | 0 | 0 | 5 | 0.500 | 0.079 |
| Hedypnois cretica | HEDCR | Asteraceae | 1 | 1 | 0 | 1 | 11 | 0.427 | 0.660 |
| Holcus annuus | HOLAN | Poaceae | 0 | 0 | 0 | 1 | 3 | 0.289 | 1.000 |
| Hordeum murinum | HORMU | Poaceae | 0 | 1 | 1 | 1 | 14 | 0.614 | 0.397 |
| Hypochaeris glabra | HYPGL | Asteraceae | 1 | 1 | 0 | 0 | 5 | 0.337 | 0.594 |
| Hypochaeris radicata | HYPRA | Asteraceae | 0 | 1 | 0 | 0 | 2 | 0.289 | 1.000 |
| Lamarckia aurea | LAMAU | Poaceae | 0 | 1 | 0 | 1 | 8 | 0.289 | 1.000 |
| Lathyrus angulatus | LATAN | Fabaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Leontodon taraxacoides | LEOTA | Asteraceae | 1 | 1 | 0 | 0 | 5 | 0.456 | 0.503 |
| Logfia gallica | LOGGA | Asteraceae | 1 | 1 | 0 | 0 | 5 | 0.289 | 1.000 |
| Lolium perenne | LOLPE | Poaceae | 1 | 0 | 0 | 1 | 6 | 0.612 | 0.013 |
| Lolium rigidum | LOLRI | Poaceae | 0 | 0 | 0 | 1 | 3 | 0.577 | 0.008 |
| Medicago arabica | MEDAR | Fabaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Medicago polymorpha | MEDPO | Fabaceae | 0 | 0 | 0 | 1 | 3 | 0.500 | 0.049 |
| Mentha pulegium | MENPU | Lamiaceae | 0 | 0 | 0 | 1 | 3 | 0.289 | 1.000 |
| Ornithopus compressus | ORNCO | Fabaceae | 0 | 0 | 1 | 1 | 10 | 0.573 | 0.215 |
| Ornithopus pinnatus | ORNPI | Fabaceae | 1 | 1 | 0 | 1 | 11 | 0.333 | 0.896 |
| Phalaris coerulescens | PHACO | Poaceae | 0 | 0 | 1 | 1 | 10 | 0.289 | 1.000 |
| Plantago coronopus | PLACO | Plantaginaceae | 1 | 1 | 0 | 1 | 11 | 0.289 | 1.000 |
| Plantago lagopus | PLALA | Plantaginaceae | 0 | 0 | 1 | 1 | 10 | 0.630 | 0.018 |
| Plantago lanceolata | PLALN | Plantaginaceae | 0 | 0 | 1 | 0 | 4 | 0.639 | 0.004 |
| Poa annua | POAAN | Poaceae | 1 | 0 | 1 | 1 | 13 | 0.373 | 0.731 |
| Polycarpon tetraphyllum | POLTE | Caryophyllaceae | 0 | 0 | 1 | 0 | 4 | 0.289 | 1.000 |
| Polypogon maritimus | POLMA | Poaceae | 0 | 0 | 0 | 1 | 3 | 0.289 | 1.000 |
| Pulicaria paludosa | PULPA | Asteraceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Ranunculus bullatus | RANBU | Ranunculaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Raphanus raphanistrum | RAPRA | Brassicaceae | 1 | 0 | 0 | 1 | 6 | 0.442 | 0.214 |
| Rumex bucephalophorus | RUMBU | Polygonaceae | 1 | 1 | 1 | 1 | 15 | 0.645 | NA |
| Rumex pulcher | RUMPU | Polygonaceae | 1 | 0 | 0 | 0 | 1 | 0.605 | 0.005 |
| Scirpoides holoschoenus | SCIHO | Cyperaceae | 0 | 0 | 1 | 0 | 4 | 0.289 | 1.000 |
| Scorpiurus muricatus | SCOMU | Fabaceae | 0 | 1 | 1 | 0 | 9 | 0.456 | 0.143 |
| Scorpiurus vermiculatus | SCOVE | Fabaceae | 0 | 1 | 1 | 0 | 9 | 0.632 | 0.016 |
| Senecio vulgaris | SENVU | Asteraceae | 1 | 1 | 1 | 1 | 15 | 0.791 | NA |
| Sherardia arvensis | SHEAR | Rubiaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Silene gallica | SILGA | Caryophyllaceae | 1 | 1 | 1 | 0 | 12 | 0.408 | 0.583 |
| Sonchus oleraceus | SONOL | Asteraceae | 1 | 1 | 0 | 0 | 5 | 0.289 | 1.000 |
| Spergula arvensis | SPEAR | Caryophyllaceae | 1 | 1 | 0 | 0 | 5 | 0.605 | 0.042 |
| Stachys arvensis | STAAR | Lamiaceae | 0 | 1 | 1 | 0 | 9 | 0.354 | 0.595 |
| Stellaria media | STEME | Caryophyllaceae | 0 | 1 | 0 | 0 | 2 | 0.289 | 1.000 |
| Tolpis barbata | TOLBA | Asteraceae | 1 | 0 | 0 | 1 | 6 | 0.289 | 1.000 |
| Torilis arvensis | TORAR | Apiaceae | 0 | 1 | 1 | 0 | 9 | 0.289 | 1.000 |
| Trifolium campestre | TRICA | Fabaceae | 1 | 1 | 0 | 0 | 5 | 0.456 | 0.144 |
| Trifolium cernuum | TRICE | Fabaceae | 1 | 1 | 1 | 1 | 15 | 0.736 | NA |
| Trifolium glomeratum | TRIGL | Fabaceae | 1 | 1 | 1 | 0 | 12 | 0.687 | 0.014 |
| Trifolium michelianum | TRIMI | Fabaceae | 1 | 1 | 1 | 1 | 15 | 0.777 | NA |
| Trifolium resupinatum | TRIRE | Fabaceae | 0 | 0 | 0 | 1 | 3 | 0.408 | 0.229 |
| Trifolium subterraneum | TRISU | Fabaceae | 0 | 1 | 0 | 0 | 2 | 0.500 | 0.051 |
| Urospermum picroides | UROPI | Asteraceae | 0 | 1 | 0 | 0 | 2 | 0.289 | 1.000 |
| Urtica membranacea | URTME | Urticaceae | 0 | 0 | 1 | 0 | 4 | 0.289 | 1.000 |
| Urtica urens | URTUR | Urticaceae | 1 | 1 | 0 | 0 | 5 | 0.377 | 0.432 |
| Veronica arvensis | VERAR | Plantaginaceae | 0 | 0 | 1 | 0 | 4 | 0.408 | 0.231 |
| Vicia lutea | VICLU | Fabaceae | 1 | 0 | 0 | 0 | 1 | 0.289 | 1.000 |
| Vulpia geniculata | VULGE | Poaceae | 0 | 1 | 1 | 1 | 14 | 0.849 | 0.015 |
References
- Reinermann, S.; Asam, S.; Kuenzer, C. Remote sensing of grassland production and management—A review. Remote Sens. 2020, 12, 1949. [Google Scholar] [CrossRef]
- Serrano, J.; Roma, L.; Shahidian, S.; Belo, A.D.F.; Carreira, E.; Paniagua, L.L.; Moral, F.; Paixão, L.; Marques da Silva, J. A technological approach to support extensive livestock management in the Portuguese Montado ecosystem. Agronomy 2022, 12, 1212. [Google Scholar] [CrossRef]
- Wachendorf, M.; Fricke, T.; Möckel, T. Remote sensing as a tool to assess botanical composition, structure, quantity and quality of temperate grasslands. Grass Forage Sci. 2017, 73, 1–14. [Google Scholar] [CrossRef]
- Salmerón-Sánchez, E.; Mendoza-Fernández, A.J.; Lorite, J.; Mota, J.F.; Peñas, J. Plant conservation in Mediterranean-type ecosystems. Mediterr. Bot. 2021, 42, e71333. [Google Scholar] [CrossRef]
- Lambert, M.; Clark, D.; Litherland, A. Advances in pasture management for animal productivity and health. N. Z. Vet. J. 2004, 52, 311–319. [Google Scholar] [CrossRef]
- Pinto-Correia, T.; Guiomar, N.; Ferraz-de-Oliveira, M.I.; Sales Baptista, E.; Rabaça, J.; Godinho, C.; Ribeiro, N.; Sá Sousa, P.; Santos, P.; Silva, C.S.; et al. Progress in identifying High Nature Value montados: Impacts of grazing on hardwood rangeland biodiversity. Rangel. Ecol. Manag. 2018, 71, 612–625. [Google Scholar] [CrossRef]
- Moscatelli, S.; Pesaresi, S.; Wikelski, M.; Tardella, F.M.; Catorci, A.; Quattrini, G. Influence of pasture diversity and NDVI on sheep foraging behavior in Central Italy. Geographies 2025, 5, 26. [Google Scholar] [CrossRef]
- Bretas, I.; Dubeux, J.; Priscila, J.; Oduor, K.; Queiroz, L.; Valente, D.; Chizzotti, F. Precision livestock farming applied to grazingland monitoring and management—A review. Agron. J. 2024, 116, 1164–1186. [Google Scholar] [CrossRef]
- Guimarães, M.; Pinto-Correia, T.; Freitas, M.; Ferraz-de-Oliveira, I.; Sales-Baptista, E.; Veiga, J.; Marques, T.; Pinto Cruz, C.; Godinho, C.; Belo, A. Farming for nature in the Montado: The application of ecosystem services in a results-based model. Ecosyst. Serv. 2023, 61, 101524. [Google Scholar] [CrossRef]
- Efe Serrano, J. Pastures in Alentejo: Technical Basis for Characterization, Grazing and Improvement; Universidade de Évora—ICAM, Ed.; Gráfica Eborense: Évora, Portugal, 2006; pp. 165–178. (In Portuguese) [Google Scholar]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisoils derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Cienc. Agrar. 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J.; Moral, F.; Carvajal-Ramirez, F.; Carreira, E.; Pereira, A.; Carvalho, M. Evaluation of the effect of dolomitic lime application on pastures—Case study in the Montado Mediterranean ecosystem. Sustainability 2020, 12, 3758. [Google Scholar] [CrossRef]
- Costa, J.P.; Mesquita, M.L.R. Floristic and phytosociology of weeds in pastures in Maranhão State, Northeast Brazil. Rev. Cien. Agron. 2016, 47, 414–442. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Machado, E.; Paniagua, L.L.; Carreira, E.; Moral, F.; Pereira, A.; de Carvalho, M. Floristic composition: Dynamic biodiversity indicator of tree canopy effect on dryland and improved Mediterranean pastures. Agriculture 2021, 11, 1128. [Google Scholar] [CrossRef]
- Ara, I.; Harrison, M.T.; Whitehead, J.; Waldner, F.; Bridle, K.; Gilfedder, L.; Da Silva, J.M.; Marques, F.; Rawnsley, R. Modelling seasonal pasture growth and botanical composition at the paddock scale with satellite imagery. in silico Plants 2021, 3, diaa013. [Google Scholar] [CrossRef]
- Fenetahun, Y.; You, Y.; Fentahun, T.; Xinwen, X.; Yong-dong, W. Effects of grazing intensity on forage nutritive value of dominant grass species in Borana rangelands of Southern Ethiopia. PeerJ 2021, 9, e12204. [Google Scholar] [CrossRef]
- Shoko, C.; Mutanga, O.; Dube, T. Optimal season for discriminating C3 and C4 grass functional types using multi-date Sentinel 2 data. GISCI Remote Sens. 2020, 57, 127–139. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Costa, F.; Carreira, E.; Pereira, A.; Carvalho, M. Can soil pH correction reduce the animal Supplementation needs in the critical autumn period in Mediterranean Montado ecosystem? Agronomy 2021, 11, 514. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Resources Report 106; Food and Agriculture Organization (FAO): Rome, Italy, 2015; 188p. [Google Scholar]
- AOAC. Official Method of Analysis of AOAC International, 18th ed.; AOAC International: Arlington, TX, USA, 2005. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grudlage für die Beurteilung des Nahrstoffzunstandes der Boden. II: Chemische extraktion methoden zur phosphor- und kalium-bestimmung. K. Lantbrhogsk. Annlr. 1960, 26, 199–216. (In German) [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Castroviejo, S. Flora Iberica; Real Jardín Botánico, CSIC: Madrid, Spain, 1986–2021; Volume I–XXI. [Google Scholar]
- Bear, D.A.; Russell, J.R.; Tufekcioglu, M.; Isenhart, T.M.; Morrical, D.G.; Kovar, J.L. Stocking rate and riparian vegetation effects on physical characteristics of riparian zones of Midwestern Pastures. Rangel. Ecol. Manag. 2012, 65, 119–128. [Google Scholar] [CrossRef][Green Version]
- Xiao, X.; Zhang, T.; Angerer, J.P.; Hou, F. Grazing seasons and stocking rates affects the relationship between herbage traits of Alpine meadow and grazing behaviors of Tibetan sheep in the Qinghai–Tibetan plateau. Animals 2020, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Avdiu, B.; Aliu, S.; Fetahu, S.; Zeka, D.; Rusinovci, I. The floristic composition of the natural pastures in massive of Novobërba. Agric. For. 2018, 64, 235–241. [Google Scholar] [CrossRef]
- Mendes, P.; Meireles, C.; Vila-Viçosa, C.; Musarella, C.; Pinto-Gomes, C. Best management practices to face degraded territories occupied by Cistus ladanifer shrublands—Portugal case study. Plant Biosyst. 2015, 149, 494–502. [Google Scholar] [CrossRef]
- Graß, R.; Malec, S.; Wachendorf, M. Biomass performance and competition effects in an established temperate agroforestry system of willow and grassland—Results of the 2nd rotation. Agronomy 2020, 10, 1819. [Google Scholar] [CrossRef]
- Paço, A.; da-Silva, J.R.; Torres, D.P.; Glick, B.R.; Brígido, C. Exogenous ACC Deaminase is key to improving the performance of pasture legume-rhizobial symbioses in the presence of a high manganese concentration. Plants 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P. Beals smoothing revisited. Oecologia 2008, 156, 657–669. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Halim, N.; Abdullah, R.; Karsani, S.; Osman, N.; Panhwar, Q.; Ishak, C. Influence of soil amendments on the growth and yield of rice in acidic soil. Agronomy 2018, 8, 165. [Google Scholar] [CrossRef]
- Li, G.D.; Conyers, M.K.; Helyar, K.R.; Lisle, C.J.; Poile, G.J.; Cullis, B.R. Long-term surface application of lime ameliorates subsurface soil acidity in the mixed farming zone of south-eastern Australia. Geoderma 2019, 338, 236–246. [Google Scholar] [CrossRef]
- Solefack, M.C.M.; Fedoung, E.F.; Temgoua, L.F. Factors determining floristic composition and functional diversity of plant communities of Mount Oku forests, Cameroon. J. Asia Pac. Biodiver. 2018, 11, 284–293. [Google Scholar] [CrossRef]
- Wild, M.; Gauly, M.; Zanon, T.; Isselstein, J.; Komainda, M. Tracking free-ranging sheep to evaluate interrelations between selective grazing, movement patterns and the botanical composition of alpine summer pastures in northern Italy. Pastor. Res. Policy Pract. 2023, 13, 25. [Google Scholar] [CrossRef]
- Porqueddu, C.; Melis, R.A.M.; Franca, A.; Sanna, F.; Hadjigeorgiou, I.; Casasús, I. The role of grasslands in the less favoured areas of Mediterranean Europe. Grassl. Sci. Eur. 2017, 22, 3–22. [Google Scholar]
- De Miguel, J.M.; Acosta-Gallo, B.; Gómez-Sal, A. Understanding Mediterranean pasture dynamics: General tree cover vs. specific effects of individual trees. Rangel. Ecol. Manage. 2013, 66, 216–223. [Google Scholar] [CrossRef]
- Chen, Y.; Guerschman, J.; Shendryk, Y.; Henry, D.; Harrison, M.T. Estimating pasture biomass using Sentinel-2 imagery and machine learning. Remote Sens. 2021, 13, 603. [Google Scholar] [CrossRef]
- Monteiro, A.T.; Alves, P.; Carvalho-Santos, C.; Lucas, R.; Cunha, M.; Marques da Costa, E.; Fava, F. Monitoring plant diversity to support Agri-environmental schemes: Evaluating statistical models informed by satellite and local factors in Southern European Mountain pastoral systems. Diversity 2022, 14, 8. [Google Scholar] [CrossRef]
- Yang, M.; Chen, A.; Zhang, M.; Gu, Q.; Wang, Y.; Guo, J.; Yang, D.; Zhao, Y.; Huang, Q.; Ma, L.; et al. Relationship between plant species diversity and aboveground biomass in alpine grasslands on the Qinghai–Tibet Plateau: Spatial patterns and the factors driving them. Front. Ecol. Evol. 2023, 11, 1138884. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, B.; Xue, J.; Shi, Y.; Zhao, M.; Geng, X.; Yan, Z.; Shen, H.; Fang, J. Mapping forage biomass and quality of the Inner Mongolia grasslands by combining field measurements and Sentinel-2 observations. Remote Sens. 2023, 15, 1973. [Google Scholar] [CrossRef]
- Hayes, S.; Cawkwell, F.; Bacon, K.L.; Wingler, A. Remote sensing of grassland plant biodiversity and functional traits. Ecol. Evol. 2025, 15, e71829. [Google Scholar] [CrossRef]




| Date | Oct 2015 | Oct 2018 | Oct 2018 | Mar 2020 | Mar 2020 | Jun 2023 | Jun 2023 | Oct 2024 | Oct 2024 |
|---|---|---|---|---|---|---|---|---|---|
| Fields | All | 1 and 2 | 3 and 4 | 1 and 2 | 3 and 4 | 1 and 2 | 3 and 4 | 1 and 2 | 3 and 4 |
| pH | 5.4 ± 0.3 | 5.3 ± 0.2 | 5.6 ± 0.2 | 5.6 ± 0.2 | 5.8 ± 0.4 | 5.5 ± 0.2 | 6.0 ± 0.4 | 5.6 ± 0.4 | 6.4 ± 0.4 |
| Mg (mg kg−1) | 95.6 ± 43.7 | 73.3 ± 34.6 | 82.9 ± 32.1 | 71.3 ± 16.8 | 108.1 ± 22.3 | 93.8 ± 25.6 | 106.1 ± 37.5 | 80.2 ± 18.1 | 110.6 ± 23.8 |
| Mn (mg kg−1) | 76.4 ± 44.9 | 66.8 ± 31.4 | 33.6 ± 16.1 | 51.6 ± 24.6 | 32.5 ± 13.6 | 66.1 ± 11.5 | 33.3 ± 36.4 | 48.3 ± 12.5 | 30.5 ± 26.5 |
| Ratio Mg Mn−1 | 1.3 | 1.1 | 2.5 | 1.4 | 3.3 | 1.4 | 3.2 | 1.7 | 3.6 |
| Soil Parameters | T1 (WDL/LSR) | T2 (WDL/HSR) | T3 (DL/HSR) | T4 (DL/LSR) |
|---|---|---|---|---|
| Sand (%) | 79.2 ± 1.6 a | 77.5 ± 2.9 a | 76.0 ± 3.0 a | 79.1 ± 4.0 a |
| Silt (%) | 11.0 ± 1.4 b | 11.3 ± 1.6 b | 13.0 ± 2.5 a | 10.7 ± 2.1 b |
| Clay (%) | 9.8 ± 1.2 a | 11.2 ± 3.6 a | 11.0 ± 1.6 a | 10.1 ± 2.8 a |
| OM (%) | 4.5 ± 1.2 a | 3.5 ± 0.6 b | 3.0 ± 0.4 b | 3.9 ± 1.0 ab |
| pH | 5.5 ± 0.2 b | 5.5 ± 0.2 b | 6.1 ± 0.4 a | 5.9 ± 0.4 a |
| P2O5 (mg kg−1) | 90.7 ± 39.8 a | 50.3 ± 22.1 b | 45.3 ± 27.7 b | 63.8 ± 38.9 ab |
| K2O (mg kg−1) | 89.9 ± 62.1 a | 100.8 ± 65.1 a | 94.8 ± 67.0 a | 129.5 ± 69.2 a |
| Mg (mg kg−1) | 95.8 ± 25.8 a | 90.8 ± 26.9 a | 110.4 ± 29.6 a | 101.7 ± 45.6 a |
| Mn (mg kg−1) | 64.6 ± 32.3 a | 67.5 ± 40.5 a | 39.7 ± 13.4 ab | 26.7 ± 9.5 b |
| CEC (cmol kg−1) | 12.0 ± 8.7 a | 6.6 ± 1.4 b | 5.7 ± 0.8 b | 5.9 ± 1.1 b |
| Sampling Dates | T1 (WDL/LSR) | T2 (WDL/HSR) | T3 (DL/HSR) | T4 (DL/LSR) |
|---|---|---|---|---|
| 5 December 2023 | 9.1 ± 5.1 b | 9.0 ± 4.1 b | 10.4 ± 5.8 ab | 12.9 ± 7.3 a |
| 18 January 2024 | 11.6 ± 7.0 ab | 6.2 ± 3.7 c | 10.4 ± 7.4 b | 16.3 ± 9.5 a |
| 29 February 2024 | 17.8 ± 9.4 ab | 10.9 ± 3.4 c | 16.0 ± 5.6 b | 20.7 ± 10.1 a |
| 19 March 2024 | 19.6 ± 10.9 ab | 11.9 ± 4.7 c | 16.9 ± 6.6 b | 26.2 ± 10.3 a |
| 17 April 2024 | 24.8 ± 11.3 ab | 17.9 ± 7.7 c | 20.8 ± 6.9 bc | 32.1 ± 12.4 a |
| 10 May 2024 | 31.7 ± 14.6 a | 19.1 ± 8.4 b | 19.8 ± 13.7 b | 29.2 ± 14.6 a |
| 11 June 2024 | 29.3 ± 11.7 a | 17.9 ± 10.2 b | 14.4 ± 13.9 b | 33.4 ± 26.5 a |
| Pasture Parameters | T1 (WDL/LSR) | T2 (WDL/HSR) | T3 (DL/HSR) | T4 (DL/LSR) |
|---|---|---|---|---|
| Bare soil | 6.8 ± 5.7 a | 8.0 ± 6.7 a | 9.4 ± 5.8 a | 11.8 ± 4.7 a |
| Total coverage | 92.2 ± 6.9 a | 92.0 ± 6.7 a | 90.6 ± 5.8 a | 88.2 ± 4.7 a |
| Green coverage | 86.8 ± 10.5 a | 88.5 ± 14.1 a | 88.8 ± 8.3 a | 87.8 ± 5.6 a |
| Tree leaf litter | 18.9 ± 31.7 a | 6.4 ± 14.5 b | 3.5 ± 11.5 b | 1.7 ± 2.7 b |
| Treatments | Based on Species | Based on Families | ||||
|---|---|---|---|---|---|---|
| t | p (perm) | n (perms) | t | p (perm) | n (perms) | |
| T1 vs. T2 | 1.2415 | 0.128 ns | 998 | 1.3387 | 0.133 ns | 998 |
| T1 vs. T3 | 1.6164 | 0.010 * | 999 | 1.8927 | 0.009 ** | 996 |
| T1 vs. T4 | 1.4818 | 0.016 * | 997 | 1.5290 | 0.066 ns | 999 |
| T2 vs. T3 | 1.3747 | 0.047 * | 999 | 1.6376 | 0.021 * | 999 |
| T2 vs. T4 | 1.6485 | 0.017 * | 999 | 1.5455 | 0.073 ns | 999 |
| T3 vs. T4 | 1.7458 | 0.002 ** | 999 | 1.6953 | 0.034 * | 997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.; Matono, P.; Carreira, E.; Shahidian, S.; Moral, F.J.; Paniagua, L.L.; Charneca, R.; Pereira, A.; Belo, A. Pasture Floristic Composition as an Indicator of Soil pH Correction and Sheep Stocking Rate in Montado Ecosystem. Environments 2025, 12, 385. https://doi.org/10.3390/environments12100385
Serrano J, Matono P, Carreira E, Shahidian S, Moral FJ, Paniagua LL, Charneca R, Pereira A, Belo A. Pasture Floristic Composition as an Indicator of Soil pH Correction and Sheep Stocking Rate in Montado Ecosystem. Environments. 2025; 12(10):385. https://doi.org/10.3390/environments12100385
Chicago/Turabian StyleSerrano, João, Paula Matono, Emanuel Carreira, Shakib Shahidian, Francisco J. Moral, Luís L. Paniagua, Rui Charneca, Alfredo Pereira, and Anabela Belo. 2025. "Pasture Floristic Composition as an Indicator of Soil pH Correction and Sheep Stocking Rate in Montado Ecosystem" Environments 12, no. 10: 385. https://doi.org/10.3390/environments12100385
APA StyleSerrano, J., Matono, P., Carreira, E., Shahidian, S., Moral, F. J., Paniagua, L. L., Charneca, R., Pereira, A., & Belo, A. (2025). Pasture Floristic Composition as an Indicator of Soil pH Correction and Sheep Stocking Rate in Montado Ecosystem. Environments, 12(10), 385. https://doi.org/10.3390/environments12100385








